Abstract
Intravascular sickling and lysis of red blood cells, a hallmark feature of sickle cell disease (SCD), releases hemoglobin (Hb) into the circulation. Increased cell-free Hb has been linked to vasculopathy and in vitro lipid oxidation. Scavenger plasma proteins haptoglobin (Hp) and hemopexin (Hpx) can attenuate cell-free Hb and total plasma heme lipid-oxidative capacity but are depleted in SCD. Here, we isolated lipids from BERK-SS mice, guinea pigs (GP) infused with heme-albumin, and patients with SCD undergoing regular exchange transfusion therapy and evaluated the level of lipid oxidation. Malondialdehyde formation, an end product of lipid peroxidation, was increased in BERK-SS mice, purified lipid fractions of the heme-albumin infused GP, and patients with SCD compared with controls. In humans, the extent of lipid oxidation was associated with the absence of Hp as well as decreased Hpx in plasma samples. Postmortem pulmonary tissue obtained from patients with SCD demonstrated oxidized LDL deposition in the pulmonary artery. The relationship between no Hp and low Hpx levels with greater LDL and HDL oxidation demonstrates the loss of protection against cell-free Hb and total plasma heme-mediated lipid oxidation and tissue injury in SCD. Strategies to protect against plasma lipid oxidation by cell-free Hb and total plasma heme (e.g., therapeutic Hp and Hpx replacement) may diminish the deleterious effects of cell-free Hb and total plasma heme toward the vascular system in SCD.
Keywords: heme, hemopexin depletion, lipoprotein oxidation, sickle cell disease, vascular injury
INTRODUCTION
Sickle cell disease (SCD) is a hereditary monogenic disorder affecting millions of people worldwide, with a high prevalence in sub-Saharan Africa (38). Defined by a single point mutation in the β-globin gene (Glu6Val), hemoglobin (Hb) S is produced in place of normal HbA (46). HbS polymerizes under low oxygen tension and results in rigid, malformed red blood cells with a proclivity for lysis and vascular obstruction (46). Plasma cell-free Hb, the direct byproduct of intravascular hemolysis, is readily oxidized by reactions with nitric oxide (NO) and hydrogen peroxide (H2O2). The oxidation of Hb from the ferrous (Fe2+) iron moiety to the ferric (Fe3+) state results in the dissociation of heme as a consequence of weakening the heme-globin interaction (39). Once free, the hydrophobic molecule hemin is transferred to plasma proteins, e.g., albumin and hemopexin (Hpx), lipoproteins, and cell membrane components, making significant quantities of free hemin in plasma unlikely (5, 41).
Plasma lipoproteins enable the transport of otherwise insoluble lipids, such as cholesterol esters and triglycerides. Nonpolar lipids are carried in the central core of lipoproteins with a surrounding surface monolayer of polar lipids, such as free cholesterol, phospholipids, and apolipoproteins. Of the seven classes of plasma lipoproteins, low-density lipoprotein (LDL) carry the majority of the plasma cholesterol. Each LDL particle has one apolipoprotein B-100 (ApoB-100) molecule as the main structural surface protein. High-density lipoprotein (HDL) refers to a group of small, heterogeneous particles containing the major structural apolipoprotein A-1 (Apo A-1). HDL is involved in reverse cholesterol transport and exhibits antiatherogenic, antioxidant, and anti-inflammatory properties (10). Heme can rapidly intercalate into the hydrophobic core of lipoproteins, suggesting that LDL and HDL are relevant pathophysiological end points of heme transfer and potential targets of oxidation in plasma (30). Free radicals initiate lipid peroxidation by abstracting a hydrogen atom from their carbon double bond and in conjunction with oxygen radicalic substitution of unsaturated lipids. The oxidative degradation of lipids results in the formation of lipid hydroperoxides and their secondary end products, such as malondialdehyde (MDA), which are analytes for the initial evaluation of general lipid oxidation (9). The susceptibility of LDL to oxidative modification by Hb and heme has been demonstrated in vitro and affects both lipid and ApoB components of LDL (3, 28, 29). Oxidation of LDL in the endothelial wall and its subsequent uptake by macrophages has been considered as an early event in the development of atherosclerotic lesions (27). Repeated exposure to intravascular hemolysis was linked to the clinical manifestation of pulmonary hypertension (PH) and is believed to contribute to many prototypical vascular complications in SCD (21, 22).
The lipid profile of patients with SCD differs from that of age- and race-matched controls. Patients with SCD demonstrate lower total cholesterol, LDL and HDL levels (48, 50). Haptoglobin (Hp) and Hpx are plasma scavenger proteins that bind cell-free Hb and heme, respectively, thereby limiting toxicity from intravascular hemolysis (42). In SCD, intravascular hemolysis leads to a decrease or depletion of these two key scavenger proteins (41, 45). We hypothesized that oxidation of LDL and HDL occurs in SCD and is aggravated by repeated exposure to cell-free Hb/heme and periods of Hp/Hpx depletion. We therefore evaluated the extent of lipid oxidation in Berkeley (BERK-SS) mice, purified lipid fractions from heme-albumin infused guinea pigs (GP), and patients with SCD in relation to control groups. BERK-SS mice approximate human SCD (25). The model is defined by deletions of murine α- and β-globins (α−/−, β−/−) and a transgene containing human α-, βs-, Aγ-, Gγ-, and β-globins (25, 36). The lipid profile of mice differs from humans in terms of HDL and LDL distribution, with higher levels of HDL than LDL in plasma (11). Human SCD plasma was collected from individuals undergoing regular exchange transfusion therapy for end-organ injury prevention (6). In human SCD samples, Hp and Hpx were depleted. However, Hpx levels were still detectable and further correlated with heme bound to Hb/total plasma heme. We also evaluated postmortem tissue (pulmonary artery and lung parenchyma) from patients with SCD and PH for the extent of oxidized LDL (oxLDL) deposition. Taken together, the results presented herein support evaluating plasma lipoprotein oxidation as a biomarker in SCD, particularly in patients with more severe hemolytic anemia posing a greater risk for end-organ injury, such as PH and right ventricular failure (22).
MATERIALS AND METHODS
Animal studies.
