Abstract
Reduced ventilatory control stability (elevated loop gain) is a key nonanatomical, pathological trait contributing to obstructive sleep apnea (OSA), yet the mechanisms responsible remain unclear. We sought to identify the key factors contributing to elevated loop gain in OSA (controller vs. plant contributions) and to examine whether abnormalities in these factors persist after OSA treatment. In 15 males (8 OSA, 7 height, weight- and age -matched controls), we measured loop gain, controller gain, and plant gain using a pseudorandom binary CO2 stimulation method during wakefulness. Factors potentially influencing plant gain were also assessed (supine lung volume via helium dilution and spirometry). Measures were repeated 2 and 6 wk after initiating continuous positive airway pressure treatment. Loop gain (LG) was higher in OSA versus controls (LG at 1 cycle/min 0.28 ± 0.04 vs. 0.16 ± 0.04, P = 0.046, respectively), and the controller exhibited a greater peak response to CO2 and faster roll-off in OSA. OSA patients also exhibited reduced forced expiratory volume in the first second and forced vital capacity compared with controls (92.2 ± 1.7 vs. 102.9 ± 3.5% predicted, P = 0.021; 93.4 ± 3.1 vs. 106.6 ± 3.6% predicted, P = 0.015, respectively). There was no effect of treatment on any variable. These findings confirm loop gain is higher in untreated OSA patients than in matched controls; however, this was not affected by treatment.
NEW & NOTEWORTHY Elevated loop gain contributes to obstructive sleep apnea (OSA) pathophysiology. However, whether loop gain is inherently elevated in OSA or induced by OSA itself, whether it is elevated due to increased chemoreflex sensitivity or obesity-dependent reduced lung volume, and whether it is treatment reversible, are all currently uncertain. This study found loop gain was elevated in OSA versus age-, sex-, height-, and weight-matched controls. However, this was not altered by 6-wk continuous positive airway pressure treatment.
Keywords: continuous positive airway pressure, loop gain, obstructive sleep apnea
INTRODUCTION
Unstable ventilatory chemoreflex control, quantified as loop gain, is accepted as a key nonanatomical pathophysiological trait contributing to obstructive sleep apnea (OSA) (34). Potential treatments to reduce loop gain are being keenly investigated as part of new treatment strategies for OSA, which are based on identification and treatment of the specific causes of an individual’s apnea (6). However, the mechanisms causing elevated loop gain in OSA and whether these are inherent or induced traits remains unclear (20, 37). Consequently, a better understanding of the mechanisms causing elevated loop gain is required to guide the development of treatments aimed at reducing loop gain.
Loop gain is an engineering concept used to quantify the stability of negative feedback control systems, which can be usefully applied to ventilatory chemoreflex control. Loop gain is the ratio of the magnitude of a ventilatory response to the magnitude of the disturbance that elicited it and is the product of two components: the controller gain and the plant gain. In the context of ventilatory control, these components predominantly reflect chemoreflex sensitivity and the ability of the lung gas stores to dampen changes in blood gases driven by ventilation, respectively (11).
Although many studies in OSA patients support that loop gain contributes to OSA pathophysiology, relatively few have included non-OSA controls to clearly establish if loop gain is consistently elevated in OSA (35, 39). In five published studies of which we are aware to have compared loop gain between adult OSA patients and non-OSA controls (2, 5, 8, 10, 25), three reported that loop gain was not different between OSA patients and controls (2, 5, 25). In contrast, Hudgel et al. (10) found loop gain was higher in OSA and speculated this was due to obesity-dependent reduced lung volume and elevated plant gain in the obese OSA patients versus the lean controls; a finding supported by Sands et al. (25), who found that loop gain was only higher in obese individuals versus lean controls and was not different between OSA patients and obese BMI-matched non-OSA controls. These findings support that obesity is a key factor influencing loop gain via plant effects. On the other hand, Gederi et al. (8) found the opposite: that loop gain was higher in OSA due solely to higher controller gain, implying that controller disturbances may also be important.
More traditional chemoreflex studies that have compared OSA patients to body mass index (BMI)-matched control participants consistently report that OSA patients exhibit abnormalities in chemoreflex control, which increase loop gain and which normalize with continuous positive airway pressure (CPAP) treatment (15, 24, 27). Intermittent hypoxia induces lasting, but reversible, neuroplastic changes at multiple sites within the ventilatory neural network, which cause the same changes to ventilatory chemoreflex control as seen in OSA patients (4, 9, 16). In both animals and humans, intermittent hypoxia-induced ventilatory neuroplasticity has also been shown to ameliorate several days after returning to stable normoxic breathing (3, 21–23). Additionally, intermittent hypoxia-induced neuroplasticity in OSA patients causes an increase in apnea hypopnea index (AHI) (30, 32, 36) in conjunction with a greater degree of hyperventilation following apnea termination (36).
