Abstract
NO is a neurotransmitter released from enteric inhibitory neurons and responsible for modulating gastrointestinal (GI) motor behaviour. Enteric neurons express nNOS (NOS1) that associates with membranes of nerve varicosities. NO released from neurons binds to soluble guanylate cyclase in post‐junctional cells to generate cGMP. cGMP‐dependent protein kinase type 1 (PKG1) is a major mediator but perhaps not the only pathway involved in cGMP‐mediated effects in GI muscles based on gene deletion studies. NOS1+ neurons form close contacts with smooth muscle cells (SMCs), interstitial cells of Cajal (ICC) and PDGFRα+ cells, and these cells are electrically coupled (SIP syncytium). Cell‐specific gene deletion studies have shown that nitrergic responses are due to mechanisms in SMCs and ICC. Controversy exists about the ion channels and other post‐junctional mechanisms that mediate nitrergic responses in GI muscles. Reduced nNOS expression in enteric inhibitory motor neurons and/or reduced connectivity between nNOS+ neurons and the SIP syncytium appear to be responsible for motor defects that develop in diabetes. An overproduction of NO in some inflammatory conditions also impairs normal GI motor activity. This review summarizes recent findings regarding the role of NO as an enteric inhibitory neurotransmitter.
Linked Articles
This article is part of a themed section on Nitric Oxide 20 Years from the 1998 Nobel Prize. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.2/issuetoc
Abbreviations
- [Ca2+]i
intracellular Ca2+
- [K+]o
external K+ concentration
- CaCC
Ca2+‐activated Cl− channel
- CaV1.2
L‐type Ca2+ channel
- CPA
cyclopiazonic acid
- EDRF
endothelial‐derived relaxing factor
- EFS
electrical field stimulation
- GI
gastrointestinal
- ICC
interstitial cells of Cajal
- ICC‐DMP
interstitial cells of Cajal at the level of the deep muscular plexus
- ICC‐IM
intramuscular interstitial cells of Cajal
- IJP
inhibitory junction potential
- IP3
inositol 1,4,5‐trisphosphate
- IP3R1
IP3 receptor type 1 (encoded by Itpr1)
- IRAG
inositol 1,4,5‐trisphosphate receptor‐associated cGMP kinase substrate
- KCa1.1
large conductance Ca2+‐activated K+ channel
- LES
lower oesophageal sphincter
- L‐NAME
NG‐nitro‐L‐arginine methyl ester
- L‐NNA
NG‐nitro‐L‐arginine
- MLCP
myosin light chain phosphatase
- PKG1
cGMP‐dependent protein kinase
- Rp 8‐Br PET cGMPS
bromo‐3,4‐dihydro‐3‐[3,5‐O‐[(R)‐mercaptophosphinylidene]‐β‐D‐ribofuranosyl]‐6‐phenyl‐9H‐Imidazo[1,2‐a]purin‐9‐one sodium salt
- SDK
stretch‐dependent K+
- SMC
smooth muscle cell
- STIC
spontaneous transient inward current
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
Introduction
Thirty years have passed since the introduction of the idea that NO is an inhibitory non‐adrenergic, non‐cholinergic (NANC) neurotransmitter in visceral smooth muscles. Building on findings that NO has similar relaxant properties on smooth muscles as endothelial‐derived relaxing factor (EDRF) (Gruetter et al., 1979), a substance like EDRF is produced by cerebellar neurons (Garthwaite et al., 1988), and arginine analogues inhibit synthesis of EDRF (Palmer et al., 1987), the group of John Gillespie reported that NG‐monomethyl L‐arginine (l‐NMMA) raised tone and blocked inhibitory responses to nerve stimulation in rat anococcygeous muscles, and excess L‐arginine reversed this effect (Gillespie et al., 1989). These authors concluded that the NANC neurotransmitter was likely to be NO, and they also noted that the enzymes responsible for synthesizing NO in NANC neurotransmission had somewhat different properties than the enzymes responsible for EDRF.
The concept of NO as an inhibitory neurotransmitter was introduced into the enteric nervous system by a seminal study showing that NANC relaxation of the canine ileocolonic junction is due to a soluble factor with properties and pharmacology like NO (Bult et al., 1990). Later, the non‐purinergic portion of electrophysiological responses to NANC nerve stimulation (inhibitory junction potentials; IJPs) was found to be NO, and responses were mimicked by exogenous NO and NO donors in gastrointestinal (GI) muscles of laboratory animals and humans (Dalziel et al., 1991; Stark et al., 1991; Thornbury et al., 1991; Stark et al., 1993). It was then found that NO comes from enteric neurons with Dogiel type 1 morphology, and it is synthesized by the cerebellar isoform of NOS [neuronal NOS (nNOS) also known as NOS1] (Bredt et al., 1990; Ward et al., 1992; Young et al., 1992).
The new hypothesis regarding NO as an enteric inhibitory neurotransmitter, however, met with considerable resistance from advocates of vasoactive intestinal polypeptide (VIP) in this role (Goyal et al., 1980; Grider et al., 1992). These authors proposed an indirect role for NO, as a secondary, paracrine‐like substance generated by smooth muscle cells (SMCs) or released ‘in series’ from neurons in response to VIP (He and Goyal, 1993; Murthy et al., 1995; Teng et al., 1998). This controversy was debated in a lively manner during the 1990s before NO was generally accepted as an enteric inhibitory neurotransmitter (Sanders and Ward, 1992; Furness et al., 1995; Murthy et al., 1996). This review focuses on progress towards ascertaining the role and mechanisms of NO as an enteric neurotransmitter that have emerged since the Nobel Prize for NO in 1998.
Prejunctional localization and activity of nNOS in enteric neurons
Neuronal NOS protein co‐localizes with VIP in enteric neurons in cell bodies and varicose processes that innervate muscle bundles in GI muscles (Ward et al., 1992; Young et al., 1992). Co‐localization of these transmitters supports the current idea that there is a single population of inhibitory motor neurons in GI muscles. There is no evidence suggesting that NO is stored in nerve varicosities. NO appears to be produced on‐demand when intracellular Ca2+ ([Ca2+]i) is elevated in motor neurons, and release of NO is blocked by antagonists of neuronal Ca2+ channels (Mashimo et al., 1996). At least six 5′‐splice variants of nNOS occur in human GI muscles; three variants of nNOSα, two of nNOSβ and one of nNOSγ (Saur et al., 2000). The α isoforms contain a domain that facilitates membrane associations. The presence of membrane‐associated and cytosolic pools of nNOS suggest possible multiple biological functions or warehousing of excess nNOS that can be rapidly recruited into service upon demand.
An interesting question is whether nNOS protein distributed along motor neurons is catalytically active or whether specialized regions of catalytically active nNOS exist in varicosities. Such punctate sources of NO within muscles may spatially limit the effective concentration of NO. Studies of this question with isolated enteric nerve varicosities suggest that active and inactive pools of nNOS are present (Rao et al., 2008). Inactive cytosolic nNOSα associates with proteins, such as dynein light chain 8 (Rodriguez‐Crespo et al., 1998), and may, through association with myosin Va, translocate to the plasma membrane (Chaudhury et al., 2011). Mice with partial loss‐of‐function of myosin Va displayed reduced association of nNOSα with varicosity membranes, attenuated inhibitory junction potentials (IJPs) and reduced hyperpolarization responses to diethylenetriamine‐NO in gastric muscles. The nNOS may be localized at varicosity membranes to facilitate interactions with regulatory proteins and a source of Ca2+ required for activation of NO synthesis (Chaudhury et al., 2009).
Strains of mice with genetic deletion of Nos1 (encoding nNOS) have been used to elucidate the role of NO in neurotransmission. The initial knockout targeted exon 2 and produced a phenotype with an enlarged stomach and a hypertrophic pyloric sphincter that was likened to juvenile pyloric stenosis (Huang et al., 1993). Alterations in colonic migrating motor complexes were also noted in this knockout mouse (Dickson et al., 2010). However, residual NOS activity (5%) was present in these mice, and it was later realized that nNOSβ and nNOSγ splice variants do not contain exon 2. Thus, part of the residual NO production after deletion of exon 2 in neuronal tissues may be due to retained nNOS function. Deletion of exon 6, encoding the haem‐binding and catalytic domain in nNOS, produced more complete deletion of nNOS and greatly reduced the conversion of [3H]‐arginine to [3H]‐citrulline (i.e. 0.3% of wild type) (Gyurko et al., 2002). These mice reproduced the phenotype of hypertrophic pyloric stenosis, but, probably due to the feeding and breeding difficulties with these sexually dimorphic mice, further phenotyping of GI abnormalities has not occurred.
Apparatus to transduce NO signals in post‐junctional cells
Mice lacking the β1 subunit of soluble guanylate cyclase (sGCβ1) fail to respond to NO donors or to NO released from enteric motor neurons (Groneberg et al., 2011). Blockade of sGC with ODQ inhibits nitrergic IJPs and blocks the inhibition of contractions caused by the release of NO from nerves and NO donors (Franck et al., 1997). ODQ does not affect responses to 8‐Br‐cGMP. Such observations suggest that sGC is the sole receptor for NO in post‐junctional cells. The receptor for NO is not G‐protein coupled, but cytoplasmic, and composed of α (α1, α2) and β (β1) subunits of sGC, α1β1 being the most common (Koesling et al., 2004). Binding of NO to the N‐terminal haem group of sGC causes a conformational change and generation of cGMP from the catalytic domain at the C‐terminus (Ignarro, 1990).
