Abstract
The prevalence of obesity and type 2 (T2D) diabetes is a major health concern in the United States and around the world. T2D is a complex disease characterized by pancreatic β-cell failure in association with obesity and insulin resistance in peripheral tissues. Although several genes associated with T2D have been identified, it is speculated that genetic variants account for only <10% of the risk for this disease. A strong body of data from both human epidemiological and animal studies shows that fetal nutrient factors in utero confer significant susceptibility to T2D. Numerous studies done in animals have shown that suboptimal maternal environment or placental insufficiency causes intrauterine growth restriction (IUGR) in the fetus, a critical factor known to predispose offspring to obesity and T2D, in part by causing permanent consequences in total functional β-cell mass. This review will focus on the potential contribution of the placenta in fetal programming of obesity and TD and its likely impact on pancreatic β-cell development and growth.
Keywords: diabetes, fetal programming, insulin secretion, islet, IUGR
INTRODUCTION
One of the primary factors affecting fetal nutrition is the maternal nutrient supply to the fetus, which is directly dependent on fetal placental growth and function. When the placenta malfunctions, it is unable to supply an adequate amount of oxygen, nutrients, and other factors to the growing fetus, and without this vital support, the fetus cannot grow and thrive to its full potential. Placental insufficiency can lead to intrauterine growth restriction (IUGR), a condition in which the fetus in utero fails to achieve its genetic full potential for growth and size. IUGR affects ~4–15% of pregnancies (125, 134), and maternal malnutrition during pregnancy is a risk factor for IUGR (13). A strong body of data from both human epidemiological and animal studies shows that fetal nutrient factors in utero confer significant susceptibility to type 2 diabetes (T2D). In most IUGR models, malprogramming of specific nutrient-sensitive tissues, such as the pancreatic β-cell, has been implicated (4, 153). The goal of this review is to provide a practical assessment of current models of IUGR with specific focus on the emerging role of placental insufficiency and its impact on insulin-producing β-cell development and growth in the fetus.
Placental insufficiency occurs when the placenta fails to provide the fetus sufficient nutrients and secreted factors. The inability of the placenta to meet fetal demand can be affected by a number of factors including altered maternal or fetal blood flow, reduced nutrient transport capabilities, changes in placental circulating hormone levels, and the content of exosomes secreted from the placenta to the fetus (42, 93, 149, 172). A mounting body of evidence suggests that IUGR is a critical determinant of neonatal morbidity and mortality (151), and the risk for chronic and metabolic disease throughout the life span (6, 162). Despite a wealth of investigations, the molecular mechanisms linking IUGR and susceptibility to metabolic diseases, such as obesity and T2D (4, 69, 92, 109, 144), remain poorly understood. As noted above, this review will focus on the impact of the placenta in fetal programming of T2D and on IUGR programming in β-cell dysfunction (21, 58).
We and others have shown that maternal nutritional stress, such as low-protein (LP) diet or total calorie restriction (CR) during pregnancy and uteroplacental insufficiency, are causes of IUGR and lead to long-term consequences in β-cell mass and function (4, 34, 50, 153, 155). β-Cell mass in adult humans is achieved during the first decade of postnatal life (64), and the β-cell population is maintained in adulthood by a very low rate of self-replication of existing β-cells (44). During development, β-cells are exquisitely sensitive to the fetal nutrient environment, which can ultimately impact final adult mass (156) and the risk for T2D later in life (3, 156). Accordingly, IUGR at birth is strongly associated with reduced β-cell mass, in part, due to decreased proliferation and increased apoptosis, and glucose intolerance in the adult offspring (3, 45, 74, 120). It is plausible to hypothesize, therefore, that total β-cell mass developed during these early stages can provide some measure of protection from or against risk for T2D, although there are no human data to directly support this concept.
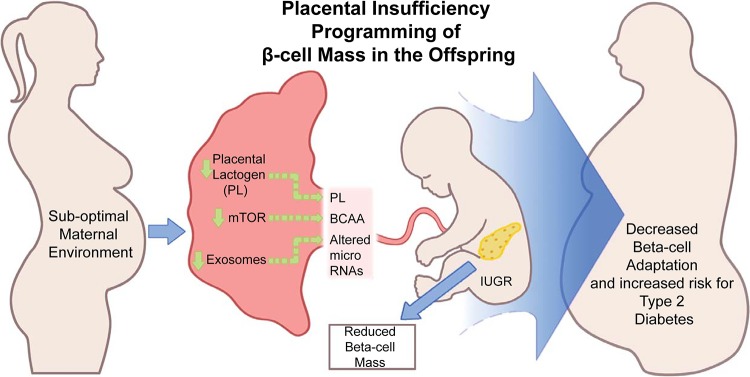
Unfortunately, there are limited data on human β-cell mass in IUGR-individuals. However, IUGR at birth is strongly associated with altered insulin sensitivity in children and adults (111), although it is important to point out that impaired insulin sensitivity is not sufficient to cause T2D by itself. In the state of insulin resistance, T2D only develops when there is a significant loss of functional β-cell mass and the amount of insulin secreted is no longer adequate to meet the demand. Thus the loss of β-cell mass and insulin secretion defects are the primary factors that determine whether an individual will develop T2D or not (52). Therefore, we will discuss the emerging roles of the nutrient-sensor kinase mechanistic target of rapamycin (mTOR) in the placenta, as well as placental lactogen (PL) and microRNAs exported by placental exosomes, in the regulation of fetal programming of β-cell mass across various animal models of placental insufficiency (Fig. 1). We focus on mTOR because this kinase functions to integrate both nutrient and growth factor signals in individual cells, including the β-cell, and more globally in organs such as the placenta, to promote growth and function.
Fig. 1.
Placental insufficiency programming of β-cell mass in the offspring. Fetal intrauterine growth restriction (IUGR) is a result of changes in suboptimal maternal intrauterine environment, which influences placental function. This includes several integrated factors, including but not limited to reduced activity of mechanistic target of rapamycin (mTOR) signaling pathways, specific hormones such as placental lactogen levels, and exosomal content (i.e., microRNAs). All of these factors may contribute to fetal IUGR and the development of pancreatic β-cell mass in the fetus. BCAA, branched chain amino acid.
FETAL UNDERNUTRITION MODELS
In this section, we will focus on key models of fetal undernutrition: uteroplacental insufficiency and maternal diet restriction. We will discuss current available data on the impact of these models on placental growth and pancreatic β-cell development and function and postulate possible mechanistic links between placental function and β-cell growth.
Placental Insufficiency
Animal and human epidemiological studies have shown a strong correlation between small birth size and chronic health diseases in adulthood (1). These include, but are not limited to, risk of developing cardiovascular disease, hypertension, obesity, and T2D (10). Placental insufficiency occurs secondary to impairments in trophoblast invasion, uteroplacental blood flow, and nutrient uptake, which all contribute to fetal undernutrition and IUGR. IUGR itself is a result of multiple and complex changes in the maternal intrauterine environment, which influence placental function, potentially including hypoxic and oxidative cell stress, metabolic dysfunction, and nutrient deprivation, and the downregulation of key placental proteins such as those in the mTOR signaling pathways and key regulators of placental development, nutrient uptake, and cellular stress response. We will discuss several animal studies to better understand the mechanisms underlying the impact of placental insufficiency on IUGR and the development of pancreatic β-cell mass.
Preeclampsia.
in humans.
