Abstract
Nitric oxide (NO) is a gas that induces relaxation of smooth muscle cells in the vasculature. Because NO reacts with oxyhaemoglobin with high affinity, the gas is rapidly scavenged by oxyhaemoglobin in red blood cells and the vasodilating effects of inhaled NO are limited to ventilated regions in the lung. NO therefore has the unique ability to induce pulmonary vasodilatation specifically in the portions of the lung with adequate ventilation, thereby improving oxygenation of blood and decreasing intrapulmonary right to left shunting. Inhaled NO is used to treat a spectrum of cardiopulmonary conditions, including pulmonary hypertension in children and adults. However, the widespread use of inhaled NO is limited by logistical and financial barriers. We have designed, developed and tested a simple and economic NO generation device, which uses pulsed electrical discharges in air to produce therapeutic levels of NO that can be used for inhalation therapy.
Linked Articles
This article is part of a themed section on Nitric Oxide 20 Years from the 1998 Nobel Prize. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.2/issuetoc
Abbreviations
- AKI
acute kidney injury
- ARDS
acute respiratory distress syndrome
- CPB
cardiopulmonary bypass
- HBOCs
Hb‐based oxygen carriers
- HEPA
high‐efficiency particulate absorption
- RBCs
red blood cells
- SCD
sickle cell disease
- STEMI
ST‐elevation myocardial infarction
Introduction
NO is a gas that is produced in the body by a family of three NOS. The enzymes use oxygen and L‐arginine to produce NO and L‐citrulline. NO stimulates soluble guanylate cyclase (sGC) to synthesize cGMP, which activates cGMP‐dependent PKG, leading to vascular relaxation. PDEs catabolize cGMP, thereby limiting its activity (Ichinose and Zapol, 2017b). In the presence of oxygenated haemoglobin (Hb), NO is rapidly metabolized to form nitrate and methaemoglobin. In erythrocytes, methaemoglobin reductase converts methaemoglobin to ferrous‐Hb.
Drugs that generate NO, such as nitroglycerin and sodium nitroprusside (SNP), have long been used to reduce blood pressure (BP) and treat angina pectoris. NO‐donor compounds can also dilate the pulmonary vasculature, but their efficacy is limited by systemic hypotension. In patients with lung injury, NO‐donor compounds given systemically may induce vasodilatation in regions of the lung that are poorly ventilated, thereby increasing ventilation‐perfusion mismatching and leading to systemic arterial hypoxaemia. Frostell and colleagues reasoned that NO administered via inhalation would relax the pulmonary vasculature but, upon reaching the bloodstream, would be rapidly scavenged by Hb thereby preventing systemic vasodilatation (Frostell et al., 1993). These investigators observed that inhalation of NO produced a dose‐dependent decrease in pulmonary artery pressure and pulmonary vascular resistance in awake sheep with pulmonary hypertension. No effect of inhaled NO was observed in sheep with normal pulmonary vascular tone (Frostell et al., 1991). The pulmonary vasodilator effects of breathing NO were readily reversible upon discontinuation of breathing the gas. Breathing NO did not alter systemic arterial BP. The selective dilation of the pulmonary vasculature induced by NO has been observed in a wide range of species, including man. Pilot studies in critically ill newborns with acute pulmonary hypertension showed that inhaled NO improved oxygenation without causing systemic hypotension (Kinsella et al., 1992; Roberts et al., 1992). Subsequent randomized, placebo‐controlled studies confirmed these results and led to the approval of inhaled NO to treat hypoxic newborns by the US Food and Drug Administration in 1999, by the European Medicine Evaluation Agency and European Commission in 2001 and by the Ministry of Health, Labour and Welfare of Japan in 2008 (NINOS group, 1997; Kinsella et al., 1997; Roberts et al., 1997; Clark et al., 2000).
A number of studies now indicate that inhaled NO has an important role in treating pulmonary hypertension of paediatric and adult patients with respiratory and cardiac failure (Kinsella et al., 1992; Roberts et al., 1992; Krasuski et al., 2000; Cockrill et al., 2001; Ozturk et al., 2016). Inhaled NO can also be used during cardiac catheterization to determine the vasodilatory capacity of the pulmonary vascular bed in patients with pulmonary hypertension. A data meta‐analysis was conducted on 1240 preterm infants who received either placebo (nitrogen gas) or NO >5 p.p.m. for a minimum of 7 days (Askie et al., 2018). This study suggested that inhaled NO prevented bronchopulmonary dysplasia (BPD) in preterm African American infants and was presented as an example of a racially customized therapy for infants with BPD. Recent studies suggest inhaled NO may prevent ischaemia‐reperfusion injury and reduce haemolysis‐induced vasoconstriction and renal failure after prolonged cardiopulmonary bypass (Lei et al., 2018). This review article will focus on recent developments in the use of NO inhalation therapy to treat patients with acute respiratory distress syndrome (ARDS) and the vascular consequences of haemolytic diseases. In addition, advances in the development and testing of an inexpensive, lightweight portable NO generating device from air will be described. The use of inhaled NO in other clinical areas including paediatric and adult pulmonary hypertension and chronic obstructive pulmonary disease have been reviewed elsewhere (Ichinose and Zapol, 2017a).
