Abstract
The purpose of this study was to examine the effects of aerobic lifelong exercise (LLE) on maximum oxygen consumption (V̇o2max) and skeletal muscle metabolic fitness in trained women (n = 7, 72 ± 2 yr) and men (n = 21, 74 ± 1 yr) and compare them to old, healthy nonexercisers (OH; women: n = 10, 75 ± 1 yr; men: n = 10, 75 ± 1 yr) and young exercisers (YE; women: n = 10, 25 ± 1 yr; men: n = 10, 25 ± 1 yr). LLE men were further subdivided based on intensity of lifelong exercise and competitive status into performance (LLE-P, n = 14) and fitness (LLE-F, n = 7). On average, LLE exercised 5 day/wk for 7 h/wk over the past 52 ± 1 yr. Each subject performed a maximal cycle test to assess V̇o2max and had a vastus lateralis muscle biopsy to examine capillarization and metabolic enzymes [citrate synthase, β-hydroxyacyl-CoA dehydrogenase (β-HAD), and glycogen phosphorylase]. V̇o2max had a hierarchical pattern (YE > LLE > OH, P < 0.05) for women (44 ± 2 > 26 ± 2 > 18 ± 1 ml·kg−1·min−1) and men (53 ± 3 > 34 ± 1 > 22 ± 1 ml·kg−1·min−1) and was greater (P < 0.05) in LLE-P (38 ± 1 ml·kg−1·min−1) than LLE-F (27 ± 2 ml·kg−1·min−1). LLE men regardless of intensity and women had similar capillarization and aerobic enzyme activity (citrate synthase and β-HAD) as YE, which were 20%–90% greater (P < 0.05) than OH. In summary, these data show a substantial V̇o2max benefit with LLE that tracked similarly between the sexes, with further enhancement in performance-trained men. For skeletal muscle, 50+ years of aerobic exercise fully preserved capillarization and aerobic enzymes, regardless of intensity. These data suggest that skeletal muscle metabolic fitness may be easier to maintain with lifelong aerobic exercise than more central aspects of the cardiovascular system.
NEW & NOTEWORTHY Lifelong exercise (LLE) is a relatively new and evolving area of study with information especially limited in women and individuals with varying exercise intensity habits. These data show a substantial maximal oxygen consumption benefit with LLE that tracked similarly between the sexes. Our findings contribute to the very limited skeletal muscle biopsy data from LLE women (>70 yr), and similar to men, revealed a preserved metabolic phenotype comparable to young exercisers.
Keywords: aging, masters athletes, maximal oxygen uptake, metabolic health
INTRODUCTION
The exercise boom of the late 1960s and early 1970s spawned a generation of lifelong exercisers who engaged in structured physical activity ranging from recreational to competitive endeavors. Historically significant events that helped trigger this era can be traced back to female pioneers Roberta Gibb and Kathrine Switzer for their Boston marathon finishes in 1966 and 1967 (11) and Frank Shorter for winning the gold medal in the 1972 Olympic marathon. Together, these events coincided with a drastic rise in the popularity of recreational running (50). Equally important was the conception of Title IX in 1972, which increased the opportunities for women to participate in sports (19, 34). These monumental events changed the culture of exercise as a hobby and paved the way for a generation of women and men who are now in their eighth decade of life and have participated in structured aerobic exercise throughout their lifespan. This large and unique cohort of individuals provides new opportunities to investigate the effects that lifelong aerobic exercise has on cardiovascular and skeletal muscle health with aging.
Maximal oxygen consumption (V̇o2max) is an integrative assessment that involves cardiovascular and skeletal muscle systems and has been shown to decline ~10% per decade after 30 yr of age (12, 25, 27, 33, 48). The age-related reduction in V̇o2max is directly associated with an increasing risk of multiple comorbidities, all-cause mortality, and loss of independence (49). V̇o2max has been studied previously in male Masters athletes, showing that individuals who continued to train at a high level maintain a greater V̇o2max than their sedentary age-matched counterparts (25, 42, 55). The higher V̇o2max results in a greater physiological reserve above the frailty threshold [5 metabolic equivalents (METs)] which is the prognostic exercise capacity for increased mortality risk (37). However, limited knowledge exists for women who have engaged in lifelong exercise at a relatively high level. Furthermore, little information is available on individuals with varying lifelong exercise habits and how this might impact cardiovascular and skeletal muscle health once beyond 70 yr of age. A greater understanding of the health benefits of exercise in women and men may lead to solutions that help to mitigate the economic liability associated with aging and enhance quality of life in later years (23).
Our overarching intent was to study the potential benefits of lifelong exercise on indices of cardiovascular and skeletal muscle health. We were fortunate to recruit a cohort of lifelong exercising women, which is novel because there are very little data [and only 2 subjects with muscle biopsy data (44)] in the literature on female Masters athletes greater than 70 yr old. The men were in larger numbers, likely because of the cultural aspects of men more commonly participating in sports in the 1960s and 1970s. As a result, we were able to evaluate two groups of men with different exercise habits (performance vs. fitness) over the past 50+ years. For comparison, we included young exercising women and men as well as old, healthy women and men with no history of structured exercise. Our general working hypothesis was that a hierarchal pattern [young exercisers (YE) > lifelong exercisers (LLE) > old healthy (OH)] would be present in cardiovascular and skeletal muscle health. This paper provides the framework for the study and presents detailed exercise histories, medical profiles, V̇o2max, and skeletal muscle (capillarization and metabolic enzymes) profiles of the various study cohorts.
METHODS
Overall study design.
This study was conducted at Ball State University’s Human Performance Laboratory (Muncie, IN) and the Indiana University Health Ball Memorial Hospital (MRI only). Subjects were screened and studied over nine visits spanning approximately 6 wk. Evaluations included anthropometrics and body composition, medical and exercise histories, fasting screening blood draws, blood pressure, resting and exercise electrocardiogram, V̇o2max, daily physical activity assessment, MRI to assess muscle mass of the lower extremities, knee extensor whole-muscle function familiarization and testing, and finally an exercise bout with resting and strategically timed postexercise muscle biopsies intended to capture molecular events of interest within the exercise-challenged muscle (6, 35, 47, 62). Data related to some of these measurements are outside the scope of the current report and will be presented in subsequent papers by our research team. The study was approved by the Institutional Review Board of Ball State University. All study procedures, risks, and benefits were explained to the subjects before obtaining written consent to participate.
Subjects.
Six groups of individuals were recruited from the greater Muncie, Indiana area using newspaper advertisements, mailed flyers, and personal interactions: female and male LLE (n = 28, 21 men), age-matched, OH female and male controls who did not report any history of structured exercise (n = 20, 10 men), and female and male YE (n = 20, 10 men). Subject characteristics can be found in Table 1 whereas a clinical profile, including medications and select blood variables for general health can be found in Table 2.
Table 1.