Morphological investigation of the guinea pig GI tract showed the expression of sGCβ1 in interstitial cells of Cajal (ICC), PDGFRα + cells and enteric neurons within ganglia (Iino et al., 2008). Nearly the same distribution was obtained for sGCα1. Immunochemical labelling of sGCβ1 was typically unresolvable in SMCs, but areas lacking intermuscular ICC (ICC‐IM) displayed weak sGCβ1‐like immunoreactivity in SMCs. The expression of sGC subunits in post‐junctional cells in the mouse internal anal sphincter was evaluated, and the relative expression profiles of sGCα1 and sGCβ1 genes were PDGFRα+ cells >ICC >>SMC (Cobine et al., 2014). Levels of cGMP before and after nitrergic stimulation were investigated in canine colonic muscles (Shuttleworth et al., 1993). Stimulation of muscles with exogenous NO increased cGMP in multiple cell types, including SMCs, but electrical field stimulation (EFS) of intrinsic neurons induced cGMP immunoreactivity in ICC, and these responses were blocked by NG‐nitro‐L‐arginine (L‐NNA).
The dominant α subunit expressed in GI muscles is sGCα1, and gastric muscles of Gucy1a1 knockouts had reduced responses to EFS, NO and BAY 41‐2272, an sGC agonist. However, inhibitory responses sensitive to ODQ were retained in gastric muscles of Gucy1a1 −/− mice (Vanneste et al., 2007). The effects of ODQ and BAY41‐2272 are consistent with responses to NO being mediated by sGC, so it appears that sGCα1 is not the only α isoform of sGC expressed in gastric muscles. Transcriptomic data from expression analysis agree with this conclusion and show a high expression of Gucy1a1 and much lower expression of Gucy1a2 (Lee et al., 2017). Despite its low level of expression, Gucy1a2 may sustain a portion of nitrergic responses when Gucy1a1 is deactivated.
The increase in cGMP in response to NO is linked to several effectors in cells, including the cGMP‐dependent protein kinase, PKG1, PDE and nucleotide‐gated ion channels (Francis et al., 2010). The expression and abundance of these effectors tend to define the mechanism of action of NO in specific types of cells. Two major splice variants of PKG1 occur, PKG1α and PKG1β (Lincoln et al., 1988). Post‐junctional responses to NO are commonly attributed to PKG1. For example, relaxation responses to NO donors and 8‐Br‐cGMP were greatly attenuated in gastric fundus muscles of Prkg1 −/− mice (Ny et al., 2000). Another study, however, investigated nitrergic responses in a different Prkg1 −/− mouse and found that significant NO‐ and ODQ‐dependent responses were retained in the mouse internal anal sphincter (Cobine et al., 2014), suggesting that downstream effectors other than PKG1 contribute to nitrergic relaxation. The effectiveness of the deactivation of Prkg1 in these studies was demonstrated by parallel positive control experiments performed on mouse aorta. It should be recognized that several PDEs are also expressed in post‐junctional cells, including the dual substrate PDE3A (Chen et al., 2007) that is expressed in ICC and directly inhibited by cGMP via competition with cAMP for the active site (Maurice et al., 2003). PDE3A could be a target for cGMP, and therefore, part of the downstream mechanisms causing the inhibitory effects of NO might be cAMP‐dependent.
Postjunctional cells that respond to enteric inhibitory neurotransmission
Morphology and connectivity of enteric inhibitory neurons and postjunctional cells
The GI tract is complicated by the fact that there are at least three types of cells that might contribute to the transduction of nitrergic signals. These cells are SMCs, ICC and PDGFRα+ cells. ICC and PDGFRα+ cells are electrically coupled to SMCs, forming an electrical syncytium, known as the SIP syncytium (Sanders et al., 2012). Changes in conductances in any of the SIP cells can influence voltage‐dependent processes in the other cells, so neurotransmitter responses can be generated in any of the cells and conduct to other SIP cells. ICC and PDGFRα+ are wound around varicose processes of enteric motor neurons and in close contact with varicosities of excitatory and inhibitory neurons, including those expressing nNOS. Thus, each SIP cell may be exposed to NO released from motor neurons, and there has been a significant effort to determine which cells mediate nitrergic responses.
After discovering that Kit is expressed in ICC in the GI tract, it was found that mutations in Kit (W locus in mice) negatively impact the development of ICC (Maeda et al., 1992; Ward et al., 1994; Huizinga et al., 1995; Torihashi et al., 1995). Severe W mutations result in complete loss of the tyrosine kinase activity of c‐Kit (Nocka et al., 1990), and homozygotic W mutants (W/W) typically die in utero (Russell, 1979). Compound heterozygotes, incorporating less severe mutations in the W locus, such as W/W V, have one allele that encodes a partially functional tyrosine kinase and offspring with this genotype survive to adulthood (Nocka et al., 1990; Chi and Powley, 2003). These mice have compromised development of ICC and have been used extensively to explore the role of ICC in GI motility (Sanders, 1996).
Morphological studies have shown close contacts between enteric motor neurons and ICC (e.g. Daniel and Posey‐Daniel, 1984; Rumessen et al., 1992). This relationship was also observed in the murine gastric fundus where enteric neurons make contacts with ICC‐IM (Burns et al., 1996). Immunofluorescence shows extensive tracking of ICC with enteric motor neurons in rodent and primate GI muscles (Wang et al., 1999; Salmhofer et al., 2001; Blair et al., 2012), as illustrated for nNOS+ neurons in Figure 1. Sites of close contact between nerve varicosities and ICC deep muscular plexus (DMP) display pre‐ and post‐junctional specializations. Similar areas of specialization occur between enteric neurons and SMCs; however, these are more rare (Daniel and Posey‐Daniel, 1984). Close, synaptic‐like contacts between ICC‐IM and nNOS+ neurons were also found in the colons of guinea pigs, and contacts of this sort were less common with SMCs (Wang et al., 2000). These observations support the idea that intramuscular types of ICC (ICC‐IM and ICC‐DMP) are innervated by nNOS+ neurons; however, the presence of similar contacts with SMCs suggests parallel innervation of these cells.
Figure 1.
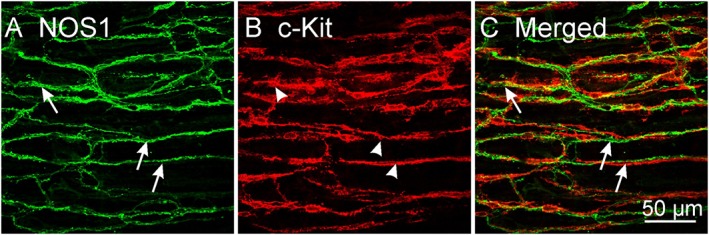
Confocal images of whole‐mount preparations showing close relationship between ICC and nNOS+ varicose nerve fibres in the murine proximal colon. Double labelling of the intramuscular class of ICC in colon (ICC‐IM) showing varicose processes of (A) nNOS+ neurons (NOS1; arrows) and (B) c‐Kit+ ICC‐IM (arrow heads). nNOS+ neurons track along ICC‐IM for distances greater than 250 μm and form close contacts. (C) Merged image showing the close relationship between nNOS+ neurons and ICC‐IM (arrows). Scale bar = 50 μm applies to all panels.
Synapse‐like connections between enteric neurons and ICC‐IM and cells lining septa separating smooth muscle bundles were investigated in canine antrum (Horiguchi et al., 2003b). Varicosities containing neurotransmitter vesicles were in close contact with ICC (<20 nm), and pre‐ and post‐synaptic densities were observed. Gap junctions were also observed between ICC and SMCs, suggesting that signaling that develop in ICC can be conducted to SMCs. Contacts between enteric nerve varicosities displayed ultrastructural features similar to nerve‐nerve varicosities in the CNS. Presynaptic densities contain a complex of proteins, including SNAP‐25, syntaxin, synaptobrevin and synaptogamin that are part of the machinery needed for neurotransmitter release via exocytosis (Chapman et al., 1994). SNAP‐25 and synaptogammin were co‐expressed in the varicose processes of nNOS+ motor neurons, and these varicosities adorned ICC‐IM along their lengths, suggesting multiple sites of innervation for each cell (Beckett et al., 2005). While vesicular transport is likely not needed for NO, the presence of synaptic proteins and nNOS suggests that release of NO occurs near release sites of other inhibitory neurotransmitters. ICC express postsynaptic density proteins PSD93 and PSD95, and recent transcriptome analysis of ICC from small bowel and colon confirms expression of genes that encode these proteins (i.e. Dlg2 and Dlg4, respectively) (Lee et al., 2017). Transcripts of Dlg2 and Dlg4 are found in whole muscles and are depressed significantly in W/W V muscles with reduced ICC (Beckett et al., 2005). Some authors have favoured the idea that neurotransmission occurs by ‘volume transmission’ in GI muscles (i.e. diffusion of neurotransmitters through the interstitium without synaptic specializations), but the presence of synapse‐like morphological structures, pre‐ and postjunctional specializations and membrane‐specific localization of nNOSα (Chaudhury et al., 2009, 2011) suggest that specialized neuro‐ICC junctions may be important for enteric motor neurotransmission.
Nitrergic responses in the lower oesophageal sphincter
The lower oesophageal sphincter (LES) maintains tone to restrict movement of gastric contents into the oesophagus. Tone is inhibited during swallowing to allow food to enter the stomach. The main inhibitory neurotransmitter causing relaxation of tone in the swallowing reflex is NO (Kim et al., 1999). The LES contains intramuscular ICC (ICC‐IM) that are closely associated with enteric motor neurons. Failure of these cells to develop in W/W V mice impairs nitrergic relaxation, suggesting that a portion of the nitrergic response is transduced by ICC‐IM (Ward et al., 1998). Cell‐specific genetic deactivation of sGC in SMCs or ICC increased LES tone, suggesting that both types of cells are innervated by nitrergic neurons and contribute to regulation of tone (Groneberg et al., 2015). However, the drop in tone induced by swallowing was compromised only when sGC was knocked down in ICC, suggesting that ICC are important transducers in the swallowing reflex.
