Abstract
Curing cancer has been one of the greatest conundrums in the modern medical field. To reduce side-effects associated with the traditional cancer therapy such as radiotherapy and chemotherapy, photothermal therapy (PTT) has been recognized as one of the most promising treatments for cancer over recent years. PTT relies on ablation agents such as nanomaterials with a photothermal effect, for converting light into heat. In this way, elevated temperature could kill cancer cells while avoiding significant side effects on normal cells. This theory works because normal cells have a higher heat tolerance than cancer cells. Thus, nanomaterials with photothermal effects have attracted enormous attention due to their selectivity and non-invasive attributes. This review article summarizes the current status of employing nanomaterials with photothermal effects for anti-cancer treatment. Mechanisms of the photothermal effect and various factors affecting photothermal performance will be discussed. Efficient and selective PTT is believed to play an increasingly prominent role in cancer treatment. Moreover, merging PTT with other methods of cancer therapies is also discussed as a future trend.
Keywords: Nanomaterials, Photothermal therapy, Cancer
1. Introduction
Cancer is a continuing crisis to public health worldwide due to its mortality and invasion. At present, two commonly used therapies, radiotherapy and chemotherapy, are usually accompanied by broad collateral damage. Drugs for chemotherapy may also cause collateral damage to normal cells via oxidative stress while killing cancer cells [1], and radiation used in the radiotherapy may result in sequelae like radiodermatitis during or after treatment [2]. Consequently, studies on invasive and selective therapies have been hot topics in the medical field. One of the most promising therapies is light stimulation therapy, including photothermal therapy (PTT) and photodynamic therapy (PDT).
Photothermal-induced elevated body temperature could be beneficial for recovery from certain diseases [3–6]. When the body temperature rises, pathogens may slow down growth and the enzymes in viruses may become inactivated because the temperature of the internal environment is no longer optimal for them. Accordingly, the thermal effect has been utilized as an anti-bacterial [7–10] and an anti-cancer [11,12] medical treatment. Thermal treatments against cancer rely on the susceptibility of cancer cells to heat. In other words, heat causes irreversible damage to cancer cell membranes and initiates protein denaturation [7–13]. Compared to normal cells, cancer cells have a lower ability to endure elevated heat [14]; this precondition makes thermal treatment a selective treatment. This means that thermal treatments can be considered a promising and selective treatment for various cancers; this holds especially true for photothermal therapy.
Laser irradiation was applied as a cancer treatment at an early stage; however, heat produced only by an external laser device may burn lesions as well as surrounding healthy tissues [15,16]. This limited selectivity restricted its clinical use. Thus, the photothermal effect used to induce local heating has started attracting great attention. Some nanomaterials called ablation agents can initiate the photothermal effect by absorbing particular light irradiation and converting this energy to heat [17–28]. With the use of ablation agents, it is possible to generate heat in the lesions themselves, making the treatment selective and non-invasive to surrounding healthy tissues. To generate heat internally, the light irradiation utilized should be able to go through healthy tissues without causing any side effect. Unlike ultraviolet light and visible light, near-infrared (NIR) light can penetrate deep tissues while doing relatively mild damage [29–31]. In more detail, the NIR region is known as the biological window [32], where water and biomolecules absorb minimal amounts of light, making NIR advantageous to PTT. On the other hand, the ablation agents should be biocompatible, allowed to congregate in tumors and should be capable of converting NIR light energy to heat effectively. Various nanomaterials can be remarkable candidates as the result of their photothermal effect in the NIR region and passive localization in tumors.
It must be considered that PTT has its own limits. Nanomaterials are able to pass through cancer cell membranes [33], leading to accumulation of nanomaterials in lesions and heightening treatment efficacy. Nevertheless, there may be accumulation of nano-materials in other healthy tissues during and after PTT as well. A concern that still needs further research is the possible long-term cytotoxicity of accumulated and aggregated nanomaterials, since it is still quite difficult to detect and eliminate nanoscale materials. As for most nanomaterials, the surface property plays a great role in the photothermal effect and other physicochemical properties [34–39]. Surface modification is usually required to satisfy PTT demand and biocompatibility. After surface modification process, nanomaterials used for PTT could own better biocompatibility and greater NIR conversion efficiency, which would avoid side effect from nanomaterials and make it unnecessary to use a large density of laser beyond standard.
Our article will review the development of nanomaterials including noble metal, semiconductor, carbon-based, and conducting polymers for PTT; additionally, mechanisms of their photothermal effect are introduced. At the end of this review article, synergic therapies containing PTT are predicted as promising cancer treatments in the near future. Although the development of individual nanomaterials for PTT has been reviewed by other groups [40–42], this review emphasizes using composite nanomaterials for a greater photothermal effect or synergic light-responsive systems for a better anti-cancer effect.
2. Light-tissue interaction
Light has been applied in the medical field for imaging diagnosis and surgery for quite a long time [43–46]. The invasion of light should be minimized during imaging diagnosis, while operations for lesion removal need the energy of a laser to ‘cut’ the targeted lesion. Both applications are related to the interaction between light and tissues. Thus, it is very important to cognize the interaction between light and tissues. Similar to the situation in an external environment, the reflection, scattering, absorption and transmission of light may occur during the light’s penetration through tissues [47]. Light may affect biological activities by, for example, oxygen consumption [48]. Light irradiation may result in the thermal effect [47] or the catalysis effect [49], which could influence biological activities by changing enzyme activity. Given these realities, interaction between light and tissues should be considered before the use of light in clinical treatments.
The interaction between light and tissues depends on the optical property of tissues and the wavelength of light. Since the optical property of tissues and biomolecules is barely modifiable, selection of light of a particular wavelength for medical use should be the primary focus. As reported [47–49], reflection, absorption and scattering are the main optical activities in the interaction between light and tissues. The absorption of light could accumulate energy from light irradiation, making it a useful tool for lesion ablation during surgery, but unnecessary thermal effect may do harm to normal tissues as well. Moreover, these three optical activities could be detected and used for diagnosis purposes.
Here we will discuss three commonly used lights in different wavelengths, X-ray, ultraviolet light (UV) and NIR; light in these specific wavelengths are used to analyze the interaction between light and tissues. X-ray has been increasingly used since the inception of computed tomography (CT) since the 1970s [50]. Though it is well-known that overexposure to X-rays may increase the risk on patient health, it is still widely used under strict guidelines because of its ability to provide three-dimensional views of deep body structure [51–54]. X-ray regions refer to the wavelength between 0.001 and 10 nm. X-rays have a relatively high frequency and high energy, leading to the high penetrability of X-rays. X-rays are applied in the scanning of different body structures, for example, bones and soft tissues. The density and thickness of target objects greatly affect the absorption of X-rays, resulting in differentiated images between bones and organs for diagnostic imaging applications. A dual-energy X-ray system, which could produce low and high energy X-rays, is applied for the measurement of bone density [55] or muscle mass [56]. It should be noted that, metal with high density can also be detected by the use of X-rays [57], thus, X-ray systems can be used to monitor ablation agents like gold nanomaterials [57,58]. However, during the interaction between X-rays and tissues, X-rays with high energy could generate primary and secondary electrons, which are considered a major cause of damage in creatures [59]. In more detail, the electron excited by an X-ray may cause the production of radicals, which could result in damage to DNA and the development of cancer [60], therefore X-rays should be used under strict guidelines. As for ultraviolet light, liquid water shows a high affinity for absorption [61]; this indicates that UV can barely penetrate through tissues. Therefore, both the application of and protection from UV is focused on human skin and eyes. UV light could be subdivided into three wavelengths, including ultraviolet A (UVA, 320–400 nm), ultraviolet B (UVB, 280–320 nm) and ultraviolet C (UVC, 200–280 nm) [62]. Similarly, the wavelengths of these UV lights determine their properties. For example, UVA is barely filtered by the ozone layer in the atmosphere due to its long wavelength and low energy, making it the main UV content in sunlight that reaches the earth’s surface [62–64]. Among solar radiation, UVA can penetrate relatively deep into skin while UVB can only penetrate the upper layers of the epidermis. UVC can barely go through the ozone layer in the atmosphere or reach the earth’s surface, but the high energy of UV light, especially UVC, may lead to burns on the skin or eye [63,64]. Furthermore, long-term exposure of UV light may result in sunburn, aging of skin, erythema, suppression of the immune system of skin, wrinkling, carcinoma and other symptoms. Various methods have been applied to shelter people from sunlight or UV light; conversely, UV light has been employed in phototherapy. As reported, UV light may induce the in vivo production of cytokines, neuropeptides and other natural molecules, which could help improve anti-inflammatory effect or immunosuppressive effects of human skin [65–68]. According to previous research (Fig. 1), light in NIR region has a relatively low absorption coefficient and deep penetration ability [30]. In fact, the NIR region is known as the biological window, where biological systems have minimal absorption [69,70]. Therefore, NIR is considered a useful tool for diagnosis and therapy for deep-seated lesions. According to relevant research [71], in the NIR region the light with longer wavelengths has deeper penetration in tissues. NIR light exhibits a higher level of penetration in tumor tissues than in normal tissues. For example, wavelengths of 630 nm penetrate through normal brain tissue for about 0.9 mm, while the penetration through lung carcinoma is about 1.6 mm. From this information it can be reasoned that NIR light can be used in selective phototherapy [71].
Fig. 1.
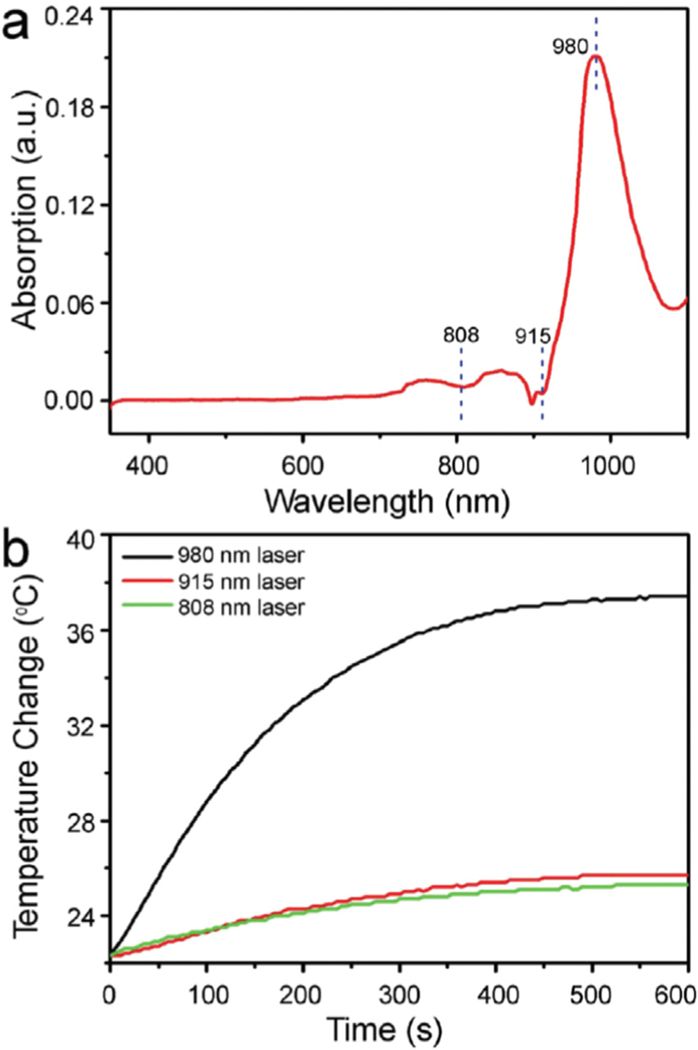
(a) UV-vis absorption spectrum of the pure water. (b) Temperature elevation of the pure water under the irradiation of 808 nm, 915 nm and 980nm lasers with the same power density of 1.0Wcm−2 for 10min [73].
Apart from wavelength, the power of laser light also plays a great role in the interaction between light and tissues. With regard to NIR light (the NIR lasers most commonly used in PTT are 808 and 980 nm), the safe power density limit is ~0.33 Wcm−2 for the 808 nm NIR laser and ~0.726 Wcm−2 for the 980 nm NIR laser according to the American National Standard for the Safe Use of Lasers [72,73]. It is important to note that exposure time and focal spot size can also affect the interaction. In practical application, all of the above mentioned parameters should be taken into account for safety.
3. Light stimulation therapy
Current anti-cancer treatments, such as chemotherapy and radiotherapy, have severe side-effects [1,2]. These treatments cause damage to normal cells while killing cancer cells. To lower the side-effects of cancer treatment, specific target therapies against cancer cells have been proposed [74]; target therapies like nanomedicines have been widely studied for selectively killing cancer cells [75]. To precisely control the expression of nanomedicine, stimulation-responsive nano-systems have been suggested. Since tumors may be deep-seated, it is a challenge to stimulate nano-systems in lesions and avoid overexpression in normal tissues. Overall, NIR light is considered a fine candidate as a stimulation source; this is because low power NIR light could penetrate tissues deeply without causing serious damage or absorption by body fluids [69,70]. Therefore, NIR induced imaging and selective treatment has recently attracted a lot of attention (see Fig. 2). NIR fluorescence has been applied to diagnosis and labeling due to the high penetrability of NIR light through tissues [76–80]. As for anti-cancer treatment, PTT [81] and PDT [82] are two current NIR-induced therapies and have been applied for causing damage to cancer cells with heat and reactive oxygen species (ROS), respectively. In addition, NIR could induce the photothermal effect, which leads to the transformation of heat-transducing nanoparticles, in order to trigger controllable nano-systems for drug delivery [83].
Fig. 2.
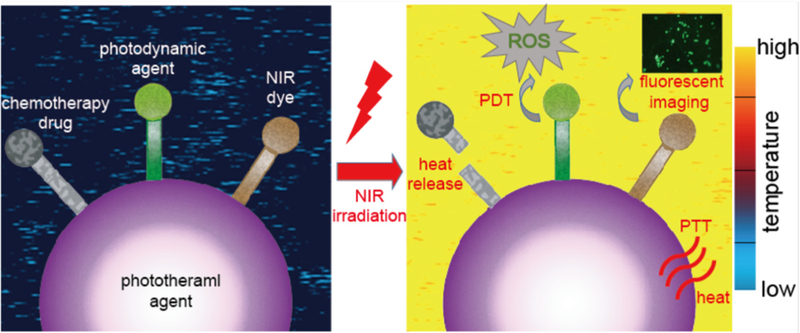
Schematic illustration of photothermal therapy and the synergic medical applications combined with phtotothermal therapy and other light- induced treatment, such as light-induced release of drugs for chemotherapy, photodynamic therapy and NIR fluorescent imaging. The color of background indicates the change of temperature during the photothermal therapy according to the color map on the right.
4. Nanomaterials for photothermal therapy
The current research on ablation agents mainly focuses on nanomaterials. Nanomaterials have many different properties compared to their macroscopic counterparts. It is important to note that the photothermal effect is related to the nanoscale properties of materials. For example, due to surface plasmon resonance (SPR), gold nanoparticles (NPs) are able to convert excited state photon energy into heat [11,84]. In addition, nanomedicines have an advantage in pasive tumor targeting during tumor treatment through a mechanism of nanoparticle induced endothelial leakiness (NanoEL) [85–87] or enhanced permeability and retention (EPR) of NPs for immature and mature tumors, respectively. In NanoEL, NPs with a certain size and density would interact with the endothelial cells’ adherens junction protein and induce micro-sized gaps between endothelial cells, improving the permeability and accumulation of the nanomedicines [85–87]. The size [34], shape [37] and surface chemistry [38,39] of the NPs play a great role in the bio-compatibility and photothermal performance. In the past, noble metal nanomaterials [26,37], as one of the first PTT ablation agents, were widely studied. In recent years, many new nanomaterials, like nanoarchitectured conducting polymers [88–90], have been developed for the application of PTT.
4.1. Noble metal nanomaterials for photothermal therapy
In the past few decades, research on agents for PTT have mainly focused on noble metal nanomaterials, such as platinum-based nanomaterials [91] and gold-based nanomaterials [37,84]. Noble metal nanomaterials have a strong optical absorption property and a relatively high photothermal conversation efficiency without causing quick photobleaching problems; this is a common phenomenon in the use of organic dyes as ablation agents [92]. As mentioned previously, SPR results in the photothermal effect of noble metal nanomaterials [11,84]. When interacting with light, noble metal nanomaterials convert light energy into heat if their oscillation is resonant at the practical frequency of the light [93]. For instance, M. Manikandan et al. studied the photothermal effect of platinum NPs [91]. They found that platinum NPs with sizes ranging from 5 to 6nm could be used as ablation agents for photo-thermal ablation of Neuro 2A cells.
Recently, the studies about the use of gold nanomaterials for PTT have attracted attention among noble metal nanomaterials. Gold NPs without modification, for example, have an SPR effect in the region of visible light [11,94]. Pitsillides et al. [94] used 20 ns laser pulses at 532 or 565 nm for the light irradiation. Although these wavelengths were not exactly in the biological window, the short laser pulses were applied to avoid over diffusion of heat. However, the SPR effect strongly depends on the size and the shape of gold nanomaterials according to the Mie theory [34,95], making it possible to shift the SPR wavelength to the NIR region. In order to tune the SPR and enhance the photothermal performance, different gold nanomaterials have been developed (Table 1), including gold nanorods (NRs) [32], gold nanoshells [96] and gold nanohexapods [37].