Inhaled NO in acute respiratory distress syndrome
ARDS is characterized by pulmonary hypertension and increased intrapulmonary shunting of blood through hypoventilated regions. Pulmonary hypertension contributes to pulmonary oedema and can cause right ventricular dysfunction and heart failure. The use of inhaled NO for the treatment of ARDS is one of the most widely studied pharmacological interventions over the past three decades. In 10 patients with severe ARDS, inhalation of NO from 5–20 p.p.m. for 3 to 53 days reduced pulmonary arterial pressure, decreased intrapulmonary shunting and improved arterial oxygenation without producing systemic vasodilatation (Rossaint et al., 1993). Benzing and colleagues demonstrated that inhalation of 40 p.p.m. NO vasodilated pulmonary vasculature and thereby lowered pulmonary capillary pressure in patients with acute lung injury (Benzing and Geiger, 1994). However, subsequent clinical trials reported disappointing results, in that inhalation of NO did not improve the survival rate in patients with ARDS (Troncy et al., 1998; Gerlach et al., 2003; Taylor et al., 2004). These randomized, controlled trials were unfortunately performed in the 1990s, before low‐volume ventilation was shown to be beneficial in patients with ARDS. With the widespread adoption of the low tidal volume ventilation strategy, Bronicki et al. enrolled 55 paediatric patients with ARDS in a prospective, randomized placebo‐controlled trial of inhaled NO that showed a significant reduction in duration of mechanical ventilation and a significantly greater survival without the need for using extracorporeal membrane oxygenation (Bronicki et al., 2015). In 161 children with ARDS, Dowell and coworkers demonstrated that inhalation of NO, for at least 1 h, within 3 days of ARDS onset was associated with a decrease in the average number of days that patients required ventilator support (Dowell et al., 2017). In an open‐label prospective crossover pilot study, breathing 20 p.p.m. of NO significantly improved oxygenation in 15 adult patients with ARDS (Albert et al., 2017). Accumulating evidence suggests that NO inhalation therapy is beneficial in patients with ARDS. Large randomized trials are needed to determine whether inhaled NO improves survival of adult patients with ARDS.
Inhaled NO in haemolysis
Endothelial cells produce NO, which acts as a potent dilator of vascular smooth muscle cells. NO depletion can lead to vasoconstriction, impaired tissue perfusion and inflammation. During haemolysis, plasma NO is consumed by circulating plasma free oxyhaemoglobin, which is transformed into methaemoglobin by the dioxygenatoin reaction. Breathing NO converts circulating cell‐free oxyhaemoglobin to methaemoglobin, thereby reducing the ability of oxyhaemoglobin to scavenge intrinsic NO. Minneci and coworkers used water‐induced haemolysis in dogs to investigate the effect of free Hb on vascular tone and renal function. By scavenging endothelium‐derived NO, free Hb in plasma induced vasoconstriction and decreased creatinine clearance (Minneci et al., 2005). Dogs that were treated with inhalation of 80 p.p.m. NO had decreased haemolysis‐induced hypertension and renal dysfunction. Based in part on these observations, it has been proposed and in many cases proven that NO inhalation attenuates the vasoconstriction that is associated with clinical haemolysis, including haemolysis induced by, or associated with, prolonged cardiopulmonary bypass, sickle cell anaemia, malaria and blood transfusion.
Cardiac surgery
Pulmonary hypertension is a recognized risk factor for mortality in cardiac surgery. In a murine model of cardiac arrest, Kida and coworkers showed that inhaled NO exerts protective effects and improves outcomes after cardiac arrest and cardiopulmonary resuscitation with or without therapeutic hypothermia (Kida et al., 2014). In 33 paediatric patients who underwent a palliative surgical procedure to treat univentricular heart, Latus and colleagues found that inhalation of NO increased pulmonary and systemic blood flow, demonstrating beneficial effects on cardiac output and tissue perfusion (Latus et al., 2016). Elmi‐Sarabi and colleagues conducted a meta‐analysis of 10 studies including 434 patients, to compare the efficacy of inhaled aerosolized vasodilators (including NO) in the treatment of pulmonary hypertension during cardiac surgery (Elmi‐Sarabi et al., 2017). The authors concluded that inhaled NO improved right ventricular performance when compared to i.v. administered agents. Janssens and coworkers conducted a multicentre, double‐blind, randomized controlled trial of inhalation of NO in 250 patients with ST‐elevation myocardial infarction (STEMI) (Janssens et al., 2018). Inhalation of NO at 80 p.p.m. for 4 h in these patients after cardiac catheterization was safe, and there was a tendency towards decreased rates of adverse events at 4 months (P = 0.10) and 1 year (P = 0.06) in patients who received NO. The results suggest that further studies of the potential benefits of inhaled NO in patients with STEMI are needed.
The most common complication associated with prolonged cardiopulmonary bypass (CPB) is acute kidney injury (AKI), which markedly increases postoperative mortality (Karkouti et al., 2009; Wrobel et al., 2015). Prolonged CPB causes haemolysis with high levels of circulating plasma Hb that scavenges NO via the dioxygenatoin reaction, depleting endogenous NO and causing vasoconstriction, proximal renal tubular injury and AKI. Lei and colleagues conducted a single centre, prospective, randomized, double‐blind controlled trial involving 217 patients with normal kidney function, who underwent elective multiple valve replacement surgery that required prolonged CPB (Lei et al., 2018). The incidence of AKI in patients treated with NO decreased from 63 to 50% (P < 0.05). Treatment with inhaled 80 p.p.m. NO during (via oxygenator) and for 24 h after the operation was safe, with blood methaemoglobin levels remaining below 10%. Based on these observations, inhalation of NO may prove to be beneficial for patients undergoing prolonged cardiac surgery.
Sickle cell disease and cerebral malaria
Sickle cell disease (SCD) is an autosomal‐recessive disorder caused by mutations in the β‐globin gene. Mutant Hb S polymerizes in erythrocytes, altering the shape of red blood cells and causing occlusion of small blood vessels. Patients with SCD experience episodes of severe pain (vaso‐occlusive crisis), with subsequent damage to major organs, and premature death. There has been considerable interest in the possible contribution of NO depletion to the pathogenesis of SCD and a potential role for inhaled NO as a treatment for SCD. Case reports (Atz and Wessel, 1997; Sullivan et al., 1999; Head et al., 2010) and a single‐institution, placebo‐controlled study (Weiner et al., 2003) suggested beneficial effects of NO inhalation in patients with SCD. However, Gladwin and colleagues performed an 11 centre, double‐blind, randomized, placebo‐controlled clinical trial involving 150 SCD patients and found that breathing 80 p.p.m. NO for up to 72 h, with pulsatile inspiratory delivery of NO through nasal prongs, did not decrease the duration of painful crisis (Gladwin et al., 2011). Maitre and colleagues conducted a prospective, double‐blind, randomized, placebo‐controlled clinical trial in 100 adult SCD patients with acute chest syndrome, which is characterized by fever and/or respiratory symptoms accompanied by new abnormalities on their chest radiograph. Inhalation of 80 p.p.m. NO for 3 days did not reduce the rate of mortality in this group of patients (Maitre et al., 2015). Future trials should target more severely ill SCD patients with hypoxaemia and/or acute pulmonary hypertension and investigate whether inhalation of NO provides benefit to this subgroup of patients.
