Abstract
Older people are four times more likely to develop pneumonia than younger people. As we age, many components of pulmonary innate immunity are impaired, including slowing of mucociliary clearance. Ciliary beat frequency (CBF) is a major determinant of mucociliary clearance, and it slows as we age. We hypothesized that CBF is slowed in aging because of increased oxidative stress, which activates PKCε signaling. We pharmacologically inhibited PKCε in ex vivo mouse models of aging. We measured a slowing of CBF with aging that was reversed with inhibition using the novel PKC inhibitor, Ro-31-8220, as well as the PKCε inhibitor, PKCe141. Inhibition of PKCε using siRNA in mouse trachea also returned CBF to normal. In addition, antioxidants decrease PKCε activity and speed cilia. We also aged wild-type and PKCε KO mice and measured CBF. The PKCε KO mice were spared from the CBF slowing of aging. Using human airway epithelial cells from younger and older donors at air-liquid interface (ALI), we inhibited PKCε with siRNA. We measured a slowing of CBF with aging that was reversed with siRNA inhibition of PKCε. In addition, we measured bead clearance speeds in human ALI, which demonstrated a decrease in bead velocity with aging and a return to baseline after inhibition of PKCε. In summary, in human and mouse models, aging is associated with increased oxidant stress, which activates PKCε and slows CBF.
Keywords: aging, airway epithelial cells, ciliary beat frequency, elderly, mucociliary clearance, PKCε pneumonia, siRNA
INTRODUCTION
Older people are more susceptible to lower respiratory tract infections such as pneumonia. Those over the age of 65 are four times more likely to develop pneumonia than younger patients (25), and lower respiratory tract infections are the eighth leading cause of death overall (33a). Aging is an independent risk factor for pneumonia mortality, even when controlling for comorbid conditions and severity (34).
Many physiological changes occur in the human lung with aging, which predispose individuals to pneumonia, including a decrease in elastic recoil (6), a decrease in lung compliance (39), a decrease in respiratory muscle strength (36), and a decrease in mucociliary clearance (42). Mucociliary clearance serves as the first line of defense against lower respiratory tract infection. The cilia beat in a coordinated manner to propel mucus and trapped particles and bacteria up and out of the lung.
Aging is known to impair mucociliary clearance. Human upper airway ciliary beat frequency (CBF) is slowed with aging (52) and is correlated with prolonged nasal saccharine transit times in women and men (8, 22, 41). Nasal saccharine transit times, however, may not always correlate with lower respiratory tract mucociliary clearance. Others have measured lower respiratory CBF directly and demonstrated ciliary slowing in aging humans (47), mice (1, 16), dogs (51), and guinea pigs (26). Ciliary slowing is associated with an impairment of human lower airway clearance of radioactive tracers with aging (13, 24, 42). Despite a literature base replete with examples of mucociliary clearance slowing with aging, little is known about the mechanisms of this slowing.
Oxidative stress has long been recognized as a consequence of aging. How oxidative stress contributes to lung aging is an emerging field of study (20). Exogenous oxidative stress has long been known to slow CBF (5, 10, 11, 28). However, little is known about how oxidative stress causes cilia slowing and even less about the cilia slowing of aging. We have previously shown that PKCε mRNA, protein, and activity are increased nearly threefold in aged BALB/c and C57BL/6 mice and that we can recapitulate the ciliary slowing of aging by using a PKCε agonist (1). We hypothesized that the increased oxidative stress associated with aging activates PKCε, which slows cilia. We designed experiments that inhibit PKCε in mice in vivo and ex vivo using pharmacological and genetic methods. In addition, we investigated the role of oxidative stress in the activation of PKCε. To increase the translatability of our findings, we also used human airway epithelial cells cultured at air-liquid interface (ALI) to test this hypothesis.
METHODS
Cell culture.
Primary, normal human bronchial epithelial cells (NHBEs) were isolated from deidentified human lungs that were rejected for transplantation. We accepted lungs from the International Institute for the Advancement of Medicine and the Nebraska Organ Retrieval System. We excluded donors with a history of any lung disease, current smoking, ≥20 pack-year history of smoking, and heavy alcohol use. The protocol was approved by the International Institute for the Advancement of Medicine and Nebraska Organ Retrieval System ethics committees and the University of Nebraska Medical Center Institutional Review Board.
Airway epithelial cells were isolated using a method previously described (3, 12). Briefly, the large airways were dissected out and protease digested. After 36–48 h, the airway lumens were scraped, and the resulting cells were plated on collagen-coated plates in bronchial epithelial growth medium (Lonza, Basel, Switzerland). They were subsequently transferred to collagen-coated 6.5-mm transwell inserts (0.4-µm pore) (Corning, Kennebunk, ME) and grown at ALI using commercially available medium (PneumaCult ALI media, StemCell Technologies, Inc., Cambridge, MA) according to the manufacturer’s directions.
Cells from a minimum of five different donors/age group were used in each experiment. Cells were only passaged one time before seeding ALI inserts. We did not use higher passage numbers to avoid phenotype drift or potentially poor ciliation.
Murine models.
All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and used in accordance with National Institutes of Health guidelines.
BALB/c mice aged 2 and 22–24 mo were obtained from the National Institute on Aging rodent colony (Bethesda, MD). The mice were euthanized using isoflurane and cervical dislocation, and the tracheas were removed and placed in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher, Waltham, MA) containing penicillin/streptomycin (Thermo Fisher) and fungizone (Thermo Fisher). Tracheal rings were cut with a scalpel to ~0.5-mm thickness and placed into DMEM to be maintained at 37°C, 5% carbon dioxide overnight to equilibrate.
A breeding pair of PKCε knockout (KO) mice (strain B6.129S4-PRKCεtm1Msg/J) was purchased from Jackson Laboratories (Bar Harbor, ME). This strain was created by disrupting a 1.2-KB region of the PRKCε gene, including the exon encoding the translation initiation codon using a targeting vector containing neomycin resistance and herpes simplex virus thymidine kinase genes (30). PKCε KO males were mated with PKCε heterozygote females, because PKCε KO females produce small litters. The resulting pups were genotyped using PCR analysis of tail DNA (Wizard Plus SV, Promega, Madison, WI). The following primers were used (5′ to 3′): wild-type (WT): forward: CCA CAA GGT GTA GCG AGT GA; reverse: CCG ATA GGA GCG TCT TGA AA; KO: forward: CTG AAT GAA CTGCAG GAC GA; reverse: ATA CTT TCT CGG CAG GAG CA. PKCε KO and WT littermate controls were aged side by side in our animal facility for up to 24 mo.
RNA silencing.
Tracheal rings were cut as above, and 1–2 were placed in 500 µl of Opti-MEM medium (Thermo Fisher) with 100 nM of mouse SMARTpool PKCε siRNA or nontargeting siRNA (Dharmacon, Lafayette, CO) and Lipofectamine RNAiMAX (7.5 µl) (Thermo Fisher). CBF and efficiency of protein knockdown were measured at 48 h.
