Abstract
In vitro and in vivo anesthetized studies led to the conclusion that “deficiencies in one neuromodulator are immediately compensated by the action of other neuromodulators,” which suggests an interdependence among neuromodulators. This concept was the focus of the 2018 Julius H. Comroe Lecture to the American Physiological Society in which I summarized our published studies testing the hypothesis that if modulatory interdependence was robust, breathing would not decrease during dialysis of antagonists to G protein-coupled excitatory receptors or agonists to inhibitory receptors into the ventral respiratory column (VRC) or the hypoglossal motor nuclei (HMN). We found breathing was not decreased during unilateral VRC dialyses of antagonists to excitatory muscarinic, serotonergic, and neurokinin-1 receptors alone or in combinations nor was breathing decreased with unilateral VRC dialysis of a µ-opioid receptor agonist. Analyses of the effluent dialysate revealed locally increased serotonin (excitatory) during muscarinic receptor blockade and decreased γ-aminobutyric acid (inhibitory) during dialysis of opioid agonists, suggesting an interdependence of neuromodulators through release of compensatory neuromodulators. Bilateral dialysis of receptor antagonists or agonist in the VRC increased breathing, which does not support the concept that unchanged breathing with unilateral dialyses was due to contralateral compensation. In contrast, in the HMN neither unilateral nor bilateral dialysis of the excitatory receptor antagonists altered breathing, but unilateral dialysis of the opioid receptor agonist decreased breathing. We conclude: 1) there is site-dependent interdependence of neuromodulators during physiologic conditions, and 2) attributing physiologic effects to a specific receptor perturbation is complicated by local compensatory mechanisms.
Keywords: breathing, compensation, neuromodulation
TRIBUTE TO DR. COMROE
It is indeed a great honor to receive an award in memory of Dr. Julius H. Comroe, Jr., who impacted the respiratory discipline as a physician, physiologist, and educator. My research activities in the control of breathing led me to review many of Dr. Comroe’s research publications in the 1930s and 1940s. I have been particularly impressed by his bold, definitive conclusions such as those stated in a 1938 publication, that “carotid body reflexes constitute an accessory mechanism, brought into action by emergencies such as foreign chemicals, anoxemia, and unusually great increases in the CO2 tension of the blood, rather than an essential part of the normal respiratory regulating system” (5). These types of statements made by strong leaders such as Dr. Comroe serve to stimulate further research by others to gain additional insights into physiologic mechanisms. Eventually, he modified this view allowing that during physiologic wakefulness and sleep, the carotid chemoreceptors may provide an important contribution to the control of breathing, which was shown by results of several studies by others. I have also learned a great deal about Dr. Comroe from reading his “Retrospectroscope,” in which he expressed grave concern about the micromanagement of research by government and business directing research to specific medical needs. He favored individual-initiated basic science research, which led him to review 529 publications between 1940 and 1975 related to clinical cardiovascular and pulmonary medicine, and he concluded 41% of the publications were strictly from individual-initiated research. Dr. Comroe was an outstanding educator adhering to the principle that in lectures to medical students, “it is better to make it clear than to make it perfectly correct.” Finally, I am proud of my connection to Dr. Comroe through Dr. John Rankin, who was a colleague of Dr. Comroe while they were both at the University of Pennsylvania. Dr. Rankin eventually came to the Department of Medicine at the University of Wisconsin and established a research laboratory in which I completed my PhD research under the primary mentorship of Dr. Jerome Dempsey but also that of Dr. Rankin.
BACKGROUND
There have been major advances in understanding the control of breathing since Dr. Comroe’s conclusion in a 1938 manuscript that “the control of breathing under ordinary conditions is accomplished entirely by the direct effects of chemical stimuli (mainly CO2) upon the cells of the center” (5). For example, in spite of subsequent findings supporting the concept that CO2 is the primary drive for breathing (9), it is now recognized that many other factors are major determinants of breathing, including neuromodulation of respiratory neurons (7, 22, 42). In addition, rather than a somewhat vague reference by Dr. Comroe to a “respiratory center,” it is now recognized that respiratory control is a network phenomenon distributed among neurons in the ventral respiratory column (VRC) and the dorsal respiratory group in the medulla and the pontine respiratory group in the dorsolateral pons (11, 47). Studies summarized herein were completed to gain insight into neuromodulation of rhythm- and pattern-generating neurons of the pre-Bötzinger complex (preBötC) region within the VRC and to gain insight into neuromodulation of neurons controlling airway diameter within the hypoglossal motor nucleus (HMN). The HMN is not considered part of the dorsal respiratory group even though it is located within the dorsal medulla.
Brezina (3) has elegantly distinguished neuromodulation from neurotransmission. He writes that “whether a signaling agent functions as a transmitter or a modulator depends ultimately on the spatial and temporal scale on which the agent acts. If the agent acts so locally in both space and time that it merely communicates the current activity of one component of the neuronal network to another component, then it is acting as a transmitter that merely implements one of the connections of the neuronal wiring diagram. A modulator, in contrast, acts globally on multiple components, over longer times, or usually both so that its effects are not directly correlated with the current activity of any particular component or connection of the neuronal network: the modulator does not mediate the activity but rather modifies it.” The major transmitters are acetylcholine (Ach), γ-aminobutyric acid (GABA), and glutamate released presynaptically to open postsynaptic ligand-gated ion channels at discrete synapses. In contrast, biogenic amines, neuropeptides, and other classes of molecules provide a global modulatory role through metabotropic receptors coupled to second-messenger cascades and slow intracellular signaling processes that modulate every physiological variable, every process, at all levels of organization of the nervous system. Each modulator exerts multiple actions, and the same action is exerted by multiple modulators forming an interconnected network; thus a single modulator will “spread” throughout the neuronal network to perturb many of its components.
INTERDEPENDENCE OF NEUROMODULATORS
The above description predicts there is interdependence of neuromodulators, whereby as stated by Doi and Ramirez (8): “A modulator’s action is determined by the concurrent modulation and interaction with other neuromodulators. Deficiencies in one neuromodulator are immediately compensated by the action of other neuromodulators.” This specific conclusion, as it applies to the respiratory control network, was based on findings in rodents that administration of a specific antagonist to one major excitatory receptor for breathing had no or minimal effects unless antagonists to one or two other excitatory receptors had previously been administered. Marder (28) and Brezina et al. (3) essentially reached the same conclusions in studies on neuromodulation in the crustacean stomatogastric ganglion or the neuromuscular junction in aplysia, respectively. They used the terms “redundant” and “degenerate” neural control networks to account for an apparent interdependence and/or interaction of neuromodulators. The studies by Doi and Ramirez (8), Marder (28) and Brezina et al. (3) have all been completed in in vitro- or in vivo-anesthetized preparations. Thus Doi and Ramirez (8) stated that “a major task of future research will be to unravel the differential activation of different receptor systems in more physiological context” and that “these studies may arrive at conclusions that are unexpected from the in vitro experiments.”
Over the past couple of decades, my laboratory has developed and utilized a goat model to test during physiologic wakefulness and/or sleep, hypotheses that were generated from in vitro or in other reduced preparation. Studies utilizing this goat model has enhanced understanding of basic mechanisms of respiratory control including rhythm and pattern generation (20, 53), chemoreceptor responsiveness (10, 32), and the exercise hyperpnea (4, 29). Herein, I summarize published data (24–26, 35–38) from studies in which I and my colleagues utilized this awake and sleeping goat model to test the hypothesis that breathing would not decrease during dialyses into the VRC or the HMN of mock cerebral spinal fluid (mCSF) with antagonists to G protein-coupled excitatory receptors or agonists to inhibitory receptors. These studies addressed five questions (listed below) related to an interdependence of neuromodulators, the rationale for each provided at the beginning of each section:
-
1.
What are the effects of dialysis into the VRC of antagonists to receptors for “excitatory” neuromodulators?
-
2.
Do the effects of antagonists to muscarinic, peptidergic, and serotonergic receptors differ between dialysis into the VRC and HMN?
-
3.
What are the effects of dialysis into the VRC of agonists to a µ-opioid G protein-coupled receptor?
-
4.
Do the effects of dialysis of agonists to a µ-opioid G protein-coupled “inhibitory” receptor differ between dialysis into the VRC and HMN?
-
5.
What are the effects of dialysis into the VRC of an agonist to an ionotropic “inhibitory” receptor?
METHODS
All methods have been previously published in detail (24–26, 35–38); thus only a brief summary of methods will be provided herein. All protocols and procedures utilized were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. All studies were on female adult goats weighing between 35 and 55 kg. The goats were housed and studied in an environmental chamber with fixed ambient temperature and 12:12-h light-dark cycle.
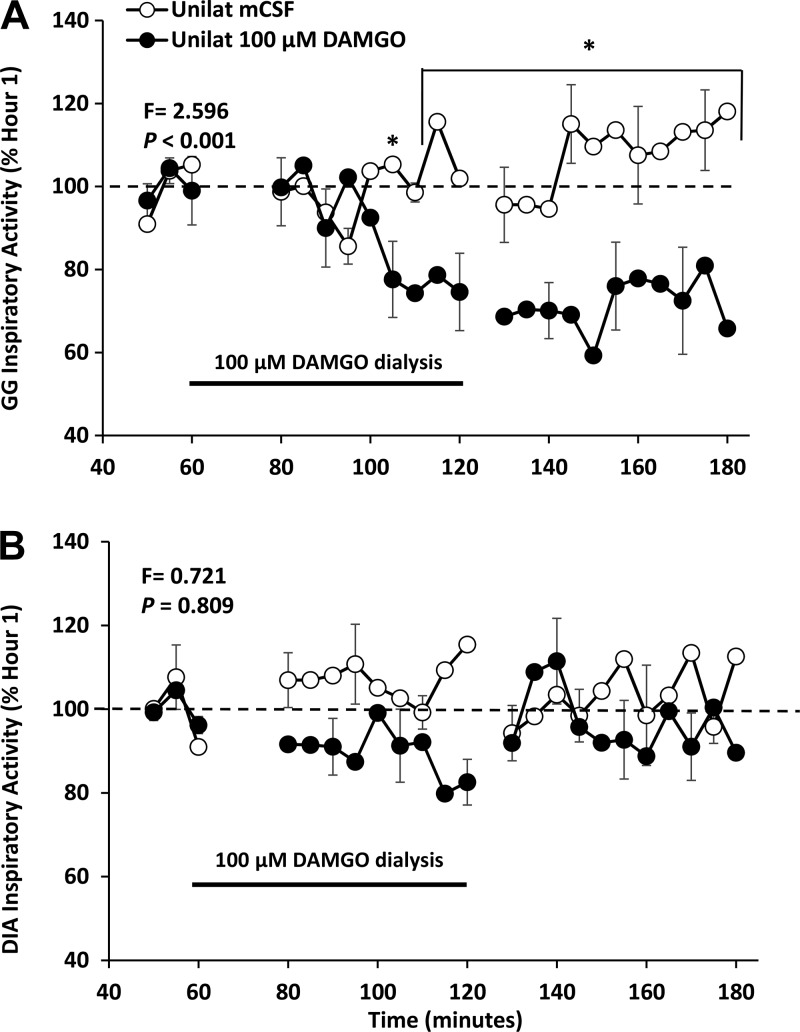
Before the studies, the carotid arteries were surgically relocated to just beneath the skin for ease of catheterization for blood sampling and recording blood pressure and heart rate. Electrodes were also surgically implanted into respiratory pump and airway muscle for recording muscle activity and into the head to obtain recordings of electrical activity to distinguish sleep state. Two weeks later, 70 mm-long stainless-steel microtubules were bilaterally surgically implanted and secured using dental acrylic into either the VRC or HMN using coordinates established by our laboratory for goats. The target site for microtubule placement into the VRC included the preBӧtC, which in goats is 2.5 to 3.5 mm rostral to obex, 4.0 to 5.0 mm lateral to the midline, and 2.5 to 3.5 mm from the dorsal surface (53). For the HMN, the microtubules were placed within 3 mm caudal to 3.5 mm rostral to obex, 0.5 to 1.5 mm lateral to the midline, and 2 to 3.5 mm from the dorsal surface. Placement of the microtubules into the preBӧt/VRC was subsequently verified while goats were awake by determining the ventilatory response to injections of a glutamate receptor agonist (NMDA, 100 mM, 500 nl), which we (53) and others (31, 48) have shown induces a distinctive hyperpnea when injected into the preBӧtC. The location of the microtubules into the HMN was verified while awake by a decrease in genioglossal muscle activity after dialysis of a µ-opioid receptor agonist {[d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO)} (see Fig. 16). The location of the microtublules was also verified using histochemistry analyses of tissue harvested after completion of all studies. For publication of work summarized herein, the histology section of the results section provides awake and histochemistry findings indicating that placement of the microtububles likely resulted in dialyzed substances reaching the target sites. However, it is likely that the substances diffused beyond the target site.
