Abstract
Habitual aerobic exercise enhances physiological function and reduces risk of morbidity and mortality throughout life, but the underlying molecular mechanisms are largely unknown. The circulating proteome reflects the intricate network of physiological processes maintaining homeostasis and may provide insight into the molecular transducers of the health benefits of physical activity. In this exploratory study, we assessed the plasma proteome (SOMAscan proteomic assay; 1,129 proteins) of healthy sedentary or aerobic exercise-trained young women and young and older men (n = 47). Using weighted correlation network analysis to identify clusters of highly co-expressed proteins, we characterized 10 distinct plasma proteomic modules (patterns). In healthy young (24 ± 1 yr) men and women, 4 modules were associated with aerobic exercise status and 1 with participant sex. In healthy young and older (64 ± 2 yr) men, 5 modules differed with age, but 2 of these were partially preserved at young adult levels in older men who exercised; among all men, 4 modules were associated with exercise status, including 3 of the 4 identified in young adults. Exercise-linked proteomic patterns were related to pathways involved in wound healing, regulation of apoptosis, glucose-insulin and cellular stress signaling, and inflammation/immune responses. Importantly, several of the exercise-related modules were associated with physiological and clinical indicators of healthspan, including diastolic blood pressure, insulin resistance, maximal aerobic capacity, and vascular endothelial function. Overall, these findings provide initial insight into circulating proteomic patterns modulated by habitual aerobic exercise in healthy young and older adults, the biological processes involved, and their relation to indicators of healthspan.
NEW & NOTEWORTHY This is the first study to assess the relation between plasma proteomic patterns and aerobic exercise status in healthy adults. Weighted correlation network analysis identified 10 distinct proteomic modules, including 5 patterns specific for exercise status. Additionally, 5 modules differed with aging in men, two of which were preserved in older exercising men. Exercise-associated modules included proteins related to inflammation, stress pathways, and immune function and correlated with clinical and physiological indicators of healthspan.
Keywords: blood pressure, inflammation, SomaLogic, weighted correlation network analysis
INTRODUCTION
Habitual aerobic exercise is associated with a myriad of health benefits, including improved physiological function and reduced risk of chronic disease and disability; however, the molecular mechanisms that produce these favorable effects of exercise remain uncertain (22, 46, 53). Recently, a charge has been made to identify the “molecular transducers” of the health benefits of physical activity in humans (46). It is hoped that such efforts may uncover novel therapeutic targets for the prevention and treatment of chronic clinical disorders (46, 53). Among healthy adults, regular exercise exerts perhaps its strongest influence in promoting optimal physiological aging and extending healthspan (10, 58), but insights into the molecular underpinnings of these effects in humans are only beginning to emerge (51).
Interrogating networks of molecules with the use of high-throughput analyses may provide important insight into the biological mechanisms influencing physiological function and disease risk (22). Broad-based analysis of protein signatures (proteomics) is a promising approach for identifying potential molecular transducers of the health benefits of exercise, in part because of the deterministic influence of proteins on physiological function (45). Network analysis identifies groups of associated proteins that may have physiological relevance and allows for more sensitive detection of proteomic changes compared with individual proteins while also greatly reducing multiple testing burden (3, 67). Moreover, because exercise produces a strong systemic physiological stimulus, the blood, i.e., the tissue with the greatest direct contact with all of the cells and organs of the body, may yield unique molecular footprints associated with the biological effects of physical activity (46, 53). That blood and its fluid component, plasma, are accessible with minimally invasive procedures in humans is another advantage of this approach in that any putative biomarkers identified could be assessed in larger clinical and epidemiological research settings.
Some information is available on the effects of acute exercise and shorter-term exercise training on the circulating proteome in humans (54) and regarding the influence of habitual exercise on proteins in other tissues, such as skeletal muscle (57) or urine (56). However, many of the health benefits of exercise are likely induced via long-term adaptations to chronic physical training. To our knowledge, the influence of chronic aerobic exercise on the circulating proteome has not been investigated in healthy young adults or with aging. Similarly, we lack any information as to the relation between exercise-associated effects on the circulating proteome and clinical and/or physiological indicators of healthspan.
The goal of this exploratory analysis was to gain initial insight into the association of habitual aerobic exercise with circulating proteomic signatures in healthy adults, including the impact of exercise on age-related proteomic patterns. A secondary aim was to identify relations between exercise-associated proteomic markers and indicators of healthspan. To accomplish this, we assessed the plasma proteome of groups of healthy sedentary and aerobic exercise-trained young men and women and middle-aged/older men using the SOMAscan multiplex assay, a targeted proteomics platform that measured abundance of 1,129 proteins in a highly sensitive manner. We then employed weighted correlation network analysis to identify modules (patterns) of highly correlated proteins and associated them with exercise status, sex, and age. Finally, we assessed proteomic modules associated with exercise for biological pathways of interest and related the modules to multiple indicators of healthspan.
Our analysis revealed 10 proteomic modules, 9 of which were associated with exercise status, biological sex, and/or age. The modules associated with exercise featured altered proteomic patterns related to inflammation, regulation of apoptosis, and stress and immune responses. These “exercise modules” correlated with several physiological and clinical indicators of healthspan, including diastolic blood pressure, insulin resistance, maximal aerobic capacity (V̇o2max), and vascular endothelial function. These findings provide initial insight into circulating proteomic patterns modulated by habitual aerobic exercise in healthy young and older adults, the physiological pathways involved, and the relation between proteomic patterns and indicators of healthspan.
MATERIALS AND METHODS
Participants
Participant data and plasma samples from our laboratory’s database were used. A total of 47 healthy men and women from Boulder County, Colorado, and the surrounding area were studied. Specifically, 31 young (aged 19–32 yr) inactive (INAC, n = 16) and aerobic exercise-trained (AEX, n = 15) men and women were included to initially assess the effects of habitual aerobic exercise on the circulating proteome of healthy young adults. Sixteen additional healthy older (aged 55–77 yr) inactive (O-INAC, n = 8) and aerobic exercise-trained (O-AEX, n = 8) men were included to assess the effects of habitual aerobic exercise on age-related changes in the circulating proteome compared with young inactive (Y-INAC, n = 8) and aerobic exercise-trained (Y-AEX, n = 8) men from the initial analysis. Because of the limit of <50 total samples eligible for the SOMAscan analysis and the exploratory nature of this study, older women were not included in the aging analysis, and this important population will need to be addressed in future studies.
Inclusion criteria consisted of lack of medication use; non-smoking; body mass index <40 kg/m2; fasted plasma glucose <126 mg/dl; and absence of chronic diseases (including peripheral artery disease: ankle brachial index >0.9) as determined by medical history, physical examination, blood chemistries, and blood pressure and electrocardiogram at rest and during an incremental treadmill exercise test. Premenopausal women were not pregnant, as determined by a pregnancy test, and were not on birth control. Participants were characterized as inactive if they had performed <2 days of exercise/wk for at least the preceding 2 yr and as aerobically exercise trained if they performed vigorous aerobic exercise ≥5 days/wk for 45 min/session for at least the preceding 5 yr. Participants included were of non-Hispanic Caucasian (n = 42), non-Hispanic Asian (n = 4), or Hispanic Caucasian (n = 1) ethnicity and race. All procedures were reviewed and approved by the Institutional Review Board at the University of Colorado Boulder. The nature, risks, and benefits of all study procedures were explained to volunteers, and their written informed consent was obtained before participation.
Measurements
All measurements were made after an overnight >12-h fast from food and caffeine (water allowed) and >24-h refrainment from physical activity and alcohol. All testing on premenopausal women was performed during the early follicular phase of their menstrual cycle to reduce variations in measurements affected by circulating hormones.
Participant Characteristics
Body mass, body mass index, and waist-to-hip ratio were determined with anthropometry (38). Body percent fat was assessed by dual-energy X-ray absorptiometry (GE Lunar Prodigy Advance). Fasting plasma glucose and insulin were determined by reflective spectrophotometry (Ortho Clinical Diagnostics) and radioimmunoassay (Millipore), respectively. Fasting serum blood lipids were measured by standard assays. Plasma oxidized low-density lipoprotein was assessed by ELISA (Mercodia) and serum high-sensitivity C-reactive protein by immunoturbidimetry (Beckman Coulter). Leisure time physical activity was assessed by the Modifiable Activity Questionnaire (47).