Preeclampsia involves the onset of new or increased hypertension during pregnancy often with proteinuria. Approximately 2–8% of pregnancies involve the development of preeclampsia (27a). Incomplete placentation and superficial trophoblast invasion of the maternal decidua lead to ischemic conditions for the placenta (99), which drive maternal proinflammatory cytokines, antiangiogenic factors, and agonistic autoantibodies against the angiotensin II type I receptor, possibly by activation of the angiotensin II type I receptor, promoting hypertension in the mother (72, 91). Malfunction at the uteroplacental interface means a reduction of placental blood flow of 50–70% (99), as well as a reduction in placental integrity due to increase in apoptosis of syncytiotrophoblast cells (77). These changes can lead to a reduction in nutrient supply and altered transport of numerous factors across the placental barrier (67, 117), translating to an increased risk of preeclamptic birth and susceptibility to various chronic and metabolic diseases later in life. IUGR is found in 39% of preeclamptic pregnancies (167). Children of preeclamptic pregnancies have also been shown to have elevated blood pressure (159), smaller hearts with increased resting heart rate (54), increased body mass index (166), and increased rates of hospitalization for endocrine, nutritional and metabolic diseases (169). To our knowledge, the impact of preeclampsia on β-cell mass in human or animal model newborns has not been reported. However, it is important to note that IUGR, a common outcome of preeclampsia, is commonly associated with insulin resistance in humans (70, 111). From longitudinal and genome-wide association studies, evidence suggests that the failure of β-cells to meet the insulin demand is the tipping point for the progression to T2D (3). Thus it will be important to assess β-cell mass in human or animal model newborns of preeclamptic pregnancies.
in animals.
The reduced uterine perfusion pressure model (RUPP) in rats is commonly used to mimic human preeclampsia. Typically utilized in Sprague-Dawley rats, this model of preeclampsia restricts blood flow to the uterus through surgical occlusion of the uterine and ovarian arteries (typically done on day 14 of pregnancy on a 21-day gestation), creating placental ischemia. Uteroplacental blood flow is reduced by 40%, and maternal blood pressure is increased by 20–30 mmHg (33). Offspring of RUPP pregnancies demonstrate IUGR (5, 63) and have been observed to develop glucose intolerance in adulthood (74). Loss of β-cell mass or a defect in insulin secretion could explain the glucose intolerance phenotype, but β-cell mass and function phenotype in fetal RUPP offspring are unknown. Presently, there are very few studies defining the causes of the increased risk for T2D in the RUPP-preeclampsia model.
Maternal uterine artery ligation.
A model of uteroplacental insufficiency involving surgical ligation of an artery or arteries supplying blood to the placenta has also been shown to induce IUGR in the offspring. These procedures induce a partial infarction of the placenta, reducing the nutrient transfer capacity of the placenta and leading to an IUGR phenotype in the fetus. Sprague-Dawley rats, when subjected to uterine artery ligation on day 18 of a 21.5-day gestation, had IUGR offspring showing decreased placental weight with reduced plasma concentrations and fetal-to-maternal ratios of amino acids (leucine, isoleucine, and valine) and reduced circulating insulin levels (115). When ligation was performed on day 19, the IUGR offspring developed obesity, glucose intolerance, insulin resistance, and T2D in adulthood (153). However, normal β-cell mass was observed between IUGR and control animals early in life (1 and 7 wk of age), suggesting a critical time window of programming to impact permanent basal β-cell mass. Interestingly, despite normal mass in early life, the β-cell mass was significantly reduced relative to controls, to 50% by 15 wk of age and to ~30% by 26 wk of age (153). An age-associated decline in β-cell function has also been identified involving multiple defects in islets, from vascularization (78), amino acid metabolism, and ion channel expression to mitochondrial health (128, 152). Epigenetic regulation of gene expression (e.g., Pdx1) was reported as one mechanism by which uteroplacental insufficiency can lead to loss of β-cell mass and function (119, 123). Interestingly, placental expression of glucose transporter proteins in the pregnant rat was unchanged with uteroplacental insufficiency induced at a gestational day 19 (133). It would be of interest in the future to assess nutrient transporter activity when uteroplacental insufficiency is induced for a longer time frame of the gestational period.
Maternal hyperthermia in sheep.
Placental insufficiency is induced in sheep through exposure to increased ambient temperature, starting after 40 days into term. Hyperthermic treatment results in increased body temperature (39–40°C) in pregnant ewes. To maintain thermal homeostasis, blood flow is driven toward the peripheral vascular system, decreasing uterine and umbilical arterial pressure. Reduction in uteroplacental blood flow is sufficient to reduce birth weights of offspring and drive chronic dysfunction in metabolic tissue (11, 141). Basal plasma insulin concentrations were lower in IUGR compared with control (102), and this was associated with reduced β-cell mass (35) and improved insulin sensitivity (96). More recently, Kelly et al. (87) provided some insight into mechanisms underlying reduced β-cell growth and proliferation and defective insulin secretion in islets from IUGR fetuses by performing unbiased RNA sequencing. In this study, they reported that reduction of proliferation and dysregulated metabolism involved multiple genes that regulate cell cycle, growth factor signaling, mitochondrial function, and the exocytosis machinery. This study further implicated the novel role of Wnt signaling and the proteasome as other possible mechanisms of β-cell dysfunction. The impacts of maternal hypertension-induced IUGR on islet development and function in this model are reviewed extensively by Boehmer et al. (21).
Maternal Diet Restriction
Nutrient availability and dietary behavior of the mother during pregnancy control the nutrients that are being sensed or transported into the fetus and may contribute significantly to fetal programming. Mostly in animal models, the role of maternal diet restriction at different time points during or postpregnancy has been studied. For example, studies have attempted to manipulate maternal diet anywhere from the last week of pregnancy only to controlling diet throughout the entire pregnancy and lactation period. This varied approach is helpful to tease apart any critical “developmental windows” and to ascertain whether a certain phase of development (early embryogenesis, midorganogenesis, and later maturation) is more sensitive to the maternal environment in utero. Below we will discuss the impact of maternal diet manipulation in human and in rodents and their impact on the pancreatic β-cell mass.
in humans.
There are very few studies in humans that directly investigate the contribution of the placenta in the relationship of maternal malnutrition on fetal programming. One study showed that placental abnormalities have been associated with cases of IUGR, using available data arising from consequences of the Dutch famine (1944–1945). This investigation included the long-term tracking of 94,800-famine cohorts and 212,900-control cohorts. Data arising from this large cohort study showed strong correlations between low birth weight and susceptibility to obesity and T2D. Women who experienced malnourishment during the third trimester of pregnancy and during the first month after birth had children with significantly lower obesity rates (130). However, children whose mothers experienced malnourishment during the first half of pregnancy showed a higher rate of obesity (130). Famine exposure during the second or third trimester of pregnancy significantly lowered the ratio of placental area to birth weight when compared with nonexposed babies or babies exposed only during the first trimester (129). The placental area was reduced irrespective of the exposure period pregnancy (118). Moreover, the adult offspring of women during the Dutch famine showed higher mean glucose concentrations, especially individuals who were exposed to the famine in midgestation and late gestation. Individuals who were reported to be born as IUGR babies showed glucose intolerance, mainly when they became obese in adulthood (129). The perinatal exposure group also showed a significant increase in fasting proinsulin and mean insulin concentrations (129). It was suggested that the impaired glucose tolerance seen in the mid- and late-gestation group may have been due to defects in insulin secretion (37). IUGR babies have been shown to have reduced insulin sensitivity (164) and fail to compensate for their reduced insulin sensitivity when they become obese in adulthood, putting them at a higher risk for developing T2D (25). The pancreatic β-cell fraction in IUGR human fetuses was only slightly lower with no differences in the percentage of β-cell reduction in fetal pancreatic tissues (16). However, a loss of β-cell number was reported in a small group of pregnancies complicated by severe fetal growth restriction (161). Hence, a role for pancreatic β-cells cannot be left out of the equation when addressing defects in insulin sensitivity of IUGR babies.
in mice.
The two most common maternal dietary manipulations to induce IUGR in the offspring are low-protein (LP) diet and total calorie restriction (CR) during pregnancy alone or until the end of lactation. Isocaloric reduction of protein concentration in the maternal diet during gestation has been demonstrated to not alter the food intake or the body weight of the dams (4). However, the effect of LP on offspring body weight has been variable in mice. Alejandro et al. (3) showed no alteration in body weight at birth, while others (31, 158) reported lowered body weight in the offspring group at days 1 and 7. It is important to note that the reduction of body weight observed was independent of litter size at birth. In some studies, IUGR offspring were reported to experience catch-up growth (see review in Ref. 84), showing a recovered body weight around day 14 (31), but Su et al. (158) reported persistent decreased body weight in LP offspring throughout adulthood. Despite a strong reduction in calorie intake, no differences in litter size and only about an 8% lower birth weight was observed in the CR mice (30). In a CR model (50% reduction in calorie intake), a significant decrease of the placental weight was seen at day 11.5 (148) or at both days 16 and 19 in a more severe CR study (80% reduction in calorie intake) (30). In the latter study, reduced placental weight was associated with decreased junctional zone volume and placental expression of glucose transporters, suggesting potential changes in placental nutrient transfer. In the same CR study, the placenta underwent morphological and functional adaptations, including long-term epigenetic changes and an alteration of the transcriptome (30, 60), to maintain fetal growth.