Table 1.
Molar extinction coefficient of indocyanine green (molecular dye) and some common gold nanomaterials, including gold nanospheres, gold nanorods, gold nanoshells and gold nanohexapods.
| Photosensitizers | Dimension (nm) | Molar extinction coefficient (M −1x cm −1) | Wavelength (nm) | Ref. |
|---|---|---|---|---|
| Indocyanine green | Molecular | 1.1 × 104 | 778 | [17] |
| Gold nanospheres | Radius = 20 | 7.7 × 109 | 528 | [34] |
| Gold nanorods | Length = 27 | 1.9 × 109 | 695 | [96] |
| Width = 10 | ||||
| Gold nanoshells | Outer radius = 65 | 2 × 1011 | 800 | [11] |
| Inner radius = 55 | ||||
| Gold nanohexapods | Octahedral core = 25 | |||
| Arm length = 16 | 5.5 × 109 | 805 | [37] | |
| Arm width=14 | ||||
Gold NRs have two characteristic optical absorptions [97], the transverse and the longitudinal, corresponding to the diameter and the length of the rods, respectively. Thus, by means of adjusting the aspect ratio (i.e., length/diameter ratio), the SPR region could be tuned and shifted to the NIR region for PTT. Link et al. studied the relationship between the absorption maximum resulting from the longitudinal plasmon resonance and the aspect ratio [97]. According to their research, though the transverse plasmon absorption is located in the visible light region like traditional gold NPs, the longitudinal plasmon absorption could be shifted to 740 nm when the average aspect ratio reached 3.3 (Fig. 3). With tunable longitudinal plasmon absorption, the photothermal effect of these gold NRs could be induced by NIR, making it more suitable for medical application.
Fig. 3.
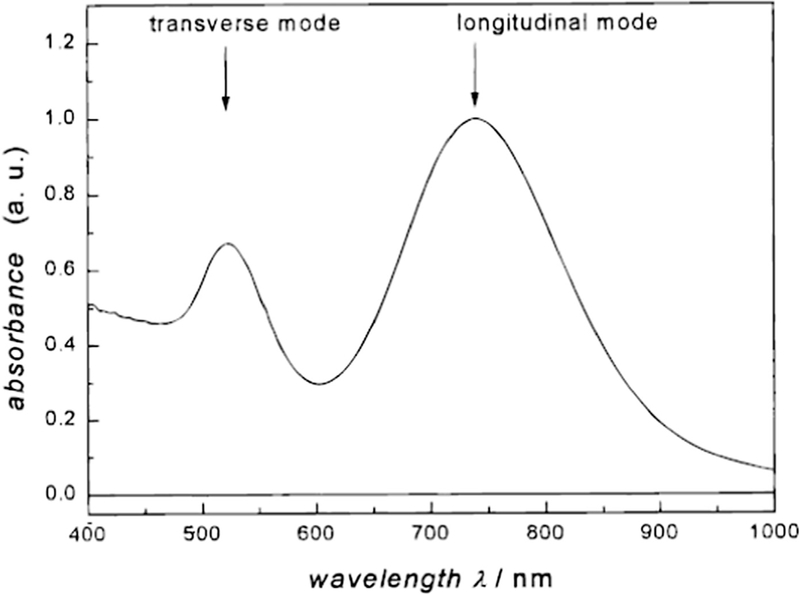
UV-vis absorption spectrum of gold nanorods with an average aspect ratio of 3.3. The band at 740 nm is referred to as the longitudinal plasmon absorption [97].
Apart from the gold nanomaterials with various shapes, dendritic gold nanoparticles have also been studied for possible application on photothermal therapy, because their properties vary according to their hyperbranched nanostructures. The connection between photothermal property and degree of branching of gold nanodendrites has been studied [98]. It is found that the optical property of gold nanodendrites was tunable and the gold nanodendrites with relatively low degree of branching owned better photothermal therapy efficiency within the second NIR window.
Additionally, gold nanomaterials can be employed not only for PTT but also for other medical applications. Gold nanomaterials have been synthesized as drug carriers and imaging contrast agents [99–101], implying their potential role in serving as multifunctional medical agents. A hollow gold nanostructure shape, for example, the gold nanocage, was proposed as a PTT ablation agent with further medical value. The hollow gold nanomaterials possess great NIR absorption for PTT and the unique hollow nanostructure is likely what made them able to act as the drug carriers and/or the contrast agents for imaging [102]. Yavuz et al. [103] covered gold nanocages with a poly(N-isopropylacrylamide)-co-polyacrylamide polymer coating. When heated by the photothermal effect of gold nanocages, the polymer coating would undergo a phase transition and form open pores, allowing the drugs inside the gold nanocages to diffuse. Wang et al. [104] designed drug-loaded gold NRs for combinational chemo-photothernal therapy against cancer. It was reported that the gold NRs were coated with DNA via the self-assembly method of electrostatic interaction. Hence, the NRs could load DOX. Gold NRs played a role as ablation agents as well as DOX release thermal-trigger in the combinational chemo-photothermal therapy (Fig. 4). Recently, gold-shelled silica NPs [105] and gold-shelled Fe3O4 NPs [106] were also proposed. These composites exhibited high optical absorption in NIR region because of the hollow nanostructure of gold nanoshells. In addition, the core composition has the potential to play a great role in further medical applications. For example, the silica nanocores could act as nanosized drug carriers with high thermal stability and the Fe3O4 magnetic NPs could help improve thermal performance.
Fig. 4.
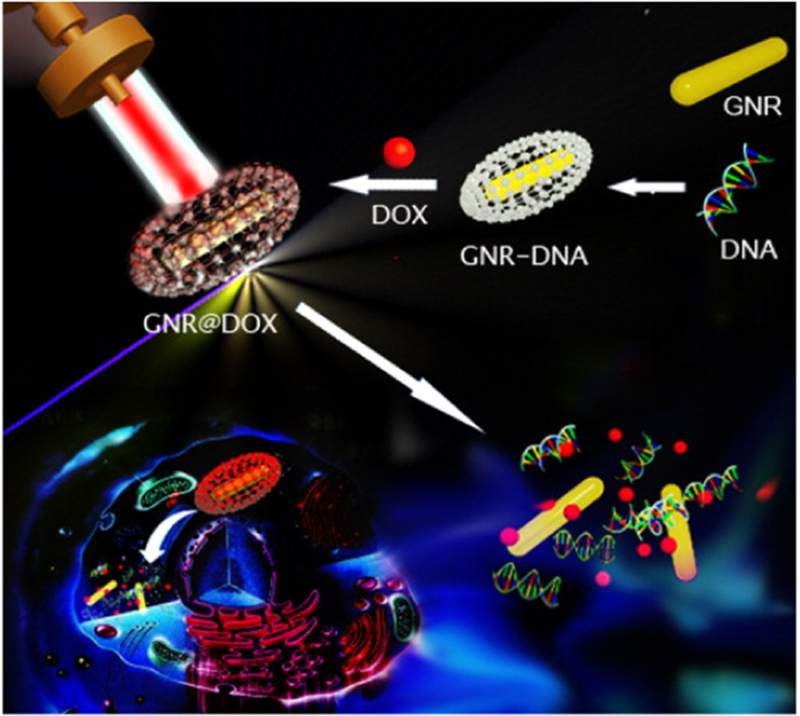
Representative scheme illustrating the fabrication of DNA-wrapped GNRs with DOX loading, and cellular mechanism for combinational chemo and PTA cancer therapy [104].
Though noble metal nanomaterials have provided an outstanding photothermal effect for cancer treatment, some problems remain to be solved. For example, gold nanomaterials are non-biodegradable and thus further detection on gold nanomaterials after treatment is necessary. The photostability of gold nanomaterials is also a concern, since some structures are relatively weak due to the ‘melting effect’ [96]. When exposed to NIR irradiation continuously, some specific gold nanomaterials deform and their photothermal effect reduces. Thus, further study on noble metal nanomaterials viability is needed.
4.2. Semiconductor nanomaterials for photothermal therapy
Besides noble metal nanomaterials, some semiconductor nanomaterials, like semiconductor copper chalcogenide nanomaterials [107,108], have gained their recognition as PTT ablation agents by reason of their low cost and low cytotoxicity. However, under the common NIR light of 808 nm, the relatively low photothermal conversation efficiency of some Cu-based nanomaterials may require higher NIR power to achieve tumor ablation. For example, the use of CuS NPs as ablation agents could cause cell death by photothermal effect under the irradiation of 808 nm NIR light as high as 16 and 24 W cm−2 [108,109]. To surmount this obstacle, NIR light of 980 nm is often selected as an alternative for some Cu-based NPs. 980 nm NIR light proves to be a relatively high limit for human skin exposure and its penetration through biological tissues could reach several centimeters [72,110]. In this aspect, the power of NIR laser could be reduced while the effect of the treatment can be retained.
The size and the shape of Cu-based nanomaterials play a prominent role in the photothermal performance [111–115]. Thus, to improve the photothermal performance of Cu-based semiconductors, adjustment of the size and shape of the Cu-based nanomaterials has been proposed. Hu’s group [116] successfully synthesized CuS NPs with uniform 3-dimensional flower-like morphology, which helped improve the photothermal conversion efficiency. It was hypothesized that such superstructures could serve as the laser-cavity mirrors of a 980 nm laser, leading to the improvement of reflection and subsequent enhancement of photothermal conversion efficiency. They also developed hydrophilic Cu9S5 plate-like nanocrystals with photothermal conversion efficiency up to 25.7%, slightly higher than that of gold NRs under the same 980 nm light irradiation [117]. Besides, a self-assembly process was applied to construct the shuttle-like CuS bundles nanostructures for the better photothermal conversion efficiency (Fig. 5) [118]. Accordingly, when undergoing the self-assembly process, the CuS NPs would form primarily CuS nanorods, and finally shuttle-like CuS bundles. Due to the change of interparticle distance and generation of new electronic structures, the molar extinction coefficient of shuttle-like CuS bundles had a notable increase when compared with the one of CuS NPs, which suggested that the shuttle-like CuS had the better photothermal conversion efficiency than CuS NPs. According to these studies, the nano-architectures of Cu-based materials play a great role in photothermal performance.
Fig. 5.
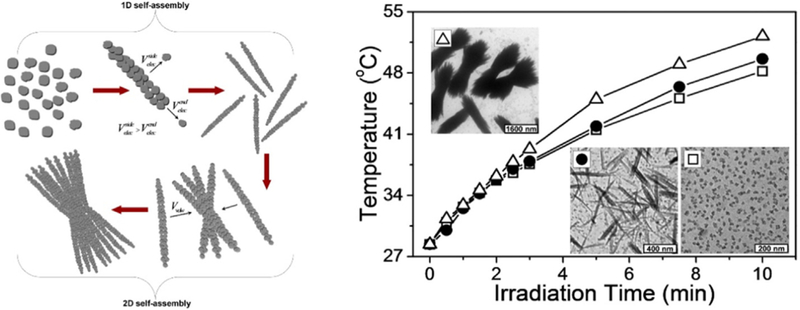
Schematic Illustration of the Stepwise Self-Assembly of CuS NPs to Primary Nanorods and Secondary Shuttle-like Bundles and the photothermal effect improvement from CuS NPs to Primary Nanorods and Secondary Shuttle-like Bundles [118].
In terms of photothermal mechanisms, the NIR light absorption of CuS NPs has been theorized to be intrinsic and derives from the d-d transition of Cu2+. Therefore, the optical absorption property of CuS NPs would not be affected by environment. On the contrary, optical absorption of noble metal NPs would be affected by the environment because it is related to the dielectric constant of the surrounding matrix, which probably results in the difference of maximum absorption peaks between in vivo and in vitro [116]. Since the light absorption ability is derived from the energy band transition of Cu2+, the maximum absorption peak could not be shifted along with the change of particle morphology, but relies instead on the state of the Cu atom. This implies that the enhancement of the photothermal conversion efficiency is related to the ‘utilizing rate’ of NIR, which may result from the NIR light reflection or other optical behavior. For example, as discussed before, the flower-like CuS superstructure could serve as a laser-cavity mirror for the 980 nm laser [116], which may result in improvements on light reflection and absorption, and finally the rise of photothermal efficiency. Recently, Burda et al. discussed the NIR absorption of Cu2-xS [119]. The optical absorption of Cu2-xS was assigned to the Cu deficiencies, which led to a high density of holes and exhibited strong free carrier absorption. According to their study [119], the NIR absorbance of all samples resulted from free carriers, making it dependent on stoichiometry. Furthermore, along with the decrease of x, the degree of doping decreased, which led to the loss of the available energy band; this process resulted in a drop of absorption intensity consequently (Fig. 6). When x was tuned down to 0, the valence band of Cu2-xS (or Cu2S) was filled and no free carrier absorption occurred.
Fig. 6.
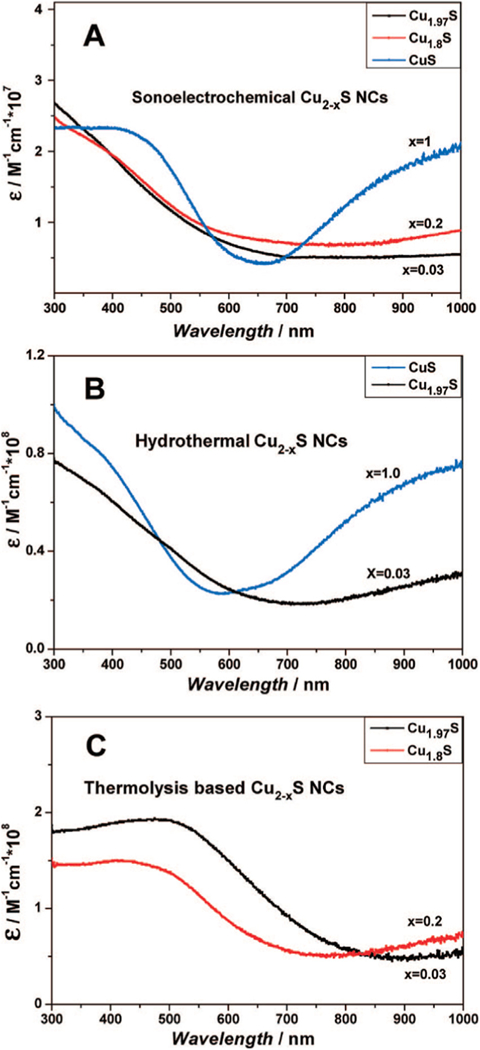
UV-vis spectra of Cu2-xS NCs prepared by (A) sonoelectrochemical, (B) hydrothermal, and (C) thermolysis methods [119].
As for the practical use of such nanomaterials for PTT, CuS NPs have been used in research involving the effects of PTT on Hela cells [108]. The dependent effect of the laser dose and CuS particle concentration was then studied. In this research, though a NIR laser (808nm) as high as 24 W cm - 2 was applied, relatively specific cancer killing was achieved. The NIR laser or CuS NPs alone showed mild effect on killing cells. In addition, the low cytotoxicity of CuS NP was verified individually in this research, implying that CuS NP-induced PTT was non-invasive for normal cells. Moreover, modified Cu7.2S4 nanocrystals with a photothermal conversion efficiency of up to 56.7% were prepared [120]. Such Cu7 2S4 nanocrystals were capable of killing cancer cells efficiently by a low dose concentration and with a low power 980 nm laser. With specific ablation effect, low cytotoxicity and low cost considered, CuS NPs could be applied for PTT as an exceptional ablation agent [108,118,120].
Still, there are some concerns on the PTT use of Cu-based nanomaterials. As reported, some Cu-based nanomaterials require a high powered NIR laser when the stimulation wavelength is 808 nm, which explains why studies have started focusing on PTT with 980 nm NIR. However, 980 nm NIR can be absorbed by water, which means temperature elevation will occur in exposed tissues, healthy or not, under 980 nm NIR without ablation agents. It was reported that temperature elevation of a PBS solution was about 10 °C under irradiation of 980 nm NIR light with a power density of 0.7 W cm - 2 [73]. It is a lurking peril that needs to be investigated before the practical use of 980 nm NIR laser in PTT can be implemented.
4.3. Carbon-based nanomaterials for photothermal therapy
Carbon nanotubes (CNTs) have drawn tremendous attention in the optoelectronics industry [121,122]. Recently, due to the optical properties and excellent thermal behaviors [122,123], CNTs have been studied in the medical fields for use in thermal treatment [124,125]. Applications of CNTs in the medical field have included drug carriers [126,127] and bioimaging probes [128,129]. CNTs can also be either covalently or noncovalently modified with different chemical groups. By binding or wrapping, CNTs can be easily functionalized with other medical molecules [130], like magnetic NPs [131] and anti-cancer drugs [132]. In this way, the photothermal performance of CNTs could be improved and synergic therapies can be developed.
Various factors, like size, dose, surface chemistry and elemental components, are relevant to the toxicity of nanomaterials, including CNTs [133–135]. As mentioned previously, CNTs can be modified with different surface chemical properties, making them valuable for the catalysis industry [136], but it’s possible for CNTs to produce a high dose of toxic compounds [133,135]. Therefore, surface functionalization for a decrease in toxicity must be addressed.