Select Healthspan Indicators
The following well-established healthspan indicators were chosen because they reflect functional status while also predicting future risk of clinical disease and mortality (2, 30, 36, 66). Resting systolic and diastolic blood pressures were measured in triplicate over the brachial artery with a semiautomated device (Dinamap XL, Johnson & Johnson). Insulin resistance was estimated with the homeostasis model of insulin resistance (HOMA-IR) formula: [fasting plasma glucose (mg/dl) × fasting plasma insulin (μU/ml)]/405 (41). V̇o2max was determined during an incremental treadmill exercise test (Balke protocol) with open-circuit spirometry (15). Endothelial function was measured by brachial artery flow-mediated dilation using high-resolution ultrasonography (Power Vision 6000) as described in detail previously (9, 14, 21). In this procedure, the brachial artery diameter change was measured after a 5-min forearm cuff occlusion distal to the probe. Flow-mediated dilation is reported as percent change from baseline diameter.
Proteomics
Plasma samples were sent to SomaLogic Inc. for analysis of circulating proteins with the SOMAscan v3 assay (SomaLogic Inc., Boulder, CO) as previously described (19). Briefly, the SOMAscan v3 assay is a well-established platform composed of single-stranded DNA aptamers with chemically modified side chains (SOMAmers) that have been selected to target specific proteins (1,129 available). Resulting SOMAmer concentration is proportional to the concentration of the corresponding protein in the sample, and the dynamic range of the assay spans over 8 logs (femtomolar to micromolar). The SOMAmer signal is quantified with DNA microarray and expressed as relative fluorescence units. Samples were processed under Good Laboratory Practice (60) for intra-run normalization and calibration and passed all quality control requirements.
Weighted Correlation Network Analysis and other Statistical Analysis
Statistical analysis was performed with R version 3.3.2. Outliers were identified as protein values ≥ 3 standard deviations from the mean and were removed. Protein values from all groups underwent a weighted (gene) correlation network analysis (WGCNA) using the WGCNA R software package to group similarly expressed proteins into modules, which has the additional benefit of reducing multiple testing burden (33). Briefly, the WGCNA package constructs an adjacency matrix based on pairwise correlations raised to a soft-threshold power, resulting in a network that is then analyzed for tightly interconnected groups of proteins referred to as modules (33). Modules are assigned an arbitrary color for identification (e.g., yellow module) and are characterized by a module “eigenprotein” (the first principal component of protein expressions in that module). Each participant’s individual protein levels then contribute to their module score, a weighted average of their expression levels for proteins within that module (33). A soft-threshold power of 7 was used, along with a minimum module size of 5 proteins. Module robustness was investigated by establishing that average module adjacency was greater than that expected by chance. Specifically, the average adjacency of each module was compared with the mean average adjacency of 1,000 random permutations of the data (all modules P < 0.01 correcting for multiple testing) (34).
Protein lists for each module were analyzed for associated Gene Ontology (GO) biological processes using the Cytoscape plug-in ClueGO, providing an interpretation of the general pathways implicated by the proteomic content of each module (4). Because of the exploratory nature of this study, significance for all subsequent analyses was set at an uncorrected α < 0.05. However, with consideration of multiple testing burden, we comment if results (factorial ANOVAs for each WGCNA-generated module, and correlations between modules and healthspan indicators) survive a Benjamini-Hochhberg correction to a false discovery rate of 0.05.
Raw individual protein levels and participant data can be found online in the Supplemental Data File (Supplemental Material for this article is available online at the Journal website). Outlier data points removed from analyses are bolded.
RESULTS
Ten modules were generated with the following corresponding number of proteins assigned to each module: black 19, blue 37, brown 36, green 24, magenta 16, pink 16, purple 14, red 21, turquoise 579, and yellow 36. Remaining uncorrelated proteins were not used for further analysis.
Habitual Aerobic Exercise and Circulating Proteomic Patterns in Young Adults
Participant characteristics and healthspan indicators.
All young participant characteristics were within normal healthy ranges (Table 1). As expected, young AEX adults had lower body fat indices (body mass, body mass index, body fat percent) and circulating triglycerides compared with the young INAC individuals (all P < 0.05). Additionally, young AEX subjects had higher activity levels (leisure time physical activity) and aerobic exercise capacity (V̇o2max; both P < 0.0001) compared with their inactive counterparts.
Table 1.
Young participant characteristics and healthspan indicators
| INAC | AEX | |
|---|---|---|
| Participant characteristics | ||
| n, men/women | 8/8 | 8/7 |
| Age, yrL | 23 ± 1 | 24 ± 1 |
| Body mass, kg | 81 ± 4 | 67 ± 3* |
| Body mass index, kg/m2 L | 27 ± 1 | 22 ± 1* |
| Body fat, % | 33 ± 2 | 17 ± 2* |
| Waist-to-hip ratio, U | 0.81 ± 0.02 | 0.77 ± 0.01 |
| Fasting glucose, mg/dl | 88 ± 2 | 88 ± 2 |
| Fasting insulin, µU/mlL | 10 ± 2 | 6 ± 1 |
| Total cholesterol, mg/dl | 166 ± 8 | 160 ± 8 |
| High-density lipoprotein, mg/dl | 49 ± 3 | 56 ± 2 |
| Low-density lipoprotein, mg/dl | 94 ± 7 | 89 ± 7 |
| Triglycerides, mg/dl | 110 ± 9 | 73 ± 5* |
| C-reactive protein, mg/lL | 0.6 ± 0.1 | 0.6 ± 0.2 |
| Oxidized low density lipoprotein, U/l | 36 ± 3 | 35 ± 4 |
| LTPA, MET-hr/wkL | 29 ± 6 | 136 ± 17* |
| Select indicators of healthspan | ||
| Systolic blood pressure, mmHg | 110 ± 3 | 116 ± 3 |
| Diastolic blood pressure, mmHg | 64 ± 2 | 59 ± 2 |
| HOMA-IR, UL | 2.1 ± 0.4 | 1.4 ± 0.2 |
| V̇o2max, ml·kg−1·min−1 | 36 ± 2 | 54 ± 2* |
| Endothelial function, FMD %Δ | 8.1 ± 0.6 | 7.5 ± 0.6 |
Data are means ± SE. Δ, change; AEX, aerobic exercise trained; FMD, flow-mediated dilation; HOMA-IR, homeostasis model assessment-insulin resistance; INAC, inactive; LTPA, leisure time physical activity; MET, metabolic equivalent; V̇o2max, maximal aerobic capacity.
Data log transformed for statistical analysis;
P < 0.05 vs. INAC.
Influence of aerobic exercise training status on circulating proteomic patterns.
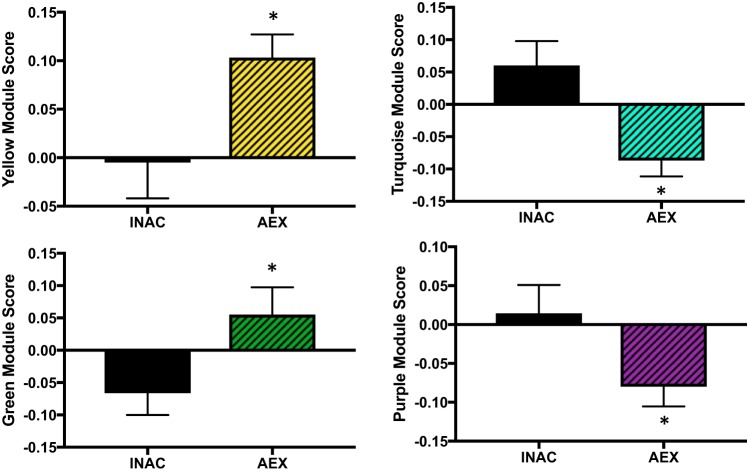
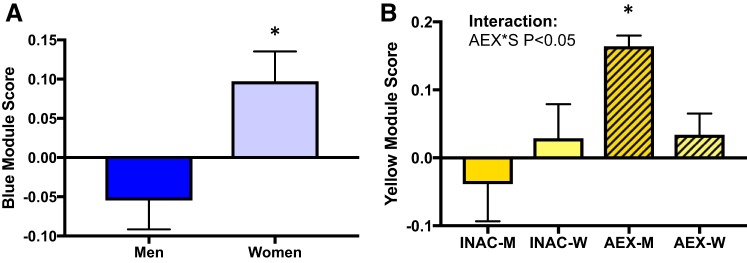
We sought to determine if aerobic exercise status influenced circulating proteomic patterns (modules) in young adults. Sex has been shown to influence the plasma proteome (29, 59), and INAC versus AEX groups showed differences in body fat percent. Taking this into consideration, the effect of habitual aerobic exercise status was assessed with a factorial ANOVA (sex: men vs. women; exercise status: inactive vs. exercise trained, controlling for body fat percent as a covariate). Of the 10 modules generated by WGCNA, 4 modules were different between the young INAC and AEX men and women (yellow, turquoise, green, and purple modules; P = 0.008, P = 0.004, P = 0.03, and P = 0.04, respectively; yellow and turquoise strictly significant; Fig. 1). Although not the primary focus of this study, one module was different between men and women (blue module; P = 0.007), and the yellow module had an exercise-sex interaction (P = 0.047; Fig. 2). Young AEX men had a higher yellow module score compared with all other groups (all P < 0.05) suggesting that exercise status has a different effect on the yellow module’s circulating proteome pattern in men compared with women.