Cerebral malaria is the most severe neurological complication of infection with Plasmodium falciparum, with a mortality rate of approximately 20%. Survivors of cerebral malaria often experience long‐term cognitive and neurological deficits (Idro et al., 2007; Serghides et al., 2011; Postels et al., 2012). In a murine model of cerebral malaria, treatment with 40 p.p.m. NO improved survival by inactivating NO‐scavenging by free Hb in the plasma (Gramaglia et al., 2006). Serghides and colleagues demonstrated that mice treated with NO during infection had reduced systemic inflammation and endothelial cell activation, decreased intercellular adhesion molecule 1 (ICAM‐1) expression, preserved integrity of the blood–brain barrier and decreased parasite accumulation in the brain (Serghides et al., 2011). In addition, inhaled NO co‐administered with artesunate (a medication currently used to treat malaria), starting 5.5 days after infection, improved the murine survival rate compared to treatment with artesunate therapy alone (Serghides et al., 2011). Hawkes and colleagues performed a randomized, blinded, placebo‐controlled trial in which standard artesunate treatment was supplemented with either 80 p.p.m. NO in air (delivered by nonrebreathing mask) or air alone. In 180 children with severe malaria, inhaled NO did not significantly decrease angiopoietin‐2 levels, an endothelial biomarker of malarial severity. There was also no observed effect of NO treatment on clinical outcomes, including mortality (Hawkes et al., 2015). Mwanga‐Amumpaire and colleagues performed a randomized open‐label, phase II, controlled trial of breathing 80 p.p.m. NO in air or air alone in 92 children with cerebral malaria in Uganda (Hawkes et al., 2011; Mwanga‐Amumpaire et al., 2015). Although this trial did not demonstrate a reduction in mortality or neurological impairment in children treated with NO, breathing NO for 48 h was safe and was associated with an increase in methaemoglobin and plasma nitrate levels (Mwanga‐Amumpaire et al., 2015). In the future, it may be valuable to focus NO inhalation therapy on cerebral malaria patients with laboratory evidence of NO scavenging and pulmonary or systemic vasoconstriction.
Blood transfusion
For many decades, Hb‐based oxygen carriers (HBOCs) have been investigated as substitutes for the use of red blood cells in blood transfusions. One of the major obstacles hindering the successful clinical development of HBOCs is systemic vasoconstriction. Yu and colleagues demonstrated in mice that HBOCs cause systemic vasoconstriction by scavenging NO produced by endothelial NOS (also known as NOS3; Yu et al., 2008). Administration of inhaled NO before an i.v. infusion of HBOCs prevented systemic vasoconstriction without causing methaemoglobinaemia in mice and sheep (Yu et al., 2008, 2009a). Inhalation of NO, at a concentration as low as 5 p.p.m., prevented HBOC‐induced pulmonary vasoconstriction in healthy awake lambs. Yu and coworkers studied the effects of HBOC infusion on mice with endothelial dysfunction caused by diabetes mellitus or by consuming a high‐fat diet for 4–6 weeks. The endothelial damage induced by these conditions resulted in decreased NO bioavailability and increased susceptibility to HBOC‐induced vasoconstriction (Yu et al., 2010). Inhaled NO can prevent the systemic vasoconstriction induced by infusion of HBOCs in these mice with endothelial dysfunction. In a case report, Marrazzo and colleagues described an 87‐year‐old patient with acute life‐threatening anaemia (Hb level at 40 g·L−1) (Marrazzo et al., 2018). This patient had a history of an anti‐Jk3 alloantibody and could only receive packed red blood cells lacking the Jk3 antigen, which is an extremely rare phenotype in most populations. Therefore, two units of HBOC were administered for compassionate use. The administration of inhaled NO combined with HBOC infusion improved cardiac output, arterial oxygen content, lactate clearance and reduced vasopressor requirement in this patient. In the future, treatment with inhaled NO may prove to be a novel strategy that permits the use of HBOC transfusion without causing systemic and pulmonary hypertension.
During ex vivo storage, red blood cells (RBCs) undergo numerous biochemical, structural and functional alterations, which are collectively termed the ‘storage lesion’. Transfusion of blood that has been stored for more than 2 weeks was associated with an increased rate of infection and multiorgan failure, longer hospitalizations and increased mortality (Koch et al., 2008; Weinberg et al., 2008; Zimrin and Hess, 2009). In diabetic mice, inhalation of 80 p.p.m. NO prevented systemic vasoconstriction and hypertension associated with transfusion of RBCs stored for 14 days (Yu et al., 2012). Lei and colleagues used a murine model of haemorrhagic shock, in the setting of hyperlipidaemia‐induced endothelial dysfunction and decreased NO bioavailability, to investigate the effects of NO on stored‐blood induced injury. Inhalation of NO during transfusion of 14‐day‐old blood decreased tissue injury, inflammation and mortality (Lei et al., 2012). Baron and colleagues developed a model of autologous blood transfusion in lambs and found that inhaled NO attenuated the pulmonary hypertension induced by transfusion of red blood cells stored for 40 days (Baron et al., 2012). Furthermore, in a sheep model of haemorrhagic shock, inhalation of NO attenuated the pulmonary hypertension and inflammation associated with transfusion of 40‐day‐old red blood cells (Baron et al., 2013). Berra and coworkers demonstrated in human volunteers that inhalation of NO prevented pulmonary hypertension associated with the transfusion of autologous leukoreduced blood stored for 40 days (Berra et al., 2014). The results of these studies suggest that inhaled NO should be considered as an adjunctive therapy for blood transfusion, especially in critically ill patients with pulmonary hypertension, endothelial dysfunction and acute lung injury.