Like other ablation agents [107,137], carbon-based nanomaterials are usually functionalized by PEG coatings. Generally speaking, PEG coatings can help improve biocompatibility, avoid aggregation and prolong blood circulation time [138,139], which is considered as advantageous in nanomaterials applied as ablation agents. Liu et al. [38] investigated the effect of a PEG-PMHC18 coating on PTT using single-walled carbon nanotubes (SWNTs). It turned out that the polymer coating played a substantial role in extending the blood circulation half-life of the SWNTs, which is related to the biodistribution of the SWNTs. It was found to be noteworthy that with the extension of the blood circulation half-life, the SWNTs tumor uptake increased while the SWNTs skin uptake also showed increasing trends (Fig. 7). Such instances as an increase of SWNTs in skin uptake may possibly cause skin damage and reduce treatment efficacy, though high tumor uptake was thought to be positive for the treatment. Therefore, it was highlighted that adjustment of blood circulation half-life needed greater attention for the balance of SWNTs uptake.
Fig. 7.
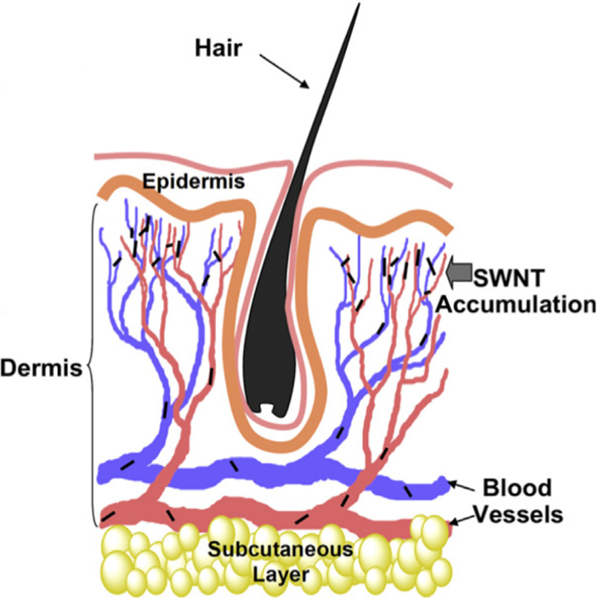
A proposed scheme to explain SWNT skin accumulation. The micro-vessels with diameters as small as 5 mm enriched in the skin dermis may tend to trap SWNTs overtime once nanotubes have an ultra-long blood circulation half-life [38].
To demonstrate the mechanism of PTT mediated by CNTs, Burke et al. [140] characterized the response of breast cancer stem cells (BCSCs) to hyperthermia using water bath or photothermal effect induced by multi-walled carbon nanotubes (MWNTs). It turned out that across a range of temperatures BCSCs were resistant to hyperthermia; but the photothermal effect induced by multi-walled CNTs could overcome the resistance by promoting necrotic cell death. This was hypothetically theorized from the interaction between cancer cell membranes and NIR-stimulated MWNTs with high surface temperature.
Graphene, a planar sheet of carbon atoms, is a relatively new carbon nanomaterial with excellent mechanical and physical properties [141–143]. Gradually, the application of graphene as an anti-tumor option has been demonstrated as better understanding has been obtained [144–147]. Similar to CNTs, graphene-based nanomaterials have a strong optical absorption in NIR region as well as high surface activity [148–151], making it a promising PTT ablation agent with the potential use in the synergic therapies. To clarify the mechanism of PTT induced by graphene, Markovic et al. [152] analyzed the type of cell death resulting from graphene- induced PTT. According to their characterizations, cells died by both necrosis and apoptosis. Despite necrosis, the general cell death modality triggered by PTT, hyperthermia would potentiate oxidative stress/superoxide production and finally cause apoptosis as well (see Fig. 8).
Fig.8.
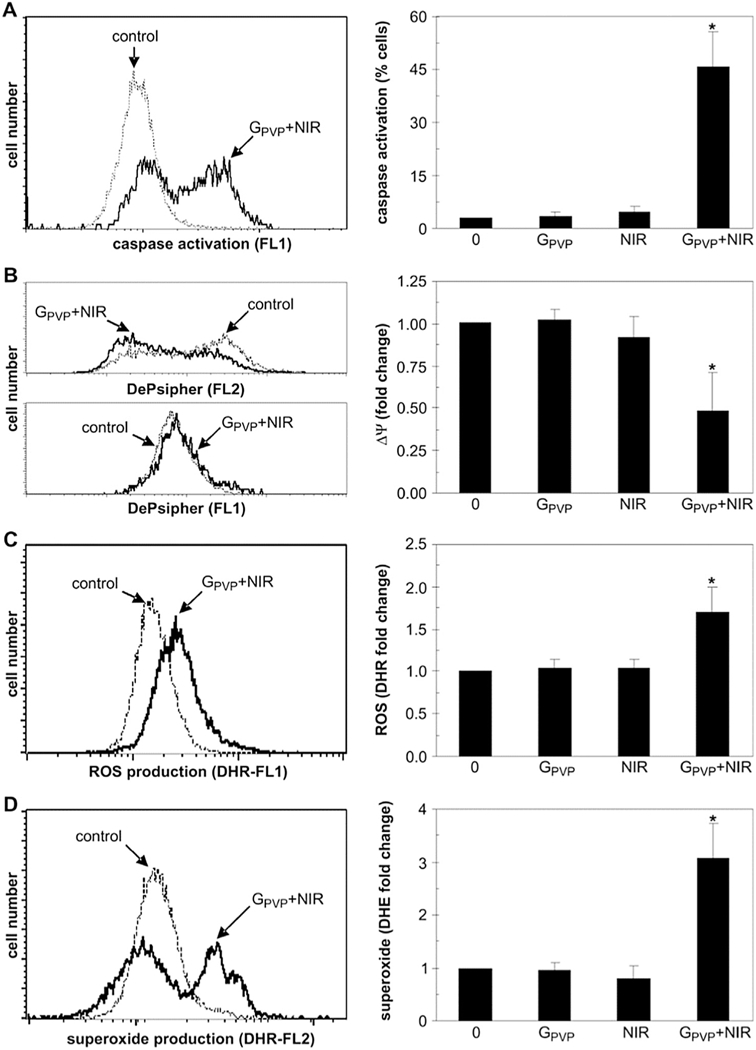
The intracellular mechanisms of graphene-induced photothermal cell death. (A-D) U251 cells were exposed for 3 min to NIR laser (808 nm, 2Wcm−2) in the absence or presence of GPVP (10mg/ml). After 24h, flow cytometry was used to assess caspase activation (ApoStat staining; A), while the mitochondrial membrane potential (ΔΨ) (DePsipher staining; B), production of ROS (DHR staining; C) and superoxide (DHE staining; D) were measured after 4 h. The representative photomicrographs, histograms and dot plots are shown. The results are mean ± SD values from three experiments (*p < 0.05 refers to untreated cells and cells treated with NIR or GPVP alone; ANOVA). [152]
Overall, both CNTs and graphene have shown similar features. Their surface activities could result in further functionalization as well as harmful degeneration in contrast. Thus, both CNTs and graphene need further modification to be functionalized for synergic therapies. As for the mechanism, it has been suggested that photothermal effects mediated by CNTs and graphene could result in necrosis and apoptosis, showing greater anti-cancer effect when compared with single hyperthermia.
4.4. Conducting polymers for photothermal therapy
The conducting polymers (CPs), such as polyaniline (PANI) [153,154] and polypyrrole (PPy) [155], have been used in bioelectronics and biomedical applications. In addition, their optical absorption abilities make it possible to employ CPs for use in PTT. CPs as a PTT ablation agent has attracted significant attention, because they show lower cost than noble metal nanomaterials and better biocompatibility than inorganic nanomaterials [156–158]. The mechanism to induce the photothermal effect of CP-based nanomaterials is thought to be quite different from the inorganic nanomaterials. When excited by specific light stimulation, a bio- polaron is formed that subsequently decays into a photon band while generating heat [159,160]. Furthermore, nanoarchitectured CPs could shift their optical absorption peak during the doping process [161–163]. In more detail, dopants would help generate an interband gap that could finally result in the change of the excitation-energy level, which is often applied to improve the conductivity of conducting polymers. It is suggested that such a doping process would also change the optical properties of CPs. For example, the water soluble PANI NPs were synthesized by the chemical oxidative polymerization method and the doping-dedoping process [164]. According to this research, the PANI NPs could result in tumor ablation when under the irradiation of NIR. The NIR absorption ability of these PANI NPs was dependent on pH. Moreover, the transformation of PANI NPs from the emeralidine base state to the emer- alidine salt state during NIR stimulation was confirmed (Fig. 9). It has been reported that these CPs can convert the NIR light energy into heat. However, as some studies mentioned [164,165], the photothermal conversion efficiency of PANI was relatively low and bare Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) NPs were found to be toxic to cells. Therefore, some modifications like PEG-coating [165] have been utilized and more novel CPs-based nanomaterials are under research.
Fig. 9.
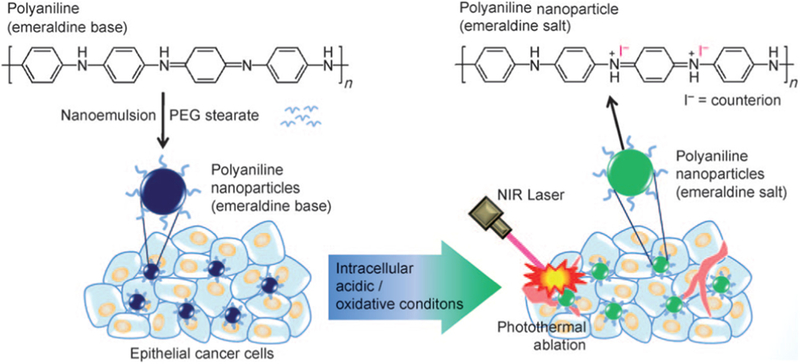
Schematic illustration of the preparation of organic photothermal agents based on polyaniline nanoparticles and their application for the photothermal ablation of epithelial cancer cells by NIR laser irradiation [164].
To improve the performance of PEDOT:PSS NPs, Cheng’s group synthesized PEG-coated PEDOT:PSS NPs [165]. Their research demonstrated that these NPs owned a long blood circulation half lifetime (21 h) and a high tumor uptake (28%), resulting in an impressive effect on tumor ablation according to the in vivo experiment. Recently, PPy nanomaterials have been utilized as a PTT ablation agent. Among the CPs, PPy nanomaterials have a relatively fine biocompatibility and have been studied in medical fields as biosensors and neural probes [144,166–168]. PPy nanomaterials have fine photostability and high photothermal conversion efficiency. As reported, sub-100 nm PPy NPs showed strong optical absorption at 808 nm (2.38 × 1010M−1cm−1) and a high photothermal conversion efficacy (44.7%) [89]. Dai’s group [88] studied the cancer cell ablation ability of PPy NPs. They synthesized uniform PPy NPs with average size of 46 nm via aqueous dispersion polymerization. In vitro study showed that Hela cells incubated with PPy NPs were destroyed when exposed to the irradiation of NIR (Fig. 10). These studies demonstrate that PPy NPs could work as an ablation agent to offer a selective therapy with low side effects.
Fig. 10.
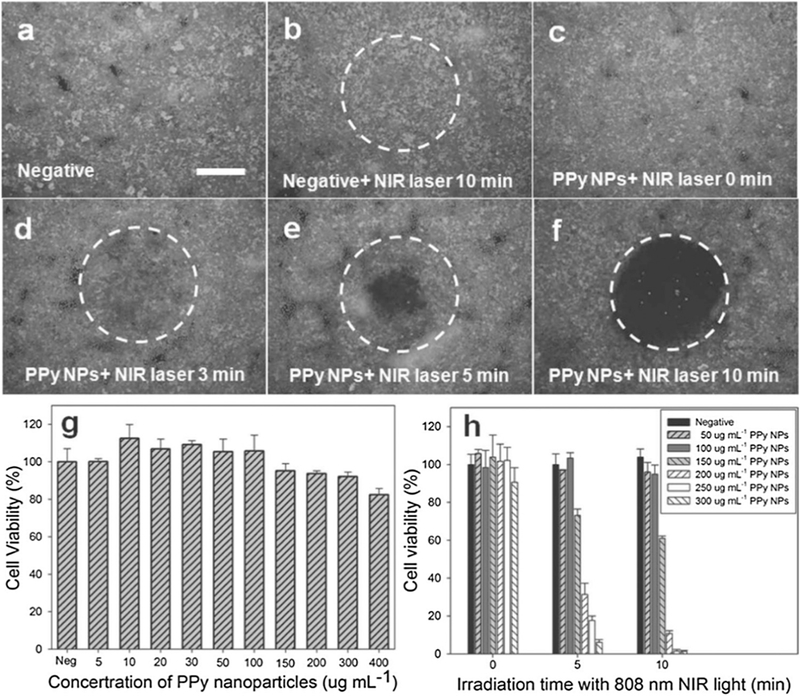
Photothermal destruction of Hela cells with or without PPy NPs and NIR laser (808 nm, 6Wx m−2) treatments (a, b, c, d, e and f). White circle indicates the laser spot; live/dead stain for viability. Scale bar = 800 μm. (g) Cell viability of HUVECs with 24 h exposure to various concentrations of PPy NPs. (h) Cell viability after treatment with different concentrations of PPy NPs and different NIR laser irradiation time [86].
PPy is relatively biocompatible, stable and easily synthesized [166–168]; thus, PPy could be an outstanding candidate as polymer coating for other nano-drugs. As mentioned previously, gold NPs have a strong SPR effect in the region of visible light, but not in the exact NIR window without further modification. It limits the use of gold NPs in PTT. To tune the responsive region to NIR, PPy-coated chainlike gold NP architectures were produced [169]. This PPy-gold composite had greater photothermal transduction in the NIR region and showed specific effect in inhibiting tumor growth (Fig. 11). Regarding this study as an example, PPy nanomaterials could be used as a photothermal coating for synergic treatments or multiple PTT.
Fig. 11.
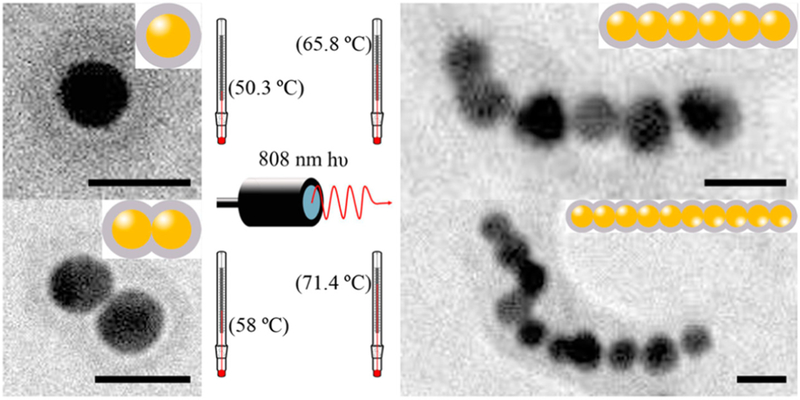
Schematic illustration of photothermal effect of PPy-coated chainlike gold NPs [169].
In a conclusion, CP-based nanomaterials have shown remarkable photothermal abilities and potential use in clinical PTT due to their high photostability. Similar to gold-based nanomaterials, nanoarchitectured PPy nanomaterials could work as drug carriers. Alternately, various synthesis methods, like chemical oxidation [88] and electrochemical deposition [170], were applied to synthesize nanoscale PPy-functionalized coating for enhanced medical systems. Thus, PPy nanomaterials could work as outstanding ablation agents as well as accessories for modified medical systems involving synergic therapies.
Overall, the nanomaterials as ablation agents cover noble metals, semiconductors, carbon-based materials, conducting polymers and so on (Table 2). The diversity makes it possible to fabricate composite ablation agents with different nanomaterials for greater photothermal effect.
Table 2.
Nanomaterials as ablation agents for PTT.
| Nanomaterials | Size (nm) | Shape | Wavelength (nm) | application | Ref. |
|---|---|---|---|---|---|
| Au nanoparticles | 10–50 | Nanospheres | 528 | Cervical carcinoma | [34] |
| Nanorods | 695 | [96] | |||
| Nanoshells | 800 | Breast epithelial carcinoma, etc. | [11] | ||
| Nanohexapods | 805 | [37] | |||
| Pt nanoparticles | 1–20 | Nanospheres | 1064 | Neuroblastoma | [91] |
| CuS nanoparticles | 1–20 | Nanospheres | 808 | Cervical carcinoma | [108] |
| [109] | |||||
| 500–800 | Flower-like | 980 | Glioblastoma, etc. | [116] | |
| [120] | |||||
| carbon nanotubes | 50–500 | Multiwalled/ | 1064 | Breast cancer | [140] |
| single-walled nanotubes | 808 | [38] | |||
| Graphene | 50–360 | Nanospheres | 808 | Glioblastoma | [152] |
| PANI | 100–150 | Nanospheres | 808 | Epidermoid carcinoma | [164] |
| PEDOT:PSS | 80–130 | Nanospheres | 808 | Breast cancer | [165] |
| PPy | 40–60 | Nanospheres | 808 | Breast cancer | [88] |
| [89] | |||||
5. Further development of photothermal therapy
Traditional therapies like radiation therapy and chemotherapy have been clinically used in cancer treatment [171–173] whereas PTT is a relatively new method showing great promise for anti-cancer capabilities. However, because the power of light may decrease along with the penetration through tissues, the therapeutic effect of PTT may decay in practical use [174]. Therefore, for a thorough cure, it’s reasonable to combine PTT with traditional therapies. Here we emphasized the synergic therapies containing PTT and other therapies. First of all, synergic therapies help lower side effects because the dose of each therapy can be reduced, while the total treatment effect can be maintained [175–177]. Interaction among therapies could lead to greater efficiency; for example, heat produced by the photothermal effect could improve the effect of some anti-cancer drugs [176,177]. The thermal effect, called hyperthermia in medical fields, may not directly cause tumor ablation by heat, but could improve the efficiency of other therapies. Furthermore, some nanomaterials as ablation agents, like CP based nanomaterials and gold nanomaterials, which could be stimulated by electricity [178] or irradiation [179], could work as controllable carriers for anti-cancer drug delivery. Thus, many nanosystems have been fabricated for synergic therapies and some actively responsive nanosystems have been developed.
