Abstract
During acute bronchoconstriction, the airway epithelium becomes mechanically compressed, as airway smooth muscle contracts and the airway narrows. This mechanical compression activates airway epithelium to promote asthmatic airway remodeling. However, whether compressed airway epithelium can feed back on the cause of bronchoconstriction has remained an open question. Here we examine the potential for epithelial compression to augment proliferation and contraction of airway smooth muscle, and thus potentiate further bronchoconstriction and epithelial compression. Well-differentiated primary human bronchial epithelial (HBE) cells maintained in air-liquid interface culture were mechanically compressed to mimic the effect of bronchoconstriction. Primary human airway smooth muscle (HASM) cells were incubated with conditioned media collected from mechanically compressed HBE cells to examine the effect of epithelial-derived mediators on HASM cell proliferation using an EdU assay and HASM cell contraction using traction microscopy. An endothelin receptor antagonist, PD-145065, was employed to probe the role of HBE cell-derived endothelin-1 on the proliferation and contraction of HASM cells. Conditioned media from compressed HBE cells increased HASM cell proliferation, independent of the endothelin-1 signaling pathway. However, conditioned media from compressed HBE cells significantly increased HASM cell basal contraction and histamine-induced contraction, both of which depended on the endothelin-1 signaling pathway. Our data demonstrate that mechanical compression of bronchial epithelial cells contributes to proliferation and basal contraction of airway smooth muscle cells and that augmented contraction depends on epithelial cell-derived endothelin-1. By means of both airway smooth muscle remodeling and contractility, our findings suggest a causal role of epithelial compression on asthma pathogenesis.
Keywords: airway hyperresponsiveness, airway smooth muscle, asthma, bronchoconstriction, endothelin-1, mechanotransduction
INTRODUCTION
One of the cardinal features of asthma is airway remodeling, but its cause remains unknown (6). Airway remodeling is directly linked to irreversible airflow limitation and airway hyperresponsiveness (AHR). AHR is caused by increases in both airway smooth muscle (ASM) mass and ASM contraction (1, 5), which have traditionally been attributed to chronic inflammation (1). However, recent evidence, including failures of clinical trials targeting inflammation, suggest that AHR can develop through inflammation-independent mechanisms (11, 14). Therefore, an important unresolved question is the extent to which inflammation-independent factors, and physical forces in particular, can drive airway remodeling.
During bronchoconstriction, ASM excessively contracts and airway caliber is reduced, resulting in buckling of the airway wall (13). Airway epithelial cells within buckled airways experience mechanical compression of ~30 cmH2O pressure (30). Analogous mechanical compression applied to well-differentiated human bronchial epithelial (HBE) cells maintained in air-liquid interface (ALI) culture recapitulates key features of airway remodeling, including secretion of asthma-associated mediators, increased collagen deposition, and goblet cell hyperplasia (15, 20, 21, 27–29). Furthermore, these in vitro data have been validated in patients with mild asthma (10). This evidence suggests that bronchoconstriction itself can promote asthmatic airway remodeling through the activation of the compressed airway epithelium. However, the direct effect of mechanical compression of HBE cells on ASM remodeling, including ASM cell proliferation and contraction, is unknown. The goal of the current study was therefore to test the hypothesis that mechanically compressed bronchial epithelial cells produce pathological mediators that cause human airway smooth muscle (HASM) cell proliferation and contraction. Our data indicate the potential existence of a positive feedback loop, whereby mediators secreted from compressed bronchial epithelial cells promote HASM cell proliferation and contraction. These effects in turn may further promote asthmatic bronchoconstriction, closing the feedback loop.
MATERIALS AND METHODS
Culture of primary human cells.
Primary HBE cells, prepared as described previously (7), were a kind gift from Dr. Scott H. Randell (Marisco Lung Institute, The University of North Carolina, Chapel Hill, NC). The cells were obtained under protocol No. 03-1396 approved by the University of North Carolina Biomedical Institutional Review Board. Nonasthmatic cells were from nonsmokers with no history of chronic lung disease. Asthmatic cells were from three cases of fatal asthma and two cases with asthma in the medical history. Passage 2 HBE cells from nine nondiseased and five asthmatic donors were cultured in ALI conditions (19–21). For the compression experiments, described below, we used HBE cells on days 14–16 in ALI culture, when the cells were well-differentiated as determined by markers for goblet and ciliated cells (Fig. 1, A and B). Primary HASM cells were a kind gift from Dr. Reynold Panettieri (The University of Pennsylvania), and passage 3 to 8 HASM cells from five nondiseased donors were used (18).