A strong correlation between placental insufficiency/IUGR and a loss of pancreatic β-cell mass also exists in mice. The offspring of Balb/c mice treated with an isocaloric LP (8%) diet from embryonic day 0.5 of gestation up until birth showed no impact on newborn β-cell mass but increased β-cell mass on day 14 (only female offspring) (31). However, all female offspring eventually developed glucose intolerance with a similar level of β-cell mass by day 130 (31). In utero LP-diet treatment also resulted in a reduced ability of both male and female offspring to recover their β-cell mass following streptozotocin-induced β-cell death (31). The offspring of C57BL/6J dams treated with isocaloric LP (9%, embryonic day 0.5 to birth) showed a reduction in β-cell area at birth but normalized mass in adulthood (4). Despite normalization of β-cell mass in these mice, offspring of the LP group demonstrated mild glucose intolerance and improved insulin sensitivity (4). The offspring of C57BL/6J dams treated with LP diet from embryonic day 0.5 to the end of the lactation period showed adult glucose intolerance due to decreased β-cell mass and a defect in insulin secretion (158). It is important to note that the varying impact on β-cell mass suggests that the strain and the duration of LP treatment matter and that these parameters should be considered carefully when interpreting data.
in rats.
More studies have been done in rats in regard to maternal diet restrictions. The LP diet from embryonic day 2 throughout pregnancy increased total food intake in dams but significantly reduced maternal, fetal, and placental weight gain at embryonic day 21 (51, 79). Maternal undernourishment during the first 2 wk of gestation resulted in obesity and adipocyte abnormalities in offspring during the postnatal period (85). However, a shorter duration of dietary restriction had no effect on offspring adiposity (14), especially if treatment was done during the early phase of gestation (days 0–7) when the placenta is not yet fully developed and functional. LP offspring developed hyperphagia, a risk factor for obesity and a symptom associated with the development of diabetes (165). LP treatment of Wister rats throughout pregnancy and lactation resulted in a smaller birth weight in the offspring and increased development of insulin resistance and diabetes in adulthood (51). In Sprague-Dawley rats, there was no change in the fetal weight at birth but stunted growth of the offspring was reported due to undernutrition (LP 9% from birth to lactation) (157).
Wister rat dams fed LP diet (5%) during the last week of gestation showed alterations in placental weight associated with reduced insulin content and β-cell mass (17). Low-protein (8%) diet during pregnancy also resulted in growth-restricted offspring due to increased placental volume without a compensatory increase in vascularization of the placenta (43). Dams treated with 50% food restriction from gestational days 10 to 20 showed reduced placental weight and hypotrophy in placental regions (basal and labyrinth zones) (12). Together these data suggest that maternal diet restrictions impact placental development and thus could contribute to the metabolic phenotypes observed in the offspring.
In female Wistar rats, isocaloric LP (8%) during pregnancy reduced β-cell mass due to reduced proliferation, islet size, islet vascularization, and pancreatic insulin content and resulted in defects in islet insulin secretion in response to arginine or leucine (34). When the offspring were kept under LP diet even past the postnatal period, they had lower fasting insulin levels with normal circulating glucose (34). Sprague-Dawley rats treated with LP (9%) also demonstrated a reduction of islet area and the downregulation of insulin and relevant genes (FoxO1, Pdx1, and MafA) for islet formation and maturation (171). Multiple studies have implicated reduced expression of Pdx1 mRNA leading to decreased β-cell proliferation and β-cell size at days 2 (94), 15, or 21 (45) and epigenetic regulation of genes such as Hnf4a associated with the development of T2D (145). Similarly, pregnant rats restricted to only 50% of their food from day 15 of gestation resulted in IUGR offspring with significant reductions in β-cell mass and insulin content at postnatal days 1 and 21 (57, 106). No change in pancreatic weight at postnatal day 21 was observed, thus suggesting a specific impact on β-cell development (57, 106). When their diet restriction started at day 11 of the gestation period, IUGR rats developed glucose intolerance and insulin resistance in adulthood, along with defects in pancreas development (56, 170). See Table 1 for a selected list of studies highlighting the impacts of maternal diet during pregnancy and uteroplacental insufficiency models on placental weight, β-cell mass, and glucose intolerance in the offspring.
Table 1.
Summary of the effects of IUGR animal models in utero
| Model | Placental Weight | Fetal Weight | β-Cell Mass Or Area | β-Cell Function | GTT | ITT | AA Transport |
|---|---|---|---|---|---|---|---|
| Mouse | |||||||
| Restricted diet (LP and CR at e0.5–e19) | ↓ (29, 30, 148) | ↓ (30, 31) | ↔ (31) ↓ (4) | ↓ (4, 158) | ↓ (4, 31, 158) | ↑ (4) ↔ (158) | ↓ (149) |
| Rat | |||||||
| RUPP (e14.5–e19.5) | ? | ↓ (63) | ? | ? | ↓ (74) | ? | ? |
| Uterine artery ligation (e19–e21) | ? | ↓ (168) | ↓ (36) | ↓ (153) | ↓ (153) | ↓ (153) | ↓ (112) |
| Restricted diet (LP and CR) |
↓ (51) ↔ (155) | ↓ (51, 155) ↔ (157) | ↓ (34, 45, 57, 106, 155) | ↓ (34, 46) | ↓ (145) | ↓ (145) | ↓ (139) |
| Sheep | |||||||
| Hyperthermia-induced placental insufficiency | ↓ (97) | ↓ (95) | ↓ (95) | ↓ (97) | ? | ↑ (96) | ↓ (140) |
IUGR, intrauterine growth restriction; AA, amino acid; GTT, glucose tolerance test; ITT, insulin tolerance test; CR, calorie restriction; LP, low-protein diet; e, embryonic day; RUPP, reduced uterine perfusion pressure.
MOLECULAR MECHANISMS THAT MAY INFLUENCE β-CELL DEVELOPMENT AND PROLIFERATION IN UTERO
In humans, β-cell mass is achieved during the first decade of postnatal life (64) and the differentiated and mature pool of β-cells is maintained by a very low rate of self-replication in adulthood (44). Pregnancy and obesity are two physiological triggers of differentiated β-cell proliferation. MicroRNAs have been shown to regulate the expression of critical genes associated with β-cell proliferation, function, and death (4, 53, 101). As discussed above, studies suggest a correlation between uteroplacental insufficiency-induced IUGR offspring and a loss of β-cell mass in the offspring. However, the mechanistic link between placental function and β-cell development is not clear. Loss of β-cell mass is attributed to a reduction in β-cell proliferation rate and cell size (95, 158, 160) and, in some cases, increased apoptosis. Epigenetic marking of critical genes such as Pdx1 (119) and Hnf4a (145) has been reported as well as the downregulation of essential β-cell proteins like mTOR, PDK1, and cyclin D1/2 via microRNA regulation (4, 46, 158). The contribution of epigenetic mechanisms is very important and has been reviewed by Pinney and Simmons (122, 123). Below, we will discuss the impact of amino acid transporters, and PL hormone (8), and further speculate on the potential effects of microRNAs from placental exosome cargo that may be exported to the fetal pancreas to affect β-cell development and proliferation (7). We are specifically focusing on these potential mechanisms because they have been shown to affect β-cell proliferation and their levels are altered in IUGR-associated models.
