Abstract
Recent findings indicate that monkeys can report their confidence in perceptual decisions and that this information is encoded in neurons involved in making decisions, including the lateral intraparietal area (LIP) and the supplementary eye field (SEF). A key issue to consider when studying confidence is that decision accuracy often correlates with confidence reports; when we are performing well, we generally feel more confident. Expanding on work performed in humans, we designed a novel task for monkeys that dissociates perceptual information leading to decisions from perceptual information leading to confidence reports. Using this task, we recently showed that decoded ensemble activity recorded from the superior colliculus (SC) reflected decisions rather than confidence reports. However, our previous population level analysis collapsed over multiple SC neuronal types and therefore left open the possibility that first, individual discharge rates might encode information related to decision confidence, and second, different neuronal cell types within the SC might signal decision confidence independently of decision accuracy. We found that when decision accuracy and decision confidence covaried, modulation occurred primarily in neurons with prelude activity (buildup neurons). However, isolating decision confidence from decision accuracy uncovered that only a few, primarily buildup neurons showed signals correlating uniquely with decision confidence and the effect sizes were very small. Based on this work and our previous work using decoding methods, we conclude that neuronal signals for decision confidence, independent of decision accuracy, are unlikely to exist at the level of single or populations of neurons in the SC. Our results together with other recent work call into question normative models of confidence based on the optimal readout of decision signals.
NEW & NOTEWORTHY Models of decision confidence suggest that our sense of confidence is an optimal readout of perceptual decision signals. Here, we report that a subcortical area, the superior colliculus (SC), contains neurons with activity that signal decisions and confidence in a task in which decision accuracy and confidence covary, similar to area lateral intraparietal area in cortex. The signals from SC occur primarily in the neurons with prelude activity (buildup neurons). However, in a task that dissociates decision accuracy from decision confidence, we find that only a few individual neurons express unique signals of confidence. These results call into question normative models of confidence based on optimal readout of perceptual decision signals.
Keywords: confidence, electrophysiology, metacognition, superior colliculus
INTRODUCTION
Confidence is a feeling about the validity of our thoughts, knowledge, or performance (Grimaldi et al. 2015; Luttrell et al. 2013). Recent findings from neuronal recordings made in the monkey lateral intraparietal area (LIP; Kiani and Shadlen 2009) and supplementary eye fields, (SEF; Middlebrooks and Sommer 2012) support the idea that sensorimotor areas involved in perceptual decision-making are also involved in evaluating confidence. Finding such as these, underlie normative models of decision confidence proposing that confidence is an optimal readout of perceptual decision signals (Hangya et al. 2016; Mainen et al. 2016; Pouget et al. 2016).
One difficulty in studying confidence experimentally is that in most tasks decision confidence and decision accuracy tend to be correlated, so if neuronal activity correlates with decision accuracy, then it is unsurprising if it also correlates with decision confidence. In other words, the correlation of neuronal activity with confidence may not be causal to the sense or the report of confidence. For example, imagine you are asked to calculate the sum of 2 + 2. Because this decision is easy, your performance will likely be high and, therefore, so will your confidence. However, what if the decision is more difficult: for instance, if you are asked what is the square root of π? Your accuracy may be reduced and so would your confidence report. Likewise, for perceptual decisions, when sensitivity, as measured by d′, is high, confidence is also likely to be high and when sensitivity is low, confidence is also likely to be low. In this case, confidence can be directly predicted from the stimulus, even though the stimulus is clearly not part of the neural mechanism giving rise to confidence.
We recently developed a novel task for monkeys in which we dissociated perceptual information leading to decisions from perceptual information leading to confidence reports. Our task stems from the finding that, in the random dot motion task, people base their decisions on the relative amount of motion evidence in favor of or against a decision, whereas they base their assessment of confidence mainly on the amount of evidence in favor of the correct decision; here referred to as positive evidence (PE; Koizumi et al. 2015; Zylberberg et al. 2012). In our task, perceptual information used for the decision remains constant, and the information used to report confidence (PE) varies, leading to a dissociation between performance or decision accuracy and confidence. In our recent work (Odegaard et al. 2018), we recorded from neurons in a sensorimotor area related to decision-making, the superior colliculus (SC; Horwitz and Newsome 1999; Kim and Basso 2008, 2010; McPeek and Keller 2004; Ratcliff et al. 2003) of monkeys while they performed this task and also while they performed a task similar to the task used by Kiani and Shadlen (2009) to study confidence in area LIP. In our previous work, we found that a multivariate decoder reliably predicted confidence reports only when confidence correlated with decision accuracy. When decision accuracy was held constant and confidence varied, the multivariate decoder failed to predict confidence (Odegaard et al. 2018).
Here, we reasoned that since population analyses collapse information over all neuron types, the population level analysis we previously performed may mask the types of neuron in the SC that may be involved in decision confidence. Moreover, it is possible that individual neurons may contain signals related to decision accuracy and confidence reports that went undetected in our population analysis. Therefore, in this report, we expand on our previous work and describe the results in more detail, focusing on analyses of single SC neurons in these two decision tasks; one in which decision accuracy and confidence covary, to replicate previous reports in LIP, and the second in which decision accuracy and confidence are decoupled, to assess whether there are signals in the SC unique to decision confidence. In the first experiment, trained monkeys performed a decision task like that used by Kiani and Shadlen (2009) in which an opt-out (OO) choice option appeared on 50% of trials of the random dot motion discrimination task (Stimulus-Matched). In the second experiment, we manipulated the amount of evidence for and against a decision to obtain two stimulus conditions in which monkeys showed matched d′ but different levels of confidence (Sensitivity-Matched). In this second experiment, we could assess whether neuronal activity in the SC correlates uniquely with decision accuracy as measured by d′, or with decision confidence reports. The results indicate that buildup neurons in the SC contain signals correlating with decision confidence, but only when decision confidence and decision accuracy (d′) covary. With experimental dissociation of decision confidence and accuracy, SC neurons signal only decision accuracy. Consistent with our population level analyses previously reported, we conclude that individual neuron or population level signals in the SC during decision-making are best explained by decision accuracy and not decision confidence. These results together with other recent reports (Koizumi et al. 2015; Komura et al. 2013; Zylberberg et al. 2012) call into question normative models of decision confidence.
MATERIALS AND METHODS
Experimental Design and Statistical Analyses
The experiment consists of the tasks described in the following sections,
Delayed-saccade task.