For ALI cells, siRNA silencing was performed at the time of seeding the NHBE for ALI as outlined by McCray (43). Briefly, 75 nM SMARTpool human PKCε siRNA or nontargeting siRNA was added to the cells along with Lipofectamine RNAiMAX transfection reagent at the time of plating and remained for 48 h.
Western blot analysis.
Tracheal rings were homogenized in cell lysis buffer (Cell Signaling, Danvers, MA) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). NHBE cells were collected by scraping in ice-cold radioimmunoprecipitation assay buffer (Cell Signaling) containing protease inhibitor cocktail (Sigma) and incubated on ice for 15 min. The samples were clarified using centrifugation then heated to 100°C for 5 min in 2× Laemmli sample buffer (Bio-Rad, Hercules, CA) with β-mercaptoethanol (Sigma). Equal amounts of protein lysates were loaded onto 8–16% gradient SDS-PAGE gels. Proteins were transferred onto nitrocellulose membranes and were blocked for 1 h at room temperature in PBS with 0.1% TWEEN 20 and 5% blotto. The membranes were incubated overnight at 4°C with PKCε primary antibody (cat. no. 22B10, Cell Signaling) diluted 1:1,000.
Membranes were washed with PBS with 0.1% TWEEN 20, then incubated with horse radish peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no. 111-035-046, Jackson ImmunoResearch) for 1 h at room temperature. Membranes were washed, then incubated in SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher), and developed using standard film-based techniques. The specificity of the PKCε antibody has been previously published (37), showing no cross-reactivity to other PKC isoforms, and we confirmed this in PKCε KO tissues (see Fig. 4B).
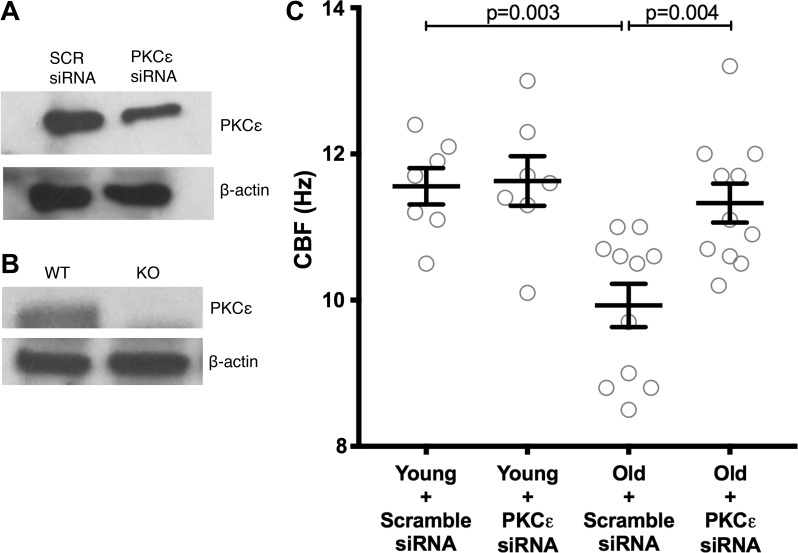
Fig. 4.
siRNA knockdown of PKCε speeds cilia beat frequency (CBF) in old tracheal rings. A: tracheal rings were prepared from young (2 mo) and old (22–24 mo) C57BL/6 mice. The rings were treated with a nonspecific (scramble) siRNA or an siRNA targeted to PKCε. At baseline, the old tracheal rings had a slowed CBF compared with the young tracheal rings (P = 0.003). After treatment with PKCε siRNA, the young rings had no change in their CBF, whereas the old rings had a significant increase in CBF (P = 0.004). B: representative blot showing knockdown of PKCε in tracheal rings. On average, in the old mice, PKCε was decreased to 49 ± 6% SE of the nontargeting control (n = 11, P = 0.002). C: at baseline, the young mice treated with scramble siRNA had a CBF of 11.56 ± 0.24 Hz, whereas the old mice treated with scramble siRNA were significantly slower at 9.92 ± 0.29 Hz (P = 0.003).
CBF measurements.
CBF was measured as previously described using Sisson-Ammons video analysis (SAVA) (49). Briefly, tracheal rings were placed in medium on a thermostatically controlled stage to maintain a constant temperature between 25 and 27°C. The beating cilia were observed using an inverted phase-contrast microscope with a ×20 objective lens. The entire lumen of each tracheal ring was used to measure CBF. Whole field video images were digitally recorded, and CBF was calculated using the SAVA system. The CBF speed is reported as cycles/second (Hz) ± SE.
Mucociliary transport time measurements.
A fluorescent bead clearance assay was used to measure the ability of the young and old cells to clear particles roughly the same size as bacteria, as previously described (35). Briefly, cells were grown at ALI until they were fully ciliated. The mucus was carefully washed off, and the cells were allowed to recover to baseline CBF. Fluorescent beads, FluoSpheres (carboxylate modified crimson, 1 µm in diameter; Thermo Fisher) were diluted and added to the apex of the cell and allowed to equilibrate for 5 min. High-speed videos (25 frames/s) were taken of bead motion. Tracker 4.11.0 (http://physlets.org/tracker/), an open source video analysis and modeling tool, was used to measure bead velocity. A total of 10 randomly chosen beads were tracked in each well, and the velocities were averaged and reported in mm/s ± SE.
PKCε activity assay.
PKCε activity was determined in whole cell cytosolic and particulate fractions similar to methods described previously (55, 56). Briefly, NHBE cells were flash-frozen in cell lysis buffer. The following reaction mix was prepared in Tris·HCl buffer:Phorbol 12-myristate 13-acetate (24 µg/ml), dithiotreitol (30 mM), ATP (150 µM), Mg-acetate (45 mM), PKCε-specific substrate peptide (ERMRPRKRQGSVRRRV; Calbiochem, San Diego, CA), and [γ-32P]ATP (10 µCi/ml). Cell lysate samples were incubated with the reaction mix for 15 min at 30°C. This mixture was then spotted onto P-81 phosphocellulose papers (Whatman, Clinton, NJ) to halt the reaction, then washed in phosphoric acid, washed in ethanol, dried, and counted in nonaqueous scintillant (National Diagnostics, Atlanta, GA). PKCε activity is expressed as picomoles of phosphate incorporated per minute per milligram of total cellular protein.
Glutathione measurement.
