Abstract
The metabolism of arachidonic acid and other polyunsaturated fatty acids produces eicosanoids, a family of biologically active lipids that are implicated in homeostasis and in several pathologies that involve inflammation. Inflammatory processes mediated by eicosanoids promote carcinogenesis by exerting direct effects on cancer cells and by affecting the tumor microenvironment. Therefore, understanding how eicosanoids mediate cancer progression may lead to better approaches and chemopreventive strategies for the treatment of cancer. The matricellular protein thrombospondin-1 is involved in processes that profoundly regulate inflammatory pathways that contribute to carcinogenesis and metastatic spread. This review focuses on interactions of thrombospondin-1 and eicosanoids with the purpose of understanding the complex interactions in the microenvironment that promote carcinogenesis and how the microenvironment can be targeted for cancer prevention and to increase curative responses of cancer patients.
Introduction
Eicosanoids are a family of bioactive lipid molecules that include prostaglandins, thromboxanes and lipoxins. Eicosanoids are produced from the enzymatic action of cyclooxygenase, lipoxygenase and p450 pathways. Eicosanoids are not normally stored in cells but rather are synthetized in response to injury and, as they are short-lived, serve as localized signaling molecules. However, dysregulated lipid and arachidonic acid metabolism associated with diseases like cancer can cause chronic inflammation that potentiates tumor growth and metastasis. The mechanisms of eicosanoid regulation are very complex and include interactions with matrix proteins that are released during inflammation and could be effectors of eicosanoid activation and regulators of their signaling. The matricellular protein thrombospondin-1 (TSP1) is found in the circulation at low levels and stored in platelets α-granules. Injury and inflammatory molecules can trigger TSP1 release from platelets and de novo synthesis by a variety of cell types. TSP1 regulates cellular physiology through ligation with its cellular receptors and association with other extracellular matrix proteins. The Thrombospondin family consists of 5 genes that are highly conserved among all vertebrates [1]. Thrombospondin-1 and -2 are matricellular proteins, playing an important regulatory role in the extracellular matrix [2]. Each member of the thrombospondin family has distinct roles and effects, and there appears to be little redundancy in function despite structural similarity [3].
Through binding to several signaling receptors, TSP1 regulates angiogenesis, platelet activation, immune responses, synaptogenesis, and plays roles in maintaining mitochondrial homeostasis, cell proliferation, and survival [4]. TSP1 associates with membrane proteins including the high affinity receptor CD47, the fatty acid translocase CD36, proteoglycans, and several integrins. Through these receptor interactions TSP1 can play both pro-and anti-inflammatory effects. This duality is mediated in part by peroxisome proliferator-activated receptor (PPAR) and TSP1-mediated activation of latent transforming growth factor beta-1 (TGFβ). These in turn can be regulated by eicosanoid synthesis; PPAR can be activated by members of the prostanoid family PGJ2 that in turn can regulate TSP1 downstream actions [5]. Moreover, lipoxins and prostaglandin synthase metabolites can regulate expression of TGFβ and TSP1 in the resolution of proinflammatory processes [6]. Therefore the bioactive role of these lipid molecules is closely associated to regulation of TSP1 and downstream effects. These complex interactions enable TSP1 to regulate the function of monocytes, macrophages, dendritic cells, T cells, and natural killer cells and profoundly regulate angiogenesis [7–10], all of which play roles in carcinogenesis and metastatic spread.
Expression of TSP1 is known to delay carcinogenesis in several animal models. TSP1 has been identified as an inhibitor of progression, and loss of THBS1 gene expression by hypermethylation serves as a poor prognostic factor in some cancers [11]. On the other hand, increased TSP1 expression has also been implicated in supporting carcinogenesis under specific conditions. The anti-tumor effects of TSP1 are historically attributed to its role as endogenous angiogenesis inhibitor but also includes modulation of tumor-associated macrophages [12]. The pro-tumor effects are more complex and include indirect and direct actions on inflammation that can be linked to regulation of eicosanoid signaling. We recently reported that the absence of TSP1 negatively regulates eicosanoid metabolism in a model of colorectal cancer in which we observed that absence of TSP1 reduced tumor multiplicity [13]. Therefore, the actions of TSP1 in carcinogenesis are intimately regulated to eicosanoid metabolism. Here we discuss to the current understanding of these spatiotemporal interactions and potential applications to develop effective strategies that impact the signaling that promotes cancer growth.
