Abstract
Acute respiratory distress syndrome (ARDS) is characterized by unrelenting polymorphonuclear neutrophil (PMN) inflammation and vascular permeability. The matrikine proline-glycine-proline (PGP) and acetylated PGP (Ac-PGP) have been shown to induce PMN inflammation and endothelial permeability in vitro and in vivo. In this study, we investigated the presence and role of airway PGP peptides in acute lung injury (ALI)/ARDS. Pseudomonas aeruginosa-derived lipopolysaccharide (LPS) was instilled intratracheally in mice to induce ALI, and increased Ac-PGP with neutrophil inflammation was noted. The PGP inhibitory peptide, arginine-threonine-arginine (RTR), was administered (it) 30 min before or 6 h after LPS injection. Lung injury was evaluated by detecting neutrophil infiltration and permeability changes in the lung. Pre- and posttreatment with RTR significantly inhibited LPS-induced ALI by attenuating lung neutrophil infiltration, pulmonary permeability, and parenchymal inflammation. To evaluate the role of PGP levels in ARDS, minibronchoalveolar lavage was collected from nine ARDS, four cardiogenic edema, and five nonlung disease ventilated patients. PGP levels were measured and correlated with Acute Physiology and Chronic Health Evaluation (APACHE) score, to (P/F), and ventilator days. PGP levels in subjects with ARDS were significantly higher than cardiogenic edema and nonlung disease ventilated patients. Preliminary examination in both ARDS and non-ARDS populations demonstrated PGP levels significantly correlated with P/F ratio, APACHE score, and duration on ventilator. These results demonstrate an increased burden of PGP peptides in ARDS and suggest the need for future studies in ARDS cohorts to examine correlation with key clinical parameters.
Keywords: acute lung injury, ARDS, LPS, PGP
INTRODUCTION
Acute respiratory distress syndrome (ARDS) remains a frequent cause of morbidity and mortality among patients admitted to the intensive care unit (ICU) (39). It is estimated that 5–20% of all mechanically ventilated patients in the ICU have ARDS (6, 7). Despite improvements in the management of ARDS with the use of lung protective ventilation strategies, mortality of severe ARDS remains high (38). There are currently no effective biomarkers and pharmacological targets to apply for the disease progression in patients with ARDS (2, 22).
Previous studies have shown that ARDS is associated with neutrophil-mediated inflammation of the lungs and dysregulated vascular permeability (35, 50). Proline-glycine-proline (PGP), a collagen breakdown product, shares structural homology with ELR+ chemokines such as CXCL8 and activates the CXCR1/2 pathway, thus enhancing neutrophil chemotaxis (41). In addition, CXCR2 activation leads to induction of vascular permeability (5, 48). PGP and its acetylated form (Ac-PGP) (termed PGP peptides) have been reported in several chronic inflammatory lung diseases such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) (14, 17, 26, 44). Recent work by our group showed that critical metalloproteinases (MMP-8 and MMP-9) that are required for PGP peptide generation were elevated in airway samples of subjects with severe pediatric ARDS (18). Subsequent studies showed the serum levels of PGP peptides were elevated in ARDS subjects and could activate human endothelial cells via the CXCR2 pathway, leading to monolayer permeability (13). These findings suggest that PGP peptides are operative in airway inflammation and might have an important role in the ongoing neutrophil influx and pulmonary capillary permeability characterized in the airspaces of ARDS. However, it is unclear if airway PGP peptide levels are elevated in ARDS subjects and if these peptides associate with clinical disease phenotypes. We therefore hypothesized that direct measurements of increased PGP peptides in the bronchial lavage, as an indicator for neutrophil infiltration, might signify the development of ongoing lung injury.
METHODS AND MATERIALS
Murine Experiments
Reagents.
Pseudomonas aeruginosa-derived lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO), Ac-PGP (Bachem, Torrance, CA), and l-arginine-threonine-arginine (RTR) (Bachem, Torrance, CA) were used for the murine experiments. Both Ac-PGP and RTR were checked for purity by high-performance liquid chromatography and mass spectrometry-mass spectrometry.
Murine model protocol.
