Abstract
The asthma-obesity syndrome represents a major public health concern that disproportionately contributes to asthma severity and induces insensitivity to therapy. To date, no study has shown an intrinsic difference between human airway smooth muscle (HASM) cells derived from nonobese subjects and those derived from obese subjects. The objective of this study was to address whether there is a greater response to agonist-induced calcium mobilization, phosphorylation of myosin light chain (MLC), and greater shortening in HASM cells derived from obese subjects. HASM cells derived from nonobese and obese subjects were age and sex matched. Phosphorylation of MLC was measured after having been stimulated by carbachol. Carbachol- or histamine-induced mobilization of calcium and cell shortening were assessed in HASM cells derived from nonobese and obese donors. Agonist-induced MLC phosphorylation, mobilization of calcium, and cell shortening were greater in obese compared with non-obese-derived HASM cells. The MLC response was comparable in HASM cells derived from obese nonasthma and nonobese fatal asthma subjects. HASM cells derived from obese female subjects were more responsive to carbachol than HASM cells derived from obese male subjects. Insulin pretreatment had little effect on these responses. Our results show an increase in agonist-induced calcium mobilization associated with an increase in MLC phosphorylation and an increase in ASM cell shortening in favor of agonist-induced hyperresponsiveness in HASM cells derived from obese subjects. Our studies suggest that obesity induces a retained phenotype of hyperresponsiveness in cultured human airway smooth muscle cells.
Keywords: asthma, body mass index, obesity, remodeling, wheezing
INTRODUCTION
Asthma and obesity evoke major public health concerns. The Centers for Disease Control and Prevention (CDC) estimate the cost of asthma at $56 billion per year and the cost of obesity at $147 billion per year in the United States (9, 12), and the prevalence of both diseases has increased over the years (2). In addition to serving as a risk factor for asthma (19, 41), obesity is also associated with severe disease, decreased response to therapy, and increased hospitalization rates (6, 14, 21, 29).
In the era of precision medicine, research efforts have focused on the classification of asthma phenotypes into clusters (22). The asthma-obesity cluster includes two phenotypes: early onset asthma associated with obesity in younger patients, a mostly Th2 dominant response, and a late-onset asthma associated with obesity in older women, a mostly Th1 dominant response (36). Multiple mechanisms have been postulated to explain these two phenotypes, including: an endocrine mechanism (insulin resistance) (13, 20), decreased adiponectin (24), and epigenetic (34) and genetic mechanisms (16).
Asthma, characterized by airway hyperresponsiveness and inflammation, can induce airway remodeling. Airway smooth muscle (ASM) acts as the pivotal tissue regulating bronchomotor tone. ASM cells also play a role in inflammation and remodeling (25). Carbachol and histamine activate a family of muscarinic and histamine receptors, respectively, increasing intracellular calcium levels. Increased intracellular calcium ([Ca2+]i) then stimulates myosin light chain kinase that phosphorylates myosin light chain (MLC) and that ultimately evokes ASM cell shortening. This shortening is amplified and sustained through actin polymerization and calcium sensitization pathways mediated by activation of phosphoinositide 3-kinase (PI3K), extracellular-regulated kinase (ERK) and protein kinase B (Akt), and Rho-associated protein kinase (ROCK) that inhibit MLC phosphatase. Given the pivotal role of ASM in mediating hyperresponsiveness, we hypothesized that human airway smooth muscle cells (HASM) isolated from obese patients intrinsically retain a hyperresponsive phenotype compared with those isolated from nonobese subjects. Our primary objective was to determine whether HASM cells derived from obese subjects retain hyperresponsiveness as measured by agonist-induced calcium release, phosphorylation of MLC, and single cell shortening by the fluorescently labeled elastomeric contractible surfaces (FLECS) method. Our secondary objective was to explore the existence of a distinct HASM cell phenotype by demonstrating increased hyperresponsiveness in obese females. Finally, we evaluated the impact of insulin treatment on agonist-induced excitation-contraction coupling in HASM cells.
METHODS
Materials.
Carbamylcholine chloride, histamine dihydrochloride, and human recombinant insulin were purchased from Sigma-Aldrich (St. Louis, MO). All PAGE/immune blotting supplies were purchased from Life Technologies (Grand Island, NY). Odyssey blocking buffer and secondary antibodies were purchased from Li-Cor (Lincoln, NE). Antibodies for detection of MLC-2, clone 19D3.1, were purchased from Millipore (Temecula, CA). Antibodies for detection of phosphorylated (p) MLC 2 (Thr18/Ser19), pAkt (S473)(D9E), Akt (pan)(40D4), P-p44/42 MAPK (T202/Y204)(D13.14.4E) (pERK), and P44/42 MAPK (Erk)(3A7) were purchased from Cell Signaling Technologies (Danvers, MA). The Fluo-8 No Wash Calcium Assay kit was purchased from Abcam (Cambridge, MA).
Isolation and culture of HASM cells.
HASM cells were isolated from tracheas obtained from the National Disease Research Interchange (Philadelphia, PA) and from the International Institute for the Advancement of Medicine (Edison, NJ). HASM cell culture was performed as described previously (26). The cells were cultured in Ham's F-12 medium supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; this medium was replaced every week. HASM cells isolated from obese and nonobese and from nonasthmatic and fatal asthma donors from 2010 to 2016 were kept in liquid nitrogen and revived for the purpose of this study. The donors were age and sex matched. In all experiments, HASM cells were used within four passages and serum starved for 48 h before experimental treatments.
Immunoblot analysis.