Collectively, these data suggest that both obesity-dependent reduced lung volume and elevated plant gain, as well as intermittent hypoxia-induced neuroplastic elevation of controller gain, may underpin elevated loop gain in OSA patients. However, there is no evidence to suggest that lung volumes would differ between OSA patients and morphologically similar non-OSA controls (40), or to support that loop gain is inherently elevated in OSA patients. Perhaps more likely is that elevated loop gain in OSA is acquired due to intermittent hypoxia-induced neuroplastic elevation of controller gain, which importantly is treatment reversible. Thus, discrepancies between studies may be due to lack of matching for morphological traits known to alter lung volumes and, consequently, plant gain. Also, some loop gain studies have been conducted in OSA participants that have been already CPAP treated for months to years [Hudgel et al. (10) and Bokov et al. (2) do not report treatment status], so that neuroplastic elevation of controller gain may have normalized before assessment.
To help clarify potential plant versus controller effects on loop gain, this study was conducted primarily to determine whether elevated overall loop gain in OSA patients (vs. age-, height- and weight-matched controls) is inherent or acquired (and thus, treatment-reversible), and to examine the contribution of plant gain and controller gain to overall loop gain. Accordingly, loop gain parameters were estimated using repeated applications of a 63-breath pseudorandom binary sequence (PRBS) of CO2 enriched and room air breaths that allows for estimation of controller, plant, and overall loop gain (12) in newly diagnosed and untreated male OSA patients at baseline and after 6 wk of CPAP treatment. Controls matched for age, sex, height, and weight underwent the same measurements over the same period but without CPAP administration. We hypothesized that, by design, plant gain and lung volumes would not differ between OSA and body habitus-matched controls and would remain unaffected after 6 wk of CPAP treatment, while controller and overall loop gain would be higher in OSA patients and would be reduced with CPAP treatment.
METHODS
Ethical approval.
All participants gave written informed consent before participation in the study. The study was approved by the Southern Adelaide Clinical Human Research and Ethics Committee.
Participants.
Ten men with newly diagnosed and previously untreated severe OSA (AHI > 30/h) and 10 healthy non-OSA men matched for age, height, weight, and BMI were recruited from the Adelaide Institute for Sleep Health following diagnostic polysomnography to either confirm or exclude OSA, and to exclude participants with any other sleep disorder. All participants were nonsmokers (had never smoked or had quit smoking a minimum of 6 mo before commencement in the study). Spirometry was evaluated as part of the screening visit, with normal lung function while seated [forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC): ≥80% predicted; JLab software version 4.53; Compactlab, Jaeger, Wuerzburg, Germany] necessary for study inclusion. None of the participants had been born or had lived at high altitude; had any known heart, lung, or kidney disease; diabetes; or any other major health problem. Patients with mild depressive disorders were included, as long as their medication and dosage did not change throughout the study. However, none was taking any medications with sedative or myorelaxant properties, or with effects on cardiac or respiratory control.
Measurements and protocol.
Measurements were obtained in OSA patients before commencing CPAP treatment (baseline) and then were repeated approximately 2 and 6 wk after commencing CPAP, with equivalent measurements and time points in control participants, who received no intervening treatment. OSA patients received treatment as usual with conventional CPAP. CPAP machine data were downloaded after 6 wk of treatment to determine CPAP usage in fortnightly intervals.
Participants arrived in the laboratory at 6 PM after being asked to abstain from eating for the preceding 4 h, and to have avoided caffeine, alcohol, and strenuous exercise throughout the day of the study. Upon arrival, height, weight, and blood pressure were measured. Subjects then lay supine with their nose clipped for measurements of supine functional residual capacity (FRC) using a helium dilution rebreathing method via a mouth-piece (CareFusion Jaeger Masterscreen PFT Hockberg, Germany System). To avoid potential effects of hemodynamic fluid shifts on lung volume, participants lay supine for 10 min before the first measurement, with a further 10 min between each replicate measurement to ensure helium washout from the lungs between measurements. FRC was determined as the mean of three replicate measures within 200 ml.
Electroencephalograms (C4-M1 and C3-M2) and left and right electrooculograms were monitored throughout the remaining protocol to confirm wakefulness (Compumedics, E-Series, Melbourne Australia). The electrocardiogram, arterial oxyhemoglobin saturation (, via ear pulse oximetry, POET II model 602-3; Criticare Systems, Waukesha, WI), and (Capnostream20, Oridion, Israel; precalibrated before each experiment against 8% CO2, α-standard gas, BOC gases Australia), were also monitored continuously. Participants were fitted with ear phones and listened to quiet music to minimize external stimuli influencing breathing.