Nitrergic responses in the stomach
The proximal stomach serves as a reservoir that relaxes, as food is ingested. This reflex is known as accommodation, and it is driven by nitrergic neurons (Desai et al., 1991). ICC‐IM are a prominent population of cells in the gastric fundus, as in the LES. ICC‐IM are closely associated with excitatory (cholinergic) and inhibitory (nitrergic) enteric motor neurons (Burns et al., 1996). Loss of ICC‐IM has been investigated in several mouse models, the most prominent being W/W V mice. Wild‐type mice generate IJPs that are partially inhibited by NG‐nitro‐L‐arginine methyl ester (L‐NAME). IJPs are smaller in W/W V mice and insensitive to L‐NAME. EFS causes inhibition of tone in the fundus, and this response switched to a contractile response in W/W V mice. The nNOS+ neurons are distributed normally in fundus muscles of W/W V mice, so the defect in nitrergic responses appears to be related to postjunctional defects. It was also observed that the hyperpolarization response to SNP was greatly reduced or absent in W/W V fundus muscles, but exogenous NO still caused relaxation of the same magnitude as in wild‐type muscles. This observation suggests that both ICC and SMCs express sGC and have the intrinsic apparatus to respond to NO; however, bath applied NO does not simulate NO released from enteric inhibitory neurons. Results from studies of W/W V mice suggested that ICC‐IM provide a pathway for transduction of responses to NO released from motor neurons.
Reflex activation of the stomach from the CNS occurs through the vagus nerve that connects with enteric motor neurons. A preparation was developed to record intracellular electrical or mechanical activities from murine fundus or antrum during stimulation of efferent anterior and posterior vagal trunks (Beckett et al., 2017). Stimulation of vagal trunks caused IJPs in the fundus that were enhanced after atropine and nearly blocked by L‐NNA. IJPs were absent in most W/W V fundus muscles, and residual IJPs in others were unaffected by L‐NNA. Relaxation responses were caused in fundus and antrum by vagal stimulation, and these were attenuated significantly in W/W V stomachs (Figure 2).
Figure 2.
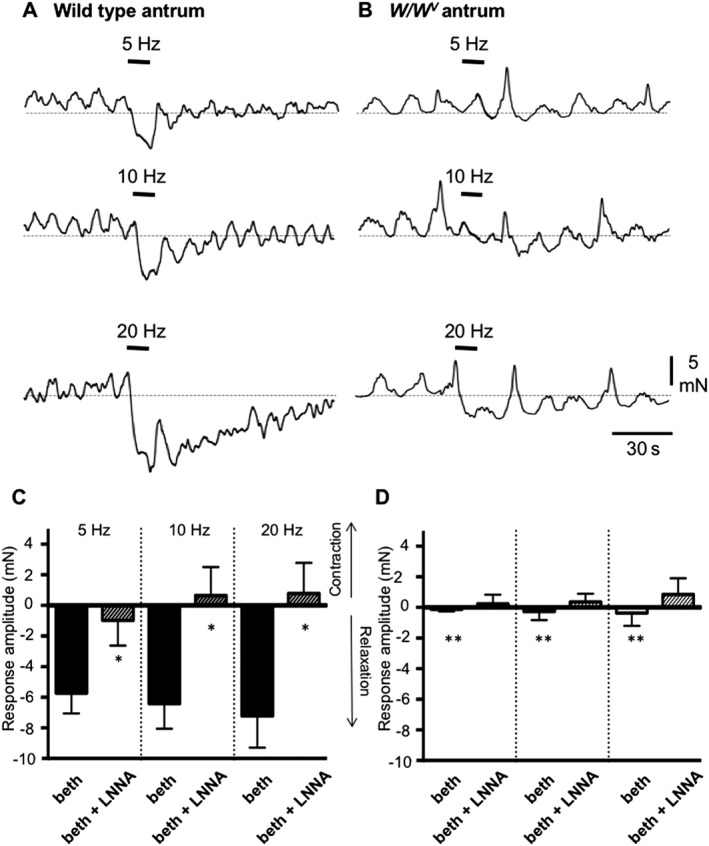
Antral responses to vagal nerve stimulation in wild‐type and W/W v mice. Contractions of wild‐type and W/W V antral muscles were measured in response to electrical vagal stimulation (EVS; 5, 10 and 20 Hz; delivered during periods denoted by black bars). Muscles were pretreated with bethanechol (3 μM). (A) EVS elicited relaxation of wild‐type muscles, and the amplitude of the relaxation increased with stimulus frequency. (B) EVS caused relaxation of W/W V muscles, as well, but the responses were of much smaller amplitude than in wild‐type muscles. (C, D) Summary of experiments showing average responses to EVS in wild‐type (n = 15) and W/W V (n = 8) antral muscles before and in the presence of L‐NNA (100 μM). Data in W/W V muscles were tested against responses under same conditions in wild‐type muscles: *P < 0.05; **P < 0.01. Redrawn from Beckett et al. (2017).
Some investigators, however, have dismissed the importance of ICC in nitrergic responses, reporting nearly normal functions in muscles with reduced ICC‐IM (Goyal, 2016). Comparisons of behaviour in W/W V muscles can be problematic because we found that lesions in ICC are not uniform, and when a substantial number of ICC remain, nitrergic inhibition is maintained (Sanders et al., 2014). A major argument against a role for ICC in nitrergic responses was that muscle tone in the LES was different in W/W V mice and Nos1 −/−. The reasoning was that nitrergic neurotransmission should be deactivated in both mice if ICC have a significant role, but the tone of the LES was hypertensive in Nos1 −/− mice and hypotensive in W/W V mice (Sivarao et al., 2001). This issue can be explained by the fact that only nitrergic responses are missing in Nos1 −/− mice, but cholinergic and nitrergic responses may be reduced in W/W V mice (Ward et al., 1998; Ward et al., 2000; Sanders et al., 2002; Sanders et al., 2010). Cholinergic input affects LES tone, so loss of this input may produce hypotensive LES muscles in W/W V mice.
The controversy about ICC versus SMCs was addressed in more detail by investigating responses of mice with cell‐specific knockouts of sGCβ1 in SMCs and ICC. Knockout of sGC in ICC of the fundus simulated the effects of L‐NAME in that relaxation responses to EFS were partially inhibited and nitrergic IJPs were essentially ablated (Groneberg et al., 2013; Lies et al., 2014a). Global knockouts displayed similar effects. These data tend to confirm results from W/W V mice, but these authors also found that specific knockout of sGCβ1 in SMCs reduced the durations of IJPs by half and also partially inhibited relaxation responses to nitrergic stimulation. These experiments suggest that cGMP‐dependent mechanisms in two cell types may be responsible for nitrergic relaxation in the murine fundus. One mechanism may be voltage‐dependent and depend upon ion channels in ICC‐IM that are either activated or deactivated in a cGMP‐dependent manner. A second mechanism may involve other types of ion channels and a non‐voltage‐dependent mechanism in which the contractile state of SMC cells, perhaps by modulation of the Ca2+ sensitivity of the contractile apparatus, is regulated.
Nitrergic responses in the small intestine
Damaging ICC by cell‐specific expression of diphtheria toxin A, caused a sustained depolarized membrane potential and loss of slow waves in small intestinal muscles (Klein et al., 2013). Neither excitatory nor inhibitory junction potentials were elicited in ICC‐depleted muscles, and these authors concluded that SMCs are denervated from enteric motor neurons when ICC are lost or damaged. Developmental studies also shed light on the relative role of ICC in nitrergic responses in the small intestine (Ward et al., 2006). ICC‐DMP develop after birth in mice, so neural inputs were compared at post‐partum day 0 (P0) and at P10. Neural responses were poorly developed at P0, but by P10, both excitatory and inhibitory responses were observed. P0 muscles were put into organ culture and treated with a neutralizing antibody against c‐Kit that restricts development of ICC. Neural responses developed in organ culture, but inclusion of the neutralizing antibody led to loss of both cholinergic and nitrergic responses, again suggesting that ICC are a primary site of neurotransmission in the small intestine.
A recent study investigated nitrergic regulation of basal contractions in the small intestine (Voussen et al., 2018). Global and SMC‐specific knockout of sGCβ1 had no effect on longitudinal muscles but increased contractions in circular muscle. Significant tone and increased contractile amplitude occurred in response to tetrodotoxin (TTX), L‐NAME and ODQ in wild‐type mice, and these responses were absent in global and SMC‐specific sGCβ1 knockout mice. These findings suggest that basal release of NO and exogenous NO affects contractions of SMCs by a mechanism intrinsic to these cells.
Nitrergic responses in the colon
The colon is also under tonic inhibitory drive, and TTX or L‐NNA depolarize and generate action potentials, suggesting that tonic inhibition comes from nitrergic neurons (Dickson et al., 2010). L‐NNA increased the frequency of contractions in wild‐type mice, and this effect of L‐NNA was absent in Nos1 −/− mice. Knockout of sGCβ1 was used as another means of blocking the activity of nitrergic neurons (Lies et al., 2015). Wild‐type mice had an irregular pattern of colon contractions, and this activity was also seen in colons with SMC‐specific knockout. Global and ICC‐specific knockouts of sGCβ1 produced contractions of regular amplitude and duration. Thus, basal release of NO mainly regulates colonic contractile behaviour through its effects on ICC.
The amplitudes of IJPs in colonic muscles were unaffected in mice with SMC‐specific knockout of sGCβ1 but reduced in ICC‐specific knockouts (Lies et al., 2014a). However, SMC‐specific knockout of sGC had a significant effect on the duration of the IJPs, and this effect was not observed in ICC‐specific knockouts. These authors hypothesized that there are two components in nitrergic IJPs in colonic muscles, a large amplitude component mediated by ICC and a slower, lower amplitude component mediated by SMCs. This observation suggests that conductances in both ICC and SMC may be targets for nitrergic regulation.