To determine MLC phosphorylation, cells were stimulated with carbachol (10 µM, 10 min). Cells were then treated with 0.6 M perchloric acid, and plates were scraped and pelleted by centrifugation. Pellets were solubilized in a solution containing RIPA buffer, reducing agent, and NuPAGE LDS sample buffer, resolved in 4–12% NuPAGE gel, and transferred to a membrane. The membrane was probed with murine anti-human MLC-2 (Millipore), Akt, and P44/42 MAPK (ERK) (Cell Signaling Technology) and rabbit anti-human p-MLC-2 (Thr18/Ser19) (pMLC), pAkt (S473), and P-p44/42 MAPK (T202/Y204) (ERK) antibodies (Cell Signaling Technology). Bands were detected using near-infrared conjugated secondary antibodies and quantified with the Li-Cor Odyssey imaging system after normalization to total MLC, total Akt, and total ERK.
Determination of [Ca2+]i in HASM cells.
Cells were treated with vehicle (hydrochloric acid) or human insulin (10 nM–1 µM) for 24 h. Cells were incubated in Ca2+-binding fluo 8 dye for 1 h. Carbachol (10 µM) and histamine (1 µM) were sequentially added as agonists, and real-time fluorescence intensity was measured in a fluorescent plate reader (Clariostar BMG Labtech). Baseline fluorescence was subtracted from each point to adjust for the cell density in wells. The area under the curve (AUC) of the agonist-induced [Ca2+]i response curve was determined in Graphad Prism software. Each donor cell line had four technical replicates per experimental condition. A second determination of agonist-induced [Ca2+]I release was done using single-cell calcium assay. Cells were incubated in Ca2+-binding fluo 8 dye for 1 h. Optical imaging (Eclipse TE2000-U Microscope; Nikon) was performed. Images were taken every second for 100 s. Carbachol (10 µM) or histamine (1 µM) was manually added to the imaged chamber after 10 frames were recorded. HASM cell fluorescence was analyzed using a cytoplasmic region of interest in 10 cells/frame, and the AUC was determined after having normalized to the baseline.
High-throughput single-cell force cytometry.
The FLECS approach (30, 31) was used to measure single HASM cell shortening. As previously described, uniform “X” shapes (70 μm diagonal by 10 μm thick) composed of fibronectin and fluorescent fibrinogen were micropatterned on soft elastomer films (30, 31). To facilitate covalent embedding of the extracellular matrix molecules in the film, these substrates were prepared using a sacrificial approach (40). Pluronic F-127 (0.5%) was used to block nonpatterned regions so that cells cannot adhere outside the fibronectin patterns. Adherent cells can create deformations of the X-shaped micropatterns when exerting tonic traction forces or stimulated contraction forces. These deformations within the micropatterns correspond to the amount of force exerted by each of the adherent cells and can be compared with the original unperturbed dimensions of the micropattern to assess the cellular contractile forces.
Before stimulation, cells were seeded on the micropatterns, allowed to adhere, and serum starved for 24 h. Cells were subsequently stimulated with histamine (100 μM) or carbachol (100 μM) to induce contraction. A baseline reading of micropatterns was taken by imaging immediately before stimulation with agonist, and further readings were taken after stimulation at intervals of 5 min. The cells were stained with Hoescht 33342 before imaging such that only patterns colocalized with one nucleus can be analyzed. Data from 400–700 cells per condition were collected. MATLAB (Mathworks, Natick, MA) was used to measure the deformations within each of the patterns and to generate distributions of the relative contractions in each of the cases. Using these distributions, we derived the median values of each of the conditions to plot the displacement over time and generated a tonality plot.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism (San Diego, CA) software. Normality was assessed using the Shapiro-Wilk normality test. Statistical significance was determined using the Student’s paired two-tail t-test or nonparametric test depending on normality. P values < 0.05 were considered significant.