Generating NO from air using pulsed electrical discharges
NO inhalation therapy requires gas cylinders and a cylinder distribution network, a complex delivery device to regulate NO and oxygen (O2) concentrations, and trained respiratory therapists. For many hospitals, inhaled NO is the most expensive drug used in neonatal medicine (Subhedar and Dewhurst, 2007). Because of the complexities and expense of delivering NO, this treatment is not available in many parts of the world and is not practical for outpatient use. Several approaches have been used to produce NO for biomedical purposes, including chemical methods and various electrical systems (Namihira et al., 2000, 2002; Stoffels et al., 2006; Kuhn et al., 2010). However, these reactions produce large amounts of toxic byproducts, such as nitrogen dioxide (NO2) and ozone, and therefore require complex purification systems (Samaranayake et al., 1999; Hu et al., 2007). Lovich and colleagues proposed that NO might be produced by catalytic conversion of liquid NO2/N2O4. However, this approach would require a large amount of highly toxic NO2 as the starting material (Lovich et al., 2014). Ren and coworkers developed a NO releasing system that used a copper (II)‐tri(2‐pyridylmethyl) amine complex to mediate electrochemical reduction of nitrite. Unfortunately, the system only produced very low levels of NO (Ren et al., 2014, 2015). Subsequent modifications, based on the electrochemical reduction of nitrite using copper (II)‐ligand as a mediator, increased the yield of NO, from 400 p.p.b. to 500 p.p.m. (Qin et al., 2017). However, the instability of the mediators and the short lifespan of the copper‐complex covered electrodes (currently less than 48 h) suggest that this approach requires further investigation and improvement.
Recently, our group designed, developed and tested a lightweight, portable and economical NO generator system that uses pulsed electrical discharges (Figures 1 and 2) (Yu et al., 2015). The NO generator produces NO in a therapeutic range (5–80 p.p.m.) at gas flow rates of 0.5 to 5 L·min−1. Iridium electrodes were found to be superior to stainless steel, nickel, carbon and tungsten electrodes in that they produced the least amount of NO2 during NO production. The small amount of potentially toxic gases and metals that were produced in the electrically generated plasma were removed by a small (12 g) in‐line, calcium hydroxide (Ca (OH)2) scavenger and a high‐efficiency particulate absorption (HEPA) filter. In lambs with acute pulmonary arterial hypertension, breathing electrically generated NO reduced pulmonary arterial pressure, as effectively as NO diluted from a conventional cylinder (Yu et al., 2015). To save energy, reduce the consumption of the scavenger and preserve the electrodes, we improved the NO generator by triggering NO production only during inspiration. The newly developed NO generator can be installed in series with the ventilator or can be used to inject NO into the airway via a transtracheal catheter.
Figure 1.
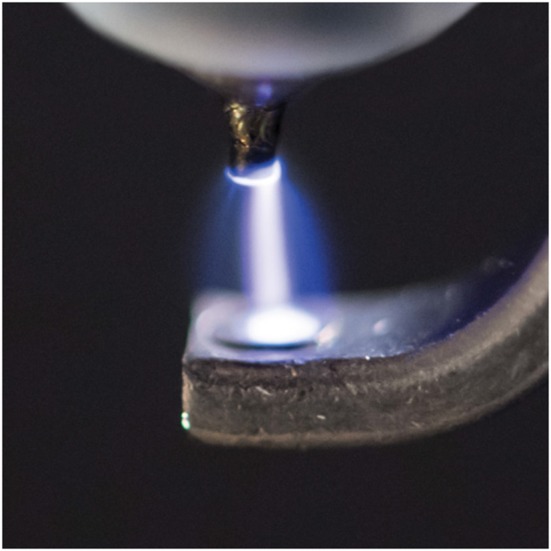
Producing NO by pulsed electrical discharge from air (from Yu et al., 2015; used with permission).
Figure 2.
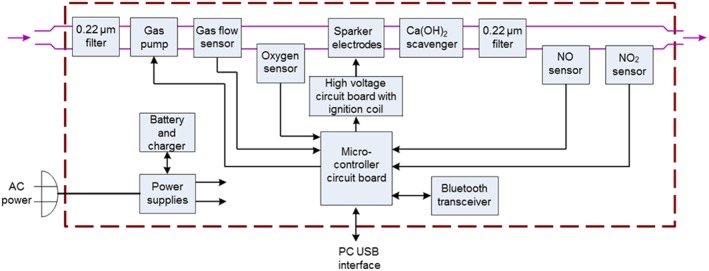
Detailed internal components of the NO generator. Purple arrows indicate gas entering and leaving the device. Black arrows indicate the sensors and pump that are connected to or controlled by the circuit board. Air or an O2/N2 mixture is pumped and filtered through a 0.22 μm high‐efficiency particulate absorption (HEPA) air filter. The gas flow rate is measured with a meter. Sensors for O2, NO and NO2 indicate the concentration of each gas. The electrodes are powered by a microcontroller circuit. The Ca(OH)2 scavenger and a 0.22 μm filter remove potential toxic gases (NO2 and O3) and metal particles before delivery of the gas. (From Yu et al., 2015 ; used with permission). O3, ozone.
Prolonged use of the NO generator resulted in erosion of the surface of the electrodes, potentially introducing contaminating metal particles into the gas stream (Yu et al., 2016). We used quadrupole mass spectroscopy to show that a single HEPA filter was sufficient to remove all metal particles from the effluent gas. Mice breathing electrically generated NO, 50 p.p.m. in air for 28 days, did not develop pulmonary inflammation or structural changes, and no trace metals were detected in the lungs of these mice (Yu et al., 2016).