Mechanisms responsible for nitrergic inhibition in GI muscles
As discussed previously, sGC is the receptor for NO and necessary for NO‐dependent relaxation in GI muscles, as shown by pharmacological experiments and global gene deactivation of sGCβ1 (Groneberg et al., 2011). The major target for cGMP produced in response to NO released from inhibitory neurons is PKG1. Global knockout of Prkg1 caused inhibition of NO‐dependent relaxation in the stomach (Pfeifer et al., 1998; Ny et al., 2000). Prkg1 occurs as at least two splice variants in smooth muscles, Prkg1α and Prkg1β, with Prkg1β being dominant (Klein et al., 2013). To generate a quantitative knockout of Prkg1, an exon common to both splice variants was targeted (Pfeifer et al., 1998). Prkg1 −/− mice had grossly distended GI tracts and gastric and pyloric hypertrophy without damage to the enteric nervous system. Nitrergic relaxation responses were absent in muscles of Prkg1 −/− mice. SMC‐specific Prkg1 −/− mice did not reproduce the phenotype of global knockout animals, so ICC‐specific Prkg1 −/− mice were produced (Klein et al., 2013). Activation of iCre driven by the endogenous promoter for c‐Kit in Prkg1 fl/fl mice resulted in loss of PKGβ1 in only about half of ICC; however, these mice displayed significant GI motor defects, including increased GI transit time, changes in contraction frequency and a reduction in the nitrergic component of IJPs (Figure 3). Results of this study support the idea that ICC transduce neural inputs to GI muscles and explain some of the controversial findings using W/W V mice (Goyal, 2016). ICC express relatively high levels of sGC subunits (Iino et al., 2008; Cobine et al., 2014), generate cGMP in response to enteric nerve stimulation (Shuttleworth et al., 1993; Iino et al., 2009) and express Prkg1 (Klein et al., 2013). If the release of NO from motor neurons is restricted spatially (possibly by amount, binding or metabolism), then the close apposition of nerve varicosities (Burns et al., 1996; Wang et al., 1999, 2000; Horiguchi et al., 2003a; Blair et al., 2012) favours responses, particularly electrophysiological responses, to be generated by ICC and conducted to SMCs. Post‐junctional mechanisms activated by nitrergic stimuli are complex and incompletely understood. Several possible pathways have been proposed and tested using patch clamp studies, deactivation of specific genes involved in proposed transduction pathways, Ca2+ imaging and intracellular electrical recording.
Figure 3.
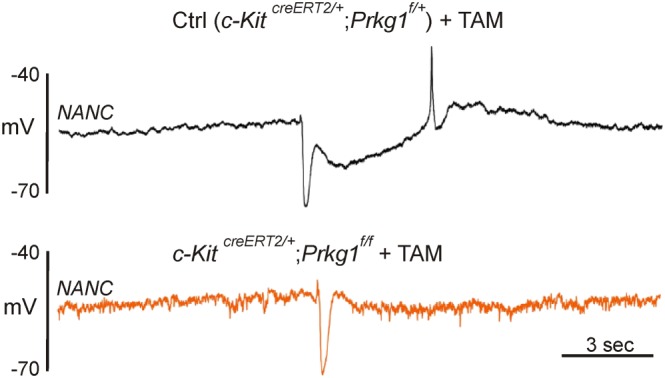
Elimination of slow (nitrergic) IJP in colon of Prkg1 −/− mice. (A) Intracellular recording of IJP in murine proximal colon from a control [tamoxifen (TAM)‐treated c‐KitCreERT2/+;Prkg1f/+] mouse. IJP, evoked by EFS, evoked by EFS, consisting of a fast IJP (fIJP) due to release of a purine, and a slow IJP (sIJP), due to release of NO. (B) IJP evoked in proximal colon of a TAM‐treated (3 days) c‐KitCreERT2/+; Prkg1f/f mouse. Expression of iCre is limited to ICC in these mice. Note reduction of sIJP in panel (B). PKG1 was not resolved in 40% of cells impaled in the c‐KitCreERT2/+; Prkg1f/f animals after 3 days of TAM treatment. Redrawn from Klein et al. (2013)
General observations about ion channels that may be affected by NO
Investigators have sought to discover the ionic conductance(s) responsible for electrophysiological responses to NO. Until only recently, it was not possible to isolate and study all three of the cell types of the SIP syncytium, so most experiments have been performed on SMCs. Cav1.2 channels, fundamental to voltage‐dependent mechanisms regulating contractions, are inhibited by cGMP‐dependent mechanisms and increased by an inhibitor of PKG1, Rp 8‐Br PET cGMPS (Ruiz‐Velasco et al., 1998). This could be a primary mechanism of nitrergic inhibition. Large conductance Ca2+‐activated K+ (KCa1.1) channels were reported to be activated by NO in a cGMP‐independent manner (Bolotina et al., 1994). However, this finding was not confirmed in studies of KCa1.1 channels from GI muscles, as NO did not affect KCa1.1 channel open probability in inside‐out patches from colon SMCs (Koh et al., 1995). KCa1.1 channels were activated significantly when membrane permeable analogues of cGMP or NO were applied to colonic SMCs (Thornbury et al., 1991; Koh et al., 1995). However, it is unlikely that KCa1.1 channels participate in postjunctional nitrergic responses, since [Ca2+]i is typically low during inhibitory stimulation, voltage‐dependent activation of KCa1.1 channels occurs at potentials more positive than membrane potentials during IJPs, and charybdotoxin does not block IJPs. Two additional K+ channels [called NO1 (82 pS) and NO2 (<4pS) due to their lack of identification in this study] were activated in canine colonic SMCs by NO or dibutyryl cGMP (Koh et al., 1995). Later studies suggested that currents from NO1 are mediated by K2P2.1 channels (Koh et al., 2001).
A role for two‐pore K+ channels in mediating postjunctional responses to NO
Colonic SMCs express stretch‐dependent K+ (SDK) channels encoded by the two‐pore K+ channel, K2P2.1 (Koh and Sanders, 2001). Membrane stretch activates 95 pS K+ channels, as does elongation of single SMCs. These channels have the same properties as the channels referred to previously as NO1 and are activated by sodium nitroprusside (SNP) or 8‐Br‐cGMP (Koh et al., 1995). Openings of SDK channels were potentiated by simultaneous application of NO or 8‐Br‐cGMP and stretch, suggesting that these stimuli could be synergistic in colonic muscles (Koh and Sanders, 2001). SDK channels, with input from NO released from nitrergic neurons, may be fundamental to maintaining a low degree of SMC excitability, providing a means for the reservoir function of this organ.
SDK channels in colonic muscles are blocked by sulfur‐containing amino acids, and L‐methionine was found to be the most selective antagonist (Park et al., 2005). L‐methionine at concentrations effective in blocking SDK channels had little or no effect on other major K+ currents in colonic SMCs. L‐methionine increased the force and frequency of colonic muscle contractions, reversed nitrergic inhibitory responses and depolarized membrane potentials. Responses to L‐methionine were blocked by pretreatment of muscles with L‐NNA. Nitrergic IJPs were also blocked by sulfur‐containing amino acids. How NO activates SDK channels was investigated by expressing K2P2.1 channels, the dominant mechanosensitive two‐pore K+ channels in colon SMCs, in COS‐7 cells (Koh et al., 2001). K2P2.1 channels are activated by stretch as were SDK channels in native cells. K2P2.1‐mediated currents were also enhanced by SNP or by 8‐Br‐cGMP. The amino acid sequence of the K2P2.1 channel contains two consensus sequences for phosphorylation by PKG1. The response to 8‐Br‐cGMP was blocked in mutated K2P2.1 channels, produced by replacing Ser351 with alanine (i.e. S351A). Thus, the proposed pathway by which NO activates K2P2.1 channels in postjunctional cells involves cGMP‐dependent activation of PKG1 and phosphorylation of K2P2.1 at Ser351.
However, other groups have disputed a role for K2P2.1 in nitrergic responses (Zhang et al., 2010; Gil et al., 2012). IJPs in oesophageal muscles were compared before and after addition of L‐methionine. L‐NAME blocked the slow phase of IJPs completely, but L‐methionine produced only a small inhibitory effect. However, such a comparison is difficult under the circumstances of this particular experiment, because L‐methionine caused substantial membrane depolarization. Thus, if IJPs are due to K+ channels, the amplitude should increase as the driving force for K+ current increases. In fact finding that the amplitude was approximately the same before and after depolarization suggests that IJPs were partially inhibited in the latter condition.
Involvement of inward current channels in nitrergic IJPs
IJPs generated by purines were compared with the IJP component attributed to NO in guinea pig colon (Hirst et al., 2004). Increasing the external K+ concentration ([K+]o), which decreases the driving force for IJPs due to a K+ conductance, decreased the amplitude of purinergic IJPs but had little effect on nitrergic IJPs. These authors concluded that purinergic IJPs were due to the opening of apamin‐sensitive K+ channels, but nitrergic IJPs are due to the suppression of the ongoing opening of an inward current. Additional experiments suggested that the inward current was dependent on Ca2+.
A mechanism for IJPs involving Ca2+‐activated Cl− channels (CaCC) has also been proposed by several authors, and molecular components necessary for such a response are available in the SIP syncytium (see section on Effects of nitrergic innervation on Ca 2+ transients in ICC). If Cl− channels are involved in nitrergic responses, this may be a means of determining which cells generate nitrergic IJPs in response to NO released from neurons, because SIP cells each express different types of ion channels. For example, CaCC are expressed in ICC, but not in SMCs and PDGFRα+ cells.