Male Hartley GP were purchased from Charles River (Wilmington, MA) and acclimated for 1 wk upon arrival to the Food and Drug Administration (FDA) Center for Biologics Evaluation and Research animal care facility (Silver Spring, MD). All animals were fed normal diets throughout the acclimation period of 1 wk and afterward fed over 12 wk with a high-fat, high-sugar diet [HD; 44% carbohydrate with 35% sugar (as sucrose) by weight, 16.2% fat (as cocoa butter) by weight, 18.6% crude protein, 13% fiber; Teklad Diets, Harlan]. The diet was gradually increased by starting with 50% HD in the first week, 75% HD in the second week, followed by a 100% HD for the remaining time. On days of surgery, GP were dosed subcutaneously with ketoprofen (5 mg/kg) for pain management and then anesthetized via the intraperitoneal route with a cocktail of ketamine HCl (100 mg/kg) and xylazine HCl (5 mg/kg; Phoenix Scientific). Sterilized PE-50 tubing catheters were placed into the left external jugular vein. HD GP (n = 4 per group) were infused with heme-albumin doses of 5 and 25 mg/kg via the jugular catheter. HD GP (n = 4 per group) were dosed with vehicle (0.9% NaCl) as a negative control. Eight hours after injection, blood samples were collected and centrifuged at 2,000 revolutions/min for 15 min, and plasma was collected and stored at −80 C° until analysis. All animal studies were approved by the FDA/Center for Biologics Evaluation and Research Institutional Animal Care and Use Committee, with all experimental procedures performed in adherence to the NIH guidelines on the use of experimental animals.
Male and female C57Bl/6 Wildtype (WT) and BERK-SS mice were obtained from a commercial vendor (Jackson, Bar Harbor, ME). BERK-SS female mice were bred with homozygous BERK-SS male mice to generate homozygous offspring. Transnetyx performed the genotyping of mice used for breeding and experiments as per Jackson protocols. Blood was collected from 20-wk-old BERK-SS (n = 9) and WT mice (n = 5), centrifuged at 2,000 revolutions/min for 15 min, and stored at −80 C° until analysis. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver Anschutz Medical Campus.
Study participants.
Plasma samples were collected from adult patients with SCD (n = 19) undergoing regular exchange transfusion therapy at the University of Colorado Anschutz Medical Campus and stored at −80 C° until analysis. The patients with SCD in this cohort were assigned to regular exchange transfusion therapy primarily for the prevention of end-organ injury (e.g., recurrent stroke) (6). Citrate-anticoagulated plasma samples were obtained at the time of automated erythrocyte apheresis therapy. The collection of blood/plasma was approved by the University of Colorado Denver Anschutz Medical Center Institutional Review Board and by FDA’s Research Involving Human Subjects Committee. Samples were compared with plasma samples from age- and race-matched healthy volunteers (n = 11).
Analysis of lipid profile, heme, Hpx, hemolysis, and endothelial dysfunction markers in human samples.
To measure total plasma heme, referring thereby to heme bound to Hb and other plasma proteins, a colorimetric assay kit (Abnova) was used. Lactate Dehydrogenase Activity (BioVision), Total Bilirubin (BioVision), soluble VCAM (Immunoassay R&D), E-Selectin/CD 62E (Quantikine ELISA, R&D), and total plasma Cholesterol, HDL, and LDL (Abcam, ab-65390), and Hpx (Abcam, ab-108859) were assessed according to the manufactures protocol.
Plasma hemoglobin analysis.
A direct spectrophotometric method was used to determine the heme bound to Hb. Plasma was diluted in 1X PBS, and Hb concentration was measured in 1-ml semimicro cuvettes using a Cary 60 spectrophotometer (Agilent Technologies, Cary 60 UV-Vis, Model G6860A). Absorbance was measured over time at every 2 nm between 350 and 650 nm with a 0.0125-s integration time per wavelength. Spectra were deconvoluted against standard extinction curves of the pure substances using a nonnegative least squares algorithm.
Non-transferrin-bound iron analysis.
Twofold diluted plasma samples (240 μl) were incubated with 24 μl of 800 mM nitrilotriacetic acid solution pH 7.0 (NTA, Sigma-Aldrich) for 30 min at room temperature (RT), and the sample was applied to a 30-kDA centrifugal filter device (Millipore, Ultracel YM-30) for 60 min at 4,000 g. To 50 μl ultrafiltrate, 25 μl of HCl (1 N, Thermo Fisher Scientific) and trichloroacetic acid (TCA, Sigma-Aldrich) 10% in equal volumes were added followed by addition of 25 μl ascorbic acid (20 mg/ml, Sigma-Aldrich) solution. In between each step, samples were mixed thoroughly. Samples were then incubated in iron detection reagent (FerroZine, HACH) for 20 min at RT, and absorbance was measured at 562 nm using a microplate reader (BioTek, Synergy).
Total iron analysis.
A total of 120 μl of HCl (1 N, Thermo Fisher Scientific) and TCA (Sigma-Aldrich) 10% in equal volumes were added to fourfold diluted plasma samples and incubated for 1 h at 60°C. Samples were centrifuged for 10 min at 15,000 revolutions/min. An amount of 40 μl of ascorbic acid (20 mg/ml, Sigma-Aldrich) solution was added to 120 μl of supernatant and mixed thoroughly. After incubation in detection reagent (FerroZine, HACH) for 20 min at RT, absorbance was measured at 562 nm using a microplate reader (BioTek, Synergy). An iron standard was run in parallel (100 mg/ml, HACH).
Hemoglobin preparation.
Hb was purified from outdated blood, as described in detail previously (17). Concentrations were determined using spectrophotometry and are given as molar concentration of total heme (1 mole Hb tetramer is therefore equivalent to 4 moles of heme). For all Hb used herein, the fraction of ferrous HbO2 was always >98%, as determined by spectrophotometry, unless otherwise stated.
Purification of HDL and LDL from human, GP, and mice plasma.
Anticoagulated plasma (500 μl) was diluted in 1,000 µl 1 mM EDTA/0.15 M NaCl and 500 µl 38% KBr solution with a final density adjustment to 1.068 g/cm3. After centrifugation at 120,000 revolutions/min for 6 h at 4°C in a Beckman Optima TM Max Ultracentrifuge (Rotor TLA 120.2, Beckman), tubes were cut with CentriTube Slicer (D7659, Beckman) at a level of 10 mm. Collected LDL from the upper part was concentrated with 50 kDa Amicon Ultra Centrifugal Filters Device (Millipore) and dialyzed against 1× dialysis buffer (150 mM NaCl, 3 mM EDTA, 2Na, 2H2O). For HDL isolation, 900 μl of 46% KBr solution was added to the remaining part to achieve a final density of 1.21 g/cm3 and centrifuged at 120,000 revolutions/min overnight at 4°C. Tubes were then cut at a level of 15 mm. The protein content was determined by the Bio-Rad Protein Assay Dye Reagent and visualized by Coomassie blue staining with detection of proteins for LDL and HDL, ApoB-100 (540 kDa), and ApoA-1 (28 kDa), respectively. Furthermore, we could observe a loss of the typical yellow color (caused by retinoids) in purified SCD HDL and LDL fractions in contrast to control.
Heme-albumin preparation.
A 4 mM heme-albumin stock solution was prepared by dissolving 65 mg Hemin (Sigma) in 100 mM NaOH at 37°C and incubating for 1 h at 37°C with 20% human serum albumin. The pH was carefully adjusted to 7.45 with 14 mM orthophosphoric acid/317 mM NaCl followed by sterile filtration.