Fig. 16.
Increased activation of µ-opioid inhibitory receptors by unilateral dialysis of 100 µM [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) into the hypoglossal motor nuclei decreased activation of the genioglossal muscle (closed circles), but did not alter activation of the diaphragm (open circles). F and P values are from two-way ANOVA comparing dialysis of DAMGO during hour 3 and dialysis of mCSF all 3 h. *P < 0.001 comparisons to the pre DAMGO dialysis period by post hoc analysis (Holm-Sidak).
Before and for 2 wk after each surgery, the goats were accustomed to the experimental procedures. For each study, a mask with breathing valve was taped to the snout for continuous recording of inspired and expired ventilation, an indwelling carotid artery catheter was connected to a transducer for continuous recording of heart rate and blood pressure and sampling of blood at regular intervals, and EMG and EEG electrodes were connected to recording equipment to assess awake state and muscle activity. Microdialysis probes (CMA, membrane diameter of 0.5 mm, 29 kDa) were inserted into the microtubules so that only the 2 mm of membrane penetrated the tissue. After 30 min were allowed for stabilization, mCSF was perfused into the probe for 3 h at a rate of 25 µl/min. Hours 1 and 3 were mCSF alone while hour 2 was mCSF with or without individual or combinations of agonists or antagonists of excitatory or inhibitory receptors. The effluent mCSF was collected each hour separately for subsequent HPLC analysis of serotonin (5-HT), glycine, GABA, glutamine, dopamine, norepinephrine, and epinephrine and its metabolites (3,4-dihydroxyphenylacetic acid, hydroxyindole-3-acetic acid, and homovanillic acid. This panel was chosen because it was feasible with our sample size and the capabilities of our departmental Analytical Chemistry Core Laboratory. At least 36 h separated each physiologic study. Breath-by-breath recordings were binned into 1-, 5-, and 15-min intervals and statistically analyzed with one- or two-way ANOVAs, as appropriate. Comparisons were always made between studies in which mCSF alone was dialyzed for all 3 h and studies in which receptor antagonists or agonists were dialyzed during hour 2. Data are expressed as absolute values or as percent of hour 1 across the 3-h protocol.
EFFECTS OF DIALYSIS OF ANTAGONISTS TO RECEPTORS FOR EXCITATORY NEUROMODULATORS IN THE VRC
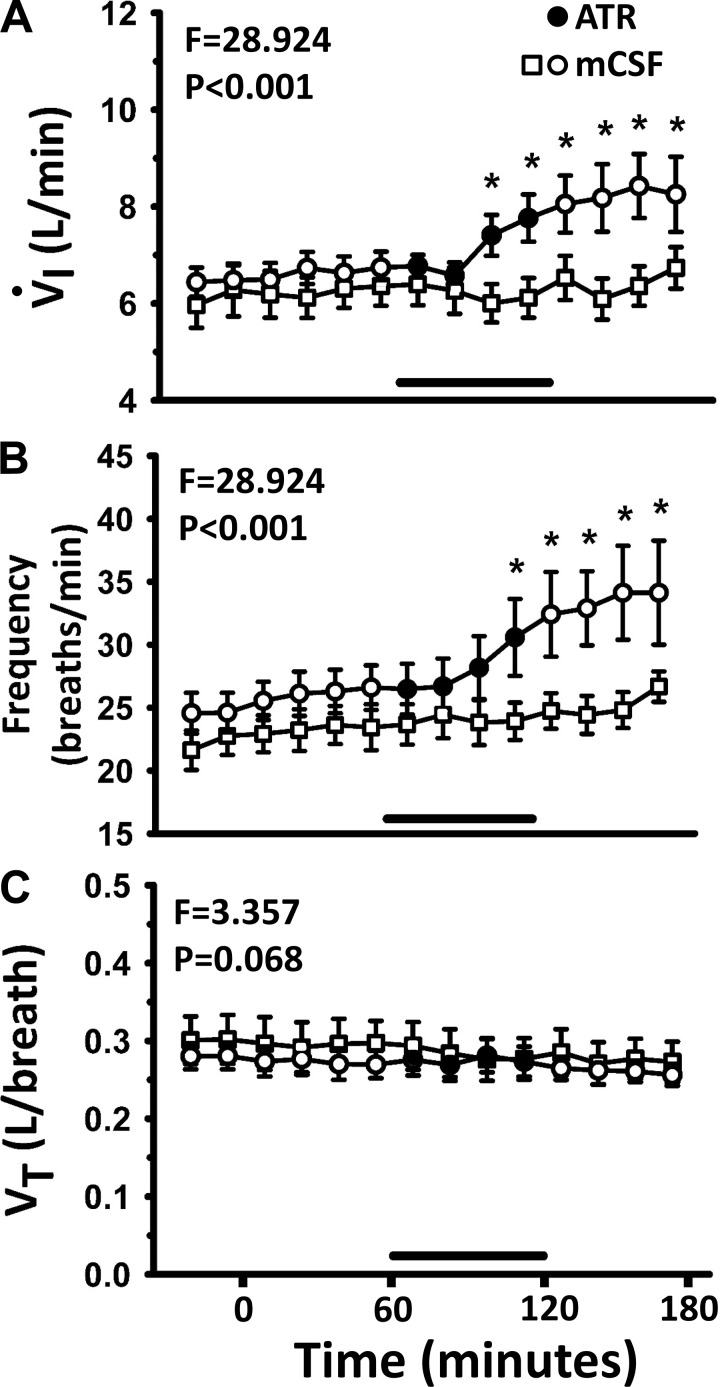
Blockade of muscarinic Ach receptors within the respiratory control network suggest an excitatory role of Ach as a neuromodulator. Shao and Feldman (45) found that application of the broad spectrum muscarinic receptor blocker atropine in a rhythmic medullary slice preparation (which included the preBötC) decreased the frequency of respiratory motor activity. Consistent with a depressant effect of atropine on respiratory neurons, we previously found that dialysis of 50 mM atropine into the pontine respiratory group of unanesthetized goats decreased pulmonary ventilation (V̇I) and breathing frequency (f) during wakefulness and during nonrapid eye movemnt sleep (2). Accordingly, we hypothesized that dialysis of 50 mM atropine into the preBötC region of the VRC would either decrease V̇I and f, or alternatively, atropine dialysis would not alter V̇I and f if there was a robust compensation from other neuromodulators (neuromodulator interdependence). However, we found that blocking muscarinic receptors unilaterally in the VRC with dialysis of 50 mM atropine during wakefulness (during the day) significantly (P < 0.001) increased V̇I and f above control (Fig. 1) (35). Tidal volume (Vt) was not altered during atropine dialyses nor in any additional studies described in this section; thus Vt will not be presented hereafter.
Fig. 1.
Unilateral dialysis of 50 mM atropine (ATR; a muscarinic receptor antagonist) into the pre-Bötzinger complex (preBötC) during the day increased (P < 0.001) pulmonary ventilation (V̇I; A) and breathing frequency (B) above dialysis of mock cerebrospinal fluid (mCSF), but tidal volume (VT; C) was not altered. The F and P values are from two-way repeated measures ANOVA. *Holm-Sidak post hoc test. Shown here (and in subsequent figures) are data binned at 15-min intervals, and the x-axis is time in minutes from start of dialysis. Open circles are data when mCSF was dialyzed over the entire period, and open squares are when 50 mM atropine was dialyzed between 60 and 120 min (closed squares and black line indicate period of atropine dialysis).
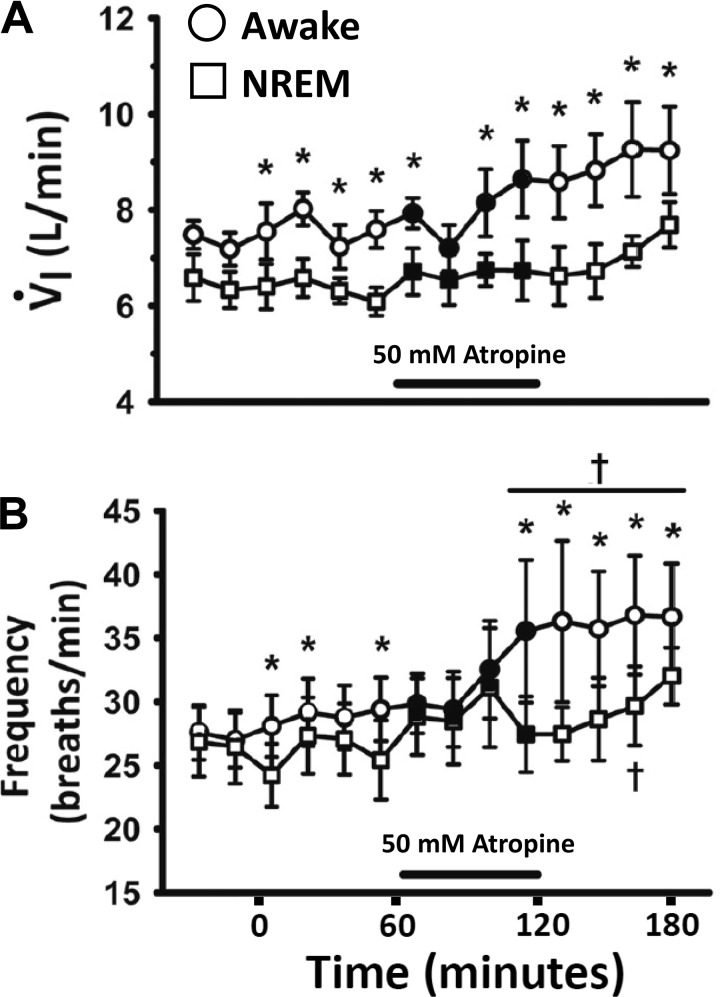
We previously found that when adult goats were only instrumented for EEG and EOG (sleep state) recordings, they were awake ~60% of the night, while they spent 30% in NREM sleep and 10% in rapid eye movement (REM) sleep states (30). The periods of both NREM and REM sleep rarely lasted longer than 5–10 min. However, herein, when microtubules were chronically implanted into the VRC or HMN, REM sleep was rare, and usually the goats were either awake or in NREM sleep about 60 and 40% of the total time, respectively, where V̇I and f were significantly lower during NREM than while awake. Unilateral dialysis of 50 mM atropine showed state-dependent effects during night studies, where V̇I and f were also significantly (P = 0.008) lower during NREM than wakefulness throughout the studies (Fig. 2) (35), but the effects of atropine to increase V̇I and f above control (P < 0.001) were solely during periods of wakefulness and not different during NREM sleep (P = 0.294) (35).
Fig. 2.
