ABSTRACT
Our aim of this study is to compare the thigh muscle thickness measurements obtained using ultrasound and bioelectrical impedance analysis (BIA) methods, and to investigate the validity and cutoff value of the ultrasonography. We analyzed a total of 201 participants (99 male and 102 female participants, mean age, 66.2 years) participated in the annual health checkup in the Yakumo Study, 2014. Thigh muscle thickness (TMT, sum of the rectus femoris and vastus intermedius muscle thickness) was measured using ultrasound at mid-thigh in the sitting position. Appendicular skeletal muscle mass (aSMI) was measured using BIA. Cutoff value of TMT was determined through the receiver operating characteristic analysis. We defined sarcopenia with the diagnostic algorithm of Asian Working Group for Sarcopenia. TMT was significantly reduced in subject with sarcopenia than in those without sarcopenia in both gender. Muscle measurements obtained using the BIA methods (aSMI) and ultrasound methods (TMT) showed a significant correlation, with a correlation coefficient of 0.38 (P < 0.001). Cutoff value, sensitivity, and specificity of TMT in diagnosis of muscle loss were 36 mm, 72.0%, and 73.9%, respectively, for the male participants, and 34 mm, 72.2%, and 72.4%, respectively, for the female participants. In conclusion, the ultrasonography for thigh muscle might be a simple diagnostic method for sarcopenia.
Key Words: ultrasonography, thigh muscle thickness, sarcopenia, community-dwelling people, cut-off value
INTRODUCTION
As the population continues to age, sarcopenia, the age-related muscle loss and increased frailty, are major socioeconomic and medical problems. Sarcopenia causes a decrease of physical activity and imbalance, which result in subsequent fractures.1) Sarcopenia potentially affects systemic problems, such as insulin resistance,2) obesity,3) infection,4) and mortality of malignant tumor.5) Identification of sarcopenia could be important as it may help improve risk assessment, and treatments for sarcopenia such as exercise and nutrition can improve muscle mass and strength even among the frailest older adults.6) The diagnostic parameters of sarcopenia are the muscle mass and its function. Several methods are recommended for measuring muscle mass, although those methods have limitations in terms of invasion and accessibility. On the other hand, in recent years, the diagnostic imaging technique using ultrasound has been improved. Ultrasonography has the advantage of being less invasive.7) However, the muscle mass measurement method for sarcopenia diagnosis using ultrasound has not yet been established.
The aim of this study was to compare the thigh muscle thickness measurement obtained using ultrasound with that obtained using BIA, which is a conventional method, and to investigate the validity and cutoff value of the ultrasound method.
MATERIALS AND METHODS
Subjects
Imagama et al. reported conducting epidemiological studies since 1983 — called the Yakumo Study — in the Yakumo Town of Hokkaido, Japan, through annual public health checkups.8-13) The current study was conducted on community-dwelling people aged ≥40 years who participated in the Yakumo Study for health checkup in 2014. Participants who had a surgical history of the lower extremity were excluded. In total, 201 subjects (mean age, 66.2 years) were analyzed. All participants provided written informed consent; the study protocol was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (No. 2014-0207), and the study procedures were carried out in accordance with the principles of the Declaration of Helsinki.
