Abstract
Bronchopulmonary dysplasia (BPD), the most common chronic lung disease in infants, is associated with long-term morbidities, including pulmonary hypertension (PH). Importantly, hyperoxia causes BPD and PH; however, the underlying mechanisms remain unclear. Herein, we performed high-throughput transcriptomic and proteomic studies using a clinically relevant murine model of BPD with PH. Neonatal wild-type C57BL6J mice were exposed to 21% oxygen (normoxia) or 70% oxygen (hyperoxia) during postnatal days (PNDs) 1–7. Lung tissues were collected for proteomic and genomic analyses on PND 7, and selected genes and proteins were validated by real-time quantitative PCR and immunoblotting analysis, respectively. Hyperoxia exposure dysregulated the expression of 344 genes and 21 proteins. Interestingly, hyperoxia downregulated genes involved in neuronal development and maturation in lung tissues. Gene set enrichment and gene ontology analyses identified apoptosis, oxidoreductase activity, plasma membrane integrity, organ development, angiogenesis, cell proliferation, and mitophagy as the predominant processes affected by hyperoxia. Furthermore, selected deregulated proteins strongly correlated with the expression of specific genes. Collectively, our results identified several potential therapeutic targets for hyperoxia-mediated BPD and PH in infants.
Keywords: bronchopulmonary dysplasia, hyperoxia, proteomics, pulmonary hypertension, transcriptomics
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common chronic lung disease in preterm infants resulting from interrupted lung development (21). Pulmonary hypertension (PH) develops in 17% of patients with BPD and increases BPD-associated mortality and morbidity (2). However, this complex disease lacks specific therapies, and the molecular mechanisms underlying BPD and PH remain unclear.
Supplemental oxygen and mechanical ventilation are life-sustaining therapies administered to infants with respiratory failure. However, oxidative stress and inflammation associated with these therapies disrupt normal lung development and lead to BPD and PH in both infants and mice (21, 30, 36). Murine lungs at birth are equivalent to the developmental lung stage of a 25- to 26-wk-old infant (42). Therefore, mice are the most commonly used laboratory animal to model human BPD. Several preclinical studies, including ours, have shown that exposure of neonatal mice to prolonged hyperoxia leads to a phenotype that models BPD and PH in infants (3, 19, 35).
Gene expression microarray and reverse-phase protein array (RPPA) allow quantification of the transcriptome and proteome, respectively. These powerful tools can identify novel genes, proteins, biological pathways, and their interactions and can help us understand the molecular mechanisms underlying disease processes. Gene-based assays such as RNA microarrays and RNA sequencing have been commonly utilized to identify the molecular mechanisms of BPD (1, 5–8, 12, 17, 26, 31, 40); however, proteomic assays are rarely used (15, 25, 38) to unravel BPD pathogenesis. Proteomic studies are important for several reasons. First, proteins are the final mediators of biological processes; therefore, they represent meaningful therapeutic targets. Second, mRNA levels may not always correlate with the level and function of corresponding proteins because of processes such as posttranscriptional modifications. Finally, combined transcriptomic and proteomic analysis can further elucidate the molecular mechanisms underlying disease. Hence, the objective of this study was to determine and correlate gene and protein expression profiles in a clinically relevant mouse model of hyperoxia-induced lung injury, to identify suitable therapeutic targets for infants with BPD with PH. Using neonatal C57BL6J wild-type (WT) mice, we tested the hypothesis that hyperoxia exposure during the saccular and alveolar development phase alters the level of genes and proteins necessary for optimal lung development and repair.
MATERIALS AND METHODS
Animals, hyperoxia experiments, and tissue collection.
This study was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted as per American Physiological Society (2010–2011) guidelines for animal studies. C57BL6J WT male and female pups from multiple litters were pooled before being randomly and equally redistributed to the dams before being exposed to 21% oxygen (normoxia; controls) or 70% oxygen (hyperoxia) during postnatal days (PNDs) 1–7. There were 10 pups in each experimental group (array experiments: n = 4/group; nonarray experiments: n = 6/group). Hyperoxia experiments were conducted in plexiglass chambers into which oxygen was delivered continuously through an oxygen blender to achieve a constant level of 70% oxygen. The dams were rotated between air- and hyperoxia-exposed litters every 24 h during the hyperoxia exposure period to prevent oxygen toxicity in the dams and to control for maternal effects between the groups (35). At PND 7, the animals were euthanized with intraperitoneal injections of 200 mg/kg of sodium pentobarbital, and the lung tissues were collected for genomic and proteomic studies.
Microarray analysis.
RNA from mouse lung tissue exposed to normoxia or hyperoxia (n = 4 per group) was purified using the Direct-zol RNA MiniPrep kit (Zymo Research, CA). Microarray experiments were performed using the Illumina Sentrix Beadchip Array Mouse Ref8_V2. The BeadStudio analysis software (Illumina, CA) was used to apply the standard quality control thresholds and analyze the data.