Subject characteristics
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women |
YE, n = 10 | LLE |
OH, n = 10 | |||||
| YE, n = 10 | LLE, n = 7 | OH, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | |||
| Age, yr | 25 ± 1 | 72 ± 2 | 75 ± 1 | 25 ± 1 | 74 ± 1 | 74 ± 1 | 75 ± 2 | 75 ± 1 |
| Height, cm | 167 ± 2 | 164 ± 2 | 157 ± 2 | 181 ± 2 | 180 ± 2 | 179 ± 2 | 182 ± 3 | 177 ± 2 |
| Weight, kg | 60 ± 2 | 61 ± 4 | 65 ± 1 | 75 ± 3 | 79 ± 2 | 77 ± 2 | 83 ± 5 | 88 ± 3 |
| BMI, kg/m2 | 21 ± 1 | 23 ± 1 | 27 ± 1 | 23 ± 1 | 24 ± 1 | 24 ± 1 | 25 ± 1 | 28 ± 1 |
| Body fat, % | 23 ± 1* | 30 ± 2† | 41 ± 2 | 18 ± 2* | 24 ± 1† | 22 ± 1‡ | 27 ± 1 | 32 ± 1 |
| Steps per day | 11,518 ± 1,404* | 7,463 ± 683 | 6,801 ± 823 | 9,404 ± 635 | 9,560 ± 619 | 9,369 ± 725 | 10,006 ± 1,265 | 5,813 ± 488* |
Values are means ± SE. BMI, body mass index; LLE, lifelong exercisers; OH, old healthy; YE, young exercisers.
P < 0.05 vs. other groups;
P < 0.05 vs. OH;
P < 0.05 vs. LLE Fitness.
Table 2.
Prescribed medications and blood markers for general health
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women |
YE, n = 10 | LLE |
OH, n = 10 | |||||
| YE, n = 10 | LLE, n = 7 | OH, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | |||
| Medications, % of group | ||||||||
| Beta Blockers | — | — | 10 | — | 10 | — | 29 | 30 |
| Blood Pressure§ | — | 29 | 20 | — | 43 | 36 | 57 | 70 |
| Thyroid | 10 | 43 | 40 | — | 14 | 7 | 29 | 20 |
| Cholesterol | — | 14 | 30 | — | 52 | 57 | 43 | 70 |
| TG, mg/dl | 79 ± 13 | 105 ± 16 | 123 ± 14 | 86 ± 6 | 78 ± 5† | 73 ± 5 | 88 ± 10 | 112 ± 15 |
| HDL, mg/dl | 79 ± 7 | 83 ± 7 | 62 ± 5 | 56 ± 5 | 65 ± 3† | 66 ± 3 | 63 ± 4 | 51 ± 2 |
| Glucose, mg/dl | 91 ± 3 | 101 ± 5 | 100 ± 4 | 90 ± 2 | 95 ± 2 | 93 ± 1‡ | 100 ± 3 | 105 ± 4 |
| Hemoglobin, g/dl | 12.4 ± 0.3 | 12.7 ± 0.2 | 13.1 ± 0.3 | 14.8 ± 0.2 | 14.5 ± 0.2 | 14.7 ± 0.2 | 13.9 ± 0.4 | 14.4 ± 0.6 |
| Hematocrit, % | 38 ± 1 | 38 ± 1 | 40 ± 1 | 44 ± 1 | 43 ± 1 | 43 ± 1‡ | 41 ± 1 | 42 ± 2 |
| Total testosterone, ng/dl | 38 ± 9* | 14 ± 3 | 14 ± 3 | 580 ± 41 | 554 ± 52 | 591 ± 73 | 479 ± 50 | 418 ± 85 |
| Estrogen, pg/ml | 178 ± 30* | 83 ± 16 | 105 ± 10 | — | — | — | — | — |
Values are means ± SE. HDL, high-density lipoprotein; LLE, lifelong exercisers; OH, old healthy; TG, triglycerides; YE, young exercisers.
P < 0.05 vs. other groups.
P < 0.05 vs. OH;
P < 0.05 vs. LLE Fitness;
not including β blockers.
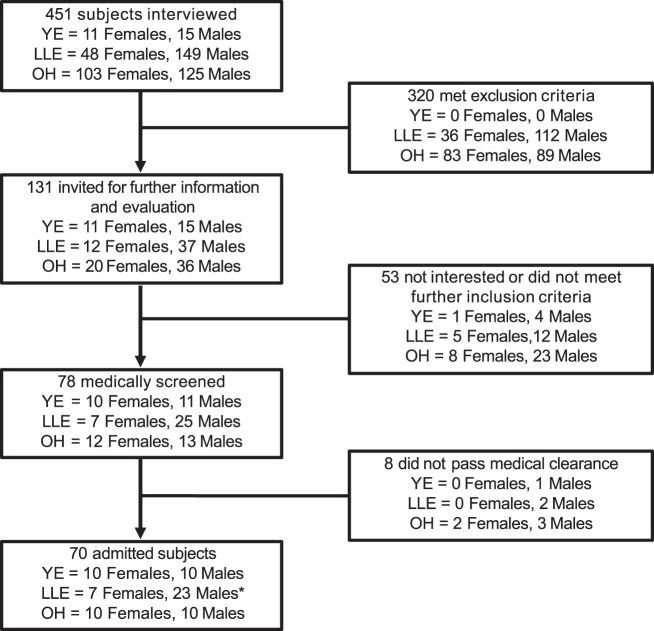
Figure 1 outlines the extensive screening process of the 451 potential subjects that inquired about the study. Ultimately, 70 subjects (16%) were admitted into the study. These individuals were free from acute or chronic illnesses (cardiac, pulmonary, liver, or kidney abnormalities, cancer, uncontrolled hypertension, insulin- or noninsulin-dependent diabetes, or other known metabolic disorders), orthopedic limitations (including any artificial joints), and did not smoke or participate in other forms of tobacco use.
Fig. 1.
Study recruitment flowchart for female and male young exercisers (YE), lifelong exercisers (LLE), and old healthy (OH) participants. *Two of the 23 LLE men were not included in analysis because of different training background (weight training), providing for 21 LLE men.
As part of the screening process outlined in Fig. 1, subjects were also extensively screened via questionnaire and interviewed by the investigative team for exercise history. Subjects of the LLE group were aerobic exercisers (primarily runners and cyclists), who had a history of participating in structured exercise 4–6 day/wk for ~7 h/wk for 52 ± 1 yr (Table 3). Exercise history of LLE subjects was reviewed for frequency, duration, intensity, and athletic achievements. In doing so, two clear male LLE subgroups emerged: one group that participated in moderate intensity training for physical fitness (fitness; LLE-F; n = 7) and another group that trained more vigorously and often participated in competitive events (performance; LLE-P; n = 14). The LLE-P group included several national caliber cyclists, a Race Across America record holder, a previous National Collegiate Athletic Association cross-country All-American, and many that continued to compete in endurance related events at a local, regional, and national level. Because of small sample size (n = 7), LLE women were not subdivided and ranged from fitness to performance. Included in the LLE female group were several individuals who continue to compete in local, regional, and national races, including a national level Masters track and field athlete and 1 subject that cycled ~4,000 miles a year at time of testing.
Table 3.