Monkeys performed a delayed-saccade task to determine the location and extent of the response field (RF) of the recorded SC neurons. For the delayed-saccade task, a fixation spot appeared initially at the center of the screen and monkeys remained fixating for 500–1,000 ms. Then, a second spot appeared peripherally on the screen and monkeys were required to remain fixating for another 200–400 ms before the fixation spot disappeared. The position of the saccade target was determined by moving a mouse and could occur anywhere on the screen. The delay times were randomized and drawn from an exponential distribution to prevent prediction. Then, the fixation spot disappeared, and this cued the animal to make a saccade to the peripheral target. If the monkeys made the saccade to the target correctly, within a 2° square electronic window, they received 0.1 ml of fluid reward.
Assessment of lateral stimulus interactions.
Because our confidence tasks required multiple stimuli on the screen (see Confidence tasks) and it is well known that SC neuronal activity is influenced by lateral stimulus interactions (Basso and Wurtz 1998; Rizzolatti et al. 1973), monkeys performed a simple odd-ball selection task while we recorded from SC neurons to assess the influence of lateral stimulus interactions. After fixation of a spot located at the center of the screen was acquired, two or four possible isoluminant targets appeared; one gray (the target) and the rest red (distractors). The number of possible targets, 2 or 4, and their position were randomly interleaved on each trial. The position of the target was always either in the center of the RF or opposite to it (see Fig. 4A). After a delay of 1,000–1,200 ms the fixation spot disappeared and cued the monkeys to make a saccade to the target. If the monkeys made the saccade correctly with a 2° square electronic window, they received a fluid reward of 0.1 ml.
Fig. 4.
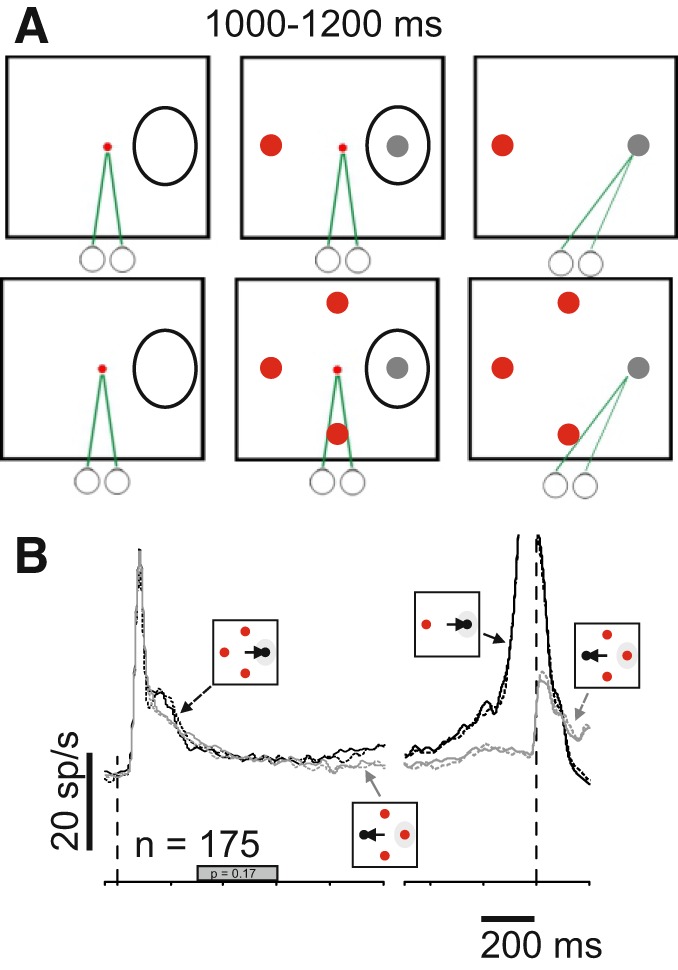
Lateral inhibition fails to explain confidence activity in the superior colliculus (SC). A: schematic of the spatial arrangement and timing of the odd-ball task used to assess stimulus display interaction effects on SC neuronal activity. The squares show the screen and the black ovals indicate the response field (RF) of the recorded neurons. The circles below the squares and the green lines show the direction of gaze. On randomly interleaved trials, either 2 or 4, isoluminant red distractor(s) and a gray target appeared on the screen. After a delay of 1,000–1,200 ms, the centrally located fixation spot (small red circle) disappeared, cueing the monkey to shift its gaze to the odd-ball target. The colors in figure are for display purposes only. During the experiment the background of the screen was black, fixation spot was red, the distractors were red and the target gray, to match the luminance of the distractors (materials and methods). B: average spike density function (SDF) plotted over time for the 175 single and multiple SC neurons recorded while monkeys performed the odd-ball task shown in A. Iconography indicates the trial types. Solid black lines show SDFs for correct choices into the RF with 2 possible targets and dashed black lines show SDFs for correct choices into the RF with 4 possible targets. Solid gray lines show SDFs for correct choices away from the RF for 2 possible targets, and dashed gray lines show SDFs for correct choices with 4 possible targets. The filled gray bar on the abscissa indicates the interval of discharge that was quantified. Note that there is no difference between 2 and 4 possible targets because there is no uncertainty in the saccade choice (P > 0.05; Basso and Wurtz 1997, 1998).
Confidence tasks.