Murine lungs were homogenized in two volumes of 0.5 N perchloric acid in a Bullet Blender. The extracts were centrifuged at 10,000 revolutions/min for 5 min and the supernatants treated with a 10% solution of tri-n-butylphosphine in dimethylformamide to reduce and deconjugate the thiols. After deproteinization, the thiols were derivatized with 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonamide (7), filtered through a 0.22-µm membrane filter, and high performance liquid chromatography (HPLC) was used to determine the GSH level, as previously described (29). A Varian HPLC system was equipped with a Prostar 210 HPLC pump, coupled to a Prostar 410 autosampler that was connected to a Phenomenex Luna 5-µm C18 analytical column (250 mm × 4.6 mm). A C18 5-µm guard column (Phenomenex) was used in front of the analytical column; a Prostar 363 Fluorescence detector was operated at an excitation wavelength of 385 nm and an emission wavelength of 515 nm. The mobile phase, pumped at 1.5 ml/min, consisted of 0.1 mol/l potassium dihydrogen phosphate (adjusted to pH 2.1 with orthophosphoric acid) containing 10% acetonitrile.
Statistical analysis.
The data is represented as scatter plots with error bars representing standard error (SE). Statistical analysis was performed using GraphPad Prism software. Either a Student’s t-test or analysis of variance with Tukey test for multiple comparisons was performed as appropriate. A P value of <0.05 was considered significant.
RESULTS
Pharmacological inhibition of PKCε in old mouse tracheal rings speeds cilia.
We prepared tracheal rings from BALB/c mice aged 2 mo (young; n = 10) and 22–24 mo (old; n = 13). We measured CBF using SAVA before and after treatment with the novel PKC inhibitor Ro-31-8220 for 4 h. The novel PKC isoforms include PKC epsilon (ε), delta (δ), theta (θ), and eta (η). Of the novel PKC isoforms, PKCε plays the largest role in the mediation of cilia slowing (2). The baseline CBF in young mice was 18.47 ± 0.37 Hz and dropped to 12.92 ± 0.32 Hz in the old mice (P < 0.0001) (Fig. 1). After treatment with Ro-31-8220 for 4 h, the young tracheal rings remained stable at 17.82 ± 0.54 (P = 0.64), whereas the old tracheas increased to 17.02 ± 0.22 Hz (P < 0.0001) (Fig. 1). There was no difference in CBF between the young and old Ro-31-8220-treated rings (P = 0.43) (Fig. 1).
Fig. 1.
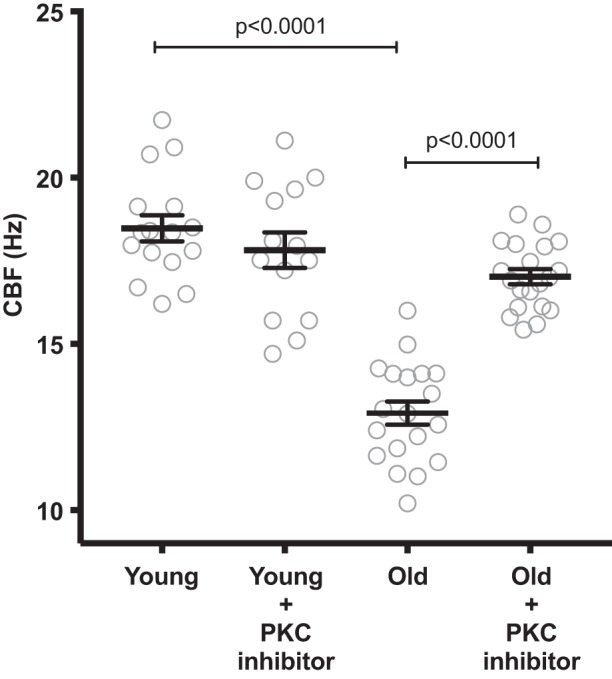
Inhibition of novel PKC isoforms restores ciliary beat frequency (CBF) in old mice. Tracheal rings were prepared from young (2 mo; n = 16) and old BALB/c mice (22–24 mo; n = 20). CBF was measured at baseline and after 4 h of treatment with Ro-31-8220 (100 µM). At baseline, the old tracheal rings had a significantly slowed CBF (P < 0.0001). Ro-31-8220 has no effect on the cilia from young tracheas but significantly speeds cilia in old mice (P < 0001).
Because Ro-31-8220 blocks not only PKCε but all of the novel PKC isoforms (δ, θ, η), we also treated the mouse trachea with a more specific inhibitor, PKCε 141 (Millipore, Billerica, MA). PKCε 141 is a thienoquinoline that disrupts the interaction between PKCε and its anchoring protein, RACK2 (45). This mechanism allows it to be a more specific inhibitor than Ro-31-8220. BALB/c tracheal rings were collected from young and old mice. Young mice had a baseline CBF of 17.68 ± 0.64 Hz, whereas old mice were slower at 11.51 ± 0.86 (P < 0.001). After treatment with PKCε 141, the young had no change (P > 0.99) in CBF, whereas the old had a 4.6-Hz increase to 16.11 ± 0.25 Hz (P < 0001) (Fig. 2).
Fig. 2.
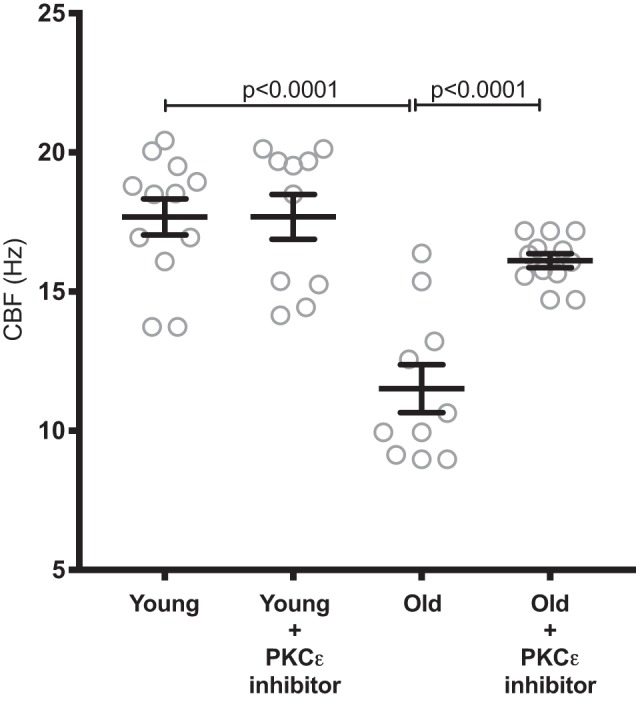
Inhibition of the interaction between PKCε and RACK2 restores cilia beat frequency (CBF) in old mice. Tracheal rings were prepared from young (2 mo; n = 12) and old (22–24 mo; n = 10) BALB/c mice. Once again, the old mice had significantly slowed CBF compared with young (P < 0.001). PKCε 141 (100 µM), which inhibits the interaction between PKCε and RACK2, restored the CBF slowing in the old tracheal rings after 4 h of treatment (P < 0.0001).
PKCε KO mice are protected from the ciliary slowing of aging.