Placental Transporter System
As an endocrine organ, the placenta adapts and responds to any alterations in the microenvironment by modulating transporter systems located on syncytiotrophoblasts, a two-layered epithelium placental barrier. These systems include distinct transporters for amino acids, fatty acids, and glucose and are expressed in both the microvillous membrane (maternal side) and the basal plasma membrane (fetus side) of syncytiotrophoblasts. To date, at least 21 amino acid transporter genes have been reported for their expression in human placenta and are mainly classified as accumulators and exchangers (28). Among these, the three main transporter systems are system A, system L, and system β. System A transporters are responsible for the uptake of nonessential amino acids and mostly linked to the microvillous membrane of syncytiotrophoblasts. Their activities are increased during pregnancy (39). Maternal LP diet was correlated to a downregulation of placental system A transporters occurring before fetal underdevelopment (149), suggesting that reduced activity of this placental protein in the offspring is a contributing factor for IUGR (61). System A transporter activity was significantly reduced in hypoxic conditions (10–13%) in pregnant mice (149). Essential amino acids are exchanged for nonessential amino acids through system L transporters and their expressions were reported to be the same as system A transporters during pregnancy (116). IUGR showed reduced leucine transport across the placenta due to reduced activity of system L transporters (82). Their activity was also reduced in a CR mouse model (55) and protein-restricted rat model (139). System β transporters regulate taurine uptake, and their activity is reduced in IUGR offspring leading to significantly lower plasma concentrations of taurine in fetuses (113). Amino acid levels (especially branched-chain amino acid like leucine) have been shown to significantly impact pancreatic progenitor cells and subsequently β-cell mass (49, 126) while supplementation of taurine was shown to normalize proliferation and apoptosis in IUGR rat pancreatic islets (22). Taurine supplementation during late gestation also improved postnatal growth of the IUGR rat offspring, but insulin resistance still developed later in adulthood (76). Fatty acid transporters in the placental barrier include lipases, fatty acid transport proteins, and fatty acid binding proteins. IUGR placentas demonstrated reduction in the mRNA expression of lipoprotein lipase and endothelial lipase (59) with reduced activity of lipoprotein lipase in preterm placenta (103). Although maternal obesity in mice has been linked to altered fatty acid binding protein expression in the placenta, leading to increased fetal lipid accumulation and growth (41), (104), its direct impact on β-cell development and function is not known. In the placenta, transport of glucose is mostly facilitated by GLUT1. Other glucose transporters, including GLUT3, GLUT4, GLUT8, GLUT9, GLUT10, and GLUT12, are also expressed in the placenta (89), but their expression did not change in IUGR (83). In rodents, both maternal undernutrition and high-fat diet resulted in increased GLUT1 placental expression (149), suggesting the ability of the placenta to adapt to the maternal nutrition environment. Both circulating glucose and oxygen availability to the growing fetal pancreas can have a great impact on β-cell growth and development (66, 73).
mTOR Signaling and Branched-Chain Amino Acids
mTOR is a nutrient-sensor that integrates diverse nutritional and environmental cues, including downstream signals of growth factor receptors, amino acids, energy levels, and cellular stress, to promote cellular growth. Existing in two complexes (mTORC1 and mTORC2), mTOR kinase is an ideal candidate linking the fetal environment to obesity and T2D susceptibility. First, reduced placental mTOR signaling is correlated to decreased fetal placental function in human and rodent models of IUGR (136, 137). Multiple studies involving both animal and human IUGR samples (LP diet, hypoxia, second-hand smoke, and obesity) show decreased placental mTOR activity (81, 88, 90, 107). Second, increased placental mTOR signaling is positively correlated with fetal overgrowth induced by maternal obesity in humans (42, 80, 147), suggesting that placental mTOR activity regulates placental function and nutrient transport. Despite these association studies done in human samples, there are limited studies investigating the molecular link between the increased risk for T2D after uteroplacental insufficiency-induced IUGR and specifically β-cell dysfunction. Although the mechanisms underlying this response are not clear, it may include mTORC1 regulation of placental growth and function, such as amino acid transport. Jonsson et al. (80) showed that mTOR protein was expressed in the transporting epithelium of the human placenta and in human IUGR placenta and that the activities of both the A and L placental amino acid transporter systems were decreased (136). mTOR has been shown to positively regulate system A transport of amino acids (137, 138) and the leucine transporter system in both villous explants of normal term placentae (136) and cultured trophoblast cells (137) at the posttranscriptional level (89). These data suggest that mTOR regulates placental amino acid transport and provide a possible mechanistic explanation for the changes in leucine transport in IUGR (137). On the other hand, activation of placental mTORC1 signaling and amino acid transporters was elevated in women who were obese and had given birth to large for gestational age babies (80). These studies show a strong correlation between mTOR signaling and birth weight, supporting the idea that mTOR functions as a placental nutrient sensor.
mTOR is ideally positioned to couple growth factor signaling and nutrient availability to match fetal growth by regulating placental nutrient transport. However, no causal studies exploring these mechanisms have been put forward to date. Indeed, decreased levels of most essential amino acids are also associated with IUGR fetuses (26, 27) in experimental dams of maternal isocaloric LP diet model in mice (18). Impairment in the placental transport of amino acids, with reduced utilization by the fetus, has been observed in the hyperthermia-induced ovine model of placental insufficiency (38, 140). In humans, maternal concentration of amino acids in IUGR-complicated pregnancies are as low as levels in nonpregnant women (40), and increasing the maternal amino acid concentration through intravenous infusion significantly increased the uptake of essential amino acids into fetuses (135). Although there is no direct evidence from animal studies to link fetal placental mTOR activity in utero to the fetal environment and β-cell dysfunction and susceptibility to T2D, we speculate that specific amino acids circulating to the fetus could have a potential impact on the developing pancreas and β-cell development. Supporting this hypothesis, a positive correlation was seen between mTOR activity and increased embryonic levels of branched-chain amino acid in the blastocyst cavity fluid of diabetic rabbits (68). Specific amino acids such as leucine that can activate mTOR had also been demonstrated to directly impact pancreatic progenitors and β-cell development in vitro (49, 126). With the use of genetic mouse models, mTOR and associated proteins of this pathway have been shown directly to regulate β-cell development, mass, and function (2, 20, 49, 127). Islets of LP offspring also showed reduced mTOR protein and activity, in part due to increased expression of microRNA 199 and 7 that target the mTOR pathway (4). Reconstituting mTOR signaling in β-cells during the last week of gestation in LP offspring (4) was sufficient to rescue the β-cell dysfunction in these mice. With the availability of genetic models with temporal regulation and new technology with high target specificity, such as CRISPR-Cas 9, future studies can directly test the requirement of placental mTOR [or protein(s) in the pathway] for the transfer of specific amino acids from the placenta to fetus, and whether supplementation of specific amino acids can reverse the impact of maternal diet restriction on β-cell mass in the fetus.
Effects of PL in β-Cell Proliferation and Development
PL, a hormone secreted solely by the placenta, could play an important role in the regulation of β-cell programming in response to suboptimal intrauterine environment. Indeed, PL is noted to play a pivotal role in maternal islet adaptations during pregnancy by potentiating β-cell function and proliferation (24). PL is known to stimulate insulin secretion and islet function in rodents and humans (23, 62, 163), independent of growth hormone (105) and insulin growth factor-I (19). To our knowledge, there is no direct evidence that PL could regulate β-cell development in the fetus during gestation. However, there is some evidence that PL is detected in the fetal circulation. PL was detected over the course of gestation, from 6 wk up to 30 wk, at levels of 1 g/day near term (150). However, the concentration in the fetal umbilical cord right after birth was found to be much lower (80–125 ng/ml) (86). PL was also detected at this lower concentration (80–120 ng/ml) in fetal circulation and at an even more depressed level (55–70 ng/ml) in the amniotic fluid (71). Thus, during midgestation in both human and sheep, plasma concentration of PL in the fetus exceeded the PL concentration in the umbilical cord at term (62). This suggests a direct secretion of PL into the fetal circulation. In regard to the direct effect of PL on β-cell proliferation in the growing fetus, it is unclear whether the concentration of PL that reaches the fetal pancreatic milieu is sufficient to activate their receptors in a way that would impact β-cell growth.