MDA assay.
An amount of 40 µl of plasma or purified LDL or HDL with a final protein concentration of 2 and 2.5 µg/µl diluted in PBS (Gibco) was mixed with 200 µl of 750 mM TCA reagent (Sigma-Aldrich) in 1 M HCl followed by brief vortexing. After addition of 160 µl 25 mM solution of 2-thiobarbituric acid (Sigma-Aldrich) in 1 M NaOH, samples were incubated at 80°C for 1 h and then centrifuged at 13,500 revolutions/min for 10 min. MDA in the supernatant was quantified by fluorimetric measurement with excitation/emission bandwidth of 520–545 and 573–608 nm, respectively (BioTek Synergy HT microplate reader). An MDA (1,1,3,3-tetramethoxypropane 98%, A-11418, Alfa Aesar) standard was run in parallel.
Size exclusion chromatography analysis of Hb:Hp complex.
Hb was added to plasma of patients with SCD and control plasma samples to detect free Hp (resulting in Hb:Hp complex formation) by size exclusion chromatography (SEC). An amount of 50 μl of hemoglobin (4 mg/ml) was added to 200 μl of plasma, and the 405 nm Hb:Hp complex peak was detected by SEC. Test sample (50 μl) was injected into a BioSep-SEC-S3000 (600 × 7.5 mm) SEC column (Phenomenex, Torrance, CA). The SEC column was attached to a Waters 2535 Quaternary Gradient Module pump and Waters 2998 Photodioide Array Detector controlled by a Waters 600 controller using Empower 2 software (Waters, Milford, MA).
Histopathology of human lung, right ventricle, and pulmonary artery from postmortem tissue samples.
Human lung, right ventricle, and pulmonary artery were obtained from Kings and St. Thomas Hospital, London, UK, or the University of Colorado Anschutz Medical Center from deceased patients with SCD. Tissues were fixed in 10% formalin for 24 h and stored in 100% isopropanol, then embedded in paraffin, and 2–5-μm sections were prepared. Sections were dewaxed and rehydrated. Citrate antigen retrieval was performed by microwave treatment for 15 min, and tissue sections were cooled for 30 min at RT. A step for blocking endogenous peroxidase activity was performed using dilute H2O2 according to Itabe et al. (18). To reassure that no artificial H2O2 oxidation occurred during this step, we repeated the method on separate tissue sections without the H2O2 blocking step according to Rosenfeld et al. (40). No differences in oxLDL immunoreactivity were observed (data not shown). Furthermore, sections were blocked with 10% normal horse serum (Vector) for 1 h at RT in a humidified chamber before overnight incubation with a rabbit polyclonal antibody to LDL (Copper oxidized, ab-14519, Abcam) at 4°C. After being washed in PBS-Tween, slides were incubated in alkaline phosphatase anti-rabbit IgG antibody (Vector) for 1 h at RT. The signal was developed by Vector red alkaline phosphatase substrate (Vector) and counterstained with Gill’s II hematoxylin. Masson’s trichrome staining was used to evaluate collagen deposition in heart tissue (23).
Morphometric/pathology evaluation.
All images were acquired using an Olympus IX73 inverted microscope equipped with an Olympus DP80 digital camera. Images were captured for ×4 and ×20 magnification for pulmonary artery, ×20 and ×60 magnification for lung, and ×4 and ×10 magnification for right heart ventricle. When taking images representing all data sets, the camera and microscope settings remained unchanged to ensure image consistency.
Statistical analysis.
Data are presented as means ± SD unless otherwise noted. A two-tailed t-test was used to compare between control and SCD group. Pearson’s correlation coefficient was used to assess the relationship between different parameters in SCD individuals. A bi-exponential curve fitting function was used to plot Hpx against oxHDL and oxLDL levels. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA).
RESULTS
Augmented oxidation of lipids in a murine model of SCD and after in vitro and in vivo exposure of lipoproteins to heme-albumin.
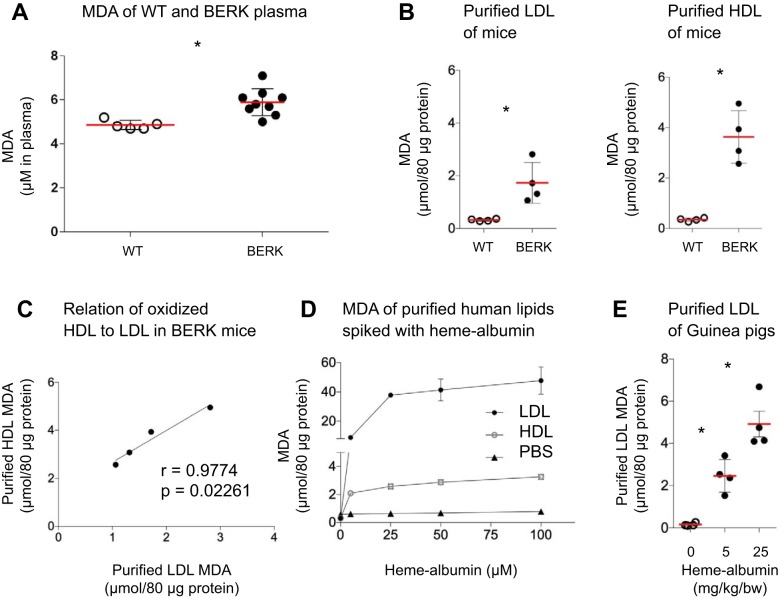
BERK-SS mice showed augmented MDA formation in plasma (n = 9, 5.8 ± 0.1 μM) compared with control animals (n = 5, 4.8 ± 0.1 μM, Fig. 1A). To verify the MDA formation was attributable to lipids and not to other components of the plasma, we purified HDL and LDL fractions from plasma. Pooled purified HDL (3.6 ± 1.0 μmol/80 μg protein) and LDL fractions (1.7 ± 0.7 μmol/80 μg protein) showed significantly increased formation of lipid oxidation end products (MDA) over WT mice (0.3 ± 0.1 μmol/80 μg protein for HDL, 0.3 ± 0.4 μmol/80 μg protein for LDL, 3–4 animals per pooled group, Fig. 1B). MDA of purified HDL to LDL correlated significantly for each pooled BERK-SS mouse group (Fig. 1C).
Fig. 1.