Berra and colleagues tested the NO generator on six healthy volunteers and six patients with chronic pulmonary hypertension (Berra et al., 2016). Each subject received 25 p.p.m. of NO for 10 min, and no adverse effects were detected. In six patients with chronic pulmonary hypertension, the acute pulmonary vasodilator haemodynamic effects of electrically generated NO were similar to those seen using NO obtained from commercially available cylinders (Berra et al., 2016).
To develop a lighter NO generator for potential portable, outpatient use, we designed a miniaturized version of the prototypic NO generator, designated the ‘mini‐NO generator’ (Yu et al., 2018). The mini‐NO generator weighs approximately 14 g and consists of two iridium electrodes within a ceramic insulator surrounded by a 3 mm aluminium housing. Two HEPA filters are used to contain the 0.8 g Ca(OH)2 scavenger and to remove potential metal particles released from the electrodes during NO generation (Figure 3). When placed adjacent to the endotracheal tube of anaesthetized rabbits with acute pulmonary hypertension, the mini‐NO generator induced selective vasodilatation of the pulmonary vasculature. We showed that a small amount of Ca(OH)2 scavenger (0.8 g) was sufficient to remove potentially toxic gases. Scanning electron microscopy and energy‐disperse X‐ray spectroscopy measurements demonstrated that a single HEPA filter was sufficient to remove all trace metal particles produced during prolonged NO generation (Yu et al., 2018). An airflow of 70 mL·min−1 was sufficient to maintain the housing of the mini‐NO device at an acceptable low temperature during prolonged NO generation.
Figure 3.
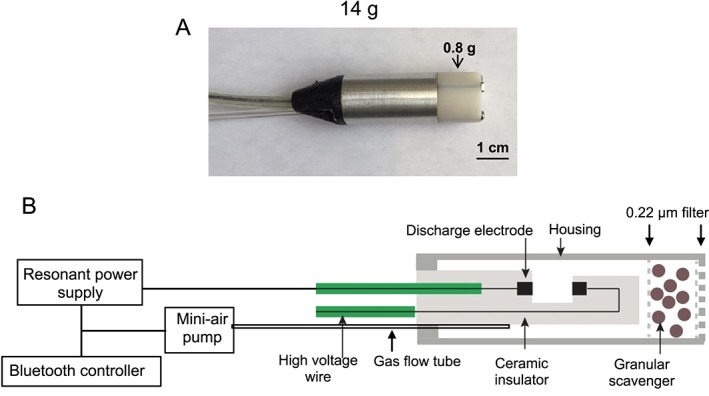
Photograph (A) and schematic (B) of the mini‐NO generator. The generator contains iridium discharge electrodes, a NO2 scavenger consisting of 0.8 g of Ca(OH)2, a 0.22 μm filter, a gas flow tube (e.g. 70 mL·min−1 airflow) to facilitate NO delivery, a mini‐air pump, resonant power supply and Bluetooth controller. The device is surrounded by a ceramic insulator. (From Yu et al., 2018; used with permission).
In summary, the electric plasma NO generator produces therapeutic levels of NO from air, with scavenging and filtration systems that effectively eliminate toxic gas and metallic impurities from the effluent gas. The device provides safe, efficient and economical NO generation, which will expand the applications of NO therapy to hospitalized and ambulatory patients around the world. Table 1 lists conditions that may benefit from treatment with inhaled NO.
Table 1.
Summary of potential therapeutic applications of inhaled NO therapy
| Cardiopulmonary diseases | Acute respiratory distress syndrome (ARDS) (Rossaint et al., 1993; Benzing and Geiger, 1994; Troncy et al., 1998; Gerlach et al., 2003; Taylor et al., 2004; Bronicki et al., 2015; Albert et al., 2017; Dowell et al., 2017) |
| Chronic obstructive pulmonary disease (COPD) (Barbera et al., 1996; Yoshida et al., 1997; Vonbank et al., 2003; Hajian et al., 2016) | |
| Bronchopulmonary dysplasia (BPD) (Schreiber et al., 2003; Ballard et al., 2006; Kinsella et al., 2006; Askie et al., 2018) | |
| Interstitial lung disease (ILD) (Blanco et al., 2011) | |
| Cardiac or lung transplantation (Stobierska‐Dzierzek et al., 2001; Fojon et al., 2005; Moreno et al., 2009; Tavare and Tsakok, 2011) | |
| Myocardial ischemia/reperfusion injury (I/R) (Hataishi et al., 2006; Janssens et al., 2018) | |
| Cardiac arrest and cardiopulmonary resuscitation (Minamishima et al., 2011; Kida et al., 2014; Derwall et al., 2015) | |
| Univentricular heart surgery (Latus et al., 2016) | |
| Pulmonary hypertension during and after cardiac surgery (Elmi‐Sarabi et al., 2017) | |
| ST‐elevation myocardial infarction (Janssens et al., 2018) | |
| Elective multiple valve replacement surgery‐prolonged CPB (Lei et al., 2018) | |
| Haemolytic diseases | Sickle cell disease (Atz and Wessel, 1997; Sullivan et al., 1999; Weiner et al., 2003; Head et al., 2010; Gladwin et al., 2011; Maitre et al., 2015) |
| Cerebral malaria (Gramaglia et al., 2006; Serghides et al., 2011; Hawkes et al., 2015; Mwanga‐Amumpaire et al., 2015) | |
| Stored blood transfusion (Yu et al., 2008, 2009b, 2010, 2012; Baron et al., 2012, 2013; Lei et al., 2012, 2018; Berra et al., 2014; Marrazzo et al., 2018) |
Conclusions
Inhaled NO is the first drug to produce selective pulmonary vasodilatation without reducing systemic arterial pressure. Inhaled NO is a life‐saving therapy in children and adults with a variety of diseases. By 2018, an estimated half a million of Americans with various causes of pulmonary hypertension had received NO inhalation therapy. With the recent breakthrough invention and testing of an electric NO generator, a simple, lightweight and economic device to produce NO from air by pulsed electrical discharge, inhaled NO will be affordable and available to patients in developing countries. Furthermore, this portable NO generator is likely to expand the indications for inhaled NO therapy, especially for patients in the ambulatory setting.