Exercise history of lifelong exercising cohorts
| Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women |
Performance |
Fitness |
|||||||||
| Age, yr | Mode | day/wk | h/wk | Intensity§ | Mode | day/wk | h/wk | Intensity§ | day/wk | h/wk | Intensity§ |
| 20–29 | Team sports, run, cycle, swim, ski, dance | 3.5 ± 0.9 | 4.4 ± 1.8 | 1.6 ± 0.4 | Team sports, run, cycle | 4.1 ± 0.7 | 6.9 ± 2.8 | 2.0 ± 0.3 | 3.6 ± 1.0 | 5.4 ± 4.2 | 1.6 ± 0.4 |
| 30–39 | Run, cycle, swim, ski, dance, yoga | 4.2 ± 0.8 | 5.4 ± 1.4 | 1.6 ± 0.4 | Team sports, run, cycle | 4.4 ± 0.5 | 7.7 ± 2.1 | 2.3 ± 0.1* | 3.6 ± 1.1 | 6.2 ± 3.2 | 1.4 ± 0.4 |
| 40–49 | Run, cycle, swim, ski, dance, yoga | 5.1 ± 0.5 | 8.3 ± 1.1 | 1.9 ± 0.2 | Run, cycle, swim | 4.4 ± 0.5 | 7.1 ± 0.9 | 2.1 ± 0.2 | 4.9 ± 0.5 | 7.5 ± 2.9 | 2.2 ± 0.2 |
| 50–59 | Run, cycle, swim, ski, dance, yoga | 4.9 ± 0.5 | 7.6 ± 1.3 | 1.9 ± 0.2 | Run, cycle, swim | 4.4 ± 0.3 | 7.2 ± 0.9 | 2.2 ± 0.1 | 5.4 ± 0.4 | 7.1 ± 1.5 | 2.0 ± 0.0 |
| 60–69 | Run, cycle, swim, ski, dance, yoga | 4.9 ± 0.6 | 7.6 ± 1.3 | 2.3 ± 0.1 | Run, cycle, swim | 4.5 ± 0.3 | 8.3 ± 1.2 | 2.2 ± 0.1 | 4.9 ± 0.6 | 5.8 ± 1.3 | 2.0 ± 0.0 |
| Current | Run, cycle, swim, ski, dance, yoga | 4.7 ± 0.4 | 6.8 ± 1.0 | 2.1 ± 0.2 | Run, cycle, swim | 4.5 ± 0.3 | 8.5 ± 1.4 | 2.2 ± 0.1* | 4.9 ± 0.7 | 7.4 ± 1.9 | 1.5 ± 0.2 |
| Lifelong average | 4.6 ± 0.3 | 6.6 ± 0.6 | 1.9 ± 0.1 | Lifelong average | 4.4 ± 0.2 | 7.6 ± 0.7 | 2.1 ± 0.1* | 4.6 ± 0.3 | 6.6 ± 0.9 | 1.8 ± 0.1 | |
Values are means ± SE. Data were gathered from a self-reported exercise history questionnaire. In the case that a subject reported more than one training intensity, the values were weighted to calculate an intensity mean (e.g., 80% of training at a 2 and 20% of training at a 3 resulted in an overall training intensity of 2.2). LLE, lifelong exerciser.
P < 0.05 vs. LLE fitness;
levels of self-reported intensity were 1 (light), 2 (moderate), and 3 (hard).
Although OH controls were not involved in any structured exercise training, participation in leisure activities (e.g., golfing, leisurely walking, and community service) was not grounds for exclusion. Young exercisers consisted of active individuals who exercised 4–6 day/wk for ~7 h/wk.
Body composition and daily physical activity.
Subjects were assessed for body composition via dual-energy X-ray absorptiometry (Lunar iDXA full body scanner, GE Healthcare, Madison, WI). Data were analyzed using enCORE 2010 V 13.40.038 software by GE Healthcare. The scanner was calibrated each day before the first scan per manufacturer guidelines.
Daily physical activity was indirectly assessed using a pedometer (Lifecorder EX, New LifeStyles, Inc., Lee’s Summit, MO). Subjects were asked to wear these devices for 2 wk and to put them on first thing in the morning and take them off just before going to bed. Total steps each day were assessed from the first to last movement recorded each day.
V̇o2max test.
Subjects performed a continuous cycle ergometer test with 12-lead ECG to volitional exhaustion. Subjects were asked to refrain from structured exercise the day of testing. Oxygen uptake was measured and averaged in 20-s intervals using indirect calorimetry via an automated open circuit system (Parvo Medics, Sandy, UT). Before each test, the gas analyzers were calibrated with standardized gases, and the pneumotach was calibrated using a standard volume of air.
The incremental protocol was adjusted based on the age and fitness of the subjects to target a test duration of 8–12 min (1). Subjects completed a 2-min warm up (25, 50, or 100 W) at a minimum of 60 revolutions/min. Following the warm up, the watt load on the cycle ergometer was increased in a ramped fashion (10, 15, or 25 W per minute) until volitional fatigue or until the subjects could not maintain a cadence of 60 revolutions/min (mean test time: 9.7 ± 0.4 min).
Maximal heart rate was determined from the 12-lead ECG and peak workload was measured from the highest watt output completed for at least 20 s. Oxygen pulse was calculated as absolute V̇o2max divided by maximal heart rate (5, 8). All subjects reached a maximal effort as evidenced by a plateau in oxygen uptake, achievement of age-predicted maximum heart rate (220–age), a respiratory exchange ratio >1.10, or a rating of perceived exertion of ≥19. The average of at least 2 of the highest consecutive readings during the last 60 s of the test was used for the measurement of V̇o2max.
Muscle biopsy.
Subjects were asked to refrain from structured exercise and physical activity outside of normal activities of daily living for 72 h before the muscle biopsy. Subjects consumed their normal evening meal and were instructed not to ingest any additional food or caloric beverage before arrival at the laboratory the following morning (~6:30 AM). Once in the laboratory, subjects rested quietly in the supine position for 30 min before undergoing a resting muscle biopsy of the vastus lateralis (7), as previously described (57). A portion of the muscle to be used for enzyme analysis was immediately frozen and stored in liquid nitrogen. A portion of the muscle to be used for capillarization measurements was oriented longitudinally in a mounting medium (tragacanth gum, Sigma, St. Louis, MO) atop a cork, frozen in isopentane cooled in liquid nitrogen, and subsequently stored in liquid nitrogen. The remaining muscle tissue was processed in a manner to complete other study objectives that will be presented in later papers from our research team.
Skeletal muscle metabolic enzymes.
Skeletal muscle metabolic enzyme activities were measured as previously described (15, 58). Briefly, muscle samples were weighed (5.9 ± 0.1 mg) at −25°C (Cahn C-35; Orion Research, Beverly, MA) and homogenized using a motorized ground-glass homogenizer (Duall; Kimble Chase, Vineland, NJ) in 100:1 volume to weight dilution of cold buffer (20 mM K2HPO4, 5 mM 2-mercaptoethanol, 0.5 mM EDTA, 0.02% BSA, 50% glycerol, pH 7.4). Glycogen phosphorylase activity was determined fluorometrically from the production of reduced NADPH. Citrate synthase activity was determined through the reduction of DTNB by the release of Coenzyme A in the cleaving of acetyl-CoA (15). β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity was determined fluorometrically from the appearance of NAD+ (15). All assays were performed in triplicate for each sample.
Skeletal muscle capillaries.