Muscle mass measurement using ultrasound
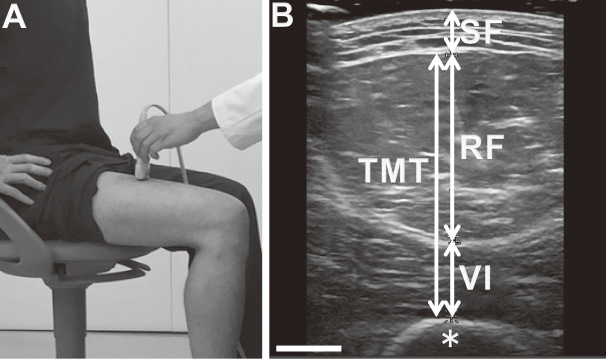
The subject sat on a chair and adjusted the height of the chair so that the hip and knee joints were at an angle of 90°. The upper edges of the patella and greater trochanter were palpated and measured using a tape measure to identify the midpoint of the top of the patella and the greater trochanter (Fig. 1a). The right thighs of all subjects were measured. An ultrasound diagnostic apparatus with a linear array probe (Noblus and L64 high frequency linear transducer 18–5 MHz, Hitachi Aloka Medical, Ltd., Tokyo) was used. The ultrasound probe was brought perpendicular to the midpoint of the front thigh, and the angle of the probe was adjusted so that the distance to the femur was the shortest; this was recorded as the axial image. So as not to affect the measurement value, we carefully set the probe on the thigh using plenty of ultrasound gel so that the probe would not directly touch the skin to push the soft tissue. An axial cross-section just above the rectus femoris muscle was drawn, and the subcutaneous fat, rectus femoris muscle, and vastus intermedius muscle thicknesses were measured. Thigh muscle thickness (TMT) was defined as the distance between the anterior fascia of the rectus femoris muscle and the posterior fascia of the vastus intermedius muscle at the axial aspect of the image (Fig. 1b). Twenty legs of ten healthy volunteers were similarly measured and evaluated to assess the reliability of the two examiners (T.H and K.I). The interclass correlation coefficients for the thickness of subcutaneous fat, rectus femoris muscle, vastus intermedius muscle, and TMT were 0.873, 0.835, 0.840 and 0.851, respectively, which showed good inter-rater reliability for the ultrasound examinations.
Fig. 1.
Measurement of thigh muscles using ultrasound.
Fig. 1a: Position of participant and ultrasound probe. The linear probe was set at midpoint of the right thigh in the sitting position.
Fig. 1b: Representative image of ultrasound. Thigh muscle thickness (TMT) was defined as the distance between the anterior fascia of rectus femoris muscle (RF) and posterior fascia of vastus intermedius muscle (VI) at the axial image. SF, subcutaneous fat; asterisk, femoral bone. Scale bar = 10 mm.
Muscle mass measurement using BIA
The appendicular skeletal muscle mass was measured using BIA (Inbody 720; Biospace Co., Ltd., Seoul, Republic of Korea), which measures the body composition according to the differences in electric impedance among biological tissues, such as fat, muscle, and bone;14) the accuracy of this method is comparable to that of the CT cross-sectional area method.15) Moreover, it enables separate measurement of the muscle mass in the upper and lower extremities, and the trunk.16) Therefore, BIA is considered a suitable method for measuring muscle mass to aid in the diagnosis of sarcopenia.17) The appendicular skeletal muscle index (aSMI) was calculated using the following formula: aSMI = arm and leg skeletal muscle mass (kg)/height2 (m2).18)
Diagnosis of sarcopenia
We diagnosed sarcopenia using the diagnostic algorithm for Asian people by the Asian Working Group for Sarcopenia based on the presence of both low muscle mass and low muscle function (low physical performance or low muscle strength) 19). Reference values for diagnosing muscle loss were aSMI of < 7.0 kg/m2 and 5.7 kg/m2 in men and women, respectively, as measured using BIA. Low physical performance was defined as gait speed less than 0.8 m/s. Low muscle strength was diagnosed with handgrip strength (< 26 kg for men and < 18 kg for women).
Statistical analysis
For the statistical examination, we used chi-square test, Fisher’s exact test, T-test, Pearson’s correlation coefficient, and partial correlation. We used the receiver operating characteristic (ROC) analysis to determine the cutoff values of muscle mass loss with the TMT at mid-thigh using ultrasound. Statistical analyses were performed using SPSS version 22 (IBM SPSS Inc., Armonk, NY, USA), and p < 0.05 was considered significant.