RESULTS
Obesity increases carbachol-induced phosphorylation of MLC in HASM cells.
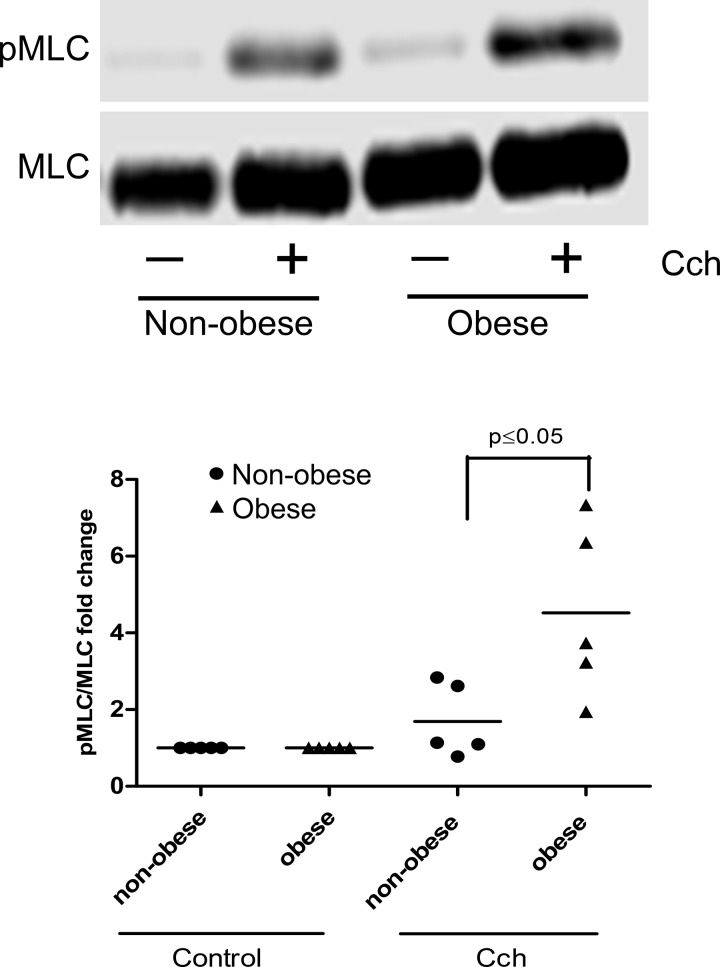
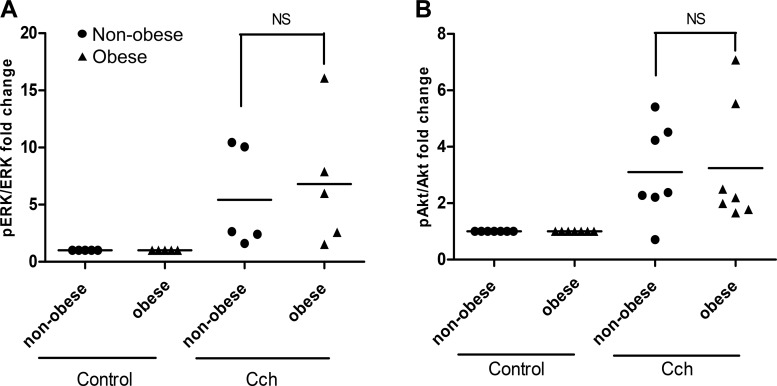
MLC phosphorylation, a pivotal signaling event, increases agonist-induced HASM shortening. Carbachol-induced phosphorylation of MLC was measured in HASM cells derived from five nonobese and five obese patients. The characteristics of the patients from which the cells were isolated (age, sex, and race) were comparable in both groups, the only difference being the body mass index (BMI; Table 1). HASM cells isolated from obese subjects showed markedly increased levels of MLC phosphorylation in response to carbachol (4.5 ± 1-fold) compared with those cells derived from age- and sex-matched nonobese subjects (1.7 ± 0.43-fold) (P < 0.05) (Fig. 1). Interestingly, carbachol-induced phosphorylation of Akt and ERK in HASM cells derived from obese subjects was not significantly increased compared with those derived from nonobese subjects (Fig. 2).
Table 1.
Initial characteristics of the nonobese and obese donors included in the carbachol-stimulated immunoblot study
| Nonobese | Obese | P Value | |
|---|---|---|---|
| Sex, W/M | 4/1 | 4/1 | 1 |
| Age, yr | 27.8 (13.72) | 41.4 (12.28) | 0.11 |
| Race, C/B | 5/0 | 4/1 | 0.29 |
| BMI, kg/m2 | 22.71 (0.87) | 44.31 (6.71) | <0.0001*** |
Data are means (SD); n = 10 subjects. W, woman; M, man; C,; B, black; BMI, body mass index.
P < 0.0001.
Fig. 1.
Immunoblot: carbachol (CCh, 10 µM, 10 min)-induced phosphorylation (p) of myosin light chain (MLC) in human airway smooth muscle (HASM) cells derived from nonobese (n = 5) and obese (n = 5) subjects. CCh induces more MLC phosphorylation in HASM cells derived from obese subjects than those derived from nonobese subjects (P ≤ 0.05).
Fig. 2.
Immunoblot: carbachol (CCh, 10 µM, 10 min)-induced phosphorylation of extracellular-regulated kinase (ERK, A) and protein kinase B (Akt, B) in human airway smooth muscle (HASM) cells derived from nonobese and obese subjects. There is no significant (NS) difference in the phosphorylation of ERK and Akt in response to CCh between nonobese and obese HASM cells.
Agonist-induced mobilization of [Ca2+]i is amplified in HASM cells derived from obese patients.
The AUC of the intracellular calcium release in response to an agonist (carbachol or histamine) was then measured in other HASM cell lines (n = 30). These HASM cell lines were comprised of HASM cells derived from nonasthma subjects (9 nonobese with a BMI <25 kg/m2, 1 overweight with 25 kg/m2 > BMI < 30 kg/m2, and 10 obese with a BMI >30 kg/m2) and 10 from subjects with fatal asthma (5 nonobese and 5 obese). The characteristics of the HASM cell donors (age, sex, and race) were comparable in the obese and nonobese groups; fatal asthma donors were significantly younger than the nonasthma donors (Table 2).
Table 2.
Initial characteristics of the nonobese, obese, nonasthma, and fatal asthma donors included in the study of analog-induced mobilization of [Ca2+]i
| Nonasthma |
Asthma |
||||||
|---|---|---|---|---|---|---|---|
| Nonobese | Obese | P Value | Nonobese | Obese | P Value |
P Value Nonasthma/Asthma |
|
| Sex, W/M | 5/4 | 5/5 | 0.809 | 3/2 | 3/2 | 1 | 0.70 |
| Age, yr | 35.22 (14.67) | 39.1 (12.24) | 0.538 | 21.6 (9.32) | 27.6 (10.76) | 0.373 | 0.03* |
| Race, C/B/H | 3/4/2 | 6/3/1 | 0.490 | 4/1/0 | 4/1/0 | 1 | 0.19 |
| BMI, kg/m2 | 21.72 (1.78) | 41.37 (8.71) | <0.0001*** | 19.09 (4.16) | 46.63 (15.39) | 0.0008** | 0.80 |
Data are means (SD); n = 29 donors. [Ca2+]i, intracellular calcium; W, woman; M, man; C, Caucasian; B, black; H, Hispanic; BMI, body mass index.
P < 0.05,
P < 0.001, and
P < 0.0001.