Prostanoids, thrombospondin-1 and Cancer
Prostaglandin G/H synthases, more commonly known as cyclooxygenase enzymes (COX) exist as two isoforms, COX1 (encoded by PTGS1) and COX2 (encoded by PTGS2), that catalyze the conversion of arachidonic acid to prostanoids, and further metabolism produces both prostaglandins and thromboxanes. Alterations in arachidonic acid metabolism are a hallmark of many cancers, with increased levels of COX2 associated with decreased survival in cancer patients. Therefore, it is not surprising that high levels of prostaglandin E2 (PGE2) are found in many cancers and that its presence is associated with poor prognosis in many cancers [14]. TSP1 receptor-mediated eicosanoid synthesis is regulated via direct TSP1 binding to CD36 [15]. TSP1 induces platelet activation via CD36, inducing a cAMP/protein kinase A signaling cascade [16]. Activation of CD36 also induces release of arachidonic acid and PGE2, leading to eicosanoid biosynthesis [17]. This may lead to a run-away cycle of inflammation. The eicosanoid product 15(S)-Hydroxyeicosatetraenoic acid in turn induces CD36 expression, resulting in foam cell formation, activation of Syk2, Pyk2, STAT-1 and ROS generation [18]. These effects can exacerbate inflammatory effects. However, TSP1 binding to and activation of latent TGF-β may also lead to inflammation resolution (reviewed in [19]). As previously noted, eicosanoids can function as inflammatory promoters and suppressors. CD36 activity is associated with eicosanoid 15-Deoxy-Δ12,14-Prostaglandin J2, which is pro-resolving of inflammation. These effects have been shown to be downstream of Nrf2-dependent expression of CD36 and subsequent macrophage phagocytosis [15]. The specific roles of eicosanoid production and effects, downstream of CD36, CD47 and TSP1-binding integrins must be further studied to understand inflammatory regulation.
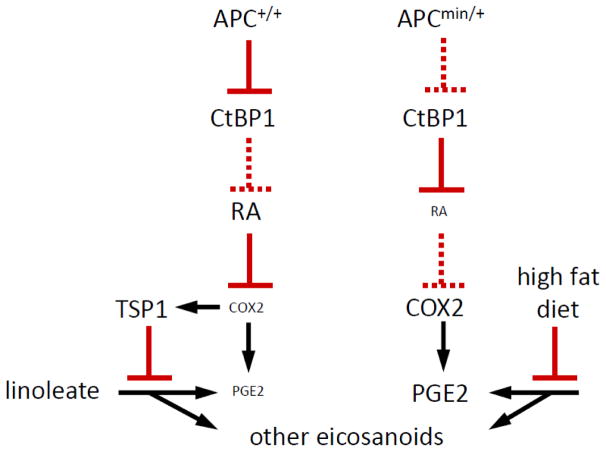
Our lab recently published evidence that TSP1 regulates, among other lipids, eicosanoid metabolism [13]. This study utilized the ApcMin/+ murine colorectal cancer model. ApcMin/+ mice are known to express increased PGE2 levels, due to over-expression of COX2 (Fig. 1). Thbs1−/− ApcMin/+ mice exhibited a further increase in eicosanoid production compared to ApcMin/+ Thbs1+/+ and Thbs1+/+ Apc+/+ mice. Thbs1−/− mouse liver tissue contained increased prostaglandin and hydroxyeicosatetranoic acid family eicosanoids [13].
Fig. 1.
Proposed mechanism for the convergent regulation of PGE2 and eicosanoid biosynthesis by APC, TSP1, and dietary fat.
Extracellular matrix expression of TSP1 is induced by inflammation [20]. Eicosanoids are known regulators of inflammation, notably through their binding and activation of PPARs [5], reviewed in [21]). Eicosanoids are known to play pro- and anti-inflammatory effects, depending on the cellular and microenvironment context [22–24]. The regulation of eicosanoid synthesis by TSP1 adds another mechanism through which TSP1 regulates inflammation. How TSP1 regulates eicosanoid synthesis is not yet understood; however, TSP1 is known to bind and regulate signaling mediated by CD36, CD47 and integrins, which have been implicated independently in regulating eicosanoid synthesis [25, 26]. Through binding these receptors, TSP1 induces expression and activation of anti-inflammatory (TGFβ1, which regulates eicosanoid inflammatory responses (reviewed in [19]).
A synthetic peptide 4N1K derived from the COOH-terminal domain of TSP1, which acts as an agonist to CD47, stimulates αvβ3-mediated spreading of C32 melanoma cells [27]. 4N1K also stimulated platelet spreading on immobilized fibrinogen. The stimulatory effects of 4N1K where inhibited by pertussis toxin administration, indicating the participation of a G protein-coupled receptor in CD47 signaling. Through this pathway TSP1 modulates cAMP levels in several cell types including melanoma cells [16, 28, 29]. Hence it could be predicted that prostaglandins would play a role in regulating this signaling mechanism. PGE1 inhibits platelet aggregation by activation of cAMP. Stimulation with PGE1 inhibited platelet spreading induced by 4N1K, thus suggesting that PGE1 suppresses signaling between CD47 and integrins and thus regulating cell spreading.