RESULTS
The baseline data of the participants are shown in Table 1. A total of 99 male participants (mean age, 66.4 years) and 102 female participants (mean age, 65.9 years) were included in this study. Age was similar between the male and female participants. There was no significant gender difference in the prevalence of sarcopenia (4.1% in male, 9.8% in female). The height and weight measurements were significantly higher in the male participants than in the female participants. Although BMI was not significantly different among the male and female participants, % body fat was significantly higher in the female participants than in the male participants. The muscle measurements of all subjects using BIA and ultrasound were shown in Table 2. All extremity muscle masses measured using BIA, including arm and leg muscle masses of the left and right sides, aSMI, arm SMI, and leg SMI, were higher in the male participants than in the female. All muscle thicknesses measured using ultrasound were significantly higher in the male participants, except for the rectus femoris muscle thickness. Subcutaneous fat thickness was significantly higher in the female participants than in the male participants. TMT measurements for male were 34.0 mm ± 4.9 mm in participant with sarcopenia and 38.9 mm ± 7.1 mm in those without sarcopenia. TMT measurements for female were 30.3 mm ± 4.8 mm in participant with sarcopenia and 36.5mm ± 7.2 mm in those without sarcopenia. TMT was significantly reduced in subject with sarcopenia in both genders (p = 0.024 in male and p < 0.001 in female).
Table 1.
Demographic data of the participants.
| Total | Male | Female | p value | ||
|---|---|---|---|---|---|
| Number of participants | 201 | 99 | 102 | ||
| Age (years) | 66.2 ± 9.8 | 66.4 ± 9.6 | 65.9 ± 10.0 | 0.71 | |
| Height (cm) | 156 ± 8 | 160 ± 6 | 151 ± 6 | < 0.001 | |
| Weight (kg) | 56.2 ± 9.5 | 64.7 ± 8.9 | 53.8 ± 8.1 | < 0.001 | |
| BMI (kg/m2) | 23.9 ± 3.1 | 24.3 ± 2.7 | 23.6 ± 3.5 | 0.096 | |
| % body fat (%) | 30.2 ± 7.9 | 26.0 ± 5.9 | 33.8 ± 7.1 | 0.035 | |
| Prevalence of sarcopenia | 7.0% | 4.1% | 9.8% | 0.165 |
Values are expressed as mean ± standard deviation.
BMI, body mass index
Table 2.
Muscle mass measurements.
| Male | Female | p value | ||
|---|---|---|---|---|
| BIA | ||||
| Arm muscle mass, right (kg) | 2.68 ± 0.44 | 1.67 ± 0.32 | < 0.001 | |
| Arm muscle mass, left (kg) | 2.65 ± 0.43 | 1.65 ± 0.32 | < 0.001 | |
| Leg muscle mass, right (kg) | 7.24 ± 1.03 | 5.25 ± 0.84 | < 0.001 | |
| Leg muscle mass, left (kg) | 7.25 ± 1.01 | 5.25 ± 0.84 | < 0.001 | |
| arm SMI (kg/m2) | 2.01 ± 0.26 | 1.45 ± 0.25 | < 0.001 | |
| leg SMI (kg/m2) | 5.45 ± 0.48 | 4.58 ± 0.51 | < 0.001 | |
| aSMI (kg/m2) | 7.46 ± 0.67 | 6.04 ± 0.71 | < 0.001 | |
| Ultrasound | ||||
| TMT (mm) | 38.7 ± 7.1 | 35.7 ± 7.2 | 0.004 | |
| Rectus femoris muscle thickness (mm) | 18.2 ± 5.0 | 16.9 ± 5.1 | 0.080 | |
| Vastus intermedius muscle thickness (mm) | 20.5 ± 4.0 | 18.8 ± 3.7 | 0.002 | |
| Subcutaneous fat thickness (mm) | 4.8 ± 2.3 | 8.5 ± 3.2 | < 0.001 | |
Values are expressed as mean ± standard deviation.