All in vivo experimental protocols were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee. C57BL/6J female mice (8 wk old; Jackson) were used for both the Ac-PGP- and LPS-induced acute lung injury (ALI) models. To establish the PGP-induced lung inflammation model, mice were anesthetized using inhaled isoflurane (5%), followed by intratracheal administration of 50 μl of Ac-PGP (250 μg). Control mice were given intratracheal instillation of 50 μl PBS instead. Mice from both the groups were euthanized at 8 h after Ac-PGP instillation. For the LPS-induced ALI model, mice were anesthetized using inhaled isoflurane (100 mg/kg), followed by intratracheal administration of 50 μl of LPS (100 μg in PBS) (47). Control mice received intratracheal instillation of 50 μl PBS. For neutrophil depletion experiments, mice received anti-Ly6G (1A8; Bio X Cell, West Lebanon, NH) or control rat IgG (2A3; Bio C Cell) via intraperitoneal (500 μg) and intratracheal (200 μg) administration at both the day preceding and the day of LPS challenge and then culled 24 h later. To study the effect of RTR, a PGP peptide inhibitor that binds PGP sequences (30), LPS-induced ALI mice were treated with intratracheal RTR (250 μg in 50 μl PBS) 30 min before LPS administration or 6 h after LPS administration. Control mice received intratracheal instillation of 50 μl PBS or 50 μl RTR (250 μg). The mice were euthanized at 24 h after LPS administration. There was no statistically significant difference in the neutrophil infiltration in the lungs of LPS-treated mice with regards to percentage of neutrophils (97.3 ± 0.9 vs. 93.9 ± 3.2%, P = 0.928) in either male or female animals, respectively. For consistency in experimental design, we have used only female mice for all aspects of the experiments.
Murine bronchoalveolar lavage analysis.
After the mouse was anesthetized by intraperitoneal injection of ketamine (120 mg/kg) and xylazine (8 mg/kg), bilateral thoracotomy was performed at the indicated time. Thereafter, bronchoalveolar lavage (BAL) was performed three times through a tracheal cannula using 1 ml of sterile PBS each time. The first 1 ml BAL fluid from each mouse was centrifuged at 400 g for 10 min at 4°C. The supernatant was separated into aliquots and frozen at −80°C until analysis. The remaining cell pellets from all three lavages were used for cell counts. After staining with trypan blue, the total number of cells per lung was counted using a hemocytometer. Differential cell counting was performed on air-dried cytospin preparations stained by the Protocol HEMA3 stain set (Thermo Fisher Scientific). Cells were identified as macrophages, neutrophils, and lymphocytes according to the standard morphology. At least 200 cells were counted, and the absolute number of neutrophils was calculated. To evaluate pulmonary vascular leakage, immunoglobulin M (IgM) in BAL was measured using a standard enzyme immunoassay (no. E-900M; Immunology Consultants Laboratory). Total MMP-9 levels in BAL were determined using standard ELISA kits (no. MMPT90; R&D System).
Histology and morphometric analysis.
To study the histological features and perform morphometric analysis of LPS-induced lung injury, the mice were euthanized with intraperitoneal injection of ketamine (120 mg/kg) and xylazine (8 mg/kg). Thereafter, the lungs were fixed with 0.5 ml 10% formalin infusion through a tracheal cannula at a constant pressure of 25 cmH2O. Animals were euthanized, and the lungs were removed and immersed in fresh fixative for at least 24 h. After paraffin embedding, 5-μm sections were cut and stained with hematoxylin and eosin according to standard methods. Slides were randomized, read blindly, and examined for tissue damage according to published criteria, and an overall ALI score between 0 and 1 was assigned (24).
Human Subjects
Sample collection.
Mini-BAL from nine ARDS, four cardiogenic edema, and five nonlung/noncardiac disease mechanically ventilated subjects, admitted to the intensive care units at the UAB and Tampa General Hospital, were collected. All ARDS subjects were secondary to gram negative sepsis. The cardiogenic edema cohort comprised of subjects who were intubated with bilateral airspace opacities on chest radiograph at the time of admission with a clinical diagnosis of congestive heart failure. These subjects also demonstrated no signs of active infection during hospitalization and had a clinical response to diuretic therapy. Nonlung/heart disease control subjects were those who had been intubated for reasons other than heart or lung failure. Patient demographics, Acute Physiology and Chronic Health Evaluation (APACHE) II score, -to- (P/F) ratio, total ventilator days, and 30-day mortality were recorded. Patients were screened at the time of inpatient admission, and informed written consent was obtained from all subjects included in the study. The study protocol was approved by the Institutional Review Boards at the UAB (protocol no.: F081016007) and the University of South Florida (protocol no.: Pro0032300). All mini-BAL samples were acquired within 24 h of initiation of mechanical ventilation. The mini-BAL was performed by administering 30 ml of sterile saline via the endotracheal tube followed by immediate suctioning using an 8-Fr suction cannula. Mini-BAL was then centrifuged at 3,500 revolutions/min for 15 min, and supernatant was stored at −80°C until analysis. Myeloperoxidase (MPO) and albumin were measured in the mini-BAL using commercially available assays [MPO kit, no. 440007 (Biolegend, San Diego, CA); albumin kit, no. E80AL (ICL, Portland, OR)]. Of note, PGP peptide measurements in the serum of some of these cases were previously reported (13) but not the mini-BAL samples.
PGP peptide measurement by electrospray ionization-liquid chromatography-tandem mass spectrometry.