IJPs evoked in opossum oesophagus were inhibited by TTX, 9‐anthroic acid and niflumic acid (Zhang and Paterson, 2002). Ca2+ store‐active drugs, such as caffeine, ryanodine and cyclopiazonic acid (CPA), blocked nitrergic IJPs in the oesophagus (Zhang and Paterson, 2003), and these authors suggested that Ca2+ released from stores may activate CaCC in SMCs. Store‐active drugs have multiple effects in intact muscles and may not work exclusively through inhibition of Ca2+ release. Caffeine, ryanodine and CPA also blocked nitrergic IJPs in murine colon, but store‐active drugs also inhibit K2P2.1 channels expressed in COS‐7 cells and native SDK currents in colonic myocytes (Hwang et al., 2008). K2P2.1 channels are not sensitive to Ca2+, so the effects of these drugs on K2P2.1 are unlikely to be due to the effects of the Ca2+ released from stores. The effects of caffeine appeared to be due to its well‐known actions as a PDE inhibitor, and its inhibitory effects were blocked by dialysis of cells with an inhibitory peptide of PKA. Suppression of CaCC by NO is an attractive hypothesis for the action of NO in GI muscles, but how NO suppresses CaCC and whether CaCC antagonists produce the same magnitude of hyperpolarization as nitrergic stimulation need to be investigated further.
Effects of nitrergic innervation on Ca2+ transients in ICC
ICC throughout the GI tract express CaCC encoded by Ano1 (Chen et al., 2007; Gomez‐Pinilla et al., 2009; Hwang et al., 2009), and a similar conductance is not present in other SIP cells. ICC also express genes that encode proteins that facilitate responses to NO, including Gucy1a1, Gucy1b1, Prkg1 and Mrvi1 (Iino et al., 2008; Baker et al., 2018), and these cells generate cGMP in response to nitrergic nerve stimulation (Shuttleworth et al., 1993; Iino et al., 2009). Isolated ICC generate spontaneous transient inward currents (STICs) (Zhu et al., 2011) linked to Ca2+ release from stores (Zhu et al., 2015). Dynamic measurements of Ca2+ transients and responses to nitrergic agonists, antagonists and NO released from enteric inhibitory neurons have been accomplished recently by imaging ICC‐DMP in small intestinal muscles in situ from mice expressing GCaMP3 in ICC (Figure 4) (Baker et al., 2018). Spatially limited Ca2+ transients occur on an ongoing basis in ICC‐DMP, the intramuscular class of ICC in the small intestine. These events are responsible for STICs in ICC‐DMP. NOS inhibitors and ODQ increased the frequency of Ca2+ transients, and NONOate and Bay 58‐2667, an sGC agonist, decreased Ca2+ transients. EFS caused inhibition of Ca2+ transients, and this effect was blocked by L‐NNA and ODQ. Taken together, these studies demonstrate that ICC‐DMP are innervated by enteric inhibitory neurons and have the molecular machinery to generate ongoing Ca2+ transients and STICs via activation of CaCC channels. Ca2+ transients are inhibited by NO, and this mechanism may provide an explanation for the nitrergic components of IJPs.
Figure 4.
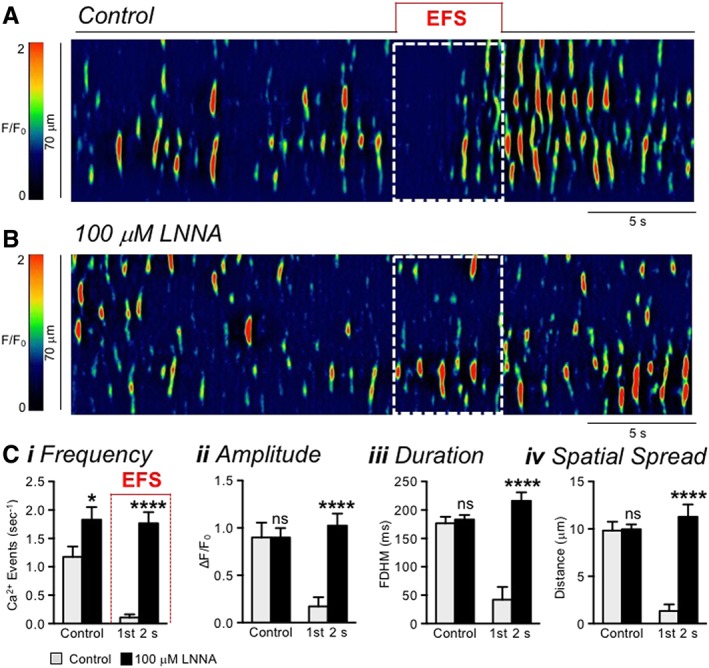
Ca2+ transients in ICC‐DMP are inhibited by nitrergic neural input. (A, B) Spatio‐temporal maps depicting Ca2+ transients in an ICC‐DMP in the mouse small intestine. Note the transient and spatially restricted nature of these events that are expected to couple to activation of CaCC channels in the plasma membrane. EFS (10 Hz, 0.5 ms pulse duration; 5 s; white dotted box) in panel (A) inhibited Ca2+ transients during the initial phase of stimulation (~2 s), and then excitatory input caused restoration of Ca2+ release. A rebound period of excitation occurred after cessation of EFS. (B) Addition of L‐NNA (100 μM) blocked the inhibition of Ca2+ transients during EFS. (C) Tabulation of effects of EFS on Ca2+ transient frequency (i), amplitude (ii), duration (iii), and spatial spread (iv) in ICC‐DMP during control conditions (pre‐EFS) and during the initial 2 s of EFS before and after L‐NNA (n = 5 animals; 15 cells). ns = P > 0.05; *P < 0.05; ****P < 0.0001. Copied with permission from Baker et al. (2018).
The cellular mechanism by which NO inhibits Ca2+ release in ICC is still under investigation. The mechanism may involve phosphorylation of inositol 1,4,5‐trisphosphate receptor‐associated cGMP kinase substrate (IRAG) by PKG1β. IRAG associates with IP3R1 and inhibits agonist‐dependent Ca2+ release (Geiselhoringer et al., 2004). Gene deactivation of IRAG generated mice with distended stomachs, possibly due to pyloric stenosis, delayed intestinal transit and reduced ability of 8‐Br‐cGMP to inhibit contractile responses. The phenotype of IRAG−/− mice was dramatic, making it likely that IRAG is involved in nitrergic inhibition. All three SIP cells express Mrvi1 (gene encoding IRAG) (Lee et al., 2017), but which cell(s) manifests the mechanism dependent upon IRAG is still not known. The expression of Ano1 in ICC and the reduction in Ca2+ release from IP3R1 in ICC in response to nitrergic signalling could be mechanisms linked to the function of NO and IRAG in GI muscles (Figures 4 and 5). Ca2+‐regulated, ongoing inward currents in SMCs that are suppressed by nitrergic mechanisms have not been identified. From the literature available to date, one must conclude that there are substantial uncertainties about the cells and mechanism mediating the Ca2+ and voltage‐dependent effects of NO in GI muscles.
Figure 5.
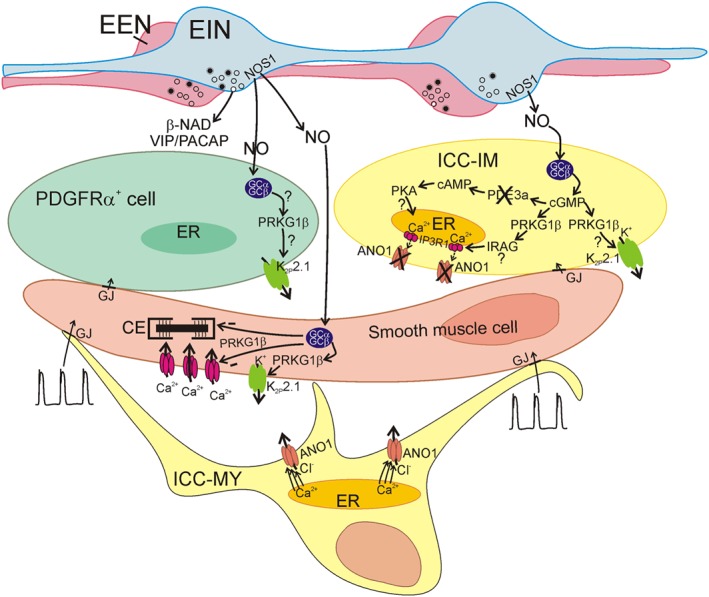
Proposed mechanisms of nitrergic neurotransmission in the SIP syncytium. GI muscles are innervated by excitatory (EEN) and inhibitory (EIN) enteric motor neurons. Major inhibitory neurotransmitters released by EIN are NO, purines (β‐NAD shown) and peptides, such as VIP and PACAP (release of β‐NAD, and VIP/PACAP is indicated, but mechanisms for these neurotransmitters are not depicted). NO is synthesized by nNOS (NOS1) expressed by EIN and released on‐demand by Ca2+ influx into varicosities. NO binds to sGC in SIP cells. In ICC‐IM, binding of NO to sGC causes formation of cGMP that can interact with PKG1β (PRKG1β) to phosphorylate and activate K2P2.1 channels and generate an outward current. PKG1β can also phosphorylate IRAG which interacts with IP3R1 (IP3R1) and inhibits Ca2+ release from stores (see Figure 4). Cessation of Ca2+ release causes deactivation of CaCC (ANO1) channels, causing a net gain in outward current in the cells. Activation of K2P2.1 channels or deactivation of CaCC (ANO1) can cause hyperpolarization of ICC‐IM and, via gap junctions (GJ) connectivity with other SIP cells, hyperpolarization of the SIP syncytium. PDE3A is also a possible target for cGMP, and its inhibitory effects on PDE3A may increase the levels of cAMP and cause downstream signalling through PKA. Effectors for this pathway have not been identified. PDGFRα+ cells also express sGC subunits, PKG1 and K2P2.1 channels. This pathway in PDGFRα+ cells is speculative at the present time. SK3 channels in PDGFRα+ cells (not shown) produce the outward current that causes the fast IJP phase of inhibitory neurotransmission (Kurahashi et al., 2011) (and see Figure 3). NO also binds to sGC in SMCs where it produces cGMP, activates PKG1β and causes Ca2+ desensitization (−) of the contractile apparatus, inhibition (−) of CaV1.2 channels and activation of K2P2.1 channels. Speculative steps are denoted by question marks. ICC‐MY are present in most areas of the GI tract and generate pacemaker activity through release of Ca2+ from stores and activation of CaCC (ANO1) channels. Pacemaker activity (depicted as slow waves) conducts to SMCs causing periodic depolarization and phasic contractions. Neural inputs are superimposed upon the pacemaker activity.