Fig. 1.
Modules different with exercise status in young inactive (INAC) and aerobic exercise-trained (AEX) men and women. Data are means ± SE; *P < 0.05.
Fig. 2.
Modules associated with sex (A) or exercise-sex interaction (B) in young inactive (INAC) and aerobic exercise-trained (AEX) men (M) and women (W). Data are means ± SE; AEX*S, aerobic exercise-trained status by sex; *P < 0.05.
Habitual Aerobic Exercise and Circulating Proteomic Patterns in Young and Older Men
Participant characteristics and healthspan indicators.
All male participant characteristics were within normal ranges and are reported in Table 2. As expected, the exercising men had lower body mass indices and higher physical activity and aerobic exercise capacity compared with their respective age-matched inactive peers (P < 0.05). The O-INAC group had higher (P < 0.05) LDL-cholesterol or tended to have higher total cholesterol than the other 3 groups.
Table 2.
Male participant characteristics and healthspan indicators: exercise-aging analysis
| Y-INAC | Y-AEX | O-INAC | O-AEX | |
|---|---|---|---|---|
| Participant characteristics | ||||
| n | 8 | 8 | 8 | 8 |
| Age, yrL | 23 ± 1 | 24 ± 2 | 66 ± 2†# | 63 ± 2†# |
| Body mass, kg | 88 ± 6 | 75 ± 2 | 84 ± 6 | 70 ± 2† |
| Body mass index, kg/m2L | 27 ± 2 | 23 ± 1 | 26 ± 1 | 23 ± 1 |
| Body fat, % | 26 ± 2 | 12 ± 2†‡ | 27 ± 4#¶ | 16 ± 1 |
| Waist-to-hip ratio, U | 0.86 ± 0.03 | 0.81 ± 0.01 | 0.94 ± 0.03# | 0.86 ± 0.01 |
| Fasting glucose, mg/dl | 88 ± 3 | 90 ± 2 | 87 ± 3 | 93 ± 5 |
| Fasting insulin, µU/mlL | 11 ± 2 | 6 ± 1 | 6 ± 1 | 4 ± 0.5† |
| Total cholesterol, mg/dl | 160 ± 10 | 159 ± 15 | 202 ± 8†# | 173 ± 8 |
| High-density lipoprotein, mg/dl | 44 ± 3 | 53 ± 3 | 50 ± 3 | 63 ± 5† |
| Low-density lipoprotein, mg/dl | 92 ± 9 | 92 ± 12 | 135 ± 6* | 94 ± 5 |
| Triglycerides, mg/dl | 118 ± 15 | 69 ± 8† | 82 ± 12 | 83 ± 7 |
| C-reactive protein, mg/lL | 0.3 ± 0.05 | 0.8 ± 0.3 | 1.6 ± 0.9 | 0.5 ± 0.1 |
| Oxidized low density lipoprotein, U/l | 36 ± 5 | 33 ± 6 | 54 ± 4 | 47 ± 6 |
| LTPA, MET-hr/wkL | 31 ± 9 | 133 ± 24†‡ | 28 ± 13 | 78 ± 12†‡ |
| Select indicators of healthspan | ||||
| Systolic blood pressure, mmHg | 115 ± 4 | 123 ± 4 | 127 ± 3 | 118 ± 4 |
| Diastolic blood pressure, mmHg | 66 ± 3 | 60 ± 3‡¶ | 76 ± 2 | 73 ± 3 |
| HOMA-IR, UL | 2.5 ± 0.6 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.9 ± 0.1† |
| V̇o2max, ml·kg−1·min−1 | 41 ± 2* | 59 ± 1* | 27 ± 1* | 50 ± 1* |
| Endothelial function, FMD %Δ | 7.7 ± 0.9 | 8.0 ± 0.8 | 4.3 ± 0.7 | 7.0 ± 1.4 |
Data are means ± SE. Δ, change; FMD, flow-mediated dilation; HOMA-IR, homeostasis model assessment-insulin resistance; LTPA, leisure time physical activity; O-AEX, older aerobic exercise trained; O-INAC, older inactive; V̇o2max, maximal aerobic capacity; Y-AEX, young aerobic exercise trained; Y-INAC, young inactive.
Data log transformed for statistical analysis;
P < 0.05 vs. Y-INAC;
P < 0.05 vs. Y-AEX;
P < 0.05 vs. O-INAC;
P < 0.05 vs. O-AEX;
P < 0.05 vs. all groups.
Influence of aging and aerobic exercise status on circulating proteomic patterns.
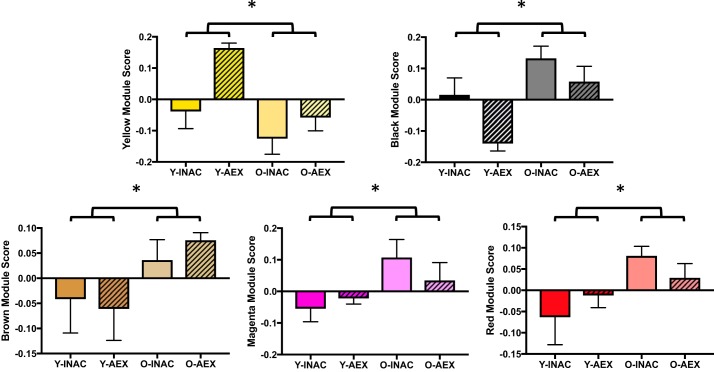
To determine if aging and aerobic exercise training status influenced circulating proteomic patterns, a factorial ANOVA was performed in men (age: young vs. older; exercise: inactive vs. trained, controlling for body fat percent as a covariate). Of the 10 modules generated by WGCNA, 5 modules were different between young and older men (yellow, black, brown, magenta, and red modules; P = 0.001, P = 0.001, P = 0.03, P = 0.03, and P = 0.04, respectively; yellow and black strictly significant; Fig. 3). Four modules were different with training status between the inactive and trained men (purple, yellow, black, and turquoise modules; P = 0.00008, P = 0.003, P = 0.01, and P = 0.03, respectively; purple, yellow, and black strictly significant; Fig. 4). Of note, the purple, turquoise, and yellow modules were also identified as influenced by exercise status in the cohort of young men and women.
Fig. 3.
Modules influenced by age in young and older men. Data are means ± SE; *P < 0.05: young vs. older men. O-AEX, older aerobic exercise-trained men; O-INAC, older inactive men; Y-AEX, young aerobic exercise-trained men; Y-INAC, young inactive men.
Fig. 4.
Modules different with exercise status in men. Data are means ± SE; *P < 0.05: inactive vs. exercise-trained men. O-AEX, older aerobic exercise-trained men; O-INAC, older inactive men; Y-AEX, young aerobic exercise-trained men; Y-INAC, young inactive men.