Differential gene expression analysis.
The effect of oxygen exposure on gene expression was assessed using a t-test. Differentially expressed genes (DEGs) were considered statistically significant at a false discovery rate (FDR)-adjusted P value < 0.01 and fold change (FC) exceeding 1.25×. Gene set enrichment analysis (GSEA) was performed using GSEA version 3. Enrichment scores ≥0.4 (positive or negative) were considered significant. Furthermore, Enrichr tool was also used for functional classification of the DEGs as described previously (10, 23). The data set will be deposited in the National Center for Biotechnology Information’s Gene Expression OMNIBUS.
Real-time quantitative PCR assays.
To validate and reproduce microarray data, we performed real-time quantitative PCR (RT-qPCR) using lung RNA samples from the microarray experiment and a separate set of samples from mice exposed to normoxia or hyperoxia (n = 6 per group). RT-qPCR analysis was performed using the TaqMan gene expression master mix and gene-specific primers (Grand Island, NY).
RPPA analysis.
Protein lysates from mice exposed to normoxia or hyperoxia (n = 3 per group) were prepared using the modified Tissue Protein Extraction Reagent (Pierce) (20). RPPA analysis was then conducted as previously described (20). Briefly, lysates were probed with a set of 204 antibodies. Antibody slides that failed quality inspection were either retested or removed. The remaining 182 antibodies (116 to detect total protein and 66 to detect phosphorylated states of proteins) were used for subsequent analyses.
Differential protein expression analysis.
The heatmap of median-centered, normalized expression values and unsupervised hierarchical clustering values was generated using the R statistical software. Significant differences in protein expression levels between groups were determined using a t-test (P < 0.05 and at least 1.2 × FC). Enrichment analysis of differentially expressed proteins (DEPs) was performed using gene ontology (GO) mapping. The global mouse gene database was used as a background for the GO enrichment analysis, and a gene set was considered significantly enriched at an FDR-adjusted P value < 0.05.
Immunoblot assays.
To validate and reproduce RPPA data, we performed immunoblot assays using protein lysates from the RPPA experiments and from a separate set of samples from mice exposed to normoxia or hyperoxia (n = 6 per group). Immunoblotting analysis was performed using primary antibodies against β-actin (Santa Cruz Biotechnology), phosphorylated (p)-AMP-activated protein kinases (p-AMPK) a[T172], p-AMPKb1[S108], SLUG, and platelet-derived growth factor receptor (PDGFR) b (Cell Signaling Technology).
Correlation between proteomic and transcriptomic data.
Correlation analysis between the relative abundance of three DEPs (p-AMPKb1[S108], PDGFRb, and SLUG), and whole-genome transcriptional profiles in normoxic and hyperoxic lungs were analyzed using RPPA and microarray data. The most significantly correlated transcripts were identified using correlation coefficient cutoffs > ± 0.95 and P < 0.05.
Statistical analyses.
The results were analyzed using the GraphPad Prism 5 software. Data are expressed as means ± standard deviations (SD). An unpaired t-test was used to determine differences in mRNA and protein levels between normoxia- and hyperoxia-exposed mice. P < 0.05 was considered significant.
RESULTS AND DISCUSSION
In the present study, we analyzed the effects of hyperoxia on lung genomic and proteomic profiles in a murine model of BPD and PH using high-throughput technologies and found significant correlations between transcriptomic and proteomic data.
WT neonatal mice exposed to 70% oxygen show persistent structural lung abnormalities up to PND 56 (28, 29, 35), PH at PND 14 (35), and PH and right ventricular dysfunction at PND 56 (28). Using this disease model, we conducted transcriptomic analysis on PND 7. Hyperoxia-mediated molecular and biochemical perturbations that lead to BPD phenotype are early events in the disease pathogenesis. Moreover, identifying these perturbations early in the disease process will help us discover biomarkers that have the potential to prevent and/or treat BPD. Therefore, we analyzed the lung tissues at PND 7 rather than at PND 14 to increase the likelihood of identifying the differentially regulated genes, proteins, and pathologic pathways. Analysis of DEGs between controls and hyperoxia-exposed mice showed 344 genes significantly regulated by hyperoxia at an FDR-adjusted P < 0.01 and FC ≥ 1.25 (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website). In hyperoxia-exposed mice, 125 genes were downregulated and 219 genes were upregulated, compared