There is a state-dependent effect on pulmonary ventilation (V̇I); A and breathing frequency (B) of a muscarinic receptor antagonist unilaterally dialyzed into the ventral respiratory column (VRC) as 50 mM atropine increased V̇I and breathing frequency in the awake state but not during nonrapid eye movement (NREM) sleep (two-way repeated-measures ANOVA). Circles are data during the awake state, and squares are data during NREM sleep. Closed symbols and black line indicate period of atropine dialysis. *P < 0.005, significant difference between state; †P < 0.05, comparing values with 1 or more points in the predialysis period by post hoc analysis (Holm-Sidak).
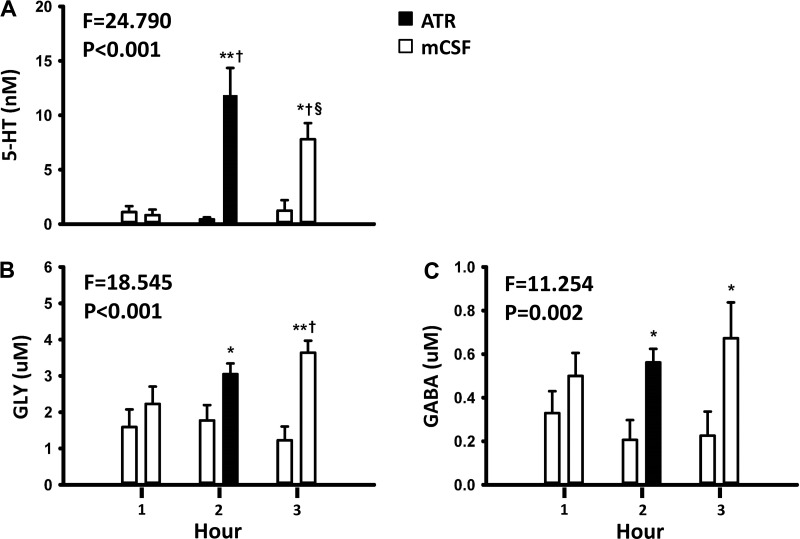
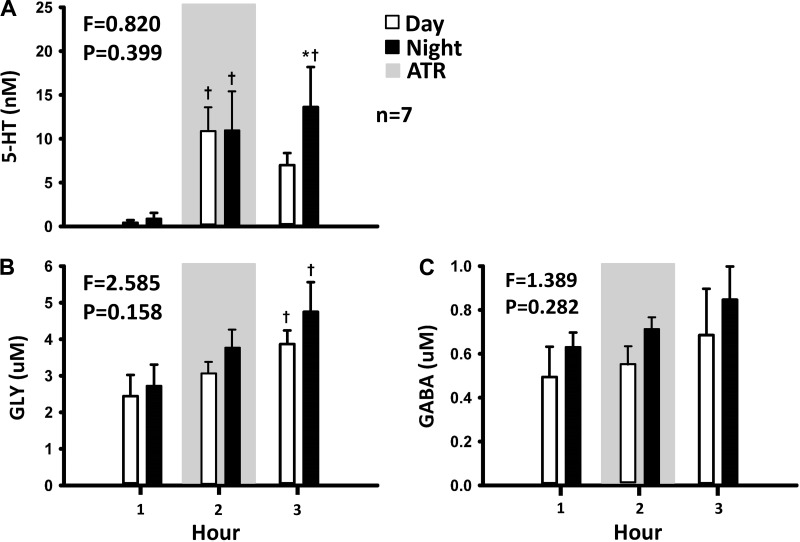
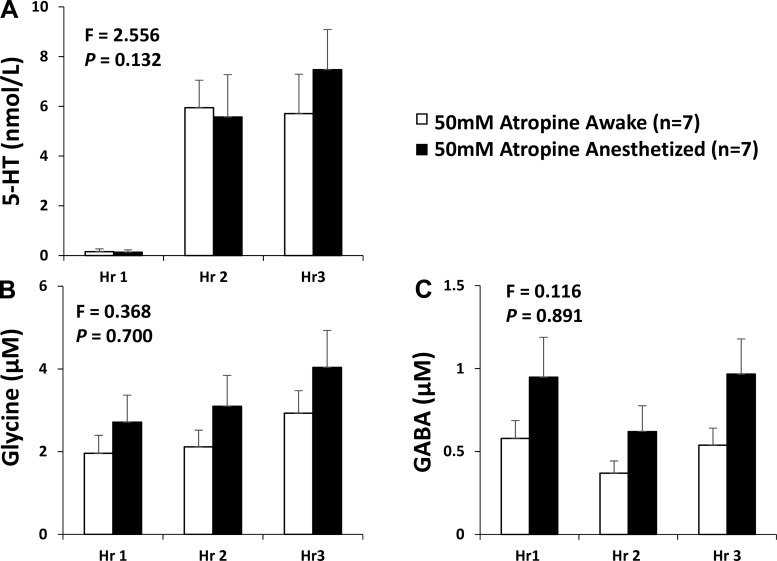
The HPLC analyses of the collected effluent mCSF revealed that dialysis of mCSF alone for 3 h did not alter the concentration of any measured neurochemical (Fig. 3) (35). However, in day studies when 50 mM atropine were dialyzed during hour 2 (drug delivery), the concentrations of 5-HT, glycine, and GABA were greater during and after atropine dialysis (hours 2 and 3; Fig. 3) (35) than before atropine dialysis (hour 1). Because of the relatively short periods of NREM sleep, the effluent mCSF collection system did not permit separation of the neurochemical data into awake and NREM sleep states; thus we were only able to compare day versus night neurochemical responses, which did not differ (Fig. 4) (35). Since the data at night were obtained during a mixture of awake and NREM sleep states, it is possible that the neurochemical responses to 50 mM atropine were state independent. However, because the dialysis is slow, differences between states might be hidden. To more directly test state dependence, we studied another group of seven goats while awake and while anesthetized with isoflurane. We found that the neurochemical responses to 50 mM atropine did not significantly (Fig. 5) (26) differ between awake and anesthetized states, indicating that this neurochemical response is indeed state- independent (26). This response during anesthesia was the same irrespective of whether arterial blood gases were maintained during anesthesia (by adjusting the ventilator settings) or arterial Pco2 was allowed to be relatively increased under anesthesia.
Fig. 3.
Dialysis of the muscarinic receptor antagonist atropine (ATR) into the ventral respiratory column (VRC) elicits apparent compensatory changes in other neuromodulators. Compared with dialysis of mock cerebral spinal fluid (mCSF) alone, the concentrations of serotonin (5-HT; A), glycine (GLY; B), γ-aminobutyric acid (GABA; C) were increased (P < 0.05) in the effluent mCSF during daytime unilateral dialysis into the VRC of 50 mM atropine. The x-axis is hour of dialysis. The 1st bar for each hour is when mCSF was dialyzed all 3 h while the 2nd bar is when 50 mM atropine was dialyzed during the 2nd hour (closed bar). F and P values are from two-way ANOVA. *P < 0.05, significant difference between treatments; **P < 0.001 between groups; †P < 0.001 comparisons with hour 1; §P < 0. 001, comparisons with hour 2 by by post hoc analysis (Holm-Sidak).
Fig. 4.
The effect of unilateral dialysis of 50 mM atropine into the ventral respiratory column (VRC) appears state-independent as shown by the finding that the mock cerebral spinal fluid (mCSF) effluent concentration of serotonin (5-HT; A), γ-aminobutyric acid (GABA; B), and glycine (GLY; C) did not differ (P > 0.05) between day and night studies even though the goats were in nonrapid eye movement for extended periods at night. The 1st and 2nd bars for each hour represent day and night studies, respectively. The shaded gray represents when 50 mM atropine (ATR) was dialyzed. F and P values are from two-way repeated measures ANOVA comparing day and night values *P < 0.05 between treatment groups, †P < 0.05 vs. hour 1.
Fig. 5.
The effect on compensatory neuromodulators of a muscarinic receptor antagonists dialyzed into the ventral respiratory column is state independent as indicated by the finding that the mock cerebral spinal fluid (mCSF) effluent concentration of serotonin (5-HT; A), glycine (B), γ-aminobutyric acid (GABA; C) did not differ (P > 0.05) between awake and isoflurane anesthesia studies. The 1st and 2nd bars for each hour represent awake and anesthetized states, respectively. F and P values are from two-way repeated measures ANOVA.
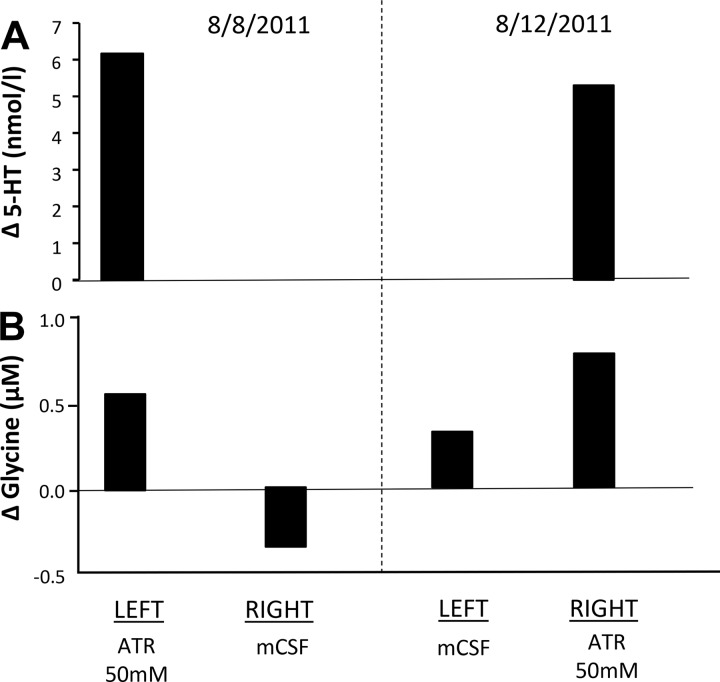
To determine whether the neurochemical responses to 50 mM atropine were global or local, we unilaterally dialyzed atropine into the VRC during hour 2 of the protocol while dialyzing mCSF for all 3 h on the contralateral site. We found that the neurochemical changes only occurred on the side of 50 mM atropine dialysis (Fig. 6), which suggests the neurochemical effects were indeed local (35).
Fig. 6.
Release of compensatory neuromodulators in response to a muscarinic receptor antagonist is locally mediated and not reflex as indicated by increases in serotonin (5-HT; A) and glycine (B) at the site of 50 mM atropine (ATR) dialysis but not at the contralateral site. Data are from an individual goat studied on 2 different dates with mock cerebral spinal fluid (mCSF) dialyzed for 3 h at one site, and on alternate days, 50 mM atropine were dialyzed at the contralateral site during hour 2 of dialysis.
To determine whether the responses to atropine were dose dependent, we unilaterally dialyzed a lower dose (5 mM) of atropine into the VRC. During the day, there was no effect on V̇I and f of 5 mM atropine dialysis (Fig. 7) (36), but at night there was a significant increase in f while awake but not NREM sleep during dialysis of 5 mM atropine (data not shown). Dialysis of 5 mM atropine did not alter concentration of any measured neurochemical. These data led to the conclusion that low-dose atropine had a small but measurable, circadian-dependent effect on breathing and had no measurable effects on local neurochemicals.
Fig. 7.
The effect on pulmonary ventilation (V̇I; A) and breathing frequency (B) of atropine dialysis is dose-dependent as indicated by the finding that in contrast to dialysis of 50 mM atropine (Fig. 1), dialysis of 5 mM atropine (this figure) into the ventral respiratory column did not affect V̇i and breathing frequency differently (P > 0.028) from dialysis of mock cerebral spinal fluid (mCSF) (two-way repeated measures ANOVA). Open circles are data when mCSF was dialyzed over the entire period, and squares are when 5 mM atropine was dialyzed between 60 and 120 min (closed squares and black line indicate period of atropine dialysis).