Tracheal rings from young and old WT and KO animals (on a C57BL/6 background) were prepared. Average CBF was as follows: young WT 13.16 ± 0.47 (n = 12), old WT 10.69 ± 0.46 Hz (n = 18), young KO 12.61 ± 0.48 Hz (n = 10), old KO 12.69 ± 0.53 Hz (n = 16) (Fig. 3). The old WT were significantly slower than the young WT (P = 0.005) and the old KO (P = 0.01). There was no difference between young WT and young KO (P = 0.90) or old KO (P = 0.91) (Fig. 3).
Fig. 3.
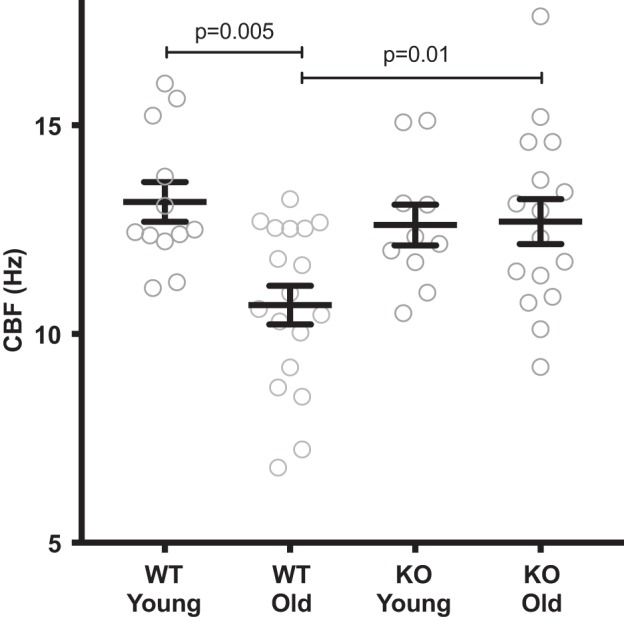
PKCε knockout (KO) mice are spared from cilia beat frequency (CBF) slowing of aging. Tracheal rings were cut from young (2–4 mo) and old (12–18 mo) wild-type (WT) and PKCε KO mice. These mice were on a C57BL/6 background and were raised side by side. There was a significant decrease in CBF in the old WT mice (P = 0.005), compared with the young WT mice. The old KO mice had the same CBF as the young KO mice. Young WT, n = 12; old WT, n = 18; young KO, n = 10; old KO, n = 16.
siRNA knock-down of PKCε in old and young murine tracheal rings.
Tracheal rings were prepared from young (2–4 mo; n = 7) and old (22–24 mo; n = 11) C57BL/6 mice. They were then treated with a nontargeting (scramble) siRNA or siRNA targeted to PKCε. Compared with scramble control, the rings treated with PKCε-targeted siRNA showed a decrease in PKCε protein of 79 ± 4% (n = 8, P = 0.001). A representative blot is shown (Fig. 4A). The antibody we used for these experiments and those used in Figs. 4 and 5 is specific to PKCε, as shown by positive staining in WT C57BL/6 mouse lung tissue at 86 Kd and no staining in the PKCε KO mouse tissue (Fig. 4B).
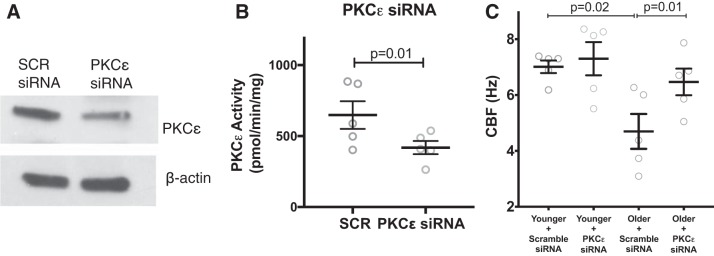
Fig. 5.
Human airway epithelial cells grown at air-liquid interface (ALI) have slowed cilia beat frequency (CBF) with aging, which is restored with PKCε siRNA. Airway epithelial cells were collected from young (age 19–40 yr; n = 5) and older (age 65–82 yr; n = 5) donors. They were cultured at ALI with nontargeting (scramble siRNA) or PKCε-targeted siRNA. Each reaction was performed in triplicate, and the mean CBF of the 3 wells is reported. A: representative blot showing knockdown of PKCε in human ALI. On average, PKCε was decreased 30 ± 4% SE compared with nontargeting control (n = 10, P = 0.0017). B: PKCε activity was significantly decreased in cells treated with PKCε siRNA compared with a scramble control (SCR). C: there was a significant decrease in CBF in the older samples at baseline, and CBF was restored by siRNA of PKCε.
At baseline, the young mice treated with scramble siRNA had a CBF of 11.56 ± 0.24 Hz, whereas the old mice treated with scramble siRNA were significantly slower at 9.92 ± 0.29 Hz (P = 0.003) (Fig. 4C). The CBF of the old mice was significantly increased after treatment with PKCε siRNA compared with scramble siRNA (P = 0.004) (Fig. 4). There was no difference between old mice treated with PKCε siRNA and young mice treated with scramble siRNA (P = 0.99).
siRNA of ALI in young and old humans.
We cultured airway epithelial cells derived from younger donors (age 19–42; n = 5) and older donors (age 65–82 yr; n = 5). The cells were treated with either a nontargeted, scramble siRNA or siRNA targeted to PKCε. We were able to decrease the expression of PKCε in the cells to 70 ± 0.04% of the nontargeted siRNA levels (n = 10) (P = 0.002). A representative blot is shown (Fig. 5A). Likewise, PKCε activity was significantly decreased with siRNA treatment compared with a scramble control (Fig. 5B). To determine whether cells from aged donors maintained elevated PKCε after multiple passages, we measured PKCε activity in submerged cultures at passage 1 and passage 5. The PKCε activity was unchanged throughout multiple passages, with an activity level of 283.3 ± 40.6 (n = 5) at passage 1 and 350.9 ± 42.1 (n = 5, P = 0.28) at passage 5.
We measured a significant decrease in CBF in the ALI in older compared with younger donors. In the samples treated with scramble siRNA, the younger donors had an average CBF of 7.01 ± 0.22, whereas the older donors had a CBF of 4.69 ± 0.62 Hz (P = 0.02). The older cells treated with PKCε siRNA increased their CBF to 6.47 ± 0.47 Hz (P = 0.01) (Fig. 5C).
Role of oxidative stress in cilia slowing of aging.