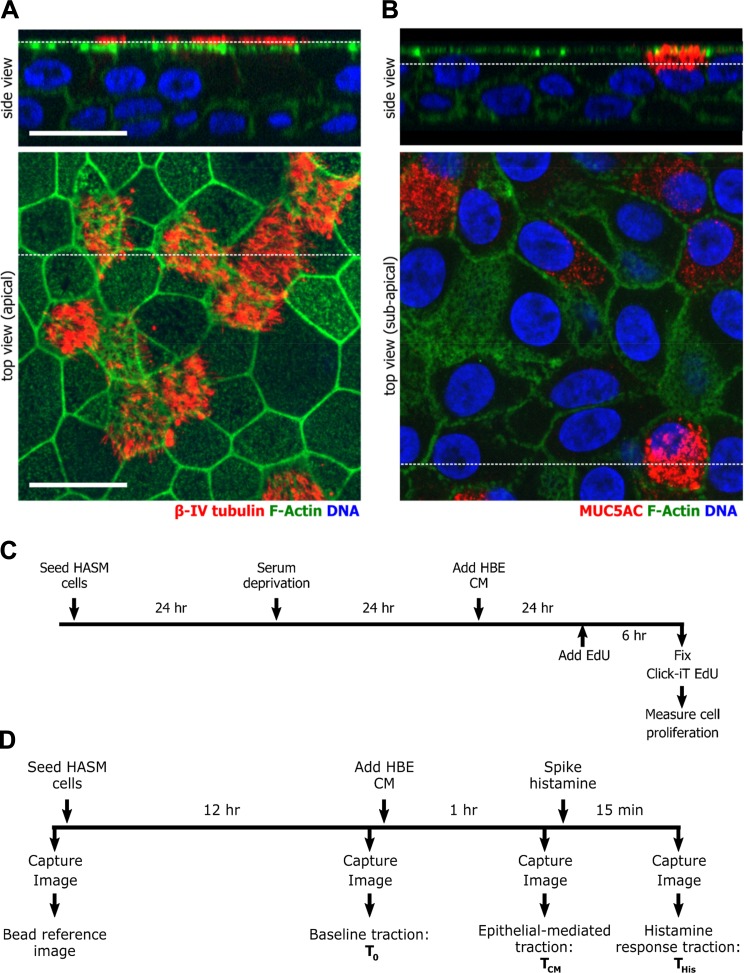
Fig. 1.
Experimental schema. A and B: primary human bronchial epithelial cells maintained in air-liquid interface culture were used for experiments when cells were well differentiated as determined by staining for βIV-tubulin (A), a ciliated cell marker, and for MUC5AC (B), a goblet cell marker. Cells were costained for F-actin and DNA (Hoechst). Broken white lines indicate the location in the corresponding image of the orthogonal cross section. The scale bar is 20 μm. C and D: timeline of the experimental procedures to measure proliferation (C) or contraction (D) of human airway smooth muscle (HASM) cells.
Mechanical compression of HBE cells.
As previously described, well-differentiated HBE cells in ALI culture were mechanically compressed by exposure to 10 or 30 cmH2O pressure for 3 h (15, 19–21), with time-matched controls exposed to zero pressure. At 20 h before initiation of compression, HBE cells were starved of bovine pituitary extract, epidermal growth factor, and hydrocortisone. Identical medium was added to empty wells in the culture plate to serve as the vehicle medium. Basolateral conditioned medium (CM) was collected at 24 h after the initiation of compression and stored at −80°C until use.
EdU cell proliferation assay.
To measure rapid proliferating cells, we used an EdU assay that is adapted from a widely accepted thymidine analog incorporation assay (24). With the use of a Click-iT EdU Alexa Fluor 488 imaging kit (Invitrogen), proliferating cells in the S phase were visualized as outlined in Fig. 1C. Nuclei were stained with Hoechst 33342 and counted as a total number of cells. To calculate the percentage of proliferating cells, the number of EdU-positive cells was divided by the total number of cells per field of view. At least four fields of view per donor for each of four donors were analyzed. The total cell numbers counted in each condition are included in the legends for Figs. 1–4.
Fig. 4.