PGP peptides in murine BAL and human mini-BAL were quantified using a MDS Sciex (Applied Biosystems) API-4000 triple-quadrupole mass spectrometer equipped with a Shimadzu high-performance liquid chromatography and a 2.0 × 150-mm Jupiter 4μ Proteo column (Phenomenex) was used. The mobile phase consisted in both sample types out of 0.1% (vol/vol) formic acid in water (A) and 0.1% formic acid in acetonitrile using gradient elution (B): 0–0.5 min 5% B/95% A, then increased linearly over 0.5–2.5 min to 100% B/0% A. During the run, samples were kept at 4°C and the column at 30°C. Positive electrospray mass transitions were at 312–140, 312–112, and 312–70 mass-to-charge ratio for Ac-PGP and 270–70, 270–116, and 270–173 mass-to-charge ratio for PGP. Peak area was measured, and PGP peptide concentrations were calculated using a relative standard curve method as previously described (41).
Statistical Analysis
Data quality and distribution assumptions were examined by the standard methods, and the appropriate test procedures were used according to the study designs and distribution assumptions. For mouse model data, one-way ANOVA and post hoc Tukey analysis for multiple-comparison tests were applied. The Mann-Whitney test was used for comparisons of the mean values of two different samples. For human subject data, Kruskal-Wallis one-way analysis of variance, post hoc Dunn’s test, and Spearman correlation were used for statistical inference. Results were expressed as means ± SD. Statistical significance was defined as P ≤ 0.05.
RESULTS
Ac-PGP Induces Neutrophilic Inflammatory Response and Permeability in the Lung
One pathological hallmark of ALI and ARDS is the transmigration of neutrophils in the lung leading to destruction of the organ. PGP peptides have been recognized to be key chemoattractant in several inflammatory diseases and seem to play as important of a role in polymorphonuclear neutrophil (PMN) influx in airway inflammatory conditions such as COPD and CF (11, 44). In C57BL/6 mice, Ac-PGP administered intratracheally induced significant neutrophil recruitment in the airways as measured in the BAL compared with control mice treated with PBS (Fig. 1, A and B). In addition to neutrophil infiltration, Ac-PGP caused a significant increase in IgM leakage in the lung (Fig. 1C). MMP-9, a critical enzyme for lung remodeling and PGP peptide generation, was also induced in the lung by Ac-PGP (Fig. 1D). These data suggest that Ac-PGP can reproduce the pathophysiological changes that occur during the development of ALI and ARDS.
Fig. 1.
Acetylated proline-glycine-proline (Ac-PGP) induced acute lung injury (ALI). C57BL/6 mice were administered it with 250 μg Ac-PGP for 8 h, and the bronchoalveolar lavage (BAL) fluid was collected for total infiltrated cells (A), neutrophils (B), immunoglobulin M (IgM, C) and total matrix metalloproteinase-9 (MMP-9, D) measurement. Statistical analysis was performed using Mann-Whitney test; n = 4–5 mice/group. All values represent means ± SD.
Ac-PGP Mediates LPS-Induced Neutrophil Recruitment in the Lung
Gram-negative bacterial pneumonia and sepsis are some of the major causes of clinical ALI and ARDS (3, 15). The CXCR2 signaling pathway plays a critical role in LPS-induced neutrophil migration in the lung (19, 33). Human IL-8, a CXCR1 and CXCR2 ligand, is associated with the development and outcomes of ALI in human subjects (20). Our previous studies showed that systemic LPS-induced microvascular permeability changes were mediated by PGP peptides generated during lung injury (13). To determine whether PGP peptide signaling is involved in the pathogenesis of ARDS, a P. aeruginosa-derived LPS-induced ALI model was used. Mice were intratracheally challenged with LPS for 24 h, and BAL was collected for Ac-PGP measurement. As shown in Fig. 2A, LPS-induced ALI mice had elevated Ac-PGP levels compared with control mice administered with PBS. Neutrophil depletion in LPS-treated mice (>98% in the LPS + Ly6G group) did not result in a significant reduction in lung Ac-PGP levels (0.029 ± 0.013 vs. 0.037 ± 0.023 ng/ml, P = 0.756, LPS + Ly6G vs. LPS).
Fig. 2.
The application of arginine-threonine-arginine (RTR) attenuates neutrophil infiltration in the lung in lipopolysaccharide (LPS)-induced acute lung injury (ALI). A: C56BL/6 mice were administered it with 100 μg LPS (Pseudomonas aeruginosa). After LPS exposure (24 h), bronchoalveolar lavage (BAL) fluid samples were collected and analyzed for proline-glycine-proline (PGP) by electrospray ionization-liquid chromatography-tandem mass spectrometry (ESI-LCMS/MS). Statistical analysis was performed using Mann-Whitney test; n = 5–7 mice/group. B and C: 24 h after initiation of LPS-induced ALI, neutrophils in BAL were determined in mice that received RTR (250 μg) at 30 min before (pre) or 6 h after (pos) induction of ALI and in control mice with ALI but no RTR. Statistical analysis was performed using 1-way ANOVA (P < 0.0001) with Tukey’s multiple-comparison posttest, n = 3–7 mice/group. All values represent means ± SD.