5.1. Imaging guided photothermal therapy
PTT can be initiated after the ablation agents are associated with the tumors through the mechanisms such as EPR or NanoEL. To detect the distribution of ablation agents and assess PTT performance on tumors, real time imaging techniques are needed.
X-ray imaging is a traditional tomographic method for diagnosis of various pathologies [180], including cancer. In some cases, when noble metal nanomaterials are applied as the ablation agents, X-ray imaging is available for PTT performance observation. Metals with a high atomic number (e.g. gold) can be detected by X-ray [181]. Other than X-rays, a number of imaging techniques have been applied for diagnosis. Although the images have a lower resolution, ultrasound imaging still has some advantages over other techniques [182–184]. Ultrasound imaging provides a low-cost and portable way for diagnosis. More importantly, ultrasound could provide instant, dynamic and multidimensional images, making it suitable for locating the PTT ablation agents and observing the ablation of tumors in real time. Contrast agents are necessary for ultrasound imaging, but not all ablation agents can act also as contrast agents and be observed by ultrasound imaging. Gold nanoshells [185] and PPy hollow microspheres [186] can be used as both ablation agents and contrast agents for ultrasound imaging simultaneously. These agents have hollow structures similar to microscale bubbles [187], the traditional contrast agents for ultrasound imaging due to their oscillation at the practical ultrasound wave. Recently, research has paid great attention to the combination of photoacoustic (PA) detection and PTT [188–190]. PA is a promising imaging method because it is non-invasive and has the capability to image deep biological tissues [191–194], where tumors are usually located. More importantly, NIR could be chosen to induce ultrasound signals in PA imaging [189]. Thus, it is possible to use NIR to induce photothermal effect as well as gain tumor ultrasound images, potentially achieving PA-guided PTT [195]. TaOX@PPy NPs with a core-shell structure was proposed for more precise detection by PA [196]. PPy shells would act as an ablation agent as well as the PA contrast agent while the TaOX core operated as an X-ray computed tomography (CT) contrast agent. In this way, bimodal imaging guided PTT could be applied (Fig. 12).
Fig. 12.
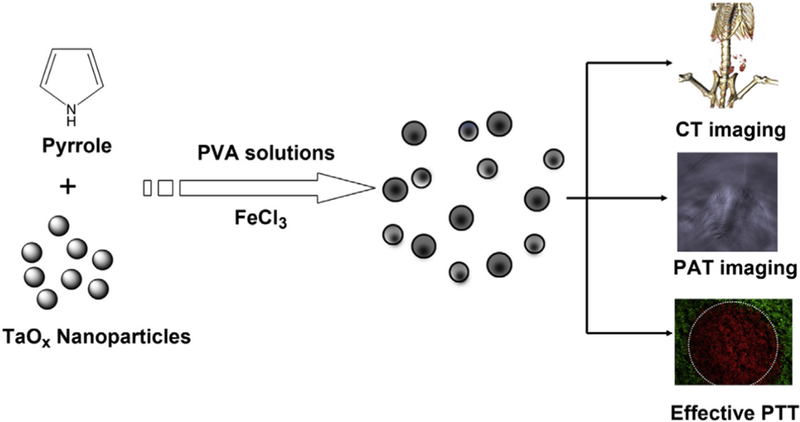
Schematic illustration of the formation of TaOx@PPy NPs for combined CT/PA imaging and PTT [196].
Apart from radiographing, surface-enhanced Raman scattering (SERS) assays have gained great attention in imaging guided PTT [197–199]. SERS can label and detect cells through NIR laser stimulation, making it a noninvasive detection method. Cai’s group [200] reported an aptamer-Ag-Au shell-core nanomaterials; the aptamer was applied in this research to distinguish A549 cells (lung adenocarcinoma cells) from normal tissue. These shell-core nanomaterials could detect A549 cells through SERS signaling, leading to the success in monitoring the PTT induced by the Au core (see Fig. 13).
Fig. 13.
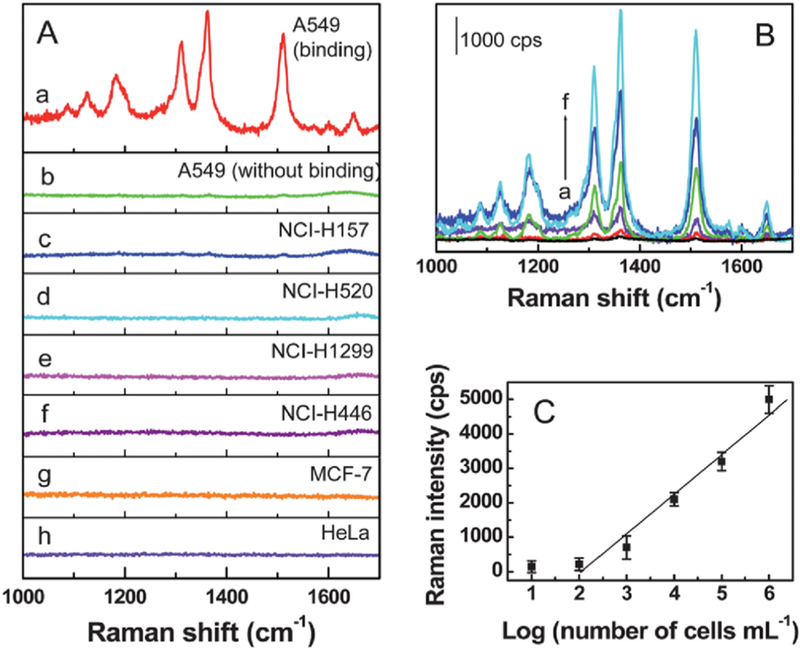
(A) SERS spectra of the A549 cells with (a) and without (b) binding of the Rh6G-labeled aptamer-Ag-Au nanostructures. (c-h) SERS spectra of the NCIH157, NCI-H520, NCI-H1299, NCI-H446, MCF-7, and HeLa cells, respectively, after incubating with the Rh6G-labeled aptamer-Ag-Au nanostructures. (B) SERS spectra of the A549 cells after binding of the Rh6G-labeled aptamer-Ag-Au nanostructures at the cell number (cells per mL) of (a) ~ 1* 101, (b) 1 * 102, (c) 1 *103, (d) 1 * 104, (e) 1 * 105, and (f) 1* 106, respectively. (C) Dependence of the SERS signals at 1363 cm−1 on the A549 cell numbers (in cells per mL) [200].
Many strategies have been proposed to achieve imaging and monitoring of PTT. SERS is considered the most promising one for practical use as it shows many advantages [200]. For example, SERS could detect targeted cells with the use of a specific aptamer. Furthermore, SERS imaging could be stimulated by NIR laser, which may cause relatively mild damage to tissues compared to X-rays. Different from NIR-based fluorescence imaging, NIR SERS imaging makes it possible to detect cancer cells as well as eradicate them with PTT simultaneously with same laser source. NIR SERS imaging could be applied as a dye-free method [201], which allows it to avoid the photobleaching problem. Therefore, we emphasized NIR SERS imaging as a promising imaging method for guiding PTT (see Fig. 14).
Fig. 14.
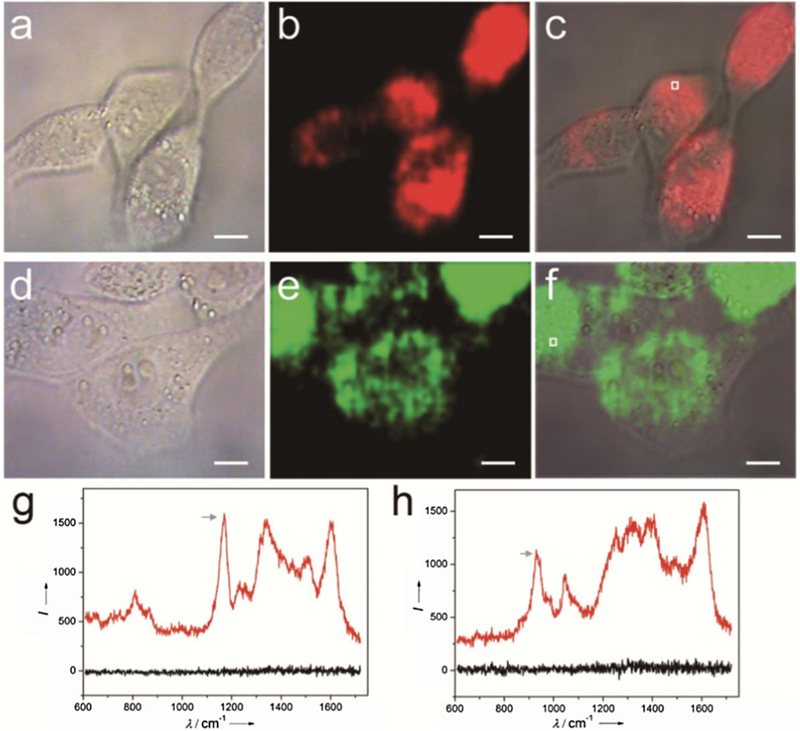
NIR SERS imaging of 4T1 cells using GNR-PANI (a-c) or GNR-PPy (d-f) as nanotags. (g and h) SERS spectral lines acquired from the 4T1 cells incubated with GNR-PANI (c) and GNR-PPy (f), respectively. Black lines are the background lines. Scale bar: 10 μm [201].
Modification on ablation agents for imaging during PTT has been proposed; detecting and tracking these nanomaterials after PTT is also needed because most nanomaterials as ablation agents (e.g. gold NPs, PPy NPs) are non-biodegradable. Observation on the metabolism and excretion of these ablation agents is necessary to avoid a potential bio-hazard. However, the follow-up survey on the toxicity of nanomaterials is usually by histological analysis. Imaging methods for real-time tracking on nanomaterials after treatment have been seldom reported. Therefore, real-time imaging and detection of nanomaterials after PTT is a topic that must be studied in order to gain a better understanding of the long term effect of these ablation materials.
5.2. Hyperthermia for improving traditional therapy
Thermal therapy can be divided into two categories. The first one is the aforementioned PTT, a treatment of ablating cancer cells by heat directly. The second one, called hyperthermia, is usually by heating below 44 °C; cell ablation is not fully expected in hyperthermia. It is applied to induce ‘fever’, elevated locally or throughout the whole body, in order to boost the treatment [202–206]. The traditional cancer treatment may cause severe side effects, for example, chemotherapy and radiotherapy kill cancer cells and normal cells at the same time. With the development of the treatments including hyperthermia, the therapies mentioned above could be improved [207–209]. Hyperthermia can enhance the effect of drugs or irradiation, resulting in improved therapeutic effect or reduced dose. For example, gold NRs were applied to induce localized mild hyperthermia [210]. This treatment could increase vascular permeability, in order to enhance transport of macromolecules for chemotherapy [210] (see Fig. 15). In the application of hyperthermia on anti-cancer treatment, heat is usually produced by photothermal agents, such as the nanomaterials capable of selectively locating and accumulating in tumors. The locally elevated temperature makes tumors more sensitive to these therapies while surrounding healthy tissues suffer mild effects. Nanomaterials with photothermal effects can act as a heat-triggered drug delivery system, making it possible to control drug release [211]. It is reported that a photo-responsive protein-- graphene-protein (PGP) capsule was constructed for heat-triggered drug delivery, which induced an enhanced therapeutic effect [212]. This work shows that such capsules could work as drug carriers with controllable delivery characteristics, improving the cell uptake of the capsule and release of the drug. The interaction of heat and drugs improved cellular drug uptake and DNA damage [212]. In summary, hyperthermia is a common phenomenon in the application of ablation agents during PTT. It is an important characteristic to consider when designing a responsive drug delivery system and a prerequisite for the synergic therapies with PTT.
Fig. 15.
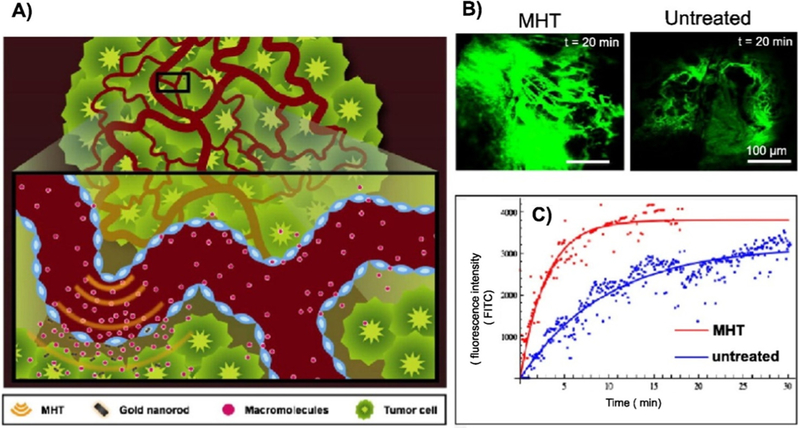
Illustration of hyperthermia-driven transport. Mild heat is generated by near infrared laser irradiation aided by heat-absorbing gold nanorods (A). Effect on tumor transport was studied by intravital microscopy with images demonstrating increased macromolecular accumulation (fluorescent intensity) in tumors receiving heat treatment versus control (B). Mathematic modeling was further used to quantify enhancements which revealed that the rate of extravasation increased by 30% while the total macromolecule accumulation increased by 200% (C) [210].
5.3. Photothermal-photodynamic synergic therapy
Both PTT and PDT are medical treatments induced by light. Under light irradiation, PDT agents could be activated and form singlet oxygen, which is cytotoxic to cells nearby due to oxidative stress [213–215]. In PDT, in terms of penetration, the NIR region is highly desired [216–218], similar to PTT. Therefore, a synergic therapy consisting of PTT and PDT could be an efficient treatment for cancer. Some PTT agents, such as gold NPs [82], could act as PTT ablation agents and the carriers of PDT agents simultaneously. Since singlet oxygen has a short half-life and relatively narrow diffusion range [219,220], the PDT agents should be ideally transported to the subcellular level before treatment. Hence, PTT agents would improve the efficiency of PDT.
A nanosystem for photodynamic-photothermal synergistic therapy was proposed [221]. Specifically, methylene blue (MB), a traditional PDT drug, was combined with magnetic core-plasmonic shell nanoparticles (Fig. 16). The magnetic core served as an imaging contrast agent whereas the plasmonic shell, known as a gold nanoshell in this study, worked as a PTT ablation agent. The study demonstrated that this nanosystem resulted in NIR-induced photodynamic-photothermal synergistic therapy.
Fig. 16.
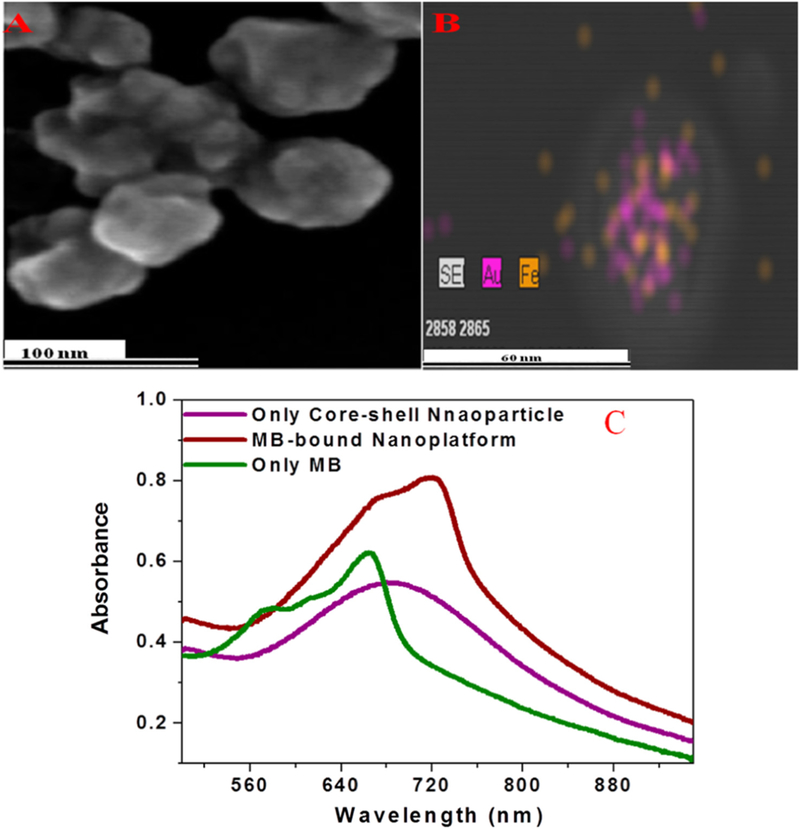
(A) SEM images of freshly prepared core-shell plasmonic nanopopcorn. (B) EDX mapping shows the presence of Fe and Au in a core-shell nanoparticle. (C) Absorption spectra of core-shell nanoparticle, methylene blue, and methylene blue conjugated nanoplatform [221].