with those in controls. GSEA revealed that hyperoxia negatively enriched the cellular compartmental process integral to the plasma membrane (Fig. 1A) and positively enriched biological and molecular functional processes such as apoptosis and oxidoreductase activity, respectively (Fig. 1, B and C). Additional functional classification of the DEGs using the Enrichr tool is shown in Supplemental Table S2. Based on their relevance in oxygen toxicity and lung development, we selected six genes [ATP binding cassette subfamily C member 3 (ABCC3), BCL2 binding component 3 (BBC3), cyclin dependent kinase 5 regulatory subunit 1 (CDK5R1), glutathione peroxidase 2 (GPX2), neurotrophic receptor tyrosine kinase 2 (NTRK2), and platelet derived growth factor receptor alpha (PDGFRA)] for microarray data validation (using microarray samples) and reproducibility (using nonarray samples; additional biological replicates) by RT-qPCR. Microarray data were validated and reproduced for five of six (83.3%) genes tested (Fig. 1D). The minor discrepancies between microarray and RT-qPCR analysis may be related to their different sensitivities and gene expression detection mechanisms. The magnitude change in expression identified by microarray correlated with that of the RT-qPCR data. Consistent with other studies (4, 6, 9, 24, 27, 33), hyperoxia dysregulated genes that modulate cell migration, proliferation and death (ASAH3L, BAX, CDKN1, COL18A1, CXCL17, GDF15, MEST, MT1, NUPR1, PDGFR, SLC19A2, TRIB3), and oxidative stress response (GPX2, GPX3, GSTA3, GSTA4, GSTM1, GSTM2, GSTM3, MGST1), which are biological processes that contribute to BPD pathogenesis. Identifying new genes that regulate these processes may enable the development of a comprehensive gene profile of biomarkers or therapeutic drug targets for infants with BPD. Therefore, we chose to validate some of these genes that are known and unknown to be dysregulated by hyperoxia. Consistent with similar studies in rodents (6, 14, 27) and infants with BPD (34), we observed that hyperoxia increased the expression of genes that regulate oxidative stress (GPX2), although decreasing the expression of those that facilitate cell proliferation and organogenesis (NTRK2 and PDGFA). Additionally, we found genes not previously shown to be dysregulated in experimental or human BPD. BCL2 binding component 3 (BBC3) expression was upregulated by hyperoxia. As a member of the proapoptotic subclass of BCL2 protein family, uninhibited BBC3 expression induces mitochondrial dysfunction and activation of apoptosis signaling cascades. Inhibition of BBC3 is shown to protect human intestinal cells against hyperoxic injury (39). On the other hand, CDK5R1, which is known to regulate neuronal maturation (13) was downregulated by hyperoxia. The decreased expression of genes that facilitate neuronal development and maturation (NTRK2 and CDK5R1) under hyperoxia is noteworthy. NTRK2 is expressed in several lung cell types and is shown to be necessary for normal alveolar and nervous system development of murine lungs (16). Furthermore, CDK5R1 is also shown to affect lung cell proliferation under hypoxic conditions (22). Therefore, decreased expression of these genes may represent some of the mechanisms through which hyperoxia contributes to interrupted development and abnormal innervation and contractile response of the lungs. It is also possible that hyperoxia could also dysregulate these genes in the brain, which would partly explain the mechanism underlying BPD-associated neurological morbidities. Therefore, optimizing the level and function of BBC3, NTRK2, and CDK5R1 in the lung cells has the potential to preserve mitochondrial function, mitigate apoptosis and abnormal airway and pulmonary vascular contractility, and facilitate optimal lung development and repair under hyperoxic conditions.
Fig. 1.
Lung transcriptomics of 1-wk-old mice exposed to hyperoxia. Lung tissues of mice exposed to normoxia or hyperoxia for 7 days were analyzed using microarrays. GSEA of microarray data showing significant negatively (A) and positively (B and C) correlated cellular compartmental and biological and molecular function processes, respectively, in the hyperoxia group (FDR < 0.01; P < 0.05). RT-qPCR quantification of ABCC3, BBC3, CDK5R1, GPX2, NTRK2, and PDGFRA mRNA levels in samples from microarray experiments (n = 3 per group) and a separate set of samples (nonarray samples; n = 6 per group) (D). Values are presented as means ± SD *Significant differences between normoxia and hyperoxia groups at P < 0.05 (t-test). FDR, false discovery rate; GSEA, gene set enrichment analysis; RT-qPCR, real-time quantitative PCR; see main text for relevant definitions of genes.