RTR is a complementary peptide that specifically antagonizes PGP peptides, in which the sequence was designed on principles of hydropathic inversion (29). To determine whether PGP peptide mediated LPS-induced lung injury, specific blockade of these peptides with RTR (30, 38) was applied 30 min before (pretreatment) or 6 h (posttreatment) after LPS-induced pulmonary inflammation. The results showed that neutrophil recruitment in the alveolar compartment significantly increased at 24 h after LPS airway exposure. Mice that were pre- and posttreated with RTR had a significant reduction in total cell count and absolute neutrophil number in their BAL compared with LPS-exposed mice without RTR treatment (Fig. 2, B and C), demonstrating that PGP peptides are involved in PMN recruitment in LPS-induced ALI.
Inhibition of Ac-PGP Attenuates LPS-Induced Lung Damage and Permeability
Changes in alveolar-capillary membrane permeability are one of the critical parameters that define the pathophysiology of ALI (21). In control mice instilled with LPS, a significant increase in pulmonary vascular leakage was observed as measured by IgM levels in the BAL. This permeability increase was reduced in mice both pre- and posttreated with RTR (Fig. 3A). MMP-9, a key protease that bridges lung remodeling and pulmonary inflammation, was also reduced after RTR application (16) (Fig. 3B). In addition to neutrophil infiltration and vascular leakage, histological evidence of lung tissue injury has been identified as an important feature of ALI (24). As shown in Fig. 4A, the PBS control mice had normal lung parenchyma without evidence of inflammatory features. In contrast, interstitial edema, neutrophil infiltration, hemorrhage, alveolar disarray, and thickness of the alveolar septum were observed in the LPS group. Lung injury scores were enhanced after LPS challenge (Fig. 4B). RTR treatment significantly diminished the alveolar wall thickness, hemorrhage, and inflammatory cell infiltration (Fig. 4A) and reduced the lung injury scores (Fig. 4B). These results suggest that RTR, a PGP peptide antagonist, has a prophylactic and therapeutic effect in LPS-induced acute lung injury.
Fig. 3.
The application of arginine-threonine-arginine (RTR) reduces airway vascular leakage and matrix metalloproteinase (MMP)-9 release in lipopolysaccharide (LPS)-induced acute lung injury (ALI). After initiation of LPS-induced ALI (24 h), immunoglobulin M (IgM, A) and matrix metalloproteinase-9 (MMP-9, B) expression in BAL fluid was determined in mice that received RTR (250 μg) and in control mice with ALI only, RTR only, or PBS only. Statistical analysis was performed using 1-way ANOVA (P < 0.0001) with Tukey’s multiple-comparison posttest; n = 3–5 mice/group. All values represent means ± SD.
Fig. 4.
The application of arginine-threonine-arginine (RTR) attenuates lipopolysaccharide (LPS)-induced acute lung injury (ALI). After initiation of LPS-induced ALI (24 h), lung histology was assessed in mice that received RTR (250 μg) at 30 min before or 6 h after induction of ALI and in control mice with ALI but no RTR. A: hematoxylin and eosin staining of representative histological images of lung sections (bar: 50 mm for ×40 and 200 mm for ×200). B: lung injury scores. Statistical analysis was performed using 1-way ANOVA (P < 0.0001) with Tukey’s multiple-comparison posttest; n = 6–10 mice/group. All values represent means ± SD.
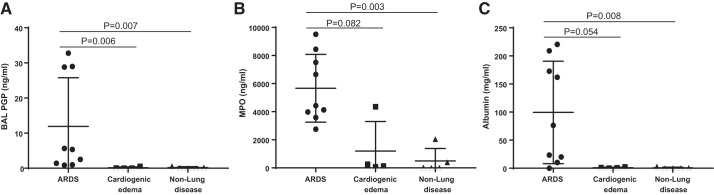
PGP Levels Are Significantly Increased in the Airway Secretions of ARDS Subjects
To evaluate the role of the airway PGP levels in patients with ARDS, mini-BAL were collected from groups of subjects mechanically ventilated for ARDS, cardiogenic edema, and nonlung/cardiac disease (Table 1). PGP levels (ng/ml) in ARDS were significantly higher than in cardiogenic edema and nonlung disease (Fig. 5A). As a surrogate of pulmonary neutrophil influx, MPO levels were measured, and the levels (ng/ml) were higher in ARDS compared with cardiogenic edema and nonlung disease, suggesting a higher neutrophil burden in ARDS (Fig. 5B). Similarly, BAL albumin levels (mg/ml) were increased more in ARDS than in cardiogenic edema and nonlung disease (Fig. 5C), confirming increased endothelial permeability in ARDS.
Table 1.