In addition, Guo et al. [222] proposed an innovative way to transport PTT and PDT agents for the photothermal/photodynamic synergic therapy. They used micelles to load Ce6 (PDT agents) and Cypate (PTT agents). Such a system could translocate both agents into subcellular environments while working as contrast agents for PA imaging and near-infrared fluorescence imaging (Fig. 17). This research developed a multi-functional platform for a dual imaging-guided and synergic light-induced treatment. Recently, Kalluru et al. [223] reported that nano-graphene oxide decorated with PEG and folate could produce singlet oxygen and achieve temperature elevation as well when under the irradiation of NIR (980 nm) light. Their work could shed light on the application of PTT-PDT synergic therapy with the use of one single photo-sensitive agent.
Fig. 17.
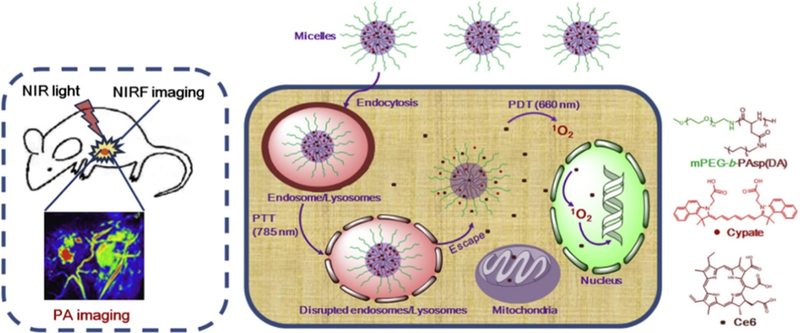
Schematic illustration of PS-loaded micelles integrating cyanine dye for dual-modal cancer imaging and synergistic therapy of PTT and PDT via an enhanced cytoplasmic delivery of PS [222].
Although PTT and PDT could be applied simultaneously with one NIR light source, theoretically, there are some unsolved problems. First, there is a possibility that the maximum absorption wavelengths among the NIR region of PTT agents and PDT agents are not exactly the same. NIR light with a single wavelength can’t fully induce both the photothermal effect and photodynamic effect at the same time, while broadband NIR light may bring an unexpected negative effect. Synergic therapies combining PTT and PDT are supposed to provide a better therapeutic effect. However, since both treatments are NIR-induced, it may be difficult to target deeply seated tumor due to the decrease of NIR during the penetration through tissues. In this case, these synergic therapies may be a total failure with inefficient NIR irradiation.
5.4. Photothermal-immuno/chemo synergic therapy
Like PTT, immunotherapy is a promising treatment for cancer. Unlike the traditional treatments such as radiotherapy and chemotherapy, immunotherapy relies on the immune system of patients themselves rather than external, fierce stimulation [224–226]. Cancer cells suppress the immune system, for example, by reducing T cells stimulation [226,227], so that the immune system is unable to respond efficiently. Immunotherapy aims at inducing an immune anti-cancer response by delivering vaccines or adjuvants. As for chemotherapy, the biggest challenge is to lower its side-effects. Targeted therapy is thought to be a useful strategy; therefore, it is reasonable to functionalize ablation agents with chemotherapy drugs, because ablation agents in nanoscale would passively target tumors through NanoEL or EPR effects for immature or mature tumor, respectively. In this case, drugs could also be delivered into tumors and reduce damage to normal tissues.
Since both ablation agents and immuno/chemo medicines have greater therapeutic effect when inside a tumor, a lot of research is focused on the development of a nanosystem for drug delivery. Chen’s group [228] invented nanocookies as a drug delivery nanosystem and applied this nanocomposite for synergic photo-chemothermal therapy (Fig. 18). This nanocomposite contained me- soporous silica as a drug carrier and amorphous carbon for improving drug delivery capacity. Alternatively, reduced graphene oxide and (S)-( + )-camptothecin (CPT) were used as PTT agents and anti-cancer drugs, respectively. Further experimentation showed that this nanocomposite had a remarkable efficacy for killing cancer cells.
Fig. 18.
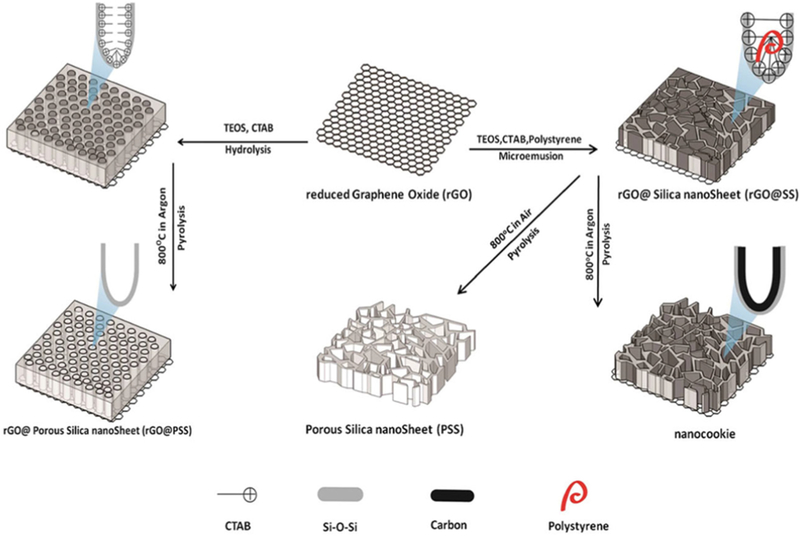
Schematic illustration of synthesis and microstructure of reduced Graphene Oxide (rGO), Porous Silica nanoSheet (PSS), rGO@Silica nanoSheet (rGO@SS), GO@Porous Silica nanoSheet and nanocookie [228].
Since some ablation agents could be applied as drug carriers, direct functionalization on ablation agents with anti-cancer drugs for synergic therapies was proposed. For example, Wang et al. [132] synthesized polyethylenimine functionalized single-walled carbon nanotubes with the remarkable sustained-release effect of doxorubicin (DOX). Carbon nanotubes in this study worked as a NIR- induced drug carrier. To achieve tumor immunotherapy and chemo-photothermal therapy, gold NRs were also applied as an ablation agent as well as a carrier for cytosineeguanosine (CpG) oligodeoxynucleotides (ODNs) and DOX by conjugation [209]. Zandberg et al. [229] demonstrated the photothermal-induced release of small molecules from gold NPs in live cells. In this study, as a model drug, a fluorescent dye was anchored on the surface of gold NPs. Heat induced by photothermal effect of gold NPs triggered the release of the fluorescent dye, the fluorescent appearance of cells would change and the success of the photothermal effect can then be detected. Furthermore, when real anti-cancer drugs are anchored on gold NPs, a nanosystem combined with ablation agents and controllable drug carriers for synergic therapy can be achieved.
In conclusion, the synergic therapy with photothermal and immunotherapy/chemotherapy is not only a simple accumulation of these treatment’s effects. First of all, hyperthermia caused by the photothermal effect could improve the treatment effect of immunotherapy/chemotherapy. Secondly, some ablation agents could be used as drug carriers, so it is reasonable to functionalize ablation agents with other anti-cancer drugs. This allows the blood circulation time of anti-cancer drug to be extended while ablation agents could be functionalized for other needs, such as better biocompatibility and specificity targeting. Moreover, when ablation agents work as drug carriers, their photothermal effect could make them a temperature responsive drug delivery system. These coupled therapy options provide prospective options for the future that should continue to be investigated and developed.
There are more and more nano-systems being constructed for synergic therapies with PTT. The main purpose is to (1) monitor the process of tumor ablation during PTT treatment, (2) improve the therapeutic effect with the use of PDT or chemical drugs and (3) improve the selectivity of the ablation agents to targeted lesions. For example, gold-coated Fe3O4 nanoroses, is another model that is being researched [230]. The nanoroses possess five medical functions for anti-cancer treatment, including magnetic resonance imaging (MRI), optical imaging, integrating aptamer-based targeting, PTT and chemotherapy (see Fig. 19). Though more specific clinical experiments are needed to verify their potential clinical use, this study highlights the importance of the combination of targeting, real-time imaging and synergic therapies against cancer.
Fig. 19.
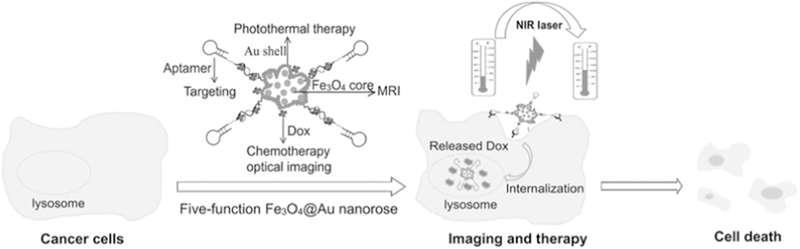
Schematic representation of five-function Fe3O4@Au nanorose for cancer cell targeting, MRI, optical imaging, photothermal and chemotherapy. Sgc8 aptamers are conjugated on the surface of nanoroses for targeting of CCRF-CEM cancer cells. Anticancer drug (Dox) is specifically delivered into cancer cells for chemotherapy and optical imaging. Fe3O4@Au nanorose is an efficient MRI agent and photothermal agent; therefore, MRI and photothermal therapy can be achieved for cancer cell imaging and therapy [230].
6. Challenges
The cytotoxicity of foreign matter, especially the one on a nanoscale, must be investigated before practical use can be explored. The ablation agents for PTT are no exception; especially since most of the ablation agents mentioned above are non-biodegradable. As a matter of fact, the cytotoxicity of nanomaterials has been one of the greatest concerns when incorporating nanomaterials into medical use [231,232]. First of all, compared with their macroscopic counterparts, nanomaterials can pass through skins, blood vessels and cell membranes easily, making them hard to track or detect; consequently, the cytotoxic nanomaterials could enter systemic circulation and reach end organs [233,234]. Secondly, nanomaterials may exhibit high surface activity due to the surface effect [235–237], which may make them easily modified by toxic compounds and harmful for cells through inflammation or production of ROS. As mentioned previously, CNTs may pose a health risk due to their surface effect. According to the pulmonary toxicity investigation [135], CNTs are more toxic than carbon black to lungs, indicating that the airborne CNTs could become a biological hazard under certain conditions. It has also been found that two commercially available carboxylated SWNTs are significantly cytotoxic [133]. The examples above suggest that CNTs must be prepared cautiously and assessed before medical use. As for gold nanomaterials, cytotoxicity of gold nanoparticles has been studied widely since they are one of earliest candidates for PTT and other medical uses. Though studies [238,239] have indicated certain kinds of gold NPs taken up by cells will not cause acute cytotoxicity, there have been conflicting opinions suggesting that the cytotoxicity of gold NPs is size dependent [240]. Thus, in order to synthesize gold NPs with low cytotoxicity, precise control of particle size is mandatory. Conversely, changes of size/shape of gold NPs could greatly affect the photothermal effect of such nanomaterials [34,37]. Therefore, we can conclude that precise synthesis and modifications to gold NPs in order to lower their cytotoxicity while maintaining or improving their photothermal effect is a great challenge. Among conducting polymers, PPy nanomaterials are considered promising candidates for PTT, due to the biocompatibility of PPy. According to the research of Zheng’s group [89], histological analysis showed that PPy NPs caused no noticeable damage to the liver, spleen or kidney after acting as ablation agents in tumor-bearing mice. Although some nanomaterials when used as ablation agents have been proven harmless or accumulated in liver and spleen through histological analysis, long-term observation for the tracking of nanomaterials and the toxicity to host is still needed. To improve the biocompatibility of ablation agents, it is common to functionalize the nanomaterials with safe coatings. For example, PEG coatings have been widely studied on the functionalization on nanomaterials [38,139], because some medical use of PEGs and medicines developed with PEGs have been approved by the Food and Drug Administration (FDA). Such coating strategies could lower the core material’s cytotoxicity but maintain their photothermal efficiency. It may also help prolong blood circulation time [139], improving the PTT effect. Although such modifications may lower cytotoxicity of ablation agents in PTT, enhanced ability to detect ablation agents is still needed in order to observe the metabolism and excretion of these ablation agents.
The surface properties of nanomaterials also affect medical performances other than biocompatibility as well. Nanomedicines could passively target immature or mature tumors through the NanoEL or EPR effect [85–87], respectively, and unstable nanomedicines may aggregate during blood circulation. The increase of size may result in the detection or absorption of ablation agents by the immune system. Moreover, the change of size may also affect the photothermal efficiency [34,37] and cytotoxicity [240]. Or even worse still, aggregation may block blood vessels, which can cause safety concerns to rise [241]. Therefore, with the unexpected increase of size, nanomedicines like ablation agents may become invalid. Under ideal circumstances, the diameter of nanomaterials should be between 20 and 100 nm [242–244], in order to avoid filtration by kidney and clearance by the reticuloendothelial system (RES). To synthesize stable nanomaterials with good aqueous dispersion, surface coatings, such as PVP ligand [241] and silica shell [245] have been applied for surface modification of nanomaterials. Furthermore, the surface properties play a great role in the medical performance of ablation agents, because surface properties, such as hydrophilicity [246,247] and surface charge, [248] affect the blood circulation time of ablation agents. In more detail, hydrophilic surfaces prevent interaction and absorption by proteins and clearance by RES [249–251]. Similarly, the surface charge also affects absorption by proteins and recognition by macrophages; it has also been reported that macrophage uptake increases with the increase of absolute value of the zeta potential of ablation agents [244,252–254]. It has been suggested that prolonged blood circulation time results in a greater chance of ablation agent accumulation in tumorous tissue before elimination of ablation agents by RES [244]. In this aspect, ablation agents with the capacity to meet the requirements above can be applied to achieve effective tumor uptake, and the risk of safety problems may decrease. Therefore, surface properties of nanomaterials for PTT should be modified and assessed before medical use.
Besides, the tumor-targeting ability of nanomaterials should be emphasized. It is a common understanding that ablation agents attached with or internalized in cancer cells will lead to greater therapy efficiency than those around the lesion. To increase the amount of the effective ablation agents is beneficial to avoid over dose of nanomaterials or NIR laser. Various methods were studied to induce greater the EPR effect for better accumulation of anti-cancer agents in tumor, such as surface charge adjustment [255,256] and the generation of nitric oxide (NO) [257]. Based on the relative results above, researchers started to focus on the surface modification and the NO release system on anti-cancer agents, in order to enhance the EPR effect and improve the therapeutic efficacy. However, the EPR effect has its own negative consequences. For example, since the EPR effect is related to the nutrients and oxygen transport, the over expression of the EPR effect could facilitate tumor growth [258]. Therefore, there is a need in a novel method to improve ablation agent accumulation in tumor for better therapeutic efficacy through NanoEL or EPR [85–87]. Moreover, to achieve this goal, we proposed a strategy for delivering the anti-cancer agents with the use of mesenchymal stem cells (MSCs) as a drug carrier [259]. It has been accepted that MSCs own a natural high tumor affinity. Besides, MSCs can be obtained from patients and re-implanted without immune rejection, which is an enormous advantage compared with the artificial compounds. By using MSCs to deliver photosensitizers, the tumor growth was inhibited after PDT treatment. It is reasonable to assume that such MSCs carriers could be applied to load different anti-cancer drugs, including the photosensitizers and ablation agents. This work may shed light on a novel approach to improve the targeting and accumulation of ablation agents in tumor. Specifically, bio-carriers could be fine candidates to help delivery of the ablation agents. Moreover, we introduced another strategy for anti-cancer drug delivery with the use of bacteriophage as a bio-carrier [260–263]. In one of these works, filamentous bacteriophage was considered as a nanowireshaped carrier that genetically displayed cancer-targeting peptide and chemically conjugated with photosensitizers (Fig. 20) [260]. In this way, targeted PDT with a bio-carrier was achieved. Such a strategy was also applied on gold NPs, the nanomaterials used in PTT [264]. Therefore, we could draw a conclusion that biological systems can be used as carriers of ablation agents.
Fig. 20.