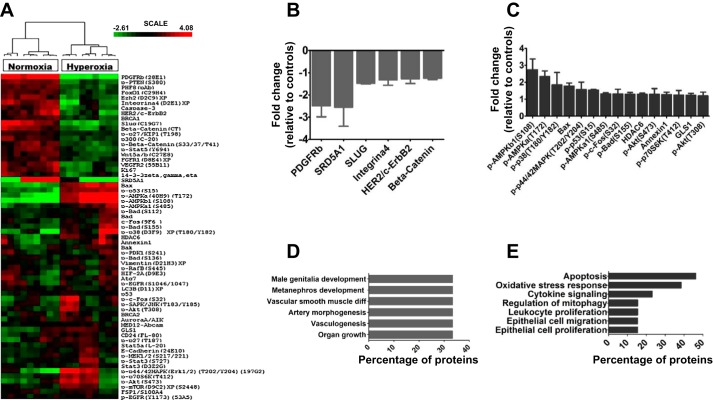
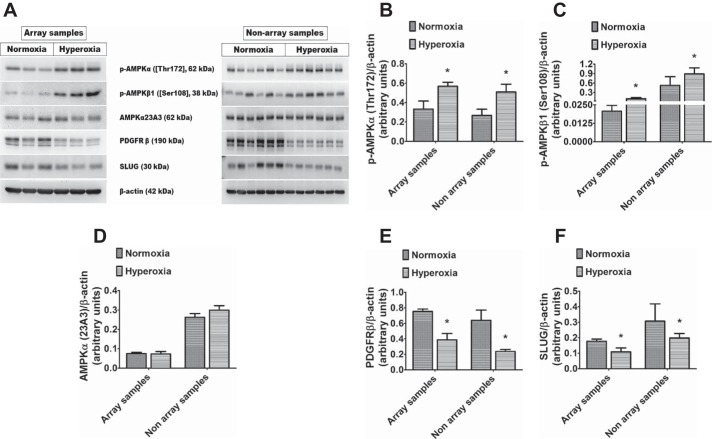
The lung proteomic profile of hyperoxia-exposed mice was assessed using RPPA on PND 7. A heatmap of the median-centered, normalized expression levels of DEPs demonstrated that lung protein expression profiles of control mice were distinguishable from those of hyperoxia-exposed mice (Fig. 2A). Analysis of DEPs between the two groups showed that 63 out of 182 proteins were differentially regulated by hyperoxia (Fig. 2A); however, only 21 out of 182 proteins were significantly regulated (P < 0.05) by hyperoxia with FC exceeding 1.2× (Supplemental Table S3). Among DEPs, 6 proteins were downregulated (Fig. 2B) and 15 were upregulated (Fig. 2C) by hyperoxia. The biological processes affected by hyperoxia were determined by performing enrichment analysis using the GO database. Downregulated proteins were enriched for organ growth, vasculogenesis, kidney development, male genitalia development, vascular smooth muscle differentiation, and vascular morphogenesis, indicating their repression by hyperoxia (Fig. 2D). In contrast, upregulated proteins were significantly enriched for processes specific to cytokine signaling, oxidative stress response, neuron apoptotic process, negative regulation of mitochondrial organization, and epithelial cell proliferation and migration, indicating activation of these pathways by hyperoxia (Fig. 2E). Based on their relevance in organ development and hyperoxic injury, we selected four DEPs (p-AMPKa[T172], p-AMPKb1[S108], SLUG, and PDGFRb) for validation (using microarray samples) and reproducibility (using nonarray samples; additional biological replicates) of the RPPA data using immunoblotting analyses. RPPA data were validated and reproduced for all tested proteins (Fig. 3, A–F), and magnitude change in protein expression was similar in RPPA and immunoblotting experiments. Hyperoxia exposure did not affect AMPKα (23A3) protein levels (Fig. 3, A and D), suggesting that hyperoxia primarily increases the phosphorylation of and not the endogenous levels of AMPKα protein. Our findings reemphasize that insults such as hyperoxia during lung development alters distinct cytoprotective, inflammatory, developmental, and reparative lung responses (6, 9, 27, 33). We observed significant activation of AMPK under hyperoxia. Recently, an AMPK agonist, metformin, was shown to decrease hyperoxic lung injury in neonatal rats (11). Although the main function of this evolutionarily conserved kinase is to maintain energy homeostasis (18), it also contributes to other cellular processes, including growth, autophagy, mitophagy, and redox regulation. Autophagy and mitophagy have been recently identified as major contributors to BPD pathogenesis (37, 41). Furthermore, endothelial AMPK expression is decreased in adults with PH, and its function within the endothelium is necessary and sufficient to protect against hypoxia-induced PH in adult mice (32). Therefore, strategies directed to increase AMPK activation has the potential to promote lung vascular health and mitigate hyperoxia-induced BPD and PH. In addition, we found that hyperoxia decreases the level of proteins critically involved in normal lung development (PDGFRb and SLUG). Although PDGFR expression was previously shown to be decreased in experimental and human BPD (34), the present study identified other novel proteins dysregulated by hyperoxia, such as SLUG.
Fig. 2.
Lung proteomics of 1-wk-old mice exposed to hyperoxia. Lung tissues of mice exposed to normoxia or hyperoxia (n = 3/group) for 7 days were analyzed using RPPA. Heatmap of unsupervised hierarchical clustering of hyperoxia-exposed mice inclusive of significantly DEPs (P < 0.05) (A). Significantly downregulated proteins (B) and upregulated proteins (C) by hyperoxia. GO analysis of significantly downregulated (D) and upregulated (E) biological processes by hyperoxia (FDR < 0.05; P < 0.05). FDR, false discovery rate; DEP, differentially expressed protein; GO, gene ontology; RPPA, reverse-phase protein array; see main text for relevant definitions of genes.
Fig. 3.