Within the placenta, human PL (hPL) has been proposed to have a role as a “maternal growth hormone” by stimulating the transfer of nutrients from mother to fetus through a potentiation of maternal glucose intolerance, lipolysis, proteolysis (65), and insulin resistance (142). When high-risk pregnant women were tested between 24 and 36 wk of gestation, their hPL concentrations were significantly lower in cases of preeclampsia and higher in gestational diabetes (48). Low maternal hPL levels have also been correlated to fetal growth restriction (9, 114). However, it remains unclear whether PL could regulate fetal islets the same way they modulate maternal mature islets (8). Thus future studies could assess the consequences of altered levels of PL or the expression of its receptor on β-cell development in IUGR offspring.
Exosomes and MicroRNAs
Exosomes are endosome-derived nanovesicles, which carry different molecules including microRNAs, noncoding RNAs, proteins, and lipids (98, 100, 131) to the extracellular environment and transport signals to distant tissues (e.g., the pancreas) to modulate cellular development, growth and function. Recently, the placenta has been found to secrete these nanovesicles into the maternal and fetal circulation during pregnancy (108). Thus it is possible to posit that exosomes exported by the placenta could reach the fetal pancreas and their cargo content (i.e., microRNAs) could affect β-cell proliferation. Interestingly, Baeyens et al. (8) suggested that placental-specific exosomes increase β-cell proliferation in human islets, suggesting that exosomes have progrowth properties. Although speculative, one can hypothesize that placental exosomes exported to the pancreas may play an active role in the proliferation and survival of β-cells both in the maternal and fetal pancreas in response to metabolic demands and developmental cues, respectively, during pregnancy. Long, noncoding RNAs have been shown to be dynamically expressed during β-cell mass expansion in the pregnant mouse and to control β-cell proliferation in vitro (154). Furthermore, increasing evidence has demonstrated that microRNAs have important roles in modulating β-cell gene expression and thereby could modify β-cell proliferation, function, and death (4, 53, 101). Secretion of exosomes substantially increases during pregnancy, and complications, including preeclampsia, are associated with even greater exosomal concentrations in maternal circulation (132). It is possible that some of the significant 50-fold increase in exosomes (108, 143, 146) during pregnancy could target β-cell development and growth. Both total and placental-derived exosomes increase with gestational age and positively correlate with placental weight (143). However, the direct relationship between secreted exosomes and their content and placental function and development is not clear (124). One can postulate that any alteration of the level and cargo composition of placental-specific exosomes, such as in preeclampsia-induced IUGR (121), may modulate β-cell proliferation and development in the growing fetus. Several reports suggest that alteration of specific microRNAs may cause a loss of β-cell mass and function in various IUGR models. For example, levels of microRNAs (miR199, miR7, miR375, and miR15b) were changed in IUGR offspring when compared with control (4, 46, 158). Dumortier and Van Obberghen (47) discussed the implication of microRNAs on the development of the pancreas and more specifically on β-cells. To date, placental-specific microRNAs have not been investigated to affect β-cell proliferation and function. Thus it will be of high scientific interest in the future to assess whether placental-exosomes are taken up by β-cells and the identity of any signaling molecules the exosomes carry and to test whether exosomes target β-cell proliferation and function.
Perspectives and Significance
The placenta as an endocrine organ plays an important role in coordinating adequate fetal growth by synthesizing and secreting critical factors that influence fetal growth and physiology. Despite decades of research, the placenta is the least understood organ, and published studies available today are particularly correlative. There is high need to develop genetic mouse models to show direct cause-effect relationship and impact of specific proteins in the placenta, such as the nutrient-sensor mTOR, that may modulate fetal programming. Subsequently, future studies should assess the efficacy of using these critical proteins as viable biomarkers for diagnosis of individuals who may be at risk for T2D. Finally, we need a greater understanding of how the placenta can communicate to the growing fetus, both in the context of health and disease. The discovery of exosomes and microRNAs as possible mechanisms of how the placenta communicates to the fetus is an important avenue to pursue. The role of placenta-derived exosomes in maternal adaptation to insulin resistance, and in particular their impact on the developing fetal pancreas, remain unknown. Future studies should be directed toward profiling placental exosomes in normal and IUGR-complicated pregnancy and assess their effects on human islet cell proliferation and death, as well as their function in vitro. Future studies could also assess the impact of exosomes in proliferation and apoptosis in vivo and induced pluripotent stem cells for β-cell differentiation. As other groups have demonstrated altered exosomes in IUGR and preeclampsia (121, 108), it is very likely that their cargo content can modulate β-cell development. The identification of specific microRNAs or other signaling molecules in exosomes is needed to allow us to identify their β-cell genes targets. This could be a significant step to discover the mechanistic link between placental function/secreted factors and β-cell proliferation and survival regulation, thereby setting up the stage for identifying molecular targets for β-cell replacement therapies.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants K01-DK-103823 and R03-DK-114465 (to E. U. Alejandro).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M., D.C.B., and E.U.A. drafted manuscript; R.M., D.C.B., and E.U.A. edited and revised manuscript; R.M., D.C.B., and E.U.A. approved final version of manuscript; E.U.A. conceived and designed research; E.U.A. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Robert Sorenson for discussion and advice. We thank Ronald Mark Barcenilla Ygona for assistance with Fig. 1.
REFERENCES
- 1.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med 41: 66–72, 2009. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- 2.Alejandro EU, Bozadjieva N, Blandino-Rosano M, Wasan MA, Elghazi L, Vadrevu S, Satin L, Bernal-Mizrachi E. Overexpression of kinase-dead mTOR impairs glucose homeostasis by regulating insulin secretion and not β-cell mass. Diabetes 66: 2150–2162, 2017. doi: 10.2337/db16-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol Aspects Med 42: 19–41, 2015. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, Bernal-Mizrachi E. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. J Clin Invest 124: 4395–4410, 2014. doi: 10.1172/JCI74237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. doi: 10.1016/S0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 6.Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr 52, Suppl 1: S34–S41, 1998. [PubMed] [Google Scholar]
- 7.Baeyens L. The Effects of Placental Exosomes on Human Islets of Various Sex and Age. American Diabetes Association 77th Scientific Sessions, San Diego, CA, 2017. [Google Scholar]
- 8.Baeyens L, Hindi S, Sorenson RL, German MS. β-Cell adaptation in pregnancy. Diabetes Obes Metab 18, Suppl 1: 63–70, 2016. doi: 10.1111/dom.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagga R, Vasishta K, Majumdar S, Garg SK. Correlation between human placental lactogen levels and glucose metabolism in pregnant women with intrauterine growth retardation. Aust NZ J Obstet Gynaecol 30: 310–313, 1990. doi: 10.1111/j.1479-828X.1990.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail 12: 819–825, 2010. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry JS, Davidsen ML, Limesand SW, Galan HL, Friedman JE, Regnault TR, Hay WW Jr. Developmental changes in ovine myocardial glucose transporters and insulin signaling following hyperthermia-induced intrauterine fetal growth restriction. Exp Biol Med (Maywood) 231: 566–575, 2006. doi: 10.1177/153537020623100511. [DOI] [PubMed] [Google Scholar]
- 12.Belkacemi L, Jelks A, Chen CH, Ross MG, Desai M. Altered placental development in undernourished rats: role of maternal glucocorticoids. Reprod Biol Endocrinol 9: 105, 2011. doi: 10.1186/1477-7827-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod 83: 325–331, 2010. doi: 10.1095/biolreprod.110.084517. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger L, Langley-Evans SC. Fetal programming of appetite by exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 109: 413–420, 2005. doi: 10.1042/CS20050127. [DOI] [PubMed] [Google Scholar]