Nitrergic effects via modulation of the Ca2+ sensitivity of the contractile apparatus
Another means by which NO regulates GI muscle contractions is by desensitization of the contractile apparatus to Ca2+. The activity of myosin light chain phosphatase (MLCP), the enzyme that dephosphorylates myosin, ends cross‐bridge cycling and causes relaxation in SMCs is regulated by G‐protein‐coupled activation of RhoA/Rho‐kinase‐dependent phosphorylation of MYPT1, a regulatory co‐factor of MLCP (Somlyo and Somlyo, 2003). Desensitization can occur by cyclic nucleotide‐dependent kinase‐dependent phosphorylation of RhoA, preventing its activation of Rho‐kinase (Somlyo and Somlyo, 2003). Another target for Ca2+‐induced desensitization by cGMP is telokin (smMLCK) which is phosphorylated at Ser13 in response to 8‐Br‐cGMP or forskolin (Walker et al., 2001). Telokin−/− mice displayed increased Ca2+ sensitivity and reduced relaxation responses to 8‐Br‐cGMP (Khromov et al., 2006). Wild‐type mice displayed basal phosphorylation at Ser13 in telokin, and phosphorylation was increased by SNP and 8‐Br‐cGMP. Telokin−/− mice showed enhanced contractile responses to elevated [K+]o, carbachol, and cholinergic neurotransmission. However, no increase in telokin phosphorylation was detected in response to nitrergic neurotransmission or with response‐matched concentrations of SNP (An et al., 2015). These data suggest that telokin phosphorylation may be recruited for responses to bath‐applied NO but may not be a factor in responses to NO released from neurons.
Mechanosensitive mechanisms linked to NO
Enteric motor neurons are organized to convey local reflexes. For example, initiation of the peristaltic reflex comes from mechanosensitive activation of afferent nerves innervating myenteric ganglia and organizing a stereotypical motor response in the colon. However, cells of the SIP syncytium also demonstrate mechanosensitive responses. For example, stretch of the proximal colon initiates a hyperpolarization response dependent upon the rate at which the stretch is applied (Won et al., 2013). This response was blocked by TTX and L‐NNA, suggesting that it might be part of a neural reflex involving activation of nitrergic neurons. However, the inhibitory response to stretch in muscles treated with L‐NNA was restored by NO donors, suggesting that a postjunctional mechanism mediating the stretch response is sensitized by NO, which is tonically released from nerves in the proximal colon. ICC appear to mediate the stretch response because muscles treated with a c‐Kit neutralizing antibody to disrupt the development of ICC lose the stretch‐dependent inhibitory response. This response was also blocked by L‐methionine, which blocks K2P2.1 channels in GI muscles (Park et al., 2005). The overall mechanism appears to be due to NO, released from nerves, sensitizing K2P2.1 channels to muscle stretch.
Defects in nNOS+ neurons or nitrergic neurotransmission lead to GI motor dysfunction
GI dysfunction occurs in a large cohort of patients with long‐standing diabetes (Lee and Hasler, 2017; Piper and Saad, 2017). At least part of the motility problems that develop have been attributed to neuropathies in extrinsic nerves and intrinsic (enteric) excitatory, inhibitory and sensory pathways that regulate smooth muscle contraction (Azpiroz and Malagelada, 2016). In keeping with the focus of this review, defects in nitrergic regulation will be discussed. Spontaneous diabetic rats (BB/W), streptozotocin‐treated rats and non‐obese diabetic (NOD) mice were found to have significantly reduced nNOS+ enteric neurons and Nos1 transcripts in tissues (Takahashi et al., 1997; Watkins et al., 2000; Choi et al., 2008). Insulin treatment restored the expression of nNOS (Watkins et al., 2000). Responses to nitrergic nerve stimulation are also diminished in GI muscles of diabetic animals and humans (Ordog et al., 2000; Watkins et al., 2000), and these authors attributed the functional effects either to loss of nNOS+ neurons or to reduced nNOS expression. Interestingly, studies of muscles from diabetic human patients found no significant loss of PGP9.5 (a pan‐neuronal marker) or of nNOS+ neurons in gastric corpus muscles of 20 diabetic patients; however, about 20% of the diabetic patients displayed a reduction in nNOS+ neurons. These studies all utilized immunofluorescence techniques to quantify nNOS+ neurons. Immunological techniques are limited by threshold‐of‐detection issues, so one must question whether reductions in nNOS+ neurons are due to reduced numbers of inhibitory enteric neurons or to reduced expression of nNOS. Co‐labelling of tissues with VIP antibodies, a peptide neurotransmitter co‐expressed in enteric inhibitory neurons, is a means of further testing the state of this population of neurons. This approach was used with both streptozotocin‐treated and NOD mice, and the data suggest that in these models, nNOS expression is reduced, but inhibitory neurons are not (Watkins et al., 2000). While a nitrergic neuropathy appears to be accepted as a cause for the GI motility disorders that accompany diabetes, it is still unresolved whether the reduction in nNOS expression in enteric motor neurons is sufficient to generate a functional reduction in NO release in response to activation of inhibitory neurons. The answer to this question awaits a comparison of NO release in normal and diabetic muscles.
An additional explanation for reduced nitrergic neural regulation of GI muscles in diabetes might be impaired connectivity between motor neurons and postjunctional target cells. As discussed in previous sections of this review, at least a portion of nitrergic neurotransduction is mediated through responses that develop in ICC (Burns et al., 1996; Lies et al., 2014b; Beckett et al., 2017; Baker et al., 2018). Loss of ICC or disrupted connectivity between enteric motor neurons and ICC is the most common histological finding in diabetic tissues of patients with gastroparesis, for example (Faussone‐Pellegrini et al., 2012). Lesions of this sort were first reported from studies of a Type I diabetic mouse model (Ordog et al., 2000), but reduced ICC have also been found in GI muscles of human patients with diabetes (He et al., 2001; Faussone‐Pellegrini et al., 2012). However, it is still unresolved whether phenotypic changes in ICC (e.g. loss of signalling molecules involved in nitrergic transduction or connectivity with SMCs) occur well before loss of cells. Such phenotypic changes might initiate GI motor defects before a significant reduction in ICC numbers occurs.
Damage to ICC in diabetes has been linked to increased oxidative stress. This appears to be related to the loss of CD206+ macrophages that express haem oxygenase (HO1). At the onset of diabetes, HO1 expression increases in resident macrophages in mice, and this is protective against ICC loss and development of delayed gastric emptying (Choi et al., 2008). Loss of CD206+ macrophages (and concomitant decrease in HO1) results in reduced ICC and the development of gastroparesis. Therapeutic strategies that restore HO1 activity might be protective against some of the GI motor problems associated with diabetes (Farrugia, 2015).
Motor defects related to overproduction of NO
NO is a potent inhibitor of GI muscle contraction, so an overabundance of NO can be detrimental to GI motility. This occurs in some inflammatory conditions. Another gene encoding NOS in cells within the wall of the GI tract is Nos2 [the gene for inducible NOS (iNOS)]. Expression of Nos2 is normally low but can be induced by exposure to pro‐inflammatory cytokines, such as TNF‐α, IL‐1 and IFN‐γ in many tissues and cells (Korhonen et al., 2005). Low concentrations of Ca2+ facilitate calmodulin binding to iNOS, creating essential conditions of constitutive activation. Abundant NO production for long periods of time can occur after induction of Nos2.
Post‐surgical ileus is one of the motility disorders that has been associated with an overproduction of NO. Disruptions in ICC networks were observed near sites of small intestinal resections within 5 h after surgery in mice (Yanagida et al., 2004). Slow waves and phasic contractions decreased in regions of muscle with ICC defects. Loss of ICC and compromised slow wave activity decreased as a function of distance from the resection. Muscles from the area near bowel resections were also poorly responsive to carbachol or transmural nerve stimulation. ICC networks and slow waves recovered spontaneously near the site of the anastomosis within 24 h. The magnitude of the ICC lesions and recovery periods decreased with pre‐surgical treatment with inhibitors of iNOS and were greatly reduced in Nos2 −/− mice (Yanagida et al., 2007).
Manipulation of the intestine, as might occur during abdominal surgical procedures unrelated to bowel surgery, activates inflammatory responses. Resident muscularis macrophages are activated by manipulation of the gut (innate response), and these cells initiate a broader inflammatory response leading to recruitment of circulating leukocytes, enhanced release of inflammatory cytokines and increased production of NO and prostanoids (Kalff et al., 2000; Bauer and Boeckxstaens, 2004). Immunohistochemistry showed that iNOS expression increases in phagocytes within the muscularis (Kalff et al., 2000). NO generation from the increased expression of iNOS causes significant inhibition of GI motor activity (Kalff et al., 2000). The overproduction of NO and effects on GI motility were limited in mice with genetic deactivation of Nos2 (Kalff et al., 2000), by treatment with iNOS inhibitors, when macrophage‐deficient mice were utilized for experimental gut manipulation (Wehner et al., 2007) and in Nos2‐deficient bone marrow chimera mice (Turler et al., 2006). These data show the importance of iNOS in the motility dysfunction elicited by surgical manipulation of the gut.
While NO has direct inhibitory effects on SMCs, it is also probable that part of the deleterious effects of overproduction of NO are mediated by damage to ICC. In a study using jejunal muscle organoids, treatment with IFN‐γ and LPS for 24 h induced expression of Nos2 and impaired the pacemaker activity of ICC (Kaji et al., 2016). Pretreatment of the organoids with iNOS inhibitors blocked the damage to ICC induced by IFN‐γ and LPS. Antioxidant treatment to reduce oxidative stress caused by NO also reduced the impairment of pacemaker activity, but no benefit was obtained with the blocker of guanylate cyclase, ODQ.