Leukotrienes, thrombospondin-1 and Cancer
The interaction of the 5-LOX enzyme with a 5-LOX activating protein known as FLAP results in the oxidation of arachidonic acid and the formation of leukotrienes and hydroxyeicosatetraenoic acids (HETEs). The 5-LOX catabolism of arachidonic acid produces an intermediate molecule known as 5-HPETE, which is converted the unstable leukotriene LTA4. LTA4 is subsequently converted to biologically active leukotrienes (LTB4, LTC4, LTD4 and LTE4), which act upon G protein-coupled receptors. Expression of these leukotrienes and their receptors are associated with induction of proliferation and inhibition of apoptosis. LTB4 and its receptors are increased in pancreatic cancer [30]. Inhibition of this metabolite results in reduction of tumor growth in preclinical models of cancer. Gene expression microarray analysis of primary effusion lymphoma cells found that the leukotriene A4 and TSP1 are strongly upregulated in these cells, impacting cell migration and chemotaxis [31]. Primary effusion lymphoma is characterized and distinguished from classical Hodgkin’s disease by the migration of cancer cells from lymph nodes to the peritoneal, pleural, or pericardial cavities. Protein and mRNA expression and release of TSP-1 in primary effusion lymphoma cells were highly upregulated when compared to classical Hodgkin disease cells [31]. Thus, TSP1 overexpression may be a chemo attractant for lymphoma cells or contribute to the invasive characteristics of this disease. The exact mechanisms of LTA4H regulation of TSP1 are not known. However, studies in Kaposi’s sarcoma suggest that LTA4H regulates PPAR, and thus up-regulation of CD36 could amplify the chemotactic response by ligation of TSP1 [15].
Eicosanoids, thrombospondin-1 and Immunosuppression
Exposure to TSP1 in PPAR-expressing cells positively regulates CD36, resulting in macrophage recruitment and pro-inflammatory response [32, 33]. TSP1 also induces superoxide production in recruited macrophages [12, 34], propagating the inflammatory response. Conversely, latent TGFβ activation by TSP1 negatively regulates CD36, limiting macrophage recruitment and inflammatory response [8]. In some contexts TSP1 inhibits eicosanoid biosynthesis. This was demonstrated using ApcMin/+ mice, which are known to alter expression of COX2, a regulator of TSP1 expression [35, 36]. ApcMin/+ mice also exhibit decreased expression of PGE2, a key enzyme in eicosanoid biosynthesis [37–39]. PGE2 expression was not altered in Thbs1−/− macrophages but elevated in Thbs1−/− liver, where increased eicosanoid levels were found [13, 40]. These data suggest that TSP1 negatively regulates eicosanoid biosynthesis by inhibiting upstream enzymes. Eicosanoids also exhibit anti-inflammatory effects, increasing the scope of TSP1 in regulating inflammatory response [41–44]. Our group recently demonstrated that TSP1 induces pro-IL1β activation by inducing NLRP3 inflammasome formation. Conversely, TSP1 inhibits expression of IL-1β mRNA in human macrophages by disrupting the interaction of CD47 and CD14, which limits activation of NFκB/AP-1 [32]. The activation of inflammasomes is associated with many chronic diseases including the progression of colorectal cancer. This adds another layer to the complexity of TSP1-induced inflammation in cancer.
A direct link between eicosanoids and TSP1 has not been established. However, eicosanoids such as PGE2 are known to induce the inflammasome NLRC4, impacting pathogen susceptibility [45, 46]. On the other hand PGE2 was shown to inhibit NLRP3 activation in human macrophages through the EP4 receptor [47]. Therefore further consideration of this pathway is needed to determine the links between TSP1, prostaglandins and formation of active inflammasomes to devise strategies to target chronic inflammation in cancer. TSP1 stimulation of collagen binding to α2β1 integrin increases platelet accumulation at a site of injury [27]. Binding of TSP1 to CD36/αvβ3 integrin mediates macrophage phagocytosis of apoptotic neutrophils, which mediates clearing of exhausted neutrophils [40, 48, 49]. Delayed phagocytosis of apoptotic neutrophils, resulting in phagocytosis of post-apoptotic bodies, leads to an exacerbated inflammatory response. Therefore, TSP1-mediated neutrophil clearance may have anti-inflammatory function [50].