Each participant wore a nasal mask (Gel mask; Respironics, Murrysville, PA) and mouth tape (with easily removable tabs) to ensure nasal breathing. The mask was fitted with a two-way nonrebreathing valve (Series 2600; Hans Rudolph, Kansas City, MO) with a pneumotachograph (PT16, Jaeger, Germany), calibrated via replicate one-liter syringe maneuvers before and after each experiment, and attached to the inspiratory side of the breathing valve for quantitative inspiratory flow and volume measurements. The common outlet port of a Gatlin-shaped four-inlet port valve system (series 2440C; Hans Rudolph) was connected to the pneumotachograph via 1 m of tubing (Hans Rudolph; 35-mm Clean-bore) for delivery of inspiratory gases. A computer-controlled solenoid system that monitored the airflow signal controlled rapid pneumatic balloon inflation and deflation to allow only one inlet port to be open at a time and ensure that all switches between ports were conducted during expiration. Three of the inlet ports were open to room air, while the fourth one was connected to a foil bag (300L; Scholle Industries, Adelaide, Australia) via three-way stop-cocks to enable closure of the bag and manual switching between the bag and the inlet port. The bag was filled with a mixture of medical-grade air and 100% CO2 blended at flow rates adjusted to achieve 4% CO2 on the precalibrated CO2 analyzer. The bag was then partially filled and flushed to discard dead space, and then filled to near capacity immediately before the experiment.
Pseudorandom binary stimulation protocol.
Participants lay supine and were instructed to keep their eyes open, to stay still and awake, and to breathe normally. Following a 10-min baseline to record stable breathing on room air, the pseudorandom binary stimulation (PRBS) protocol commenced. This consisted of a sequence of 63 breaths alternating between room air and 4% CO2 in a predetermined pseudorandom sequence spanning the full range of switching frequencies of between one and six consecutive breaths of CO2 (12). The same sequence was repeated for a total of eight times without interruption between adjacent sequences.
Data analysis.
Inspiratory, expiratory, and total breath times were determined from the flow signal, which was digitally integrated to measure inspiratory tidal volume and minute ventilation breath-by-breath. Breath-by-breath measurements of inspiratory and expiratory (end-tidal) partial pressure of CO2 were determined from the nadir and peak in mask CO2, respectively, after adjusting for gas sampling delay.
The first pseudorandom binary stimulation sequence and any sequence containing breaths contaminated by movement artifact, swallows, sighs, or periods of sleep were excluded from analysis (13). Controller gain, plant gain, overall loop gain, feedback delay, and other parameters of a simplified model of CO2 chemoreflex control of breathing were estimated by fitting breath-by-breath measures of minute ventilation (VI), total breath time (TTOT), inspiratory CO2 (), and during the PRBS test to the CO2 chemoreflex control model of breathing described by Khoo (13), with a minor modification to allow estimation of controller, plant, and overall loop gain across a range of frequencies (the MATLAB analysis script used in this study is available as supplementary material). The main outcomes of this analysis were gain parameters estimated at frequencies of 1–10 cycles/min, as well as the model-based estimated impulse response of the system over 63 breaths to a single breath increase in alveolar CO2.
Statistical analysis.
Age, height, BMI, FEV1, and FVC were compared between groups using Student’s independent samples t-tests. Linear mixed-model analysis was used to examine group, time, and interaction effects in measures repeated over time at each visit [weight, mean arterial pressure (MAP), FRC and controller gain, plant gain, and loop gain]. VI, , VT, FB, and from 5 min of supine room air breathing during the baseline period before the commencement of the PRBS protocol was also compared using linear mixed-model analyses to examine group, time, and interaction effects. Pearson’s product-moment correlation coefficient was used to explore the strength of linear correlations between FEV1, FVC, FRC, and AHI. PRBS-derived CO2 impulse responses were compared between groups and visits using linear mixed-model analysis. CPAP data were assessed in 2-wk blocks to assess CPAP compliance over time. Significant main or interaction effects were examined using Bonferroni adjusted post hoc analyses where appropriate. All statistical analysis was performed using SPSS (IBM SPSS Statistics versions 22 and 24). All data are presented as means ± SE. P values <0.05 were considered statistically significant.
RESULTS
Subjects.
After providing consent, several participants did not complete the full protocol, and one OSA patient was prescribed mandibular advancement splint treatment instead of CPAP and was excluded. One OSA patient and another control participant commenced CPAP treatment before baseline measurements could be collected. A further OSA participant withdrew before baseline measurements were complete, and another control participant hyperventilated and was unable to breathe normally while wearing the nasal mask. Consequently, baseline data from seven OSA participants and eight non-OSA controls were available for analysis. The characteristics of these participants are presented in Table 1. One further OSA participant was subsequently lost to follow-up, and technical problems led to some further missing FRC and other measurements (n shown accordingly).
Table 1.
Characteristics of OSA patients and matched controls
| OSA | Controls | |
|---|---|---|
| Age, yr | 46.0 ± 3.2 | 46.0 ± 4.7 |
| Height, cm | 177.3 ± 1.5 | 179.2 ± 2.4 |
| Weight, kg | 102.7 ± 4.5 | 97.1 ± 4.5 |
| BMI, kg/m2 | 32.6 ± 1.2 | 30.3 ± 0.9 |
| FEV1, % predicted | 92.2 ± 1.7* | 102.9 ± 3.5* |
| FVC, % predicted | 93.4 ± 3.1* | 106.6 ± 3.6* |
| AHI | 55.3 ± 6.4* | 5.2 ± 0.9* |
Values are means ± SE. Anthropometric characteristics of obstructive sleep apnea (OSA) patients (n = 7) and non-OSA controls (n = 8). Body mass index (BMI), percent predicted forced expiratory volume in the first second (FEV1), percent predicted forced vital capacity (FVC), and total sleep apnea hypopnea index (AHI).