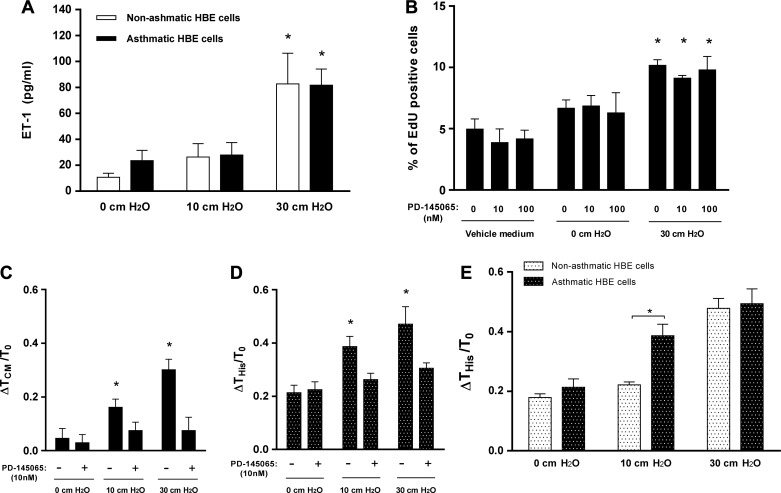
Conditioned media from compressed asthmatic human bronchial epithelial (HBE) cells induce augmented histamine response in human airway smooth muscle (HASM) cells. A: compression with 30 cmH2O pressure significantly increased endothelin-1 (ET-1) secretion from both nonasthmatic and asthmatic HBE cells (n = 4, *P < 0.05 vs. 0 cmH2O). B: conditioned media from compressed asthmatic HBE cells (30 cmH2O) significantly increased HASM cell proliferation (n = 4, *P < 0.05 vs. vehicle medium). Pretreatment with PD-145065 did not affect HASM cell proliferation. The average number of cells counted for each condition was ~3,100, and 4 donors were used. C: pretreatment of HASM cells with PD-145065 blocked the increased traction mediated by conditioned media from compressed asthmatic HBE cells (n = 4, *P < 0.05 vs. 0 cmH2O). D: conditioned media from asthmatic HBE cells compressed at either 10 or 30 cmH2O significantly increased the histamine response of HASM cells (n = 4, *P < 0.05 vs. 0 cmH2O). Pretreatment of HASM cells with PD-145065 blocked the increased histamine response. E: a significant difference induced by CM between nonasthmatic and asthmatic HBE cells was the increased contraction response [Δhistamine response traction (THis)/baseline traction (T0)] to histamine (10 μM) in the HASM cells, after incubation of HASM cells with CM from compressed HBE cells with 10 cmH2O pressure (n = 4, *P < 0.05 vs. nonasthmatic cells).
Traction microscopy.
To measure contractile force of HASM cells in response to CM from HBE cells (Fig. 1D), we used Fourier transform traction microscopy (3). This rests on the measurement of the deformation of beads embedded in a soft substrate, and to convert this to the tractions (contractile force per unit area) exerted by the cells consistent with that deformation (3). HASM cells were seeded on 4 kPa polyacrylamide gels; after 12 h, HASM cell media were replaced with vehicle medium or CM collected from HBE cells, and then incubated for 1 h. To test the response of HASM cells to a contractile agonist, CM was removed and HASM cells were washed two times with Hanks’ Balanced Salt Solution (HBSS) and then incubated with 10 μM histamine in HBSS for 15 min. Fluorescent bead images were taken at four different time points as follows: before seeding (reference image), immediately before adding CM, 1 h after adding CM, and 15 min after spiking histamine (as outlined in Fig. 1D). Images taken with cells present were compared with the reference images to compute the following tractions: baseline traction (T0), HBE cell-CM-mediated traction (TCM), and histamine response traction (THis). As described previously (18), all traction data are presented as relative change in average traction in each well. For example, the basal traction change following the incubation with CM was computed as ΔTCM/T0, where ΔTCM = TCM − T0.
Enzyme-linked immunosorbent assay.
Endothelin-1 (ET-1) concentrations in CM were quantified by enzyme-linked immunosorbent assay using a Quantikine kit (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions.
Blocking of ET-1 pathway.
Endothelin receptors, ETA and ETB, are expressed in HASM cells (9). Both receptors are known to contribute to proliferation and contraction of HASM cells (4, 17). Thus, to block the impact of HBE cell-derived ET-1 via ETA and ETB receptors, we used a well-characterized combined ETA and ETB receptor antagonist, PD-145065 (IC50 = 3.5 nM) (2). PD-145065 was added to the HASM cells 1 h before incubation with HBE cell-CM. In the pilot experiment we tested PD-145065 in a range of 10 to 1,000 nM, to determine the inhibitory concentrations of PD-145065 on the proliferation and contraction induced by HBE CM. Results were consistent across all doses used, with the maximal inhibitory effect achieved by PD-145065 at the lowest dose, 10 nM. We used 10 nM for subsequent experiments.
We performed ET-1 blocking experiment in HASM cells using CM from nonasthmatic and asthmatic HBE cells with 10 and 30 cmH2O pressure in parallel. Next, the collected data were used for two comparisons. First, the data were compared to determine the blocking effect of PD-145065 on the HASM cells after incubation with CM from nonasthmatic (Fig. 3, B and C) and asthmatic (Fig. 4, C and D) HBE cells, separately. Second, the data were compared to determine differences in the effect of CM between nonasthmatic and asthmatic HBE cells (Fig. 4E). Statistical significance was determined using all of the data together to reduce the potential for error due to multiple comparisons.
Fig. 3.