The newly developed NO generator can produce therapeutic levels of NO gas, which can be delivered through a face mask, nasal cannulas, an endotracheal tube or a ventilator. Because the device is lightweight and inexpensive, we anticipate that the device will have a wide range of applications including (i) treatment of hospitalized patients with cardiac and/or pulmonary diseases, to replace heavy and expensive tanks; (ii) treatment of outpatients with chronic respiratory illnesses, either in the ambulatory setting or at home; (iii) facilitation of research and preclinical studies; (iv) treatment of patients who are facing extreme conditions, such as those with respiratory failure who are injured on the battle field or while fighting fires; and (v) treatment of pulmonary arterial hypertension, for example, in hypoxic mountain climbers (Scherrer et al., 1996).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Conflict of interest
W.M.Z. and B.Y. have filed patents at MGH on the electric generation of NO. W.M.Z. is on the scientific advisory board of Third Pole Inc., which has licensed patents on NO generators from MGH. Other authors declare no conflicts of interest.
Acknowledgements
This study was partially supported by grants from NHLBI B‐BIC/NCAI (#U54HL119145) to W.M.Z., an NHLBI grant (#R21HL130956) to B.Y., and the National Institutes of Diabetes and Digestive and Kidney Diseases (DK082971) to D.B.B. This study was also supported by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (MGH).
Yu, B. , Ichinose, F. , Bloch, D. B. , and Zapol, W. M. (2019) Inhaled nitric oxide. British Journal of Pharmacology, 176: 246–255. 10.1111/bph.14512.
References
- Albert M, Corsilli D, Williamson DR, Brosseau M, Bellemare P, Delisle S et al (2017). Comparison of inhaled milrinone, nitric oxide and prostacyclin in acute respiratory distress syndrome. World J Crit Care Med 6: 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askie LM, Davies LC, Schreiber MD, Hibbs AM, Ballard PL, Ballard RA (2018). Race effects of inhaled nitric oxide in preterm infants: an individual participant data meta‐analysis. J Pediatr 193: 34–39 e32. [DOI] [PubMed] [Google Scholar]
- Atz AM, Wessel DL (1997). Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology 87: 988–990. [DOI] [PubMed] [Google Scholar]
- Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD et al (2006). Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 355: 343–353. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez‐Roisin R (1996). Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 347: 436–440. [DOI] [PubMed] [Google Scholar]
- Baron DM, Beloiartsev A, Nakagawa A, Martyn T, Stowell CP, Malhotra R et al (2013). Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs*. Crit Care Med 41: 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP et al (2012). Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology 116: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing A, Geiger K (1994). Inhaled nitric oxide lowers pulmonary capillary pressure and changes longitudinal distribution of pulmonary vascular resistance in patients with acute lung injury. Acta Anaesthesiol Scand 38: 640–645. [DOI] [PubMed] [Google Scholar]
- Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO et al (2014). Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med 190: 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra L, Rodriguez‐Lopez J, Rezoagli E, Yu B, Fisher DF, Semigran MJ et al (2016). Electric plasma‐generated nitric oxide: hemodynamic effects in patients with pulmonary hypertension. Am J Respir Crit Care Med 194: 1168–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco I, Ribas J, Xaubet A, Gomez FP, Roca J, Rodriguez‐Roisin R et al (2011). Effects of inhaled nitric oxide at rest and during exercise in idiopathic pulmonary fibrosis. J Appl Physiol (1985) 110: 638–645. [DOI] [PubMed] [Google Scholar]
- Bronicki RA, Fortenberry J, Schreiber M, Checchia PA, Anas NG (2015). Multicenter randomized controlled trial of inhaled nitric oxide for pediatric acute respiratory distress syndrome. J Pediatr 166: 365–369.e1. [DOI] [PubMed] [Google Scholar]
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA et al (2000). Low‐dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342: 469–474. [DOI] [PubMed] [Google Scholar]
- Cockrill BA, Kacmarek RM, Fifer MA, Bigatello LM, Ginns LC, Zapol WM et al (2001). Comparison of the effects of nitric oxide, nitroprusside, and nifedipine on hemodynamics and right ventricular contractility in patients with chronic pulmonary hypertension. Chest 119: 128–136. [DOI] [PubMed] [Google Scholar]
- Derwall M, Ebeling A, Nolte KW, Weis J, Rossaint R, Ichinose F et al (2015). Inhaled nitric oxide improves transpulmonary blood flow and clinical outcomes after prolonged cardiac arrest: a large animal study. Crit Care 19: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell JC, Thomas NJ, Yehya N (2017). Association of response to inhaled nitric oxide and duration of mechanical ventilation in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 18: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmi‐Sarabi M, Deschamps A, Delisle S, Ased H, Haddad F, Lamarche Y et al (2017). Aerosolized vasodilators for the treatment of pulmonary hypertension in cardiac surgical patients: a systematic review and meta‐analysis. Anesth Analg 125: 393–402. [DOI] [PubMed] [Google Scholar]
- Fojon S, Fernandez‐Gonzalez C, Sanchez‐Andrade J, Lopez‐Perez JM, Hermida LF, Rodriguez JA et al (2005). Inhaled nitric oxide through a noninvasive ventilation device to assess reversibility of pulmonary hypertension in selecting recipients for heart transplant. Transplant Proc 37: 4028–4030. [DOI] [PubMed] [Google Scholar]
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM (1991). Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83: 2038–2047. [DOI] [PubMed] [Google Scholar]
- Frostell CG, Blomqvist H, Hedenstierna G, Lundberg J, Zapol WM (1993). Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 78: 427–435. [DOI] [PubMed] [Google Scholar]
- Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM et al (2003). Dose–response characteristics during long‐term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med 167: 1008–1015. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L et al (2011). Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 305: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA et al (2006). Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med 12: 1417–1422. [DOI] [PubMed] [Google Scholar]