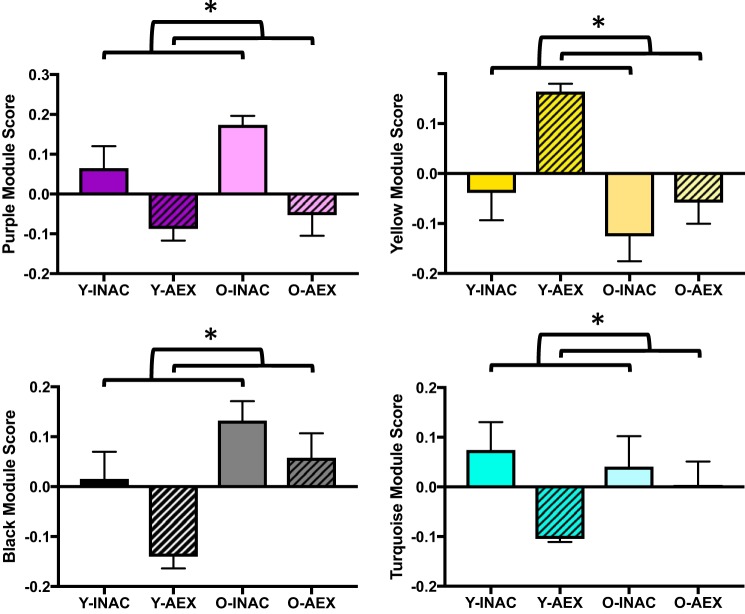
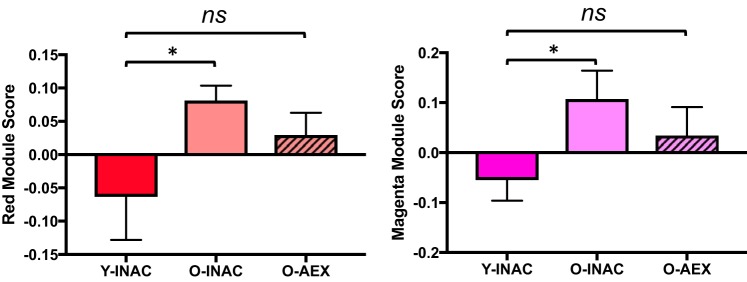
To determine if habitual aerobic exercise influences circulating protein patterns that are associated with aging, we sought to identify modules in O-AEX men that were preserved at or toward levels of young men. A multiple linear regression was used to identify modules different with age (O-INAC vs. Y-INAC, P < 0.05) but not different in O-AEX men compared with Y-INAC men (i.e., young control group). This analysis yielded 2 age-associated modules (red and magenta) that were significantly different between the Y-INAC versus O-INAC men (both P < 0.05) but not between the Y-INA versus O-AEX men (both P = 0.2) (Fig. 5).
Fig. 5.
Modules associated with age (Y-INAC vs. O-INAC) and partially preserved in older exercise-trained (O-AEX) men. Data are means ± SE; *P < 0.05. ns, not significant; O-INAC, older inactive men; Y-INAC, young inactive men.
Biological Processes Indicated in Protein Patterns
Proteins within each module were analyzed for associated GO biological processes terms using ClueGO to provide insight into the general pathways represented (1, 4, 18). Additionally, individual proteins were ranked according to their intramodular connectivity score (kME) within their respective module. Proteins with a kME closer to 1 indicate higher contribution to the module compared with proteins with a kME closer to 0. Individual proteins with a kME >0.8 up to the first 10 proteins for each module are reported, along with group means of interest, in each respective table as indicated below. The Universal Protein Resource (UniProt), a consortium for protein sequence and functional information, was used to report known individual functions of these highest contributing proteins.
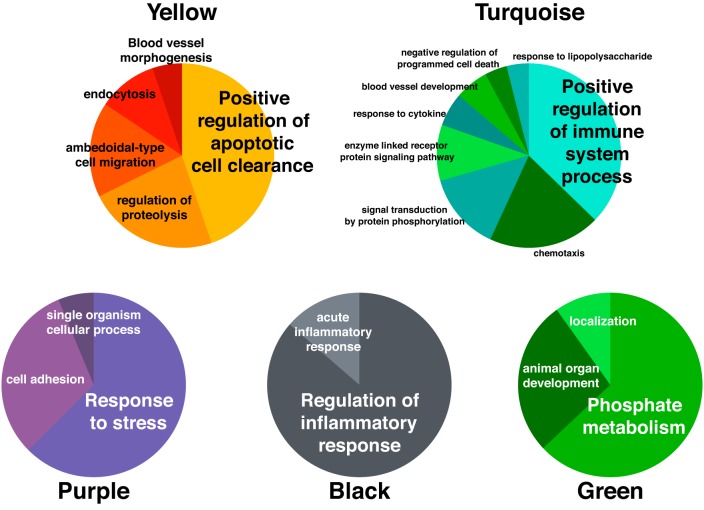
Habitual aerobic exercise influenced modules.
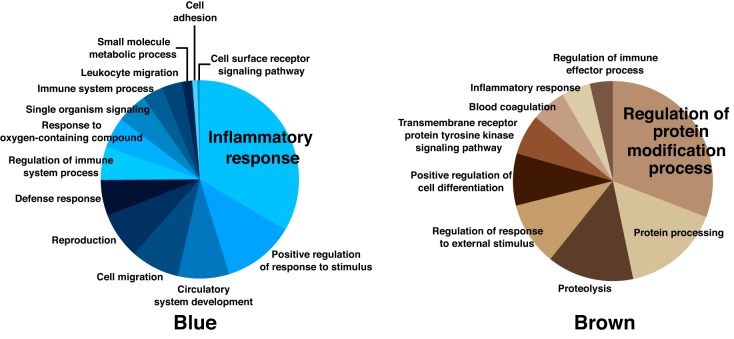
Processes in modules influenced by exercise training status (yellow, turquoise, purple, black, and green modules) are reported in Fig. 6 and include pathways primarily involved in apoptosis, immune system function, response to stress, inflammation, and phosphate metabolism, respectively. The highest ranked individual proteins within these modules are reported in Table 3. The highest contributing proteins in each exercise-related module were α-2-macroglobulin (yellow module), Ras-related C3 botulinum toxin substrate 1 (Rac1; turquoise module), fibronectin (purple module), apolipoprotein E (black module), and dual specificity mitogen-activated protein kinase 3 (green module). Although not part of the primary focus of this study, GO analysis was performed for the blue module (different with sex status), which primarily represented processes related to the inflammatory response (Fig. 7); the top ranked proteins for the blue module can be found in Table 4.
Fig. 6.
GO biological processes implicated by proteins in modules associated with habitual aerobic exercise status. GO, Gene Ontology.
Table 3.
Modules different with aerobic exercise
| Module | Protein | UniProt ID | Primary Function | Protein Level, RFU |
|
|---|---|---|---|---|---|
| INAC | AEX | ||||
| Yellow | Alpha-2-macroglobulin | P01023 | Inhibits proteinases | 20,334 ± 4,513 | 36,067 ± 4,180 |
| Complement C3b, inactivated | P01024 | Activation of immune response; triglyceride synthesis and glucose transport | 10,599 ± 2,403 | 19,385 ± 1,928 | |
| Apolipoprotein B | P04114 | Protein constituent of chylomicrons, LDL, and VLDL | 45,566 ± 11,332 | 70,167 ± 9,376 | |
| Complement 3a | P01024 | Activation of immune response; triglyceride synthesis and glucose transport | 1,562 ± 279 | 2,112 ± 163 | |
| Complement C4b | P0C0L4 | Activation of immune response | 1,071 ± 274 | 1,754 ± 240 | |
| Proprotein convertase subtilisin/kexin type 7 | Q16549 | Peptidase activity | 1,223 ± 123 | 937 ± 119 | |
| Sonic hedgehog protein | Q15465 | Development; biosynthesis, growth, and differentiation | 543 ± 58 | 329 ± 41 | |
| Insulin-like growth factor-binding protein 4 | P22692 | Cell proliferation and metabolism | 6,807 ± 374 | 6,027 ± 327 | |
| Complement 3 | P01024 | Activation of immune response; triglyceride synthesis and glucose transport | 86,286 ± 5,895 | 100,039 ± 6,183 | |
| Protein 4.1 | P11171 | Regulating membrane stability | 4,679 ± 790 | 5,376 ± 460 | |
| Turquoise | Ras-related C3 botulinum toxin substrate 1 | P63000 | GTPase | 12,974 ± 2,321 | 7,162 ± 1,529 |
| Ribosome maturation protein SBDS | Q9Y3A5 | Ribosome assembly; cellular stress resistance | 7,723 ± 1,691 | 3,286 ± 1,133 | |
| Tyrosine-protein kinase CSK | P41240 | Cell growth, differentiation, migration, and immune response | 7,214 ± 1,686 | 2,827 ± 1,006 | |
| Methionine aminopeptidase 2 | P50579 | Protein synthesis | 19,556 ± 4,087 | 7,160 ± 2,936 | |
| Triosephosphate isomerase | P60174 | Oxidoreductase activity | 8,776 ± 1,236 | 5,558 ± 833 | |
| Translationally-controlled tumor protein | P13693 | Calcium binding and microtubule stabilization | 9,238 ± 1,708 | 5,150 ± 1,120 | |
| Glycogen synthase kinase-3 alpha/beta | P49840 | Negative regulator in glucose homeostasis | 10,600 ± 2,186 | 4,759 ± 1,409 | |
| Elongation factor 1-beta | P24534 | RNA binding | 1,367 ± 195 | 870 ± 131 | |
| Cytokine receptor common subunit gamma | P31785 | Interleukin receptor | 2,749 ± 399 | 1,760 ± 282 | |
| Aflatoxin B1 aldehyde reductase member 2 | O43488 | Electron carrier activity | 2,457 ± 561 | 878 ± 335 | |
| Purple | Fibronectin | P02751 | Inhibits tumor growth, angiogenesis, and metastasis | 14,863 ± 1,556 | 8,084 ± 1,082 |
| D-dimer | P02671 | Wound repair and blood coagulation | 8,901 ± 415 | 7,464 ± 423 | |