SMI, skeletal muscle mass index; aSMI, appendicular skeletal muscle mass index; TMT, thigh muscle thickness
Fig. 2 shows the relationship between muscle measurements of aSMI using the BIA method and of TMT using the ultrasound method. Muscle measurement values using the BIA method (aSMI) and ultrasound method (TMT) were significantly related in whole participants (R = 0.38, p < 0.001) and in both genders (males, R = 0.25, p < 0.001; females, R = 0.44, p < 0.0001). After controlling for gender with partial correlation analysis, there was significant correlation between aSMI and TMT (R = 0.35, p < 0.0001).
Fig. 2.
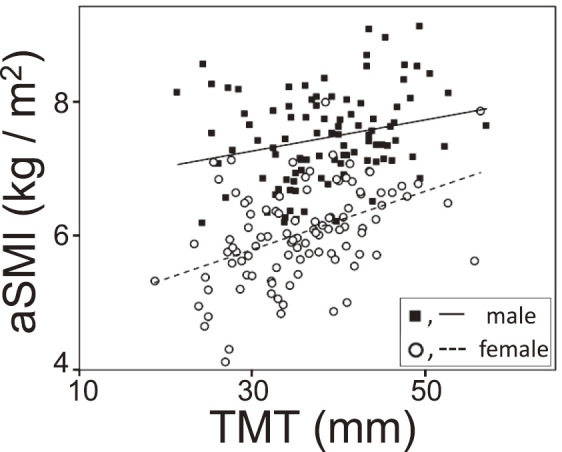
Relationship of the leg muscle measurements using bioelectrical impedance analysis (BIA) and ultrasound
Appendicular skeletal mass index (aSMI) was measured using BIA. aSMI was defined as sum of the arm and leg muscle masses divided by height squared. Thigh muscle thickness (TMT) was measured using ultrasound at midpoint of the right thigh. Males, R = 0.25, p < 0.001; females, R = 0.44, p < 0.0001.
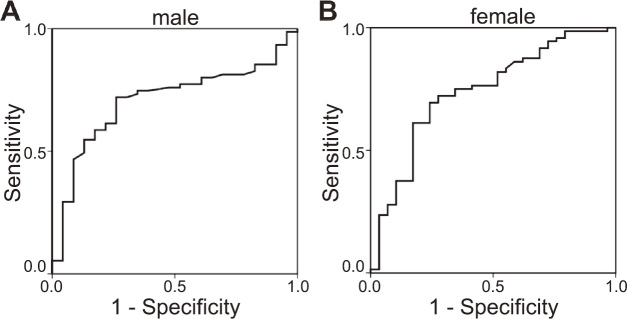
For the diagnosis of muscle loss using the BIA method, the area under curve (AUC) in the ROC analysis of the ultrasound method was 0.71 for the male participants and 0.74 for the female participants, which confirmed moderate reliability (Fig. 3). Cutoff value, sensitivity, and specificity of TMT in the diagnosis of muscle loss were 36 mm, 72.0%, and 73.9%, respectively, for the males, and 34 mm, 72.2%, and 72.4%, respectively, for the females, respectively.
Fig. 3.
Receiver operating characteristic (ROC) curve analysis to determine the corresponding muscle mass decrease with the thigh muscle thickness at mid-thigh using ultrasound
Fig. 3a: Male; area under the curve: 0.71; cutoff: 36 mm.
Fig. 3b: Female; area under the curve: 0.74; cutoff: 34 mm.
DISCUSSION
The current study presented that the TMT measured using ultrasound in the sitting position was significantly related to aSMI measured using BIA, which is the standard method in measuring the muscle mass method for the diagnosis of sarcopenia. Our findings also indicated that the cutoff value for TMT in the sitting position to diagnose muscle loss was <36 mm for the males and <34 mm for the females.