Patient demographics
| Group | n | Age (Median) | Sex, %Male | / | APACHE II Score | Mechanical Ventilation Duration | 30-Day Mortality, % |
|---|---|---|---|---|---|---|---|
| ARDS | 9 | 54.9 (11.1) | 66.7 | 128 (62.8) | 26.9 (4.5) | 16 (12.4) | 66.7 |
| Cardiogenic edema | 4 | 44.5 (19) | 25 | 213 (85.7) | 11.75 (6.8) | 1.0 (0) | Nil |
| Nonlung disease | 5 | 46.0 (11.9) | 60 | 447 (123) | 9.6 (4.4) | 2.4 (1.9) | Nil |
n, No. of subjects. APACHE II score, Acute Physiology and Chronic Health Evaluation score (estimates intensive care unit mortality based on a number of laboratory values and patient signs taking both acute and chronic disease into account); ARDS, acute respiratory distress syndrome.
Fig. 5.
Proline-glycine-proline (PGP) levels were elevated in acute respiratory distress syndrome (ARDS) compared with cardiogenic edema and nonlung disease patients. Mini-bronchoalveolar lavage (BAL) was collected from patients with ARDS (n = 9), cardiogenic edema (n = 4), and nonlung disease control (n = 5) for PGP measurement by electrospray ionization-liquid chromatography-tandem mass spectrometry (A). Myeloperoxidase (MPO, B) and albumin (C) levels in mini-BAL were detected by ELISA. Statistical analysis was performed using the Kruskal-Wallis test (P < 0.002) among groups and post hoc Dunn’s test of ARDS patients compared with the patients with cardiogenic edema and the nonlung disease control group. All values represent means ± SD.
A Marked Increase in BAL PGP Correlates with a Subsequent Deterioration in Clinical Parameters
To further investigate the impact of lung PGP levels, we examined the relationship between PGP levels and clinical risk factors for ALI/ARDS. BAL PGP showed significant positive correlation with APACHE II score and duration of mechanical ventilation and significant negative correlation with the P/F ratio (Table 2). Additionally, elevated BAL PGP correlated positively with MPO and BAL albumin concentrations. The association of PGP to MPO in mini-BAL samples corroborates its specificity to lung inflammation. No statistically significant correlation was observed with PGP level and mortality. These results suggest that elevated PGP levels are associated with metrics of inflammation and clinical derangement in ICU subjects.
Table 2.
Correlations between the PGP level in BAL and subject characteristics
| rs | P Value | |
|---|---|---|
| Clinical readout | ||
| / ratio | −0.612 | 0.0069 |
| APACHE II score | 0.849 | <0.0001 |
| Days on ventilator | 0.755 | 0.0003 |
| Biochemical readout | ||
| MPO | 0.864 | <0.0001 |
| Albumin | 0.836 | <0.0001 |
n = 18 Subjects [9 acute respiratory distress syndrome (ARDS), 4 cardiogenic edema, and 5 nonlung disease control]. PGP, proline-glycine-proline; BAL, bronchoalveolar lavage; rs, Spearman rank correlation coefficient; / ratio, the ratio of arterial oxygen partial pressure to fractional inspired oxygen; APACHE II score, Acute Physiology and Chronic Health Evaluation score (estimates intensive care unit mortality based on a number of laboratory values and patient signs taking both acute and chronic disease into account); MPO, myeloperoxidase.
DISCUSSION
Despite advances in treatment modalities, mortality associated with severe ARDS remains high (8, 38). Although several potential biomarkers and therapeutic targets have been identified for ARDS, their clinical application is uncertain (10). In this study, we found that levels of PGP, a matrikine tripeptide, were significantly elevated in airway samples of ARDS compared with human subjects with cardiogenic edema and nonlung disease. Elevated PGP levels also correlated with severity of disease in human subjects. Additionally, using a murine model of LPS-induced ALI, we demonstrated the role of PGP in regulation of neutrophilic chemotaxis and vascular permeability in the lung.
ARDS is characterized by increased neutrophilic infiltration and capillary leakage, resulting in deposition of protein-rich inflammatory fluid in the lung (40, 42, 49). Disruption of extracellular matrix during injury can lead to secretion of proinflammatory chemokines such as CXCL8 that can activate the transmigration of neutrophils to the site of injury (23). During this process, neutrophils secrete matrix-degrading enzymes such as MMP-8 and MMP-9 (43). It has been shown that alkali hydrolysis or proteolytic digestion of ECM protein by MMP-8 and -9 can lead to formation of peptide fragments that are cleaved by prolyl endopeptidase to form PGP peptides (9, 41). PGP peptides can thereafter be produced independent of the CXCL8 pathway by prolyl endopeptidase released from neutrophils, thus exponentially increasing the cascade of inflammation (27, 28). We and others have previously shown that PGP peptides can act as neutrophil chemoattractant and mediates neutrophilic injury in several inflammatory conditions (1, 9, 26). In addition to the neutrophil chemoattractant function, PGP peptides also regulate vascular permeability. Using an in vitro human endothelial model, our group previously demonstrated that PGP peptides selectively increased vascular permeability using the CXCR2 pathway via the Rac1-PAK-ERK signaling axis (13). In the same study, PGP was found to be elevated in the plasma of ARDS subjects. However, it was unclear if this was a result of the systemic inflammation related to the overall sepsis or if these findings were reflective of lung inflammation in ARDS.