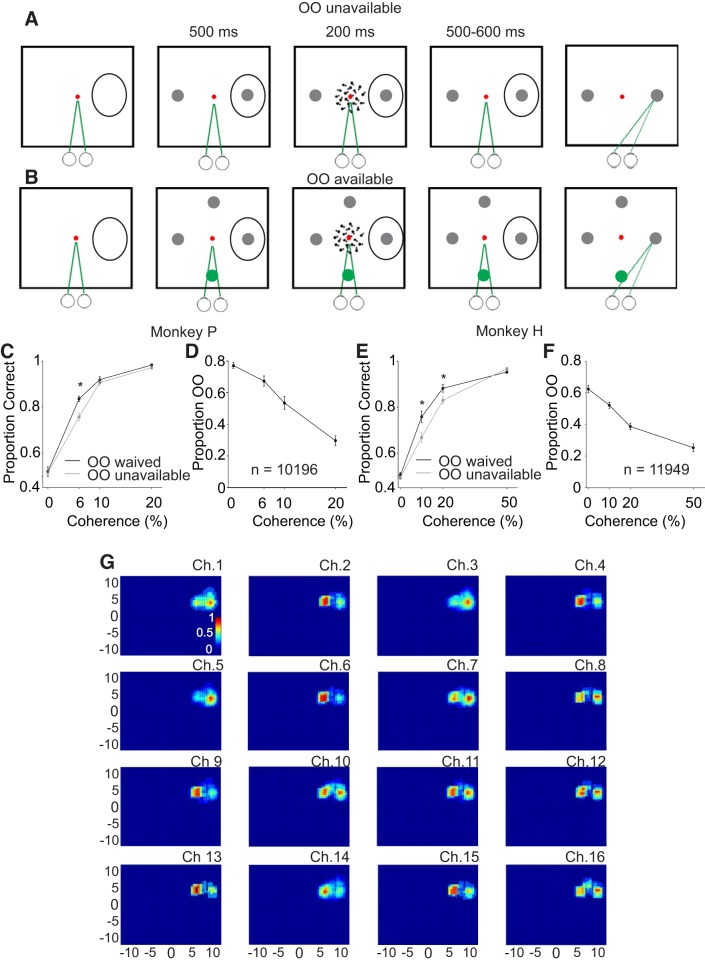
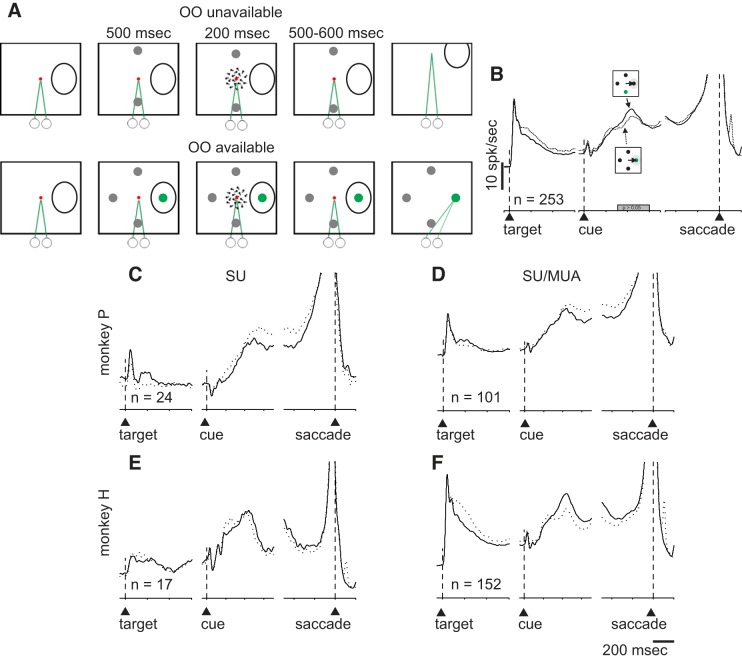
To assess confidence, we used two experimental paradigms we refer to as Stimulus-Matched and Sensitivity-Matched. For the Stimulus-Matched task, monkeys initially acquired and maintained fixation on a centrally located spot (1.0 cd/m2) and then the choice targets appeared. One choice target appeared in the center of the RF, and the other was placed symmetrically in the opposite hemifield. Five-hundred milliseconds after the choice targets appeared, a 3°-radius random dot motion stimulus appeared at the center of the screen for 200 ms. After 500–600 ms (the time was randomly drawn from an exponential distribution to avoid prediction), the fixation spot disappeared and the monkeys reported their direction decision with a saccade to one of the two choice targets. The strength of the sensory evidence, expressed as percent coherence, varied among four difficulty levels by changing the proportion of dots moving coherently. For monkey P we used 0, 6, 10, and 20% as its discrimination performance was exceptional. For monkey H, we used 0, 10, 20, and 50%. The dot size was 0.05°, and the dot density was 35 dots/°2. Every dot was displayed on the screen for one video frame (16 ms) and then displaced to give the impression of apparent motion at a speed of 5°/s. Each dot was plotted at the beginning of the stimulus and disappeared after 200 ms or when it reached the border of the stimulus. The monkeys reported the direction of motion with a saccade to the corresponding choice target, left for leftward motion and right for rightward motion. Correct decisions resulted in a 0.10-ml juice reward. Incorrect decisions resulted in no reward and a 3,000-ms timeout before the next trial commenced. On 50% of the trials and randomly interleaved, a third choice option appeared that we refer to as the opt-out choice (OO). The OO target appeared orthogonal to the left or right choice targets and when selected, always yielded a smaller reward (80–90% of the correct reward). In all trials when the OO was available, a fourth dot was presented on the screen in a location opposite to the OO to control for asymmetry in the display that could alter lateral inhibitory effects (Basso and Wurtz 1998; Rizzolatti et al. 1973). This target was irrelevant to the task and was never associated with reward. All choice targets were isoluminant (13 cd/m2). This task was the same as that used previously by Kiani and Shadlen (2009) with the exception that in our task the choice targets appeared before the onset of the motion cue and in theirs, the choice targets appeared after the motion cue onset. In a subset of trials, we placed the OO in the RF. These trials were interleaved with the other trials in blocks of ~25 trials. Because the position of the choice targets depended upon the location of the RF of the neuron being recorded (see below), the specific location of each choice target varied across days.
For the Sensitivity-Matched task, the sequence of the events and the possible choice targets were identical to that described above for the Stimulus-Matched task. The difference was the motion cue. Also, in this experiment we never placed the OO in the RF: the configuration of the stimulus was always as shown in Fig. 1A. We developed a Sensitivity-Matched stimulus display adapted from theoretical and experimental work performed in humans in which people base their decisions on the relative amount of motion evidence in favor or against a decision, whereas people based their assessment of confidence mainly on the amount of evidence in favor of their decision (Koizumi et al. 2015; Zylberberg et al. 2012). Therefore, we divided the motion stimulus into three components, motion rightward, motion leftward, and noise. We could then define these components as motion congruent with the direction of the correct choice (referred to as PE) or motion opposite to the correct choice [referred to as negative evidence (NE)] and noise, which was motion in all other directions. We created two stimulus conditions by manipulating the amount of PE and NE for individual monkeys and individual sessions empirically to obtain two stimulus conditions in which monkeys showed matched d′ and thus matched sensitivity but different levels of confidence; high confidence with high PE (HPE) and low confidence with low PE (LPE). The noise component ensured an equal number of dots for the motion cues in the different conditions. With this display we could assess whether neuronal activity was more correlated with sensitivity (defined as d′) or decision confidence (defined by proportion of OO trials). To analyze the physiological activity, we performed statistical comparisons when appropriate using ANOVA, Wilcoxon signed-rank test for paired comparisons or Kolmogorov-Smirnov test for unpaired distributions. Unless differently specified, α = 0.05 was used to determine significance. In specific cases where Bonferroni correction was used, the α-value is specified (Keppel 1991). Unless explicitly stated, we performed statistical analyses on a 300-ms epoch from 300 to 600 ms after motion onset to avoid inclusion of the dip in neuronal activity that occurred after the onset of the central cue (Li and Basso 2005). This epoch was well before the cue to make a saccade ensuing that only delay activity was included in the analysis. All statistical analyses and plots were performed using Matlab R2014 (MathWorks, Natick, MA).
Fig. 1.