Intestinal manipulation (IM) also impairs ICC networks. Twenty‐four hours after IM, the density of ICC in the myenteric region of the small intestine, as determined by c‐Kit or ANO1 immunoreactivity, decreased by about 50%, and the ICC recovered spontaneously by 48 h (Kaji et al., 2018). The speed with which this occurred appeared to be due to loss of these key functional proteins in ICC rather than cell death, as electron microscopy demonstrated retention of a gap junction‐coupled network of ICC‐like cells in the myenteric region. The appearance of cytoplasmic vacuoles in ICC at 24 h after IM may have indicated the development of autophagy that might have contributed to the decreased immunoreactivity of common ICC proteins. Treatment of muscles with aminoguanidine reduced the disruption in ICC networks, suggesting that the damage was largely due to production of NO via iNOS.
Summary and conclusions
Nitrergic regulation is extremely important in GI motility, as NO is a major inhibitory neurotransmitter in nearly every region and a mediator of inflammatory effects. NOS+ motor neurons are plentiful in GI muscles, and they release NO when activated. Postjunctional responses in cells of the SIP syncytium are activated by NO. Several defined responses have been reported in ICC and SMCs. The apparatus necessary to transduce NO signals is also available in PDGFRα+ cells (Iino et al., 2008; Iino et al., 2009; Lee et al., 2017); however, no studies have reported effects of NO specific to these cells. The majority of evidence suggests that nitrergic responses are integrated, with contributions from both ICC and SMCs. The actual mechanisms of nitrergic IJPs and relaxation are still controversial with possible contributions from activation of K+ channels (K2P2.1), suppression of activation of Cl− channels (CaCC/ANO1), reduced open probability of Ca2+ channels (CaV1.2) and Ca2+ desensitization of the contractile apparatus in SMCs. Relative contributions from these mechanisms may change from region‐to‐region in the GI tract; however, the ubiquitous expression of CaCC and Ca2+ release from stores in ICC throughout the gut suggest the suppression of CaCC activity has a prominent role in nitrergic responses in many regions. A reduced expression of nNOS has been demonstrated in animal models of diabetes and in human GI muscles of diabetic patients. Loss of nitrergic neural signalling may contribute to GI motor disorders occurring in patients with long‐standing diabetes. The increased expression of iNOS occurring in inflammatory responses may cause damage to ICC, leading to a pseudo‐obstruction‐like state or other motor abnormalities. Neither the mechanisms of the muscle inhibition nor the mechanisms causing ICC remodelling are fully understood, so there are many things yet to learn about nitrergic regulation of GI muscles.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d,e).
Author contributions
K.M.S and S.M.W. researched and discussed literature on NO in enteric inhibitory neurotransmission; K.M.S. and S.M.W. developed or redrew the figures; K.M.S. drafted the manuscript; K.M.S. and S.M.W. edited and agreed upon the final draft of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge support from the National Institute of Diabetes and Digestive and Kidney Diseases through funding of P01 DK41315 to K.M.S. and S.M.W., R37 DK40569 to K.M.S. and R01 DK57236 to S.M.W. that have provided support for all projects related to NO in our labs.
Sanders, K. M. , and Ward, S. M. (2019) Nitric oxide and its role as a non‐adrenergic, non‐cholinergic inhibitory neurotransmitter in the gastrointestinal tract. British Journal of Pharmacology, 176: 212–227. 10.1111/bph.14459.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Other ion channels. Br J Pharmacol 174: S195–S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion N, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2015/16: ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Striessnig J, Kelly E, Marrion N, Peters JA, Faccenda E et al (2017e). The Concise Guide to PHARMACOLOGY 2015/16: voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Bhetwal BP, Sanders KM, Somlyo AV, Perrino BA (2015). Role of telokin in regulating murine gastric fundus smooth muscle tension. PloS one 10: e0134876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada C (2016). Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia 59: 404–408. [DOI] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Cobine CA, Keef KD, Sanders KM (2018). Inhibitory neural regulation of the Ca (2+) transients in intramuscular interstitial cells of cajal in the small intestine. Front Physiol 9: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Boeckxstaens GE (2004). Mechanisms of postoperative ileus. Neurogastroenterol Motil 16 (Suppl. 2): 54–60. [DOI] [PubMed] [Google Scholar]
- Beckett EA, Sanders KM, Ward SM (2017). Inhibitory responses mediated by vagal nerve stimulation are diminished in stomachs of mice with reduced intramuscular interstitial cells of Cajal. Sci Rep 7: 44759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM (2005). Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol 493: 193–206. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Bayguinov Y, Sanders KM, Ward SM (2012). Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil 24: e437–e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA (1994). Nitric oxide directly activates calcium‐dependent potassium channels in vascular smooth muscle. Nature 368: 850–853. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH (1990). Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770. [DOI] [PubMed] [Google Scholar]
- Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG (1990). Nitric oxide as an inhibitory non‐adrenergic non‐cholinergic neurotransmitter. Nature 345: 346–347. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM (1996). Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A 93: 12008–12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, An S, Barton N, Jahn R (1994). SNAP‐25, a t‐SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem 269: 27427–27432. [PubMed] [Google Scholar]
- Chaudhury A, He XD, Goyal RK (2009). Role of PSD95 in membrane association and catalytic activity of nNOSalpha in nitrergic varicosities in mice gut. Am J Physiol Gastrointest Liver Physiol 297: G806–G813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, He XD, Goyal RK (2011). Myosin Va plays a key role in nitrergic neurotransmission by transporting nNOSalpha to enteric varicosity membrane. Am J Physiol Gastrointest Liver Physiol 301: G498–G507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D et al (2007). Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31: 492–509. [DOI] [PubMed] [Google Scholar]
- Chi MM, Powley TL (2003). c‐Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am J Physiol Regul Integr Comp Physiol 285: R1170–R1183. [DOI] [PubMed] [Google Scholar]
- Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T et al (2008). Heme oxygenase‐1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 135: 2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobine CA, Sotherton AG, Peri LE, Sanders KM, Ward SM, Keef KD (2014). Nitrergic neuromuscular transmission in the mouse internal anal sphincter is accomplished by multiple pathways and postjunctional effector cells. Am J Physiol Gastrointest Liver Physiol 307: G1057–G1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel HH, Thornbury KD, Ward SM, Sanders KM (1991). Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol 260: G789–G792. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Posey‐Daniel V (1984). Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol 246: G305–G315. [DOI] [PubMed] [Google Scholar]
- Desai KM, Sessa WC, Vane JR (1991). Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351: 477–479. [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK (2010). The mechanisms underlying the generation of the colonic migrating motor complex in both wild‐type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol 298: G222–G232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G (2015). Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am 44: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone‐Pellegrini MS, Grover M, Pasricha PJ, Bernard CE, Lurken MS, Smyrk TC et al (2012). Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med 16: 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD, Sibley D (2010). cGMP‐dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck H, Sweeney KM, Sanders KM, Shuttleworth CW (1997). Effects of a novel guanylate cyclase inhibitor on nitric oxide‐dependent inhibitory neurotransmission in canine proximal colon. Br J Pharmacol 122: 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WA, McConalogue K (1995). Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology 108: 554–563. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess‐Williams R (1988). Endothelium‐derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336: 385–388. [DOI] [PubMed] [Google Scholar]
- Geiselhoringer A, Werner M, Sigl K, Smital P, Worner R, Acheo L et al (2004). IRAG is essential for relaxation of receptor‐triggered smooth muscle contraction by cGMP kinase. EMBO J 23: 4222–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil V, Gallego D, Moha Ou Maati H, Peyronnet R, Martinez‐Cutillas M, Heurteaux C et al (2012). Relative contribution of SKCa and TREK1 channels in purinergic and nitrergic neuromuscular transmission in the rat colon. Am J Physiol Gastrointest Liver Physiol 303: G412–G423. [DOI] [PubMed] [Google Scholar]
- Gillespie JS, Liu XR, Martin W (1989). The effects of L‐arginine and NG‐monomethyl L‐arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol 98: 1080–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ et al (2009). Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296: G1370–G1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK (2016). CrossTalk opposing view: interstitial cells are not involved and physiologically important in neuromuscular transmission in the gut. J Physiol 594: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Rattan S, Said SI (1980). VIP as a possible neurotransmitter of non‐cholinergic non‐adrenergic inhibitory neurones. Nature 288: 378–380. [DOI] [PubMed] [Google Scholar]
- Grider JR, Murthy KS, Jin JG, Makhlouf GM (1992). Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol 262: G774–G778. [DOI] [PubMed] [Google Scholar]
- Groneberg D, Konig P, Koesling D, Friebe A (2011). Nitric oxide‐sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology 140: 1608–1617. [DOI] [PubMed] [Google Scholar]
- Groneberg D, Lies B, Konig P, Jager R, Seidler B, Klein S et al (2013). Cell‐specific deletion of nitric oxide‐sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology 145: 188–196. [DOI] [PubMed] [Google Scholar]
- Groneberg D, Zizer E, Lies B, Seidler B, Saur D, Wagner M et al (2015). Dominant role of interstitial cells of Cajal in nitrergic relaxation of murine lower oesophageal sphincter. J Physiol 593: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L (1979). Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res 5: 211–224. [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL (2002). Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143: 2767–2774. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G (2001). Loss of interstitial cells of cajal and inhibitory innervation in insulin‐dependent diabetes. Gastroenterology 121: 427–434. [DOI] [PubMed] [Google Scholar]
- He XD, Goyal RK (1993). Nitric oxide involvement in the peptide VIP‐associated inhibitory junction potential in the guinea‐pig ileum. J Physiol 461: 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bywater RA, Teramoto N, Edwards FR (2004). An analysis of inhibitory junction potentials in the guinea‐pig proximal colon. J Physiol 558: 841–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Keef KD, Ward SM (2003a). Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol 284: G756–G767. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Sanders KM, Ward SM (2003b). Enteric motor neurons form synaptic‐like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res 311: 299–313. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC (1993). Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75: 1273–1286. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A (1995). W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR et al (2009). Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, O'Kane N, Singer C, Ward SM, Sanders KM, Koh SD (2008). Block of inhibitory junction potentials and TREK‐1 channels in murine colon by Ca2+ store‐active drugs. J Physiol 586: 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ (1990). Haem‐dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol 67: 1–7. [DOI] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Nojyo Y (2008). Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide‐sensitive guanylate cyclase in the guinea‐pig gastrointestinal tract. Neuroscience 152: 437–448. [DOI] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM (2009). Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenter Motil 21: 542–550, e512–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji N, Horiguchi K, Iino S, Nakayama S, Ohwada T, Otani Y et al (2016). Nitric oxide‐induced oxidative stress impairs pacemaker function of murine interstitial cells of Cajal during inflammation. Pharmacol Res 111: 838–848. [DOI] [PubMed] [Google Scholar]