- Hajian B, De Backer J, Vos W, Van Holsbeke C, Ferreira F, Quinn DA et al (2016). Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 11: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S et al (2006). Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 291: H379–H384. [DOI] [PubMed] [Google Scholar]
- Hawkes M, Opoka RO, Namasopo S, Miller C, Conroy AL, Serghides L et al (2011). Nitric oxide for the adjunctive treatment of severe malaria: hypothesis and rationale. Med Hypotheses 77: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes MT, Conroy AL, Opoka RO, Hermann L, Thorpe KE, McDonald C et al (2015). Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar J 14: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head CA, Swerdlow P, McDade WA, Joshi RM, Ikuta T, Cooper ML et al (2010). Beneficial effects of nitric oxide breathing in adult patients with sickle cell crisis. Am J Hematol 85: 800–802. [DOI] [PubMed] [Google Scholar]
- Hu H, Liang H, Li J, Zhao Q, He J (2007). Study on production of inhaled nitric oxide for medical applications by pulsed discharge. IEEE Trans. Plasma Sci. 35: 619–625. [Google Scholar]
- Ichinose F, Zapol WM (2017a). Inhaled nitric oxide—current practice and future potential uses and development In: Ignarro LJ, Freeman BA. (eds). Nitric Oxide, 3rd edn, Vol. 1 Elsevier: London, pp. 339–353. [Google Scholar]
- Ichinose F, Zapol WM (2017b). Inhaled pulmonary vasodilators in cardiac surgery patients: correct answer is “NO”. Anesth Analg 125: 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley J et al (2007). Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA 297: 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens SP, Bogaert J, Zalewski J, Toth A, Adriaenssens T, Belmans A et al (2018). Nitric oxide for inhalation in ST‐elevation myocardial infarction (NOMI): a multicentre, double‐blind, randomized controlled trial. Eur Heart J 39: 2717–2725. [DOI] [PubMed] [Google Scholar]
- Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M et al (2009). Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 119: 495–502. [DOI] [PubMed] [Google Scholar]
- Kida K, Shirozu K, Yu B, Mandeville JB, Bloch KD, Ichinose F (2014). Beneficial effects of nitric oxide on outcomes after cardiac arrest and cardiopulmonary resuscitation in hypothermia‐treated mice. Anesthesiology 120: 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C et al (2006). Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 355: 354–364. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Neish SR, Shaffer E, Abman SH (1992). Low‐dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340: 819–820. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE et al (1997). Randomized, multicenter trial of inhaled nitric oxide and high‐frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131 (1 Pt 1): 55–62. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T et al (2008). Duration of red‐cell storage and complications after cardiac surgery. N Engl J Med 358: 1229–1239. [DOI] [PubMed] [Google Scholar]
- Krasuski RA, Warner JJ, Wang A, Harrison JK, Tapson VF, Bashore TM (2000). Inhaled nitric oxide selectively dilates pulmonary vasculature in adult patients with pulmonary hypertension, irrespective of aetiology. J Am Coll Cardiol 36: 2204–2211. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Bibinov N, Gesche R, Awakowicz P (2010). Non‐thermal atmospheric pressure HF plasma source: generation of nitric oxide and ozone for bio‐medical applications. Plasma Sources Sci Technol 19: 1–8. [Google Scholar]
- Latus H, Gerstner B, Kerst G, Moysich A, Gummel K, Apitz C et al (2016). Effect of inhaled nitric oxide on blood flow dynamics in patients after the Fontan procedure using cardiovascular magnetic resonance flow measurements. Pediatr Cardiol 37: 504–511. [DOI] [PubMed] [Google Scholar]
- Lei C, Berra L, Emanuele R, Yu B, Dong H, Yu S et al (2018). Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med. 10.1164/rccm.201710-2150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM (2012). Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology 117: 1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovich MA, Fine DH, Denton RJ, Wakim MG, Wei AE, Maslov MY et al (2014). Generation of purified nitric oxide from liquid N2O4 for the treatment of pulmonary hypertension in hypoxemic swine. Nitric Oxide 37: 66–72. [DOI] [PubMed] [Google Scholar]
- Maitre B, Djibre M, Katsahian S, Habibi A, Stankovic Stojanovic K, Khellaf M et al (2015). Inhaled nitric oxide for acute chest syndrome in adult sickle cell patients: a randomized controlled study. Intensive Care Med 41: 2121–2129. [DOI] [PubMed] [Google Scholar]
- Marrazzo F, Larson G, Sherpa Lama TT, Droghi MT, Joyce M, Ichinose F et al (2018). Inhaled nitric oxide prevents systemic and pulmonary vasoconstriction due to hemoglobin‐based oxygen carrier infusion: a case reprot. J Crit Care, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima S, Kida K, Tokuda K, Wang H, Sips PY, Kosugi S et al (2011). Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation 124: 1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM et al (2005). Hemolysis‐associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115: 3409–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I, Vicente R, Mir A, Leon I, Ramos F, Vicente JL et al (2009). Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc 41: 2210–2212. [DOI] [PubMed] [Google Scholar]
- Mwanga‐Amumpaire J, Carroll RW, Baudin E, Kemigisha E, Nampijja D, Mworozi K et al (2015). Inhaled nitric oxide as an adjunctive treatment for cerebral malaria in children: a phase II randomized open‐label clinical trial. Open Forum Infect Dis 2: ofv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namihira T, Katsuki S, Hackam R, Akiyama H, Okamoto K (2002). Production of nitric oxide using a pulsed arc discharge. IEEE Trans Plasma Sci 30: 1993–1998. [Google Scholar]
- Namihira T, Tsukamoto S, Wang D, Katsuki S, Hackam R, Okamoto K et al (2000). Production of nitric monoxide using pulsed discharges for a medical application. IEEE Trans Plasma Sci 28: 109–114. [Google Scholar]
- Ozturk E, Haydin S, Tanidir IC, Ozyilmaz I, Ergul Y, Erek E et al (2016). Use of inhaled nitric oxide in pediatric cardiac intensive care unit. Turk Kardiyol Dern Ars 44: 196–202. [DOI] [PubMed] [Google Scholar]