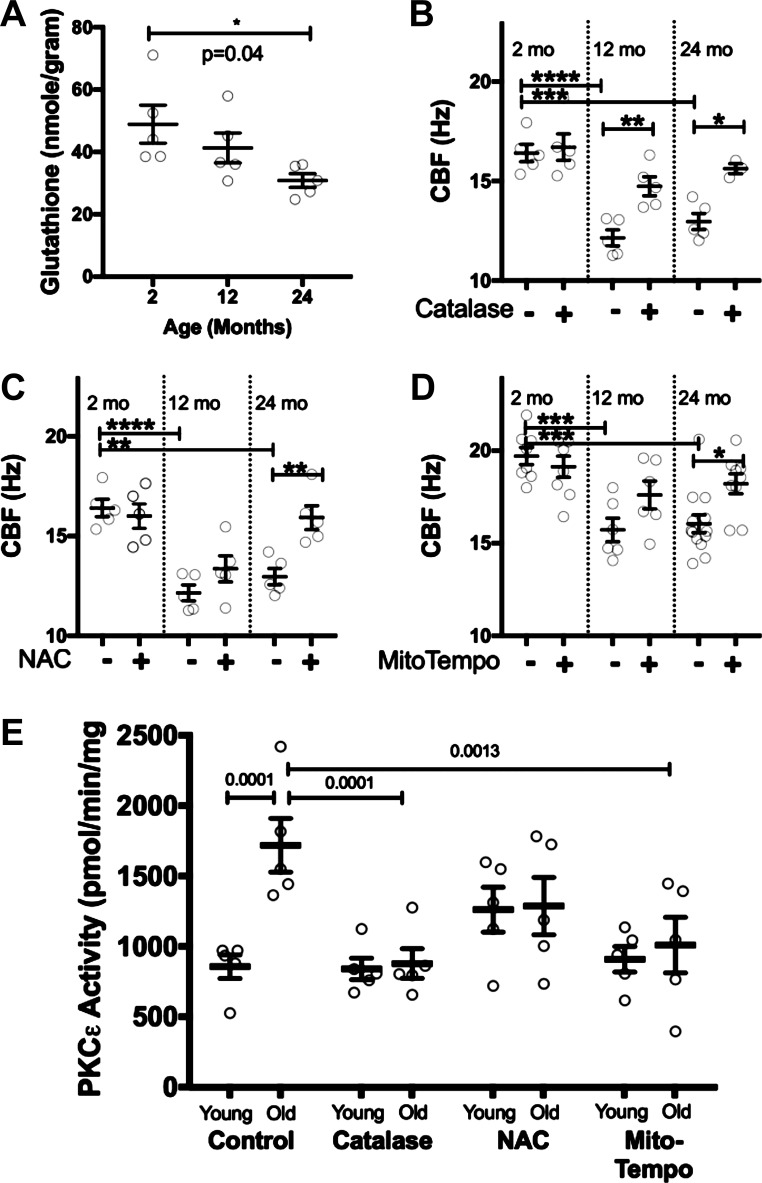
Oxidative stress is known to increase with aging. To determine whether changes in lung oxidant burden were occurring in our murine model of aging, we measured glutathione levels from whole lung homogenate from 2-, 12-, and 24-mo old BALB/c mice. Glutathione is a naturally occurring antioxidant. We measured a significant decline in glutathione between the 2- mo-old (48.9 ± 6.1 nmol/gram) and 24-mo-old mice (30.8 ± 2.2 nmol/gram; P = 0.04) (Fig. 6A). This decrease in antioxidant protection occurs at the same age that we measure decreases in CBF and increases in PKCε activity (1).
Fig. 6.
Antioxidants improve cilia beat frequency (CBF) and PKCε activity. Glutathione, a naturally occurring antioxidant, was measured in 2-, 12-, and 24-mo-old mice (A). There was a significant decline in glutathione in the older mice. This indicates a higher oxidant burden in the older mice. CBF was measured from 2-, 12-, and 24-mo-old BALB/c tracheas at baseline and 4 h after treatment with catalase (1,400 units/ml) (B), N-acetyl-l-cysteine (NAC; 100 µM) (C), or MitoTempo (100 µM) (D), a mitochondrial-specific antioxidant. With each treatment, there was an increase in CBF in the older animals (n = 5–12/group *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001.) Normal human bronchial epithelial (NHBE) cells from older human donors were treated with catalase (1, 400 units/ml), NAC (100 µM), or MitoTempo (100 µM) for 1 h, and PKCε activity was measured (E). We noted a decrease in PKCε activity in the older cells treated with antioxidants. This was statistically significant for catalase and MitoTempo (n = 5).
To determine whether decreasing oxidative stress could improve CBF in older mice, we treated 2-, 12-, and 24-mo-old BALB/c mouse tracheal rings with several antioxidants, including catalase, N-acetyl-l-cysteine (NAC), and MitoTempo. Each of these antioxidants had no effect on the CBF of young mice but increased the CBF of older mice (Fig. 6, B–D). Catalase increased CBF ~2 Hz in both the 12- and 24-mo-old mice. NAC increased CBF ~2 Hz in the 24-mo-old mice. To determine whether diminishing oxidative stress would decrease PKCε, we treated NHBE from “younger” (age 19–40 yr; n = 5) and “older” donors (age 65–82 yr; n = 5) with catalase, NAC, and MitoTempo for 1 h, then measured PKCε activity. We noted a decrease in PKCε activity in the older cells treated with antioxidants. Treatment with catalase for 1 h in older cells significantly decreased PKCε activity (P = 0.0001, n = 5). MitoTempo also decreased PKCε This suggests that antioxidants increase CBF through decreasing PKCε activity.
Mucociliary transport velocity is diminished in ALI derived from older humans.
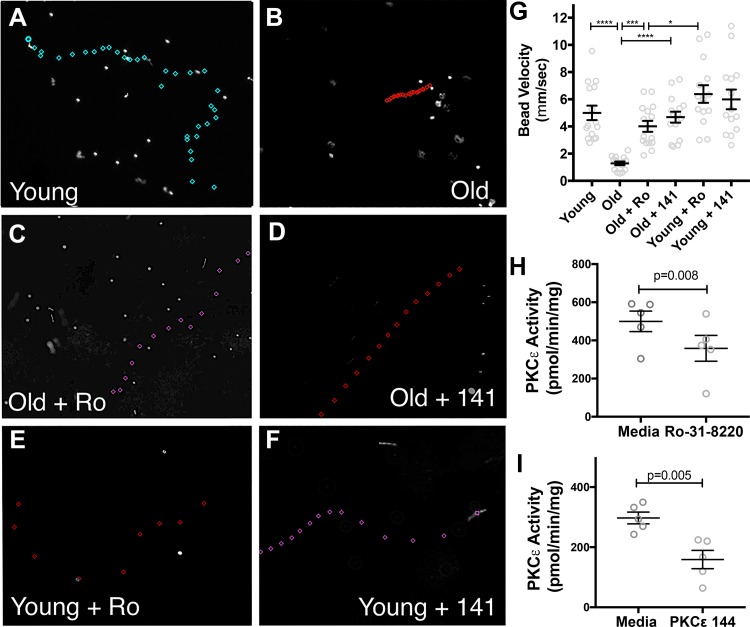
Mucociliary transport velocity was measured in ALI derived from younger (age 19–40 yr; n = 5) and older (age 65–82 yr; n = 5) donors in triplicate. A representative image of the path traveled by one bead is shown (Fig. 7, A–F). The position of the bead is marked every frame (0.04 s), and the velocity is calculated. Longer distances between marks indicate faster velocities. The cells derived from younger donors traveled significantly faster, at an average of 5.0 ± 0.53 mm/s, whereas the cells derived from older donors had a significantly slower velocity at 1.28 ± 0.51 mm/s (P < 0.0001) (Fig. 7G). After treatment with Ro-33-8220 (10 µM for 4 h), CBF of the older cells increased to 4.01 ± 0.39 mm/s (P = 0.003). After treatment with PKCε 141 (200 µM for 4 h), the bead velocity of the older cells increased to 4.68 ± 0.39 (P < 0.0001). We confirmed that PKCε activity was decreased after treatment with Ro-33-8220 and PKCε 141 (Fig. 7, H and I). The younger cells did not have a significant change in bead velocity with Ro-33-8220 (6.38 ± 0.64 mm/s) or PKCε 141 (5.99 ± 0.72 mm/s).