| Fibronectin 1.3 | P02751 | Inhibits tumor growth, angiogenesis, and metastasis | 1,084 ± 95 | 670 ± 69 | |
| Fibrinogen gamma chain | P02679 | Blood coagulation | 42,871 ± 1,903 | 35,581 ± 1,727 | |
| Fibrinogen | P02671 | Blood coagulation | 107,145 ± 3,654 | 95,775 ± 2,966 | |
| Fibronectin 1.4 | P02751 | Inhibits tumor growth, angiogenesis, and metastasis | 40,453 ± 2,990 | 29,988 ± 1,850 | |
| Black | Apolipoprotein E (isoform E4) | P02649 | Binding, internalization, and catabolism of lipoprotein particles | 44,233 ± 3,775 | 34,336 ± 3,351 |
| Apolipoprotein E (isoform E3) | 58,933 ± 5,220 | 49,640 ± 4,388 | |||
| Apolipoprotein E | 4,576 ± 502 | 3,628 ± 429 | |||
| Apolipoprotein E (isoform E2) | 149,140 ± 10,250 | 130,004 ± 7,750 | |||
| Complement C4 | P0C0L4 | Activation of immune response | 80,951 ± 7,475 | 72,075 ± 5,994 | |
| Green | Dual specificity mitogen-activated protein kinase 3 | P46734 | Activates immune response and metabolic pathways in response to cytokines and environmental stress | 2,348 ± 86 | 2,623 ± 95 |
| Serine/threonine-protein kinase MRCK beta | Q9Y5S2 | Regulates cytoskeletal reorganization | 1,299 ± 46 | 1,410 ± 48 | |
| Interleukin-5 | P05113 | Immune, differentiation, and signaling activity | 2,889 ± 122 | 3,359 ± 142 | |
| Breast cancer anti-estrogen resistance protein 3 | O75815 | Regulates metabolic activity, signaling, development | 1,083 ± 29 | 1,214 ± 37 | |
| UMP-CMP kinase | P30085 | Pyrimidine nucleotide biosynthesis | 4,040 ± 162 | 4,473 ± 183 | |
| Macrophage scavenger receptor types I and II | P21757 | Macromolecule endocytosis; pro-atherosclerotic | 922 ± 34 | 1,016 ± 43 | |
| Serine protease 27 | Q9BQR3 | Peptidase activity | 6,689 ± 371 | 8,943 ± 588 | |
| Cadherin-15 | P55291 | Cell adhesion and differentiation | 1,933 ± 60 | 2,013 ± 59 | |
Data are means ± SE. AEX, aerobic exercise trained; INAC, inactive; RFU, relative fluorescence units.
Fig. 7.
GO biological processes implicated by proteins in modules associated with sex (blue) or age (brown). GO, Gene Ontology.
Table 4.
Modules associated with only sex or aging
| Protein Level, RFU |
|||||
|---|---|---|---|---|---|
| Module | Protein | UniProt ID | Primary Function | Young men | Young women |
| Module different with sex | |||||
| Blue | Calcineurin subunit B type 1 | P63098 | Regulates calcium sensitivity | 2,615 ± 192 | 3,548 ± 205 |
| cGMP-dependent 3′,5′-cyclic phosphodiesterase | O00408 | Regulates mitochondrial cAMP levels and respiration | 3,995 ± 258 | 5,087 ± 277 | |
| Kallikren-14 | Q9P0G3 | Peptidase activity in reproductive process | 3,859 ± 251 | 5,267 ± 285 | |
| Cadherin-6 | P55285 | Cell adhesion proteins | 492 ± 32 | 603 ± 34 | |
| Opioid-binding protein/cell adhesion molecule | Q14982 | Binds opioids | 1,714 ± 88 | 2,064 ± 86 | |
| Ephrin type-B receptor 4 | P54760 | Heart morphogenesis and angiogenesis | 3,926 ± 230 | 4,709 ± 246 | |
| Apolipoprotein D | P05090 | Lipid transporter | 2,904 ± 169 | 3,676 ± 144 | |
| Dickkopf-like protein 1 | Q9UK85 | Signal transducer activity | 4,165 ± 355 | 5,568 ± 451 | |
| Basigin | P35613 | Mannose binding; spermatogenesis, and development | 3,648 ± 235 | 4,361 ± 247 | |
| Retinol-binding protein 4 | P02753 | Retinol delivery | 531 ± 27 | 563 ± 27 | |
| Module different only with age | Young men | Older men | |||
| Brown | Thrombin | P00734 | Blood homeostasis, inflammation and wound healing | 15,883 ± 4,280 | 3,979 ± 1,911 |
| Inter-α-trypsin inhibitor heavy chain H4 | Q14624 | Inflammatory responses | 11,262 ± 1,527 | 16,103 ± 1,023 | |
| Tyrosine-protein kinase JAK2 | O60674 | Cell growth/differentiation and innate/adaptive immunity | 5,709 ± 1,067 | 9,589 ± 744 | |
| Plasma serine protease inhibitor | P05154 | Proteolytic activity; blood coagulation | 35,557 ± 7,842 | 51,063 ± 4,617 | |
| Bone morphogenetic protein 7 | P18075 | Calcium regulation and bone formation | 1,181 ± 181 | 763 ± 96 | |
| Aspartate aminotransferase, cytoplasmic | P17174 | Amino acid biosynthesis and glutamate regulator | 9,363 ± 1,340 | 6,723 ± 949 | |
| Interleukin-2 | P60568 | Activation of immune response | 2,264 ± 339 | 1,911 ± 209 | |
| Growth/differentiation factor 2 | Q9UK05 | Angiogenesis inhibition | 1,502 ± 192 | 1,050 ± 129 | |
| NAD-dependent protein deacetylase sirtuin-2 | Q8IXJ6 | Intracellular protein regulator | 12,610 ± 1,972 | 7,488 ± 1,427 | |
Data are means ± SE. RFU, relative fluorescence units.
Aging modules and age-related modules partially preserved with exercise.
We identified five modules different with age in men (yellow, black, brown, magenta, and red modules), with 4 of these modules also influenced by exercise status (discussed above). The lone exception was the brown module, which included proteins associated with the regulation of covalent protein modification, as shown in Fig. 7; the highest ranked individual proteins in this module are listed in Table 4.
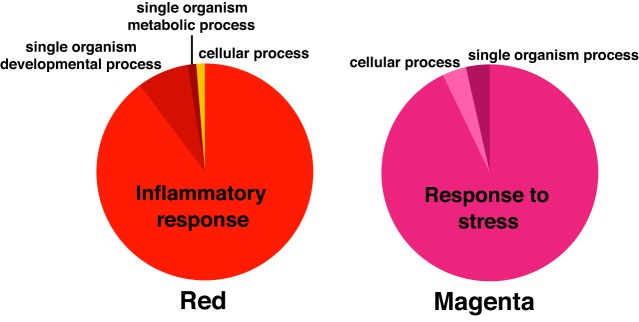
Additionally, the 2 age-related modules found in the inactive men that were at least partially preserved in older exercise-trained men, i.e., red and magenta, included proteins associated with the inflammatory response and response to cell stress, respectively, as shown in Fig. 8; the highest-ranking individual proteins are listed in Table 5 with the highest contributing protein for the red module being serine/threonine-protein kinase PAK 3 and for the magenta module leukocyte antigen CD97 (CD97).
Fig. 8.
GO biological processes implicated by proteins in modules partially preserved in habitually trained older men to levels of young men. GO, Gene Ontology.
Table 5.