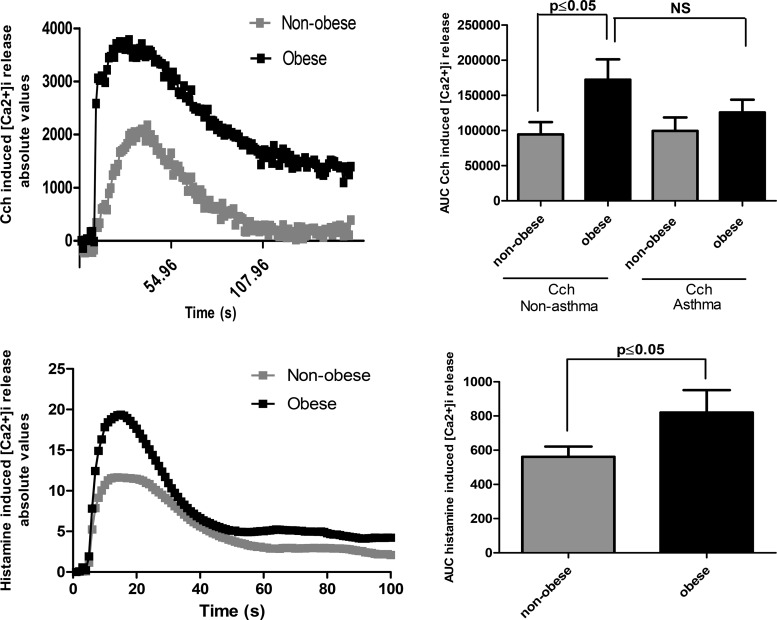
The AUC of carbachol-induced intracellular calcium release was significantly greater in HASM cells isolated from obese, nonasthmatic than in those from nonobese nonasthmatic donors (P < 0.05). There was no significant difference between the intracellular calcium response to carbachol in HASM cells without asthma derived from obese donors and those isolated from fatal asthma donors (P = 0.6). Histamine-induced calcium release in nonobese and obese HASM cells was then determined using single-cell calcium analysis in six of these nonasthmatic cell lines. The AUC of histamine-induced intracellular calcium release was significantly greater in HASM cells isolated from obese than in those isolated from nonobese donors (P < 0.05) (Fig. 3).
Fig. 3.
Carbachol (CCh, 10 µM)- and histamine (1 µM)-induced mobilization of intracellular calcium ([Ca2+]i) in human airway smooth muscle (HASM) cells derived from obese and nonobese and nonasthma (n = 19) and fatal asthma (n = 10) subjects. Data are expressed as means and SE. HASM cells derived from obese subjects have a significantly greater calcium response to CCh (P < 0.05) and histamine (P < 0.05) than those derived from nonobese (P < 0.05). HASM cells derived from obese nonasthma subjects have a response to CCh comparable to those derived from fatal asthma subjects.
Agonist-induced cell shortening is greater in HASM cells derived from obese patients.
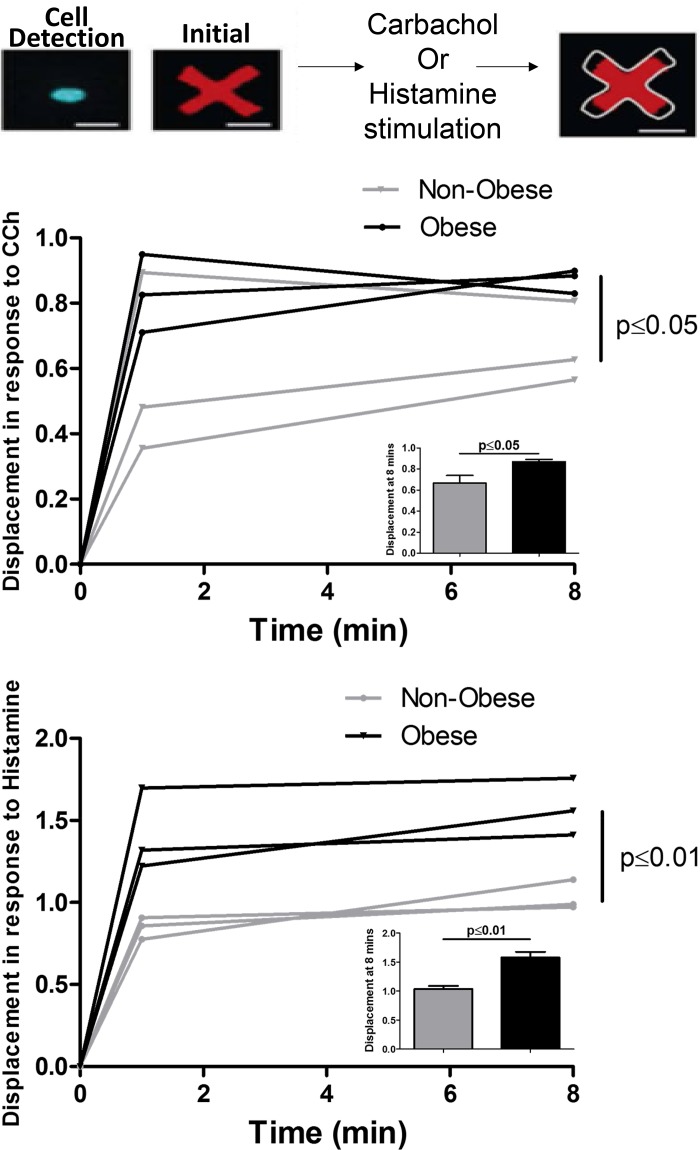
The shortening force generated by HASM cells derived from three nonobese and three obese race-, sex-, and age-matched donors in response to carbachol or histamine was determined using FLECS assay. Each cell was bound to a contractile fluorescent micropattern and stimulated with carbachol or histamine. HASM cells derived from obese donors generated a greater cell shortening in response to histamine than those derived from nonobese donors (P < 0.01). The same tendency was noted when HASM cells were stimulated with carbachol (P ≤ 0.05) (Fig. 4).
Fig. 4.