Skeletal muscle capillaries were measured as previously described (2, 3, 58). Briefly, for each biopsy sample, transverse sections (10 μm) for histochemical analysis were cut on a microtome-cryostat (HM 525, Microm, Walldorf, Germany) at −20°C. Capillary staining was completed with 5-min fixation in Carnoy’s, followed by a 30-min incubation (37°C) in 1% amylase and then periodic acid-Schiff staining (2). Section images were captured with a microscope (BX51, Olympus, Tokyo, Japan) and camera (DP26, Olympus) interfaced to a computer with specialized software (cellSens Entry 1.11, Olympus). Images were calibrated with a micrometer calibration slide and analyzed on a Macintosh computer with NIH ImageJ (1.47) software.
Capillary density, capillary-to-fiber ratio, and capillaries in contact with each fiber (CCEF) were determined from approximately four separate 300 μm × 300-μm artifact-free regions per subject. Capillary density was calculated as the number of capillaries in this defined area (capillaries/mm2). Capillary-to-fiber ratio was calculated as the number of capillaries divided by the number of muscle fibers in this defined area. CCEF was determined by counting the number of capillaries touching each muscle fiber in the defined area. Collectively, these three different methodological approaches provide a comprehensive view of muscle capillarization that no one method can capture alone. All capillary measurements were completed by two independent investigators and averaged to represent each sample (5.8% coefficient of variation across all three measurements).
Statistical analysis.
Statistical analyses were performed using Statistical Analysis Software (SAS version 9.3; Cary, NC), and statistical tests yielding P values less than or equal to 0.05 were considered statistically significant. Intrasex comparisons between YE, LLE, and OH were conducted using a one-way ANOVA with Tukey’s post hoc test used to compare groups means in the event of a statistically significant omnibus F-test. LLE-P and LLE-F were further compared using an independent two-tailed t-test with a Levene’s Test for equality of variance. No statistical differences in V̇o2max parameters were observed by excluding individuals on beta-blockers and were thus included in the group analysis. All data are presented as means ± SE.
Although chronological age is fixed by date of birth, physiological age can vary based upon numerous health metrics. Here, we used the maximal values achieved during the V̇o2max test to estimate physiological age of the older participants tested in the current study. To do this, linear regression analysis (R2 > 0.98) was performed using the 50th percentile of absolute V̇o2max, maximal heart rate, and peak power across the lifespan from the fitness registry and the importance of exercise national database (FRIEND) (28). The FRIEND was selected because it is cycle specific, used gas analysis for V̇o2max measures (as opposed to estimates), and has >4,400 female and male participants allowing for sex-specific physiological age calculations. In this way, we were able to gain additional insight into the physiological state of the participants from the current study. The following equations were used for females: physiological age = (−2.5605 + absolute V̇o2max)/−0.0211, physiological age = (−198.57 + heart rate)/−0.8731, physiological age = (−210.07 − peak power)/−1.8731. The following equations were used for men: physiological age = (−4.0968 + absolute V̇o2max)/−0.033, physiological age = (−200.11 + heart rate)/−0.9207, physiological age = (−348.21 + peak power)/−2.8921.
RESULTS
Maximal aerobic capacity in women.
Maximal exercise test data are shown in Table 4. A hierarchical pattern was observed (YE > LLE > OH) in V̇o2max, ventilation, and heart rate. Absolute V̇o2max (l/min) in YE was 68% greater than LLE and 131% greater than OH (P < 0.05), whereas LLE was 37% greater than OH (P < 0.05). Similarly, V̇o2max relative to body mass (ml·kg−1·min−1) in YE was 69% greater than LLE and 144% greater than OH (P < 0.05), whereas LLE was 44% greater than OH (P < 0.05). The YE cohort had a maximal heart rate of 24 beats/min (15%) greater than LLE and 36 beats/min (24%) greater than OH (P < 0.05), whereas LLE was 12 beats/min (8%) greater than OH (P < 0.05). Maximal oxygen pulse was 4.6 to 6.6 ml O2/beat greater in YE compared with LLE and OH (P < 0.05), with no differences between LLE and OH. Peak power achieved by YE during the maximal cycle test was 93 W (67%) greater than LLE and 140 W (154%) greater than OH (P < 0.05), whereas LLE was 47 W (52%) greater than OH (P < 0.05).
Table 4.
Maximal exercise data
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women |
YE, n = 10 | LLE |
OH, n = 10 | |||||
| YE, n = 10 | LLE, n = 7 | OH, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | |||
| V̇o2max, l/min | 2.66 ± 0.14* | 1.58 ± 0.15† | 1.15 ± 0.05 | 3.92 ± 0.17* | 2.67 ± 0.10† | 2.89 ± 0.10‡ | 2.24 ± 0.12 | 1.95 ± 0.16 |
| V̇o2max, ml·kg−1·min−1 | 44 ± 2* | 26 ± 2† | 18 ± 1 | 53 ± 3* | 34 ± 1† | 38 ± 1‡ | 27 ± 2 | 22 ± 1 |
| V̇o2max, ml·kg LBM−1·min−1 | 61 ± 3* | 39 ± 2 | 32 ± 1 | 67 ± 2* | 47 ± 2† | 51 ± 1‡ | 40 ± 2 | 34 ± 1 |
| Ventilation, l/min | 101 ± 5* | 61 ± 5† | 42 ± 2 | 139 ± 6* | 100 ± 4† | 108 ± 4‡ | 84 ± 4 | 71 ± 6 |
| Heart rate, beats/min | 188 ± 2* | 164 ± 2† | 152 ± 5 | 193 ± 3* | 157 ± 5 | 167 ± 4‡ | 138 ± 10 | 144 ± 6 |
| O2 pulse, ml O2/beat | 14.2 ± 0.8* | 9.6 ± 0.9 | 7.6 ± 0.4 | 20.4 ± 0.9* | 17.2 ± 0.6† | 17.4 ± 0.5 | 16.7 ± 1.4 | 13.7 ± 1.2 |
| RER | 1.21 ± 0.02 | 1.27 ± 0.05 | 1.24 ± 0.03 | 1.25 ± 0.02 | 1.20 ± 0.01 | 1.21 ± 0.01 | 1.18 ± 0.03 | 1.24 ± 0.02 |
| Peak power, W | 231 ± 10* | 138 ± 11† | 91 ± 5 | 332 ± 11* | 222 ± 9† | 240 ± 9‡ | 185 ± 8 | 151 ± 8 |
Values are means ± SE. LBM, lean body mass; LLE, lifelong exercisers; OH, old healthy; RER, respiratory exchange ratio; V̇o2max, maximal oxygen consumption; YE, young exercisers.
P < 0.05 vs. other groups;
P < 0.05 vs. OH;
P < 0.05 vs. LLE fitness.
Maximal aerobic capacity in men.
Measurements of V̇o2max and ventilation also demonstrated a hierarchical pattern (YE > LLE > OH) among men. Absolute V̇o2max in YE was 47% greater than LLE and 102% greater than OH (P < 0.05), whereas LLE was 38% greater than OH (P < 0.05). Similarly, V̇o2max relative to body mass in YE was 56% greater than LLE and 141% greater than OH (P < 0.05), whereas LLE was 55% greater than OH (P < 0.05). Maximal heart rate was 36 to 49 beats/min greater in YE compared with LLE and OH (P < 0.05), with no differences between LLE and OH. Maximal oxygen pulse in YE was 3.2 ml O2/beat (19%) greater than LLE and 6.7 ml O2/beat (49%) greater than OH (P < 0.05), whereas LLE was 3.5 ml O2/beat greater (26%) than OH (P < 0.05). Peak power achieved by YE during the maximal cycle test was 110 W (50%) greater than LLE and 181 W (120%) greater than OH (P < 0.05), whereas LLE was 71 W (47%) greater than OH (P < 0.05).