An opt-out (OO) task to assess decision confidence in monkeys. Schematic of the spatial arrangement of the behavioral task. A: OO-unavailable condition. Squares show the screen viewed by the monkeys. The small circles and green lines indicate the direction of gaze and required eye position. The duration of each task event in ms appears above the squares. The red spot shows the centrally located fixation spot and the black ovals are schematics of the response fields (RF) of the recorded neurons. Note that the exact position of the choice targets depended upon the RF of the recorded neuron (materials and methods). After an initial period of fixation, 2 choice targets appeared, 1 in the RF, and 1 in the opposite hemifield (gray circles). Then, the motion cue appeared for 200 ms. Monkeys maintained fixation for another 500–600 ms and then the fixation point disappeared, cueing the monkey to report its motion direction decision. B: in randomly interleaved trials, a 3rd choice (OO available) appeared 90° from the 2 choice targets (green circle). To control for lateral inhibition, a fourth, behaviorally irrelevant target appeared opposite to the OO choice target (see materials and methods). The colors in A and B are for representation purpose only. During the experiment, the background of the screen was black, fixation spot was red, the OO was green, and the choice targets were gray to match the luminance of the OO (materials and methods). C: proportion correct for monkey P plotted against motion coherence for the OO available but waived trials (black points and lines) and the OO-unavailable trials (gray points and lines). *P < 0.005. D: proportion of trials in which monkey P selected the OO target plotted against motion coherence. There were 10 sessions and 10,196 trials. E: same as C for monkey H. F: same as D for monkey H. There were 10 sessions and 11,949 trials. G: 1 example recording from the 16 channel V probe. Ch# is the channel or contact number (1–16), and the discharge rate measured during a saccade epoch is plotted normalized to maximum discharge for this same epoch for each contact individually. The result is plotted as a heat map in Cartesian coordinates with warmer colors indicating higher discharge; 0.0 indicates the center for the visual screen.
Surgical Procedures
Two male rhesus macaque monkeys (9–13 kg) were prepared for electrophysiological recordings and measurement of eye movements. A headpost was implanted to secure the head, and an MRI-compatible recording chamber (Crist Instruments) was placed at AP +3, ML 0 and angled 38° posteriorly to access the SC. Precise positioning of the headpost and the recording chamber was obtained using MRI-guided surgical software (BrainSight; Rogue Research, Montreal, CA). To track eye movements, one monkey (monkey H) was implanted with eye loops to measure eye position (Judge et al. 1980; Fuchs et al. 1966). For the other monkey (moneky P), eye position was monitored with an iView camera (Sensomotoric instruments, Boston, MA). All surgical procedures were performed while monkeys were under general anesthesia using aseptic procedures. Anesthesia was induced with ketamine and midazolam (5.0 and 0.2 mg/kg im). Atropine (0.04 mg/kg im) was provided to reduce salivation. Monkeys were intubated and maintained under general anesthesia with isoflurane. One hour before the procedure, animals received buprenorphine (0.01 mg/kg im) and the antibiotic Excede (20 mg/kg im; 7 days, slow release) and then meloxicam (0.3 mg/kg im) at the conclusion of the procedure. Meloxicam (0.2 mg/kg im) and buprenorphine (0.01 mg/kg im) were administered for 3 days postsurgically for multimodal analgesia. All experimental protocols were approved by the University of California, Los Angeles Chancellor’s Animal Research Committee and complied with and generally exceeded standards set by the Public Health Service policy on the humane care and use of laboratory animals.
Eye Movement Recording
We used a QNX-based real-time experimental data acquisition system (REX) and Windows-based visual stimulus generation system (VEX; Laboratory of Sensorimotor Research, National Eye Institute, Bethesda, MD; Hays et al. 1982) to create the behavioral paradigm, display the visual stimulus and acquire eye position signals. Voltage signals proportional to horizontal and vertical components of eye position were filtered (8-pole Bessel −3 dB, 180 Hz), digitized at 16-bit resolution and sampled at 1 kHz (PCI-6036E; National Instruments, Austin, TX). The camera acquired eye position signals were filtered digitally using a built-in bilateral filter. We used an automated procedure to define the onset of saccadic eye movements using eye velocity (20°/s) and acceleration criteria (5,000°/s2), respectively. The adequacy of the algorithm was verified and adjusted as necessary on a trial-by-trial basis by the experimenter.
Electrophysiological Data Acquisition and Processing
We recorded individually isolated single neurons (n = 109) and multineuron activity (n = 811), from a total number of 672 recording contacts, in the SC with a 16-channel platinum/iridium V Probe coated with polyimide (Plexon, Dallas, TX), with an impedance of 275 (±50) kΩ for all contacts. The V Probe was inserted through a guide tube, perpendicular to the surface of the SC, positioned with a grid system (Crist et al. 1988), and advanced using an electronic microdrive system controlled by a graphical user interface (Nan Instruments). Action potential waveforms were bandpass filtered (250 Hz to 5 kHz; 4-pole Butterworth), and amplified using the BlackRock NSP hardware system controlled by the Cerebus software suite (BlackRock Microsystems). Neuronal waveform data were digitized at 16-bit resolution and sampled at 30 kHz and saved to disk for offline analysis using Plexon offline sorting algorithms (Plexon, Dallas, TX). When possible, neurons were also isolated online using time and amplitude windowing criteria. The times of occurrence of action potentials were digitized at 16-bit resolution and sampled at 1 kHz and saved to disk.
We mapped the RF of SC neurons online by moving a spot around the monitor and having monkeys make delayed saccades to the different spots, as described above. We listened for maximal discharge rate and considered the center of the RF of 1 contact on the 16-contact probe to be the location at which a saccade was associated with maximal audible discharge. We confirmed the center of the RF by plotting the discharge rate as a heat map in Cartesian coordinates. Only neurons with RF eccentricities between 7 and 20° were selected for study to ensure no overlap of the RF with the centrally located motion cue stimulus. The position of the chamber ensured that the electrode penetrations were orthogonal to the SC surface, and as such, there were negligible differences in the RFs of simultaneously recorded neurons at different contacts along the shaft of the V Probe. Nevertheless, we optimized the RF for at least one recording site on each experimental day using the audible changes in dischare associated with saccades.