Quantification of human airway smooth muscle (HASM) cell contraction in response to carbachol (CCh) and histamine in nonobese and obese donors using high-throughput single-cell force cytometry (fluorescently labeled elastomeric contractible surfaces) technology (n = 3 nonobese, n = 3 obese). The inset bar graphs show means ± SE of single cell shortening force at 8 min (P ≤ 0.05 for CCh and P < 0.01 for histamine). Representative images of a single HASM cell bound to a contractible fluorescent micropattern and of the resulting decrement in the area of the pattern once the cell contracts in response to carbachol or histamine. At 8 min the displacement of the micropattern in response to histamine was significantly higher in HASM cells isolated from obese donors than in those isolated from nonobese donors (P < 0.01). The same trend was noted after stimulating the HASM cells with CCh (P ≤ 0.05).
Insulin alone has little effect on agonist-induced [Ca2+]i.
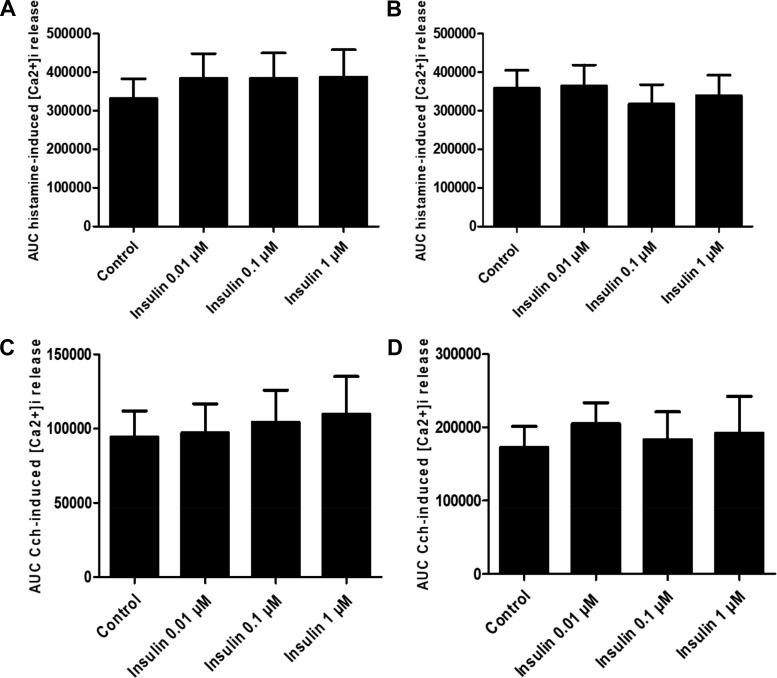
To determine whether obesity modulates insulin effects on agonist-induced calcium mobilization, HASM cell lines were treated with varying concentrations of insulin (0.01, 0.1, and 1, μM) for 24 h. Insulin had little effect on agonist (carbachol or histamine)-induced calcium mobilization regardless of subjects’ BMI (Fig. 5).
Fig. 5.
Area under the curve (AUC) of histamine (1 µm, A and B)- and carbachol (10 µM ,CCh, C and D)-induced mobilization of intracellular calcium ([Ca2+]i) in human airway smooth muscle (HASM) cells derived from obese (n = 10, B–D) and nonobese (n = 9, A–C) subjects, with insulin pretreatment. Data are expressed as means ± SE; 24 h insulin pretreatment has no effect on histamine-induced mobilization of [Ca2+]i in HASM cells isolated from nonobese (A) and obese (B) donors or CCh-induced mobilization of [Ca2+]i in HASM cells isolated from nonobese (C) and obese (D) donors.
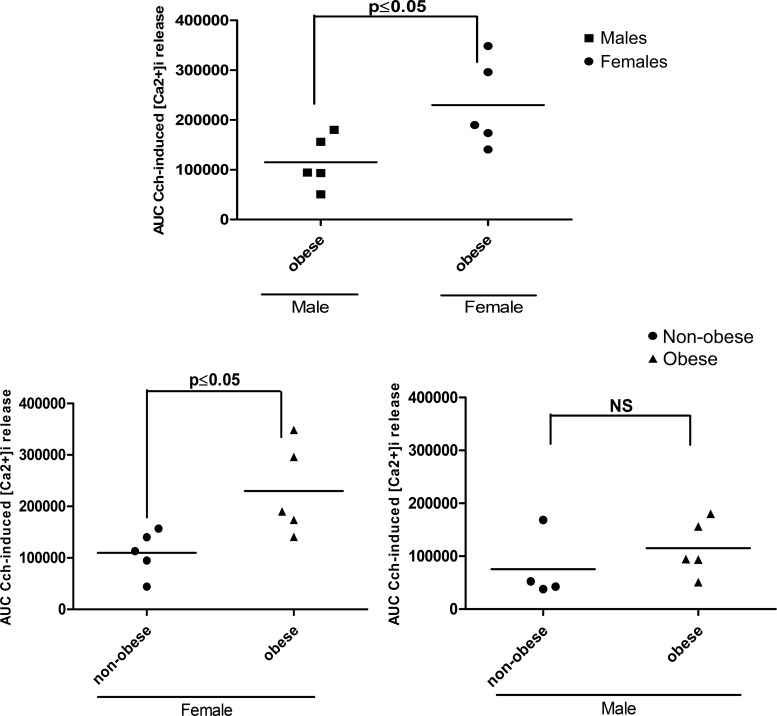
Sex modulates HASM cell responsiveness to carbachol.
To address whether sex and obesity modulate agonist-induced responses, HASM cells were isolated from comparable nonobese and obese women and men (Table 3). HASM cells from obese females had significantly greater intracellular calcium release (AUC) in response to carbachol than HASM cells from obese males (P = 0.04). The AUC of calcium release in response to carbachol was significantly higher among HASM cells derived from obese compared with those from nonobese females (P < 0.05); however, responses from HASM cells derived from obese and nonobese males were not significantly different (Fig. 6).