Performance versus fitness in men.
Upon separation of the LLE male cohort to LLE-P and LLE-F, V̇o2max both absolute and relative to body mass was 29% and 40% greater in LLE-P compared with LLE-F, respectively (P < 0.05). Maximal heart rate was 29 beats/min (21%) greater in LLE-P than LLE-F (P < 0.05). No differences were observed in respiratory exchange ratio and maximal oxygen pulse. Peak power achieved was 55 W (30%) greater in LLE-P than LLE-F (P < 0.05).
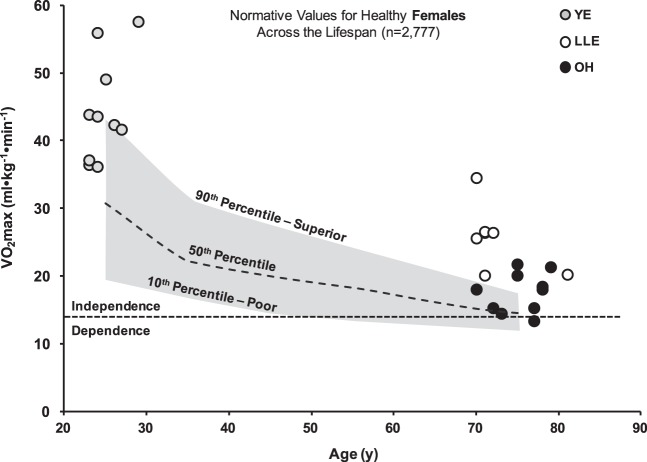
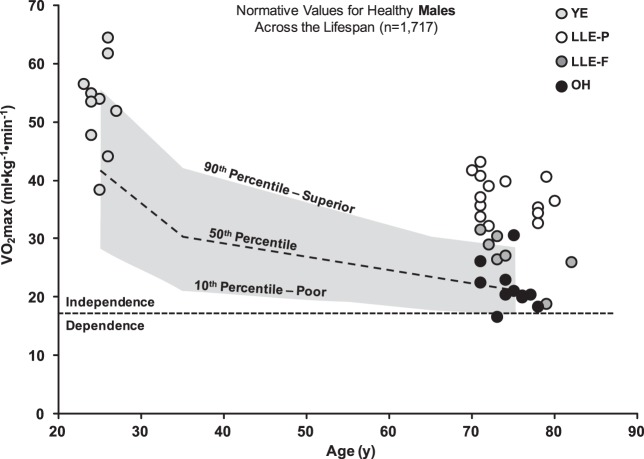
Comparison of V̇o2max to normative values.
Individual V̇o2max (ml·kg−1·min−1) data points for the female and male groups were plotted and compared with normative values for the cycle ergometer from the FRIEND database in Figs. 2 and 3, respectively (28). Linear regression analysis estimating physiological age from the FRIEND (28) indicated that the LLE women and men were physiologically ~30 yr younger than their chronological age (Table 5). Upon separation of the male LLE cohort, LLE-P and LLE-F were ~35 yr and 15 yr younger than their respective chronological ages.
Fig. 2.
Individual relative V̇o2max data of young exercisers (YE), lifelong aerobic exercisers (LLE), and old healthy untrained individuals (OH) compared with normative values from the FRIEND database (n = 2,777) (28). The FRIEND database was used due to similar graded exercise test mode (cycle), and those data were generated from direct gas analysis rather than estimates. These values are compared with an estimated female-specific frailty threshold of 14 ml·kg−1·min−1 (10, 28, 37). Although this threshold has not been as well established in women as it is in men [(17.5 ml/kg/min; 5 metabolic equivalents (METs)] (37), this estimate may be the prognostic exercise capacity necessary for an independent lifestyle in women. Furthermore, failure to remain above this threshold may result in an increased risk for mortality as described by Myers et al. (37). FRIEND, fitness registry and the importance of exercise national database; V̇o2max, maximal oxygen consumption.
Fig. 3.
Individual relative V̇o2max data of young exercisers (YE), vigorous lifelong aerobic exercisers (LLE-P), fitness trained lifelong aerobic exercisers (LLE-F), and old healthy untrained individuals (OH) compared with normative values from the FRIEND database (n = 1,717) (28). The FRIEND database was used due to similar graded exercise test mode (cycle), and those data were generated from direct gas analysis rather than estimates. These values are compared with a male-specific frailty threshold of [(17.5 ml/kg/min; 5 metabolic equivalents (METs)], which has been established as the prognostic exercise capacity necessary for an independent lifestyle in men. Furthermore, failure to remain above this threshold may result in an increased risk for mortality as described by Myers et al. (37). FRIEND, fitness registry and the importance of exercise national database; V̇o2max, maximal oxygen consumption.
Table 5.
Physiological age based on variables from the maximal exercise test
| Men |
|||||||
|---|---|---|---|---|---|---|---|
| Women |
LLE |
OH, n = 10 | |||||
| LLE, n = 7 | OH, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | |||
| Chronological age | 72 ± 2 | 75 ± 1 | 74 ± 1 | 74 ± 1 | 75 ± 2 | 75 ± 1 | |
| Physiological age | |||||||
| V̇o2max | 47 ± 7 | 67 ± 2 | 43 ± 3 | 37 ± 3 | 56 ± 4 | 65 ± 5 | |
| Heart rate | 39 ± 2 | 53 ± 6 | 47 ± 5 | 37 ± 4 | 67 ± 10 | 61 ± 7 | |
| Peak power | 39 ± 6 | 64 ± 3 | 44 ± 3 | 37 ± 3 | 56 ± 3 | 68 ± 3 | |
Values are means ± SE. LLE, lifelong exercisers; OH, old healthy; V̇o2max, maximal oxygen consumption.
Skeletal muscle capillaries.
Capillary data for women and men are shown in Table 6. In the three measurements of skeletal muscle capillarization (capillaries/mm2, capillaries/fiber, and CCEF), no differences between YE and LLE in both sexes were found, whereas both cohorts were greater than OH (P < 0.05). Between LLE male subgroups, LLE-P had 25% more capillaries/mm2 compared with LLE-F (P < 0.05), but no differences were found between subgroups in capillaries/fiber and CCEF.
Table 6.
Skeletal muscle (vastus lateralis) capillarization profile
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women |
LLE |
|||||||
| YE, n = 10 | LLE, n = 7 | OH, n = 10 | YE, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | OH, n = 10 | |
| Capillaries/mm2 | 377 ± 27 | 338 ± 22 | 281 ± 12* | 363 ± 19 | 347 ± 14 | 372 ± 13‡ | 298 ± 22 | 248 ± 15* |
| Capillaries/fiber | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.1 ± 0.1* | 2.3 ± 0.2 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 1.5 ± 0.1* |
| CCEF | 4.3 ± 0.4 | 3.8 ± 0.4 | 2.8 ± 0.2* | 5.4 ± 0.3 | 5.3 ± 0.2 | 5.4 ± 0.3 | 4.9 ± 0.3 | 3.5 ± 0.3* |
Values are means ± SE. CCEF, capillaries in contact with each fiber; LLE, lifelong exercisers; OH, old healthy; YE, young exercisers.