Validation of the RPPA results in immunoblot assays: Lung tissues of mice exposed to normoxia or hyperoxia for seven days from RPPA experiments (array samples; n = 3 per group) and a separate set of experiments (nonarray samples; n = 6 per group) were analyzed in immunoblot assays to determine the protein expression levels of p-AMPKα [Thr172], p-AMPKβ1 [Ser108], AMPKα [23A3], PDGFRβ, and SLUG (A). Densitometric analyses wherein p-AMPKα [Thr172] (B), p-AMPKβ1 [Ser108] (C), AMPKα [23A3] (D), PDGFRβ (E), and SLUG (F) band intensities were quantified and normalized to β-actin. The values shown are presented as the mean ± SD *Significant differences between normoxia and hyperoxia groups at P < 0.05 (t-test). AMPK, AMP-activated protein kinase; RPPA, reverse-phase protein array; see main text for relevant definitions of genes.
Proteins significantly dysregulated by hyperoxia are expected to be functionally associated with expression levels of genes that mediate responses to differential oxygen tension in the lungs. Identifying and understanding these functional relationships could shed light on the molecular basis of hyperoxia-mediated lung injury and enable early diagnosis and improved management. As a preliminary analysis, we selected three DEPs (p-AMPKb1[S108], PDGFRb, and SLUG) based on their relevance in developmental lung injury and performed a cross-platform transcorrelation analysis (protein/transcript) to identify genes whose expression most significantly correlated with the levels of these DEPs. This analysis was performed using RPPA and gene expression analytes from normoxic and hyperoxic lungs. We observed strong correlations (r > ± 0.95, P < 0.05) between selected DEP levels and the expression of specific genes (Tables 1 and 2). Determining whether these correlations indicate functional relationships between DEPs and gene expression is one of our future goals. The canonical functions of many of these genes suggest they may play a crucial role in the pathogenesis of hyperoxic lung injury.
Table 1.
Positively correlating gene transcripts. Gene transcripts showing a positive correlation with DEP relative abundance in normoxic and hyperoxic lungs
| Gene | Correlation Coefficient | P Value |
|---|---|---|
| p-AMPKb1 (S108) differentially expressed | ||
| Hyperoxia | ||
| ATP1B1 | 0.952 | 0.048 |
| GSTA3 | 0.953 | 0.047 |
| ALDH4A1 | 0.962 | 0.038 |
| BC048546 | 0.964 | 0.036 |
| AHNAK | 0.964 | 0.036 |
| CLEC2D | 0.964 | 0.036 |
| ALDH1A1 | 0.968 | 0.032 |
| AK1 | 0.970 | 0.030 |
| CCNG1 | 0.971 | 0.029 |
| CDKN1A | 0.972 | 0.028 |
| IFIT3 | 0.973 | 0.027 |
| FAS | 0.977 | 0.023 |
| LYVE1 | 0.982 | 0.018 |
| MDM2 | 0.985 | 0.015 |
| CCND1 | 0.987 | 0.013 |
| GAA | 0.991 | 0.009 |
| LMNA | 0.992 | 0.008 |
| HSPA9 | 0.994 | 0.006 |
| EGR1 | 0.995 | 0.005 |
| Normoxia | ||
| HOXB5 | 0.960 | 0.040 |
| LAMP2 | 0.966 | 0.034 |
| KISS1 | 0.967 | 0.033 |
| DCXR | 0.974 | 0.026 |
| MEST | 0.978 | 0.022 |
| CDCA7 | 0.985 | 0.015 |
| GSTM2 | 0.994 | 0.006 |
| GYPA | 0.996 | 0.004 |
| COX6B2 | 0.998 | 0.002 |
| PDGFRb (28E1) differentially expressed | ||
| Hyperoxia | ||
| FAHD2A | 0.987 | 0.013 |
| Normoxia | ||
| MEGF10 | 0.954 | 0.046 |
| CCNG1 | 0.957 | 0.043 |
| GPX3 | 0.960 | 0.040 |
| CYP2F2 | 0.963 | 0.037 |
| GSTA3 | 0.969 | 0.031 |
| ADCY8 | 0.976 | 0.024 |
| AOX3 | 0.980 | 0.020 |
| GLB1L2 | 0.981 | 0.019 |
| HLX | 0.984 | 0.016 |
| MGP | 0.991 | 0.009 |
| ATP1B1 | 0.998 | 0.002 |
| ABCC3 | 0.999 | 0.001 |
| Slug (C19G7) differentially expressed | ||
| Hyperoxia | ||
| ASAH3L | 0.978 | 0.022 |
| MGLL | 0.989 | 0.011 |
| Normoxia | ||
| GYPA | 0.959 | 0.041 |
| GSTM2 | 0.963 | 0.037 |
| MFAP2 | 0.966 | 0.034 |
| GJA4 | 0.969 | 0.031 |
| ASZ1 | 0.975 | 0.025 |
| MGST1 | 0.980 | 0.020 |
| MEST | 0.987 | 0.013 |
| CCND1 | 0.990 | 0.010 |
| DCXR | 0.997 | 0.003 |
| CDS1 | 0.998 | 0.002 |
See main text for relevant definitions.