- Postels DG, Taylor TE, Molyneux M, Mannor K, Kaplan PW, Seydel KB et al (2012). Neurologic outcomes in retinopathy‐negative cerebral malaria survivors. Neurology 79: 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zajda J, Brisbois EJ, Ren H, Toomasian JM, Major TC et al (2017). Portable nitric oxide (NO) generator based on electrochemical reduction of nitrite for potential applications in inhaled NO therapy and cardiopulmonary bypass surgery. Mol Pharm 14: 3762–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Colletta A, Koley D, Wu J, Xi C, Major TC et al (2015). Thromboresistant/anti‐biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry 104: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Wu J, Xi C, Lehnert N, Major T, Bartlett RH et al (2014). Electrochemically modulated nitric oxide (NO) releasing biomedical devices via copper (II)‐Tri(2‐pyridylmethyl) amine mediated reduction of nitrite. ACS Appl Mater Interfaces 6: 3779–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JD Jr, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD et al (1997). Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 336: 605–610. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Polaner DM, Lang P, Zapol WM (1992). Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340: 818–819. [DOI] [PubMed] [Google Scholar]
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM (1993). Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328: 399–405. [DOI] [PubMed] [Google Scholar]
- Samaranayake WJM, Miyahara Y, Namihira T, Katsuki S, Hackam R, Akiyama H (1999). Ozone production by pulsed power in dry air. IEEE 2: 1326–1329. [Google Scholar]
- Scherrer U, Vollenweider L, Delabays A, Savcic M, Eichenberger U, Kleger GR et al (1996). Inhaled nitric oxide for high‐altitude pulmonary edema. N Engl J Med 334: 624–629. [DOI] [PubMed] [Google Scholar]
- Schreiber MD, Gin‐Mestan K, Marks JD, Huo D, Lee G, Srisuparp P (2003). Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349: 2099–2107. [DOI] [PubMed] [Google Scholar]
- Serghides L, Kim H, Lu Z, Kain DC, Miller C, Francis RC et al (2011). Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 6: e27714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobierska‐Dzierzek B, Awad H, Michler RE (2001). The evolving management of acute right‐sided heart failure in cardiac transplant recipients. J Am Coll Cardiol 38: 923–931. [DOI] [PubMed] [Google Scholar]
- Stoffels E, Gonzalvo YA, Whitmore TD, Seymour DL, Rees JA (2006). A plasma needle generates nitric oxide. Plasma Sources Sci. Technol. 15: 501–506. [Google Scholar]
- Subhedar N, Dewhurst C (2007). Is nitric oxide effective in preterm infants? Arch Dis Child Fetal Neonatal Ed 92: F337–F341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KJ, Goodwin SR, Evangelist J, Moore RD, Mehta P (1999). Nitric oxide successfully used to treat acute chest syndrome of sickle cell disease in a young adolescent. Crit Care Med 27: 2563–2568. [DOI] [PubMed] [Google Scholar]
- Tavare AN, Tsakok T (2011). Does prophylactic inhaled nitric oxide reduce morbidity and mortality after lung transplantation? Interact Cardiovasc Thorac Surg 13: 516–520. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr et al (2004). Low‐dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 291: 1603–1609. [DOI] [PubMed] [Google Scholar]
- The Neonatal Inhaled Nitric Oxide Study (NINOS) group . (1997). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics 99: 838–845. [DOI] [PubMed] [Google Scholar]
- Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T et al (1998). Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med 157 (5 Pt 1): 1483–1488. [DOI] [PubMed] [Google Scholar]
- Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P et al (2003). Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 58: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G Jr, Griffin RL, Huynh VQ, Cherry SA 3rd, Marques MB et al (2008). Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 65: 279–282, discussion 282–274. [DOI] [PubMed] [Google Scholar]
- Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C (2003). Preliminary assessment of inhaled nitric oxide for acute vaso‐occlusive crisis in pediatric patients with sickle cell disease. JAMA 289: 1136–1142. [DOI] [PubMed] [Google Scholar]
- Wrobel K, Stevens SR, Jones RH, Selzman CH, Lamy A, Beaver TM et al (2015). Influence of baseline characteristics, operative conduct, and postoperative course on 30‐day outcomes of coronary artery bypass grafting among patients with left ventricular dysfunction: results from the Surgical Treatment for Ischemic Heart Failure (STICH) trial. Circulation 132: 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Taguchi O, Gabazza EC, Yasui H, Kobayashi T, Kobayashi H et al (1997). The effect of low‐dose inhalation of nitric oxide in patients with pulmonary fibrosis. Eur Respir J 10: 2051–2054. [DOI] [PubMed] [Google Scholar]
- Yu B, Blaesi AH, Casey N, Raykhtsaum G, Zazzeron L, Jones R et al (2016). Detection and removal of impurities in nitric oxide generated from air by pulsed electrical discharge. Nitric Oxide 60: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Bloch KD, Zapol WM (2009a). Hemoglobin‐based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med 19: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Ferrari M, Schleifer G, Wepler M, Zapol WM, Bloch DB (2018). Development of a portable mini‐generator to safely produce nitric oxide for the treatment of infants with pulmonary hypertension. Nitric Oxide 75: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM (2012). Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion 52: 1410–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Muenster S, Blaesi AH, Bloch DB, Zapol WM (2015). Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci Transl Med 7: 294ra107. [DOI] [PubMed] [Google Scholar]
- Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM (2008). Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117: 1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C et al (2010). Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin‐based oxygen carrier. Anesthesiology 112: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM (2009b). Prevention of the pulmonary vasoconstrictor effects of HBOC‐201 in awake lambs by continuously breathing nitric oxide. Anesthesiology 110: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimrin AB, Hess JR (2009). Current issues relating to the transfusion of stored red blood cells. Vox Sang 96: 93–103. [DOI] [PubMed] [Google Scholar]