Sarcopenia, an aging-induced decrease in muscle mass, is known to affect elderly individuals by decreasing activities of daily living and increasing frailty and vulnerability to various medical problems. Sarcopenia has been reported to affect more than 40% of elderly individuals aged ≥70 years, approximately 50 million people worldwide. This number is estimated to increase to 500 million people in the year 2050.17) Therefore, there has been a major interest in diagnosing sarcopenia properly to help in the treatment against attenuating the age-related decline and disability. Several consensus groups, such as the European Working Group on Sarcopenia in Older People,17) the International Working Group on Sarcopenia,20) and the Asian Working Group for Sarcopenia19) have recently published operational criteria for the diagnosis of sarcopenia. It is common in these diagnostic criteria that the measurement of muscle mass is essential for diagnosis. The following three techniques have been used for estimating muscle mass or lean body mass: cross-sectional area measured using computed tomography (CT) or magnetic resonance imaging (MRI), whole-body dual energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA).17) These conventional methods have some limitations. Analysis of cross-sectional area (CSA) of a specific muscle using CT or MRI involves manual measurements.15,21) The diagnostic reference value has not yet been established, and there are problems of cost, exposure to radiation, and measurement time.17) DXA is a traditional method for determining body composition, which is classically used for measuring bone mineral density.22) Two X-ray beams of different energy levels are aimed at the patient’s body. Unlike the bone density measurement, a special machine that can measure the whole body is necessary for muscle mass measurement so that patients are unable to access this examination easily.1,23) BIA is a non-invasive and traditional method for measuring body composition.14,24) Electrodes are attached to various parts of the body, and a small electric signal is circulated. BIA measures the impedance or resistance of muscle and fat tissues and estimates tissue content and composition. Modern BIA can separately measure each part of the body. However, accessibility issues are similar for BIA and DXA. On the other hand, ultrasonography is a simple and non-invasive method. It is used at primary care clinics of various medical departments, and it is an examination modality that can be carried out at relatively many medical institutions, with high accessibility. Thus, its application in diagnosing sarcopenia diagnosis is expected.
Several previous studies reported the ultrasonography measurements for skeletal muscles of lower extremities. Most of the past studies assessed only the relation between muscle strength and muscle thickness.25,26) These studies reported that there is a positive correlation between muscle thickness and muscle strength. Minetto et al. assessed the relationship between thickness of the rectus femoris muscle measured using ultrasound, and the appendicular muscle mass measured using whole body DXA. However, this study lacks data on aSMI and cutoff value for diagnosing sarcopenia, so that its results were not considered sufficient to diagnose sarcopenia.27)
The muscle mass thickness of the thigh was significantly larger in the male participants than in the female participants in the current study. It has been reported that the skeletal muscle mass is generally larger in men than in women,13,23,28) and similar reports are made on ultrasound measurements.27,29) We also reported the large fat thickness in the female participants in the current study, which is consistent with the past findings that reported women had more fat mass than men.30) A positive correlation was found between the muscle measurement using the BIA and that using the ultrasound method. TMT was thicker in non-sarcopenic participants than in sarcopenic participants. We also found a cutoff value for muscle loss measured using ultrasound for the diagnosis of sarcopenia. Rolland et al. reported that the sensitivity and specificity values for diagnosing muscle loss by calf circumference were 44.3% and 91.4%, respectively, and AUC was not mentioned in the paper.21) Compared to the anthropometric method, our findings using ultrasound indicate that both sensitivity and specificity values are more than 70% with moderate reliability as assessed by AUC; these findings showed that the ultrasound method might be helpful enough in diagnosing sarcopenia at clinical situations.