- 16.Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes 51: 385–391, 2002. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- 17.Bertin E, Gangnerau MN, Bellon G, Bailbé D, Arbelot De Vacqueur A, Portha B. Development of β-cell mass in fetuses of rats deprived of protein and/or energy in last trimester of pregnancy. Am J Physiol Regul Integr Comp Physiol 283: R623–R630, 2002. doi: 10.1152/ajpregu.00037.2002. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 58: 559–566, 2009. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billestrup N, Nielsen JH. The stimulatory effect of growth hormone, prolactin, and placental lactogen on beta-cell proliferation is not mediated by insulin-like growth factor-I. Endocrinology 129: 883–888, 1991. doi: 10.1210/endo-129-2-883. [DOI] [PubMed] [Google Scholar]
- 20.Blandino-Rosano M, Barbaresso R, Jimenez-Palomares M, Bozadjieva N, Werneck-de-Castro JP, Hatanaka M, Mirmira RG, Sonenberg N, Liu M, Rüegg MA, Hall MN, Bernal-Mizrachi E. Loss of mTORC1 signalling impairs β-cell homeostasis and insulin processing. Nat Commun 8: 16014, 2017. doi: 10.1038/ncomms16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehmer BH, Limesand SW, Rozance PJ. The impact of IUGR on pancreatic islet development and β-cell function. J Endocrinol 235: R63–R76, 2017. doi: 10.1530/JOE-17-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia 45: 856–866, 2002. doi: 10.1007/s00125-002-0833-6. [DOI] [PubMed] [Google Scholar]
- 23.Brelje TC, Bhagroo NV, Stout LE, Sorenson RL. Beneficial effects of lipids and prolactin on insulin secretion and beta-cell proliferation: a role for lipids in the adaptation of islets to pregnancy. J Endocrinol 197: 265–276, 2008. doi: 10.1677/JOE-07-0657. [DOI] [PubMed] [Google Scholar]
- 24.Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132: 879–887, 1993. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 25.Brufani C, Grossi A, Fintini D, Tozzi A, Nocerino V, Patera PI, Ubertini G, Porzio O, Barbetti F, Cappa M. Obese children with low birth weight demonstrate impaired beta-cell function during oral glucose tolerance test. J Clin Endocrinol Metab 94: 4448–4452, 2009. doi: 10.1210/jc.2009-1079. [DOI] [PubMed] [Google Scholar]
- 26.Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261, 1990. doi: 10.1016/0002-9378(90)90860-A. [DOI] [PubMed] [Google Scholar]
- 27.Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol 158: 120–126, 1988. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- 27a.Chappell LC, Shennan AH. Assessment of proteinuria in pregnancy. BMJ 336: 968–969, 2008. doi: 10.1136/bmj.39540.657928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol 20: 419–426, 2008. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 29.Coan PM, Vaughan OR, McCarthy J, Mactier C, Burton GJ, Constância M, Fowden AL. Dietary composition programmes placental phenotype in mice. J Physiol 589: 3659–3670, 2011. doi: 10.1113/jphysiol.2011.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 588: 527–538, 2010. doi: 10.1113/jphysiol.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox AR, Gottheil SK, Arany EJ, Hill DJ. The effects of low protein during gestation on mouse pancreatic development and beta cell regeneration. Pediatr Res 68: 16–22, 2010. doi: 10.1203/PDR.0b013e3181e17c90. [DOI] [PubMed] [Google Scholar]
- 33.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000. doi: 10.1161/01.HYP.35.1.367. [DOI] [PubMed] [Google Scholar]
- 34.Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes 40, Suppl 2: 115–120, 1991. doi: 10.2337/diab.40.2.S115. [DOI] [PubMed] [Google Scholar]
- 35.Davis MA, Macko AR, Steyn LV, Anderson MJ, Limesand SW. Fetal adrenal demedullation lowers circulating norepinephrine and attenuates growth restriction but not reduction of endocrine cell mass in an ovine model of intrauterine growth restriction. Nutrients 7: 500–516, 2015. doi: 10.3390/nu7010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Prins FA, Van Assche FA. Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Biol Neonate 41: 16–21, 1982. doi: 10.1159/000241511. [DOI] [PubMed] [Google Scholar]
- 37.de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, Bossuyt PM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care 29: 1897–1901, 2006. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 38.de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab 287: E1114–E1124, 2004. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- 39.Desforges M, Greenwood SL, Glazier JD, Westwood M, Sibley CP. The contribution of SNAT1 to system A amino acid transporter activity in human placental trophoblast. Biochem Biophys Res Commun 398: 130–134, 2010. doi: 10.1016/j.bbrc.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Giulio AM, Carelli S, Castoldi RE, Gorio A, Taricco E, Cetin I. Plasma amino acid concentrations throughout normal pregnancy and early stages of intrauterine growth restricted pregnancy. J Matern Fetal Neonatal Med 15: 356–362, 2004. doi: 10.1080/14767050410001725578. [DOI] [PubMed] [Google Scholar]
- 41.Díaz P, Harris J, Rosario FJ, Powell TL, Jansson T. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am J Physiol Regul Integr Comp Physiol 309: R1569–R1577, 2015. doi: 10.1152/ajpregu.00385.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimasuay KG, Boeuf P, Powell TL, Jansson T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol 7: 12, 2016. doi: 10.3389/fphys.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta 24: 34–38, 2003. doi: 10.1053/plac.2002.0858. [DOI] [PubMed] [Google Scholar]
- 44.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 45.Dumortier O, Blondeau B, Duvillié B, Reusens B, Bréant B, Remacle C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 50: 2495–2503, 2007. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- 46.Dumortier O, Hinault C, Gautier N, Patouraux S, Casamento V, Van Obberghen E. Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes 63: 3416–3427, 2014. doi: 10.2337/db13-1431. [DOI] [PubMed] [Google Scholar]
- 47.Dumortier O, Van Obberghen E. MicroRNAs in pancreas development. Diabetes Obes Metab 14, Suppl 3: 22–28, 2012. doi: 10.1111/j.1463-1326.2012.01656.x. [DOI] [PubMed] [Google Scholar]
- 48.Durkovi J, Bojana M. The importance of determining human placental lactogen in the third trimester of pregnancy. J Soc Med Biochem Serbia 28: 97–100, 2009. doi: 10.2478/v10011-009-0003-1. [DOI] [Google Scholar]
- 49.Elghazi L, Blandino-Rosano M, Alejandro E, Cras-Méneur C, Bernal-Mizrachi E. Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Mol Metab 6: 560–573, 2017. doi: 10.1016/j.molmet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann NY Acad Sci 1212: 78–96, 2010. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, Doherty C, James L, Gusterson B, Hales CN. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br J Nutr 90: 815–822, 2003. doi: 10.1079/BJN2003967. [DOI] [PubMed] [Google Scholar]
- 52.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90: 493–500, 2005. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 53.Filios SR, Shalev A. β-Cell microRNAs: small but powerful. Diabetes 64: 3631–3644, 2015. doi: 10.2337/db15-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fugelseth D, Ramstad HB, Kvehaugen AS, Nestaas E, Støylen A, Staff AC. Myocardial function in offspring 5-8 years after pregnancy complicated by preeclampsia. Early Hum Dev 87: 531–535, 2011. doi: 10.1016/j.earlhumdev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice. Endocrinology 153: 3995–4007, 2012. doi: 10.1210/en.2011-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garg M, Thamotharan M, Dai Y, Lagishetty V, Matveyenko AV, Lee WN, Devaskar SU. Glucose intolerance and lipid metabolic adaptations in response to intrauterine and postnatal calorie restriction in male adult rats. Endocrinology 154: 102–113, 2013. doi: 10.1210/en.2012-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garofano A, Czernichow P, Bréant B. In utero undernutrition impairs rat beta-cell development. Diabetologia 40: 1231–1234, 1997. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 58.Gatford KL, Simmons RA. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin Obstet Gynecol 56: 520–528, 2013. doi: 10.1097/GRF.0b013e31829e5b29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, Cetin I, Desoye G, Wadsack C. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 92: 2256–2263, 2007. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- 60.Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 30: 411–417, 2009. doi: 10.1016/j.placenta.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519, 1997. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Gluckman PD, Kaplan SL, Rudolph AM, Grumbach MM. Hormone ontogeny in the ovine fetus. II. Ovine chorionic somatomammotropin in mid- and late gestation in the fetal and maternal circulations. Endocrinology 104: 1828–1833, 1979. doi: 10.1210/endo-104-6-1828. [DOI] [PubMed] [Google Scholar]