- Kaji N, Nakayama S, Horiguchi K, Iino S, Ozaki H, Hori M (2018). Disruption of the pacemaker activity of interstitial cells of Cajal via nitric oxide contributes to postoperative ileus. Neurogastroenter Motil. 10.1111/nmo.13334. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ (2000). Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118: 316–327. [DOI] [PubMed] [Google Scholar]
- Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R et al (2006). Smooth muscle of telokin‐deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP‐induced relaxation. Proc Natl Acad Sci U S A 103: 2440–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CD, Goyal RK, Mashimo H (1999). Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am J Physiol 277: G280–G284. [DOI] [PubMed] [Google Scholar]
- Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R et al (2013). Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow‐wave activity. Nat Commun 4: 1630. [DOI] [PubMed] [Google Scholar]
- Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A (2004). Nitric oxide‐sensitive guanylyl cyclase: structure and regulation. Neurochem Int 45: 813–819. [DOI] [PubMed] [Google Scholar]
- Koh SD, Campbell JD, Carl A, Sanders KM (1995). Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol 489 (Pt 3): 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM et al (2001). TREK‐1 regulation by nitric oxide and cGMP‐dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem 276: 44338–44346. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM (2001). Stretch‐dependent potassium channels in murine colonic smooth muscle cells. J Physiol 533: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E (2005). Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4: 471–479. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM (2011). A functional role for the ‘fibroblast‐like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AA, Hasler WL (2017). Diabetes and the stomach. Curr Treat Options Gastroenterol 15: 441–459. [DOI] [PubMed] [Google Scholar]
- Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L et al (2017). Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PloS one 12: e0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A (2015). Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol 593: 4589–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E et al (2014a). Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 307: G98–G106. [DOI] [PubMed] [Google Scholar]
- Lies B, Groneberg D, Friebe A (2014b). Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastroenterol Motil 26: 901–912. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Thompson M, Cornwell TL (1988). Purification and characterization of two forms of cyclic GMP‐dependent protein kinase from bovine aorta. J Biol Chem 263: 17632–17637. [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K et al (1992). Requirement of c‐kit for development of intestinal pacemaker system. Development 116: 369–375. [DOI] [PubMed] [Google Scholar]
- Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK (1996). Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest 98: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR et al (2003). Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol 64: 533–546. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Grider JR, Jin JG, Makhlouf GM (1995). Interplay of VIP and nitric oxide in the regulation of neuromuscular activity in the gut. Arch Int Pharmacodyn Ther 329: 27–38. [PubMed] [Google Scholar]
- Murthy KS, Grider JR, Jin JG, Makhlouf GM (1996). Interplay of VIP and nitric oxide in the regulation of neuromuscular function in the gut. Ann N Y Acad Sci 805: 355–362. [DOI] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P et al (1990). Molecular bases of dominant negative and loss of function mutations at the murine c‐kit/white spotting locus: W37, Wv, W41 and W. EMBO J 9: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Pfeifer A, Aszodi A, Ahmad M, Alm P, Hedlund P et al (2000). Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP‐dependent protein kinase I. Br J Pharmacol 129: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM (2000). Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 49: 1731–1739. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S (1987). Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature 327: 524–526. [DOI] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD (2005). Sulfur‐containing amino acids block stretch‐dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol 144: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C et al (1998). Defective smooth muscle regulation in cGMP kinase I‐deficient mice. EMBO J 17: 3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MS, Saad RJ (2017). Diabetes mellitus and the colon. Curr Treat Options Gastroenterol 15: 460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao YM, Chaudhury A, Goyal RK (2008). Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 294: G627–G634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Crespo I, Straub W, Gavilanes F, Ortiz de Montellano PR (1998). Binding of dynein light chain (PIN) to neuronal nitric oxide synthase in the absence of inhibition. Arch Biochem Biophys 359: 297–304. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Velasco V, Zhong J, Hume JR, Keef KD (1998). Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ Res 82: 557–565. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Mikkelsen HB, Thuneberg L (1992). Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology 102: 56–68. [DOI] [PubMed] [Google Scholar]
- Russell ES (1979). Hereditary anemias of the mouse: a review for geneticists. Adv Genet 20: 357–459. [PubMed] [Google Scholar]
- Salmhofer H, Neuhuber WL, Ruth P, Huber A, Russwurm M, Allescher HD (2001). Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res 305: 331–340. [DOI] [PubMed] [Google Scholar]
- Sanders KM (1996). A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492–515. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Hwang SJ, Ward SM (2010). Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ro S, Ward SM (2012). Regulation of gastrointestinal motility – insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9: 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Salter AK, Hennig GW, Koh SD, Perrino BA, Ward SM et al (2014). Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. J Neurogastroenterol Motil 20: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM (1992). Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol 262: G379–G392. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM, Daniel EE (2002). ICC in neurotransmission: hard to swallow a lack of involvement. Gastroenterology 122: 1185–1186 author reply 1186‐1187. [DOI] [PubMed] [Google Scholar]
- Saur D, Paehge H, Schusdziarra V, Allescher HD (2000). Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology 118: 849–858. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM (1993). Immunohistochemical localization of 3′,5′‐cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience 56: 513–522. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Mashimo HL, Thatte HS, Goyal RK (2001). Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W (v) mutant mice. Gastroenterology 121: 34–42. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV (2003). Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358. [DOI] [PubMed] [Google Scholar]
- Stark ME, Bauer AJ, Sarr MG, Szurszewski JH (1993). Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology 104: 398–409. [DOI] [PubMed] [Google Scholar]
- Stark ME, Bauer AJ, Szurszewski JH (1991). Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol 444: 743–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C (1997). Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology 113: 1535–1544. [DOI] [PubMed] [Google Scholar]
- Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T et al (1998). Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol 275: G342–G351. [DOI] [PubMed] [Google Scholar]
- Thornbury KD, Ward SM, Dalziel HH, Carl A, Westfall DP, Sanders KM (1991). Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol 261: G553–G557. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM (1995). c‐kit‐dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 280: 97–111. [DOI] [PubMed] [Google Scholar]
- Turler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL et al (2006). Leukocyte‐derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg 244: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste G, Dhaese I, Sips P, Buys E, Brouckaert P, Lefebvre RA (2007). Gastric motility in soluble guanylate cyclase alpha 1 knock‐out mice. J Physiol 584: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voussen B, Beck K, Mauro N, Keppler J, Friebe A (2018). Comparison of nitrergic signaling in circular and longitudinal smooth muscle of murine ileum. Neurogastroenterol Motil 30 10.1111/nmo.13175. [DOI] [PubMed] [Google Scholar]
- Walker LA, MacDonald JA, Liu X, Nakamoto RK, Haystead TA, Somlyo AV et al (2001). Site‐specific phosphorylation and point mutations of telokin modulate its Ca2+−desensitizing effect in smooth muscle. J Biol Chem 276: 24519–24524. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM (1999). Intimate relationship between interstitial cells of cajal and enteric nerves in the guinea‐pig small intestine. Cell Tissue Res 295: 247–256. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM (2000). Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res 302: 331–342. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM (2000). Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20: 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM (1994). Mutation of the proto‐oncogene c‐kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480 (Pt 1): 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, McLaren GJ, Sanders KM (2006). Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol 573: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM (1998). Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology 115: 314–329. [DOI] [PubMed] [Google Scholar]
- Ward SM, Xue C, Shuttleworth CW, Bredt DS, Snyder SH, Sanders KM (1992). NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol 263: G277–G284. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH et al (2000). Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest 106: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A et al (2007). Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KJ, Sanders KM, Ward SM (2013). Stretch‐dependent sensitization of post‐junctional neural effectors in colonic muscles. Neurogastroenterol Motil 25: e101–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida H, Sanders KM, Ward SM (2007). Inactivation of inducible nitric oxide synthase protects intestinal pacemaker cells from postoperative damage. J Physiol 582: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida H, Yanase H, Sanders KM, Ward SM (2004). Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology 127: 1748–1759. [DOI] [PubMed] [Google Scholar]
- Young HM, Furness JB, Shuttleworth CW, Bredt DS, Snyder SH (1992). Co‐localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea‐pig intestine. Histochemistry 97: 375–378. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Miller DV, Paterson WG (2010). TREK‐1 channels do not mediate nitrergic neurotransmission in circular smooth muscle from the lower oesophageal sphincter. Br J Pharmacol 159: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG (2002). Role of Ca2+−activated Cl‐ channels and MLCK in slow IJP in opossum esophageal smooth muscle. Am J Physiol Gastrointest Liver Physiol 283: G104–G114. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG (2003). Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol 140: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O'Driscoll K et al (2011). Muscarinic activation of Ca2+−activated Cl‐ current in interstitial cells of Cajal. J Physiol 589: 4565–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung TS, O'Driscoll K, Koh SD, Sanders KM (2015). Intracellular Ca(2+) release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol 308: C608–C620. [DOI] [PMC free article] [PubMed] [Google Scholar]