Age-related modules partially preserved with aerobic exercise in men
| Module | Protein | UniProt ID | Primary Function | Protein Level, RFU |
||
|---|---|---|---|---|---|---|
| Y-INAC | O-INAC | O-AEX | ||||
| Red | Serine/threonine-protein kinase PAK 3 | O75914 | Cytoskeleton regulation, cell migration, or cell cycle regulation | 758 ± 89 | 893 ± 40 | 835 ± 55 |
| Interleukin-37 | Q9NZH6 | Suppressor of inflammation and immune responses | 739 ± 76 | 906 ± 31 | 877 ± 46 | |
| Cyclin-dependent kinase 5 | Q00535 | Anatomical structure formation; cell development, differentiation, migration, and morphogenesis | 973 ± 67 | 1,058 ± 37 | 1,023 ± 40 | |
| Tumor necrosis factor receptor superfamily member 3 | P36941 | Promotes apoptosis | 3,051 ± 223 | 3,619 ± 138 | 3,542 ± 134 | |
| Kallikrein-12 | Q9UKR0 | Peptidase activity | 3,128 ± 442 | 3,826 ± 215 | 3,687 ± 286 | |
| Glutamate carboxypeptidase 2 | Q04609 | Peptidase activity; metabolic processes | 1,326 ± 115 | 1,491 ± 63 | 1,421 ± 71 | |
| Tumor necrosis factor receptor superfamily member 11B | O00300 | Bone homeostasis; promotes apoptosis | 5,199 ± 651 | 7,535 ± 238 | 6,920 ± 635 | |
| Lymphotoxin α-2 β-1 | P01374 | Adaptive immunity; cell signaling, development, and differentiation | 1,168 ± 111 | 1,384 ± 54 | 1,304 ± 89 | |
| Arginase-1 | P05089 | Aging; metabolic processes | 436 ± 19 | 498 ± 14 | 471 ± 10 | |
| Complement C1q subcomponent | P02745 | Activation of immune response | 24,624 ± 3,660 | 29,642 ± 1,879 | 26,825 ± 2,426 | |
| Magenta | Leukocyte antigen CD97 | P48960 | Leukocyte migration | 1,514 ± 67 | 1,569 ± 81 | 1,524 ± 78 |
| Oncostatin-M | P13725 | Regulates tumor growth, cytokine production, and cell maturation | 1,401 ± 74 | 1,576 ± 80 | 1,470 ± 63 | |
| Ras GTPase-activating protein 1 | P20936 | Inhibitory regulator of Ras-cyclic AMP pathway | 882 ± 23 | 999 ± 48 | 933 ± 28 | |
| Carbonic anhydrase 4 | P22748 | Carbon dioxide hydration | 1,272 ± 55 | 1,360 ± 50 | 1,377 ± 72 | |
| C-C motif chemokine 1 | P22362 | Immune response | 2,391 ± 92 | 2,766 ± 115 | 2,730 ± 143 | |
| Tropomyosin beta chain | P07951 | Muscle contraction | 1,565 ± 94 | 1,834 ± 91 | 1,647 ± 106 | |
| Carbonic anhydrase-related protein 10 | Q9NS85 | Development | 548 ± 27 | 639 ± 26 | 570 ± 27 | |
Data are means ± SE. O-AEX, older aerobic exercise trained; O-INAC, older inactive; RFU, relative fluorescence units; Y-INAC, young inactive.
Association of Proteomic Patterns with Select Healthspan Indicators
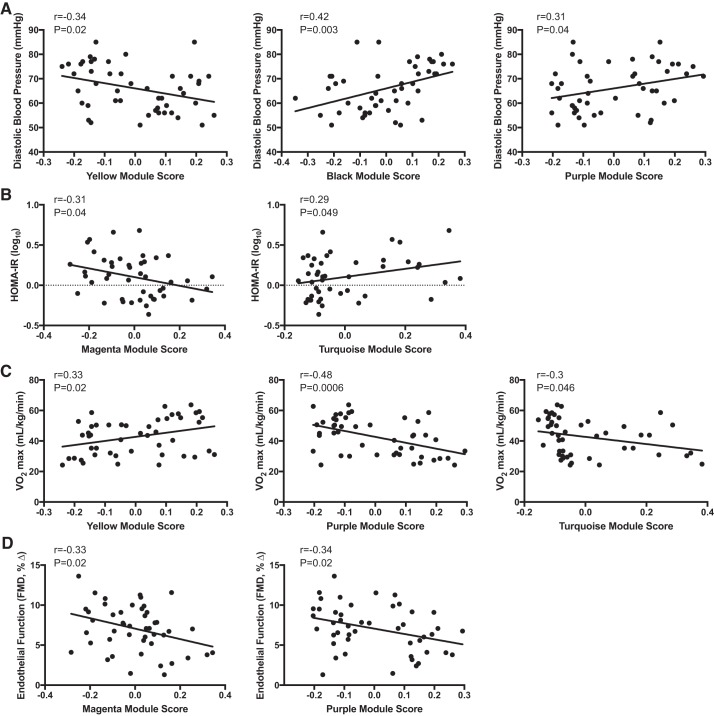
To determine if modules were clinically relevant, the 5 modules related to aerobic exercise status in adults and two modules preserved with age in older trained men were correlated with the physiological and clinical indicators of healthspan in the overall group of participants. We found that 5 of the 7 modules related to exercise status (yellow, black, purple, magenta and turquoise) were related to indicators of healthspan, including diastolic blood pressure, insulin resistance, aerobic exercise capacity and vascular endothelial function. The significance and magnitude of each nominally significant correlation (Pearson’s r) is reported in Fig. 9. The strongest correlation observed was between the purple module and aerobic exercise capacity, which also survives strict control of multiple testing burden (r = −0.48 P = 0.0006, Fig. 9C).
Fig. 9.
Modules influenced by exercise or age-related modules preserved with exercise associated with select indicators of healthspan [diastolic blood pressure (A), HOMA-IR (B), V̇o2max (C), endothelial function (D)] in all participants. FMD, flow-mediated dilation; HOMA-IR, homeostasis model assessment-insulin resistance; V̇o2max, maximal aerobic capacity.
DISCUSSION
To our knowledge this is the first study to assess circulating plasma proteomic patterns with habitual aerobic exercise and their association to indicators of healthspan in healthy adults. Through WGCNA, we found 10 distinct co-expressed plasma protein modules. Of these 10 modules, a total of 7 were influenced by habitual exercise status (i.e., different in inactive/active adults or preserved in older trained men), including 3 modules that were influenced by aerobic exercise status in both young adults and young and older men. These findings demonstrate that habitual aerobic exercise has a prominent effect on the circulating proteome, regardless of sex or age. Importantly, 5 of the exercise-influenced modules were associated with at least one clinical indicator of healthspan (diastolic blood pressure, insulin resistance, V̇o2max, or vascular endothelial function), suggesting that the protein patterns captured within these modules may have clinical relevance. Finally, exercise-related modules were associated with biological processes related to the stress response, inflammatory signaling, the immune system, and apoptosis, suggesting a potential role for these and other pathways described in transducing the beneficial impact of regular aerobic exercise on healthspan.
Proteomic Patterns and Markers Associated with Habitual Aerobic Exercise and Healthspan Indicators
Prominent plasma proteins within our exercise-related modules may represent key modulators of physiological function and could be considered for future study (3, 67). Our analysis identified 3 modules (yellow, purple, turquoise) that were associated with habitual aerobic exercise status in both young individuals and the young and older men cohort.
The yellow proteomic module was correlated with diastolic blood pressure and aerobic capacity. The highest-ranking protein within this module was α-2-macroglobulin, an anti-protease that inhibits matrix metalloproteinases (MMPs) (49), the latter being a key regulator of extracellular matrix remodeling (25, 65) and modulating influence on blood pressure (17, 63). α-2-macroglobulin also is involved in improvements in aerobic capacity with exercise training (62). Other key proteins within the yellow module are associated with pathways of apoptosis, complement system, lipid metabolism, and vascular remodeling, suggesting the importance of inflammation and immune function in the physiological benefits of habitual aerobic exercise.
The turquoise module was associated with insulin resistance and aerobic capacity. It is well established that chronic aerobic exercise is associated with clinically significant improvements in insulin resistance (35). Of note, Rac1, triosephosphate isomerase (TPI), and glycogen synthase kinase-3 α/β (GSK-3) are among the highest-ranking proteins identified in the turquoise module and have previously been shown to be involved in glucose transport, glycolysis, and increased insulin resistance, respectively (24, 61, 64).
The highest-ranking proteins of the purple module consisted of fibronectin and other proteins important for wound healing. These proteins have been shown to facilitate atherogenic and thrombogenic processes in the vascular endothelium and smooth muscle of the arterial wall (42, 44). Moreover, fibrinogen is an independent risk factor for cardiovascular disease (26, 37) and is lowered with chronic exercise (12). In the context of the beneficial effects of regular aerobic exercise, these observations are consistent with our finding of a relation between the proteomic pattern of the purple module and healthspan indicators, including vascular endothelial function, diastolic blood pressure, and V̇o2max.