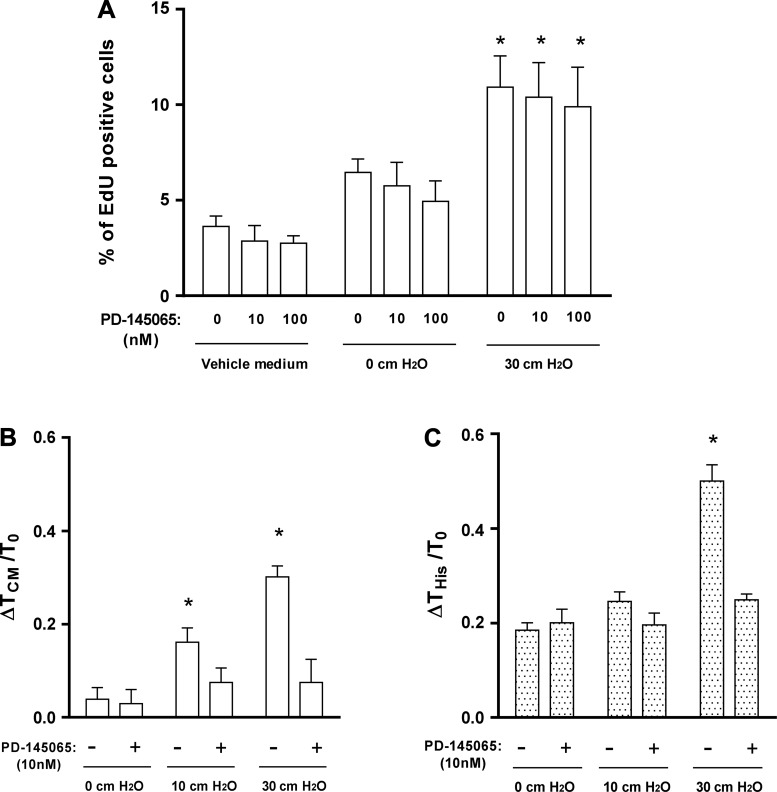
Endothelin-1 (ET-1) is responsible for compressed human bronchial epithelial (HBE) cell-induced airway smooth muscle (ASM) contraction. A: conditioned media from compressed HBE cells (30 cmH2O) significantly increased human airway smooth muscle (HASM) cell proliferation (n = 4, *P < 0.05 vs. vehicle medium). Pretreatment with PD-145065 did not affect HASM cell proliferation in any given condition. The average number of cells counted for each condition was ~3,400, and 4 donors were used. B and C: pretreatment of HASM cells with PD-145065 (10 nM) inhibited increased HASM cell traction mediated by conditioned media from compressed HBE cells (n = 4, *P < 0.05 vs. 0 cmH2O; B) and inhibited subsequent contractile response (10 μM) of HASM cells to histamine (n = 4, *P < 0.05 vs. 0 cmH2O; C).
Statistics.
All statistics were performed in Prism 5 (GraphPad Software, La Jolla, CA). Data are presented as means ± SE. An ANOVA was used for comparing all data sets, followed by a Holm-Sidak’s multiple comparison; P < 0.05 was considered statistically significant.
RESULTS
Conditioned media from compressed HBE cells induce HASM cell proliferation and contraction.
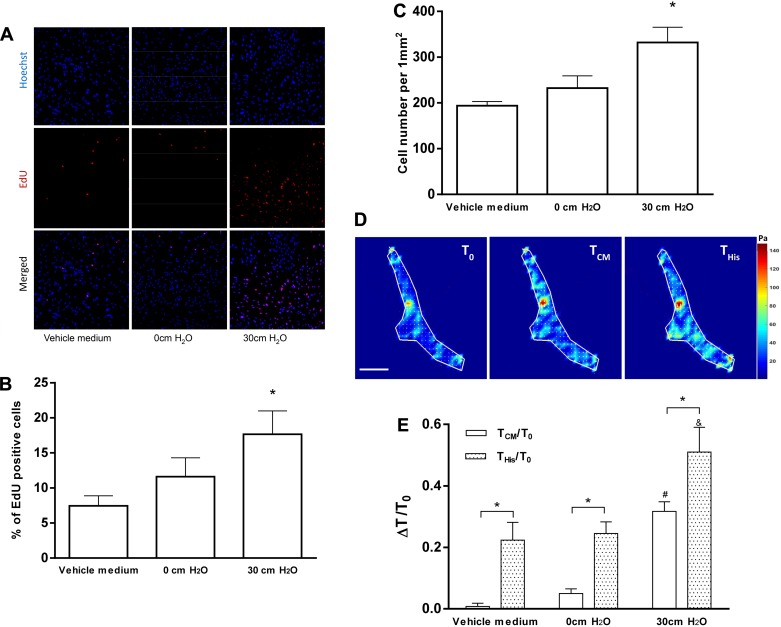
Following incubation with vehicle medium and CM from HBE cells, proliferating HASM cells were determined by the EdU assay (Fig. 2A). The percentage of EdU-positive cells was similar in the HASM cells that had been incubated with vehicle medium (7 ± 2%) and uncompressed HBE cell-CM (11 ± 3%), whereas it was significantly higher in the HASM cells that had been incubated with compressed HBE cell-CM (17 ± 3%, P < 0.05; Fig. 2B). The total cell number per field of view (1 mm2) was similar in the HASM cells that had been incubated with control media (196 ± 8 cells) and uncompressed HBE cell-CM (229 ± 25 cells), whereas it was significantly greater in the HASM cells that had been incubated with compressed HBE cell-CM (332 ± 33 cells, P < 0.05; Fig. 2C).