P < 0.05 vs. other groups;
P < 0.05 vs. LLE fitness.
Skeletal muscle enzymes.
Citrate synthase, β-HAD, and glycogen phosphorylase enzyme activity are shown in Table 7. In women, both the YE and LLE cohorts had greater citrate synthase activity than OH (61% and 37%, respectively; P < 0.05), whereas no differences among the cohorts were observed in β-HAD and glycogen phosphorylase. In men, no differences in citrate synthase and β-HAD were observed between YE and LLE. Furthermore, the LLE cohort had a greater citrate synthase (+90%) and β-HAD (+29%) activity than OH (P < 0.05). The YE and OH cohorts had similar glycogen phosphorylase activity, whereas LLE was 27% lower than YE (P < 0.05). No differences in enzyme activities were observed between the LLE subgroups.
Table 7.
Skeletal muscle (vastus lateralis) metabolic enzyme profile
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women |
LLE |
|||||||
| YE, n = 10 | LLE, n = 7 | OH, n = 10 | YE, n = 10 | All, n = 21 | Performance, n = 14 | Fitness, n = 7 | OH, n = 10 | |
| Citrate synthase | 25.8 ± 1.7 | 21.9 ± 1.7 | 16.0 ± 1.6* | 28.6 ± 1.7 | 29.7 ± 2.2 | 31.7 ± 2.9 | 25.8 ± 3.2 | 15.6 ± 1.0* |
| β-HAD | 25.5 ± 1.3 | 24.0 ± 1.9 | 22.9 ± 1.7 | 26.2 ± 1.1 | 27.9 ± 1.3 | 27.8 ± 1.3 | 28.1 ± 3.2 | 21.6 ± 1.3† |
| Glycogen phosphorylase | 36.6 ± 2.9 | 32.3 ± 4.5 | 31.8 ± 2.9 | 46.0 ± 2.8 | 33.8 ± 2.6§ | 31.5 ± 3.2 | 38.3 ± 4.3 | 41.0 ± 2.1 |
Values are means ± SE. Enzyme activity data presented in µmol·g−1·min−1. β-HAD, β-hydroxyacyl-CoA dehydrogenase; LLE, lifelong exercisers; OH, old healthy; YE, young exercisers.
P < 0.05 vs. other groups;
P < 0.05 vs. LLE;
P < 0.05 vs. YE.
DISCUSSION
The exercise boom triggered a large number of women and men to engage in structured physical activity as a lifestyle. Now, more than 50 years later, several of these individuals that remained consistent with an exercise regimen are in their eighth decade of life. This provided a unique opportunity to assess the physiological benefits of lifelong aerobic exercise on indices of cardiovascular and skeletal muscle health. The main findings from this investigation indicate a hierarchical pattern (YE > LLE > OH) in V̇o2max for women and men. Furthermore, the LLE men that exercised at a higher intensity for performance-based activities (LLE-P) had a higher V̇o2max than fitness-trained individuals (LLE-F). The benefits of lifelong aerobic exercise were also apparent in skeletal muscle as capillarization and aerobic enzyme activities among the LLE population were similar to young individuals. Interestingly, the skeletal muscle aerobic profiles were similar between LLE-P and LLE-F, suggesting that muscle metabolic fitness may be maintained with consistent exercise of lesser intensity. These data show a substantial V̇o2max benefit with LLE and suggest that skeletal muscle metabolic fitness may be easier to maintain with lifelong aerobic exercise than more central aspects of the cardiovascular system.
We identified 91 peer-reviewed papers that recorded V̇o2max in individuals in their eighth decade of life (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website), 73 of which separated male and female data. From these data, we estimated that ~3,006 individuals were assessed for V̇o2max, with 55% of them men (n = 1,649) and 45% women (n = 1,357). Of these individuals, we classified 389 men (24% of men; 13% total) as Masters athletes (competitive athletes or highly trained individuals), whereas only 60 women (4% of women; 2% total) were classified as Masters athletes. This highlights the limited amount of data available in individuals in the 70–79-yr age group who have been engaged in structured exercise at a relatively high level. Furthermore, muscle biopsy data are even more limited in lifelong exercising men and women in their eighth decade of life.
For V̇o2max (absolute and relative) and ventilation, we observed a hierarchical pattern in women and men as YE > LLE > OH (Table 4). Remarkably, one woman (at time of testing reported cycling ~4,000 miles a year) had a relative V̇o2max of 34.6 ml·kg−1·min−1, which is among the highest reported in the scientific literature for women in their eighth decade (43, 61). For the LLE men, a clear distinction was noted between LLE-P (range = 32.3 to 43.2 ml·kg−1·min−1) and LLE-F (range = 18.4 to 31.7 ml·kg−1·min−1) (Fig. 3), suggesting a relationship between higher exercise intensity throughout the lifespan and higher V̇o2max. When compared with normative values from the FRIEND, the LLE women and LLE-P men had a V̇o2max similar to an individual ~35 yr younger, whereas the LLE-F men had a V̇o2max similar to an individual ~15 yr younger (Table 5). Also noteworthy was that LLE-P recorded V̇o2max data comparable to former elite level athletes who continued to exercise throughout their life (20, 55, 60). The V̇o2max data reported here have important health implications for the sector of the population engaged in lifelong exercise habits as recently described by a statement from the American Heart Association (49).
Maximal aerobic capacity has emerged as a key health marker for all-cause mortality (24, 37, 49) with a 13%–15% reduction in risk of mortality with every 1-MET increase in V̇o2max (29). This equates to ~30% reduction in risk of mortality in the lifelong exercising women and men compared with the OH cohort. The vigorous lifelong exercisers (LLE-P) were in the lowest category of risk (≥38 ml·kg−1·min−1), which we have previously observed for elite octogenarian endurance athletes (29, 55). When put in context of the frailty threshold for men (17.5 ml·kg−1·min−1; 5 METs), the lifelong exercisers have a large physiological reserve, which may be beneficial when confronted with a health challenge (37). Although the frailty threshold has not been as well defined in women, it is likely lower (~14 ml·kg−1·min−1) based upon the available data in the literature (10, 28, 37), which would equate to similar benefits for LLE women.
The maximal heart rate data provided additional insight into the cardiovascular benefits of lifelong exercise. Maximal heart rate has been shown to decline gradually with age with little influence of exercise history on the decline (53). However, upon review of the 91 peer-reviewed papers (Supplemental Table S1), we calculated a greater maximal heart rate in similarly aged female and male Masters athletes compared with nonathletes (~158 vs. 149 beats/min, respectively). In further support of this, recent data from our laboratory (40, 55) and others (16, 20) have also indicated lifelong vigorous exercise may indeed help slow the decline in maximal heart rate with age. Contributing to this growing body of literature, the LLE women in the current study had a greater maximal heart rate (12 beats/min) than the OH cohort. This was also noted in the LLE-P men who had a 22–29 beats/min higher maximal heart rate compared with both the untrained and LLE-F cohorts. The higher maximal heart rate in LLE with vigorous exercise habits likely contributed to the greater V̇o2max measured in these individuals.