Action potential waveforms were sorted offline using the Plexon Offline Sorter (Offline Sorter; Plexon). We classified single neurons and multineuron activity into visual, visuomotor, burst and buildup neurons (Basso and Wurtz 1998; Li and Basso 2005; McPeek and Keller 2002; Munoz and Wurtz 1995). For this classification, we compared the mean discharge rate in several epochs of the trial: 200 ms before the onset of the motion cue (baseline); 175-ms epoch beginning 25 ms after the choice targets appeared (visual); 300–600 ms after motion onset (cue epoch); and the 100-ms epoch ending with the saccade onset (saccade epoch). Visual neurons (n = 13; 5 in the Stimulus-Matched experiment and 8 in the Sensitivity-Matched experiment) were defined as neurons with activity in the visual epoch that differed significantly from baseline; visuomotor neurons (n = 44; 38 in the Stimulus-Matched experiment and 6 in the Sensitivity-Matched experiment) were defined as neurons with activity in visual epoch and the saccade epoch that differered significantly from baseline; buildup neurons (n = 477; 253 in the Stimulus-Matched experiment and 224 in the Sensitivity-Matched experiment) were defined as neurons with activity during the cue epoch that differed significantly from baseline, and burst neurons (n = 188; 69 in the Stimulus-Matched experiment and 119 in the Sensitivity-Matched experiment) were defined as neurons with activity during the saccade epoch that differed signficantly from baseline. Eighty-nine neurons did not fall in any of the above-mentioned catagories and were discarded from further analysis. We discarded five visual neurons from the Stimulus-Matched and eight visual and six visuomotor neurons from the Sensitivity-Matched experiments because there were too few for meaningful statistical analysis.
To display the spike density functions (SDF), we convolved the times of occurrence of action potentials with a Gaussian (σ of 10 ms). In results, we show the raw (see Fig. 5, A and B) and normalized (see Fig. 5, C and D) SDF sorted by high and low coherence. We grouped (see Fig. 5) the two lowest coherence levels for monkey P (0 and 6%) with the two lowest coherence levels for monkey H (0 and 10%) into the low-coherence condition and the two highest coherence levels from each monkey into the high-coherence condition (10 and 20% for monkey P and 20 and 50% for monkey H).
Fig. 5.
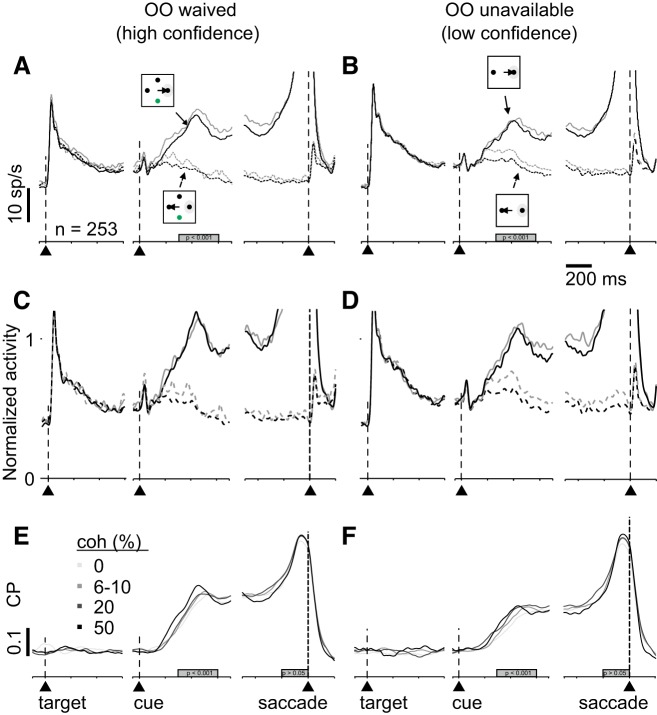
Choice probability (CP) analysis reveals confidence signals in superior colliculus (SC). spike density functions (SDFs), and CP in opt-out (OO)-waived (A, C, and E) and OO-unavailable (B, D, and F) trials. A: SDFs (σ = 10 ms) from 253 single and multiple neurons in SC plotted over time for high coherence (black lines: 10 and 20% for monkey P and 20 and 50% for monkey H) and low coherence (gray lines: 0 and 6% for monkey P and 0 and 10% for monkey H). Solid lines show trials in which monkeys chose the target in the response field (RF) and dashed lines show the SDFs from trials in which monkeys chose the opposite target in trials when the OO was available. The alignment is the same as in Fig. 3. B: same as in A for the OO-unavailable trials. C and D: normalized SDF (σ = 10 ms) of the same neurons shown in A and B. The activity was normalized to a 300-ms time window starting 300 ms after the random moving dots appeared. E: CP as a function of time sorted by each coherence in OO-waived trials. Grayscale indicates the different coherencies. Alignment is indicated by the vertical dashed lines and arrowheads. The gray rectangle indicates the time period of quantification. F: same as in E for OO-unavailable trials. The distributions of area under the curve during a time window (gray bard in E and F) are significantly different in the cue period (P < 0.001) but not in the saccade period (P > 0.05).
Choice Probability
To compute the choice probability (CP), we calculated receiver operating characteristic (ROC) curves comparing the mean discharge rate of all trials to the RF (correct and incorrect) versus all trials away from RF (Britten et al. 1992; Green and Swets 1966). We computed the probability that the discharge rate measured in the cue epoch, in a window from 300 to 600 ms after cue onset, exceeds the criterion on a trial-by-trial basis for each neuron and excluded those neurons with fewer than three trials for each condition. The criterion was incremented from the minimum to the maximum discharge rate measured in a trial in 100-ms steps. A probability was calculated for each criterion value and the ROC results from plotting the probability for each criterion for each coherence condition. ROC curves shown in results (see Fig. 6, A and C, and Fig. 10, A and C) are population ROC curves and were obtained by averaging individual ROCs calculated from each neuron. The area under the ROC curve (AUC) ranges from 0.0 to 1.0 and indicates the probability that the two distributions of discharge rates come from different distributions. An AUC of 0.5 indicates that the two populations are indistinguishable, and an AUC of 0 or 1 indicates that the two distributions are completely separable. The statistical significance of single neuron ROCs was calculated using a permutation test (n. permutations = 2,000; P < 0.05).
Fig. 6.