Since M2 receptors are mainly presynaptic (1, 13) located and inhibitory to neuronal activity, we hypothesized that dialysis of a specific M2 receptor antagonist would reduce inhibition of presynaptic release of excitatory neuromodulators and thus increase breathing. On the other hand, since M3 receptors are predominately postsynaptic (13, 27), we performed additional studies to test the hypothesis that dialysis of a specific M3 receptor antagonist should decrease breathing (36). Accordingly, in the same goats used for dialysis of 50 mM atropine, we unilaterally dialyzed a specific M2 antagonist (methoctramine hydrate, 50 mM), or a specific M3 receptor antagonist (4-diphenylacetoxy-N-methylpiperidine methiodide, 5 mM) in the awake state. Neither antagonist had any effect on V̇I or f, and furthermore did not significantly alter any of the measured neurochemicals (36). Thus the hypotheses were not supported, indicating that the effect of 50 mM atropine on V̇I and f and neurochemicals was not due to a differential effect of atropine on pre- vs postsynaptic muscarinic receptors.
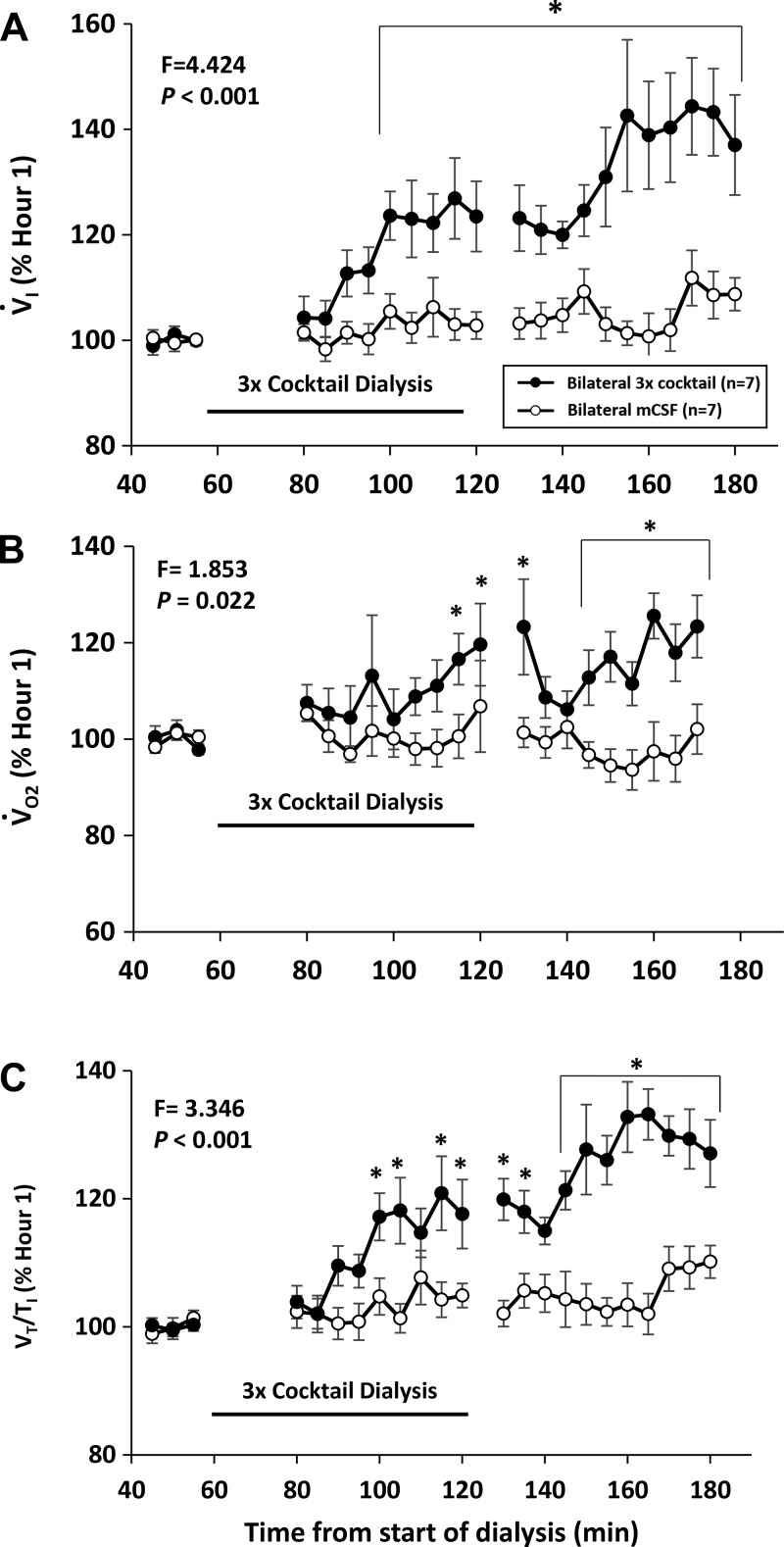
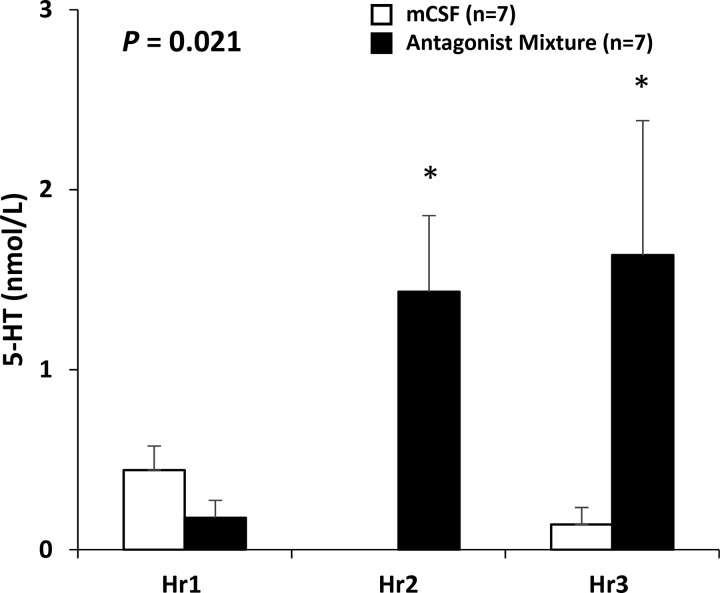
Others have shown that increased or decreased activation of neurokinin-1 (NK-1) receptors (7, 8) or 5-HT2A (14) receptors increased and decreased the respiratory activity of rodents in vitro, respectively. However, we found in awake and sleeping goats that unilateral dialysis of NK-1 receptor antagonists had no significant effect on V̇I, f, or measured neurochemicals (38). Similarly, unilateral dialysis of an antagonist to 5-HT2A receptors (MDL 11939) had no effect on V̇I, f, or measured neurochemicals (37). Moreover, we found that unilateral dialysis of mixtures of combinations of two or three antagonists to NK-1, M3, and 5-HT2A receptors had no effect on V̇I, f (Fig. 8) (37) or measured neurochemicals in awake and sleeping goats (37). Because it is possible that compensation during unilateral dialysis could occur by a reflex-mediated increase in activity on the contralateral site, bilateral dialysis was performed of the antagonists to the three excitatory receptor antagonists, which significantly (P < 0.001) increased V̇I, f, Vt/Ti (an index of ventilatory drive), and V̇o2 (metabolic rate) during day studies (Fig. 9) (37), and at night during both awake and NREM sleep (37). Effluent mCSF 5-HT was increased (P = 0.033) during both day (Fig. 10) (37) and night studies of bilateral dialysis (37). However, it is uncertain whether the increased 5-HT mediated the increased breathing because the 5-HT antagonist MDL 11939 should have blocked the activity of the excitatory 5-HT2A receptors.
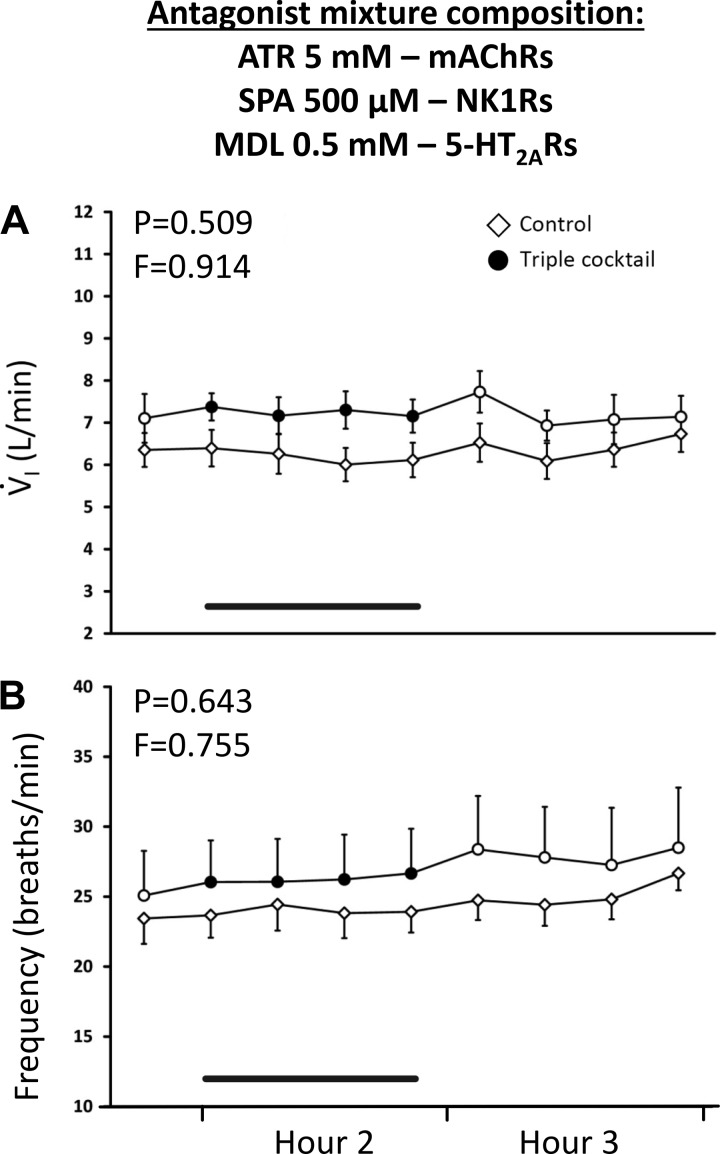
Fig. 8.
Simultaneous blockade of 3 excitatory receptor neuromodulators was insufficient to alter pulmonary ventilation (V̇I; A) and breathing frequency (B) as indicated by the finding that unilateral dialysis into the ventral respiratory column during the day of a triple mixture of antagonist [5 mM atropine (ATR), 500 µM spantide (SPA), and 0.5 mM MDL 11939] had no effect on V̇I or breathing frequency. The x-axis is time from start of dialysis. Open triangles are data when mock cerebral spinal fluid (mCSF) was dialyzed over the entire period, and circles are when the mixture was dialyzed during hour 2 of dialysis (closed squares and black line indicate period of cocktail dialysis). F and P are values from interaction term of two-way repeated measures ANOVA. mAChRs, muscarinic acetylcholine receptors.
Fig. 9.
Bilateral dialysis into the ventral respiratory column (VRC) of a triple mixture of excitatory receptor antagonists had a paradoxical effect as indicated by an increased pulmonary ventilation (V̇I; A), metabolic rate (V̇o2; B), and ventilatory drive (Vt/Ti; C). Open circles are when mock cerebral spinal fluid (mCSF) was dialyzed for 3 hours and closed circles are when the cocktail was dialyzed during hour 2 of dialysis. The x-axis is time from beginning of dialysis. F and P are values from interaction term of two-way repeated measures ANOVA. *P < 0.05, post hoc significant difference between treatments.