Fig. 2.
Conditioned media from compressed human bronchial epithelial (HBE) cells induce human airway smooth muscle (HASM) cell proliferation and contraction. A: representative images show nuclei by Hoechst staining (blue) and proliferating cells by EdU staining (red). The scale bar is 200 μm. Conditioned media from compressed HBE cells (30 cmH2O) significantly increased the percentage of EdU-positive cells (B) and total HASM cells (C) (*P < 0.05 vs. vehicle medium). The average number of cells counted for each condition was ~4,600, and 4 donors were used. D: representative traction maps of a HASM cell before (T0) and after [CM-mediated traction (TCM) and histamine response traction (THis)] incubation with compressed conditioned media (30 cmH2O). The scale bar is 20 μm, and the unit of the color scale is pascal (Pa). E: after incubation with conditioned media from compressed HBE cells (30 cmH2O), contraction of HASM cells (white bars, representing ΔTCM/T0) was significantly increased (n = 5, #P < 0.05 vs. vehicle medium). For all conditions, contractile responses to histamine (10 μM) were significantly increased (stippled bars, representing ΔTHis/T0) (n = 5, *P < 0.05 vs. without histamine). Compared with the other conditions, in HASM cells incubated with conditioned media from compressed HBE cells (30 cmH2O), contractile response to histamine was significantly increased (ΔTHis/T0 = 0.51 ± 0.08; n = 5, &P < 0.05 vs. vehicle medium).
Next, to determine the impact of HBE cell compression on the contractile force of HASM cells, we measured the tractions of HASM cells after incubation with vehicle medium and CM from HBE cells. Representative traction maps (Fig. 2D) of a HASM cell show an increase in traction after incubation with CM from compressed HBE cells. Quantification of tractions shows that neither vehicle medium nor CM from uncompressed HBE cells changed the average contractile force of HASM cells from baseline (Fig. 2E). By contrast, compressed HBE cell-CM treatment on HASM cells significantly increased average contractile force from baseline (ΔTCM/T0 = 0.32 ± 0.05, P < 0.05; Fig. 2E).
To mimic the contractility of HASM cells as occurs during AHR, the HASM cells were further stimulated with the contractile agonist histamine following the incubation of the HASM cells with HBE cell-CM as above. As expected, within all conditions, the histamine challenge significantly increased the traction exerted by HASM cells (P < 0.05; Fig. 2E). Comparing across conditions, the contractile response of HASM cells to histamine was similar in the cells that had been incubated with vehicle medium (ΔTHis/T0 = 0.22 ± 0.06) and uncompressed HBE cell-CM (ΔTHis/T0 = 0.25 ± 0.04). However, the contractile response to histamine of the HASM cells that had been incubated with compressed HBE cell-CM was significantly greater (ΔTHis/T0 = 0.51 ± 0.08, P < 0.05).
ET-1 regulates the effect of HBE conditioned media on HASM traction and histamine response.
To determine whether ET-1 was responsible for the increased HASM cell proliferation that was caused by the CM from compressed HBE cells, we blocked the ET-1 receptor activity with PD-145065 (10 and 100 nM). As in our initial experiments (Fig. 2B), CM from compressed HBE cells significantly increased HASM cell proliferation (P < 0.05; Fig. 3A). However, blocking ET-1 receptor activity in HASM cells did not affect the increased HASM cell proliferation as measured by the total cell number (data not shown) and percentage of EdU-positive cells (Fig. 3A).
To determine whether ET-1 was responsible for the increased contraction (ΔTCM/T0) and histamine response (ΔTHis/T0) in the HASM cells, we also used PD-145065. In this experiment, HBE cells were exposed to two different magnitudes of pressure, 10 and 30 cmH2O. CM from compressed HBE cells with either pressure increased the average contractile force from baseline, but CM from compressed cells with 30 cmH2O showed a higher contractile response (Fig. 3B).
Pretreatment of HASM cells with PD-145065 (10 nM) completely blocked the increased contractility induced by CM from compressed HBE cells at either 10 or 30 cmH2O pressure (Fig. 3B). Contractile response to histamine was not further increased in HASM cells incubated with HBE CM from 10 cmH2O pressure, whereas contractile response to histamine was further increased in HASM cells incubated with HBE CM from 30 cmH2O pressure (Fig. 3C). This increased contractile response was completely blocked by pretreatment of PD-145065 (Fig. 3C).