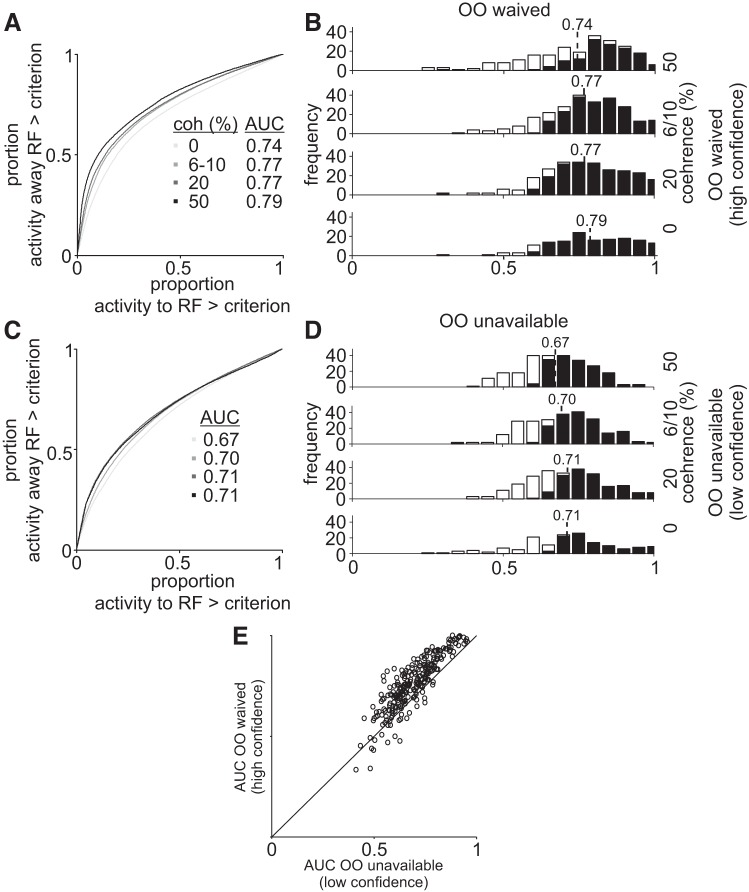
A: choice probability predicts the difficulty level in the Stimulus-Matched task. receiver operating characteristic curves plotted for different motion coherences in opt-out (OO)-waived trials in the Stimulus-Matched experiment. The probability of neuronal activity for choices toward the response field (RF) greater than criterion plotted against the probability of neuronal activity choices away from the RF greater than criterion for all motion coherences indicated by the gray scale. Lines falling along the 0.5 indicate no difference in neuronal activity for correct and incorrect choices to the RF. Values greater than 0.5 indicate higher discriminability. B: frequency distribution of the area under the curve (AUC) for each coherence (0, 6, 10, 20, and 50%) for OO-waived trials. The black bars show the number of neurons with an AUC that differs significantly from 0.50 (permutation test, P < 0.05 or P > 0.95). Open bars show the numbers of neurons with AUC that did not differ significantly from 0.50 using the permutation test. C: same as in A for the OO-unavailable trials. D: same as in B for OO-unavailable trials. E: scatter plot of the AUCs of each recording site in OO-unavailable trials (abscissa) and OO-waived trials (ordinate).
Fig. 10.
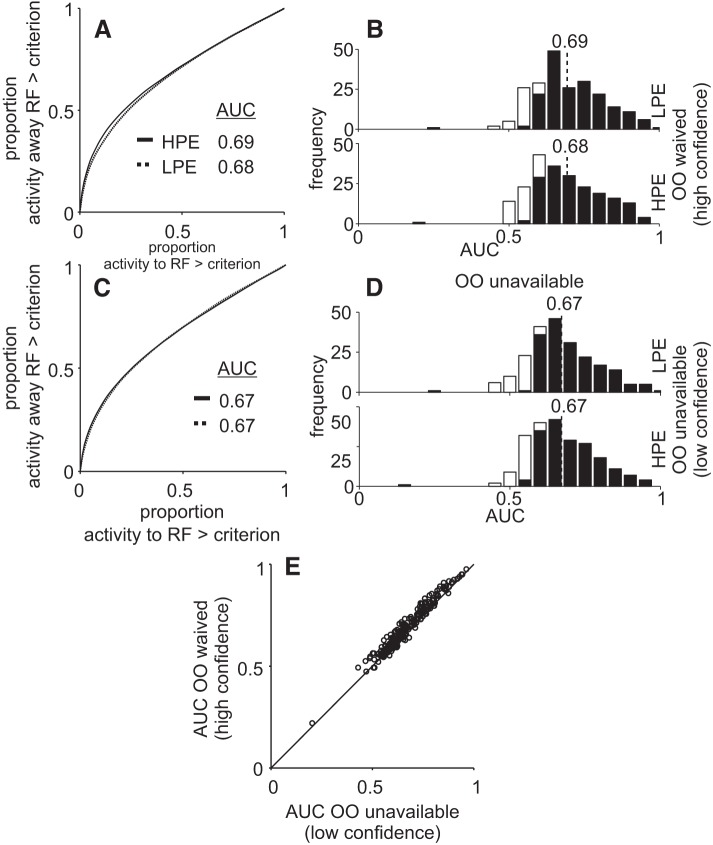
Choice probability is unable to distinguish high-positive evidence (HPE) from low-positive evidence (LPE) trails in the Sensitivity-Matched task. A and C: same as Fig. 6 for the Stimulus-Matched experiment. A: receiver operating characteristic curves for opt-out (OO)-waived trials. C: same as in C for the OO-unavailable trials. E: scatter plot of the area under the curve (AUC) of each recording site in OO-unavailable trials (abscissa) and OO-waived trials (ordinate). B and D: frequency distribution of AUC for HPE and LPE trials, in the Sensitivity-Matched experiment. B: OO-waived trials. D: OO-unavailable trials. Same conventions as in Fig. 6. The black bars show the number of neurons with an AUC that differs significantly from 0.50 (permutation test, P < 0.05 or P > 0.95). Open bars show the numbers of neurons with AUC that did not differ significantly from 0.50 using the permutation test.
Behavioral analysis
We used signal detection theory methods to quantify the sensitivity of the monkeys in our task. d′ is a measure of sensitivity or discriminability since it measures how accurately an ideal observer can distinguish a sensory stimulus either from noise in a detection task or from another sensory stimulus in the case of a single interval classification task as used here. A d′ = 0 means the observer is unable to discriminate the sensory stimuli and therefore is insensitive, whereas a d′ = infinity indicates perfect discriminability and therefore, perfect sensitivity. We calculated d′ as; d′ = Z (Hit Rate) − Z (False Alarm Rate), where Z is the function for the inverse normal distribution. To avoid artifacts due to a nonnormal distribution of Hits and False Alarm rates in OO-waived trials (near threshold trials are likely to result in an OO choice), we calculated d′ only for trials when the OO was unavailable. For the Sensitivity-Matched experiment, we defined matched trials as those with d′ values for the HPE trials and LPE trials within 0.70 d′ neurons of each other, and the probability of OO was statistically different using a signed rank test.