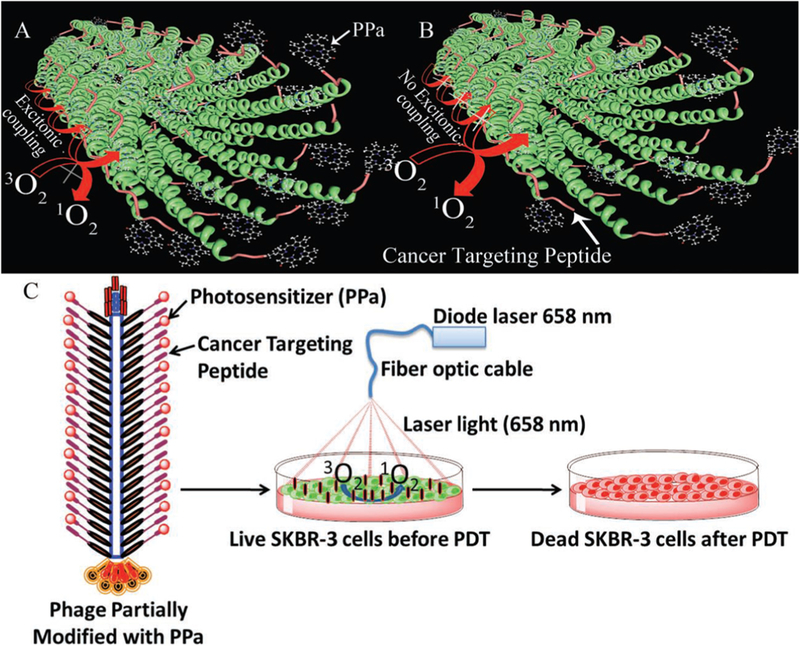
Schematic diagram of photosensitizer conjugation onto biological nanowire and its targeted photodynamic therapy [260].
As for light sources, as mentioned previously, the safe limit of power density should be taken in to consideration. It should be noted that absorption of NIR light by tissues may occur, this situation may lead to the reduction of NIR light power and unexpected thermal effects. For example, it was reported that under irradiation of a 980 nm NIR light with a power density of 0.7 Wcm−2, the temperature of PBS solution rose 10 °C [73], inferring absorption of 980 nm NIR light by PBS solution. In this case, the temperature elevation could also occur on normal tissues and the targeting function of PTT may become invalid. Moreover, though NIR light has been seen as a non-invasive light with high penetration, NIR light energy decreases along with the penetration through tissues due to light scattering and absorption, leading to lowered therapeutic efficacy [174]. To make up for the losses of therapeutic efficacy, synergic therapies combined with PTT and other light stimulation therapies have been studied [132,221,222,265].
7. Conclusion and perspective
Many nanomaterials have been introduced into PTT, showing great promise for this selective and non-invasive cancer treatment. Though the experiments are still in the early stage, some biological research for PTT has shown welcome results. Although the mechanisms of all these ablation agents are quite different, it was found that the photothermal effect could usually be tunable by adjusting the shape and the size of PTT agents. On one hand, with many promising candidates and ablation agents for PTT and with the use of nano effects, we may be capable of synthesizing a compound with better photothermal efficiency for PTT. On the other hand, the potential threat of nanomaterials to patients and the environment should not be ignored. As for the safety of the PTT agents, though different methods, such as PEG-coating, have been applied, the limitations of solubility, hydrophilicity and aggregation are still uncertain in the complicated internal environment. Precise modification to satisfy both good biocompatibility and high photothermal efficiency remains a great difficulty. Like other medical studies on nanomaterials, the tracing of nanomaterials after treatment is challenging, so an advanced technology for tracking and researching the nanomaterials over a long-period in the patients is needed. Furthermore, it is worth noting that not all ablation agents described in this article were stimulated by a NIR source with safe power according to safety limit from the American National Standard. Inappropriate light for stimulation may result in severe absorption by water and lower PTT efficiency against tumors.
In conclusion, PTT continues to be developed for clinical applications. In the near future, PTT will probably satisfy the need for anti-tumor treatments and safety concerns will be eliminated. Nevertheless, to improve curative effects, the future work should focus on PTT integration into synergic therapies. The PTT ablation agents themselves also need further evolvement to become multifunctional. As introduced previously, some PTT ablation agents possess the potential to work as contrast agents or drug carriers. In this way, real-time imaging PTT or PTT combined with chemotherapy could be achieved. According to these studies, synergic therapies containing PTT could be realized in order to avoid over-dosing concerns or secondary treatments, and achieve better anticancer abilities. Photothermal nanomaterials and their applications have been widely studied and some positive results have been emphasized. Potential synergic therapies with PTT should be valued in the future.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program) (2015AA033502), the National Natural Science Foundation of China (51541201 and 51673168), National Key Research and Development Program of China (2016YFA0100900), Science and Technology Planning Project of Guangdong Province, China (2014A010105048), the Natural Science Foundation of Guangdong Province (2015A030313493, 2016A030308014) and State Key Laboratory for Mechanical Behavior of Materials, China(Grant No. 20141607). YZ and CBM also thank the financial support from National Institutes of Health (CA200504, CA195607, and EB021339).
References
- [1].Chen Y, Jungsuwadee P, Vore M, Butterfield D, St-Clair D. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interventions 2007;7:2653–63. [DOI] [PubMed] [Google Scholar]
- [2].Edison M, Johns C. Acute and chronic cutaneous reactions to radiotherapy In: Cognetta AB, Mendenhall WM, editors. Radiation therapy for skin cancer. New York: Springer; 2013. p. 55–69. [Google Scholar]
- [3].Matteini P, Tatini F, Cavigli L, Ottaviano S, Ghini G, Pini R. Graphene as a photothermal switch for controlled drug release. Nanoscale 2014;6:7947–53. [DOI] [PubMed] [Google Scholar]
- [4].Tang S, Chen M, Zheng N. Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small 2014;10:3139–44. [DOI] [PubMed] [Google Scholar]
- [5].Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008;23:217–28. [DOI] [PubMed] [Google Scholar]
- [6].Ramasamy M, Lee SS, Yi DK, Kim K. Magnetic, optical gold nanorods for recyclable photothermal ablation of bacteria. J Mater Chem B 2014;2:981–8. [DOI] [PubMed] [Google Scholar]
- [7].Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperth 2003;19:252–66. [DOI] [PubMed] [Google Scholar]
- [8].He X, Wolkers WF, Crowe JH, Swanlund DJ, Bischof JC. In situ thermal denaturation of proteins in dunning AT-1 prostate cancer cells: implication for hyperthermic cell injury. Ann Biomed Eng 2004;32:1384–98. [DOI] [PubMed] [Google Scholar]
- [9].Tong L, Cheng JX. Gold nanorod-mediated photothermolysis induces apoptosis of macrophages via damage of mitochondria. Nanomed: Nanotechnol Biol Med 2009;4:265–76. [DOI] [PubMed] [Google Scholar]
- [10].Mocan T, Matea CT, Cojocaru I, Ilie I, Tabaran FA, Zaharie F, et al. Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J Cancer 2014;5:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA 2003;100:13549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wust P, Ahlers O, Dieing A. The cellular and molecular basis of hyperthermia. Crit Rev Oncol/Hematol 2002;43:33–56. [DOI] [PubMed] [Google Scholar]
- [13].Melamed JR, Edelstein RS, Day ES. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015;9:6–11. [DOI] [PubMed] [Google Scholar]
- [14].Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, et al. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol Immunother 2003;52:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Masters A, Bown SG. Interstitial laser hyperthermia in the treatment of tumours. Lasers Med Sci 1990;5:129–36. [Google Scholar]
- [16].Sultan RA. Tumour ablation by laser in general surgery. Lasers Med Sci 1990;5:185–93. [Google Scholar]
- [17].Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B, et al. Self-assembly synthesis, tumor cell targeting, and photothermal capabilities of antibody- coated indocyanine green nanocapsules. J Am Chem Soc 2010;132:1929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials 2012;33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tang J, Jiang X, Wang L, Zhang H, Hu Z, Liu Y, et al. Au@Pt nanostructures: a novel photothermal conversion agent for cancer therapy. Nanoscale 2014;6:3670–8. [DOI] [PubMed] [Google Scholar]
- [20].Li J, Cai R, Kawazoe N, Chen G. Facile preparation of albumin-stabilized gold nanostars for the targeted photothermal ablation of cancer cells. J Mater Chem B 2015;3:5806–14. [DOI] [PubMed] [Google Scholar]
- [21].Zheng R, Wang S, Tian Y, Jiang X, Fu D, Shen S, et al. Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Appl Mater Interf 2015;7:15876–84. [DOI] [PubMed] [Google Scholar]
- [22].Zhou Z, Sun Y, Shen J, Wei J, Yu C, Kong B, et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014;35:7470–8. [DOI] [PubMed] [Google Scholar]
- [23].Tan L, Wu Z, Wang X, Sun J. Facile synthesis of CuS mesostructures with high photothermal conversion efficiency. RSC Adv 2015;5:35317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jia G, Lou W, Cheng F, Wang X, Yao J, Dai N, et al. Excellent photothermal conversion of core/shell CdSe/Bi2Se3 quantum dots. Nano Res 2015;8:1443–53. [Google Scholar]
- [25].Fang W, Tang S, Liu P, Fang X, Gong J, Zheng N. Pd nanosheet-covered hollow mesoporous silica nanoparticles as a platform for the chemo-photothermal treatment of cancer cells. Small 2012;8:3816–22. [DOI] [PubMed] [Google Scholar]
- [26].Richardson HH, Hickman ZN, Govorov AO, Thomas AC, Zhang W, Kordesch ME. Thermooptical properties of gold nanoparticles embedded in ice: characterization of heat generation and melting. Nano Lett 2006;6:783–8. [DOI] [PubMed] [Google Scholar]
- [27].Skirtach AG, Antipov AA, Shchukin DG, Sukhorukov GB. Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir: ACS J Surf Colloids 2004;20:6988–92. [DOI] [PubMed] [Google Scholar]
- [28].Shen Y, Skirtach AG, Seki T, Yagai S, Li H, Mohwald H, et al. Assembly of fullerene-carbon nanotubes: temperature indicator for photothermal conversion. J Am Chem Soc 2010;132:8566–8. [DOI] [PubMed] [Google Scholar]
- [29].Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett 2007;7:1929–34. [DOI] [PubMed] [Google Scholar]
- [30].Weissleder R A clearer vision for in vivo imaging. Nat Biotechnol 2001;19:316–7. [DOI] [PubMed] [Google Scholar]
- [31].Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev 2003;103:577–644. [DOI] [PubMed] [Google Scholar]
- [32].Tsai MF, Chang SH, Cheng FY, Shanmugam V, Cheng YS, Su CH, et al. Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy. ACS Nano 2013;7:5330–42. [DOI] [PubMed] [Google Scholar]
- [33].Chances Riediker M. and risks of nanomaterials for health and environment In: Schmid A, Goel S, Wang W, Beiu V, Carrara S, editors. Nano-Net. Springer; Berlin Heidelberg; 2009. p. 128–33. [Google Scholar]
- [34].Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B 2006;110:7238–48. [DOI] [PubMed] [Google Scholar]
- [35].Zhao P, Zheng M, Yue C, Luo Z, Gong P, Gao G, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipidpolymer nanoparticles. Biomaterials 2014;35:6037–46. [DOI] [PubMed] [Google Scholar]
- [36].Wang Q, Wang J, Lv G, Wang F, Zhou X, Hu J, et al. Facile synthesis of hydrophilic polypyrrole nanoparticles for photothermal cancer therapy. J Mater Sci 2014;49:3484–90. [Google Scholar]
- [37].Wang Y, Black KC, Luehmann H, Li W, Zhang Y, Cai X, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013;7:2068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu X, Tao H, Yang K, Zhang S, Lee ST, Liu Z. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 2011;32:144–51. [DOI] [PubMed] [Google Scholar]
- [39].Liu X, Atwater M, Wang J, Huo Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B Biointerf 2007;58:3–7. [DOI] [PubMed] [Google Scholar]
- [40].Shanmugam V, Selvakumar S, Yeh C-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev 2014;43:6254–87. [DOI] [PubMed] [Google Scholar]
- [41].Shibu ES, Hamada M, Murase N, Biju V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J Photochem Photobiol C 2013;15:53–72. [Google Scholar]
- [42].Qiu J, Wei WD. Surface plasmon-mediated photothermal chemistry. J Phys Chem C 2014;118:20735–49. [Google Scholar]
- [43].Goldberg SN, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228:335–45. [DOI] [PubMed] [Google Scholar]
- [44].Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 2001;7:864–8. [DOI] [PubMed] [Google Scholar]
- [45].Stevenson AT Jr, Reese LM, Hill TK, McGuire J, Mohs AM, Shekhar R, et al. Fabrication and characterization of medical grade polyurethane composite catheters for near-infrared imaging. Biomaterials 2015;54:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bishara A, Meir M, Portnoy E, Shmuel M, Eyal S. Near infrared imaging of indocyanine green distribution in pregnant mice and effects of concomitant medications. Mol Pharm 2015;12:3351–7. [DOI] [PubMed] [Google Scholar]
- [47].Walsh J Basic interactions of light with tissue In: Welch AJ, van Gemert MJC, editors. Optical-thermal response of laser-irradiated tissue. Netherlands: Springer; 2011. p. 13–26. [Google Scholar]
- [48].Sarna T, Sealy RC. Photoinduced oxygen consumption in melanin systems. Action spectra and quantum yields for eumelanin and synthetic melanin. Photochem Photobiol 1984;39:69–74. [DOI] [PubMed] [Google Scholar]
- [49].Hanf R, Fey S, Schmitt M, Hermann G, Dietzek B, Popp J. Catalytic efficiency of a photoenzyme—an adaptation to natural light conditions. Chemphyschem: Eur J Chem Phys Phys Chem 2012;13:2013–5. [DOI] [PubMed] [Google Scholar]
- [50].Boyd DP. Status of diagnostic X-ray CT: 1979. IEEE Trans Nucl Sci 1979;26:2835–9. [Google Scholar]
- [51].Heiken JP, Peterson CM, Menias CO. Virtual colonoscopy for colorectal cancer screening: current status Wednesday 5 October 2005, 14:00–16:00. Cancer Imaging 2005;5:S133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005;129:328–37. [DOI] [PubMed] [Google Scholar]
- [53].Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology 2004;232:735–8. [DOI] [PubMed] [Google Scholar]
- [54].Beinfeld MT, Wittenberg E, Gazelle GS. Cost-effectiveness of whole-body CT screening. Radiology 2005;234:415–22. [DOI] [PubMed] [Google Scholar]
- [55].Johnson J, Dawson-Hughes B. Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int 1991;49:174–8. [DOI] [PubMed] [Google Scholar]
- [56].Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J Appl Physiol 1999;87:1513–20. [DOI] [PubMed] [Google Scholar]
- [57].Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, et al. Folic acid-conjugated Silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 2011;32:9796–809. [DOI] [PubMed] [Google Scholar]
- [58].Tian Y, Luo S, Yan H, Teng Z, Pan Y, Zeng L, et al. Gold nanostars functionalized with amine-terminated PEG for X-ray/CT imaging and photothermal therapy. J Mater Chem B 2015;3:4330–7. [DOI] [PubMed] [Google Scholar]
- [59].Cai Z, Cloutier P, Hunting D, Sanche L. Comparison between X-ray photon and secondary electron damage to DNA in vacuum. J Phys Chem B 2005;109:4796–800. [DOI] [PubMed] [Google Scholar]
- [60].Cheng A, Caffrey M. Free radical mediated X-ray damage of model membranes. Biophys J 1996;70:2212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Quickenden TI, Irvin JA. The ultraviolet absorption spectrum of liquid water. J Chem Phys 1980;72:4416–28. [Google Scholar]
- [62].Soehnge H, Ouhtit A, Ananthaswamy ON. Mechanisms of induction of skin cancer by UV radiation. Front Biosci 1997:d538–51. [DOI] [PubMed] [Google Scholar]
- [63].Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998;68:63–70. [PubMed] [Google Scholar]
- [64].Pastila R, Leszczynski D. Ultraviolet-A radiation induces changes in cyclin G gene expression in mouse melanoma B16-F1 cells. Cancer Cell Int 2007;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Grewe M, Gyufko K, Schöpf E, Krutmann J. Lesional expression of interferon-γ in atopic eczema. Lancet 1994;343:25–6. [DOI] [PubMed] [Google Scholar]
- [66].Grewe M, Trefzer U, Ballhorn A, Gyufko K, Henninger H, Krutmann J. Analysis of the mechanism of ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by human epidermoid carcinoma cells. J, Invest Dermatol 1993;101:528–31. [DOI] [PubMed] [Google Scholar]
- [67].Brzoska T, Scholzen T, Becher E, Luger TA. Effect of UV light on the production of proopiomelanocortin-derived peptides and melanocortin receptors in the skin In: Altmeyer P, Hoffmann K, Stücker M, editors. Skin cancer and UV radiation. Springer; Berlin Heidelberg; 1997. p. 227–37. [Google Scholar]
- [68].Luger TA, Schwarz T. Cytokines and neuroendocrine hormones as mediators of cutaneous immunity and inflammation In: Eibl M, Huber C, Peter H, Wahn U, editors. Symposium in immunology IV. Springer; Berlin Heidelberg; 1995. p. 3–23. [Google Scholar]
- [69].Maestro LM, Ramírez-Hernández JE, Bogdan N, Capobianco JA, Vetrone F, Solé JG, et al. Deep tissue bio-imaging using two-photon excited CdTe fluorescent quantum dots working within the biological window. Nanoscale 2011;4:298–302. [DOI] [PubMed] [Google Scholar]
- [70].Ju Q, Chen X, Ai F, Peng D, Lin X, Kong W, et al. An upconversion nanoprobe operating in the first biological window. J Mater Chem B 2015;3:3548–55. [DOI] [PubMed] [Google Scholar]
- [71].Stolik S, Delgado JA, Pérez A, Anasagasti L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B 2000;57:90–3. [DOI] [PubMed] [Google Scholar]