Table 2.
Negatively correlating gene transcripts. Gene transcripts showing a negative correlation with DEP relative abundance in normoxic and hyperoxic lungs
| Gene | Correlation Coefficient | P value |
|---|---|---|
| p-AMPKb1 (S108) differentially expressed | ||
| Hyperoxia | ||
| HTRA1 | −0.999 | 0.001 |
| AGT | −0.996 | 0.004 |
| HIST2H2AC | −0.994 | 0.006 |
| HOXB5 | −0.990 | 0.010 |
| CD82 | −0.989 | 0.011 |
| DNAJC15 | −0.985 | 0.015 |
| EIF5 | −0.979 | 0.021 |
| HIST1H2AO | −0.975 | 0.025 |
| HDC | −0.968 | 0.032 |
| D8ERTD82E | −0.964 | 0.036 |
| ADCY8 | −0.951 | 0.049 |
| Normoxia | ||
| MEGF10 | −0.996 | 0.004 |
| ATP6AP2 | −0.991 | 0.009 |
| ENPP5 | −0.985 | 0.015 |
| GSTA3 | −0.974 | 0.026 |
| GCNT2 | −0.963 | 0.037 |
| GUCY1B3 | −0.961 | 0.039 |
| PDGFRb (28E1) differentially expressed | ||
| Hyperoxia | ||
| BC034076 | −0.983 | 0.017 |
| KCNK3 | −0.975 | 0.025 |
| DNAIC1 | −0.974 | 0.026 |
| Normoxia | ||
| MEST | −0.986 | 0.014 |
| DLK1 | −0.983 | 0.017 |
| EXOC4 | −0.968 | 0.032 |
| EIF5 | −0.965 | 0.035 |
| LAMP2 | −0.964 | 0.036 |
| HOXB5 | −0.955 | 0.045 |
| Slug (C19G7) differentially expressed | ||
| Hyperoxia | ||
| ADAM28 | −0.996 | 0.004 |
| MEGF10 | −0.987 | 0.013 |
| CCDC85B | −0.985 | 0.015 |
| CLIC6 | −0.984 | 0.016 |
| LOC100046120 | −0.979 | 0.021 |
| CKMT1 | −0.970 | 0.030 |
| Normoxia | ||
| LMNA | −1.000 | 0.000 |
| ANGPT2 | −0.999 | 0.001 |
| ENPP5 | −0.979 | 0.021 |
| GUCY1B3 | −0.964 | 0.036 |
| HSPB8 | −0.961 | 0.039 |
| AHNAK | −0.961 | 0.039 |
| IGFBP2 | −0.956 | 0.044 |
See main text for relevant definitions.
Our study differs from others because we profiled both transcriptomic and proteomic data and performed a transcorrelation analysis between them. However, our study has a few limitations, which we plan to address in the future. First, the mouse lungs at birth are functionally different than those of a preterm infant and a murine model of hyperoxic lung injury may not accurately model human BPD. Second, omics analysis at single time-points may not identify all dysregulated genes, proteins, and pathways. Additionally, the number of quantifiable protein by RPPA in our study was rather limited to those validated by our core laboratory and is biased toward those selected for the array. Unbiased methods such as global proteomics and phosphoproteomics are required to identify all the significant pathways that are altered upon exposure to hyperoxia. Third, we did not map dysregulated genes and proteins to specific lung cell types. Finally, sex-specific effects were not evaluated.
In summary, we identified several genes and proteins that are potential biomarkers and therapeutic targets for BPD infants with PH. Additionally, our transcriptomic data may partly explain the mechanisms underlying BPD-associated neurologic morbidities. Importantly, our data reiterate that BPD with PH is a complex disease mediated by multiple pathways and may require a comprehensive approach for disease prevention and mitigation.
GRANTS
This work was supported by National Institutes of Health Grants HD-073323 (to B. Shivanna) and P30-CA-125123 (to S. Huang), Cancer Prevention & Research Institute of Texas Proteomics & Metabolomics Core Facility Support Award RP170005 (to S. Huang), American Heart Association Award BGIA-20190008 (to B. Shivanna), and American Lung Association Award RG-349917 (to B. Shivanna).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. conceived and designed research; A.K.S., R.T.M., and B.S. performed experiments; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. analyzed data; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. interpreted results of experiments; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. prepared figures; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. drafted manuscript; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. edited and revised manuscript; A.K.S., V.Y.N.G., R.T.M., J.L.H., S.H., and B.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Fuli Jia and Danli Wu (Antibody-based Proteomic Core) for helping with RPPA experiments. We also thank statisticians Kimal Rajapakshe, Cristian Coarfa, and Qianxing Mo for processing, normalizing, and analyzing the RPPA data.