Prostaglandins and TSP1 interplay also regulates immunosuppressive activities involving adaptive immunity that can impact cancer progression. Murine retinal pigment epithelial cells inhibit T-cell activation by the release of immunoregulatory factors such as TGFβ [51]. Supernatants from these cells caused the differentiation to CD4+CD25+ T regs that were also positive for CTLA-4 and Foxp3 molecules [51]. Treatment with human recombinant TGFβ2 (the most abundant isoform in the eye) results in increased TSP1 and PGE2, thus implicating prostaglandins and TSP1 in mechanisms of immunosuppression that could lead to resistance to cancer immunotherapy.
Eicosanoids, thrombospondin-1 and tumor angiogenesis
Angiogenesis, the formation of new blood vessels from preexisting vessels, is imperative for primary tumor growth and metastasis in cancer. Increased tumor vascularization allows an abundance of nutrients and oxygen to supply and promote tumor growth while removing waste products [52]. The hypoxic properties of the tumor promotes the angiogenic switch that initiates angiogenesis [53]. The tumor secretes growth factors including vascular endothelial growth factors (VEGF) and fibroblast growth factors (FGF) to activate endothelial cells through their corresponding receptors [52]. Other pro-angiogenic factors are secreted from cells within the tumor microenvironment including tumor-associated fibroblasts and cancer associated macrophages, to further enhance blood vessel growth [53]. Endothelial cells will begin proliferating and migrate to form blood vessels for the tumor [52]. Once the primary tumor is vascularized, metastasis can initiate via intravasation of tumor cells. Tumor cells thus can escape and travel to distant parts of the body through these newly formed blood vessels [53]. Metastatic cancer cells can proliferate and form new tumors throughout the body.
Tumor angiogenesis can be inhibited by anti-angiogenic factors including TSP1 [54]. During early stages of carcinogenesis, TSP1 expression is elevated [16]. TSP1 inhibits VEGF-A, FGF2, and nitric oxide signaling in endothelial cells that promote vascularization [54]. TSP1 also inhibits the tumor from releasing VEGF and FGF2 to stimulate angiogenesis [54]. Endothelial cell migration and proliferation initiated by the tumor for blood vessel formation is also inhibited by TSP1 [55]. Apoptosis of endothelial cells associated with tumor vascularization is activated by TSP1 [54]. This occurs in part through the endothelial cell receptor CD36 binding with TSP1 [56]. An alteration in the function of Src and Fyn kinase induces apoptosis. [54, 57]. Nitric oxide signaling also enhances the growth of pro-angiogenic endothelial cells [11]. The binding of TSP1 to CD47 and CD36 inhibits nitric oxide signaling, decreasing the proliferation of endothelial cells [57, 58] and limiting tumor angiogenesis. The physiological anti-angiogenic function of TSP1 is lost when cancers undergo the angiogenic switch, which causes a decrease in TSP1 abundance and function [11]. Thus, loss of the anti-angiogenic function of TSP1 is associated with progression in several cancers.
Vascularization of tumors through angiogenesis can be enhanced by eicosanoids like prostaglandins, leukotrienes and hydroxyeicosatetranoates (HETE) [59]. Eicosanoids are often present in cancerous environments to enhance angiogenesis within the tumor microenvironment. Prostaglandins cause expression and secretion of factors like VEGF to initiate angiogenesis. PGE2 has been shown to induce VEGF secretion for the formation of blood vessels in human lung fibroblasts [60]. Prostaglandins cause an infiltration of cells, like neutrophils, into the tumor microenvironment to enhance angiogenesis [59]. Leukotrienes have also been shown to enhance proliferation and migration of endothelial cells to promote angiogenesis through VEGF stimulation [59]. A variety of HETE are involved in angiogenesis for tumor progression. Some metastases have an association with 12-HETE. 12-HETE stimulates angiogenesis, thus providing a pathway for the proliferation, migration and eventual metastasis of tumor cells [61].
During the early stages of cancer progression before the angiogenic switch, the abundance of anti-angiogenic TSP1 can inhibit synthesis and function of pro-angiogenic eicosanoids. Elevated COX2 has been seen in cancers like human colorectal cancer [13], which is a cancer where progression is associated with loss of TSP1 [56]An increase in COX2 causes an upregulation of eicosanoids synthesis, furthering cancer progression through inflammation and angiogenesis. PGE2 can be regulated by TSP1 [13]. When TSP1 is present in mice, a decrease in PGE2 function occurred possibly providing a protective barrier and inhibiting functions of PGE2, like promoting angiogenesis, from occurring. Disruption of Thbs1 in mice was associated with an increase in the secretion and function of several eicosanoids and their metabolites, including PGE2 [13]. Based on these mouse experiments, it is evident that TSP1 is involved in the regulation of eicosanoids within the tumor microenvironment [13]. By regulating eicosanoids, angiogenesis is also regulated. Vascularization of the tumor is a necessary pathway for tumor cells to escape and cause metastasis. Therefore, the anti-angiogenic effects of TSP1 can indirectly regulate tumor metastasis by inhibiting pro-angiogenic eicosanoid synthesis and function.