Our study has several limitations. We compared aSMI and TMT without controlling by height. There are various arguments currently in controlling muscle mass by height.31) Height decreases with age at senescence due to spinal degenerative kyphosis, decrease of vertebral disc height, or asymptomatic vertebral fractures.32) There is a bias of overestimating muscle mass when controlled with height, particularly in old population. Screening criteria sometimes does not control by height in order to give priority to simplicity. For example, a waist diameter is used as one of diagnostic criteria for metabolic syndrome, but not controlled by height. Further analysis would benefit from including TMT controlled by physical indicators such as thigh length or arm span. We measured TMT only in the sitting position and not in the supine position. Obtaining measurements using ultrasound at primary care clinics is easier for most patients in the sitting position. However, there may be a small number of patients who cannot maintain the sitting position in clinical situation. Because the measurement may change depending on the position of the limbs, we would like to consider obtaining further data in the supine position. Another limitation is that we included only healthy volunteers in the study, and no hospitalized patients or patients with severe complications participated. Health conditions, such as the degree of edema of the patient, may affect ultrasound measurements. Therefore, it might be necessary to investigate in the future whether the diagnostic method using ultrasound is effective also in patients with complications.
In conclusion, ultrasound measurement of TMT was correlated with aSMI measured using BMI. The cutoff value of TMT for muscle loss in sarcopenia was 36 mm for the males and 34 mm for the females. The ultrasonography for thigh muscle might be a simple diagnostic method for sarcopenia.
ACKNOWLEDGEMENTS
This study was funded by research grants from the Foundation for Total Health Promotion in 2012, the Tateisi Science and Technology Foundation in 2013 (No. 2031014), the Japan Osteoporosis Foundation in 2013 and 2014, the Japan Orthopaedics and Traumatology Research Foundation, Inc. (No. 307) in 2014, the Research Foundation for the Electrotechnology of Chubu in 2015 (R-27213), and Chukyo Longevity Foundation in 2015, and Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering in 2016. The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article. The authors wish to thank Ms. Makiko Noda, Ms. Marie Miyazaki, and Ms. Hiroko Ino for their assistance in data collection.
CONFLICTS OF INTERST
None.
REFERENCES
- 1).Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int, 2013; 13: 413–420. [DOI] [PubMed]
- 2).Bijlsma AY, Meskers CG, van Heemst D, Westendorp RG, de Craen AJ, Maier AB. Diagnostic criteria for sarcopenia relate differently to insulin resistance. Age (Dordr), 2013; 35: 2367–2375. [DOI] [PMC free article] [PubMed]
- 3).Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE, 2010; 5: e10805. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0010805 Accessed on May 7, 2018. [DOI] [PMC free article] [PubMed]
- 4).Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr, 2006; 96: 895–901. [DOI] [PubMed]
- 5).Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg, 2013; 100: 1523–1530. [DOI] [PubMed]
- 6).Bonnefoy M, Cornu C, Normand S, Boutitie F, Bugnard F, Rahmani A, et al. The effects of exercise and protein–energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br J Nutr, 2007; 89: 731. [DOI] [PubMed]
- 7).Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med, 2011; 364: 749–757. [DOI] [PubMed]
- 8).Imagama S, Hasegawa Y, Ando K, Kobayashi K, Hida T, Ito K, et al. Staged decrease of physical ability on the locomotive syndrome risk test is related to neuropathic pain, nociceptive pain, shoulder complaints, and quality of life in middle-aged and elderly people - The utility of the locomotive syndrome risk test. Mod Rheumatol, 2017; 27: 1051–1056. [DOI] [PubMed]