Fig. 10.
Bilateral dialysis into the ventral respiratory column of the triple mixture of excitatory receptor antagonists increased the concentration of 5-HT in the effluent mock cerebral spinal fluid (mCSF) over values when mCSF was dialyzed for 3 h. The 1st bar for each hour is when mCSF was dialyzed all 3 h while the 2nd bar is when the triple mixture was dialyzed during the 2nd hour (closed bar). *During the hour of and the hour after antagonists dialysis, 5-HT was significantly increased above mCSF dialysis.
Summary and Conclusions
First, no single or combination of unilateral or bilateral dialysis of three excitatory receptor antagonists decreased breathing, which suggests there may have been an increase in another excitatory neuromodulator or decrease in an inhibitory neuromodulator to compensate for the presumed antagonist-induced decreased excitation. Second, the significant increases in 5-HT in the effluent mCSF during both 50 mM atropine dialysis and the bilateral dialysis of the triple mixture of excitatory receptor antagonists support the concept that increased release of compensatory neuromodulators is one mechanism of interdependence of neuromodulators. Third, during unilateral dialysis of excitatory receptor antagonists, the changes in compensatory neuromodulators were at the site of dialysis but not at the contralateral site, which suggests that the 5-HT release is not mediated by reflex mechanism. Fourth, unilateral dialysis 50 mM atropine dialysis increased breathing frequency in the awake state but not during NREM sleep nor when the goats were anesthetized, but there was increased 5-HT during awake, anesthetized and at night during a mixture of awake and sleep states. These date suggest that the ventilatory but not the 5-HT release are affected by mechanisms that have a depressant effect on the brain. Fifth, the absence of a change in breathing or any measured neurochemical during unilateral dialysis of the 5-HT2A antagonist or the NK-1 antagonist alone or in combination suggests unmeasured neurochemicals or mechanisms downstream of these receptors may have compensated for the presumed decreases in excitatory receptor activity. Finally, bilateral dialysis of the mixture of antagonists to three excitatory receptors increased breathing with only a relatively small compensatory increase in 5-HT in the effluent mCSF. This finding does not support the hypothesis that the compensation during unilateral dialysis was in part due to increased reflex activity on the contralateral site. We speculate these data indicate there was a compensatory increase in some unmeasured neuromodulator or that compensation occurred through mechanisms downstream from neuromodulators.
SITE-SPECIFICITY OF EFFECTS ON BREATHING OF ANTAGONISTS TO MUSCARINIC, PEPTIDERGIC, AND SEROTONERGIC RECEPTORS
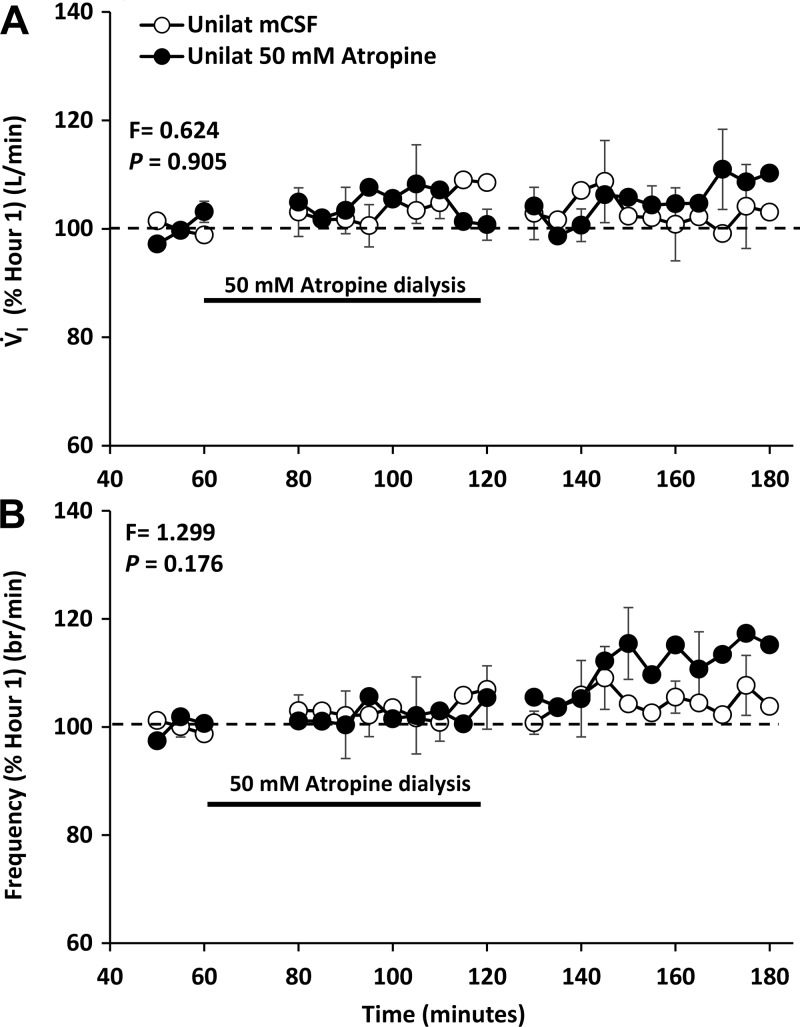
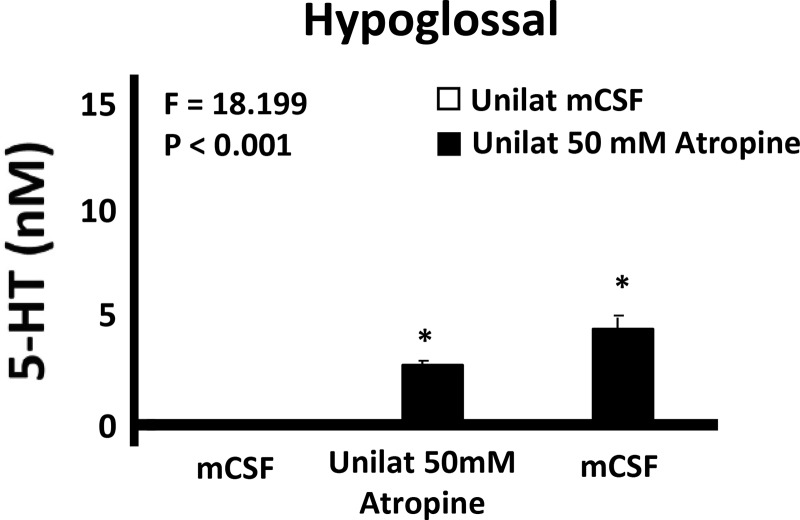
We chose the HMN to test site specificity because this nucleus is composed primarily of motoneurons in contrast to the respiratory rhythm- and pattern-generating neurons of the VRC. In addition, others have previously studied neuromodulation of the HMN (1, 12, 18) and found state-dependent and reflex drive to the upper airway in rodents (15). Thus we reasoned it would be valuable to study state dependence of neuromodulator interdependence in the HMN in our intact goat model. Based on previous findings that muscarinic receptors in the HMN are primarily inhibitory through M2 receptors (1, 13), we tested the hypothesis that dialysis of 50 mM atropine into the HMN would increase breathing and upper airway motor activity, or consistent with the concept of neuromodulator interdependence, atropine would not alter breathing or motor activity. Similarly, since during the awake state in rats it has been shown that antagonists to 5-HT receptors have minimal effect on breathing (49), we hypothesized that the mixture of antagonist to 5-HT2A, muscarinic, and NK-1 receptors would not alter breathing. Indeed, we found that neither 50 mM atropine (Fig. 11) (25) nor the mixture of excitatory receptor antagonists significantly altered V̇I, f, or VT in the awake state (25). However, at night during NREM sleep, VT was significantly (P = 0.017) decreased (data not shown) during and after unilateral dialysis of the antagonist mixture. We found that 5-HT in the effluent mCSF during 50 mM atropine dialysis (Fig. 12) (25) significantly (P < 0.001) increased to ~50% of the increase during 50 mM atropine dialysis (Fig. 3) (35) into the VRC (25, 35). This 5-HT increase during atropine dialysis may have offset the presumed disinhibition of cholinergic M2 receptors through stimulation of 5-HT1B receptors, which have been found to be inhibitory to upper airway muscle activity (15, 46). No other measured neurochemical was altered during dialysis into the HMN of 50 mM atropine or the mixture of three antagonists.
Fig. 11.
There is a site- dependent effect on pulmonary ventilation (V̇I; A) and breathing frequency (B) of unilateral dialysis of 50 mM atropine as dialysis into the hypoglossal motor nuclei did not (P = 0.905) alter V̇I and breathing frequency which contrast to the marked hyperpnea after atropine dialysis into the ventral respiratory column (Fig. 1). The black line denotes when atropine was dialyzed.
Fig. 12.
Unilateral dialysis of 50 mM atropine has an apparent site-independent effect on release of 5-HT ventral respiratory column as indicated by increased 5-HT after atropine dialysis into both the hypoglossal motor nuclei (this figure) and the ventral respiratory column (Fig. 3). Values are presented for the 3 h of dialysis when atropine was dialyzed during hour 2. *P < 0.001, comparisons with hour 1 by post hoc analysis (Holm-Sidak),
Summary and Conclusions
First, the presumed attenuated inhibitory M2 receptor activity during dialysis of 50 mM atropine into the HMN did not increase V̇I, f, VT, or hypoglossal muscle activity, which suggests there may have been a compensatory increase in another inhibitory neuromodulator or a decrease in an excitatory neuromodulator. Second, dialysis of 50 mM atropine increased the content of 5-HT in the effluent mCSF. Through stimulation of inhibitory 5-HT1B receptors (46), the 5-HT increase presumably compensated for the presumed reduced M2 inhibition. This increased 5-HT in the effluent mCSF during and after 50 mM atropine dialysis along with the increased 5-HT during atropine dialysis into the VRC indicates that this robust 5-HT response is not specific to a single brainstem region, a single neuronal subtype, or to a specific change in neuronal activity. Fourth, the reduced VT during dialysis of the excitatory receptor antagonist mixture at night during NREM sleep but not in the awake state supports the findings of Horner et al. (15) that reflex drives to upper airway muscles are state dependent. Finally, the absence of any change in neurochemicals in the effluent mCSF during dialysis of the triple antagonist mixture suggests compensation must be by unmeasured neurochemicals or by mechanisms downstream of neuromodulator receptors.
EFFECTS OF DIALYSIS OF AN AGONIST TO A µ-OPIOID G PROTEIN-COUPLED INHIBITORY RECEPTOR IN THE VRC
The physiologic effects of administration of an agonist to a µ-opioid G protein-coupled inhibitory receptor were studied for two reasons: first, the exponential increase over the last few years in human deaths resulting from an apparent overdose from synthetic opioids, likely as a result of opioid-induced respiratory depression, emphasizes the need to better understand the affected sites in the brain, and by what mechanism opioids depress breathing. Second, the role of the preBötC region of the VRC in respiratory depression of breathing is somewhat controversial. In previous studies, we found in awake goats that injections bilaterally into VRC of the µ-opioid agonists DAMGO has no effect on eupneic breathing (21). On the other hand, Montandon et al. (34) have shown that dialysis of DAMGO into the preBötC of awake and sleeping rats depresses breathing and in anesthetized rats causes apnea. Different still are the findings of Mustapic et al. (39) that dialysis of DAMGO into the preBötC of decerebrate dogs increases breathing. These differences in the effects of opioids within the VRC have been a subject of a point-counterpoint review article (33), further suggesting that more research in this area is a major need in the field.