Dietary effects on inflammation and cancer
The chronic inflammation caused by obesity is an underlying element in transformation and carcinogenesis. While obesity-induced inflammation shares many traits of acute and chronic inflammation, it is distinguished in part by onset of metabolic syndrome, migration of macrophages to adipose tissue, and production of TNFα that ultimately leads to insulin insensitivity [62]. All of these alterations lead to metabolic changes that result in increased production of reactive oxygen species, mitochondrial dysfunction, and endoplasmic reticulum stress [62]. To test the role of TSP1 in cancer associated obesity we developed a model of colorectal cancer by crossing Thbs1−/− with ApcMin/+ mice and compared effects of feeding a high or low fat diet on carcinogenesis [13]. Examination of liver tissue by global metabolomics showed that all strains of mice fed the high-fat diet had accumulation of various medium-chain and long-chain saturated, monounsaturated and polyunsaturated free fatty acids [13]. Dietary consumption of a high-fat diet was associated with liver accumulation of fatty acids, which can affect cell energy metabolism and signaling to promote carcinogenesis. Both fatty acid oxidation and the observed increases in ketogenic amino acids provide sources of acetyl-CoA. ApcMin/+:Thbs1−/− mice fed the low-fat diet had a 1.8-fold elevated level relative to ApcMin/+ mice of the ketone body 3-hydroxybutyrate (BHBA). This elevation was lost in ApcMin/+:Thbs1−/− mice fed a high-fat diet, representing a significant reduction. BHBA is synthesized in the liver from excess acetyl-CoA and utilized by extra hepatic tissues to meet energy demands during periods of starvation. BHBA has been identified as a serum biomarker of colorectal carcinoma and esophageal carcinoma.
Lipid metabolites involved in eicosanoid biosynthesis showed strong dependences on dietary fat as well as TSP1 expression. The ApcMin mutation is known to increase COX2 expression and consequently prostaglandin PGE2 levels via a CtBP1-dependent mechanism[38], and COX2 is known to regulate TSP1 expression (Fig. 1), but no change in PGE2 expression was reported in Thbs1−/− macrophages. However, Thbs1−/− liver showed strong elevation in PGE2 levels as well as in PGF2 and 5- and 15-hydroxyeicosatetraenoic acid levels, This indicates a general inhibitory effect of TSP1 on eicosanoid biosynthesis from n6-polyunsaturated fatty acids. Notably, the high-fat diet suppressed these elevations in Thbs1−/− liver. The high-fat diet was also inhibitory in the other genotypes, except for ApcMin/+:Thbs1−/− mice, where levels were low in mice fed both diets.
These changes resulted an increase in adenoma formation in the small intestine of ApcMin/+:Thbs1−/− fed a low fat diet and increase in adenoma formation in their small and large intestines when compared with ApcMin/+ mice fed the same diet. Adenoma formation in the small intestine increased in mice of both genotypes fed a high-fat diet. Moreover, ApcMin/+:Thbs1−/− mice fed a high-fat diet had an over 30% increase in adenoma formation when compared with mice of the same genotype fed a low-fat diet. When fed a high-fat diet, however, lesion formation in the small intestine was not significantly different between mice of these two genotypes. More relevant to human APC-dependent colon cancers, ApcMin/+:Thbs1−/− mice fed a high-fat diet had a 48% increase in adenoma formation in the large intestine when compared with ApcMin/+ mice fed the same diet. Therefore dietary fat consumption overcomes the protective role of TSP1 in formation of colon cancer lesions. In ApcMin/+:Thbs1−/ high levels of 1-Methylnicotinamide were found in the liver of mice fed a low fat diet when compared to ApcMin/+:Thbs1−/− fed a high fat diet. 1-Methylnicotinamide is an anti-inflammatory COX-dependent nicotinamide catabolite. Therefore the loss of the protective effect of TSP1 may come from regulation of COX dependent pathways.