Among the proteins identified in the exercise-related modules were several previously indicated in the literature as being altered with exercise, including brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6), secreted protein acidic and rich in cysteine (SPARC), decorin, heat shock proteins 60 (Hsp60) and 70 (Hsp70), and superoxide dismutase (SOD) from the turquoise module and interleukin-8 (IL-8) from the purple module (7, 28, 54). These proteins represent biological pathways involved in neurogenesis, glucose metabolism, muscle hypertrophy, mitochondrial protein transport, protection from thermal or oxidative stress, and angiogenesis (5, 7, 16, 22, 28, 31, 40, 55). All of these processes are known to participate in mediating the broad, systemic health benefits of regular exercise.
We would expect to distinguish proteomic factors relating to cardiorespiratory fitness between trained and untrained individuals, and as discussed above, the yellow, turquoise, and purple modules were associated with V̇o2max. However, the proteins in these modules primarily included proteins related to apoptosis, immune, and stress pathways rather than exhibiting markers clearly linked to V̇o2max or its direct determinants, such as cardiac output or blood volume. Still, some proteins in these exercise- or V̇o2max-correlated modules have been shown to be related to oxygen transport capacity or cardiac contractility and hypertrophy, including hemoglobin, cAMP-specific 3′, 5′-cyclic phosphodiesterase 4D, and GSK-3 (8, 13, 27, 32), although only GSK-3 is among the top 10 ranking proteins within its respective module. The limitations in identifying proteins directly related to V̇o2max may be due to this particular SomaLogic assay not being designed to target those pathways, but it is also plausible that proteins directly related to determinants of cardiorespiratory fitness may not be as different in habitually trained individuals assessed at rest. Such signals might only be evident in response to acute bouts of exercise or with dynamic changes in fitness over time. A key goal of future research should be to identify novel biomarkers in these physiological states.
Age-Related Proteomic Patterns and Markers Partially Preserved with Exercise
In the present analysis, we identified 2 age-related modules (red and magenta) that were preserved in older exercise-trained men, suggesting that exercise may partially preserve age-related proteomic patterns. Chronic aerobic exercise promotes optimal physiological aging and extends healthspan (6, 20, 53), suggesting that preserved proteomic patterns in older trained adults may help elucidate molecular transducers of these benefits of exercise. The red and magenta modules included proteins associated with inflammatory and cell stress responses, respectively. Although not a high-ranking protein in the red module, chordin-like protein 1, an antagonist of bone morphogenic protein 4 (involved in bone formation), has previously been shown to differ with age using the same SOMAscan proteomics platform (43). Here we found that chordin-like protein 1 was part of the red module, which differed with age but also was preserved in older exercise-trained men.
The magenta module was associated with insulin sensitivity and vascular endothelial function. The 2 highest-ranking proteins in this module are CD97 and oncostatin M. CD97 is a membrane protein on inflammatory cells that plays an important role in intercellular signaling in the immune system through regulation of cell adhesion and migration (52). CD97 is upregulated in individuals with metabolic syndrome and is related to insulin resistance (48). Oncostatin M is a pro-inflammatory cytokine in the IL-6 family and is believed to be pro-atherosclerotic (50) and involved in mediating insulin resistance (11, 23, 39). These findings provide preliminary identification of proteins related to inflammation and stress response that are partially preserved in older aerobic-trained adults, but future studies are needed to further investigate their roles.
Limitations and Future Directions
Because of the combination of rigorous screening required to exclude clinical disease and study only healthy older adults, the deep physiological phenotyping of our participants that required broad and extensive technical demands, and the high per-sample cost of the SOMAscan proteomic analyses, the size of our subject sample was limited. However, the present analyses and results were intended to represent an initial, hypothesis-generating effort to establish preliminary insight as to the feasibility of the model and to identify putative proteomic targets for a future larger-scale study. We believe that we accomplished these goals in the present investigation. We also recognize that the SOMAscan analysis employed here does not survey the complete circulating proteome and that more research is needed to discover additional circulating proteins and the means to assess them.
It should be noted that the present study is but one component of an assessment of the spectrum of adaptations to aerobic exercise, where in the present study we assessed individuals at a relative physiological steady state (at rest) who have been habitually exercising for years. This timepoint we assessed should be taken into context for the circulating proteome markers identified, as they do not reflect changes in the proteome during or immediately after an acute bout of exercise or dynamic changes in fitness going from the untrained to the trained state. Future studies are needed to complete the overall picture of the effects of exercise, including assessment of dynamic changes in the proteome with acute exercise (the hours before, during, and after exercise), exercise interventions (weeks to months training modulating fitness), and how these changes in the proteome are tied to improvements in physiological and clinical measures (e.g., V̇o2max). In addition, other tissues (e.g., muscle) and omic pathways (e.g., genomics, epigenetics, metabolomics, etc.) should be included to accurately and holistically capture the local and systemic effects of exercise, as blood and proteomics only provides one small piece of a larger picture and may or may not reflect what is happening in tissue.
Finally, although we identified a proteomic pattern influenced by a sex and exercise status interaction in young adults, additional studies will be needed to further investigate potential sex differences in the circulating proteome with habitual exercise and, in particular, differences with age.
Summary and Conclusions
In summary, employing a SOMAscan assay, we have conducted an initial analysis of >1,000 plasma proteomic markers associated with aerobic exercise status in young men and women, as well as in healthy older men. Using weighted correlation network analysis to identify clusters of highly co-expressed proteins, we then identified 10 distinct plasma proteomic modules (patterns) in our participants, identified the individual protein markers most strongly represented in those patterns, and linked the proteomic composition of the modules to their respective biological pathways using the GO reference database. Finally, we correlated proteomic patterns with numerous clinical and physiological indicators of human healthspan.
We were able to characterize 5 specific proteomic patterns associated with aerobic exercise status in adults, as well as 2 modules that were preserved with aging in regularly exercising men. Habitual exercise-associated proteomic patterns were related to biological pathways involved in wound healing, regulation of apoptosis, glucose-insulin and cellular stress signaling, and inflammation/immune responses. Several of the exercise-related proteomic patterns were associated with physiological and clinical indicators of healthspan, including diastolic blood pressure, insulin resistance, V̇o2max, and vascular endothelial function. Overall, these findings provide initial insight into circulating proteomic patterns modulated by habitual aerobic exercise in healthy young and older adults, the biological processes involved, and the relation between proteomic patterns and clinical and physiological indicators of human healthspan.
GRANTS
This work was supported by NIH awards R37 AG013038, Colorado Clinical and Translational Science Award UL1 TR001082, and T32 AG000279-14S1.