RESULTS
Two trained monkeys reported their perceived direction of motion of a random dot-motion (RDM) stimulus by making a saccade to one of two possible choice targets located in the left or right visual field, corresponding to the two possible motion directions (Fig. 1A). In 50% of the trials only two choice targets appeared on the screen and the monkeys made a decision about the direction of motion in the display (single interval classification task, Fig. 1A). In the other randomly interleaved 50% of the trials, a third choice target appeared (OO; Fig. 1B, green circle). Correct choices resulted in a 0.10-ml sip of juice reward whereas incorrect choices resulted in no reward. The OO choice target always yielded a reward smaller than a correct choice (80–90% of the correct reward). We reasoned that, as previously shown by Kiani and Shadlen (2009), confident monkeys choose one of the two targets corresponding to the motion direction whereas less confident monkeys choose the smaller but guaranteed reward from the OO choice target. In all trials with the OO choice target available, a fourth stimulus also appeared on the screen (Fig. 1B). This stimulus was irrelevant to the task and if the monkey chose it, the trial was aborted. This stimulus was introduced to maintain symmetry of the visual display and avoid lateral interactions (Basso and Wurtz 1998; Rizzolatti et al. 1973). We recorded from a total of 811 multineurons and single neurons (390 neurons in monkey P and 421 neurons in monkey H), using a 16 contact V Probe (Plexon) while monkeys performed this task and one of the choice targets always appeared at the center of the RF of at least one recorded neuron (see materials and methods; Fig. 1G). Out of 811 neurons, 13 were visual, 44 visuomotor, 188 burst, and 477 buildup neurons. Eighty-nine neurons did not fall into any of these categories and were not used for further analysis. In the Stimulus-Matched task, we included only visuomotor, buildup, and burst neurons due to the low number of visual neurons (n = 5). In the Sensitivity-Matched task we recorded 8 visual neurons, 6 visuomotor neurons, 199 burst, and 477 buildup neurons. We include only buildup and burst neurons due to the small number of visual and visuomotor (n = 8 and 6).
SC Neuronal Activity Correlates with Decision Confidence when Confidence and Accuracy Covary
Trained monkeys performed a motion direction discrimination task in which we varied the difficulty of the motion direction decision by presenting RDM stimuli containing four different motion strengths: 0, 6, 10, and 20% for monkey P and 0, 10, 20 (Fig. 1, C and D), and 50% for monkey H (Fig. 1, E and F). Different coherences were used because monkey P had exceptional discrimination performance. Figure 1C shows the proportion of correct trials as a function of coherence for monkey P averaged across 10 recording sessions in trials when the OO was unavailable (Fig. 1C, gray line) or available but waived (Fig. 1C, black line). Figure 1E shows the same for monkey H. The performance was ~50% correct (chance) for 0% coherence and steadily increased as the motion strength increased. Note that in both monkeys, performance was better for trials in which the OO target was available and waived compared with trials in which the OO target was unavailable, for the same coherence (Fig. 1, C and E, signed rank test, P < 0.005, a point we return to later). Figure 1, D and F, shows that monkeys chose the OO target more often for the weak motion signal trials compared with the strong motion trials. As the motion strength increased, the probability of opting out decreased. These data show that both monkeys use motion evidence to guide their decisions and that they report being less confident by choosing to OO on trials in which decisions contain weaker motion evidence.
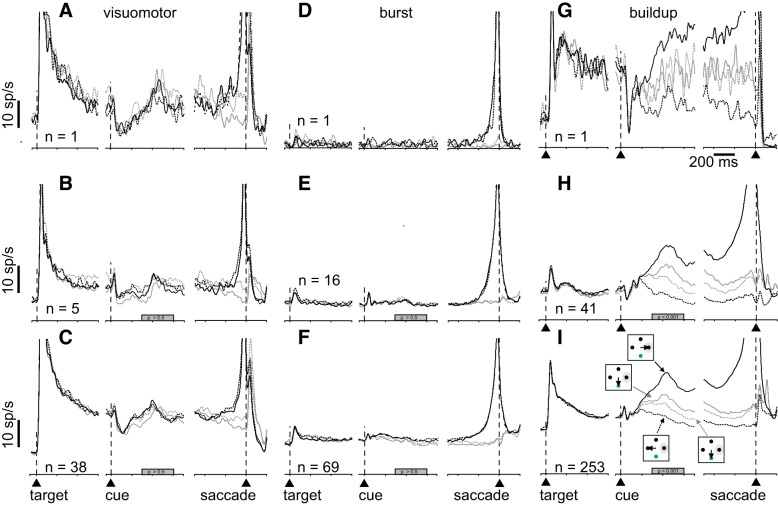
Figure 2 shows the activity of visuomotor (Fig. 2, A–C), burst (Fig. 2, D–F), and buildup neurons (Fig. 2, G–I) recorded from the SC during performance of the Stimulus-Matched task collapsed over all coherences for both monkeys. Figure 2, A, D, and G shows example single neurons; Fig. 2, B, E, and H, shows averages of single neurons; and Fig. 2, C, F, and I, shows the average of single and multineurons.
Fig. 2.
Superior colliculus (SC) neuronal activity correlates with confidence reports in an opt out confidence task. A: spike density functions (SDF; σ = 10 ms) plotted over time for an example visuomotor SC neuron recorded during performance of the Stimulus-Matched task. Iconography in I shows the different trial types. Solid black lines show SDFs for correct choices into the response field (RF), opt-out (OO) waived; dashed black lines show SDFs for correct choices away from the RF, OO waived; solid gray lines show SDFs for trials in which the monkey selected the OO choice with motion direction toward the RF; and dashed gray lines show SDFs for trials in which the monkey selected the OO choice with motion direction away from the RF. The SDF panels are aligned to the onset of the choice targets (leftmost; arrowhead and vertical dashed line), when the motion direction cue appeared (middle, arrowhead and vertical dashed line), and when the choice was reported (rightmost, arrowhead and vertical dashed line). D and G: same as in A for burst (D) and buildup neurons (G). B, E, and H: same as in A, D, and G for the average single neurons recorded from 2 monkeys. C, F, and I: same as in A, D, and G, and B, E, and H: for neurons recorded as mixed single neurons and multiple neurons from 2 monkeys. The filled gray bars on the abscissas in B and C indicate the interval of discharge that was quantified (P > 0.5 in C and F; P < 0.05 in I). Vertical scale bar = 10 sp/s; horizontal scale bar = 200 ms.