Increased lipid oxidation is observed in purified lipids of BERK-SS mice, heme-albumin-infused guinea pigs and heme-albumin-spiked human plasma samples: Augmented MDA formation of plasma and purified lipoproteins in BERK-SS mice and heme-albumin-spiked human lipoproteins as well as in purified lipids of heme-albumin-infused guinea pigs. A: MDA formation in plasma of BERK-SS mice (n = 9) over WT (n = 5). B: comparison of MDA end products formation of purified LDL and HDL fractions from pooled BERK mice to WT mice (n = 3–4 animals per pooled group). C: relation of purified oxidation of HDL to LDL in pooled BERK mice (n = 3–4 animals per pooled group). D: MDA formation of purified LDL and HDL fractions of human Ctrl samples (n = 5) spiked with different heme-albumin concentrations (0, 5, 25, 50, and 100 μM heme-albumin, respectively). E: MDA of purified LDL fraction from HD GP (n = 4 per group) sampled 8 h after infusion with 0, 5, or 25 mg/kg body wt heme-albumin, respectively. The data are represented in means ± SD or Pearson correlation coefficient (r). *P < 0.05, significant difference compared with Ctrl group. Ctrl, control; MDA, malondialdehyde; WT, wild type.
To demonstrate the capacity of heme to induce lipid oxidation, purified LDL and HDL isolated from healthy control subjects (n = 5) were spiked with different heme-albumin concentrations (0, 5, 25, 50, and 100 μM heme-albumin). Heme-albumin spiked LDL and HDL showed a dose-dependent increase in the formation of MDA after 24 h incubation at 37°C. A negative control reaction consisting of PBS spiked with heme-albumin did not elicit MDA formation (Fig. 1D). To confirm our in vitro observations, we then evaluated the heme-albumin capability to induce lipid oxidation in HD GP, an animal model demonstrating elevated total cholesterol and lipoprotein fractions. HD GP infused with either 5 or 25 mg/kg body wt heme-albumin revealed significantly more MDA accumulation in purified LDL fractions (2.5 ± 0.8 μmol/80 μg protein and 4.9 ± 1.2 μmol/80 μg protein, respectively) compared with HD GP control animals (0.2 ± 0.08 μmol/80 μg protein, Fig. 1E).
These findings support our hypothesis that heme either bound to Hb or other plasma proteins (e.g., albumin) is capable of inducing oxidation of lipids in vitro as well in vivo.
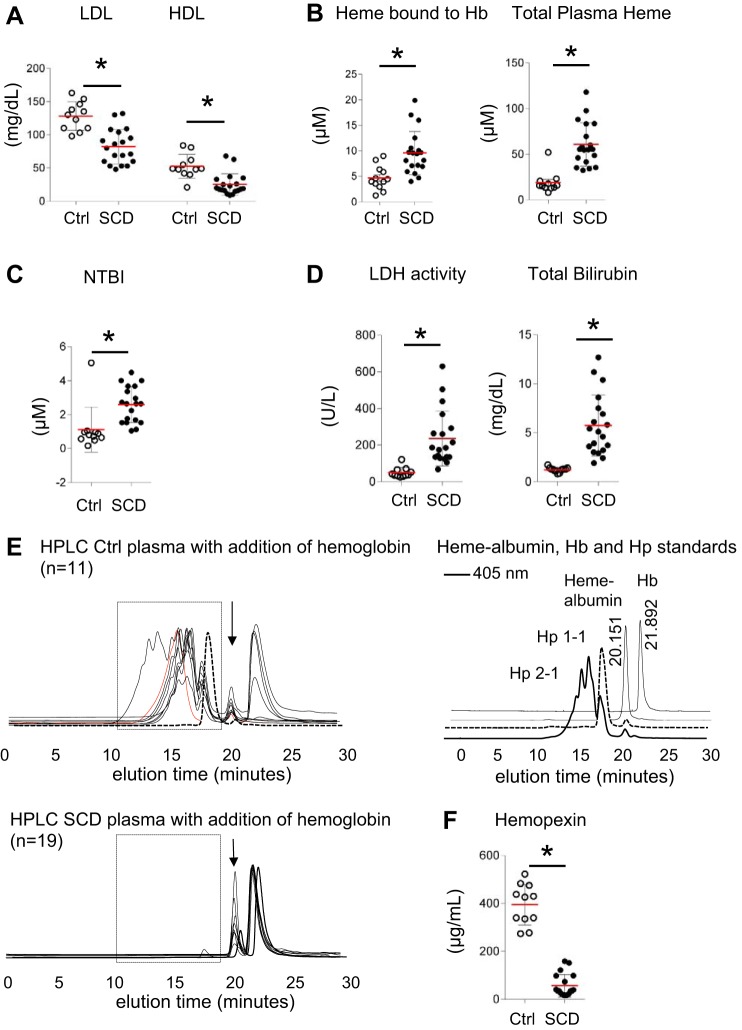
Decreased scavenger protein (Hp and Hpx) levels in plasma of human SCD result in diminished lipid peroxidation defense.
The lipid profile of human SCD plasma revealed significantly lower total HDL and LDL plasma levels (25 ± 16 and 82 ± 26 mg/dl, respectively) as compared with control plasma (Ctrl, 53 ± 18 and 128 ± 21 mg/dl, Fig. 2A). We found significantly increased heme bound to Hb (9.5 ± 4 μM) and total plasma heme levels (60 ± 23 μM) in SCD plasma compared with control plasma (4.6 ± 4.2 μM and 19 ± 11 μM, respectively, Fig. 2B), reflecting a basal level of hemolysis in this SCD cohort. Although total iron was similar in both groups (data not shown), the level of non-transferrin-bound iron in patients with SCD was significantly elevated compared with controls (2.6 ± 1.3 versus 1 ± 1.0 μM, Fig. 2C), a likely result of transfusion in these patients. Lactate dehydrogenase and bilirubin, prototypical markers of hemolysis, were expectedly elevated in SCD plasma (236 ± 150 U/l and 5.7 ± 3 mg/dl) compared with controls (49 ± 28 U/l and 1.2 ± 0.2 mg/dl, Fig. 2D). In contrast to control plasma (Fig. 2E, rectangular box, top panel), the Hb:Hp HPLC peak was not detectable in patients with SCD (Fig. 2E, rectangular box, bottom panel), indicating deficient cell-free Hb scavenging capability in SCD plasma. The Hpx and heme-albumin peak at 20 min elution time were similar in both control and SCD plasma (Fig. 2E, arrow). However, Hpx levels were significantly lower in SCD plasma (56 ± 46 μg/ml) relative to control (394 ± 85 μg/ml, Fig. 2F).
Fig. 2.