Another aspect observed in this study was that dietary supplementation affected xenobiotic metabolite levels in the liver[13]. It is evident that dietary intake can impact microbiota which in turn can influence cancer susceptibility and even response to therapeutics. ApcMin/+ and ApcMin/+:Thbs1−/− had differences in benzoate metabolism regardless of diet. In particular lower levels of hippurate were observed in ApcMin/+ when compared to WT[13]. Hippurate, is glycine conjugate of benzoic acid, normally excreted in the urine and is associated with the microbial degradation of specific dietary components[63]. The excretion hippurate was found to be lower in patients with ulcerative colitis which suggest a regulation during inflammation[64]. On the other hand ApcMin/+:Thbs1−/− levels of hippurate were not significantly regulated when compared to WT but were higher when compared to ApcMin/+ when fed a low fat diet[13]. Interestingly the levels of hippurate were reduced when ApcMin/+:Thbs1−/− were fed a high fat diet when compared to the same genotype fed a low fat diet. ApcMin/+:Thbs1−/− fed the high fat diet had increased levels of prostaglandins and thus suggest that increase in these metabolites may regulate microbiome and may be accountable for the loss of the protective effect of TSP1 during high fat diet supplementation. The peroxidase activity of COX-2 is known to metabolize xenobiotics which in turn can impact microbiota[65]. Therefore further studies are warranted to understand the interplay of TSP1 expression and inflammation regulated by eicosanoids and microbiota.
Remaining questions and therapeutic applications
Thrombospondin 1 is a multifaceted protein that regulates, cell survival, migration, invasion, angiogenesis and inflammation. The effects of TSP1 expression in cancer may be a double-edged sword, and pathological studies suggest that TSP1 expression has different repercussions depending on the cancer type. TSP1 is the first endogenous anti-angiogenic factor discovered, and mimetic drugs were synthetized focusing on this role [66]. A preclinical study demonstrated that ABT-510 could mimic the antiangiogenic and anti-inflammatory activities of TSP1 in a mouse model inflammatory bowel disease, which suggest a potential cancer preventive activity [67]. In clinical trials the TSP1 mimetic peptide ABT-510 consistently reduced circulating levels of VEGF-A and VEGF-C. While ABT-510 was deemed safe, the dismal overall responses rate led to the discontinued investigation of this agent [68]. A second generation TSP1 mimetic ABT-898 was shown to also have anti-angiogenic activity and strong anti-immunoinflammatory activity [69]. Even though many studies link eicosanoids and TSP1 further studies are needed to determine whether these TSP1 mimics modulate eicosanoid responses. Therefore strategies are needed combining these TSP1 mimetics and eicosanoid targeting compounds reduce inflammatory signaling that fuels carcinogenesis and metastasis. In other instances it has been shown that microRNA regulation can increase elevate inflammation thorough COX-2 increasing levels of VEGFA, COX-2, PGE2, and TSP1 [70]. Using an inhibitor of microRNA 101 reduced endometrial carcinogenesis and was associated with a reduction of TSP1. Therefore, the types of cancer or instances were TSP1 can be pro-inflammatory need to be understood in particular the regulation of biosynthesis of pro inflammatory eicosanoids. The patient’s co-morbidities may also need to be considered. Accumulation of TSP1 is considered inflammatory during aging and is associated with increase tissue injury in models of cardiovascular disease [10]. Moreover, in our studies lack of TSP1 was associated with reduced colon tumor multiplicity in the ApcMin/+ mice, however high fat diet overcame the protective activity of TSP1 which was associated with an increase in arachidonic acid metabolites [13]. Moreover levels of xenobiotics where affected by dietary fat and presences or absence of TSP1[13]. Therefore aspects such as microbiome impact and comorbidities should be considered for the design of effective strategies to target inflammation through modulation of TSP1 and pro-inflammatory eicosanoid production.
Acknowledgments
Funding: DSP is funded by the NCI Career Transition Award K22 (1K22CA181274-01A1), the Metavivor foundation Young Investigator Award. MUR is supported by NIGMS grant number 5K12GM102773-05. DDR is supported by the NIH Intramural Research Program (ZIASC009172). The authors wish to acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197.