- 63.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9: 147–160, 2002. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 64.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 97: 3197–3206, 2012. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grumbach MM, Kaplan SL, Sciarra JJ, Burr IM. Chorionic growth hormone-prolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the “growth hormone” of the 2d half of pregnancy. Ann NY Acad Sci 148, 2 Growth Hormon: 501–531, 1968. doi: 10.1111/j.1749-6632.1968.tb20372.x. [DOI] [PubMed] [Google Scholar]
- 66.Guillemain G, Filhoulaud G, Da Silva-Xavier G, Rutter GA, Scharfmann R. Glucose is necessary for embryonic pancreatic endocrine cell differentiation. J Biol Chem 282: 15228–15237, 2007. doi: 10.1074/jbc.M610986200. [DOI] [PubMed] [Google Scholar]
- 67.Guillemette L, Lacroix M, Allard C, Patenaude J, Battista MC, Doyon M, Moreau J, Ménard J, Ardilouze JL, Perron P, Côté AM, Hivert MF. Preeclampsia is associated with an increased pro-inflammatory profile in newborns. J Reprod Immunol 112: 111–114, 2015. doi: 10.1016/j.jri.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Gürke J, Hirche F, Thieme R, Haucke E, Schindler M, Stangl GI, Fischer B, Navarrete Santos A. Maternal diabetes leads to adaptation in embryonic amino acid metabolism during early pregnancy. PLoS One 10: e0127465, 2015. doi: 10.1371/journal.pone.0127465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35: 595–601, 1992. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 70.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 71.Handwerger S, Freemark M. Role of placental lactogen and prolactin in human pregnancy. Adv Exp Med Biol 219: 399–420, 1987. doi: 10.1007/978-1-4684-5395-9_19. [DOI] [PubMed] [Google Scholar]
- 72.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 130: 409–419, 2016. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heinis M, Simon MT, Ilc K, Mazure NM, Pouysségur J, Scharfmann R, Duvillié B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 59: 662–669, 2010. doi: 10.2337/db09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heltemes A, Gingery A, Soldner EL, Bozadjieva N, Jahr KN, Johnson BK, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Exp Biol Med (Maywood) 235: 892–899, 2010. doi: 10.1258/ebm.2010.009357. [DOI] [PubMed] [Google Scholar]
- 76.Hultman K, Alexanderson C, Mannerås L, Sandberg M, Holmäng A, Jansson T. Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol 579: 823–833, 2007. doi: 10.1113/jphysiol.2006.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 186: 158–166, 2002. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 78.Jaeckle Santos LJ, Li C, Doulias PT, Ischiropoulos H, Worthen GS, Simmons RA. Neutralizing Th2 inflammation in neonatal islets prevents β-cell failure in adult IUGR rats. Diabetes 63: 1672–1684, 2014. doi: 10.2337/db13-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576: 935–946, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab 98: 105–113, 2013. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol 56: 591–601, 2013. doi: 10.1097/GRF.0b013e3182993a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res 44: 532–537, 1998. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 23: 392–399, 2002. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 84.Jimenez-Chillaron JC, Patti ME. To catch up or not to catch up: is this the question? Lessons from animal models. Curr Opin Endocrinol Diabetes Obes 14: 23–29, 2007. doi: 10.1097/MED.0b013e328013da8e. [DOI] [PubMed] [Google Scholar]
- 85.Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science 215: 1518–1519, 1982. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 86.Kaplan SL, Grumbach MM. Serum chorionic “growth hormone-prolactin” and serum pituitary growth hormone in mother and fetus at term. J Clin Endocrinol Metab 25: 1370–1374, 1965. doi: 10.1210/jcem-25-10-1370. [DOI] [PubMed] [Google Scholar]
- 87.Kelly AC, Bidwell CA, McCarthy FM, Taska DJ, Anderson MJ, Camacho LE, Limesand SW. RNA sequencing exposes adaptive and immune responses to intrauterine growth restriction in fetal sheep islets. Endocrinology 158: 743–755, 2017. doi: 10.1210/en.2016-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimball R, Wayment M, Merrill D, Wahlquist T, Reynolds PR, Arroyo JA. Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR). Physiol Rep 3: e12651, 2015. doi: 10.14814/phy2.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy 2012: 179827, 2012. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lager S, Samulesson AM, Taylor PD, Poston L, Powell TL, Jansson T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol Rep 2: e00242, 2014. doi: 10.1002/phy2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol 11: 175–179, 2011. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lango H, Palmer CN, Morris AD, Zeggini E, Hattersley AT, McCarthy MI, Frayling TM, Weedon MN; UK Type 2 Diabetes Genetics Consortium . Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes 57: 3129–3135, 2008. doi: 10.2337/db08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewis RM, Demmelmair H, Gaillard R, Godfrey KM, Hauguel-de Mouzon S, Huppertz B, Larque E, Saffery R, Symonds ME, Desoye G. The placental exposome: placental determinants of fetal adiposity and postnatal body composition. Ann Nutr Metab 63: 208–215, 2013. doi: 10.1159/000355222. [DOI] [PubMed] [Google Scholar]
- 94.Lim JS, Lee JA, Hwang JS, Shin CH, Yang SW. Non-catch-up growth in intrauterine growth-retarded rats showed glucose intolerance and increased expression of PDX-1 mRNA. Pediatr Int 53: 181–186, 2011. doi: 10.1111/j.1442-200X.2010.03204.x. [DOI] [PubMed] [Google Scholar]
- 95.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 96.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 97.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 98.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol 44: 11–15, 2012. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 99.Lunell NO, Lewander R, Mamoun I, Nylund L, Sarby S, Thornström S. Uteroplacental blood flow in pregnancy induced hypertension. Scand J Clin Lab Invest 44, Suppl 169: 28–35, 1984. doi: 10.3109/00365518409085374. [DOI] [PubMed] [Google Scholar]
- 100.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T, Takizawa T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 81: 717–729, 2009. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 101.Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56: 2938–2945, 2007. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- 102.Macko AR, Yates DT, Chen X, Shelton LA, Kelly AC, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology 157: 2104–2115, 2016. doi: 10.1210/en.2015-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab 89: 4607–4614, 2004. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- 104.Makkar A, Mishima T, Chang G, Scifres C, Sadovsky Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta 35: 802–807, 2014. doi: 10.1016/j.placenta.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin JM, Friesen H. Effect of human placental lactogen on the isolated islets of Langerhans in vitro. Endocrinology 84: 619–621, 1969. doi: 10.1210/endo-84-3-619. [DOI] [PubMed] [Google Scholar]
- 106.Matveyenko AV, Singh I, Shin BC, Georgia S, Devaskar SU. Differential effects of prenatal and postnatal nutritional environment on ß-cell mass development and turnover in male and female rats. Endocrinology 151: 5647–5656, 2010. doi: 10.1210/en.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mejia C, Lewis J, Jordan C, Mejia J, Ogden C, Monson T, Winden D, Watson M, Reynolds PR, Arroyo JA. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res 367: 387–395, 2017. doi: 10.1007/s00441-016-2496-5. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol 213, Suppl: S173–S181, 2015. doi: 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, et al.; Wellcome Trust Case Control ConsortiumMeta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) InvestigatorsGenetic Investigation of ANthropometric Traits (GIANT) ConsortiumAsian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) ConsortiumSouth Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44: 981–990, 2012. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?–A systematic review. Diabet Med 20: 339–348, 2003. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 112.Nitzan M, Orloff S, Schulman JD. Placental transfer of analogs of glucose and amino acids in experimental intrauterine growth retardation. Pediatr Res 13: 100–103, 1979. doi: 10.1203/00006450-197902000-00003. [DOI] [PubMed] [Google Scholar]
- 113.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res 44: 233–238, 1998. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 114.Obiekwe BC, Sturdee D, Cockrill BL, Chard T. Human placental lactogen in pre-eclampsia. Br J Obstet Gynaecol 91: 1077–1080, 1984. doi: 10.1111/j.1471-0528.1984.tb15079.x. [DOI] [PubMed] [Google Scholar]
- 115.Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism 35: 970–977, 1986. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 116.Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol 282: C196–C204, 2002. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- 117.Ophir E, Dourleshter G, Hirsh Y, Fait V, German L, Bornstein J. Newborns of pre-eclamptic women: a biochemical difference present in utero. Acta Obstet Gynecol Scand 85: 1172–1178, 2006. doi: 10.1080/00016340600697272. [DOI] [PubMed] [Google Scholar]