REFERENCES
- 1.Ahmad A, Bhattacharya S, Sridhar A, Iqbal AM, Mariani TJ. Recurrent copy number variants associated with bronchopulmonary dysplasia. Pediatr Res 79: 940–945, 2016. doi: 10.1038/pr.2016.23. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghanem G, Shah P, Thomas S, Banfield L, El Helou S, Fusch C, Mukerji A. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J Perinatol 37: 414–419, 2017. doi: 10.1038/jp.2016.250. [DOI] [PubMed] [Google Scholar]
- 3.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180: 1122–1130, 2009. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao TP, Wu R, Cheng HP, Cui XW, Tian ZF. Differential expression of long non-coding RNAs in hyperoxia-induced bronchopulmonary dysplasia. Cell Biochem Funct 34: 299–309, 2016. doi: 10.1002/cbf.3190. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, Pryhuber GS. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Zhou Z, Yee M, Chu CY, Lopez AM, Lunger VA, Solleti SK, Resseguie E, Buczynski B, Mariani TJ, O’Reilly MA. The genome-wide transcriptional response to neonatal hyperoxia identifies Ahr as a key regulator. Am J Physiol Lung Cell Mol Physiol 307: L516–L523, 2014. doi: 10.1152/ajplung.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucherat O, Franco-Montoya ML, Thibault C, Incitti R, Chailley-Heu B, Delacourt C, Bourbon JR. Gene expression profiling in lung fibroblasts reveals new players in alveolarization. Physiol Genomics 32: 128–141, 2007. doi: 10.1152/physiolgenomics.00108.2007. [DOI] [PubMed] [Google Scholar]
- 8.Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, Hershenson MB. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev 20: 1995–2007, 2011. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambellan A, Cruickshank PJ, McKenzie P, Cannady SB, Szabo K, Comhair SA, Erzurum SC. Gene expression profile of human airway epithelium induced by hyperoxia in vivo. Am J Respir Cell Mol Biol 35: 424–435, 2006. doi: 10.1165/rcmb.2005-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Walther FJ, Sengers RM, Laghmani H, Salam A, Folkerts G, Pera T, Wagenaar GT. Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response. Am J Physiol Lung Cell Mol Physiol 309: L262–L270, 2015. doi: 10.1152/ajplung.00389.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuna A, Halloran B, Faye-Petersen O, Kelly D, Crossman DK, Cui X, Pandit K, Kaminski N, Bhattacharya S, Ahmad A, Mariani TJ, Ambalavanan N. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am J Respir Cell Mol Biol 53: 60–73, 2015. doi: 10.1165/rcmb.2014-0160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Thonel A, Ferraris SE, Pallari HM, Imanishi SY, Kochin V, Hosokawa T, Hisanaga S, Sahlgren C, Eriksson JE. Protein kinase Czeta regulates Cdk5/p25 signaling during myogenesis. Mol Biol Cell 21: 1423–1434, 2010. doi: 10.1091/mbc.e09-10-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J, Carey WA, Abel S, Collura C, Jiang G, Tomaszek S, Sutor S, Roden AC, Asmann YW, Prakash YS, Wigle DA. MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics 13: 204, 2012. doi: 10.1186/1471-2164-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floen MJ, Forred BJ, Bloom EJ, Vitiello PF. Thioredoxin-1 redox signaling regulates cell survival in response to hyperoxia. Free Radic Biol Med 75: 167–177, 2014. doi: 10.1016/j.freeradbiomed.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Suárez O, Pérez-Pinera P, Laurà R, Germana A, Esteban I, Cabo R, Silos-Santiago I, Cobo JL, Vega JA. TrkB is necessary for the normal development of the lung. Respir Physiol Neurobiol 167: 281–291, 2009. doi: 10.1016/j.resp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Go H, La P, Namba F, Ito M, Yang G, Brydun A, Igarashi K, Dennery PA. MiR-196a regulates heme oxygenase-1 by silencing Bach1 in the neonatal mouse lung. Am J Physiol Lung Cell Mol Physiol 311: L400–L411, 2016. doi: 10.1152/ajplung.00428.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilman RP, Lagoski MB, Lee KJ, Taylor JM, Kim GA, Berkelhamer SK, Steinhorn RH, Farrow KN. Right ventricular cyclic nucleotide signaling is decreased in hyperoxia-induced pulmonary hypertension in neonatal mice. Am J Physiol Heart Circ Physiol 308: H1575–H1582, 2015. doi: 10.1152/ajpheart.00569.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holdman XB, Welte T, Rajapakshe K, Pond A, Coarfa C, Mo Q, Huang S, Hilsenbeck SG, Edwards DP, Zhang X, Rosen JM. Upregulation of EGFR signaling is correlated with tumor stroma remodeling and tumor recurrence in FGFR1-driven breast cancer. Breast Cancer Res 17: 141, 2015. doi: 10.1186/s13058-015-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Serebreni L, Fallica J, Hamdan O, Wang L, Johnston L, Kolb T, Damarla M, Damico R, Hassoun PM. Cyclin-dependent kinase five mediates activation of lung xanthine oxidoreductase in response to hypoxia. PLoS One 10: e0124189, 2015. doi: 10.1371/journal.pone.0124189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44: W90–W97, 2016. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingappan K, Maity S, Jiang W, Wang L, Couroucli X, Veith A, Zhou G, Coarfa C, Moorthy B. Role of cytochrome P450 (CYP)1A in hyperoxic lung injury: analysis of the transcriptome and proteome. Sci Rep 7: 642, 2017. doi: 10.1038/s41598-017-00516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magagnotti C, Matassa PG, Bachi A, Vendettuoli V, Fermo I, Colnaghi MR, Carletti RM, Mercadante D, Fattore E, Mosca F, Andolfo A. Calcium signaling-related proteins are associated with broncho-pulmonary dysplasia progression. J Proteomics 94: 401–412, 2013. doi: 10.1016/j.jprot.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 26.McAdams RM, Vanderhoeven J, Beyer RP, Bammler TK, Farin FM, Liggitt HD, Kapur RP, Gravett MG, Rubens CE, Adams Waldorf KM. Choriodecidual infection downregulates angiogenesis and morphogenesis pathways in fetal lungs from Macaca nemestrina. PLoS One 7: e46863, 2012. [Erratum in PLoS One 8: 2013.] doi: 10.1371/journal.pone.0046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath-Morrow SA, Lauer T, Collaco JM, Lopez A, Malhotra D, Alekseyev YO, Neptune E, Wise R, Biswal S. Transcriptional responses of neonatal mouse lung to hyperoxia by Nrf2 status. Cytokine 65: 4–9, 2014. doi: 10.1016/j.cyto.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon RT, Shrestha AK, Reynolds CL, Barrios R, Shivanna B. Long-term pulmonary and cardiovascular morbidities of neonatal hyperoxia exposure in mice. Int J Biochem Cell Biol 94: 119–124, 2018. doi: 10.1016/j.biocel.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon RT, Shrestha AK, Shivanna B. Hyperoxia exposure disrupts adrenomedullin signaling in newborn mice: Implications for lung development in premature infants. Biochem Biophys Res Commun 487: 666–671, 2017. doi: 10.1016/j.bbrc.2017.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mourani PM, Abman SH. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol 42: 839–855, 2015. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, Sunamura S, Tatebe S, Aoki T, Sugimura K, Miyata S, Hoshikawa Y, Okada Y, Shimokawa H. Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ Res 119: 197–209, 2016. doi: 10.1161/CIRCRESAHA.115.308178. [DOI] [PubMed] [Google Scholar]
- 33.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696, 2003. doi: 10.1165/rcmb.4692. [DOI] [PubMed] [Google Scholar]
- 34.Popova AP, Bentley JK, Cui TX, Richardson MN, Linn MJ, Lei J, Chen Q, Goldsmith AM, Pryhuber GS, Hershenson MB. Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 307: L231–L239, 2014. doi: 10.1152/ajplung.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds CL, Zhang S, Shrestha AK, Barrios R, Shivanna B. Phenotypic assessment of pulmonary hypertension using high-resolution echocardiography is feasible in neonatal mice with experimental bronchopulmonary dysplasia and pulmonary hypertension: a step toward preventing chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 11: 1597–1605, 2016. doi: 10.2147/COPD.S109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 37.Sureshbabu A, Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front Physiol 4: 384, 2013. doi: 10.3389/fphys.2013.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veerappan A, Thompson M, Savage AR, Silverman ML, Chan WS, Sung B, Summers B, Montelione KC, Benedict P, Groh B, Vicencio AG, Peinado H, Worgall S, Silver RB. Mast cells and exosomes in hyperoxia-induced neonatal lung disease. Am J Physiol Lung Cell Mol Physiol 310: L1218–L1232, 2016. doi: 10.1152/ajplung.00299.2015. [DOI] [PubMed] [Google Scholar]
- 39.Vitiello PF, Staversky RJ, Keng PC, O’Reilly MA. PUMA inactivation protects against oxidative stress through p21/Bcl-XL inhibition of bax death. Free Radic Biol Med 44: 367–374, 2008. doi: 10.1016/j.freeradbiomed.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801, 2004. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Yue L, Yao H. Mitochondrial dysfunction in inflammatory responses and cellular senescence: pathogenesis and pharmacological targets for chronic lung diseases. Br J Pharmacol 173: 2305–2318, 2016. doi: 10.1111/bph.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoetis T, Hurtt ME. Species comparison of lung development. Birth Defects Res B Dev Reprod Toxicol 68: 121–124, 2003. doi: 10.1002/bdrb.10014. [DOI] [PubMed] [Google Scholar]