- [72].Robinson JT, Welsher K, Tabakman SM, Sherlock SP, Wang H, Luong R, et al. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res 2010;3:779–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li B, Zhang Y, Zou R, Wang Q, Zhang B, An L, et al. Self-assembled WO3-x hierarchical nanostructures for photothermal therapy with a 915 nm laser rather than the common 980 nm laser. Dalton Trans 2014;43:6244–50. [DOI] [PubMed] [Google Scholar]
- [74].Barinaga M Designing therapies that target tumor blood vessels. Science 1997;275:482–4. [DOI] [PubMed] [Google Scholar]
- [75].Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther 2007;322:80–8. [DOI] [PubMed] [Google Scholar]
- [76].Povrozin YA, Markova LI, Tatarets AL, Sidorov VI, Terpetschnig EA, Patsenker LD. Near-infrared, dual-ratiometric fluorescent label for measurement of pH. Anal Biochem 2009;390:136–40. [DOI] [PubMed] [Google Scholar]
- [77].Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626–34. [DOI] [PubMed] [Google Scholar]
- [78].Fu Y, Li X, Sun C, Ren Z, Weng W, Mao C, et al. pH-triggered SrTiO3: Er nano fibers with optically monitored and controlled drug delivery functionality. ACS Appl Mater Interf 2015;7:25514–21. [DOI] [PubMed] [Google Scholar]
- [79].Liu H, Fu Y, Li Y, Ren Z, Li X, Han G, et al. A fibrous localized drug delivery platform with NIR-triggered and optically monitored drug release. Langmuir: ACS J Surf Colloids 2016;32:9083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li X, Zhang Q, Ahmad Z, Huang J, Ren Z, Weng W, et al. Near-infrared luminescent CaTiO3:Nd3+ nanofibers with tunable and trackable drug release kinetics. J Mater Chem B 2015;3:7449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].von Maltzahn G, Park J-H, Agrawal A, Bandaru NK, Das SK, Sailor MJ, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res 2009;69:3892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pech O, Gossner L, May A, Rabenstein T, Vieth M, Stolte M, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc 2005;62:24–30. [DOI] [PubMed] [Google Scholar]
- [83].Tan L, Liu J, Zhou W, Wei J, Peng Z. A novel thermal and pH responsive drug delivery system based on ZnO@PNIPAM hybrid nanoparticles. Mater Sci Eng C 2014;45:524–9. [DOI] [PubMed] [Google Scholar]
- [84].Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 2006;128:2115–20. [DOI] [PubMed] [Google Scholar]
- [85].Setyawati MI, Tay CY, Bay BH, Leong DT. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 2017;11:5020–30. [DOI] [PubMed] [Google Scholar]
- [86].Tay CY, Setyawati MI, Leong DT. Nanoparticle density: a critical biophysical regulator of endothelial permeability. ACS Nano 2017;11:2764–72. [DOI] [PubMed] [Google Scholar]
- [87].Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat Commun 2013;4:1673. [DOI] [PubMed] [Google Scholar]
- [88].Zha Z, Yue X, Ren Q, Dai Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv Mater 2013;25:777–82. [DOI] [PubMed] [Google Scholar]
- [89].Chen M, Fang X, Tang S, Zheng N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem Commun 2012;48:8934–6. [DOI] [PubMed] [Google Scholar]
- [90].Yang K, Xu H, Cheng L, Sun C, Wang J, Liu Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv Mater 2012;24:5586–92. [DOI] [PubMed] [Google Scholar]
- [91].Manikandan M, Hasan N, Wu H-F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013;34:5833–42. [DOI] [PubMed] [Google Scholar]
- [92].Jiang R, Cheng S, Shao L, Ruan Q, Wang J. Mass-based photothermal comparison among gold nanocrystals, PbS nanocrystals, organic dyes, and carbon black. J Phys Chem C 2013;117:8909–15. [Google Scholar]
- [93].Link S, El-Sayed MA. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 1999;103:8410–26. [Google Scholar]
- [94].Pitsillides CM, Joe EK, Wei X, Anderson RR, Lin CP. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys J 2003;84:4023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mie G Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann Phys 1908;330:377–445. [Google Scholar]
- [96].Vankayala R, Lin CC, Kalluru P, Chiang CS, Hwang KC. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014;35:5527–38. [DOI] [PubMed] [Google Scholar]
- [97].Link S, Mohamed MB, El-Sayed MA. Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J Phys Chem B 1999;103:3073–7. [Google Scholar]
- [98].Qiu P, Yang M, Qu X, Huai Y, Zhu Y, Mao C. Tuning photothermal properties of gold nanodendrites for in vivo cancer therapy within a wide near infrared range by simply controlling their degree of branching. Biomaterials 2016;104:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wei M, Chen N, Li J, Yin M, Liang L, He Y, et al. Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide-conjugated gold nanoparticles. Angew Chem Int Ed 2012;124:1202–6. [DOI] [PubMed] [Google Scholar]
- [100].Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013;7:3926–38. [DOI] [PubMed] [Google Scholar]
- [101].Jang B, Park J-Y, Tung C- H, Kim I- H, Choi Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011;5:1086–94. [DOI] [PubMed] [Google Scholar]
- [102].Li S, Zhang L, Wang T, Li L, Wang C, Su Z. The facile synthesis of hollow Au nanoflowers for synergistic chemo-photothermal cancer therapy. Chem Commun 2015;51:14338–41. [DOI] [PubMed] [Google Scholar]
- [103].Yavuz MS, Cheng Y, Chen J, Cobley CM, Qiang Z, Rycenga M, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater 2009;8:935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang D, Xu Z, Yu H, Chen X, Feng B, Cui Z, et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials 2014;35:8374–84. [DOI] [PubMed] [Google Scholar]
- [105].Liu H, Chen D, Li L, Liu T, Tan L, Wu X, et al. Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed 2011;50:891–5. [DOI] [PubMed] [Google Scholar]
- [106].Mohammad F, Balaji G, Weber A, Uppu RM, Kumar CSSR. Influence of gold nanoshell on hyperthermia of superparamagnetic iron oxide nanoparticles. J Phys Chem C 2010;114:19194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Huang Y, Lai Y, Shi S, Hao S, Wei J, Copper Chen X. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. Chem-Asian J 2015;10:370–6. [DOI] [PubMed] [Google Scholar]
- [108].Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomed Nanotechnol Biol Med 2010;5:1161–71. [DOI] [PubMed] [Google Scholar]
- [109].Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, et al. A chelator-free multifunctional [Cu-64]CuS nanoparticle platform for simultaneous micro-PET/ CT imaging and photothermal ablation therapy. J Am Chem Soc 2010;132:15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chen Z, Zhang L, Sun Y, Hu J, Wang D. 980-nm laser-driven photovoltaic cells based on rare-earth up-converting phosphors for biomedical applications. Adv Funct Mater 2009;19:3815–20. [Google Scholar]
- [111].Sigman MB, Ali G, Tobias H, Saunders AE, Frank L, Korgel BA. Solventless synthesis of monodisperse Cu2s nanorods, nanodisks, and nanoplatelets. J Am Chem Soc 2003;125:16050–7. [DOI] [PubMed] [Google Scholar]
- [112].Puspitasari I, Gujar TP, Jung KD, Joo OS. Simple chemical preparation of CuS nanowhiskers. Mater Sci Eng B 2007;140:199–202. [Google Scholar]
- [113].Zhang HT, Wu G, Chen XH. Controlled synthesis and characterization of covellite (CuS) nanoflakes. Mater Chem Phys 2006;98:298–303. [Google Scholar]
- [114].Feng X, Li Y, Liu H, Li Y, Cui S, Wang N, et al. Controlled growth and field emission properties of CuS nanowalls. Nanotechnology 2007;18:776–89. [Google Scholar]
- [115].Liu Y, Qin D, Wang L, Cao Y. A facile solution route to CuS hexagonal nanoplatelets. Mater Chem Phys 2007;102:201–6. [Google Scholar]
- [116].Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, et al. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv Mater 2011;23:3542–7. [DOI] [PubMed] [Google Scholar]
- [117].Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, et al. Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011;5:9761–71. [DOI] [PubMed] [Google Scholar]
- [118].Bu X, Zhou D, Li J, Zhang X, Zhang K, Zhang H, et al. Copper sulfide self-assembly architectures with improved photothermal performance. Langmuir: ACS J Surf Colloids 2014;30:1416–23. [DOI] [PubMed] [Google Scholar]
- [119].Zhao Y, Pan H, Lou Y, Qiu X, Zhu J, Burda C. Plasmonic Cu(2-x)S nanocrystals: optical and structural properties of copper-deficient copper(I) sulfides. J Am Chem Soc 2009;131:4253–61. [DOI] [PubMed] [Google Scholar]
- [120].Li B, Wang Q, Zou R, Liu X, Xu K, Li W, et al. Cu7.2S4 nanocrystals: a novel photothermal agent with a 56.7% photothermal conversion efficiency for photothermal therapy of cancer cells. Nanoscale 2014;6:3274–82. [DOI] [PubMed] [Google Scholar]
- [121].Consales M, Cutolo A, Penza M, Aversa P, Cassano G, Giordano M, et al. Carbon nanotubes coated acoustic and optical VOCs sensors: towards the tailoring of the sensing performances. IEEE Trans Nanotechnol 2007;6:601–12. [Google Scholar]
- [122].Peng L-M, Zhang Z, Wang S, Li Y. Carbon nanotube (CNT)-based high-performance electronic and optoelectronic devices In: Zhai T, Yao J, editors. Onedimensional nanostructures: principles and applications. John Wiley & Sons, Inc.; 2012. p. 321–38. [Google Scholar]
- [123].Yang DJ, Wang SG, Zhang Q, Sellin PJ, Chen G. Thermal and electrical transport in multi-walled carbon nanotubes. Phys Lett A 2004;329:207–13. [Google Scholar]
- [124].Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 2009;3:3707–13. [DOI] [PubMed] [Google Scholar]
- [125].Neves LFF, Krais JJ, Van Rite BD, Rajagopal R, Resasco DE, Harrison RG. Targeting single-walled carbon nanotubes for the treatment of breast cancer using photothermal therapy. Nanotechnology 2013;24:375104–15. [DOI] [PubMed] [Google Scholar]
- [126].Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007;1:50–6. [DOI] [PubMed] [Google Scholar]
- [127].Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc 2008;130:11467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, et al. Single-walled carbon nanotubes in the intact organism: near-IR imaging and biocompatibility studies in Drosophila. Nano Lett 2007;7:2650–4. [DOI] [PubMed] [Google Scholar]
- [129].Zavaleta C, de la Zerda A, Liu Z, Keren S, Cheng Z, Schipper M, et al. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett 2008;8:2800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Arsawang U, Saengsawang O, Rungrotmongkol T, Sornmee P, Wittayanarakul K, Remsungnen T, et al. How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system? J Mol Graph Model 2011;29:591–6. [DOI] [PubMed] [Google Scholar]
- [131].Georgakilas V, Tzitzios V, Gournis D, Petridis D. Attachment of magnetic nanoparticles on carbon nanotubes and their soluble derivatives. Chem Mater 2005;17:1613–7. [Google Scholar]
- [132].Wang L, Shi J, Jia X, Liu R, Wang H, Wang Z, et al. NIR-/pH-responsive drug delivery of functionalized single-walled carbon nanotubes for potential application in cancer chemo-photothermal therapy. Pharm Res 2013;30:2757–71. [DOI] [PubMed] [Google Scholar]
- [133].Wang R, Mikoryak C, Li S, Bushdiecker D, Musselman IH, Pantano P, et al. Cytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharm 2011;8:1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kostarelos K The long and short of carbon nanotube toxicity. Nat Biotechnol 2008;26:774–6. [DOI] [PubMed] [Google Scholar]
- [135].Lam CW, James JT, Mccluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci Off J Soc Toxicol 2004;77:126–34. [DOI] [PubMed] [Google Scholar]
- [136].Gonsalves AG, Figueiredo JL, Órfao JJM, Pereira MFR. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon 2010;48:4369–81. [Google Scholar]
- [137].Liu X, Huang N, Li H, Wang H, Jin Q, Ji J. Multidentate polyethylene glycol modified gold nanorods for in vivo near-infrared photothermal cancer therapy. ACS Appl Mater Interf 2014;6:5657–68. [DOI] [PubMed] [Google Scholar]
- [138].Gravel E, Tanguy C, Cassette E, Pons T, Knittel F, Bernards N, et al. Compact tridentate ligands for enhanced aqueous stability of quantum dots and in vivo imaging. Chem Sci 2012;4:411–7. [Google Scholar]
- [139].Liu X, Huang N, Wang H, Li H, Jin Q, Ji J. The effect of ligand composition on the in vivo fate of multidentate poly(ethylene glycol) modified gold nanoparticles. Biomaterials 2013;34:8370–81. [DOI] [PubMed] [Google Scholar]
- [140].Burke AR, Singh RN, Carroll DL, Wood JCS, D’Agostino RB Jr., Ajayan PM, et al. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012;33:2961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, Evmenenko G, et al. Preparation and characterization of graphene oxide paper. Nature 2007;448:457–60. [DOI] [PubMed] [Google Scholar]
- [142].Korkut S, Roy-Mayhew JD, Dabbs DM, Milius DL, Aksay IA. High surface area tapes produced with functionalized graphene. ACS Nano 2011;5:5214–22. [DOI] [PubMed] [Google Scholar]
- [143].Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, et al. Superior thermal conductivity of single-layer graphene. Nano Lett 2008;8:902–7. [DOI] [PubMed] [Google Scholar]
- [144].Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale 2011;3:1252–7. [DOI] [PubMed] [Google Scholar]
- [145].Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc 2008;130:10876–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 2008;1:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett 2010;10:3318–23. [DOI] [PubMed] [Google Scholar]
- [148].Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011;5:3693–700. [DOI] [PubMed] [Google Scholar]
- [149].Zhang W, Guo Z, Huang D, Liu Z, Guo X, Zhong H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011;32:8555–61. [DOI] [PubMed] [Google Scholar]
- [150].Robinson JT, Tabakman SM, Liang Y, Wang H, Sanchez Casalongue H, Vinh D, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc 2011;133:6825–31. [DOI] [PubMed] [Google Scholar]
- [151].Kim SH, Lee JE, Sharker SM, Jeong JH, In I, Park SY. In vitro and in vivo tumor targeted photothermal cancer therapy using functionalized graphene nanoparticles. Biomacromolecules 2015;16:3519–29. [DOI] [PubMed] [Google Scholar]
- [152].Markovic ZM, Harhaji-Trajkovic LM, Todorovic-Markovic BM, Kepic DP, Arsikin KM, Jovanovic SP, et al. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011;32:1121–9. [DOI] [PubMed] [Google Scholar]
- [153].Guo S, Dong S, Wang E. Polyaniline/Pt hybrid nanofibers: high-efficiency nanoelectrocatalysts for electrochemical devices. Small 2009;5:1869–76. [DOI] [PubMed] [Google Scholar]
- [154].Aussawasathien D, Dong JH, Dai L. Electrospun polymer nanofiber sensors. Synth Met 2005;154:37–40. [Google Scholar]
- [155].Ghanbari K, Bathaie SZ, Mousavi MF. Electrochemically fabricated polypyrrole nanofiber-modified electrode as a new electrochemical DNA biosensor. Biosens Bioelectron 2008;23:1825–31. [DOI] [PubMed] [Google Scholar]
- [156].Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater 2011;10:324–32. [DOI] [PubMed] [Google Scholar]
- [157].Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett 2011;11:2560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhou J, Lu Z, Zhu X, Wang X, Liao Y, Ma Z, et al. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013;34:9584–92. [DOI] [PubMed] [Google Scholar]
- [159].Chen X, Inganäs O. Three-step redox in polythiophenes: evidence from electrochemistry at an ultramicroelectrode. J Phys Chem 1996;100:15202–6. [Google Scholar]
- [160].Di B, Meng Y, Wang YD, Liu XJ, An Z. Electroluminescence enhancement in polymer light-emitting diodes through inelastic scattering of oppositely charged bipolarons. J Phys Chem B 2011;115:9339–44. [DOI] [PubMed] [Google Scholar]
- [161].Stejskal J, Gilbert RG. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl Chem 2002;74:857–68. [Google Scholar]
- [162].Dimitriev OP. Doping of polyaniline by transition-metal salts. Macromolecules 2004;37:3388–95. [Google Scholar]
- [163].Gizdavic-Nikolaidis M, Travas-Sejdic J, Bowmaker GA, Cooney RP, Kilmartin PA. Conducting polymers as free radical scavengers. Synth Met 2004;140:225–32. [Google Scholar]
- [164].Yang J, Choi J, Bang D, Kim E, Lim E-K, Park H, et al. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells. Angew Chem Int Ed 2011;50:441–4. [DOI] [PubMed] [Google Scholar]
- [165].Cheng L, Yang K, Chen Q, Liu Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 2012;6:5605–13. [DOI] [PubMed] [Google Scholar]
- [166].Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials 2003;24:777–87. [DOI] [PubMed] [Google Scholar]
- [167].George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, et al. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005;26:3511–9. [DOI] [PubMed] [Google Scholar]