Conditioned media from compressed asthmatic HBE cells led to augmented-histamine response in HASM cells.
We next assessed the impact of the disease state of the donor of the HBE cells on the HASM cell remodeling. First, we measured ET-1 concentration in the CM collected from HBE cells after exposure to either 0, 10, or 30 cmH2O pressure. ET-1 levels were similar in CM between nonasthmatic and asthmatic HBE cells in all conditions and were significantly elevated only after compression with 30 cmH2O pressure (Fig. 4A). Compared with uncompressed CM, compressed CM (30 cmH2O) from asthmatic HBE cells significantly increased the proliferation of HASM cells (P < 0.05; Fig. 4B). However, there was no difference in the magnitude of increased HASM cell proliferation caused by compressed CM between nonasthmatic (Fig. 3A) and asthmatic (Fig. 4B) HBE cells. Furthermore, blocking ET-1 receptor activity did not affect the increased proliferation of HASM cells as measured by the total cell number (data not shown) and percentage of EdU-positive cells (Fig. 4B).
To determine the role of ET-1 in the HASM cell response to CM from asthmatic donors, we used PD-145065 as above. Compared with uncompressed CM, compressed CM from asthmatic HBE cells significantly increased the contractile force of HASM cells (ΔTCM/T0) in a magnitude of pressure-dependent manner (P < 0.05; Fig. 4C). Pretreatment of HASM cells with PD-145065 completely blocked the elevated basal contraction observed after incubation with CM from compressed asthmatic HBE cells with either 10 or 30 cmH2O pressure (Fig. 4C). The HASM cells were further stimulated with histamine (10 μM) following incubation with CM from asthmatic HBE cells. As observed with nonasthmatic HBE CM from 30 cmH2O pressure (Fig. 3C), the contractile response to histamine was further significantly increased in HASM cells incubated with asthmatic HBE CM from either 10 or 30 cmH2O pressure (ΔTHis/T0 = 0.39 ± 0.04 and 0.47 ± 0.07, respectively, P < 0.05; Fig. 4D). Pretreatment of HASM cells with PD-145065 completely blocked the elevated histamine responses observed after incubation with CM from compressed asthmatic HBE cells with either 10 or 30 cmH2O pressure (Fig. 4D).
As with proliferation, there was also no difference between the magnitude of HASM cell traction increased by CM from nonasthmatic and asthmatic HBE cells. However, after subsequent histamine challenge, the contraction response was significantly different between HASM cells incubated with CM from nonasthmatic compared with asthmatic HBE cells after compression with 10 cmH2O pressure (P < 0.05; Fig. 4E).
DISCUSSION
The goal of this study was to test the hypothesis that bronchoconstriction-induced mechanical compression of airway epithelial cells can cause HASM cell remodeling, specifically manifested in two ways: through increased proliferation and through increased contractility. To test our hypothesis, we employed three distinct in vitro approaches. To mimic the compressive mechanical force imposed to airway epithelium during asthmatic bronchoconstriction, we applied compression similar to that predicted during maximum airway constriction during an asthma exacerbation to well-differentiated primary HBE cells in ALI culture (Fig. 1, A and B) (21, 27). Following this, basolateral media collected from compressed HBE cells were transferred to HASM cells to determine the effect of mediators secreted from compressed HBE cells on two critical HASM cell functions, proliferation and contraction (Fig. 1, C and D). Second, to determine proliferation of HASM cells, we used an EdU incorporation assay (23). Third, to determine the contraction of HASM cells at baseline or in response to the contractile agonist histamine, we used Fourier transform traction cytometry (3).
Our data indicate that basolateral CM from compressed HBE cells induced proliferation, basal contraction, and histamine-induced contraction of HASM cells (Figs. 2–4). This is the first study showing that compressed airway epithelial cells resulting from bronchoconstriction can cause phenotypical changes in HASM cells, in the absence of inflammatory cells. This is a critical observation that has implications for the pathobiology of asthma, since it demonstrates that communication between these two types of lung structural cells can augment pathophysiological responses. Although airway epithelium was previously shown to modulate both contraction and relaxation of ASM (25), it was previously unknown if contraction and relaxation are impacted by the airway epithelium that is structurally deformed because of bronchoconstriction. We have found this to be true.
In well-differentiated airway epithelial cells cultured from nonasthmatic human donors, Tschumperlin et al. previously demonstrated that mechanical compression mimicking bronchoconstriction induces mRNA expression and secretion of ET-1 (28). Increased levels of ET-1 in bronchoalveolar fluid have been associated with AHR and severity of asthma (8, 22). Although ET-1 is a well-known mitogen and contractile agonist for smooth muscle from studies in mice and humans (4, 12, 17, 26), the biological function of ET-1 derived from HBE cells on airway smooth muscle cells has not been tested. Therefore, we hypothesized that ET-1 was responsible for increased proliferation and contraction of HASM cells after incubation with CM from compressed HBE cells. Our data demonstrate that ET-1 is not responsible for compressed HBE cell-mediated proliferation of HASM cells, but it is responsible for compressed HBE cell-mediated contraction of HASM cells (Fig. 3).