Inflammation is the initial driver of inflammation in pathogen-related acute lung injury/ARDS (23, 34). Thereafter, vascular injury leads to leakage of cytokine-rich fluid in the lung parenchyma (23, 31). Our murine experiments show that intratracheal instillation of Ac-PGP led to increased neutrophil recruitment and protein leakage. These results confirm that PGP peptides mediate the local pulmonary inflammation in ALI and ARDS. PGP peptides have previously been shown to be produced in the lung, which plays a role in cellular responses to LPS (41). We used a previously published mouse model of lung injury using intratracheal LPS instillation to cause lung inflammation and injury to study PGP peptides in the direct setting of ARDS (25, 32). The purpose of using intratracheal LPS to cause lung injury in this model was to mimic the inflammatory response seen in pneumonia caused by gram-negative bacteria, a common cause of ARDS in patients (45). The results in this study showed that bacterial LPS-induced ALI was mediated by PGP peptides. The neutralization of neutrophils via Ly6G antibody led to no significant decrease in Ac-PGP levels. Accordingly, neutrophilic infiltration did not seem to impact the generation of PGP in this model. These results suggest that either structural cells (i.e., epithelial cells) or other immune cell populations (i.e., macrophages) may play a role in early PGP generation in this model of LPS-induced lung injury.
Previous studies have indicated that the PGP peptide inhibitor RTR has protective effects in several types of disease models, including smoke-induced lung injury, Ac-PGP-induced lung emphysema, dextran sodium sulfate-induced colitis, and alkali-injured rabbit cornea ulceration (4, 12, 17, 30, 37). In LPS-induced ALI mice, pre- and post-LPS administration with RTR decreased the number of infiltrating inflammatory cells (such as neutrophils) and reduced inflammation observed in LPS-treated mice, suggesting that RTR can attenuate pulmonary injury. MMP-9 is one of proteases related to pathogenesis of ARDS (16) and released from activated neutrophils, also involved in PGP peptides generation (9, 41) and PGP-mediated feedforward cycle of the matrix-derived chemokine (matrikine) augmenting its generation (46). These results suggest that MMP-9 is a key protease that bridges lung remodeling and pulmonary inflammation. MMP-9 was noted to be reduced after RTR application (Fig. 3B). RTR-related decrease in MMP-9 may explain how RTR attenuates PGP peptides generation and improves experimental ARDS. We also demonstrated that RTR significantly improves histopathological features and ameliorates pulmonary edema and lung vascular leakage in the LPS-induced ALI model, although it is important to note that PGP peptides did not entirely account for these effects. Regardless, these results demonstrate that RTR pre- and post-LPS administration reduced LPS-induced ALI and highlight that PGP peptides function as multifaceted matrikines in LPS-induced ALI.
Airway fluid from human subjects demonstrated increased neutrophilic burden (increased MPO levels) and vascular leakage (increased albumin) in ARDS, which paralleled with significantly elevated PGP levels in ARDS subjects. Unlike ARDS, cardiogenic edema is not mediated by neutrophilic inflammation and occurs because of increased hydrostatic pressure in the pulmonary vasculature, resulting in leakage of albumin-deficient fluid in the lung (36). The difference in pathophysiology of ARDS and cardiogenic edema may explain the variation in airway fluid PGP levels that was observed, although it is too early to know if PGP may serve as a biomarker to discern between these two conditions. The association of PGP to MPO in the airway fluid also corroborates its specificity to lung inflammation. Larger studies with serial PGP peptide measurements during the course of ARDS are required to further validate its utility as a reproducible biomarker.
There are a few limitations in this study. Our human subject sample size was small, since this project was intended as a pilot study to understand the role of PGP in ARDS. Nevertheless, the significant results with samples derived from two separate institutions along with appropriate controls and the confirmation of our hypothesis using in vivo experiments provide strong validity to our observations. The samples in our study were collected via a mini-BAL and not a traditional BAL in human subjects. However, elevation of PGP in the mini-BAL reflects its practical usability as a biomarker because of the relative ease of obtaining a mini-BAL sample compared with a traditional BAL in ARDS subjects.
In conclusion, PGP peptides are elevated in LPS-induced ALI/ARDS and may be important to the ongoing inflammatory response observed in the condition (Fig. 6). Inhibition of PGP peptides in ALI/ARDS with an inhaled RTR could represent a potential pharmacological approach to treat patients with ARDS. PGP elevated in the airway fluid of ARDS subjects compared with cardiogenic edema and nonlung disease subjects regulates neutrophilic inflammation and vascular permeability, leading to respiratory failure in the absence of cardiac failure. These results provide important preliminary evidence for the role of PGP peptides in the pathophysiology of ARDS.