References
- 1.Bentley AA, Adams JC. The evolution of thrombospondins and their ligand-binding activities. Mol Biol Evol. 2010;27:2187–2197. doi: 10.1093/molbev/msq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondins expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, Campbell SC, Bedford DF, Nelius T, Veliceasa D, Shroff EH, Henkin J, Schneider A, Bouck N, Volpert OV. Peroxisome proliferator-activated receptor gamma ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Mol Cancer Res. 2004;2:541–550. [PubMed] [Google Scholar]
- 6.Brennan EP, Nolan KA, Borgeson E, Gough OS, McEvoy CM, Docherty NG, Higgins DF, Murphy M, Sadlier DM, Ali-Shah ST, et al. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFbetaR1. J Am Soc Nephrol. 2013;24:627–637. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Thrombospondins: A Role in Cardiovascular Disease. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18071540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50:212–230. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008;68:7090–7099. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto-Pantoja DR, Sipes JM, Martin-Manso G, Westwood B, Morris NL, Ghosh A, Emenaker NJ, Roberts DD. Dietary fat overcomes the protective activity of thrombospondin-1 signaling in the Apc(Min/+) model of colon cancer. Oncogenesis. 2016;5:e230. doi: 10.1038/oncsis.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W, Lee HN, Jang JH, Kim SH, Lee YH, Hahn YI, Ngo HK, Choi Y, Joe Y, Chung HT, et al. 15-Deoxy-Delta(12,14)-Prostaglandin J2 Exerts Proresolving Effects Through Nuclear Factor E2-Related Factor 2-Induced Expression of CD36 and Heme Oxygenase-1. Antioxid Redox Signal. 2017;27:1412–1431. doi: 10.1089/ars.2016.6754. [DOI] [PubMed] [Google Scholar]
- 16.Roberts W, Magwenzi S, Aburima A, Naseem KM. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood. 2010;116:4297–4306. doi: 10.1182/blood-2010-01-265561. [DOI] [PubMed] [Google Scholar]
- 17.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J Biol Chem. 2011;286:17785–17795. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotla S, Singh NK, Traylor JG, Jr, Orr AW, Rao GN. ROS-dependent Syk and Pyk2-mediated STAT1 activation is required for 15(S)-hydroxyeicosatetraenoic acid-induced CD36 expression and foam cell formation. Free Radic Biol Med. 2014;76:147–162. doi: 10.1016/j.freeradbiomed.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65:79–84. [PubMed] [Google Scholar]
- 21.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 22.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 24.Furuyashiki T, Narumiya S. Roles of prostaglandin E receptors in stress responses. Curr Opin Pharmacol. 2009;9:31–38. doi: 10.1016/j.coph.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Asch AS, Tepler J, Silbiger S, Nachman RL. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991;266:1740–1745. [PubMed] [Google Scholar]
- 27.Chung J, Wang XQ, Lindberg FP, Frazier WA. Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in alpha2beta1-mediated platelet activation. Blood. 1999;94:642–648. [PubMed] [Google Scholar]
- 28.Guo N, Zabrenetzky VS, Chandrasekaran L, Sipes JM, Lawler J, Krutzsch HC, Roberts DD. Differential roles of protein kinase C and pertussis toxin-sensitive G-binding proteins in modulation of melanoma cell proliferation and motility by thrombospondin 1. Cancer Res. 1998;58:3154–3162. [PubMed] [Google Scholar]
- 29.Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2011;63:13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X, Zhu C, Qiang H, Zhou X, Zhou G. Enhancing antitumor effects in pancreatic cancer cells by combined use of COX-2 and 5-LOX inhibitors. Biomed Pharmacother. 2011;65:486–490. doi: 10.1016/j.biopha.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Arguello M, Paz S, Hernandez E, Corriveau-Bourque C, Fawaz LM, Hiscott J, Lin R. Leukotriene A4 hydrolase expression in PEL cells is regulated at the transcriptional level and leads to increased leukotriene B4 production. J Immunol. 2006;176:7051–7061. doi: 10.4049/jimmunol.176.11.7051. [DOI] [PubMed] [Google Scholar]
- 32.Stein EV, Miller TW, Ivins-O’Keefe K, Kaur S, Roberts DD. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1beta Production and Activation through CD47. Sci Rep. 2016;6:19684. doi: 10.1038/srep19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stawski L, Haines P, Fine A, Rudnicka L, Trojanowska M. MMP-12 deficiency attenuates angiotensin II-induced vascular injury, M2 macrophage accumulation, and skin and heart fibrosis. PLoS One. 2014;9:e109763. doi: 10.1371/journal.pone.0109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Manso G, Navarathna DH, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, Roberts DD. Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS One. 2012;7:e48775. doi: 10.1371/journal.pone.0048775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, Delespesse G, Sarfati M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–1283. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar AM, Checchin D, Brault S, Gobeil F, Beauchamp MH, Mwaikambo B, Courtois Y, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108:198–204. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 37.Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 38.Hull MA, Faluyi OO, Ko CW, Holwell S, Scott DJ, Cuthbert RJ, Poulsom R, Goodlad R, Bonifer C, Markham AF, Coletta PL. Regulation of stromal cell cyclooxygenase-2 in the ApcMin/+ mouse model of intestinal tumorigenesis. Carcinogenesis. 2006;27:382–391. doi: 10.1093/carcin/bgi236. [DOI] [PubMed] [Google Scholar]