Oxygen pulse, a surrogate for stroke volume, was used to assess the effects of lifelong exercise on cardiac function. Previous research has shown an increased left ventricular mass, compliance, distensibility, and diastolic filling, as well as an increased total blood volume with lifelong endurance exercise (4, 9, 13, 22, 26, 39, 41), all of which would improve stroke volume. The female LLE cohort was similar to OH, whereas both older groups were ~40% lower than YE. Conversely, in the males, a hierarchical pattern appeared (YE > LLE > OH), with LLE-P similar to LLE-F. These data suggest a potential sex difference, which warrants further investigation as previous lifelong exercise studies combined the sexes (9, 13), potentially concealing any sex difference. Additionally, intensity appears not to play a role in preserving stroke volume in men, which may be explained by stroke volume plateauing during exercise at 50%–75% of V̇o2max (36, 38, 45). Because LLE-P and LLE-F generally exercised above 50% V̇o2max throughout their life, maximal stroke volume was likely obtained quite frequently during exercise in both subgroups, which may have resulted in similar adaptations. In further support of this idea, previous research suggests frequency of exercise (≥4 days per week) may be the critical threshold to preserve stroke volume (9, 13), with no further improvements seen in greater intensity of exercise training. As shown in Table 3, LLE-P and LLE-F continued to exercise for at least 4 days per week throughout their lifespan, thus further supporting frequency and not greater intensity above the stroke volume threshold as the impetus for improvements in stroke volume compared with sedentary individuals.
To complement the V̇o2max assessment, we also sought to determine the effects of lifelong exercise on skeletal muscle metabolic fitness by measuring capillarization and metabolic enzyme activities. Unlike the decline in V̇o2max, these data suggest a full preservation in skeletal muscle capillarization (Table 6) and aerobic enzymes (Table 7) in both women and men with 50+ years of lifelong exercise (YE = LLE > OH). These data are in agreement with previous research in younger and similarly aged Masters athletes (18, 30, 44, 46, 56) and suggest capillary supply can be maintained in individuals >70 yr that maintain quality exercise habits. The higher capillary density observed in the LLE-P compared with the LLE-F is in agreement with previous Masters athlete research from our laboratory in highly trained former elite distance runners (56) and suggest that vigorous exercise may provide some benefit for capillarization above already-preserved levels. The preservation of skeletal muscle capillarization may provide benefits for athletic performance and skeletal muscle health by enhancing blood flow to the muscle for increased oxygen and substrate delivery and improve the ability for the muscle to communicate with other tissues in the body (21, 32, 51, 52, 59).
Similar to the capillary profile, citrate synthase was preserved with lifelong aerobic exercise in both sexes as YE was similar to LLE, which has been previously reported in male Masters athletes (18, 30, 46). Furthermore, no differences were found between LLE-P and LLE-F in the measured metabolic enzymes, suggesting greater intensity in lifelong exercise may not provide further benefit for skeletal muscle aerobic enzymatic activity. In contrast, neither aging nor exercise influenced glycogen phosphorylase and β-HAD activity in women, as YE, LLE, and OH were similar. However, in the men, LLE had lower glycogen phosphorylase than YE and greater β-HAD activity than OH. Whereas most other variables we measured tracked similarly between the women and the men, the differential pattern for glycogen phosphorylase and β-HAD is not clear. Generally, it appears that the LLE women did not have the same magnitude of aerobic enzyme activity as the men, which may be related to the lifelong exercise habits that we were not able to tease out with our exercise history documentation. It is plausible that a sex difference in muscle metabolic aging may occur as has been suggested by others (17, 31, 54) and warrants more research.
Limitations and future directions.
We had the unique opportunity to assess the V̇o2max and skeletal muscle aerobic profile in individuals that have been consistently exercising for the last ~50 yr; however, we acknowledge several limitations to our study design. Because of the cross-sectional nature of this study, we were unable to track precisely training volume and intensity throughout the lifespan of these lifelong exercisers as self-reported data from the last five decades were collected through questionnaires, training logs, and interviews. With the increasing use of technology and digital tracking during exercise, more detailed exercise profiles may be attainable in future aging exercise investigations. Furthermore, because of the increasing presence of prescription medications in health care, particularly with aging (14), we acknowledge that some of these subjects were on medications that may have impacted the variables presented. Separate statistics were completed with and without including subjects on certain medications (e.g., beta blockers). No differences in our statistical analysis appeared, and the subjects were thus included in the analysis. Future research should examine various aspects of health with lifelong exercise at the whole body to gene level (i.e., functional genomics), in multiple tissues across the lifespan, between sexes, and across modes of exercise (e.g., aerobic and resistance exercise).
In summary, lifelong aerobic exercise provided substantial benefits for V̇o2max and skeletal muscle metabolic fitness among women and men in their eighth decade of life. The greater V̇o2max in the LLE cohort compared with OH remarkably decreases the relative risk of mortality and provides for a large physiological reserve above the frailty threshold, with further benefit from vigorous lifelong training (29, 37). For skeletal muscle, 50+ years of lifelong aerobic exercise fully preserve skeletal muscle capillarization and aerobic enzymes, regardless of intensity. These data support the idea that skeletal muscle metabolic fitness may be easier to maintain with lifelong aerobic exercise than more central aspects of the cardiovascular system. The conservation of the skeletal muscle presented here may translate to other biological signatures of skeletal muscle health and warrants further investigation.
GRANTS
This research was supported by the National Institutes of Health Grant No. AG-038576.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.R., L.A.K., T.A.T., and S.T. conceived and designed research; K.J.G., U.R., R.K.P., K.M.L., B.S.O., L.A.K., T.A.T., and S.W.T. performed experiments; K.J.G., U.R., R.K.P., K.M.L., B.S.O., B.G., W.H.F., T.A.T., and S.T. analyzed data; K.J.G., U.R., L.J.D., W.H.F., T.A.T., and S.T. interpreted results of experiments; K.J.G. and S.T. prepared figures; K.J.G. and S.T. drafted manuscript; K.J.G., U.R., R.K.P., K.M.L., B.S.O., L.J.D., B.G., W.H.F., L.A.K., T.A.T., and S.T. edited and revised manuscript; K.J.G., U.R., R.K.P., K.M.L., B.S.O., L.J.D., B.G., W.H.F., L.A.K., T.A.T., and S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the many Human Performance Lab (HPL) graduate students and support staff involved with various aspects of data collection and interaction with the volunteers. We are grateful to all the volunteers who graciously gave their time, energy, and support to this project.
Dr. Leonardo D’Acquisto was a visiting scholar at the HPL and is a professor in the Department of Health Sciences at Central Washington University, Ellensburg, WA.