Fig. 6.
A model of proline-glycine-proline (PGP)-mediated acute lung injury. Infection and tissue injury leads to activation of matrix-degrading enzymes from either structural cells or immune cells and subsequent breakdown of extracellular matrix. As a result, several bioactive matrikines are released. PGP and its acetylated form (Ac-PGP) mediate neutrophil chemotaxis and increase endothelial permeability through the CXCR2 pathway, leading to profound inflammation and tissue injury. ARDS, acute respiratory distress syndrome.
GRANTS
These studies were funded in part by American Heart Association Grant 16SDG27040000 (to X. Xu); National Heart, Lung, and Blood Institute Grant HL-07783, HL-090999, and HL-087824 (to J. E. Blalock), HL-102371 (to A. Gaggar), and HL-126596 (to J. E. Blalock and A. Gaggar); Cystic Fibrosis Foundation Therapeutics Grant GAGGARA0 (to A. Gaggar); Veterans Administration Grant 1 I01 BX001756 (to A. Gaggar); Ismail Moustapha Scholar Fund (to A. Gaggar); University of South Florida Startup Funds (to N. S. Sharma); and the National Science Foundation Grant DMS1701433 (to X.-Y. Lou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S.S., J.E.B., A.G., and X.X. conceived and designed research; N.S.S., J.-d.L., X.-y.L., L.V., T.A., R.K., J.S., P.K., A.S.-C., S.W., P.L.J., A.G., and X.X. performed experiments; N.S.S., J.-d.L., X.-y.L., T.A., R.K., R.R.-J., A.S.-C., S.W., P.L.J., J.E.B., A.G., and X.X. analyzed data; N.S.S., C.V.L., J.-d.L., X.-y.L., T.A., J.S., R.R.-J., A.S.-C., S.W., P.L.J., A.G., and X.X. interpreted results of experiments; N.S.S., J.-d.L., A.S.-C., A.G., and X.X. prepared figures; N.S.S., A.G., and X.X. drafted manuscript; N.S.S., C.V.L., J.E.B., A.G., and X.X. edited and revised manuscript; N.S.S., C.V.L., J.-d.L., X.-y.L., L.V., T.A., R.K., J.S., R.R.-J., A.S.-C., S.W., P.L.J., J.E.B., A.G., and X.X. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Michael Brown, Ali Jiwani, and German Luy for assistance in the sample collection and patient recruitment. Most importantly, we thank the patients who agreed to enroll in this clinical study.
REFERENCES
- 1.Akthar S, Patel DF, Beale RC, Peiró T, Xu X, Gaggar A, Jackson PL, Blalock JE, Lloyd CM, Snelgrove RJ. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat Commun 6: 8423, 2015. doi: 10.1038/ncomms9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin Z, Rahmawati FN. Recent insight into potential acute respiratory distress syndrome. Saudi Med J 38: 344–349, 2017. doi: 10.15537/smj.2017.4.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes. Clin Chest Med 27: 549–557, 2006. doi: 10.1016/j.ccm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol 300: L255–L265, 2011. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer J, Hebda JK, Le Guelte A, Galan-Moya EM, Smith SS, Azzi S, Bidere N, Gavard J. Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS One 7: e45562, 2012. doi: 10.1371/journal.pone.0045562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ; Mechanical Ventilation International Study Group . Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287: 345–355, 2002. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 7.Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, Pozo M, Gómez A, Baredes N, Jannello G, Osatnik J. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med 30: 2450–2456, 2002. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38: 1573–1582, 2012. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 9.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 180: 5662–5669, 2008. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaggar A, Olman MA. Biologic markers of mortality in acute lung injury. Clin Chim Acta 372: 24–32, 2006. doi: 10.1016/j.cca.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Gaggar A, Rowe SM, Matthew H, Blalock JE. Proline-glycine-proline (PGP) and high mobility group box protein-1 (HMGB1): potential mediators of cystic fibrosis airway inflammation. Open Respir Med J 4: 32–38, 2010. doi: 10.2174/1874306401004010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddox JL, Pfister RR, Sommers CI, Blalock JE, Villain M. Inhibitory effect of a complementary peptide on ulceration in the alkali-injured rabbit cornea. Invest Ophthalmol Vis Sci 42: 2769–2775, 2001. [PubMed] [Google Scholar]
- 13.Hahn CS, Scott DW, Xu X, Roda MA, Payne GA, Wells JM, Viera L, Winstead CJ, Bratcher P, Sparidans RW, Redegeld FA, Jackson PL, Folkerts G, Blalock JE, Patel RP, Gaggar A. The matrikine N-α-PGP couples extracellular matrix fragmentation to endothelial permeability. Sci Adv 1: 1, 2015. doi: 10.1126/sciadv.1500175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol 182: 4423–4431, 2009. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilberath JN, Carlo T, Pfeffer MA, Croze RH, Hastrup F, Levy BD. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3. FASEB J 25: 1827–1835, 2011. doi: 10.1096/fj.10-169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu AT, Barrett CD, DeBusk GM, Ellson CD, Gautam S, Talmor DS, Gallagher DC, Yaffe MB. Kinetics and role of plasma matrix metalloproteinase-9 expression in acute lung injury and the acute respiratory distress syndrome. Shock 44: 128–136, 2015. doi: 10.1097/SHK.