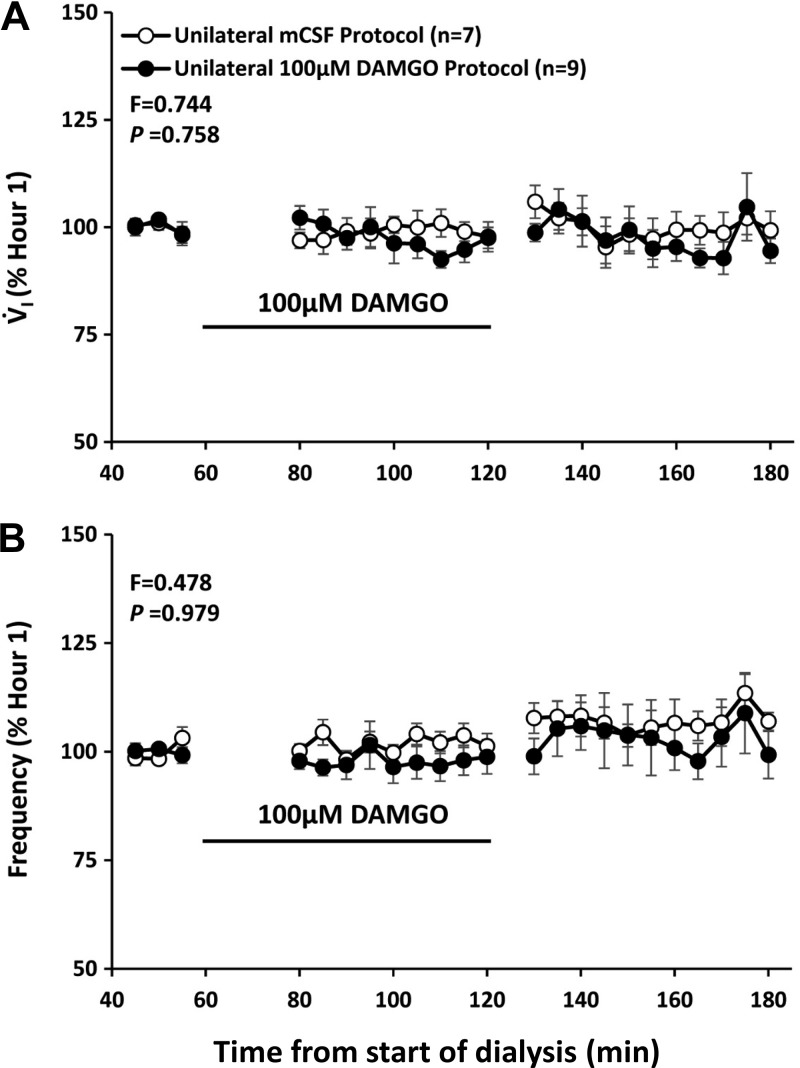
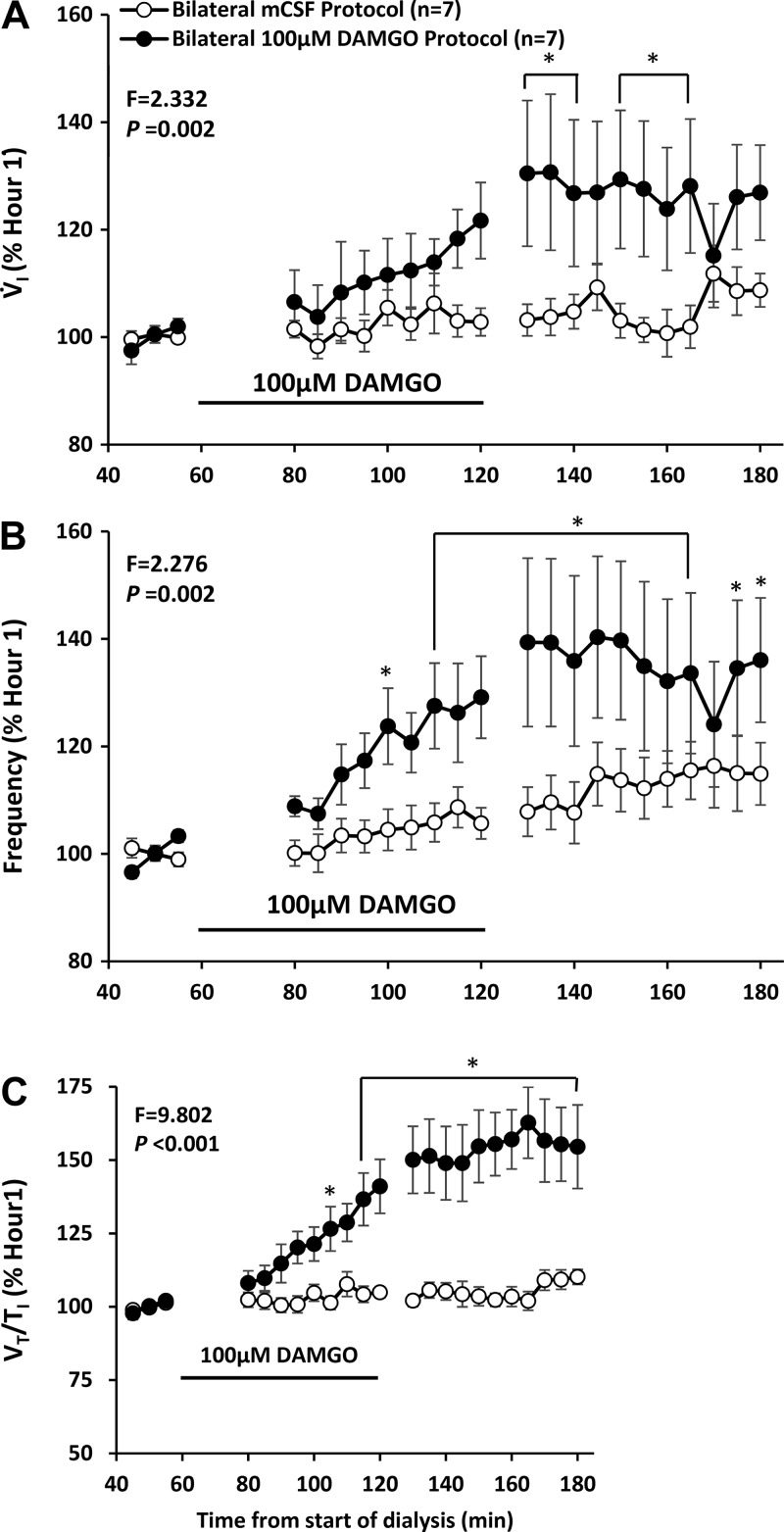
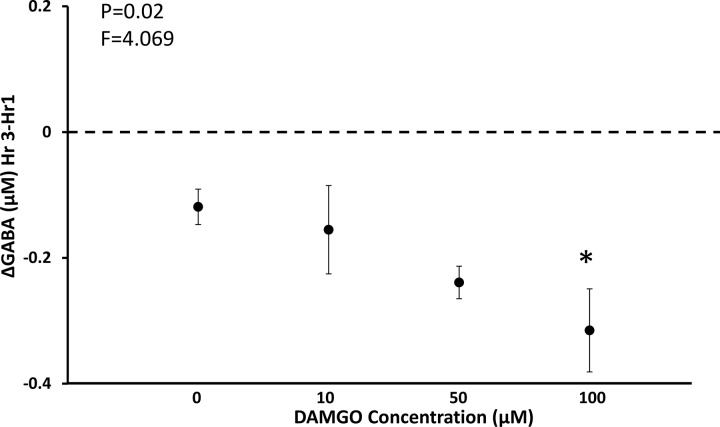
Our published studies showed that unilateral dialysis of 10, 50, and 100 µM DAMGO in the VRC did not significantly (P = 0.758) alter V̇I or f compared with dialysis of mCSF alone (Fig. 13) (24). However unilateral DAMGO dialysis in the VRC did cause irregular breathing in several goats, and bilateral dialysis of 100 µM DAMGO in this region induced significantly (P < 0.001) irregular breathing in all goats (24). Moreover, bilateral dialysis of 100 µM DAMGO in the VRC significantly increased V̇I, f, and Vt/Ti while awake during the day (Fig. 14) (24) and at night and while asleep at night (24). During unilateral dialysis during day studies, we found a significant (P = 0.042), dose-dependent decrease in the concentration of GABA in the dialyzed effluent mCSF (Fig. 15) (24), suggesting that a decrease in this endogenous inhibitory neurotransmitter could offset, to some degree, the increased DAMGO-induced exogenous inhibition. However, during bilateral 100 µM DAMGO dialysis during the day and at night, there was no statistically significant change in GABA or any other measured neurochemical (24).
Fig. 13.
Increased activation of µ-opioid inhibitory receptors by unilateral dialysis of 100 µM of [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO; closed circles) into the ventral respiratory column did not (P = 0.758) alter pulmonary ventilation (V̇i; A) and breathing frequency (B) compared with dialysis of mock cerebral spinal fluid (mCSF) (open circles). The black line denotes when DAMGO was dialyzed.
Fig. 14.
Increased activation of µ-opioid inhibitory receptors by bilateral dialysis of 100 µM d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) into the ventral respiratory column paradoxically increased pulmonary ventilation (V̇i; A), breathing frequency (B), and ventilatory drive (Vt/Ti; C). Open circles are when mCSF was dialyzed for 3 h and closed circles are when DAMGO was dialyzed during hour 2 of dialysis. F and P are values from interaction term of two-way repeated measures. *P < 0.001 comparisons to the pre DAMGO dialysis period by post hoc analysis (Holm-Sidak).
Fig. 15.
There was a dose-dependent decrease in the concentration of the endogenous inhibitory receptor agonist GABA when the exogenous inhibitory receptor agonist [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) was dialyzed into the ventral respiratory column. F and P values are from a one-way ANOVA.
Summary and Conclusions
First, unilateral dialysis of DAMGO into the VRC did not alter any measured ventilatory variable during the day or at night. Second, bilateral dialysis of DAMGO into the VRC increased V̇I, f, and Vt/Ti, which presumably indicates a DAMGO-induced increased excitability of neurons rather than the prevalent concept that opioids depress neuronal excitability. While this overall excitatory effects of opioids may be indirect, others have elucidated a cellular mechanism whereby opioids directly increase excitability of neurons. For example, Zhang et al. (55) found in rats that DAMGO depresses inhibitory synaptic neurotransmission in the ventral tegmental area and in the periaqueductal gray. In addition, Lalley and Mifflin (23) found, in anesthetized rats, that threshold doses of the µ-opioid receptor agonist fentanyl induced increased excitability of VRC neurons, as indicated by lowering of the threshold for firing, through the enhancement of spike train oscillations and via prolongation of discharges. Also, Sánchez-Blázquez et al. (43) have provided data indicating disruption of a µ-opioid receptor-NMDA receptor complex with administration of opioids, which enhanced postsynaptic NMDA receptor currents and could provide the increase excitability suggested by our studies under physiologic conditions. Accordingly, our working hypothesis is that bilateral µ-opioid-induced increased excitability of VRC neurons through decreased endogenous GABA inhibition by a presynaptic opioid effect to decrease release of GABA and thereby decrease postsynaptic inhibition or the increased excitation could be due to increased postsynaptic activity through disruption of the µ-opioid receptor-NMDA receptor complex that increases NMDA receptor activity.