SCD patient and control plasmas demonstrate differences in lipid levels, cell-free Hb, total plasma heme, hemolytic parameters, and the scavenger proteins of Hb and heme (Hp, Hpx): Lipid, cell-free Hb, total plasma heme, NTBI, hemolysis parameters, and scavenger proteins (Hp, Hpx) differences of SCD to control plasma. A–D: comparison of SCD (n = 19) to control (Ctrl, n = 11) plasma samples for total LDL and HDL (A), cell-free Hb (heme bound to Hb), total plasma heme, and NTBI levels (B and C), and hemolysis parameters LDH and bilirubin (D). E: Hp 2-1, 2-2, and 1-1 (in black, red, and dashed black lines, respectively) genotype were detectable in control samples (rectangular box, top) but not in individuals with SCD (rectangular box, bottom) by SEC. At 20 min elution time, the Hpx and heme-albumin peak was detectable in both Ctrl and SCD plasma samples (black arrow). On the right, HPLC standards at 405 nm for Hb (elution peak at approximately 22 min) and heme-albumin (elution peak at approximately 20 min) as well as for Hp 1-1 (dashed black line) and Hp 2-1 (thick black line) are shown. F: Hpx levels of SCD compared with Ctrl plasma. Data are represented in means ± SD or Pearson correlation coefficient (r). *P < 0.05 for statistical comparisons to Ctrl group. Hb, hemoglobin; Hp, haptoglobin; Hpx, hemopexin; NTBI, non-transferrin-bound iron; SCD, sickle cell disease; SEC, size exclusion chromatography.
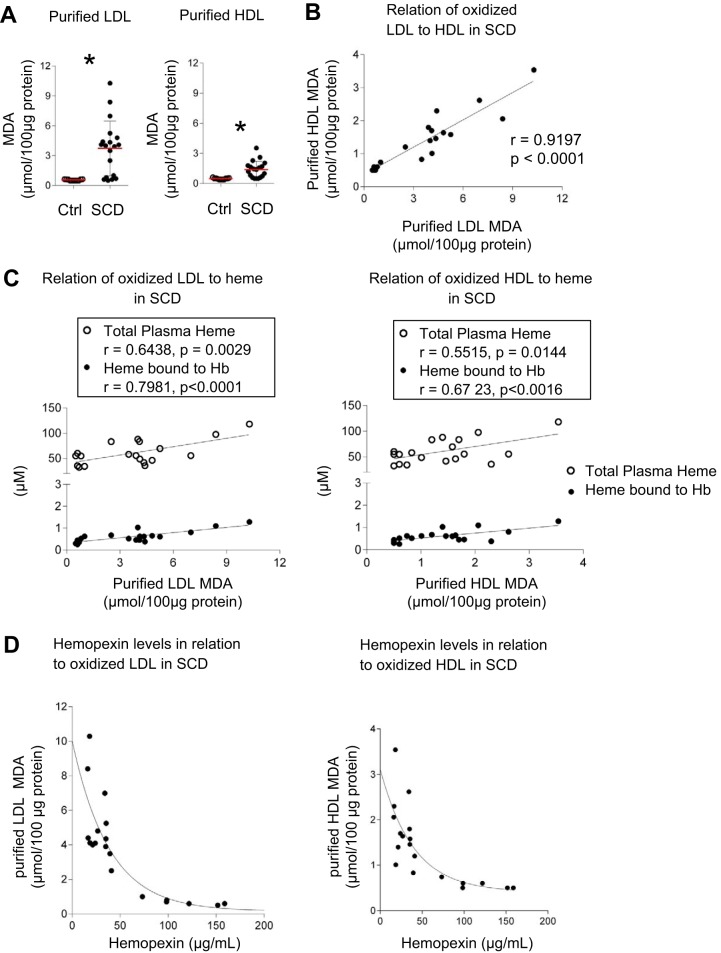
Extent of lipid oxidation in purified LDL and HDL fractions is associated with heme accumulation in SCD.
Significantly more MDA generation was found in LDL fractions purified from human SCD plasma relative to controls (3.7 ± 2.7 vs. 0.6 ± 0.07 μmol/100 μg protein). This was also observed in HDL fractions (1.4 ± 0.8 vs 0.5 ± 0.07 μmol/100 μg protein, Fig. 3A). The level of lipid oxidation in purified HDL and LDL fractions significantly matched (r = 0.91917, P < 0.0001, Fig. 3B). The quantity of oxidized lipids in purified HDL and LDL fractions significantly correlated with corresponding total plasma heme (LDL, r = 0.6438, P = 0.0029; HDL, r = 0.5515, P = 0.0144) and heme bound to Hb (LDL, r = 0.7981, P < 0.0001; HDL, r = 0.6726, P < 0.0016, Fig. 3C). These observations are consistent with depleted Hp and the observation that Hpx levels in patients with SCD negatively correlated with the extent of lipid oxidation in HDL and LDL fractions (Fig. 3D).
Fig. 3.
Relationships between levels of oxidation in purified LDL and HDL from patients with SCD compared with total plasma heme, cell-free Hb, and Hpx levels in plasma: Oxidation of purified LDL and HDL from SCD compared with control and its correlation to total plasma heme, cell-free Hb, and Hpx in plasma. A: MDA formation of purified LDL and HDL fractions of SCD (n = 19) compared with control (n = 11). B: relation of purified oxidation of HDL to LDL in each individual with SCD. C: correlation of purified LDL and HDL oxidation to total plasma heme and cell-free Hb in plasma of individuals with SCD. D: Hpx levels of patients with SCD in relation to purified oxLDL and oxHDL levels. Data are represented as means ± SD or Pearson correlation coefficient (r). A bi-exponential curve fitting function was used to plot Hpx against oxHDL and oxLDL levels. *P < 0.05, significant difference compared with Ctrl group. Hb, hemoglobin; Hpx, hemopexin; MDA, malondialdehyde; oxHDL, oxidized HDL; oxLDL, oxidized LDL; SCD, sickle cell disease.
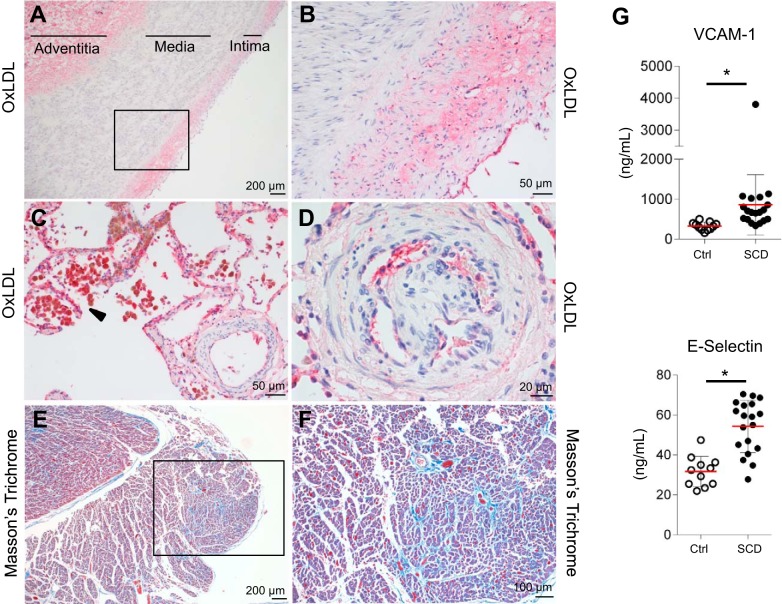
Postmortem pathology in SCD: evidence for oxLDL deposition in the pulmonary artery and endothelium, right ventricular fibrosis, and increased endothelial dysfunction markers.