Significant difference between groups (P < 0.05).
Consistent with case-matching between groups, there were no statistically significant differences in age, height, weight, or BMI between OSA patients and non-OSA controls (Table 1). However, FEV1 (P = 0.021) and FVC (P = 0.015) were significantly lower in the OSA compared with non-OSA group. There were no significant differences between groups, visits, or group by visit interaction effects in weight, mean arterial pressure or FRC (Table 2).
Table 2.
Measurements repeated at each visit in OSA patients and matched controls
| Baseline | 2 wk | 6 wk | |
|---|---|---|---|
| OSA | |||
| FRC, liter | 1.8 ± 0.1 (6) | 2.1 ± 0.2 (4) | 1.9 ± 0.2 (5) |
| Weight, kg | 102.7 ± 4.5 (7) | 103.3 ± 5.3 (7) | 105.2 ± 5.7 (6) |
| MAP, mmHg | 101.9 ± 5.6 (6) | 99.8 ± 3.4 (7) | 101.1 ± 4.0 (6) |
| Controls | |||
| FRC, liter | 2.2 ± 0.2 (8) | 2.2 ± 0.2 (8) | 2.3 ± 0.2 (8) |
| Weight, kg | 97.1 ± 4.5 (8) | 96.7 ± 4.5 (8) | 97.1 ± 4.5 (8) |
| MAP, mmHg | 94.3 ± 2.9 (8) | 94.1 ± 2.0 (8) | 97.8 ± 2.4 (8) |
Values are means ± SE in obstructive sleep apnea (OSA) patients and non-OSA controls at baseline, 2 wk, and 6 wk. Supine functional residual capacity (FRC), weight, and mean arterial pressure (MAP). The number of participants contributing to each measure is shown in parentheses.
Baseline ventilation.
There was no significant difference between groups and no difference from the first to the third visit in either group in VI, , VT, FB, or during 5 min of the room air baseline breathing period before commencement of the PRBS protocols (Table 3).
Table 3.
Room air baseline ventilatory measurements
| Baseline | 2 wk | 6 wk | |
|---|---|---|---|
| OSA | |||
| VI, l/min | 9.2 ± 0.4 | 9.3 ± 0.5 | 9.8 ± 0.9 |
| , mmHg | 40.8 ± 0.9 | 39.9 ± 1.0 | 41.7 ± 0.9 |
| VT, liter | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| FB, beats/min | 13.5 ± 1.2 | 14.6 ± 0.7 | 13.9 ± 4.6 |
| , % | 96.6 ± 0.5 | 96.5 ± 0.5 | 97.1 ± 0.4 |
| Controls | |||
| VI, l/min | 9.0 ± 0.2 | 9.0 ± 0.2 | 9.5 ± 0.5 |
| , mmHg | 39.4 ± 0.7 | 38.5 ± 0.5 | 39.1 ± 0.8 |
| VT, liter | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.0 |
| FB, beats/min | 13.9 ± 1.0 | 12.9 ± 1.0 | 13.9 ± 0.9 |
| , % | 95.9 ± 0.5 | 96.7 ± 0.2 | 96.9 ± 0.2 |
Values are expressed as means ± SE in OSA patients and non-OSA controls at baseline, 2 wk, and 6 wk. Minute ventilation (VI), end-tidal partial pressure CO2 (), tidal volume (VT), breathing frequency (FB), and oxyhemoglobin saturation (). Group, time, and group × time significance for all variables, P > 0.05.
CPAP data.
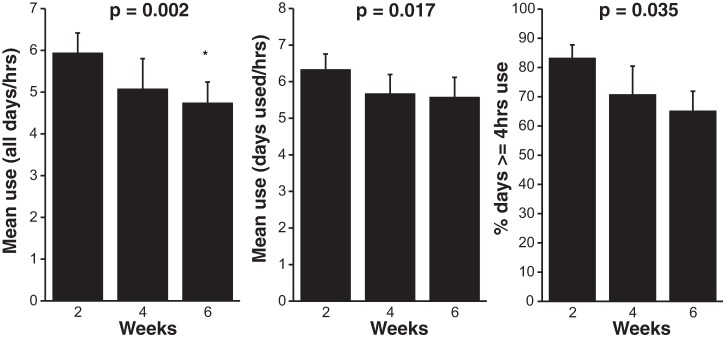
CPAP usage on all days, days used, and the percentage of days with ≥4 h use were consistent with normal clinical use, but significantly declined over the 6-wk period of study (Fig. 1). However, there was no significant effect of visit on the percentage of days used (84.0 ± 5.4%), time spent in large leak (5.5 ± 3.4 min), residual AHI (3.8 ± 0.6 events/h), or CPAP pressure (12.3 ± 1.1 cmH2O).
Fig. 1.