- 9).Tsuboi M, Hasegawa Y, Matsuyama Y, Suzuki S, Suzuki K, Imagama S. Do musculoskeletal degenerative diseases affect mortality and cause of death after 10 years in Japan? J Bone Miner Metab, 2011; 29: 217–223. [DOI] [PubMed]
- 10).Imagama S, Matsuyama Y, Hasegawa Y, Sakai Y, Ito Z, Ishiguro N, et al. Back muscle strength and spinal mobility are predictors of quality of life in middle-aged and elderly males. Eur Spine J, 2011; 20: 954–961. [DOI] [PMC free article] [PubMed]
- 11).Imagama S, Hasegawa Y, Seki T, Matsuyama Y, Sakai Y, Ito Z, et al. The effect of beta-carotene on lumbar osteophyte formation. Spine (Phila Pa 1976), 2011; 36: 2293–2298. [DOI] [PubMed]
- 12).Imagama S, Hasegawa Y, Matsuyama Y, Sakai Y, Ito Z, Hamajima N, et al. Influence of sagittal balance and physical ability associated with exercise on quality of life in middle-aged and elderly people. Arch Osteoporos, 2011; 6: 13–20. [DOI] [PMC free article] [PubMed]
- 13).Hida T, Imagama S, Ando K, Kobayashi K. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: the Yakumo Study. Mod Rheumatol, 2017; 28: 345–350. [DOI] [PubMed]
- 14).Hoffer EC, Meador CK, Simpson DC. Correlation of whole-body impedance with total body water volume. J Appl Physiol, 1969; 27: 531–534. [DOI] [PubMed]
- 15).Yamada Y, Ikenaga M, Takeda N, Morimura K, Miyoshi N, Kiyonaga A, et al. Estimation of thigh muscle cross-sectional area by single- and multifrequency segmental bioelectrical impedance analysis in the elderly. J Appl Physiol, 2014; 116: 176–182. [DOI] [PubMed]
- 16).Roubenoff R, Baumgartner RN, Harris TB, Dallal GE, Hannan MT, Economos CD, et al. Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci, 1997; 52: M129–136. [DOI] [PubMed]
- 17).Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing, 2010; 39: 412–423. [DOI] [PMC free article] [PubMed]
- 18).Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr, 1990; 52: 214–218. [DOI] [PubMed]
- 19).Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc, 2014; 15: 95–101. [DOI] [PubMed]
- 20).Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc, 2011; 12: 249–256. [DOI] [PMC free article] [PubMed]
- 21).Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc, 2003; 51: 1120–1124. [DOI] [PubMed]
- 22).Ito K, Tsushita K, Muramoto A, Kanzaki H, Nohara T, Shimizu H, et al. Cross-calibration of pencil-beam (DPX-NT) and fan-beam (QDR-4500C) dual-energy X-ray absorptiometry for sarcopenia. Nagoya J Med Sci, 2015; 77: 647–652. [PMC free article] [PubMed]
- 23).Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J, 2016; 25: 3424–3431. [DOI] [PubMed]
- 24).National_Institutes_of_Health. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr, 1996; 64: 524S-532S. [DOI] [PubMed]
- 25).Radaelli R, Bottaro M, Wilhelm EN, Wagner DR, Pinto RS. Time course of strength and echo intensity recovery after resistance exercise in women. J Strength Cond Res, 2012; 26: 2577–2584. [DOI] [PubMed]
- 26).Watanabe Y, Yamada Y, Fukumoto Y, Ishihara T, Yokoyama K, Yoshida T, et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging, 2013; 8: 993–998. [DOI] [PMC free article] [PubMed]
- 27).Minetto MA, Caresio C, Menapace T, Hajdarevic A, Marchini A, Molinari F, et al. Ultrasound-based detection of low muscle mass for diagnosis of sarcopenia in older adults. Pm r, 2016; 8: 453–462. [DOI] [PubMed]
- 28).Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev, 1999; 107: 123–136. [DOI] [PubMed]
- 29).Berger J, Bunout D, Barrera G, de la Maza MP, Henriquez S, Leiva L, et al. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch Gerontol Geriatr, 2015; 61: 33–38. [DOI] [PubMed]
- 30).Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab, 2000; 279: E366–375. [DOI] [PubMed]
- 31).Kasai T, Ishiguro N, Matsui Y, Harada A, Takemura M, Yuki A, et al. Sex- and age-related differences in mid-thigh composition and muscle quality determined by computed tomography in middle-aged and elderly Japanese. Geriatr Gerontol Int, 2014 2015; 15:700–706. [DOI] [PubMed]
- 32).Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in the elderly. I. A 25-year longitudinal study of a population aged 70 to 95 years. Eur J Clin Nutr, 1999; 53: 905–914. [DOI] [PubMed]