OxLDL is rapidly cleared from the circulation (24). Consequently, measurements of oxLDL and HDL in the peripheral blood could underestimate the extent of lipoprotein oxidation in SCD. Therefore, we also assessed for evidence of oxLDL deposition and end-organ vasculopathy in the pulmonary artery, the right heart ventricle, and lung vasculature from postmortem SCD tissue. The cause of death in the selected patients was PH with right heart failure, ultimately leading to arrhythmia and sudden death. Staining of the pulmonary artery for oxLDL revealed deposition of oxidized lipids mainly within the enlarged intimal layer and adventitia but not in the thickened media (Fig. 4A). Figure 4B shows a magnified image of the rectangular box in Fig. 4A. In the lung tissue, oxLDL was strongly positive in macrophages (Fig. 4C). Endothelium of precapillary lung vessels stained positive for oxLDL (Fig. 4D). Pulmonary fibrosis was observed in precapillary lung vessels, particularly those demonstrating unregulated endothelial proliferation and increased muscularization (Fig. 4D). These alterations seen in precapillary lung vessels are consistent with plexiform lesions, a hallmak of PH (15, 20). Areas of fibrosis were seen in Masson's trichrome staining of the right heart ventricle (Fig. 4E). Figure 4F represents a higher magnification of the rectangular box of Fig. 4E. These histopathologic findings indicate the existence of pulmonary vasculopathy in SCD. This is further supported by measurements of endothelial dysfunction markers across our cohort of 19 patients with SCD and control samples. Both E-selectin and soluble VCAM were significantly elevated in plasma of patients with SCD (54 ± 13 and 857 ± 755 ng/ml) over controls (31 ± 7.6 and 325 ± 101 ng/ml, respectively, Fig. 4G).
Fig. 4.
Vascular injury localized with lipid oxidation is observed in the pulmonary artery, lung tissue, and right heart ventricle of deceased patients with SCD. This coincided with observations of endothelial dysfunction markers in plasma of SCD and control patients. Vascular injury in pulmonary artery, lung tissue, and right heart ventricle of deceased patients with SCD and endothelial dysfunction markers in plasma of SCD to control group. A and B: thickened intimal and medial compartments of the pulmonary artery of deceased patients with SCD showed positive staining for oxLDL in the enlarged intimal layer and adventitia but not in the thickened media compartment. C: macrophage accumulation around lung vessels with intensified staining for oxLDL. D: endothelium of precapillary vessels stained positive for oxLDL and showed unregulated endothelial proliferation. E and F: right heart ventricle fibrosis was seen in MT staining. G: endothelial dysfunction markers such as soluble VCAM-1 and E-Selectin were significantly elevated in plasma of SCD over Ctrl. *P < 0.05, significant difference compared with Ctrl group. Ctrl, control; oxLDL, oxidized LDL; MT, Masson’s trichrome; SCD, sickle cell disease.
DISCUSSION
Intravascular hemolysis with elevation of cell-free Hb and total plasma heme is a hallmark feature of SCD (37). Scavenger proteins Hp and Hpx are depleted in SCD, rendering cell-free Hb and heme harmful to its immediate surroundings during intravascular hemolysis (42, 45). The capability of heme to induce lipid oxidation in vitro is well described and has been confirmed by our in vitro spiking of isolated lipoprotein fractions with heme-albumin (3, 28). However, to our knowledge we describe for the first time elevated levels of lipid oxidation end products in purified LDL and HDL fractions of both BERK-SS mice and patients with SCD. In addition, our human data indicate a link between the extent of HDL and LDL oxidation to increased heme bound to Hb and other plasma proteins such as albumin. Our data demonstrate an important link between lipid oxidation and heme exposure resulting from depleted Hp and reduced Hpx levels in plasma of patients with SCD.
Prior in vitro studies have indicated that lipoproteins contribute significantly to the consumption of plasma heme and lipid oxidation (30). Chronic hemolysis and episodic increases in hemolytic rate occur in SCD in the setting of depleted scavenger proteins (Hp and Hpx), which likely renders lipoproteins more prone to oxidative modification and contributes to a higher ratio of oxidized to reduced lipids in the plasma of patients with SCD (1, 4).
Consistent with the findings of others, LDL and HDL levels in plasma of patients with SCD were decreased compared with healthy control individuals (48, 50). However, MDA formation was significantly elevated in the purified LDL and HDL fractions of patients with SCD and correlated with increased Hb and total plasma heme levels. The capability of heme-albumin to induce lipid oxidation in vivo was demonstrated by our HD GP study. Furthermore, BERK-SS mice, an animal model with similarities to human SCD in terms of intravascular hemolysis and erythrocyte sickling, showed an augmented level of oxidized lipids relative to WT mice (25, 36).
Cell-free Hb is described as a molecule with pro-oxidative properties in SCD (41). Here we suggest that heme, either bound to Hb representing cell-free Hb or other plasma proteins such as heme-albumin, may be a direct oxidizer of lipoproteins in SCD. Furthermore, our findings indicate that lipid peroxidation reflects the ongoing hemolysis in patients with SCD. The linear relationship between MDA formation in individual HDL and LDL samples suggests a similar degree of lipid oxidation in the two lipoproteins by cell-free Hb, heme, or heme-albumin (47). The overall higher levels of LDL compared with HDL in plasma as well the greater protein to lipid ratio in HDL compared with LDL are possible causes for lower MDA formation seen in purified HDL compared with LDL in humans. The opposite observation was made in BERK-SS mice with a known reversed distribution profile of HDL and LDL in plasma compared with humans (11).
The scavenger proteins Hp and Hpx, respectively, are depleted in SCD (41). No residual Hp level was detectable by SEC-HPLC in any of our SCD patient plasma samples, demonstrating that the Hp system is initially overwhelmed by hemolysis, consistent with our own findings and those of Muller-Eberhard et al. (7, 32). In contrast to Hp, Hpx is rarely completely depleted but is significantly lower in plasma of patients with SCD, reflecting a loss of Hpx as a functional antioxidant of plasma lipids against heme (7, 32). The quantitative negative relationship observed between Hpx depletion and lipid oxidation suggests that the Hpx system is severely stressed but not completely overwhelmed in most patients with SCD.