Table 3.
Initial characteristics of the female and male donors included in the study of analog-induced mobilization of [Ca2+]i
| Men | Women | P Value | |
|---|---|---|---|
| BMI, kg/m2 | 30.96 (11.19) | 32.56 (12.42) | 0.76 |
| Age, yr | 40.67 (10.92) | 35.64 (14.89) | 0.41 |
Data are means (SD); n = 20 donors. BMI, body mass index.
Fig. 6.
Area under the curve of carbachol (CCh)-induced intracellular calcium ([Ca2+]i) in human airway smooth muscle (HASM) cells derived from obese and nonobese male and female subjects. HASM cells derived from obese females mobilize significantly more [Ca2+]i in response to CCh than HASM cells isolated from nonobese females (P < 0.05). The same trend is not noted in obese men [not significant (NS), P > 0.05]. The obese female phenotype is more responsive to CCh than the obese male phenotype (P < 0.05).
DISCUSSION
Current evidence suggests that the asthma-obesity syndrome constitutes a major public health problem because of the increasing prevalence of both diseases (2) and the difficulty in treating such patients (6, 7, 23, 36). The nature of the association between these two diseases has given rise to many hypotheses, but the molecular mechanism remains elusive. This is the first study that shows differential responses of HASM cells to agonists depending on the BMI and sex of the subject. We have shown higher intracellular calcium release, a larger increase in the phosphorylation of MLC, and a greater cell shortening in response to an agonist in HASM cells derived from obese patients. Carbachol and histamine induce HASM cell shortening by increasing calcium release from the cytosolic stores that in turn stimulates the phosphorylation of MLC, leading to the generation of a contractile force. Collectively, our data suggest that BMI and sex modulate hyperresponsiveness to an agonist as determined by calcium mobilization, pMLC, and single cell shortening. As a preliminary approach to the mechanistic pathway involved in obesity-associated hyperresponsiveness, our results also suggest that the calcium sensitization pathway through Akt and ERK is not significantly involved in this response. The negative results related to Akt and ERK suggest that hyperresponsiveness seen in obese donor cells does not involve the M3-Gα12-PI3K-ROCK-Akt (44) calcium sensitization pathway but most likely involves an increase in intracytosolic calcium release through inositol triphosphate.
From 2011 to 2014, the CDC reported the prevalence of asthma among adults to be 8.8% but noted great disparities between normal-weight individuals (7.1%) and obese adults (11.1%). Among obese adults, women were more affected than men (14.6 vs. 7.1%) (2). This sex disparity trend was mirrored in our study, in which HASM cells isolated from obese female patients had a significantly greater calcium release in response to an agonist than HASM cells isolated from obese male patients. A distinctive phenotype of obese asthmatic females, with obesity preceding the development of airway hyperreactivity, has been supported by current evidence (17, 22, 42, 45). This phenotype should be distinguished from early onset asthma associated with obesity that appears to be a dominant Th2-driven response (42). The distinction between these two phenotypes is important and could explain the lack of difference in hyperresponsiveness between HASM cells isolated from nonobese and obese asthmatic donors in our study, since the asthmatic donors were significantly younger (Table 3). In addition, our study was not primarily designed to investigate the difference in hyperresponsiveness in HASM cells isolated from asthmatic patients, and a lack of power can also explain our failure to find difference between nonasthmatic and asthmatic donors and nonobese and obese asthmatic donors. Moore et al. characterized different asthmatic phenotypes in unbiased clusters analyses. Cluster 3 comprised older women with high BMI and late onset of asthma. This group was also characterized by uncontrolled asthma despite high-dose inhaled corticosteroids and long-acting β-agonists, high healthcare utilization, and frequent use of oral corticosteroids (22). The obese patients are at greater risk for developing asthma (19, 41) and also manifest a higher risk of severe asthma, decreased disease control (6, 19), and decreased response to corticosteroid therapy (14, 29, 39).
Several mechanisms may explain the association between obesity and asthma. The pathophysiology includes increased work of breathing with reduced functional residual capacity, lower lung volumes of breathing, increased collapsibility of airways, and airway dysanapsis in children (15, 18, 27, 43).
Metabolic syndrome and insulin resistance have also been reported to be associated with obesity-induced asthma (3, 8, 13, 20). Insulin treatment induced hyperresponsiveness in bovine airway smooth muscle trachea through insulin-induced laminin through PI3K and Rho kinase-dependent pathways (11, 35). In our study, 24 h insulin treatment of HASM cells had little effect on agonist-induced calcium release. However, we cannot completely rule out an action of insulin on airway hyperresponsiveness. We only studied a long-term effect of insulin (24 h treatment) and measured response to an agonist rather than a change in the baseline intracellular calcium that could be modulated by insulin (38).
A potential mechanism inducing airway hyperresponsiveness in obesity arises from the systemic and airway proinflammatory state that obesity induces. Previous studies have reported an increase in inflammatory mediators mediating the Th1 response in both pediatric (32, 33) and late-onset asthma-obesity syndrome, such as TNF-α, IL-17 (10), IL-6 (28), IL-13, interferon-γ (45), decreased adiponectin (4, 5, 24), and increased leptin (36, 37).