Continuous positive airway pressure (CPAP) usage across the 6 wk of treatment. Use all days (h/night) is expressed as means ± SE. CPAP use was measured in hours per night across all nights of the study, including 0 h on nights of no use. Use on days used (h/night) is means ± SE. CPAP use is expressed as hours per night only on nights when CPAP was used. % days with ≥4 h use is the mean ± SE percent of days with ≥4 h of use. P values indicate main effect of visits. *Significant post hoc difference versus 2 wk (P = 0.003). n = 6.
Loop gain.
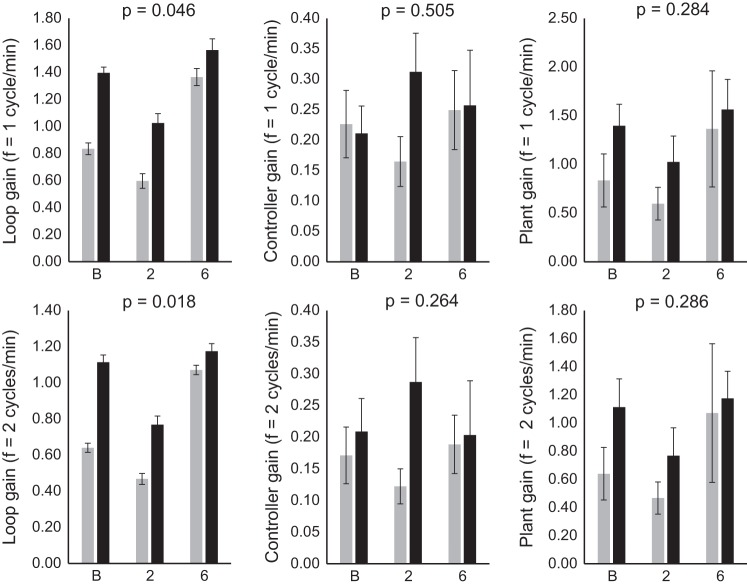
Loop gain was significantly higher in OSA patients at frequencies 1–7 cycles/min across all visits. The most physiologically relevant frequencies of 1 and 2 cycles/min (30- and 60-s intervals), which span the range most relevant to obstructive apnea and hypopnea cycles (typically 30–60 s), are presented (Fig. 2). Across all visits, LG at 1 cycle/min was 0.28 ± 0.04 in OSA patients versus 0.16 ± 0.04 in control participants, P = 0.046. LG at 2 cycles/min was 0.18 ± 0.03 in OSA patients versus 0.09 ± 0.02 in control participants; P = 0.018. However, there were no significant differences between groups in controller or plant gain and no treatment effect in any gain parameter.
Fig. 2.
Loop gain parameters in obstructive sleep apnea (OSA) and control participants at each visit. Loop gain, controller gain, and plant gain were calculated at one (top) and two (bottom) cycles/min measured at baseline (B), and after 2 and 6 wk of continuous positive airway pressure (CPAP) use in OSA patients (black) and in age- and body habitus-matched controls without CPAP treatment (gray). P values indicate main significance between groups. Values are expressed as means ± SE; n = 7 OSA; n = 8 non-OSA.
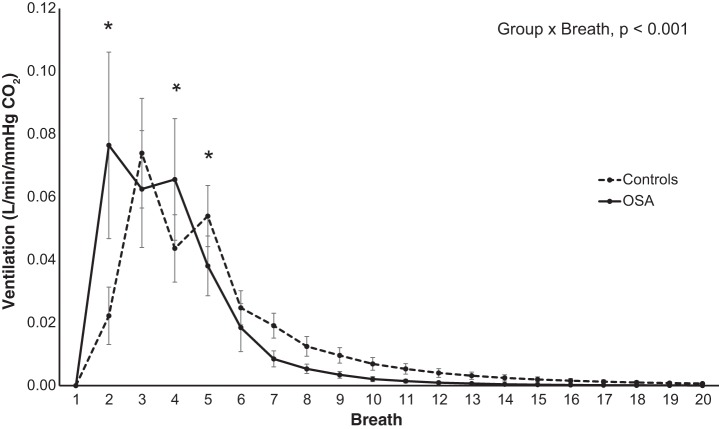
The estimated impulse response to a single breath of increased alveolar CO2 was significantly different between groups (P < 0.001; Fig. 3 shows all visits combined), with a faster and greater peak response in OSA patients compared with controls (0.077 ± 0.030 vs. 0.022 ± 0.009 l·min−1·mmHg−1 [] on the second breath, P < 0.001). The ventilatory response was also different between groups at breaths 4 and 5 (Fig. 3). However, there was no significant effect of CPAP treatment.
Fig. 3.
Estimated impulse responses to a sudden change in CO2. Estimated chemoreflex impulse response across visits over the first 20-breath period for obstructive sleep apnea (OSA) patients (solid) and matched controls (dashed). *Significant post hoc differences between groups (P < 0.05). Values are expressed as means ± SE; n = 7 OSA; n = 8 non-OSA.
Correlations.
There was no correlation between AHI and any loop gain, anthropomorphic, or spirometric measure.