When we compared the effect of CM from nonasthmatic and asthmatic HBE cells after compression, we did not observe any differences in ET-1 concentration, proliferation, and baseline contraction of HASM cells (Figs. 3 and 4). However, when we compared the histamine response of HASM cells after incubation with CM from HBE cells compressed at a lower magnitude (10 cmH2O), CM only from asthmatic HBE cells significantly augmented the contractile response of HASM cells to histamine (Fig. 4, D and E). Stated another way, a smaller magnitude of compression (10 cmH2O) to the asthmatic HBE cells elicited the same effect as a larger magnitude of compression (30 cmH2O), despite the similar level of ET-1 concentration. We speculate that, when HBE cells are exposed to a lower pressure (10 cmH2O), nonasthmatic HBE cells might produce inhibitory mediators that protect against histamine-induced contraction as a normal defense function while asthmatic HBE cells are impaired in the production of inhibitory mediators. Another possible origin of this discrepancy is that asthmatic HBE cells readily produce additional cofactors (19, 28, 29) in response to even a low magnitude of pressure (10 cmH2O). Therefore, those cofactors might elicit synergistic or potentiating effects together with ET-1, which might be necessary for the contraction of HASM cells. Taken together, these data support the notion that asthmatic airway epithelial cells are more sensitive to insult or injury or impaired in protective functions (16) and further suggest that asthmatic HBE cells are more susceptible to mechanical compression resulting from bronchoconstriction.
In summary, our data demonstrate that mechanical compression of HBE cells contributes to proliferation and contraction of HASM cells, importantly, even in the absence of inflammatory cells. Our results provide mechanistic evidence that ET-1 secreted by compressed HBE cells in turn induces and sustains HASM cell contraction, as a direct consequence of bronchoconstriction. Our findings imply that airway epithelial compression by itself can perpetuate airway remodeling through a positive feedback loop.
GRANTS
This work was supported by a Parker B. Francis Fellowship (J.-A. Park), American Heart Association Scientist Development Grant 13DG14320004 (J.-A. Park), National Institutes of Health (NIH) Grants HL-007118, R01-HL-107561, and P01-HL-120839, an operating grant from the Canadian Institution of Health (MOP-13505 and MOP-97988; W. C. Cole), and an Eye’s High Fellowship from the University of Calgary (B. Lan). HBE cells from Dr. Randell were supported by Cystic Fibrosis Foundation Grant BOUCHE15R0 and NIH Grant P30-DK-065988.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
B.L., J.A.M., M.J.O., J.P.B., and J.-A.P. conceived and designed research; B.L., J.A.M., M.J.O., C.Y.P., J.H.K., and J.-A.P. performed experiments; B.L., J.A.M., M.J.O., J.P.B., and J.-A.P. analyzed data; B.L., J.A.M., M.J.O., C.Y.P., J.H.K., W.C.C., J.P.B., and J.-A.P. interpreted results of experiments; B.L., J.A.M., and J.-A.P. prepared figures; B.L., J.A.M., W.C.C., J.P.B., and J.-A.P. drafted manuscript; B.L., J.A.M., M.J.O., C.Y.P., J.H.K., W.C.C., J.P.B., and J.-A.P. edited and revised manuscript; B.L., J.A.M., M.J.O., C.Y.P., J.H.K., W.C.C., J.P.B., and J.-A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Reynold Panettieri for providing primary human airway smooth muscle cells and Dr. Scott Randell for providing primary human bronchial epithelial cells. We thank Drs. Jeffrey Fredberg and Jeffrey Drazen for critical reading.