Fig. 7.
Human air-liquid interface (ALI) from older donors has impaired bead clearance. Airway epithelial cells were collected from young (age 19–40 yr; n = 5) and older (age 65–82 yr; n = 5) donors and were cultured at ALI in triplicate. When they were fully ciliated, all mucus was washed off, and fluorescent beads were placed on the apex of the ciliated cells. High-speed video of the bead motion was collected, and the velocity of the beads was measured. In each frame, the position of the bead was marked, and the images were superimposed to show the path of the bead. Longer distances between the marks indicate faster speeds. Representative images of younger (A), older (B), older treated with the PKCε inhibitor Ro-31-8220 (Ro; 10 µM for 4 h) (C), and older cells treated with the PKCε inhibitor PKCε 141 (141; 200 µM for 4 h) are shown (D). Younger cells treated with Ro (10 µM for 4 h) (E). Younger cells treated with 141 (200 µM for 4 h) (F). The average velocity of the beads for each condition (n = 15) (****P < 0.0001, ***P = 0.003, *P = 0.01) (G). The bead velocity is slowed in the older cells and returns to baseline with both inhibitors of PKCε. Inhibition of PKCε in younger cells has little effect on bead transport velocity. H: PKCε activity is significantly decreased in cells from older donors with treatment with Ro (10 µM for 1 h). I: PKCε activity is significantly decreased in cells from older donors with treatment with PKCε141 (200 µM for 1 h).
DISCUSSION
In this series of experiments, we show that multiple methods of inhibiting PKCε lead to improvements in the ciliary slowing of aging. We have shown that this is true in ex vivo and in vivo mouse models of aging as well as in cells derived from younger and older human donors. We also demonstrate an association of cilia slowing with oxidant burden in our murine aging model. This complements our previous findings that CBF is slowed in aging in both C57BL/6 and BALB/c mice (1) and correlates with increases in PKCε mRNA, protein, and activity (1). In addition, we have shown that these mechanisms are important in human as well as mouse aging.
We demonstrated that inhibition of the novel PKC isoforms (PKCε, δ, θ and η) with the compound Ro-31-8220 had no effect on cilia from young mice but sped the cilia of old mice (Fig. 1). The more specific inhibitor PKCε 141, which inhibits the interaction between RACK2 and PKCε, similarly increased the CBF in aged mouse cilia back to baseline and had no effect on the young cilia. This is consistent with our previous work showing that there is no RACK1 targeting protein in human or bovine ciliated cells (50). This allows a RACK2 inhibitor to be especially effective. Likewise, siRNA knockdown of PKCε in old mouse tracheal rings increases CBF, despite relatively inefficient knockdown of PKCε in these thick tracheal rings. We have also shown that PKCε KO mice are spared the ciliary slowing of aging. This suggests that in the mouse, this phenomenon is specific to PKCε, and other PKC isoforms do not compensate for its loss over time.
To determine how PKCε is upregulated in aging, we first measured glutathione levels in the lungs of young and old mice. Not surprisingly, we found that glutathione levels are decreased in aging (Fig. 6A). Others have measured decreased glutathione levels in old rat lungs (9, 15) and old mouse lungs (18). Glutathione dietary supplementation has been shown to protect from alcohol-induced ciliary dysfunction in mice fed alcohol in their drinking water (48). We have also shown that treatment with antioxidants such as NAC, catalase, and MitoTempo can increase baseline CBF in older tracheal rings (Fig. 6, B–D). NAC has been shown to both increase and decrease CBF, depending on the concentration (38). However, some of that effect may be because of changing mucus rheology. Our experiments were done in a mucus-free state to avoid this. Catalase has been shown to be protective against ciliary slowing in other oxidative stress situations such as infection (21). Our work shows that it is also effective in aging.
Adding to the translational importance of our work, we show that the ciliary slowing of aging is maintained in human airway epithelial cells after culture at ALI (Fig. 5). The baseline CBF in the young human cells are lower than other reports of human CBF for several reasons. First, to be consistent with our mouse methods, the CBF was read at room temperature. In addition, the siRNA transfection also contributes to some ciliary slowing; however, we think that including a scramble control is essential to this experiment.
Over the last 40 years, several groups have reported an age-related slowing of CBF in both the upper (22, 31) and lower respiratory tract of humans (47) and several experimental animal models, including mice (1, 16), dogs (51), and guinea pigs (26). In contrast, others have reported no changes in CBF with aging. All of these reports have been focused on nasal cilia. Jorissen et al. (27) reported no change in nasal CBF with aging; however, these patients were being evaluated for ciliary dyskinesia, so cannot be considered normal subjects. Another study measured nasal mucociliary transport using a gamma camera and did not measure any age-associated changes. This study, however, reports diminished clearance in all subjects, young and old, compared with other studies.
CBF is reported to directly correlate with mucociliary transfer speed. As early as 1977, Goodman et al. (14) reported that mean tracheal mucus velocity was significantly slower in nonsmoking older humans (age 55–70) (5.7 mm/min) compared with younger (age 19–28) (9.7 mm/min), as measured by bronchoscopic observation of test particle movement. They further report that 10% of older subjects had no motion at all of the test particles (14). This work is consistent with the dramatic slowing of CBF and bead velocity we see in our older human samples (Fig. 7).
Our work begins to dissect out the mechanism of why CBF slows as we age. Activation of PKC is implicated in the slowing of mammalian cilia (32, 33, 35, 54). More recently, PKCε has been shown to slow cilia in a variety of circumstances, including bacterial infection (2, 44, 53), coexposure to cigarette smoke and alcohol (57), and aging (1). We speculate that the oxidative stress associated with aging upregulates PKCε not only at the level of kinase activity but also at a transcriptional level. This is supported by our previous findings that PKCε mRNA and protein are increased in aged mice (1). Moreover, this elevation of PKCε activity appears to be conserved after several passages, suggesting that it may be upregulated at an epigenetic level. Further study is required to determine whether this occurs because of changes in histone modifications, DNA methylation, or DNA damage, which can also be associated with aging.