- 39.Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, Moretto J, Mendes F, Smith AP, Bennington JL, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y, et al. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014;7:440–448. doi: 10.1038/mi.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoxha M. A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Adv Med Sci. 2017;63:22–29. doi: 10.1016/j.advms.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Tahir A, Bileck A, Muqaku B, Niederstaetter L, Kreutz D, Mayer RL, Wolrab D, Meier SM, Slany A, Gerner C. Combined Proteome and Eicosanoid Profiling Approach for Revealing Implications of Human Fibroblasts in Chronic Inflammation. Anal Chem. 2017;89:1945–1954. doi: 10.1021/acs.analchem.6b04433. [DOI] [PubMed] [Google Scholar]
- 43.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J, Gebicki J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152:230–239. doi: 10.1038/sj.bjp.0707383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wozniacka A, Wieczorkowska M, Gebicki J, Sysa-Jedrzejowska A. Topical application of 1-methylnicotinamide in the treatment of rosacea: a pilot study. Clin Exp Dermatol. 2005;30:632–635. doi: 10.1111/j.1365-2230.2005.01908.x. [DOI] [PubMed] [Google Scholar]
- 45.Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and -8. Immunity. 2017;46:649–659. doi: 10.1016/j.immuni.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Shaw DK, Hammond HL, Sutterwala FS, Rayamajhi M, Shirey KA, Perkins DJ, Bonventre JV, Velayutham TS, Evans SM, et al. The Prostaglandin E2-EP3 Receptor Axis Regulates Anaplasma phagocytophilum-Mediated NLRC4 Inflammasome Activation. PLoS Pathog. 2016;12:e1005803. doi: 10.1371/journal.ppat.1005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolowska M, Chen LY, Liu Y, Martinez-Anton A, Qi HY, Logun C, Alsaaty S, Park YH, Kastner DL, Chae JJ, Shelhamer JH. Prostaglandin E2 Inhibits NLRP3 Inflammasome Activation through EP4 Receptor and Intracellular Cyclic AMP in Human Macrophages. J Immunol. 2015;194:5472–5487. doi: 10.4049/jimmunol.1401343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuang PC, Wu MH, Shoji Y, Tsai SJ. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol. 2009;219:232–241. doi: 10.1002/path.2588. [DOI] [PubMed] [Google Scholar]
- 50.Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- 51.Horie S, Sugita S, Futagami Y, Yamada Y, Mochizuki M. Human retinal pigment epithelium-induced CD4+CD25+ regulatory T cells suppress activation of intraocular effector T cells. Clin Immunol. 2010;136:83–95. doi: 10.1016/j.clim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015;21:267–273. doi: 10.1097/PPO.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakanishi M, Sato T, Li Y, Nelson AJ, Farid M, Michalski J, Kanaji N, Wang X, Basma H, Patil A, et al. Prostaglandin E2 stimulates the production of vascular endothelial growth factor through the E-prostanoid-2 receptor in cultured human lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46:217–223. doi: 10.1165/rcmb.2010-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valderrama JA, Durante-Rodriguez G, Blazquez B, Garcia JL, Carmona M, Diaz E. Bacterial degradation of benzoate: cross-regulation between aerobic and anaerobic pathways. J Biol Chem. 2012;287:10494–10508. doi: 10.1074/jbc.M111.309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res. 2013;12:1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 65.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 66.Haviv F, Bradley MF, Kalvin DM, Schneider AJ, Davidson DJ, Majest SM, McKay LM, Haskell CJ, Bell RL, Nguyen B, et al. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J Med Chem. 2005;48:2838–2846. doi: 10.1021/jm0401560. [DOI] [PubMed] [Google Scholar]
- 67.Punekar S, Zak S, Kalter VG, Dobransky L, Punekar I, Lawler JW, Gutierrez LS. Thrombospondin 1 and its mimetic peptide ABT-510 decrease angiogenesis and inflammation in a murine model of inflammatory bowel disease. Pathobiology. 2008;75:9–21. doi: 10.1159/000113790. [DOI] [PubMed] [Google Scholar]
- 68.Baker LH, Rowinsky EK, Mendelson D, Humerickhouse RA, Knight RA, Qian J, Carr RA, Gordon GB, Demetri GD. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J Clin Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 69.Gutierrez LS, Ling J, Nye D, Papathomas K, Dickinson C. Thrombospondin peptide ABT-898 inhibits inflammation and angiogenesis in a colitis model. World J Gastroenterol. 2015;21:6157–6166. doi: 10.3748/wjg.v21.i20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Li H, Zhao C, Jia H. MicroRNA-101 inhibits angiogenesis via COX-2 in endometrial carcinoma. Mol Cell Biochem. 2018 doi: 10.1007/s11010-018-3313-0. [DOI] [PubMed] [Google Scholar]