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde AA, Jones CW, Jackson PL, Blalock JE, Sparidans RW, Kruijtzer JA, Garssen J, Folkerts G, Kraneveld AD. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut 63: 578–587, 2014. doi: 10.1136/gutjnl-2012-303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong MY, Li Y, Oster R, Gaggar A, Clancy JP. Early elevation of matrix metalloproteinase-8 and -9 in pediatric ARDS is associated with an increased risk of prolonged mechanical ventilation. PLoS One 6: e22596, 2011. doi: 10.1371/journal.pone.0022596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konrad FM, Reutershan J. CXCR2 in acute lung injury. Mediators Inflamm 2012: 740987, 2012. doi: 10.1155/2012/740987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krupa A, Kato H, Matthay MA, Kurdowska AK. Proinflammatory activity of anti-IL-8 autoantibody:IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 286: L1105–L1113, 2004. doi: 10.1152/ajplung.00277.2003. [DOI] [PubMed] [Google Scholar]
- 21.Martin TR, Matute-Bello G. Experimental models and emerging hypotheses for acute lung injury. Crit Care Clin 27: 735–752, 2011. doi: 10.1016/j.ccc.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM; Acute Lung Injury in Animals Study Group . An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res 10: 38, 2009. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol 217: 51–54, 2009. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overbeek SA, Henricks PA, Srienc AI, Koelink PJ, de Kruijf P, Lim HD, Smit MJ, Zaman GJ, Garssen J, Nijkamp FP, Kraneveld AD, Folkerts G. N-acetylated proline-glycine-proline induced G-protein dependent chemotaxis of neutrophils is independent of CXCL8 release. Eur J Pharmacol 668: 428–434, 2011. doi: 10.1016/j.ejphar.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfister RR, Haddox JL, Blalock JE, Sommers CI, Coplan L, Villain M. Synthetic complementary peptides inhibit a neutrophil chemoattractant found in the alkali-injured cornea. Cornea 19: 384–389, 2000. doi: 10.1097/00003226-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Pfister RR, Sommers CI. L-Arginine-threonine-arginine (RTR) tetramer peptide inhibits ulceration in the alkali-injured rabbit cornea. Cornea 25: 1187–1192, 2006. doi: 10.1097/ICO.0b013e31802ca33a. [DOI] [PubMed] [Google Scholar]
- 31.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 27: 304–312, 1999. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 32.Puig F, Herrero R, Guillamat-Prats R, Gómez MN, Tijero J, Chimenti L, Stelmakh O, Blanch L, Serrano-Mollar A, Matthay MA, Artigas A. A new experimental model of acid- and endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 311: L229–L237, 2016. doi: 10.1152/ajplung.00390.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 289: L807–L815, 2005. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 34.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16: 1713–1720, 2002. doi: 10.1096/fj.02-0331com. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 150: 113–122, 1994. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 36.Szidon JP. Pathophysiology of the congested lung. Cardiol Clin 7: 39–48, 1989. doi: 10.1016/S0733-8651(18)30455-7. [DOI] [PubMed] [Google Scholar]
- 37.van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G. Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB J 22: 3403–3408, 2008. doi: 10.1096/fj.07-096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM; ALIEN Network . The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37: 1932–1941, 2011. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 39.Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol 4: 159–169, 2012. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 41.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 12: 317–323, 2006. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 42.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis 133: 218–225, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol 44–46: 122–129, 2015. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, Gaggar A, Rennard SI, Jackson PL, Blalock JE. An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 190: 51–61, 2014. doi: 10.1164/rccm.201401-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564, 2007. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One 6: e15781, 2011. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan C, Ward PA, Wang X, Gao H. Myeloid depletion of SOCS3 enhances LPS-induced acute lung injury through CCAAT/enhancer binding protein δ pathway. FASEB J 27: 2967–2976, 2013. doi: 10.1096/fj.12-225797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarbock A, Allegretti M, Ley K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br J Pharmacol 155: 357–364, 2008. doi: 10.1038/bjp.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J, Yu H, Liu Y, Gibson SA, Yan Z, Xu X, Gaggar A, Li PK, Li C, Wei S, Benveniste EN, Qin H. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 311: L868–L880, 2016. doi: 10.1152/ajplung.00281.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman GA, Renzetti AD, Hill HR. Functional and metabolic activity of granulocytes from patients with adult respiratory distress syndrome. Evidence for activated neutrophils in the pulmonary circulation. Am Rev Respir Dis 127: 290–300, 1983. [DOI] [PubMed] [Google Scholar]