SITE-SPECIFICITY OF EFFECTS OF AN AGONIST TO A µ-OPIOID G PROTEIN-COUPLED INHIBITORY RECEPTOR IN THE HMN
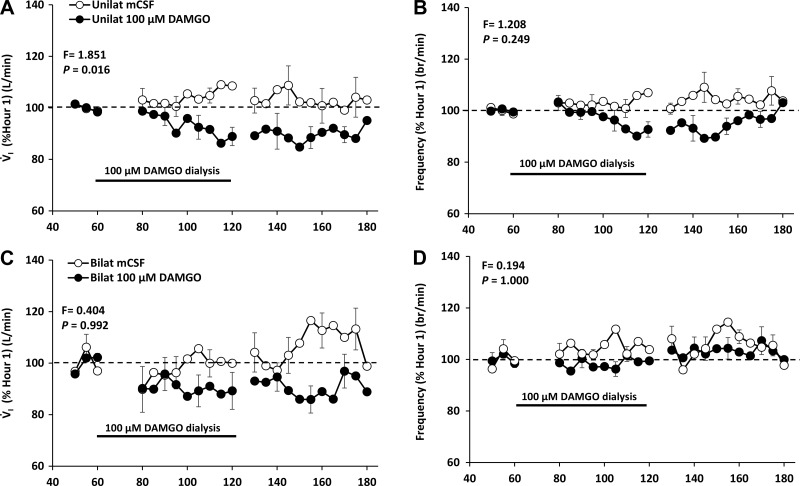
Others have found that dialysis of µ-opioid receptor agonists into HMN depressed respiratory output and genioglossal muscle activity (12). Therefore, we tested the hypothesis that unilateral and bilateral dialysis of DAMGO into the HMN would depress respiratory output and genioglossal activity in awake and sleeping goats. A finding of depressed respiratory output would suggest an absence of compensatory neuromodulation, and so we also hypothesized that DAMGO dialysis in the HMN would not elicit changes in neuromodulators measured in the effluent mCSF. Unilateral (Fig. 16) (25) and bilateral dialysis of 100 µM DAMGO into the HMN decreased (P < 0.001) activity of the genioglossal muscle (25). This finding provides physiological evidence that the dialysis was into the HMN. However dialysis of DAMGO into the HMN did not alter activity of the diaphragm (Fig. 16) (25).
Unilateral dialysis of 100 µM DAMGO into the HMN during the day significantly (P < 0.001) decreased V̇I but not f or VT whereas bilateral dialysis of DAMGO did not change (Fig. 17) (25) any measured ventilatory variable (25). In addition, unilateral but not bilateral 100 µM DAMGO significantly (P < 0.01) decreased metabolic rate and heart rate (data not shown), which suggests that the dialysate may have diffused beyond the HMN (into the neighboring nucleus tractus solitarii, for example). At night there were no significant effects of unilateral dialysis of 100 µM DAMGO in either awake and NREM sleep states (25). DAMGO was not dialyzed bilaterally at night into the HMN. Finally, dialysis of DAMGO into the HMN did not alter the concentration of any neurochemical measured in the effluent mCSF.
Fig. 17.
There is a site-dependent effect on pulmonary ventilation (V̇i; A and C) and breathing frequency (B and D)of dialysis of a µ-opioid inhibitory receptor agonist. Unilateral (A and B) and bilateral (C and D) dialysis of the agonist (closed circles) into the HMN decreased P < 0.016) or did not change (P = 462) V̇i and breathing frequency. These findings contrast to unchanged V̇i and breathing frequency (Fig. 13) or increased values (Fig. 14) during dialysis into the ventral respiratory column (VRC). The black line denotes when DAMGO was dialyzed.
Summary and Conclusions
First, a major finding from unilateral dialysis of DAMGO into the HMN during the day was a decrease in genioglossal muscle activity with no change in diaphragm activity. These data coupled with a decrease in V̇I during unilateral DAMGO dialysis suggests increased airway resistance may have caused the reduce airflow. Second, there were no changes in other ventilatory variables or in measured neurochemicals in the effluent mCSF during unilateral dialysis. Third, there were no significant changes in any ventilatory variables or measured neurochemicals during bilateral DAMGO dialysis. The difference in effects between unilateral and bilateral dialysis of DAMGO could be due to greater diffusion of DAMGO to neighboring nuclei (such as nucleus tractus solitarii) during bilateral dialysis. This lack of physiologic changes during bilateral DAMGO dialysis differs greatly from the overall excitatory effects after bilateral dialysis of DAMGO into the VRC. Also differing from VRC studies was that 100 µM DAMGO did not significantly affect ventilatory variables at night during either awake or NREM sleep. Overall, these findings do not support the concept of neuromodulator interdependence at this brainstem site. This major differences in effects of dialysis of µ-opioids into the VRC and HMN suggests site-specificity of mechanism of interdependence of neuromodulators. In other words, the cellular mechanisms summarized in effects of dialysis of an agonist to a µ-opioid g protein-coupled inhibitory receptor in the vrc that potentially account for the increased excitability with bilateral DAMGO dialysis into the VRC apparently are not characteristic of the HMN.
EFFECTS OF AN AGONIST TO AN IONOTROPIC INHIBITORY RECEPTOR
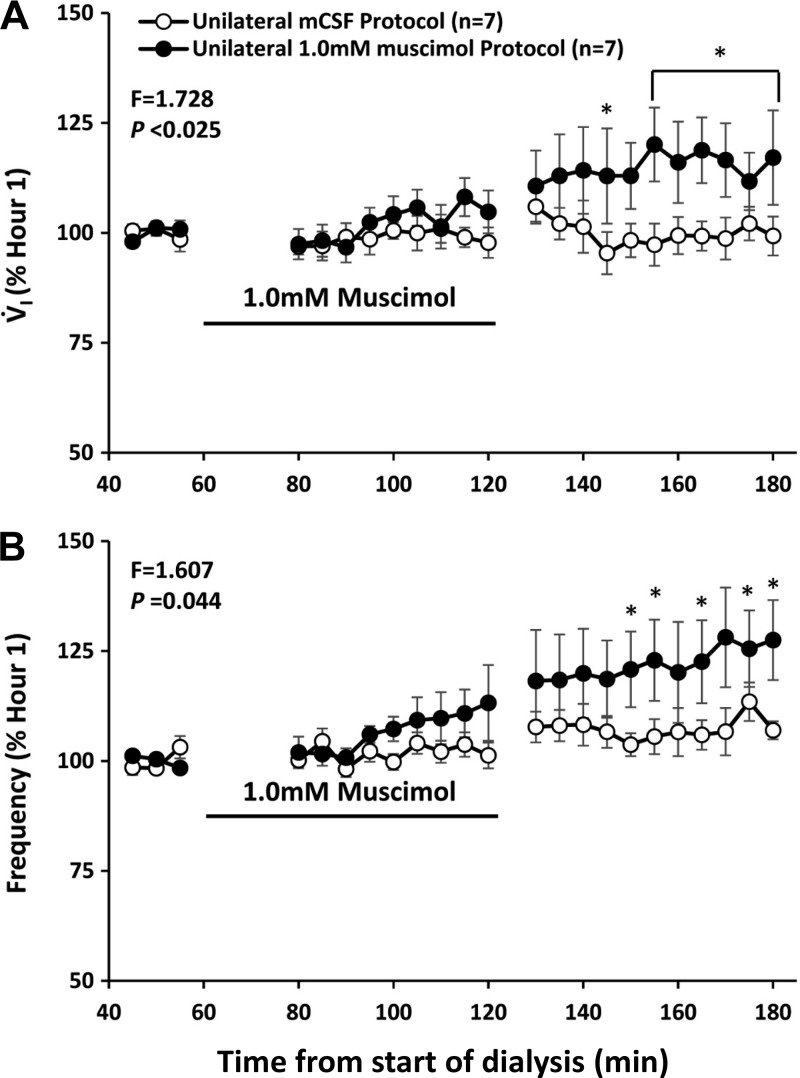
It is unknown whether neuromodulator interdependence occurs when the activity of ionotropic receptors is altered and whether compensation occurs by similar or different mechanisms to increases in inhibitory signaling through G protein-coupled receptors. Acting through GABAA ionotropic receptors, GABA decreases neuronal activity by altering intracellular chloride (51). There are conflicting reports on whether muscimol, a GABAA agonist, increases or decreases breathing (6, 41, 50, 54) when administered to the respiratory control network. Accordingly, we tested the hypothesis that dialysis of muscimol into the VRC of awake goats would decrease breathing, but there would be no change in any of the measured neurochemicals in the effluent mCSF. We unilaterally dialyzed muscimol at concentrations of 0.5, 1.0, and 10.0 mM, which is over the range others (cited above) have used in studies on awake and sleeping rats and piglets. We found that when ventilatory variables during and after muscimol dialysis were expressed as a percent of the periods just before muscimol dialysis, there was a significantly increased V̇I (P < 0.025), increased f (P < 0.044) (Fig. 18 for 1.0 mM), and decreased expiratory time (P < 0.001) comparing all muscimol doses and/or when each elevated muscimol concentration was individually compared with the mCSF control study (24). We found no significant change in any measured neurochemical with muscimol dialysis, with the exception that in four of six goats there was an increased norepinephrine concentration in the effluent mCSF during dialysis of 10.0 mM muscimol. The concentration of muscimol used also elicited varied degrees of behavioral excitability, nystagmus, spastic limb movements, and postural instability lasting up to 4 h after dialysis. These effects were not seen with agonists/antagonists to G protein-coupled receptors previously tested. In three of nine goats, these effects were too severe to warrant continuation of the study beyond the second hour.
Fig. 18.
Unilateral dialysis into the ventral respiratory column of 1 mM muscimol (closed circles), an ionotropic GABA receptor agonist paradoxically increased pulmonary ventilation (V̇i; A) and breathing frequency (B) compared with dialysis of mCSF (open circles). The black line denotes when muscimol was dialyzed. *P < 0.001, comparisons with the pre DAMGO dialysis period by post hoc analysis (Holm-Sidak).
Summary and Conclusions
Herein we found what could be interpreted as overcompensation for increases in inhibitory ionotropic GABAA receptor activity, and an indication that increased norepinephrine could contribute to these responses. Thus our overall hypotheses were not verified. A mechanism of GABAA receptor-mediated increases in norepinephrine has been proposed by Scatton and Bartholini (44), who found that administration of a GABAA receptor agonist in the rat hypothalamus activated noradrenergic neurons and increased norepinephrine turnover. Noteworthy is that our studies on GABAA receptor activation are the only studies in which we have observed an increase in norepinephrine. It is also possible that muscimol acts presynaptically on GABA receptors to increase release of glutamate and postsynaptic neuronal activity to thereby increase breathing (17, 19). The behavioral responses to muscimol were very similar to the previously observed behavioral responses of goats to injections into the VRC of ibotenic acid, which is an agonist of glutamate receptors that causes irreversible excitation to induce neuronal death (53). Both ibotenic acid and muscimol are derived from the mushroom known as Amanita Muscaria (52). It likely the metabolism of muscimol results in increased ibotenic acid, which then increased stimulation of glutamate receptors that resulted in increased V̇I and behavioral excitation. Irrespective of the mechanism and/or pathway, the ventilatory responses to muscimol indicate that compensation and/or secondary mechanisms exist that can prevent respiratory depression during perturbations of inhibitory ionotropic receptors.
CAVEATS AND LIMITATIONS OF THESE STUDIES
As stated in all our published manuscripts (cited earlier), a limitation of our studies is uncertainty regarding the exact placement of microtubules and the uncertainty regarding diffusion of dialyzed substances. Nevertheless, in all published articles we provide physiologic and immunohistochemistry data indicating that placement of the microtubules likely resulted in dialyzed substance reaching the target site.