- 118.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20: 345–352, 2005. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 119.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118: 2316–2324, 2008. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 140: 4861–4873, 1999. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 121.Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 46: 18–25, 2016. doi: 10.1016/j.placenta.2016.08.078. [DOI] [PubMed] [Google Scholar]
- 122.Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab 21: 223–229, 2010. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pinney SE, Simmons RA. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr Diab Rep 12: 67–74, 2012. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 124.Poirier C, Desgagné V, Guérin R, Bouchard L. MicroRNAs in pregnancy and gestational diabetes mellitus: emerging role in maternal metabolic regulation. Curr Diab Rep 17: 35, 2017. doi: 10.1007/s11892-017-0856-5. [DOI] [PubMed] [Google Scholar]
- 125.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol 35: 99–107, 1992. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 126.Rachdi L, Aïello V, Duvillié B, Scharfmann R. L-leucine alters pancreatic β-cell differentiation and function via the mTor signaling pathway. Diabetes 61: 409–417, 2012. doi: 10.2337/db11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic βcells induces β cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci USA 105: 9250–9255, 2008. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rashid CS, Lien YC, Bansal A, Jaeckle-Santos LJ, Li C, Won KJ, Simmons RA. Transcriptomic analysis reveals novel mechanisms mediating islet dysfunction in the intrauterine growth-restricted rat. Endocrinology 159: 1035–1049, 2018. doi: 10.1210/en.2017-00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351: 173–177, 1998. doi: 10.1016/S0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 130.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295: 349–353, 1976. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 131.Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta 35: 297–302, 2014. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta 29, Suppl : 73–77, 2008. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 133.Reid GJ, Lane RH, Flozak AS, Simmons RA. Placental expression of glucose transporter proteins 1 and 3 in growth-restricted fetal rats. Am J Obstet Gynecol 180: 1017–1023, 1999. doi: 10.1016/S0002-9378(99)70675-7. [DOI] [PubMed] [Google Scholar]
- 134.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 6, Suppl 3: 332–336, 2009. [PubMed] [Google Scholar]
- 135.Ronzoni S, Marconi AM, Paolini CL, Teng C, Pardi G, Battaglia FC. The effect of a maternal infusion of amino acids on umbilical uptake in pregnancies complicated by intrauterine growth restriction. Am J Obstet Gynecol 187: 741–746, 2002. doi: 10.1067/mob.2002.124291. [DOI] [PubMed] [Google Scholar]
- 136.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449–459, 2007. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]
- 138.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 297: C723–C731, 2009. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 139.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 152: 1119–1129, 2011. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol Endocrinol Metab 270: E491–E503, 1996. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- 141.Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW Jr. Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res 65: 72–78, 2009. doi: 10.1203/PDR.0b013e318189358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab 67: 341–347, 1988. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 143.Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 9: e98667, 2014. doi: 10.1371/journal.pone.0098667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sandovici I, Hammerle CM, Ozanne SE, Constância M. Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes. Cell Mol Life Sci 70: 1575–1595, 2013. doi: 10.1007/s00018-013-1297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, Ito Y, Jones RH, Marquez VE, Cairns W, Tadayyon M, O’Neill LP, Murrell A, Ling C, Constância M, Ozanne SE. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA 108: 5449–5454, 2011. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12: 204, 2014. doi: 10.1186/1479-5876-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sati L, Soygur B, Celik-Ozenci C. Expression of mammalian target of rapamycin and downstream targets in normal and gestational diabetic human term placenta. Reprod Sci 23: 324–332, 2016. doi: 10.1177/1933719115602765. [DOI] [PubMed] [Google Scholar]
- 148.Schulz LC, Schlitt JM, Caesar G, Pennington KA. Leptin and the placental response to maternal food restriction during early pregnancy in mice. Biol Reprod 87: 120, 2012. doi: 10.1095/biolreprod.112.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol 7: 33, 2016. doi: 10.3389/fphys.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shalimov VV, Dolzhenko VF. [Clinical physiological indices in calves at a high air temperature]. Veterinariia 9: 92–94, 1974. [PubMed] [Google Scholar]
- 151.Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr 10: 67–83, 2016. doi: 10.4137/CMPed.S40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Best Pract Res Clin Endocrinol Metab 26: 701–708, 2012. doi: 10.1016/j.beem.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 154.Sisino G, Zhou AX, Dahr N, Sabirsh A, Soundarapandian MM, Perera R, Larsson-Lekholm E, Magnone MC, Althage M, Tyrberg B. Long noncoding RNAs are dynamically regulated during β-cell mass expansion in mouse pregnancy and control β-cell proliferation in vitro. PLoS One 12: e0182371, 2017. doi: 10.1371/journal.pone.0182371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57: 107–118, 1990. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 156.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445: 886–891, 2007. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 157.Strakovsky RS, Zhou D, Pan YX. A low-protein diet during gestation in rats activates the placental mammalian amino acid response pathway and programs the growth capacity of offspring. J Nutr 140: 2116–2120, 2010. doi: 10.3945/jn.110.127803. [DOI] [PubMed] [Google Scholar]
- 158.Su Y, Jiang X, Li Y, Li F, Cheng Y, Peng Y, Song D, Hong J, Ning G, Cao Y, Wang W. Maternal low protein isocaloric diet suppresses pancreatic β-cell proliferation in mouse offspring via miR-15b. Endocrinology 157: 4782–4793, 2016. doi: 10.1210/en.2016-1167. [DOI] [PubMed] [Google Scholar]
- 159.Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res 59: 320–324, 2006. doi: 10.1203/01.pdr.0000196734.54473.e3. [DOI] [PubMed] [Google Scholar]
- 160.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. doi: 10.1055/s-0031-1275516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 162.Varvarigou AA. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J Pediatr Endocrinol Metab 23: 215–224, 2010. doi: 10.1515/JPEM.2010.23.3.215. [DOI] [PubMed] [Google Scholar]
- 163.Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275: 15399–15406, 2000. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 164.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab 87: 4657–4661, 2002. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 165.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 166.Washburn L, Nixon P, Russell G, Snively BM, O’Shea TM. Adiposity in adolescent offspring born prematurely to mothers with preeclampsia. J Pediatr 162: 912–7.e1, 2013. doi: 10.1016/j.jpeds.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Weiler J, Tong S, Palmer KR. Is fetal growth restriction associated with a more severe maternal phenotype in the setting of early onset pre-eclampsia? A retrospective study. PLoS One 6: e26937, 2011. doi: 10.1371/journal.pone.0026937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wigglesworth JS. Experimental growth retardation in the foetal rat. J Pathol Bacteriol 88: 1–13, 1964. doi: 10.1002/path.1700880102. [DOI] [PubMed] [Google Scholar]
- 169.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol 201: 269.e1–269.e10, 2009. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 170.Yuan Q, Chen L, Liu C, Xu K, Mao X, Liu C. Postnatal pancreatic islet β cell function and insulin sensitivity at different stages of lifetime in rats born with intrauterine growth retardation. PLoS One 6: e25167, 2011. doi: 10.1371/journal.pone.0025167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang L, Chen W, Dai Y, Zhu Z, Liu Q. Detection of expressional changes induced by intrauterine growth restriction in the developing rat pancreas. Exp Biol Med (Maywood) 241: 1446–1456, 2016. doi: 10.1177/1535370216638771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang S, Regnault TR, Barker PL, Botting KJ, McMillen IC, McMillan CM, Roberts CT, Morrison JL. Placental adaptations in growth restriction. Nutrients 7: 360–389, 2015. doi: 10.3390/nu7010360. [DOI] [PMC free article] [PubMed] [Google Scholar]