Clinical manifestation of pulmonary arterial hypertension occurs in ~6–11% of patients with SCD (16). The World Health Organization categorizes SCD PH into Group 5 (PH with unclear or multifactorial mechanisms), and it is generally characterized by symptoms of pulmonary arterial pressures greater than 25 mmHg upon pulmonary artery catheterization, endothelial dysfunction, adventitial inflammation, fibrosis, as well as vascular smooth muscle cell and right ventricular hypertrophy (26, 31, 44). Consistent with the definition of PH for SCD, the endothelial dysfunction markers E-selectin and soluble VCAM were elevated in our cohort of patients with SCD. In postmortem tissue sections obtained from patients with SCD, we also observed fibrosis of precapillary pulmonary arteries and of the right heart ventricle. Both observations are consistent with right heart dysfunction described in SCD (13).
Oxidative damage to LDL has been described as a critical process in the initiation and progression of atherosclerotic vascular disease (35). However, patients with SCD rarely develop typical atherosclerotic lesions (8). Reasons for this might be lower total plasma lipid levels, transient upregulation of tissue heme-oxygenase (Hmox1) in response to increased heme levels, and possible protection of the vasculature by bilirubin (33, 34). Nevertheless, proliferation and thickening of the intimal and medial vascular compartments develop in patients with SCD at sites of high flow turbulence, such as the pulmonary artery (8). We found oxLDL deposition in the thickened intimal layer and adventitia of the pulmonary artery and in the endothelium of lung vessels of deceased patients with SCD. This was accompanied by strong staining for oxLDL in macrophages surrounding blood vessels in the lung.
PH in patients with SCD has previously been related to NO consumption by cell-free Hb (14, 15). However, oxLDL itself has been linked to PH and acts as a relevant biological reactive oxygen species generator and inducer of vasoconstriction through inactivation of NO (43). Moreover, oxLDL is removed at a faster rate from plasma by peripheral macrophages than native LDL and supports macrophage proliferation, differentiation, and foam cell formation (5, 49). In addition to the adverse effects of oxLDL, conversion of HDL properties from anti-inflammatory to proinflammatory by increased cell-free Hb and markers of liver disease has been reported in patients with SCD (2, 19). Bioactive lipids such as lysophosphatidylcholine are formed during oxidation of lipids and enhance superoxide (O2·−) formation in human endothelial cells and vascular smooth muscle cells (12). The formed products react further with a wide variety of biological molecules through heme-lipoprotein interactions with either HDL or LDL. This chain reaction persuaded by lipid peroxidation most likely results in enhanced cytotoxicity to the endothelium and surrounding environment leading to the progression of fundamental pathophysiology in SCD.
A limitation of our study remains auto-oxidation of lipids by lipoprotein-associated heme that may occur during sample processing. However, we also found increased MDA formation in unfractionated and immediately frozen plasma samples of SCD mice, suggesting that sample processing may at worst lead to a quantitative overestimation of lipid oxidation in purified lipids without changing the fundamental assumptions. In addition, the negative relationship between oxidized lipoproteins and the concentration of soluble plasma Hpx reassures reliability of the proposed concept. Nevertheless, future approaches include collecting plasma samples in ice cold methanol with addition of 0.5% butylated hydroxytoluene before processing the samples to quench in vitro lipid oxidation precipitated by heme or other prooxidants and using more specific and sensitive methods for measuring lipid oxidation. Human SCD plasma samples were obtained during scheduled red cell exchange transfusion. Although we believe that the contribution to lipid oxidation is primarily the result of lipid oxidation occurring during events of SCD hemolysis, as a limitation to the study we cannot rule out the contribution of transfusion-related prooxidative components (iron, heme, and hemoglobin) from transfused red blood cells.
In summary, these observations indicate that a window of opportunity may exist for a laboratory assessment of oxidized lipid content in plasma as an experimental biomarker to determine the risk for heme/Hb-induced end-organ injury in SCD. Furthermore, this may help determine a need for therapeutic intervention with Hp as well Hpx replacement in patients with SCD and allow for monitoring the progress of Hp/Hpx therapy after its initiation.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant 1R01-HL-125642 (D. C. Irwin).
DISCLOSURES
The authors declare that they have no conflicts of interest relevant to the manuscript submitted.
AUTHOR CONTRIBUTIONS
A.Y., J.W.D., D.J.S., and P.W.B. conceived and designed research; A.Y., J.W.D., R.C.H., J.H.B., and P.W.B. performed experiments; A.Y., J.W.D., R.C.H., J.H.B., K.H., K.R., D.C.I., D.J.S., and P.W.B. analyzed data; A.Y., J.W.D., R.C.H., J.H.B., K.H., K.R., D.C.I., D.J.S., and P.W.B. interpreted results of experiments; A.Y., J.W.D., J.H.B., K.H., and P.W.B. prepared figures; A.Y., R.C.H., K.R., D.C.I., D.J.S., and P.W.B. drafted manuscript; A.Y., J.W.D., R.C.H., J.H.B., K.H., K.R., D.C.I., D.J.S., and P.W.B. edited and revised manuscript; A.Y., J.W.D., R.C.H., J.H.B., K.H., K.R., D.C.I., D.J.S., and P.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sebastian Lucas (King’s College and Guy’s and St. Thomas Hospital, London, UK) for providing tissues, Dr. Lucia Rohrer (Institute of Clinical Chemistry, University Hospital Zurich, Zurich, Switzerland) for assisting with the purification of LDL and HDL fractions from human plasma samples, and Dr. Luisa Gregori (US Food and Drug Administration, Center for Biologics Evaluation and Research, Silver Spring, MD) for providing equipment for lipid purification.