DISCUSSION
The main findings of this study were a higher loop gain in male OSA patients compared with an age- and body habitus-matched control group of males, and an altered ventilatory response to normoxic CO2, consistent with less stable CO2 chemoreflex control in OSA patients. Contrary to previous reports (15, 24), this study found no evidence to support a change in chemoreflex control over 6 wk of CPAP use. There was also no significant difference between groups or treatment effect in controller gain, plant gain, or FRC, although these findings could potentially reflect type II error.
Induced and reversible high controller gain.
Compared with non-OSA controls, OSA patients have previously been shown to exhibit an elevated hypoxic ventilatory response (18), reduced eupnic , and increased CO2 sensitivity below eupnea (24), and an elevated ventilatory response to combined hypercapnic hypoxia (38), all of which normalize with CPAP treatment (15, 24, 27). Therefore, we expected to observe a higher controller gain in OSA (vs. matched controls) that would normalize following 6 wk of CPAP treatment. Although loop gain was higher in the OSA patients and the controller impulse response (ventilatory response to a sudden change in CO2) exhibited a larger and faster peak response compared with non-OSA control participants, our summary measures of controller gain were not different between groups. There were also no changes in either the controller impulse response or controller gain following 6 wk of CPAP treatment. Our findings that loop gain and the controller impulse response were different in OSA but did not change with CPAP treatment indicate that these differences are unlikely to be an acquired phenomenon (e.g., intermittent hypoxia-induced neuroplasticity). Indeed, in our study, there were no group differences in mean ventilation or at baseline (before PRBS) that are typically a harbinger of intermittent hypoxia-induced neuroplasticity (4, 9, 16, 19, 24).
There are several factors to consider when comparing our results to those of prior studies that found CPAP-related changes in chemoreflex control in OSA patients. Our study was performed during wakefulness and, therefore, abnormalities in chemoreflex control below eupnea and the position of eupnea itself, which have been observed during sleep in OSA patients, would likely be obscured by the presence of waking drive to breathe (24). Additionally, our study examined ventilatory responses to inspired CO2. Our findings are, therefore, similar to those by Spicuzza et al. (27), who observed no effect of CPAP treatment on steady-state hypercapnic responses in OSA; rather, differences were exclusive to the hypoxic ventilatory response. It is also possible that previous findings of reduced chemosensitivity with CPAP treatment may have been driven by hypoxic chemoreflex contributions. Loewen et al. (15) found a reduction in the dynamic ventilatory response to both CO2 in air and hypercapnic hypoxic gas mixtures post-CPAP. However, as the study was conducted in Calgary at an altitude of ~3,450 ft, the reduced ventilatory response to CO2 in air following CPAP treatment may be driven by a dampened hypoxic rather than hypercapnic response. Further research to explore the role of the hypoxic chemoreflex on loop gain in OSA is warranted.
Obesity, lung volume, and plant gain.
We are aware of only one other published study that has separately assessed plant and controller gain components in OSA versus non-OSA control participants. Although a similar CO2 PRBS test was used to evaluate chemoreflex control, Hudgel et al. (10) did not provide values of controller, plant, or their product loop gain. However, the authors found a greater peak and faster post-peak recovery in the closed-loop response (reflecting the combined controller and plant gain feedback behavior of the system), but no difference in the open-loop response (reflecting controller gain behavior without feedback effects) in obese OSA patients compared with normal-weight non-OSA controls (10). Although lung volume was not measured, given the absence of weight and BMI matching, the authors speculated that less stable control in the closed-loop response in OSA patients may have been secondary to reduced lung volume in obese patients versus lean controls (10). These findings are supported by Sands et al. (25), who compared loop gain between OSA patients and similar BMI non-OSA controls and found that loop gain was elevated in both obese OSA patients and obese non-OSA controls compared with lean non-OSA controls, but there was no difference between obese OSA patients and obese controls. These findings support a role of obesity-dependent reduced lung volume and increased plant gain as a mechanism increasing loop gain in OSA patients.
Unlike the findings of previous loop gain studies discussed above that suggest obesity is the main determinant of loop gain (10, 25), our findings of a higher loop gain in OSA patients versus matched non-OSA controls agree with other studies using more traditional chemoreflex tests (24, 38). Some of the discrepancies between studies could reflect studies in patients already treated with CPAP for several months (5, 25, 33), after which intermittent hypoxia-induced abnormalities in chemoreflex control may have normalized before assessment. We anticipated that case-control matching of morphologic traits expected to influence lung volume would minimize FRC and plant gain differences to allow direct comparison of controller gain and potential treatment effects between groups. However, while loop gain was elevated in the OSA patients and there was no significant difference between groups in FRC or plant gain, there was also no significant difference between groups in controller gain. Theoretically, for loop gain to be elevated, in the absence of conditions such as hypertension or heart disease with the potential to confound circulation time (14, 28), at least one of these factors must be elevated. Thus, the lack of between-group differences in controller gain, plant gain, and FRC likely reflects a type II error. Although the current findings support that for the same degree of obesity and waking, supine FRC loop gain is elevated in OSA patients versus non-OSA participants, it is not possible to determine the mechanisms causing elevated loop gain in OSA patients from the current findings. Further studies are needed in larger cohorts to clarify whether lung volume, plant gain, or controller gain effects dominate in OSA.