REFERENCES
- 1.American College of Sports Medicine ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 2.Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand 95: 203–205, 1975. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270: 677–690, 1977. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 5.Astrand P, Rodahl K. Textbook of Work Physiology: Physiological Bases of Exercise. New York: McGraw-Hill, 1986. [Google Scholar]
- 6.Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15: 405–411, 2012. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 1–110, 1962. [Google Scholar]
- 8.Bhambhani Y, Norris S, Bell G. Prediction of stroke volume from oxygen pulse measurements in untrained and trained men. Can J Appl Physiol 19: 49–59, 1994. doi: 10.1139/h94-003. [DOI] [PubMed] [Google Scholar]
- 9.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 64: 1257–1266, 2014. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair SN, Kohl HW III, Paffenbarger RS JR, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 11.Burfoot A. First Ladies of Running. New York: Rodale, 2016, p. 43–65. [Google Scholar]
- 12.Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc 46: 1824–1829, 1987. [PubMed] [Google Scholar]
- 13.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlesworth CJ, Smit E, Lee DS, Alramadhan F, Odden MC. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J Gerontol A Biol Sci Med Sci 70: 989–995, 2015. doi: 10.1093/gerona/glv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi MM, Hintz CS, Coyle EF, Martin WH III, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 16.Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol (1985) 75: 2125–2133, 1993. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- 17.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47: B71–B76, 1992. doi: 10.1093/geronj/47.3.B71. [DOI] [PubMed] [Google Scholar]
- 18.Coggan AR, Spina RJ, Rogers MA, King DS, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol (1985) 68: 1896–1901, 1990. doi: 10.1152/jappl.1990.68.5.1896. [DOI] [PubMed] [Google Scholar]
- 19.Cushman DM, Markert M, Rho M. Performance trends in large 10-km road running races in the United States. J Strength Cond Res 28: 892–901, 2014. doi: 10.1519/JSC.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everman S, Farris JW, Bay RC, Daniels JT. Elite distance runners: a 45-year follow-up. Med Sci Sports Exerc 50: 73–78, 2018. doi: 10.1249/MSS.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 21.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 33: 114–119, 2005. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D, Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 590: 1871–1880, 2012. doi: 10.1113/jphysiol.2011.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabriel Z, Bowling A. Quality of life from the perspectives of older people. Ageing Soc 24: 675–691, 2004. doi: 10.1017/S0144686X03001582. [DOI] [Google Scholar]
- 24.Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J, Ross R. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis 60: 11–20, 2017. doi: 10.1016/j.pcad.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol 51: 634–640, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Howden EJ, Carrick-Ranson G, Sarma S, Hieda M, Fujimoto N, Levine BD. Effects of sedentary aging and lifelong exercise on left ventricular systolic function. Med Sci Sports Exerc 50: 494–501, 2018. doi: 10.1249/MSS.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 27.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin Proc 90: 1515–1523, 2015. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the fitness registry and the importance of exercise national database (FRIEND) registry. Mayo Clin Proc 92: 228–233, 2017. doi: 10.1016/j.mayocp.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 30.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942, 2008. [Erratum in Diabetes 61: 2653, 2012]. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levadoux E, Morio B, Montaurier C, Puissant V, Boirie Y, Fellmann N, Picard B, Rousset P, Beaufrere B, Ritz P. Reduced whole-body fat oxidation in women and in the elderly. Int J Obes Relat Metab Disord 25: 39–44, 2001. doi: 10.1038/sj.ijo.0801530. [DOI] [PubMed] [Google Scholar]
- 32.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loe H, Rognmo Ø, Saltin B, Wisløff U. Aerobic capacity reference data in 3816 healthy men and women 20-90 years. PLoS One 8: e64319, 2013. [Erratum in PLoS One 8: e3115, 2013]. doi: 10.1371/journal.pone.0064319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med 19: 163–173, 2000. doi: 10.1016/S0278-5919(05)70196-4. [DOI] [PubMed] [Google Scholar]
- 35.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 36.McLaren PF, Nurhayati Y, Boutcher SH. Stroke volume response to cycle ergometry in trained and untrained older men. Eur J Appl Physiol Occup Physiol 75: 537–542, 1997. doi: 10.1007/s004210050201. [DOI] [PubMed] [Google Scholar]
- 37.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Spina RJ, Martin WH III, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992. doi: 10.1161/01.CIR.86.2.494. [DOI] [PubMed] [Google Scholar]
- 39.Opondo MA, Aiad N, Cain MA, Sarma S, Howden E, Stoller DA, Ng J, van Rijckevorsel P, Hieda M, Tarumi T, Palmer MD, Levine BD. Does high-intensity endurance training increase the risk of atrial fibrillation? A longitudinal study of left atrial structure and function. Circ Arrhythm Electrophysiol 11: e005598, 2018. doi: 10.1161/CIRCEP.117.005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozemek C, Whaley MH, Finch WH, Kaminsky LA. High cardiorespiratory fitness levels slow the decline in peak heart rate with age. Med Sci Sports Exerc 48: 73–81, 2016. doi: 10.1249/MSS.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 41.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101: 336–344, 2000. doi: 10.1161/01.CIR.101.3.336. [DOI] [PubMed] [Google Scholar]
- 42.Pollock ML, Mengelkoch LJ, Graves JE, Lowenthal DT, Limacher MC, Foster C, Wilmore JH. Twenty-year follow-up of aerobic power and body composition of older track athletes. J Appl Physiol (1985) 82: 1508–1516, 1997. doi: 10.1152/jappl.1997.82.5.1508. [DOI] [PubMed] [Google Scholar]
- 43.Pollock RD, Carter S, Velloso CP, Duggal NA, Lord JM, Lazarus NR, Harridge SD. An investigation into the relationship between age and physiological function in highly active older adults. J Physiol 593: 657–680, 2015. doi: 10.1113/jphysiol.2014.282863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollock RD, O’Brien KA, Daniels LJ, Nielsen KB, Rowlerson A, Duggal NA, Lazarus NR, Lord JM, Philp A, Harridge SDR. Properties of the vastus lateralis muscle in relation to age and physiological function in master cyclists aged 55-79 years. Aging Cell 17: e12735, 2018. doi: 10.1111/acel.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J Appl Physiol (1985) 84: 599–605, 1998. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- 46.Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78: 2033–2038, 1995. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- 47.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112: 1625–1636, 2012. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson S. Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 10: 251–323, 1938. [Google Scholar]
- 49.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134: e653–e699, 2016. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 50.Scheerder J, Breedveld K, Borgers J. Who is doing a run with the running boom? In: Running Across Europe: the Rise and Size of One of the Largest Sport Markets, edited by Scheerder J, Breedveld K, Borgers J. London: Palgrave Macmillan UK, 2015, p. 1–27. [Google Scholar]
- 51.Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 80: 115–125, 2015. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJC, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8: 267–276, 2017. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 54.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol (1985) 68: 302–308, 1990. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 55.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trappe SW, Costill DL, Fink WJ, Pearson DR. Skeletal muscle characteristics among distance runners: a 20-yr follow-up study. J Appl Physiol (1985) 78: 823–829, 1995. doi: 10.1152/jappl.1995.78.3.823. [DOI] [PubMed] [Google Scholar]
- 57.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trappe TA, Ratchford SM, Brower BE, Liu SZ, Lavin KM, Carroll CC, Jemiolo B, Trappe SW. COX inhibitor influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci 71: 1289–1294, 2016. doi: 10.1093/gerona/glv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Wilmore JH, Miller HL, Pollock ML. Body composition and physiological characteristics of active endurance athletes in their eighth decade of life. Med Sci Sports 6: 44–48, 1974. [PubMed] [Google Scholar]
- 61.Wiswell RA, Hawkins SA, Jaque SV, Hyslop D, Constantino N, Tarpenning K, Marcell T, Schroeder ET. Relationship between physiological loss, performance decrement, and age in master athletes. J Gerontol A Biol Sci Med Sci 56: M618–M626, 2001. doi: 10.1093/gerona/56.10.M618. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]