Our study shows that HASM cells from obese individuals, removed from in vivo endocrine or cytokine stimulation, retain characteristics similar to HASM cells isolated from fatal asthma patients. Because HASM cells isolated from donors with high BMI exhibit greater agonist-mediated phosphorylation of MLC, our findings suggest that epigenetic or genetic mechanisms might be at play. Granell et al. used a Mendelian randomization to uncover single nucleotide polymorphisms involved in high BMI and association with the development of asthma (16). In this prospective study involving 4,835 children, the weighted allele score was strongly associated with high BMI and asthma (mostly nonatopic). Genetic mechanisms could in part explain why HASM cells derived from obese subjects retain hyperresponsiveness. Additionally, Ahangari et al. found an increase in chitinase-3 like-1 expression in the white adipose tissue and the lung tissue of mice after exposure to a high-fat diet or allergen, underlying the possibility of epigenetic modification of the chitinase-3 like-1 gene resulting from high-fat diet in the development of asthma and obesity (1). We also found decreased methylation of promoters involved in innate immune response and nonatopic inflammation (CCL5, IL2RA, and TBX21) in the peripheral blood mononuclear cells of obese asthmatic children and increased methylation of a low-affinity IGE receptor and TGF-β1 (34). Although this observation was done in peripheral blood mononuclear cells, considering our findings in HASM cells, we postulate that such processes may modulate agonist-induced excitation-contraction coupling in HASM cells.
A main limitation of our study was related to the precise identification of the molecular pathway explaining hyperresponsiveness in HASM cells from obese donors. Defining this pathway will ultimately foster precise therapy for obese asthmatic patients and particularly for obese women with late-onset asthma.
This is the first study showing an intrinsic differential response in HASM cells derived from obese donors. The increase in agonist-stimulated calcium release associated with an increase in MLC phosphorylation and cell shortening supports a hyperresponsive phenotype in HASM cells derived from obese donors. These findings suggest genetic or epigenetic mechanisms in HASM cells may underlie the molecular mechanism mimicked by obesity. Identifying these mechanisms will provide new therapeutic targets to improve clinical outcome in asthma patients with obesity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-114471-03.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.O., J.J., G.C., D.R., and R.P. conceived and designed research; S.O., J.J., B.T.D., G.C., M.v.Z., I.P., H.E.M., R.D., and D.D.C. performed experiments; S.O., J.J., B.T.D., G.C., M.v.Z., I.P., H.E.M., R.D., D.D.C., and R.P. analyzed data; S.O., J.J., B.T.D., G.C., D.R., M.v.Z., I.P., H.E.M., R.D., D.D.C., and R.P. interpreted results of experiments; S.O., B.T.D., M.v.Z., I.P., H.E.M., R.D., and D.D.C. prepared figures; S.O., J.J., and R.P. drafted manuscript; S.O., J.J., B.T.D., G.C., D.R., M.v.Z., I.P., H.E.M., D.D.C., and R.P. edited and revised manuscript; S.O., J.J., B.T.D., G.C., D.R., M.v.Z., I.P., H.E.M., R.D., D.D.C., and R.P. approved final version of manuscript.
REFERENCES
- 1.Ahangari F, Sood A, Ma B, Takyar S, Schuyler M, Qualls C, Dela Cruz CS, Chupp GL, Lee CG, Elias JA. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med 191: 746–757, 2015. doi: 10.1164/rccm.201405-0796OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Fryar CD. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief 239: 1–8, 2016. [PubMed] [Google Scholar]
- 3.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE; North West Adelaide Health Study Team . Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol 118: 1284–1291, 2006. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne D, Scott H, MacDonald-Wicks L, Gibson PG, Wood LG. Resistin is a predictor of asthma risk and resistin:adiponectin ratio is a negative predictor of lung function in asthma. Clin Exp Allergy 46: 1056–1065, 2016. doi: 10.1111/cea.12742. [DOI] [PubMed] [Google Scholar]
- 5.Bianco A, Nigro E, Monaco ML, Matera MG, Scudiero O, Mazzarella G, Daniele A. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm Pharmacol Ther 43: 20–25, 2017. doi: 10.1016/j.pupt.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, Lenoir MA, Lurmann F, Meade K, Serebrisky D, Rodriguez-Cintron W, Kumar R, Rodriguez-Santana JR, Thyne SM, Burchard EG. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med 187: 697–702, 2013. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159: 2582–2588, 1999. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 8.Cardet JC, Ash S, Kusa T, Camargo CA Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J 48: 403–410, 2016. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Asthma facts _ CDC’s National Asthma Control Program Grantees. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2013. [Google Scholar]
- 10.Chen JH, Qin L, Shi YY, Feng JT, Zheng YL, Wan YF, Xu CQ, Yang XM, Hu CP. IL-17 protein levels in both induced sputum and plasma are increased in stable but not acute asthma individuals with obesity. Respir Med 121: 48–58, 2016. doi: 10.1016/j.rmed.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol 41: 494–504, 2009. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 28: w822–w831, 2009. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 13.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol 136: 304–311, 2015. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC; Childhood Asthma Management Program Research Group . Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol 127: 741–749, 2011. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, Cloutier MM, Canino G, Weiss ST, Litonjua AA, Celedón JC. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med 195: 314–323, 2017. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, Palmer TM, Sterne JA. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med 11: e1001669, 2014. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 127: 1486–93.e2, 2011. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King GG, Brown NJ, Diba C, Thorpe CW, Muñoz P, Marks GB, Toelle B, Ng K, Berend N, Salome CM. The effects of body weight on airway calibre. Eur Respir J 25: 896–901, 2005. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- 19.Koebnick C, Fischer H, Daley MF, Ferrara A, Horberg MA, Waitzfelder B, Young DR, Gould MK. Interacting effects of obesity, race, ethnicity and sex on the incidence and control of adult-onset asthma. Allergy Asthma Clin Immunol 12: 50, 2016. doi: 10.1186/s13223-016-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, Jung KH, Lee SY, Kim JH, Lee SY, Shin C, Shim JJ, Kang KH, Yoo SH. Asthma-like symptoms are increased in the metabolic syndrome. J Asthma 46: 339–342, 2009. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 21.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, Meade K, LeNoir MA, Avila PC, Farber HJ, Serebrisky D, Brigino-Buenaventura E, Rodriguez-Cintron W, Kumar R, Bibbins-Domingo K, Thyne SM, Sen S, Rodriguez-Santana JR, Borrell LN, Burchard EG. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest 147: 1591–1598, 2015. doi: 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program . Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181: 315–323, 2010. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muc M, Mota-Pinto A, Padez C. Association between obesity and asthma - epidemiology, pathophysiology and clinical profile. Nutr Res Rev 29: 194–201, 2016. doi: 10.1017/S0954422416000111. [DOI] [PubMed] [Google Scholar]
- 24.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol 20: 81–88, 2009. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 25.Panettieri RA Jr, Kotlikoff MI, Gerthoffer WT, Hershenson MB, Woodruff PG, Hall IP, Banks-Schlegel S; National Heart, Lung, and Blood Institute . Airway smooth muscle in bronchial tone, inflammation, and remodeling: basic knowledge to clinical relevance. Am J Respir Crit Care Med 177: 248–252, 2008. doi: 10.1164/rccm.200708-1217PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 256: C329–C335, 1989. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 27.Pasic A, Skokic F, Pasic F, Ilic M. The effect of body mass index on spirometric parameters in children with asthma. Med Arh 70: 186–190, 2016. doi: 10.5455/medarh.2016.70.186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy JV; National Heart, Lung, and Blood Institute Severe Asthma Research Program . Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 4: 574–584, 2016. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 27: 495–503, 2006. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 30.Pushkarsky I. FLECS technology for high-throughput single-cell force biology and screening. Assay Drug Dev Technol 16: 7–11, 2018. doi: 10.1089/adt.2017.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pushkarsky I, Tseng P, Black D, France B, Warfe L, Koziol-White CJ, Jester WF, Trinh RK, Lin J, Scumpia PO, Morrison SL, Panettieri RA, Damoiseaux R, Di Carlo D. Elastomeric sensor surfaces for high-throughput single-cell force cytometry. Nat Biomed Eng 2: 124–137, 2018. doi: 10.1038/s41551-018-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, Arens R. Obesity-associated asthma in children: a distinct entity. Chest 141: 895–905, 2012. doi: 10.1378/chest.11-0930. [DOI] [PubMed] [Google Scholar]
- 33.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, Khan ZS, Tesfa L, Hall CB, Macian F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med 191: 149–160, 2015. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep 3: 2164, 2013. doi: 10.1038/srep02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaafsma D, McNeill KD, Stelmack GL, Gosens R, Baarsma HA, Dekkers BG, Frohwerk E, Penninks JM, Sharma P, Ens KM, Nelemans SA, Zaagsma J, Halayko AJ, Meurs H. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol 293: C429–C439, 2007. doi: 10.1152/ajpcell.00502.2006. [DOI] [PubMed] [Google Scholar]
- 36.Sideleva O, Dixon AE. The many faces of asthma in obesity. J Cell Biochem 115: 421–426, 2014. doi: 10.1002/jcb.24678. [DOI] [PubMed] [Google Scholar]
- 37.Silva FM, Oliveira EE, Gouveia AC, Brugiolo AS, Alves CC, Correa JO, Gameiro J, Mattes J, Teixeira HC, Ferreira AP. Obesity promotes prolonged ovalbumin-induced airway inflammation modulating T helper type 1 (Th1), Th2 and Th17 immune responses in BALB/c mice. Clin Exp Immunol 189: 47–59, 2017. doi: 10.1111/cei.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, . et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol 310: L837–L845, 2016. doi: 10.1152/ajplung.00091.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland ER, Camargo CA Jr, Busse WW, Meltzer EO, Ortega HG, Yancey SW, Emmett AH, Stempel DA. Comparative effect of body mass index on response to asthma controller therapy. Allergy Asthma Proc 31: 20–25, 2010. doi: 10.2500/aap.2010.31.3307. [DOI] [PubMed] [Google Scholar]
- 40.Tseng P, Pushkarsky I, Di Carlo D. Metallization and biopatterning on ultra-flexible substrates via dextran sacrificial layers. PLoS One 9: e106091, 2014. doi: 10.1371/journal.pone.0106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinmayr G, Forastiere F, Büchele G, Jaensch A, Strachan DP, Nagel G; ISAAC Phase Two Study Group . Overweight/obesity and respiratory and allergic disease in children: international study of asthma and allergies in childhood (ISAAC) phase two. PLoS One 9: e113996, 2014. doi: 10.1371/journal.pone.0113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716–725, 2012. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 43.Yao TC, Tsai HJ, Chang SW, Chung RH, Hsu JY, Tsai MH, Liao SL, Hua MC, Lai SH, Chen LC, Yeh KW, Tseng YL, Lin WC, Chang SC, Huang JL; Prediction of Allergies in Taiwanese Children (PATCH) Study Group . Obesity disproportionately impacts lung volumes, airflow and exhaled nitric oxide in children. PLoS One 12: e0174691, 2017. doi: 10.1371/journal.pone.0174691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo EJ, Cao G, Koziol-White CJ, Ojiaku CA, Sunder K, Jude JA, Michael JV, Lam H, Pushkarsky I, Damoiseaux R, Di Carlo D, Ahn K, An SS, Penn RB, Panettieri RA Jr. Gα12 facilitates shortening in human airway smooth muscle by modulating phosphoinositide 3-kinase-mediated activation in a RhoA-dependent manner. Br J Pharmacol 174: 4383–4395, 2017. doi: 10.1111/bph.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng J, Zhang X, Zhang L, Zhang HP, Wang L, Wang G. Interactive effects between obesity and atopy on inflammation: A pilot study for asthma phenotypic overlap. Ann Allergy Asthma Immunol 117: 716–717, 2016. doi: 10.1016/j.anai.2016.09.430. [DOI] [PubMed] [Google Scholar]