Our work also has important limitations. Although mucociliary clearance is proportional to CBF (4), mucociliary clearance speeds can also be affected by mucus characteristics. This is especially apparent in diseases with thick mucus such as cystic fibrosis (46) and chronic obstructive pulmonary disease (23). There are very little data that mucus in the lung changes with human aging. There are, however, data that Muc5ac, a major secreted mucin, decreases in aging mice (16). There are also data that mucus characteristics in other organs change with aging, such as the eye (19) and gastrointestinal tract (17, 40). Our experiments were performed in a mucus-depleted state, so we are unable to determine what effects potential mucus changes in aging could have, but these experiments maximized our ability to measure the role of CBF.
In conclusion, both human and mouse CBF slows with aging. Aging is associated with increased oxidant stress, which activates PKCε and slows CBF.
GRANTS
This work was supported by National Institutes of Health Grants R01-AG-0535553 (to K. L. Bailey), R01-AA-008769 (to J. H. Sisson), and R01-AA-017663 (to T. A. Wyatt) and Veterans Affairs Grant I01-BX-003635 (to T. A. Wyatt). T. A. Wyatt is the recipient of a Research Career Scientist Award (No. IK6-BX-003781) from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L.B., K.K.K., and T.A.W. conceived and designed research; K.L.B., K.K.K., and D.M.K. performed experiments; K.L.B., K.K.K., and D.M.K. analyzed data; K.L.B., J.H.S., and T.A.W. interpreted results of experiments; K.L.B. and D.M.K. prepared figures; K.L.B. and D.M.K. drafted manuscript; K.L.B., K.K.K., D.M.K., J.H.S., and T.A.W. edited and revised manuscript; K.L.B., K.K.K., D.M.K., J.H.S., and T.A.W. approved final version of manuscript.
REFERENCES
- 1.Bailey KL, Bonasera SJ, Wilderdyke M, Hanisch BW, Pavlik JA, DeVasure J, Robinson JE, Sisson JH, Wyatt TA. Aging causes a slowing in ciliary beat frequency, mediated by PKCε. Am J Physiol Lung Cell Mol Physiol 306: L584–L589, 2014. doi: 10.1152/ajplung.00175.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey KL, LeVan TD, Yanov DA, Pavlik JA, DeVasure JM, Sisson JH, Wyatt TA. Non-typeable Haemophilus influenzae decreases cilia beating via protein kinase Cε. Respir Res 13: 49, 2012. doi: 10.1186/1465-9921-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol functionally upregulates Toll-like receptor 2 in airway epithelial cells. Alcohol Clin Exp Res 33: 499–504, 2009. doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braiman A, Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir Physiol Neurobiol 163: 202–207, 2008. doi: 10.1016/j.resp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Burman WJ, Martin WJ 2nd. Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest 89: 410–413, 1986. doi: 10.1378/chest.89.3.410. [DOI] [PubMed] [Google Scholar]
- 6.Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol Respir Environ Exerc Physiol 47: 683–691, 1979. doi: 10.1152/jappl.1979.47.4.683. [DOI] [PubMed] [Google Scholar]
- 7.Cornwell PE, Morgan SL, Vaughn WH. Modification of a high-performance liquid chromatographic method for assay of homocysteine in human plasma. J Chromatogr A 617: 136–139, 1993. doi: 10.1016/0378-4347(93)80432-4. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira-Maul JP, Barbosa de Carvalho H, Goto DM, Maia RM, Fló C, Barnabé V, Franco DR, Benabou S, Perracini MR, Jacob-Filho W, Saldiva PHN, Lorenzi-Filho G, Rubin BK, Nakagawa NK. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest 143: 1091–1097, 2013. doi: 10.1378/chest.12-1183. [DOI] [PubMed] [Google Scholar]
- 9.Farooqui MY, Day WW, Zamorano DM. Glutathione and lipid peroxidation in the aging rat. Comp Biochem Physiol B 88: 177–180, 1987. doi: 10.1016/0305-0491(87)90097-6. [DOI] [PubMed] [Google Scholar]
- 10.Feldman C, Anderson R, Cockeran R, Mitchell T, Cole P, Wilson R. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir Med 96: 580–585, 2002. doi: 10.1053/rmed.2002.1316. [DOI] [PubMed] [Google Scholar]
- 11.Feldman C, Anderson R, Kanthakumar K, Vargas A, Cole PJ, Wilson R. Oxidant-mediated ciliary dysfunction in human respiratory epithelium. Free Radic Biol Med 17: 1–10, 1994. doi: 10.1016/0891-5849(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 12.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Goodman RM, Yergin BM, Landa JF, Golivanux MH, Sackner MA. Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis. Am Rev Respir Dis 117: 205–214, 1978. doi: 10.1164/arrd.1978.117.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Goodman R, Yergin B, Landa J, Golinvaux M, Sackner M.. Tracheal mucous velocity (tmv) in non-smokers, smokers and patients with obstructive lung-disease. FASEB J 607–607, 1977. [Google Scholar]
- 15.Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med 182: 1114–1122, 2010. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol 310: L860–L867, 2016. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guslandi M, Pellegrini A, Sorghi M. Gastric mucosal defences in the elderly. Gerontology 45: 206–208, 1999. doi: 10.1159/000022088. [DOI] [PubMed] [Google Scholar]
- 18.Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. Biochem J 188: 25–30, 1980. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazlett LD, Moon MM. Ocular surface complex carbohydrates are modified with aging. Exp Eye Res 44: 89–100, 1987. doi: 10.1016/S0014-4835(87)80028-3. [DOI] [PubMed] [Google Scholar]
- 20.Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am J Physiol Lung Cell Mol Physiol 314: L642–L653, 2018. doi: 10.1152/ajplung.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirst RA, Sikand KS, Rutman A, Mitchell TJ, Andrew PW, O’Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun 68: 1557–1562, 2000. doi: 10.1128/IAI.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Sun J, Leung R, Tsang KW. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 163: 983–988, 2001. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 23.Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J 13: 1177–1188, 1999. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- 24.Incalzi RA, Maini CL, Fuso L, Giordano A, Carbonin PU, Galli G. Effects of aging on mucociliary clearance. Compr Gerontol A Suppl 3: 65–68, 1989. [PubMed] [Google Scholar]
- 25.Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis 4: 112–124, 2004. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 26.Joki S, Saano V. Influence of ageing on ciliary beat frequency and on ciliary response to leukotriene D4 in guinea-pig tracheal epithelium. Clin Exp Pharmacol Physiol 24: 166–169, 1997. doi: 10.1111/j.1440-1681.1997.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 27.Jorissen M, Willems T, Van der Schueren B. Nasal ciliary beat frequency is age independent. Laryngoscope 108: 1042–1047, 1998. doi: 10.1097/00005537-199807000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Kantar A, Oggiano N, Giorgi PL, Braga PC, Fiorini R. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung 172: 215–222, 1994. doi: 10.1007/BF00164438. [DOI] [PubMed] [Google Scholar]