DISCLAIMERS
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.S.-P. and D.R.S. conceived and designed research; J.R.S.-P. performed experiments; J.R.S.-P. and K.S.S.-P. analyzed data; J.R.S.-P., K.S.S.-P., M.B.M., C.R.M., and D.R.S. interpreted results of experiments; J.R.S.-P. and K.S.S.-P. prepared figures; J.R.S.-P. and K.S.S.-P. drafted manuscript; J.R.S.-P., K.S.S.-P., M.B.M., C.R.M., and D.R.S. edited and revised manuscript; J.R.S.-P., K.S.S.-P., M.B.M., C.R.M., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the staff of the University of Colorado Boulder Clinical and Translational Research Center for technical assistance.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care 33: 1179–1185, 2010. doi: 10.2337/dc09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet 12: 56–68, 2011. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093, 2009. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors 22: 123–131, 2004. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) 111: 1497–1504, 2011. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 7.Catoire M, Kersten S. The search for exercise factors in humans. FASEB J 29: 1615–1628, 2015. doi: 10.1096/fj.14-263699. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol 51: 607–613, 2011. doi: 10.1016/j.yjmcc.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Daskalopoulou C, Stubbs B, Kralj C, Koukounari A, Prince M, Prina AM. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 38: 6–17, 2017. doi: 10.1016/j.arr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Elks CM, Zhao P, Grant RW, Hang H, Bailey JL, Burk DH, McNulty MA, Mynatt RL, Stephens JM. Loss of oncostatin M signaling in adipocytes induces insulin resistance and adipose tissue inflammation in vivo. J Biol Chem 291: 17066–17076, 2016. doi: 10.1074/jbc.M116.739110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst E. Regular exercise reduces fibrinogen levels: a review of longitudinal studies. Br J Sports Med 27: 175–176, 1993. doi: 10.1136/bjsm.27.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschenhagen T. PDE4 in the human heart - major player or little helper? Br J Pharmacol 169: 524–527, 2013. doi: 10.1111/bph.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol (1985) 78: 1931–1941, 1995. doi: 10.1152/jappl.1995.78.5.1931. [DOI] [PubMed] [Google Scholar]
- 16.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251–262, 2002. doi: 10.1161/res.90.3.251. [DOI] [PubMed] [Google Scholar]
- 18.The Gene Ontology Consortium Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res 45: D331–D338, 2017. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, Flather D, Forbes A, Foreman T, Fowler C, Gawande B, Goss M, Gunn M, Gupta S, Halladay D, Heil J, Heilig J, Hicke B, Husar G, Janjic N, Jarvis T, Jennings S, Katilius E, Keeney TR, Kim N, Koch TH, Kraemer S, Kroiss L, Le N, Levine D, Lindsey W, Lollo B, Mayfield W, Mehan M, Mehler R, Nelson SK, Nelson M, Nieuwlandt D, Nikrad M, Ochsner U, Ostroff RM, Otis M, Parker T, Pietrasiewicz S, Resnicow DI, Rohloff J, Sanders G, Sattin S, Schneider D, Singer B, Stanton M, Sterkel A, Stewart A, Stratford S, Vaught JD, Vrkljan M, Walker JJ, Watrobka M, Waugh S, Weiss A, Wilcox SK, Wolfson A, Wolk SK, Zhang C, Zichi D. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One 5: e15004, 2010. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harridge SD, Lazarus NR. Physical activity, aging, and physiological function. Physiology (Bethesda) 32: 152–161, 2017. doi: 10.1152/physiol.00029.2016. [DOI] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 159: 738–749, 2014. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Henkel J, Gärtner D, Dorn C, Hellerbrand C, Schanze N, Elz SR, Püschel GP. Oncostatin M produced in Kupffer cells in response to PGE2: possible contributor to hepatic insulin resistance and steatosis. Lab Invest 91: 1107–1117, 2011. doi: 10.1038/labinvest.2011.47. [DOI] [PubMed] [Google Scholar]
- 24.Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr Drug Targets 7: 1435–1441, 2006. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- 25.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 258: 1183–1186, 1987. doi: 10.1001/jama.1987.03400090067035. [DOI] [PubMed] [Google Scholar]
- 27.Kanstrup IL, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc 16: 256–262, 1984. doi: 10.1249/00005768-198406000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 450: 1089–1094, 2014. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 29.Kim CX, Bailey KR, Klee GG, Ellington AA, Liu G, Mosley TH Jr, Rehman H, Kullo IJ. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One 5: e9065, 2010. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 31.Koll H, Guiard B, Rassow J, Ostermann J, Horwich AL, Neupert W, Hartl FU. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell 68: 1163–1175, 1992. doi: 10.1016/0092-8674(92)90086-R. [DOI] [PubMed] [Google Scholar]
- 32.Lal H, Ahmad F, Woodgett J, Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res 116: 138–149, 2015. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langfelder P, Luo R, Oldham MC, Horvath S. Is my network module preserved and reproducible? PLOS Comput Biol 7: e1001057, 2011. doi: 10.1371/journal.pcbi.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942, 2008. [Erratum in Diabetes 61: 2653, 2012]. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002. [Erratum in Lancet 361: 1060, 2003]. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 37.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 38.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Books, 1988. [Google Scholar]
- 39.Luo P, Wang PX, Li ZZ, Zhang XJ, Jiang X, Gong J, Qin JJ, Guo J, Zhu X, Yang S, Li H. Hepatic oncostatin M receptor β regulates obesity-induced steatosis and insulin resistance. Am J Pathol 186: 1278–1292, 2016. doi: 10.1016/j.ajpath.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Martin TA. Interleukin-8 and angiogenesis. In Growth Factors and Their Receptors in Cancer Metastasis, edited by Snyder CR, Jiang WG, Matsumoto K, Nakamura T. Dordrecht, the Netherlands: Springer, 2001, p. 51–65. [Google Scholar]
- 41.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 42.Maurer LM, Tomasini-Johansson BR, Mosher DF. Emerging roles of fibronectin in thrombosis. Thromb Res 125: 287–291, 2010. doi: 10.1016/j.thromres.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menni C, Kiddle SJ, Mangino M, Viñuela A, Psatha M, Steves C, Sattlecker M, Buil A, Newhouse S, Nelson S, Williams S, Voyle N, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Spector TD, Dobson R, Valdes AM. Circulating proteomic signatures of chronological age. J Gerontol A Biol Sci Med Sci 70: 809–816, 2015. doi: 10.1093/gerona/glu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KJ, Fisher EA. The double-edged sword of fibronectin in atherosclerosis. EMBO Mol Med 4: 561–563, 2012. doi: 10.1002/emmm.201200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair KS, Jaleel A, Asmann YW, Short KR, Raghavakaimal S. Proteomic research: potential opportunities for clinical and physiological investigators. Am J Physiol Endocrinol Metab 286: E863–E874, 2004. doi: 10.1152/ajpendo.00370.2003. [DOI] [PubMed] [Google Scholar]
- 46.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab 22: 4–11, 2015. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 29, Suppl: S1–S205, 1997. [PubMed] [Google Scholar]
- 48.Poelkens F, Lammers G, Pardoel EM, Tack CJ, Hopman MT. Upregulation of skeletal muscle inflammatory genes links inflammation with insulin resistance in women with the metabolic syndrome. Exp Physiol 98: 1485–1494, 2013. doi: 10.1113/expphysiol.2013.072710. [DOI] [PubMed] [Google Scholar]
- 49.Rehman AA, Ahsan H, Khan FH. α-2-Macroglobulin: a physiological guardian. J Cell Physiol 228: 1665–1675, 2013. doi: 10.1002/jcp.24266. [DOI] [PubMed] [Google Scholar]
- 50.Richards CD. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm 2013: 512103, 2013. doi: 10.1155/2013/512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safaee M, Clark AJ, Ivan ME, Oh MC, Bloch O, Sun MZ, Oh T, Parsa AT. CD97 is a multifunctional leukocyte receptor with distinct roles in human cancers (Review). Int J Oncol 43: 1343–1350, 2013. doi: 10.3892/ijo.2013.2075. [DOI] [PubMed] [Google Scholar]
- 53.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol 12: 504–517, 2016. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 54.Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular disease. Oxid Med Cell Longev 2016: 7239639, 2016. doi: 10.1155/2016/7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med 11: 239–246, 1991. doi: 10.1016/0891-5849(91)90119-N. [DOI] [PubMed] [Google Scholar]
- 56.Sampson DL, Broadbent JA, Parker AW, Upton Z, Parker TJ. Urinary biomarkers of physical activity: candidates and clinical utility. Expert Rev Proteomics 11: 91–106, 2014. doi: 10.1586/14789450.2014.859527. [DOI] [PubMed] [Google Scholar]
- 57.Schild M, Ruhs A, Beiter T, Zügel M, Hudemann J, Reimer A, Krumholz-Wagner I, Wagner C, Keller J, Eder K, Krüger K, Krüger M, Braun T, Nieß A, Steinacker J, Mooren FC. Basal and exercise induced label-free quantitative protein profiling of m. vastus lateralis in trained and untrained individuals. J Proteomics 122: 119–132, 2015. doi: 10.1016/j.jprot.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594: 2001–2024, 2016. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silliman CC, Dzieciatkowska M, Moore EE, Kelher MR, Banerjee A, Liang X, Land KJ, Hansen KC. Proteomic analyses of human plasma: Venus versus Mars. Transfusion 52: 417–424, 2012. doi: 10.1111/j.1537-2995.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.SomaLogic SOMAscan Technical White Paper [Online]. http://www.somalogic.com/wp-content/uploads/2016/08/SSM-002-Rev-3-SOMAscan-Technical-White-Paper.pdf [21 Sept. 2015].
- 61.Sylow L, Jensen TE, Kleinert M, Højlund K, Kiens B, Wojtaszewski J, Prats C, Schjerling P, Richter EA. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 62: 1865–1875, 2013. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Sundberg CJ. Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol 3: 19, 2005. doi: 10.1186/1741-7007-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 60: 459–466, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wierenga RK, Kapetaniou EG, Venkatesan R. Triosephosphate isomerase: a highly evolved biocatalyst. Cell Mol Life Sci 67: 3961–3982, 2010. doi: 10.1007/s00018-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5: 2145–2154, 1991. doi: 10.1096/fasebj.5.8.1850705. [DOI] [PubMed] [Google Scholar]
- 66.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao W, Langfelder P, Fuller T, Dong J, Li A, Hovarth S. Weighted gene coexpression network analysis: state of the art. J Biopharm Stat 20: 281–300, 2010. doi: 10.1080/10543400903572753. [DOI] [PubMed] [Google Scholar]