REFERENCES
- 1.Alassane D, Fatou C, Fatou GT, Fatou D, Oumar TF, Ndéné SG, Philomène LS, Diop SN, Méïssa T. Serum lipids and oxidized low density lipoprotein levels in sickle cell disease: assessment and pathobiological significance. Afr J Biochem Res 8: 39–42, 2014. doi: 10.5897/AJBR12.095. [DOI] [Google Scholar]
- 2.Ataga KI, Hinderliter A, Brittain JE, Jones S, Xu H, Cai J, Kim S, Pritchard KA, Hillery CA. Association of pro-inflammatory high-density lipoprotein cholesterol with clinical and laboratory variables in sickle cell disease. Hematology 20: 289–296, 2015. doi: 10.1179/1607845414Y.0000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb 11: 1700–1711, 1991. doi: 10.1161/01.ATV.11.6.1700. [DOI] [PubMed] [Google Scholar]
- 4.Belcher JD, Marker PH, Geiger P, Girotti AW, Steinberg MH, Hebbel RP, Vercellotti GM. Low-density lipoprotein susceptibility to oxidation and cytotoxicity to endothelium in sickle cell anemia. J Lab Clin Med 133: 605–612, 1999. doi: 10.1016/S0022-2143(99)90191-9. [DOI] [PubMed] [Google Scholar]
- 5.Camejo G, Halberg C, Manschik-Lundin A, Hurt-Camejo E, Rosengren B, Olsson H, Hansson GI, Forsberg GB, Ylhen B. Hemin binding and oxidation of lipoproteins in serum: mechanisms and effect on the interaction of LDL with human macrophages. J Lipid Res 39: 755–766, 1998. [PubMed] [Google Scholar]
- 6.Davis BA, Allard S, Qureshi A, Porter JB, Pancham S, Win N, Cho G, Ryan K; British Society for Haematology . Guidelines on red cell transfusion in sickle cell disease part II: indications for transfusion. Br J Haematol 176: 192–209, 2017. doi: 10.1111/bjh.14383. [DOI] [PubMed] [Google Scholar]
- 7.Deuel JW, Vallelian F, Schaer CA, Puglia M, Buehler PW, Schaer DJ. Different target specificities of haptoglobin and hemopexin define a sequential protection system against vascular hemoglobin toxicity. Free Radic Biol Med 89: 931–943, 2015. doi: 10.1016/j.freeradbiomed.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Elsharawy MA, Moghazy KM, Shawarby MA. Atherosclerosis in sickle cell disease - a review. Int J Angiol 18: 62–66, 2009. doi: 10.1055/s-0031-1278326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 10.Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. In Endotext, edited by De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A. South Dartmouth, MA: MDText.com, 2000. [Google Scholar]
- 11.Fernandez ML, Volek JS. Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab (Lond) 3: 17, 2006. doi: 10.1186/1743-7075-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 185: 219–226, 2006. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol 59: 1123–1133, 2012. doi: 10.1016/j.jacc.2011.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 886–895, 2004. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 15.Gordeuk VR, Castro OL, Machado RF. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood 127: 820–828, 2016. doi: 10.1182/blood-2015-08-618561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes MM, Vedamurthy A, George G, Dweik R, Klings ES, Machado RF, Gladwin MT, Wilson KC, Thomson CC; American Thoracic Society Implementation Task Force . Pulmonary hypertension in sickle cell disease. Ann Am Thorac Soc 11: 1488–1489, 2014. doi: 10.1513/AnnalsATS.201408-405CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin DC, Baek JH, Hassell K, Nuss R, Eigenberger P, Lisk C, Loomis Z, Maltzahn J, Stenmark KR, Nozik-Grayck E, Buehler PW. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 82: 50–62, 2015. doi: 10.1016/j.freeradbiomed.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itabe H, Takeshima E, Iwasaki H, Kimura J, Yoshida Y, Imanaka T, Takano T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J Biol Chem 269: 15274–15279, 1994. [PubMed] [Google Scholar]
- 19.Ji X, Feng Y, Tian H, Meng W, Wang W, Liu N, Zhang J, Wang L, Wang J, Gao H. The mechanism of proinflammatory HDL generation in sickle cell disease is linked to cell-free hemoglobin via haptoglobin. PLoS One 11: e0164264, 2016. doi: 10.1371/journal.pone.0164264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, Nickel N, Hussein K, Maus U, Lehmann U, Janciauskiene S, Welte T, Haverich A, Rische J, Kreipe H, Laenger F. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 179: 167–179, 2011. doi: 10.1016/j.ajpath.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37–47, 2007. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127: 750–760, 2017. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klimova LA, Ufimtseva AG, Bezchinskaia MI. [Cytohistological picture of the palatine tonsils in patients with chronic tonsillitis before and after the treatment by helium-neon laser irradiation]. Vestn Otorinolaringol 3: 64–67, 1987. [PubMed] [Google Scholar]
- 24.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal 13: 39–75, 2010. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood 107: 1651–1658, 2006. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maron BA, Brittain EL, Choudhary G, Gladwin MT. Redefining pulmonary hypertension. Lancet Respir Med 6: 168–170, 2018. doi: 10.1016/S2213-2600(17)30498-8. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev 7: 558–566, 2008. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry 36: 12189–12198, 1997. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 29.Miller YI, Felikman Y, Shaklai N. Hemoglobin induced apolipoprotein B crosslinking in low-density lipoprotein peroxidation. Arch Biochem Biophys 326: 252–260, 1996. doi: 10.1006/abbi.1996.0073. [DOI] [PubMed] [Google Scholar]
- 30.Miller YI, Shaklai N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim Biophys Acta 1454: 153–164, 1999. doi: 10.1016/S0925-4439(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 31.Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O. Pulmonary arterial hypertension. Orphanet J Rare Dis 8: 97, 2013. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32: 811–815, 1968. [PubMed] [Google Scholar]
- 33.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novotný L, Vítek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 228: 568–571, 2003. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 35.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol 610: 403–417, 2010. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278: 876–878, 1997. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 37.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol 308: L314–L324, 2015. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 376: 2018–2031, 2010. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 39.Rifkind JM, Nagababu E. Hemoglobin redox reactions and red blood cell aging. Antioxid Redox Signal 18: 2274–2283, 2013. doi: 10.1089/ars.2012.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenfeld ME, Palinski W, Ylä-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis 10: 336–349, 1990. doi: 10.1161/01.ATV.10.3.336. [DOI] [PubMed] [Google Scholar]
- 41.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121: 1276–1284, 2013. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaer DJ, Vinchi F, Ingoglia G, Tolosano E, Buehler PW. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol 5: 415, 2014. doi: 10.3389/fphys.2014.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma S, Ruffenach G, Umar S, Motayagheni N, Reddy ST, Eghbali M. Role of oxidized lipids in pulmonary arterial hypertension. Pulm Circ 6: 261–273, 2016. doi: 10.1086/687293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Smith A, McCulloh RJ. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol 6: 187, 2015. doi: 10.3389/fphys.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet 364: 1343–1360, 2004. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 47.Thomas MJ, Chen Q, Zabalawi M, Anderson R, Wilson M, Weinberg R, Sorci-Thomas MG, Rudel LL. Is the oxidation of high-density lipoprotein lipids different than the oxidation of low-density lipoprotein lipids? Biochemistry 40: 1719–1724, 2001. doi: 10.1021/bi0022442. [DOI] [PubMed] [Google Scholar]
- 48.VanderJagt DJ, Shores J, Okorodudu A, Okolo SN, Glew RH. Hypocholesterolemia in Nigerian children with sickle cell disease. J Trop Pediatr 48: 156–161, 2002. doi: 10.1093/tropej/48.3.156. [DOI] [PubMed] [Google Scholar]
- 49.Yui S, Sasaki T, Miyazaki A, Horiuchi S, Yamazaki M. Induction of murine macrophage growth by modified LDLs. Arterioscler Thromb 13: 331–337, 1993. doi: 10.1161/01.ATV.13.3.331. [DOI] [PubMed] [Google Scholar]
- 50.Zorca S, Freeman L, Hildesheim M, Allen D, Remaley AT, Taylor JG VI, Kato GJ. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. Br J Haematol 149: 436–445, 2010. doi: 10.1111/j.1365-2141.2010.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]