Lung function in OSA.
Reduced FEV1 and FVC in OSA patients did not appear to be explained by any systematic difference in smoking history. Inflammatory airway diseases characterized by airflow obstruction, such as asthma and chronic obstructive pulmonary disease, commonly coexist with OSA (26), so these findings could reflect common risk factors or differential body weight distribution lung volume effects. Alternatively, intermittent hypoxia-induced oxidative stress is known to exacerbate obstructive airway disease (17, 26) and potentially cause airway damage via inflammation (1, 29). Thus, our finding of reduced lung function could reflect intermittent hypoxia-induced airway damage in OSA. While there was no correlation between lung function measures and plant gain, it is possible that reduced lung function may alter loop gain in OSA patients.
Limitations.
While group differences were apparent, suggesting adequate power to detect similar magnitude changes over time within subjects, clearly Type II error cannot be ruled out to explain the lack of between-group differences in controller gain and/or plant gain and the lack of treatment effects on controller gain in the OSA group.
The CO2 PRBS method, like all currently available techniques to assess loop gain, may also have some limitations for detecting controller versus plant gain effects. Proportional assist ventilation and CPAP pressure drop techniques are not designed to separately measure controller and plant gain components of overall loop gain (25, 39). New methods using spontaneous respiratory disturbances during a diagnostic sleep study in OSA patients cannot be used in non-OSA controls (31). Thus, we elected to use PRBS using 4% CO2 during wakefulness as the only currently available method to allow controller gain, plant gain, and overall loop gain comparisons between OSA patients and non-OSA controls. However, as this method is conducted in normoxia, the contribution of increased hypoxic sensitivity to controller gain in OSA is almost certainly underestimated. As increased inspired CO2 decreases the efficacy of the lungs to excrete CO2, the PRBS method would be expected to decrease plant gain and somewhat underestimate loop gain. However, given the same sequence of breaths and concentration of CO2 were used in both groups at both time points, these effects should not have substantially influenced the main between-group and change over time comparisons in this study. Also, as PRBS is conducted during wakefulness and involves increasing rather than decreasing ventilatory drive, specific chemoreflex abnormalities limited to below eupnea would not have been captured. Future studies may benefit from new methods incorporating hypoxic chemoreflex assessment during sleep into loop gain modeling, to better evaluate sleep-dependent controller gain contributions to elevated loop gain in OSA patients.
Although CPAP use during the final fortnight of treatment remained above what would often be considered to be clinically acceptable compliance (≥4 h use on >65% of nights) (7), it is possible that treatment effects on ventilatory neuroplasticity require greater treatment compliance. However, CPAP usage was similar to that reported by Spicuzza et al. (27) (6 h/night), who found that hypoxic ventilatory responsiveness decreased following 1 mo of CPAP. Salloum et al. (24) also found 1 mo of CPAP reduced CO2 responsiveness below eupnea in a similarly sized group but did not report CPAP compliance. However, and more importantly, in the current study, controller gain was not elevated and, thus, abnormal in the OSA patients before treatment, such that CPAP would not be expected to have reduced an already normal controller gain.
Conclusion.
Our study found that male OSA patients have a greater loop gain than age-, weight-, height-, and BMI-matched male non-OSA controls, and that this greater loop gain is not reduced by CPAP treatment. The greater loop gain could not be attributed specifically to controller gain or plant gain, although interesting differences in the speed of the controller response were observed. OSA patients exhibited differences in spirometry, but these factors were not associated with increased plant gain. Our failure to illustrate (using daytime dynamic inspired CO2 administration) that controller gain is both augmented in OSA and reduced with treatment suggests that the elevated loop gain we detected in OSA patients was not due to intermittent hypoxia-induced neuroplasticity. Development of methods able to quantify the contribution of hypoxic chemoreflex control into loop gain measurements, and methods able to be conducted during sleep, will likely be necessary to adequately determine the role of induced versus inherent loop gain traits, and treatment effects, in OSA pathophysiology.
GRANTS
S. Sands was supported by the National Institute of Health Grant R01HL-102321. P. Catcheside and direct costs for this project were supported by Australian Research Council Future Fellowship (Grant FT120100510).
DISCLOSURES
Dr. Sands consults for Cambridge Sound Management, Nox Medical, and Merck. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.L.D., D.R.M., and P.G.C. conceived and designed research; N.L.D. performed experiments; N.L.D. analyzed data; N.L.D., S.A.S., D.R.M., and P.G.C. interpreted results of experiments; N.L.D. prepared figures; N.L.D. drafted manuscript; N.L.D., S.A.S., D.R.M., and P.G.C. edited and revised manuscript; N.L.D., S.A.S., D.R.M., and P.G.C. approved final version of manuscript.