- 29.Kharbanda KK, Todero SL, King AL, Osna NA, McVicker BL, Tuma DJ, Wisecarver JL, Bailey SM. Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. Int J Hepatol 2012: 962183, 2012. doi: 10.1155/2012/962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 24: 253–260, 1999. doi: 10.1016/S0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SW, Mo JH, Kim JW, Kim DY, Rhee CS, Lee CH, Min YG. Change of nasal function with aging in Korean. Acta Otolaryngol Suppl 558: 90–94, 2007. doi: 10.1080/03655230701624939. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K, Salathé M, Pratt MM, Cartagena NJ, Soloni F, Seybold ZV, Wanner A. Mechanism of hydrogen peroxide-induced inhibition of sheep airway cilia. Am J Respir Cell Mol Biol 6: 667–673, 1992. doi: 10.1165/ajrcmb/6.6.667. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Tamaoki J, Sakai N, Chiyotani A, Takizawa T. Inhibition of ciliary activity by phorbol esters in rabbit tracheal epithelial cells. Lung 167: 277–284, 1989. doi: 10.1007/BF02714957. [DOI] [PubMed] [Google Scholar]
- 33a.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Hyattsville, MD: National Center for Health Statistics, 2016, vol. 65, p. 1–121. [PubMed] [Google Scholar]
- 34.Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K; Competence Network for Community-Acquired Pneumonia study group . Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 32: 139–146, 2008. doi: 10.1183/09031936.00092507. [DOI] [PubMed] [Google Scholar]
- 35.Lee RJ, Workman AD, Carey RM, Chen B, Rosen PL, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Cohen NA. Fungal aflatoxins reduce respiratory mucosal ciliary function. Sci Rep 6: 33221, 2016. doi: 10.1038/srep33221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging 8: 1489–1496, 2013. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res 109: 1031–1043, 2011. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- 38.Melville GN, Ismail S, Sealy C. Tracheobronchial function in health and disease. Effect of mucolytic substances. Respiration 40: 329–336, 1980. doi: 10.1159/000194301. [DOI] [PubMed] [Google Scholar]
- 39.Mittman C, Edelman NH, Norris AH, Shock NW. Relationship between chest wall and pulmonary compliance and age. J Appl Physiol 20: 1211–1216, 1965. doi: 10.1152/jappl.1965.20.6.1211. [DOI] [Google Scholar]
- 40.Ouwehand AC, Isolauri E, Kirjavainen PV, Salminen SJ. Adhesion of four Bifidobacterium strains to human intestinal mucus from subjects in different age groups. FEMS Microbiol Lett 172: 61–64, 1999. doi: 10.1111/j.1574-6968.1999.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 41.Paul P, Johnson P, Ramaswamy P, Ramadoss S, Geetha B, Subhashini AS. The effect of ageing on nasal mucociliary clearance in women: a pilot study. ISRN Pulmonol 1–5: 2013, 2013. doi: 10.1155/2013/598589. [DOI] [Google Scholar]
- 42.Puchelle E, Zahm JM, Bertrand A. Influence of age on bronchial mucociliary transport. Scand J Respir Dis 60: 307–313, 1979. [PubMed] [Google Scholar]
- 43.Ramachandran S, Krishnamurthy S, Jacobi AM, Wohlford-Lenane C, Behlke MA, Davidson BL, McCray PB JR. Efficient delivery of RNA interference oligonucleotides to polarized airway epithelia in vitro. Am J Physiol Lung Cell Mol Physiol 305: L23–L32, 2013. doi: 10.1152/ajplung.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read RC, Roberts P, Munro N, Rutman A, Hastie A, Shryock T, Hall R, McDonald-Gibson W, Lund V, Taylor G, et. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol (1985) 72: 2271–2277, 1992. doi: 10.1152/jappl.1992.72.6.2271. [DOI] [PubMed] [Google Scholar]
- 45.Rechfeld F, Gruber P, Kirchmair J, Boehler M, Hauser N, Hechenberger G, Garczarczyk D, Lapa GB, Preobrazhenskaya MN, Goekjian P, Langer T, Hofmann J. Thienoquinolines as novel disruptors of the PKCε/RACK2 protein-protein interaction. J Med Chem 57: 3235–3246, 2014. doi: 10.1021/jm401605c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regnis JA, Robinson M, Bailey DL, Cook P, Hooper P, Chan HK, Gonda I, Bautovich G, Bye PT. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med 150: 66–71, 1994. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 47.Roth Y, Aharonson EF, Teichtahl H, Baum GL, Priel Z, Modan M. Human in vitro nasal and tracheal ciliary beat frequencies: comparison of sampling sites, combined effect of medication, and demographic relationships. Ann Otol Rhinol Laryngol 100: 378–384, 1991. doi: 10.1177/000348949110000506. [DOI] [PubMed] [Google Scholar]
- 48.Simet SM, Pavlik JA, Sisson JH. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol 47: 629–635, 2013. doi: 10.1016/j.alcohol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211: 103–111, 2003. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 50.Slager RE, Devasure JM, Pavlik JA, Sisson JH, Wyatt TA. RACK1, a PKC targeting protein, is exclusively localized to basal airway epithelial cells. J Histochem Cytochem 56: 7–14, 2008. doi: 10.1369/jhc.7A7249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whaley SL, Muggenburg BA, Seiler FA, Wolff RK. Effect of aging on tracheal mucociliary clearance in beagle dogs. J Appl Physiol (1985) 62: 1331–1334, 1987. doi: 10.1152/jappl.1987.62.3.1331. [DOI] [PubMed] [Google Scholar]
- 52.Whan Kim S, Mo JH, Kim JW, Kim DY, Rhee CS, Lee CH, Min YG. Change of nasal function with aging in Korean. Acta Otolaryngol Suppl 127, sup558: 90–94, 2007. doi: 10.1080/03655230701624939. [DOI] [PubMed] [Google Scholar]
- 53.Wilson R, Roberts D, Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax 40: 125–131, 1985. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong LB, Park CL, Yeates DB. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via nPKC, independently of PKA. Am J Physiol 275: C440–C448, 1998. doi: 10.1152/ajpcell.1998.275.2.C440. [DOI] [PubMed] [Google Scholar]
- 55.Wyatt TA, Forgèt MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163: 1157–1166, 2003. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyatt TA, Schmidt SC, Rennard SI, Tuma DJ, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med 225: 91–97, 2000. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- 57.Wyatt TA, Sisson JH, Allen-Gipson DS, McCaskill ML, Boten JA, Devasure JM, Bailey KL, and Poole JA. Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase C epsilon-dependent manner. Am J Pathol 181: 431–440, 2012. doi: 10.1016/j.ajpath.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]