Because of interaction or redundancy among different neuromodulators and/or downstream pathways, Brezina (3) concluded “the standard experimental paradigm that uses exogenous modulator application to identify the modulatory actions is grossly inadequate.” He added that an additional limitation and/or problem could result from “extracellular processing of enzymes transforming active modulators into other modulators with different dynamics” (3). Indeed, Huxtable et al. (16) have shown that the enzymatic breakdown of ATP to ADP and adenosine results in a dynamic interaction between the actions of ATP at P2 receptors and ATP metabolites on P2Y and P1 receptors. Similarly, in our studies, the presumed breakdown of muscimol to ibotenic acid likely resulted in a clear stimulation of breathing during dialysis of muscimol, presumably as a result of activation of glutamate receptors (53) rather than the expected direct effect of muscimol on GABAA inhibitory receptors. In addition, we have found that the muscarinic receptor antagonist atropine mixed in mCSF is degraded to form substance P in solution and the NMDA glutamate receptor antagonist AP-5 mixed in mCSF can be degraded to spontaneously form glycine in measurable amounts (unpublished observations). In both cases, the metabolites/breakdown products could potentially effect breathing and thus alter the conclusions of the study. Our HPLC analyses did not identify any other breakdown product or biproduct of experimental drugs administered, and we only measured the eight substances targeted in the assays, which highlights the limitations of an HPLC-based, targeted approach to the measurement of local neurochemicals in vivo.
Overall Summary and Conclusions
Several conclusions regarding neuromodulation are warranted from our studies under the conditions of physiologic wakefulness and sleep. First, studies dialyzing one or more receptor agonist or antagonist that did not result in altered breathing lend support to the concepts of interdependence, redundancy, degeneracy within the systems controlling neuromodulatory excitability within the respiratory control network. Second, in response to atropine-induced reductions in excitatory cholinergic neuromodulation, there was a robust compensatory increase in the excitatory neuromodulator 5-HT indicating that perhaps one mechanism of compensation is increased release of other compensatory neuromodulators. Third, as outlined by Marder et al. (28), there are multiple potential mechanisms protecting against overmodulation, yet unilateral dialysis of 50 mM atropine into the VRC and bilateral dialysis into the VRC of a triple mixture of excitatory receptor antagonist or bilateral dialysis of a µ-opioid agonists into the VRC elicited large increases in breathing indicating overcompensation; thus there appears to be limitations to the mechanisms proposed by Marder et al. to prevent overcompensation. Fourth, studies that did not identify an altered compensatory neuromodulator when perturbing neuromodulatory receptors do not necessarily indicate absence of a neurochemical change since only a select few modulators were measured, suggesting more advanced methods for large-scale screens of local changes in neurochemicals may be necessary. Furthermore, our method of neurochemical analysis (HPLC) could have been too insensitive to detect low levels of neurochemicals. Fifth, there are major site-specific physiologic and neurochemical effects of dialyzing substances that alter neuromodulatory receptor activity. These differences indicate that when multiple sites are perturbed simultaneously as occurs after intravenous injections, the physiologic responses cannot likely be attributed to any single site. Sixth, dialysis into the VRC of muscimol, an agonist of GABAA inhibitory receptors, unexpectedly increased breathing and caused behavioral arousal without significantly affecting local neurochemicals. Finally, in spite of limitations of the summarized studies where neuromodulator agonist or antagonists were dialyzed into the brain during physiological wakefulness or sleep, these studies have clearly identified compensatory changes and/or indirect effects that can occur during experimental perturbations of neuromodulators and more importantly may be occurring in our everyday lives as a result of breakdown products of ingested and absorbed food and medications.
GRANTS
The funding for studies summarized herein was from National Heart, Lung, and Blood Institute Grants HL-25739, HL-112996, HL-007852 and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.V.F. conceived and designed research; H.V.F. performed experiments; H.V.F. analyzed data; H.V.F. interpreted results of experiments; H.V.F. prepared figures; H.V.F. drafted manuscript; H.V.F. edited and revised manuscript; H.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank colleagues Dr. Lawrence Pan and Dr. Matthew Hodges; graduate students Dr. Clarissa Muere, Dr. Thomas Langer III Nicholas Burgraff, and Dr. Justin Miller; laboratory manager Suzanne Neumueller; and consultants Dr. Astrid Stucke and Dr. J. M. Ramirez for multiple contributions to the research described herein.
REFERENCES
- 1.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758–3770, 1996. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- 2.Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol (1985) 109: 159–170, 2010. doi: 10.1152/japplphysiol.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci 365: 2363–2374, 2010. doi: 10.1098/rstb.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brice AG, Forster HV, Pan LG, Brown DR, Forster AL, Lowry TF. Effect of cardiac denervation on cardiorespiratory responses to exercise in goats. J Appl Physiol (1985) 70: 1113–1120, 1991. doi: 10.1152/jappl.1991.70.3.1113. [DOI] [PubMed] [Google Scholar]
- 5.Comroe JH, Schmidt CM. The part played by reflexes from the carotid in the chemical regulation of the respiration in the dog. Am J Physiol 121: 75–97, 1938. doi: 10.1152/ajplegacy.1937.121.1.75. [DOI] [Google Scholar]
- 6.Curran AK, Chen G, Darnall RA, Filiano JJ, Li A, Nattie EE. Lesion or muscimol in the rostral ventral medulla reduces ventilatory output and the CO2 response in decerebrate piglets. Respir Physiol 123: 23–37, 2000. doi: 10.1016/S0034-5687(00)00143-2. [DOI] [PubMed] [Google Scholar]
- 7.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol 210: 459–472, 1966. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- 10.Forster HV, Ohtake PJ, Pan LG, Lowry TF, Korducki MJ, Aaron EA, Forster AL. Effects on breathing of ventrolateral medullary cooling in awake goats. J Appl Physiol (1985) 78: 258–265, 1995. doi: 10.1152/jappl.1995.78.1.258. [DOI] [PubMed] [Google Scholar]
- 11.Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173: 244–255, 2010. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol 587: 2677–2692, 2009. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada S, Houtani T, Trifonov S, Kase M, Maruyama M, Shimizu J, Yamashita T, Tomoda K, Sugimoto T. Histological determination of the areas enriched in cholinergic terminals and M2 and M3 muscarinic receptors in the mouse central auditory system. Anat Rec (Hoboken) 293: 1393–1399, 2010. doi: 10.1002/ar.21186. [DOI] [PubMed] [Google Scholar]
- 14.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol 164: 222–232, 2008. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol (1985) 116: 325–336, 2014. doi: 10.1152/japplphysiol.00531.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci 29: 14713–14725, 2009. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience 138: 25–35, 2006. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol 532: 467–481, 2001. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga H, Ishibashi H, Shimada H, Jang IS, Nakamura TY, Nabekura J. Activation of presynaptic GABAA receptors increases spontaneous glutamate release onto noradrenergic neurons of the rat locus coeruleus. Brain Res 1046: 24–31, 2005. doi: 10.1016/j.brainres.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol (1985) 106: 605–619, 2009. doi: 10.1152/japplphysiol.90966.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol (1985) 107: 1591–1599, 2009. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalley PM, Mifflin SW. Oscillation patterns are enhanced and firing threshold is lowered in medullary respiratory neuron discharges by threshold doses of a μ-opioid receptor agonist. Am J Physiol Regul Integr Comp Physiol 312: R727–R738, 2017. doi: 10.1152/ajpregu.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer TM 3rd, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV. Effects on breathing of agonists to μ-opioid or GABAA receptors dialyzed into the ventral respiratory column of awake and sleeping goats. Respir Physiol Neurobiol 239: 10–25, 2017. doi: 10.1016/j.resp.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer TM 3rd, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV. Ventilation and neurochemical changes during µ-opioid receptor activation or blockade of excitatory receptors in the hypoglossal motor nucleus of goats. J Appl Physiol (1985) 123: 1532–1544, 2017. doi: 10.1152/japplphysiol.00592.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer TM 3rd, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV. State-dependent and -independent effects of dialyzing excitatory neuromodulator receptor antagonists into the ventral respiratory column. J Appl Physiol 122: 327–338, 2016. doi: 10.1152/japplphysiol.00619.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience 63: 207–221, 1994. doi: 10.1016/0306-4522(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 28.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Pan LG, Qian B, Forster HV. The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respir Physiol Neurobiol 157: 242–251, 2007. doi: 10.1016/j.resp.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martino PF, Forster HV, Feroah T, Wenninger J, Hodges M, Pan LG. Do neurotoxic lesions in rostral medullary nuclei induce/accentuate hypoventilation during NREM sleep? Respir Physiol Neurobiol 138: 59–75, 2003. doi: 10.1016/S1569-9048(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 31.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol (1985) 115: 1088–1098, 2013. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montandon G, Horner R. Rebuttal from Gaspard Montandon and Richard Horner. J Physiol 592: 1167, 2014. doi: 10.1113/jphysiol.2013.268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31: 1292–1301, 2011. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol (1985) 114: 694–704, 2013. doi: 10.1152/japplphysiol.00634.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Evidence for respiratory neuromodulator interdependence after cholinergic disruption in the ventral respiratory column. Respir Physiol Neurobiol 205: 7–15, 2015. doi: 10.1016/j.resp.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muere C, Neumueller S, Olesiak S, Miller J, Langer T, Hodges MR, Pan L, Forster HV. Combined unilateral blockade of cholinergic, peptidergic, and serotonergic receptors in the ventral respiratory column does not affect breathing in awake or sleeping goats. J Appl Physiol (1985) 119: 308–320, 2015. doi: 10.1152/japplphysiol.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muere C, Neumueller S, Olesiak S, Miller J, Hodges MR, Pan L, Forster HV. Blockade of neurokinin-1 receptors in the ventral respiratory column does not affect breathing but alters neurochemical release. J Appl Physiol (1985) 118: 732–741, 2015. doi: 10.1152/japplphysiol.00884.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol 103: 409–418, 2010. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narimiya M, Yamada H, Matsuba I, Ikeda Y, Tanese T, Abe M. Adrenergic modulation of insulin and glucagon secretion from the isolated perfused rat pancreas. Endocrinol Jpn 28: 281–292, 1981. doi: 10.1507/endocrj1954.28.281. [DOI] [PubMed] [Google Scholar]
- 41.Nattie E, Li A. Muscimol dialysis into the caudal aspect of the nucleus tractus solitarii of conscious rats inhibits chemoreception. Respir Physiol Neurobiol 164: 394–400, 2008. doi: 10.1016/j.resp.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch 435: 485–494, 1998. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Blázquez P, Rodríguez-Muñoz M, Berrocoso E, Garzón J. The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain. Eur J Pharmacol 716: 94–105, 2013. doi: 10.1016/j.ejphar.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 44.Scatton B, Bartholini G. Drug-induced changes of epinephrine turnover in the rat hypothalamus. Life Sci 29: 1161–1170, 1981. doi: 10.1016/0024-3205(81)90205-8. [DOI] [PubMed] [Google Scholar]
- 45.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol 83: 1243–1252, 2000. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol 76: 799–807, 1996. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- 47.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- 49.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med 172: 1338–1347, 2005. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 50.Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphé region of conscious rats. Respir Physiol Neurobiol 153: 203–216, 2006. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Tillakaratne NJ, Medina-Kauwe L, Gibson KM. Gamma-aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol 112: 247–263, 1995. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 52.Tsujikawa K, Mohri H, Kuwayama K, Miyaguchi H, Iwata Y, Gohda A, Fukushima S, Inoue H, Kishi T. Analysis of hallucinogenic constituents in Amanita mushrooms circulated in Japan. Forensic Sci Int 164: 172–178, 2006. doi: 10.1016/j.forsciint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol (1985) 97: 1629–1636, 2004. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 54.Yamada KA, Hamosh P, Gillis RA. Respiratory depression produced by activation of GABA receptors in hindbrain of cat. J Appl Physiol Respir Environ Exerc Physiol 51: 1278–1286, 1981. doi: 10.1152/jappl.1981.51.5.1278. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Yang HL, Song JJ, Chen M, Dong Y, Lai B, Yu YG, Ma L, Zheng P. DAMGO depresses inhibitory synaptic transmission via different downstream pathways of μ opioid receptors in ventral tegmental area and periaqueductal gray. Neuroscience 301: 144–154, 2015. doi: 10.1016/j.neuroscience.2015.05.077. [DOI] [PubMed] [Google Scholar]