REFERENCES
- 1.Aihara K, Oga T, Harada Y, Chihara Y, Handa T, Tanizawa K, Watanabe K, Tsuboi T, Hitomi T, Mishima M, Chin K. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Respir Med 105: 939–945, 2011. doi: 10.1016/j.rmed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Bokov P, Essalhi M, Delclaux C. Loop gain in severely obese women with obstructive sleep apnoea. Respir Physiol Neurobiol 221: 49–53, 2016. doi: 10.1016/j.resp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Brugniaux JV, Pialoux V, Foster GE, Duggan CT, Eliasziw M, Hanly PJ, Poulin MJ. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans. Eur Respir J 37: 880–887, 2011. doi: 10.1183/09031936.00156009. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 108: 369–377, 2010. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188: 996–1004, 2013. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 590: 1199–1211, 2012. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gay P, Weaver T, Loube D, Iber C; Positive Airway Pressure Task Force; Standards of Practice Committee; American Academy of Sleep Medicine . Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 29: 381–401, 2006. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 8.Gederi E, Nemati S, Edwards BA, Clifford GD, Malhotra A, Wellman A. Model-based estimation of loop gain using spontaneous breathing: a validation study. Respir Physiol Neurobiol 201: 84–92, 2014. doi: 10.1016/j.resp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med 158: 1142–1149, 1998. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 11.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol 122: 167–182, 2000. doi: 10.1016/S0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 12.Khoo MC, Yang F, Shin JJ, Westbrook PR. Estimation of dynamic chemoresponsiveness in wakefulness and non-rapid-eye-movement sleep. J Appl Physiol (1985) 78: 1052–1064, 1995. doi: 10.1152/jappl.1995.78.3.1052. [DOI] [PubMed] [Google Scholar]
- 13.Khoo MCK, editor. Physiological Control Systems: Anlaysis, Simulation, and Estimation. New York: The Institute of Electrical and Electronics Engineers Press, 2000. [Google Scholar]
- 14.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation 107: 727–732, 2003. doi: 10.1161/01.CIR.0000049641.11675.EE. [DOI] [PubMed] [Google Scholar]
- 15.Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, Younes M. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep 32: 1355–1365, 2009. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol (1985) 96: 1197–1205, 2004. doi: 10.1152/japplphysiol.00573.2003. [DOI] [PubMed] [Google Scholar]
- 17.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis 51: 392–399, 2009. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999. doi: 10.1161/01.CIR.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 19.Olson EB Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985) 91: 709–716, 2001. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- 20.Orr JE, Edwards BA, Malhotra A. CrossTalk opposing view: Loop gain is not a consequence of obstructive sleep apnoea. J Physiol 592: 2903–2905, 2014. doi: 10.1113/jphysiol.2014.271841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol (1985) 96: 1236–1242, 2004. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 22.Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol (1985) 94: 2342–2349, 2003. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- 23.Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 180: 1002–1009, 2009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 24.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181: 189–193, 2010. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, Wellman A. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med 190: 930–937, 2014. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaya FT, Lin PJ, Aljawadi MH, Scharf SM. Elevated economic burden in obstructive lung disease patients with concomitant sleep apnea syndrome. Sleep Breath 13: 317–323, 2009. doi: 10.1007/s11325-009-0266-2. [DOI] [PubMed] [Google Scholar]
- 27.Spicuzza L, Bernardi L, Balsamo R, Ciancio N, Polosa R, Di Maria G. Effect of treatment with nasal continuous positive airway pressure on ventilatory response to hypoxia and hypercapnia in patients with sleep apnea syndrome. Chest 130: 774–779, 2006. doi: 10.1378/chest.130.3.774. [DOI] [PubMed] [Google Scholar]
- 28.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, Tosi C, Carlisle C, Millman RP, Buxton A. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest 132: 433–439, 2007. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundar KM, Daly SE, Willis A. Is untreated obstructive sleep apnea (OSA) a cause of COPD in non-smokers? Am J Respir Crit Care Med 194: A3023, 2016. [Google Scholar]
- 30.Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol (1985) 114: 52–65, 2013. doi: 10.1152/japplphysiol.00985.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, Sands SA. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J 45: 408–418, 2015. 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol (1985) 104: 1625–1633, 2008. doi: 10.1152/japplphysiol.01273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 110: 1627–1637, 2011. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A, White DP. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 114: 911–922, 2013. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 162: 144–151, 2008. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokhana SS, Gerst DG III, Lee DS, Badr MS, Qureshi T, Mateika JH. Impact of repeated daily exposure to intermittent hypoxia and mild sustained hypercapnia on apnea severity. J Appl Physiol (1985) 112: 367–377, 2012. doi: 10.1152/japplphysiol.00702.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Younes M. CrossTalk proposal: elevated loop gain is a consequence of obstructive sleep apnoea. J Physiol 592: 2899–2901, 2014. doi: 10.1113/jphysiol.2014.271833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol (1985) 103: 1929–1941, 2007. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 39.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 163: 1181–1190, 2001. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 40.Zerah-Lancner F, Lofaso F, Coste A, Ricolfi F, Goldenberg F, Harf A. Pulmonary function in obese snorers with or without sleep apnea syndrome. Am J Respir Crit Care Med 156: 522–527, 1997. doi: 10.1164/ajrccm.156.2.9609015. [DOI] [PubMed] [Google Scholar]