REFERENCES
- 1.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 36: 1174–1184, 2010. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 2.Battistini B, Warner TD, Fournier A, Vane JR. Characterization of ETB receptors mediating contractions induced by endothelin-1 or IRL 1620 in guinea-pig isolated airways: effects of BQ-123, FR139317 or PD 145065. Br J Pharmacol 111: 1009–1016, 1994. doi: 10.1111/j.1476-5381.1994.tb14844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers GW, Little SA, Patel KR, Thomson NC. Endothelin-1-induced bronchoconstriction in asthma. Am J Respir Crit Care Med 156: 382–388, 1997. doi: 10.1164/ajrccm.156.2.9702066. [DOI] [PubMed] [Google Scholar]
- 5.Doeing DC, Solway J. Airway smooth muscle in the pathophysiology and treatment of asthma. J Appl Physiol (1985) 114: 834–843, 2013. doi: 10.1152/japplphysiol.00950.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol 128: 439–448, 2011. doi: 10.1016/j.jaci.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. In: Epithelial Cell Culture Protocols. Totowa, NJ: Humana, p. 109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 8.Gawlik R, Jastrzebski D, Ziora D, Jarzab J. Concentration of endothelin in plasma and BALF fluid from asthmatic patients. J Physiol Pharmacol 57, Suppl 4: 103–110, 2006. [PubMed] [Google Scholar]
- 9.Goldie RG, Henry PJ, Knott PG, Self GJ, Luttmann MA, Hay DW. Endothelin-1 receptor density, distribution, and function in human isolated asthmatic airways. Am J Respir Crit Care Med 152: 1653–1658, 1995. doi: 10.1164/ajrccm.152.5.7582310. [DOI] [PubMed] [Google Scholar]
- 10.Grainge CL, Lau LCK, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 364: 2006–2015, 2011. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 11.Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, Ruse C, Hall IP, Pavord ID. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. Eur Respir J 27: 1144–1151, 2006. doi: 10.1183/09031936.06.00102605. [DOI] [PubMed] [Google Scholar]
- 12.Gregory LG, Jones CP, Mathie SA, Pegorier S, Lloyd CM. Endothelin-1 directs airway remodeling and hyper-reactivity in a murine asthma model. Allergy 68: 1579–1588, 2013. doi: 10.1111/all.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis 139: 242–246, 1989. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O’Byrne PM, Hargreave FE. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis 142: 832–836, 1990. doi: 10.1164/ajrccm/142.4.832. [DOI] [PubMed] [Google Scholar]
- 15.Mitchel JA, Antoniak S, Lee J-H, Kim S-H, McGill M, Kasahara DI, Randell SH, Israel E, Shore SA, Mackman N, Park J-A. IL-13 Augments Compressive Stress-Induced Tissue Factor Expression in Human Airway Epithelial Cells. Am J Respir Cell Mol Biol 54: 524–531, 2016. doi: 10.1165/rcmb.2015-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moheimani F, Hsu AC-Y, Reid AT, Williams T, Kicic A, Stick SM, Hansbro PM, Wark PAB, Knight DA. The genetic and epigenetic landscapes of the epithelium in asthma. Respir Res 17: 119, 2016. doi: 10.1186/s12931-016-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panettieri RA Jr, Goldie RG, Rigby PJ, Eszterhas AJ, Hay DW. Endothelin-1-induced potentiation of human airway smooth muscle proliferation: an ETA receptor-mediated phenomenon. Br J Pharmacol 118: 191–197, 1996. doi: 10.1111/j.1476-5381.1996.tb15385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CY, Zhou EH, Tambe D, Chen B, Lavoie T, Dowell M, Simeonov A, Maloney DJ, Marinkovic A, Tschumperlin DJ, Burger S, Frykenberg M, Butler JP, Stamer WD, Johnson M, Solway J, Fredberg JJ, Krishnan R. High-throughput screening for modulators of cellular contractile force. Integr Biol 7: 1318–1324, 2015. doi: 10.1039/C5IB00054H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J-A, Drazen JM, Tschumperlin DJ. The chitinase-like protein YKL-40 is secreted by airway epithelial cells at base line and in response to compressive mechanical stress. J Biol Chem 285: 29817–29825, 2010. doi: 10.1074/jbc.M110.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J-A, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, Drazen JM. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol 130: 1375–1383, 2012. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J-A, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol 41: 459–466, 2009. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redington AE, Springall DR, Ghatei MA, Madden J, Bloom SR, Frew AJ, Polak JM, Holgate ST, Howarth PH. Airway endothelin levels in asthma: influence of endobronchial allergen challenge and maintenance corticosteroid therapy. Eur Respir J 10: 1026–1032, 1997. doi: 10.1183/09031936.97.10051026. [DOI] [PubMed] [Google Scholar]
- 23.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105: 2415–2420, 2008. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaykhiev R, Beisswenger C, Kändler K, Senske J, Püchner A, Damm T, Behr J, Bals R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 289: L842–L848, 2005. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 25.Spina D. Epithelium smooth muscle regulation and interactions. Am J Respir Crit Care Med 158, Suppl 2: S141–S145, 1998. doi: 10.1164/ajrccm.158.supplement_2.13tac100a. [DOI] [PubMed] [Google Scholar]
- 26.Stewart AG, Grigoriadis G, Harris T. Mitogenic actions of endothelin-1 and epidermal growth factor in cultured airway smooth muscle. Clin Exp Pharmacol Physiol 21: 277–285, 1994. doi: 10.1111/j.1440-1681.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 27.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA 98: 6180–6185, 2001. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol 28: 142–149, 2003. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 29.Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol 282: L904–L911, 2002. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 30.Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol (1985) 83: 1814–1821, 1997. doi: 10.1152/jappl.1997.83.6.1814. [DOI] [PubMed] [Google Scholar]