- [168].Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. Biocompatibility of polypyrrole particles: an in-vivo study in mice. J Pharm Pharmacol 2007;59:311–5. [DOI] [PubMed] [Google Scholar]
- [169].Lin M, Guo C, Li J, Zhou D, Liu K, Zhang X, et al. Polypyrrole-coated chainlike gold nanoparticle architectures with the 808 nm photothermal transduction efficiency up to 70%. ACS Appl Mater Interf 2014;6:5860–8. [DOI] [PubMed] [Google Scholar]
- [170].Singh S, Jain DVS, Singla ML. One step electrochemical synthesis of gold-nanoparticles-polypyrrole composite for application in catechin electrochemical biosensor. Anal Methods 2013;5:1024–32. [Google Scholar]
- [171].Glimelius B Chemotherapy in the treatment of cancer of the pancreas. J Hepato-Biliary-Pancreatic Surg 1998;5:235–41. [DOI] [PubMed] [Google Scholar]
- [172].Carney DN, Mitchell JB, Kinsella TJ. In vitro radiation and chemotherapy sensitivity of established cell lines of human small cell lung cancer and its large cell morphological variants. Cancer Res 1983;43:2806–11. [PubMed] [Google Scholar]
- [173].Rule W, Meyer J. Current status of radiation therapy for the management of rectal cancer. Crit Rev Oncog 2012;17:331–43. [DOI] [PubMed] [Google Scholar]
- [174].Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Delivery Rev 2012;64:1005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules 2006;7:572–9. [DOI] [PubMed] [Google Scholar]
- [176].Overgaard J Combined adriamycin and hyperthermia treatment of a murine mammary carcinoma in vivo. Cancer Res 1976;36:3077–81. [PubMed] [Google Scholar]
- [177].Tang Y, McGoron AJ. Combined effects of laser-ICG photothermotherapy and doxorubicin chemotherapy on ovarian cancer cells. J Photochem Photobiol B 2009;97:138–44. [DOI] [PubMed] [Google Scholar]
- [178].Abidian MR, Kim DH, Martin DC. Conducting-polymer nanotubes for controlled drug release. Adv Mater 2006;18:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gao L, Fei J, Zhao J, Li H, Cui Y, Li J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012;6:8030–40. [DOI] [PubMed] [Google Scholar]
- [180].Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, et al. Development and applications of photo-triggered theranostic agents. Adv Drug Delivery Rev 2010;62:1094–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Liu H, Shen M, Zhao J, Zhu J, Xiao T, Cao X, et al. Facile formation of folic acid-modified dendrimer-stabilized gold-silver alloy nanoparticles for potential cellular computed tomography imaging applications. Analyst 2013;138:1979–87. [DOI] [PubMed] [Google Scholar]
- [182].Correas J-M, Bridal L, Lesavre A, Méjean A, Claudon M, Hélénon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol 2001;11:1316–28. [DOI] [PubMed] [Google Scholar]
- [183].Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol 1998;27(Suppl. 2):S157–60. [DOI] [PubMed] [Google Scholar]
- [184].Cosgrove D Ultrasound contrast agents: an overview. Eur J Radiol 2006;60:324–30. [DOI] [PubMed] [Google Scholar]
- [185].Ke H, Wang J, Dai Z, Jin Y, Qu E, Xing Z, et al. Gold-nanoshelled microcapsules: a theranostic agent for ultrasound contrast imaging and photothermal therapy. Angew Chem Int Ed 2011;50:3017–21. [DOI] [PubMed] [Google Scholar]
- [186].Zha Z, Wang J, Qu E, Zhang S, Jin Y, Wang S, et al. Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation. Sci Rep 2013;3:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Quaia E Microbubble ultrasound contrast agents: an update. Eur Radiol 2007;17:1995–2008. [DOI] [PubMed] [Google Scholar]
- [188].Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett 2008;8:3953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano 2012;6:7489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zha Z, Zhang S, Deng Z, Li Y, Li C, Dai Z. Enzyme-responsive copper sulphide nanoparticles for combined photoacoustic imaging, tumor-selective chemotherapy and photothermal therapy. Chem Commun 2013;49:3455–7. [DOI] [PubMed] [Google Scholar]
- [191].Jose J, Manohar S, Kolkman RGM, Steenbergen W, van Leeuwen TG. Imaging of tumor vasculature using Twente photoacoustic systems. J Biophotonics 2009;2:701–17. [DOI] [PubMed] [Google Scholar]
- [192].Kolkman RGM, Hondebrink E, Steenbergen W, de Mul FFM. In vivo photoacoustic imaging ofblood vessels using an extreme-narrow aperture sensor. IEEE J Sel Top Quantum Electron 2003;9:343–6. [Google Scholar]
- [193].Ku G, Wang X, Xie X, Stoica G, Wang LV. Imaging of tumor angiogenesis in rat brains in vivo by photoacoustic tomography. Appl Opt 2005;44:770–5. [DOI] [PubMed] [Google Scholar]
- [194].Wang LV. Prospects of photoacoustic tomography. Med Phys 2008;35:5758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Mou J, Li P, Liu C, Xu H, Song L, Wang J, et al. Ultrasmall Cu2-xS nanodots for highly efficient photoacoustic imaging-guided photothermal therapy. Small 2015;11:2275–83. [DOI] [PubMed] [Google Scholar]
- [196].Jin Y, Li Y, Ma X, Zha Z, Shi L, Tian J, et al. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials 2014;35:5795–804. [DOI] [PubMed] [Google Scholar]
- [197].Zeng L, Pan Y, Wang S, Wang X, Zhao X, Ren W, et al. Raman reporter-coupled Agcore@Aushell nanostars for in vivo improved surface enhanced raman scattering imaging and near-infrared-triggered photothermal therapy in breast cancers. ACS Appl Mater Interf 2015;7:16781–91. [DOI] [PubMed] [Google Scholar]
- [198].Gao Y, Li Y, Chen J, Zhu S, Liu X, Zhou L, et al. Multifunctional gold nanostar-based nanocomposite: Synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials 2015;60:31–41. [DOI] [PubMed] [Google Scholar]
- [199].Liu S, Ling X, Zhou C, Zhang J, Huang J, Yan Y. Combining superior surface enhanced Raman scattering and photothermal conversion on one platform: a strategy of ill-defined gold nanoparticles. RSC Adv 2015;5:27120–5. [Google Scholar]
- [200].Wu P, Gao Y, Lu Y, Zhang H, Cai C. High specific detection and near-infrared photothermal therapy of lung cancer cells with high SERS active aptamer-silver- gold shell-core nanostructures. Analyst 2013;138:6501–10. [DOI] [PubMed] [Google Scholar]
- [201].Liu Z, Ye B, Jin M, Chen H, Zhong H, Wang X, et al. Dye-free near-infrared surface-enhanced Raman scattering nanoprobes for bioimaging and high-performance photothermal cancer therapy. Nanoscale 2015;7:6754–61. [DOI] [PubMed] [Google Scholar]
- [202].Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res 2000;60:4440–5. [PubMed] [Google Scholar]
- [203].Bastide C, Garcia S, Anfossi E, Ragni E, Rossi D. Histologic evaluation of radiofrequency ablation in renal cancer. Eur J Surg Oncol 2006;32:980–3. [DOI] [PubMed] [Google Scholar]
- [204].Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008;44:2546–54. [DOI] [PubMed] [Google Scholar]
- [205].Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker P. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003;10:463–8. [DOI] [PubMed] [Google Scholar]
- [206].Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 2005;10:112–22. [DOI] [PubMed] [Google Scholar]
- [207].Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26. [DOI] [PubMed] [Google Scholar]
- [208].Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 2012;24:1504–34. [DOI] [PubMed] [Google Scholar]
- [209].Tao Y, Ju E, Liu Z, Dong K, Ren J, Qu X. Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo- photothermal therapy. Biomaterials 2014;35:6646–56. [DOI] [PubMed] [Google Scholar]
- [210].Kirui DK, Koay EJ, Guo X, Cristini V, Shen H, Ferrari M. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomed Nanotechnol Biol Med 2014;10:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q, et al. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016;81:114–24. [DOI] [PubMed] [Google Scholar]
- [212].Hu S-H, Fang R- H, Chen Y- W, Liao B- J, Chen IW, Chen S- Y. Photoresponsive protein-graphene-protein hybrid capsules with dual targeted heat-triggered drug delivery approach for enhanced tumor therapy. Adv Funct Mater 2014;24:4144–55. [Google Scholar]
- [213].Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol 2008;26:612–21. [DOI] [PubMed] [Google Scholar]
- [214].Saczko J, Chwilkowska A, Kulbacka J, Berdowska I, Zieliński B, Drag-Zalesińska M, et al. Photooxidative action in cancer and normal cells induced by the use of photofrin in photodynamic therapy. Folia Biol 2008;54:24–9. [PubMed] [Google Scholar]
- [215].Fukuda H, Casas A, Batlle A. Aminolevulinic acid: from its unique biological function to its star role in photodynamic therapy. Int J Biochem Cell Biol 2005;37:272–6. [DOI] [PubMed] [Google Scholar]
- [216].Zhang P, Steelant W, Kumar M, Scholfield M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J Am Chem Soc 2007;129:4526–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011;32:6145–54. [DOI] [PubMed] [Google Scholar]
- [218].Zhou A, Wei Y, Wu B, Chen Q, Xing D. Pyropheophorbide A and c(RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy. Mol Pharm 2012;9:1580–9. [DOI] [PubMed] [Google Scholar]
- [219].Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther 2004;1:279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003;3:380–7. [DOI] [PubMed] [Google Scholar]
- [221].Fan Z,Dai X, Lu Y,Yu E, Brahmbatt N, Carter N, et al. Enhancing targeted tumor treatment by near IR light-activatable photodynamic-photothermal synergistic therapy. Mol Pharm 2014;11:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Guo M, Mao H, Li Y, Zhu A, He H, Yang H, et al. Dual imaging-guided photothermal/photodynamic therapy using micelles. Biomaterials 2014;35:4656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kalluru P, Vankayala R, Chiang C-S, Hwang KC. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016;95:1–10. [DOI] [PubMed] [Google Scholar]
- [224].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [225].Sheng W-Y, Huang L. Cancer immunotherapy and nanomedicine. Pharm Res 2011;28:200–14. [DOI] [PubMed] [Google Scholar]
- [226].Weigert A, Sekar D, Brüne B. Tumor-associated macrophages as targets for tumor immunotherapy. Immunotherapy 2008;1:83–95. [DOI] [PubMed] [Google Scholar]
- [227].Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des 2009;15:1879–92. [DOI] [PubMed] [Google Scholar]
- [228].Chen Y-W, Chen P- J, Hu S- H, Chen IW, Chen S- Y. NIR-triggered synergic photo-chemothermal therapy delivered by reduced graphene oxide/carbon/meso- porous silica nanocookies. Adv Funct Mater 2014;24:451–9. [Google Scholar]
- [229].Zandberg WF, Bakhtiari ABS, Erno Z, Hsiao D, Gates BD, Claydon T, et al. Photothermal release of small molecules from gold nanoparticles in live cells. Nanomed Nanotechnol Biol Med 2012;8:908–15. [DOI] [PubMed] [Google Scholar]
- [230].Li C, Chen T, Ocsoy I, Zhu G, Yasun E, You M, et al. Gold-coated Fe3O4 nanoroses with five unique functions for cancer cell targeting, imaging, and therapy. Adv Funct Mater 2014;24:1772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol 2003;21:1166–70. [DOI] [PubMed] [Google Scholar]
- [232].Service RF. Nanotechnology grows up. Science 2004;304:1732–4. [DOI] [PubMed] [Google Scholar]
- [233].Yacobi NR, Fazllolahi F, Kim YH, Sipos A, Borok Z, Kim K-J, et al. Nanomaterial interactions with and trafficking across the lung alveolar epithelial barrier: implications for health effects of air-pollution particles. Air Qual Atmos Health 2011;4:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res 2013;46:761–9. [DOI] [PubMed] [Google Scholar]
- [235].Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005;113:823–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhalation Toxicol 2002;14:5–27. [DOI] [PubMed] [Google Scholar]
- [237].Nel A Air pollution-related illness: effects of particles. Science 2005;308:804–6. [DOI] [PubMed] [Google Scholar]
- [238].Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nat Mater 2003;2:668–71. [DOI] [PubMed] [Google Scholar]
- [239].Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005;1:325–7. [DOI] [PubMed] [Google Scholar]
- [240].Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-dependent cytotoxicity of gold nanoparticles. Small 2007;3:1941–9. [DOI] [PubMed] [Google Scholar]
- [241].Dong K, Liu Z, Li Z, Ren J, Qu X. Hydrophobic anticancer drug delivery by a 980 nm laser-driven photothermal vehicle for efficient synergistic therapy of cancer cells in vivo. Adv Mater 2013;25:4452–8. [DOI] [PubMed] [Google Scholar]
- [242].Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008;5:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discovery 2008;7:771–82. [DOI] [PubMed] [Google Scholar]
- [244].Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013;9:1521–32. [DOI] [PubMed] [Google Scholar]
- [245].Wang Y, Wang K, Zhang R, Liu X, Yan X, Wang J, et al. Synthesis of core-shell graphitic Carbon@Silica nanospheres with dual-ordered mesopores for cancer- targeted photothermochemotherapy. ACS Nano 2014;8:7870–9. [DOI] [PubMed] [Google Scholar]
- [246].Bertholon I, Vauthier C, Labarre D. Complement activation by core-shell poly(isobutylcyanoacrylate)-polysaccharide nanoparticles: influences of surface morphology, length, and type of polysaccharide. Pharm Res 2006;23:1313–23. [DOI] [PubMed] [Google Scholar]
- [247].Esmaeili F, Ghahremani MH, Esmaeili B, Khoshayand MR, Atyabi F, Dinarvand R. PLGA nanoparticles of different surface properties: preparation and evaluation of their body distribution. Int J Pharm 2008;349:249–55. [DOI] [PubMed] [Google Scholar]
- [248].Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small 2009;5:126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science 1994;263:1600–3. [DOI] [PubMed] [Google Scholar]
- [250].Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol). Langmuir: ACS J Surf Colloids 2006;22:8178–85. [DOI] [PubMed] [Google Scholar]
- [251].Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 2009;8:543–57. [DOI] [PubMed] [Google Scholar]
- [252].Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011;32:3435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Xu F, Yuan Y, Shan X, Liu C, Tao X, Sheng Y, et al. Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int J Pharm 2009;377:199–206. [DOI] [PubMed] [Google Scholar]
- [254].Thiele L, Rothen-Rutishauser B, Jilek S, Wunderli-Allenspach H, Merkle HP, Walter E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J Control Release 2001;76:59–71. [DOI] [PubMed] [Google Scholar]
- [255].Nakamura M, Davila-Zavala P, Tokuda H, Takakura Y, Hashida M. Uptake and gene expression of naked plasmid DNA in cultured brain microvessel endothelial cells. Biochem Biophys Res Commun 1998;245:235–9. [DOI] [PubMed] [Google Scholar]
- [256].Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res 2002;62:6831. [PubMed] [Google Scholar]
- [257].Akaike T, Maeda H. Nitric oxide and virus infection. Immunology 2000;101:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Delivery Rev 2011;63:136–51. [DOI] [PubMed] [Google Scholar]
- [259].Cao B, Yang M, Zhu Y, Qu X, Mao C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy. Adv Mater 2014;26:4627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Gandra N, Abbineni G, Qu X, Huai Y, Wang L, Mao C. Bacteriophage bionanowire as a carrier for both cancer-targeting peptides and photosensitizers and its use in selective cancer cell killing by photodynamic therapy. Small 2013;9:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Ngweniform P, Li D, Mao C. Self-assembly of drug-loaded liposomes on genetically engineered protein nanotubes: a potential anti-cancer drug delivery vector. Soft Matter 2009;5:954–6. [Google Scholar]
- [262].Ngweniform P, Abbineni G, Cao B, Mao C. Self-assembly of drug-loaded liposomes on genetically engineered target-recognizing M13 phage: a novel nanocarrier for targeted drug delivery. Small 2009;5:1963–9. [DOI] [PubMed] [Google Scholar]
- [263].Abbineni G, Modali S, Safiejko-Mroczka B, Petrenko VA, Mao C. Evolutionary selection of new breast cancer cell-targeting peptides and phages with the cell- targeting peptides fully displayed on the major coat and their effects on actin dynamics during cell internalization. Mol Pharm 2010;7:1629–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Liu A, Abbineni G, Mao C. Nanocomposite films assembled from genetically engineered filamentous viruses and gold nanoparticles: nanoarchitecture- and humidity-tunable surface plasmon resonance spectra. Adv Mater 2009;21:1001–5. [Google Scholar]
- [265].Lv R, Yang P, He F, Gai S, Yang G, Dai Y, et al. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature- responsive drug release. Biomaterials 2015;63:115–27. [DOI] [PubMed] [Google Scholar]