When monkeys correctly chose the target in the RF (toRF), buildup neurons showed greater activity compared with when monkeys chose the target opposite the RF (awayRF; cf. Fig. 2, G, H, and I, solid and dashed black). In trials when the monkey opted out, the activity reached levels intermediate to that seen in the toRF correct trials versus the awayRF correct trials (Fig. 2, G, H, and I, solid and dashed gray lines), consistent with a reduced confidence on these trials, and like that reported in area LIP (Kiani and Shadlen, 2009). The activity measured in a 300-ms epoch beginning 300 ms after the onset of the RDM cue, confirmed that the activity in high-confidence trials (black) versus low-confidence trials (gray) for buildup neurons was significantly different [Fig. 2H, one-way ANOVA, F(3,160) = 1.7.10−5, P < 0.001, η2 = 0.0.25; Fig. 2I, one-way ANOVA, F(3,1008) = 349.05, P < 10−9, η2 = 0.24]. This result shows that when monkeys opted out, the decision-related activity of buildup neurons shows a reduced activity compared with when monkeys choose correctly. The activity of visuomotor [Fig. 2C, one-way ANOVA, F(3,148) = 0.03, P = 0.99, η2 = 0.0005] and burst neurons [Fig. 2F, one-way ANOVA, F(3,272) = 0.1, P = 0.96, η2 = 0.0011] was not significantly modulated during the cue period.
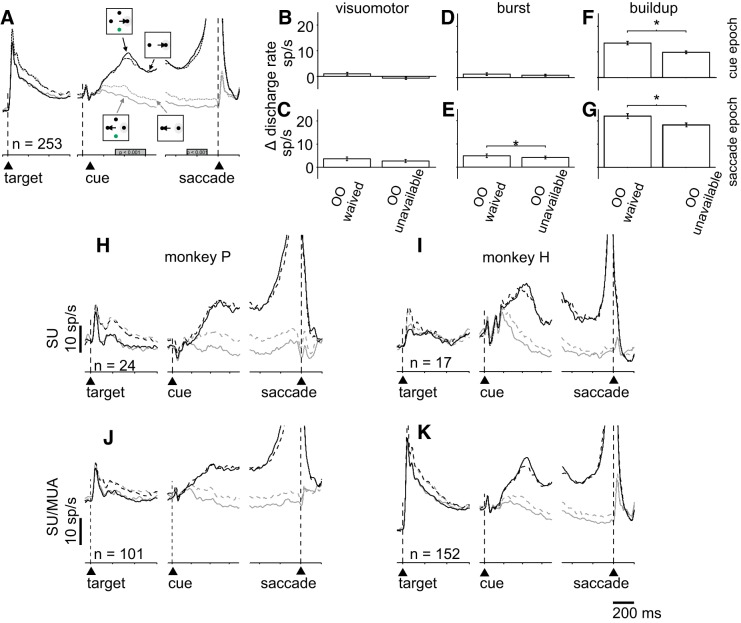
One concern with the Stimulus-Matched task is that the OO target appeared at a location that was 90° between the correct and incorrect choice targets. Therefore, the differences observed in activity of neurons in area LIP reported in Kiani and Shadlen (2009) and the SC reported above could result from different saccades rather than lower confidence per se. These authors (Kiani and Shadlen 2009) addressed this problem by providing the two or three possible choice targets after the RDM cue occurred. This way monkeys could make their decisions and presumably evaluate confidence before knowing whether the OO would be available. We took a different tack. We compared directly the neuronal activity measured on trials in which monkeys waived the OO target, to trials in which the OO target was unavailable, when each was in the RF. We reasoned that OO-waived trials are those when monkeys have high confidence, whereas OO-unavailable trials contain a mix of high- and low-confidence trials. If the neuronal activity of the toRF neurons uniquely signals confidence, then the activity should be higher in the OO-waived trials compared with the OO-unavailable trials. The SDF in Fig. 3A shows the average activity of 253 single + multiple neurons, recorded in correct trials with high confidence (OO waived) and mixed confidence (OO unavailable) for both monkeys. Comparing the black solid versus the black dashed lines of Fig. 3A show that toRF neurons do not contain a unique signal associated with confidence (Kolmogorov-Smirnov test, ksstat = 0.17, P = 0.9967, d = 0.029).
Fig. 3.
The differential activity between target in the response field (toRF) choices and target opposite the RF (awayRF) choices to the RF (Δ) is modulated by confidence. A: spike density functions (σ = 10 ms) for the average of 253 single and multiple neurons. Iconography shows the different trial types: solid black lines show the correct trials to the RF in opt-out (OO)-waived trials, solid gray line indicates correct trials away from RF in OO-waived trials, dashed gray line indicates correct trials to the RF when the OO was unavailable, dashed gray line indicates correct trials away from RF when the OO was unavailable. The filled gray bars on the abscissas in A indicate the interval of discharge that was quantified. B, D, and F: bar graphs of the differential (Δ) discharge rate of the neurons shown in A during the cue epoch of visuomotor (B), burst (D), and buildup neuros (F). ΔDischarge rate = discharge rate for correct trials to the RF − discharge rate for correct trials away from the RF in OO-waived and OO-unavailable trials, C, E, and G: Δdischarge rate in the presaccade epoch for visuomotor (C), burst (E), and buildup neurons (G). *P < 0.05, for E, F, and G. H–K: the same layout as shown in A for the single and multiple neurons shown separately for each monkey. MUA, multiunit activity; SU, single-unit activity.
Figure 3A shows that although there are no differences in the activity for the toRF condition, the neuronal activity in the awayRF condition appears different in the OO-waived and the OO-unavailable trials. We calculated a differential activity (Δdischarge rate) by taking the difference in activity for buildup neurons during a 300-ms cue epoch beginning 300 ms after RDM onset for OO trials and OO-unavailable trials. We also calculated a Δdischarge rate for a 200-ms saccade epoch beginning 300 ms before saccade onset for buildup neurons. We calculated the same two epochs of Δdischarge rates for visuomotor neurons and burst neurons. Figure 3, B, D, and F, shows the cue epoch Δdischarge rate for each neuron type, and Fig. 3, C, E, and G, shows the saccade epoch Δdischarge rate for each neuron type. We found that the Δdischarge rate was greater for OO-waived trials than OO-unavailable trials for buildup neurons during the cue period (Fig. 3F, Kolmogorov-Smirnov test, ksstat = 0.17, P = 0.0079, d = 0.433) and the saccade period (Fig. 3G, Kolmogorov-Smirnov test, ksstat = 0.13, P = 0.024 d = 0.35). All other comparisons did not reach significance (Fig. 3B, Kolmogorov-Smirnov test, ksstat = 0.23, P = 0.2, d = 0.76; Fig. 3C, Kolmogorov-Smirnov test, ksstat = 0.13, P = 0.83, d = 0.28; Fig. 3D, Kolmogorov-Smirnov test, ksstat = 0.15, P = 0.31, d = 0.14; Fig. 3E, Kolmogorov-Smirnov test, ksstat = 0.144, P = 0.43, d = 0.17). This result occurred in both monkeys individually and in both the single and multineuron recordings shown separately (Fig. 3, H–K).
We next used CP (see materials and methods) and a permutation test (2,000 permutations) to assess whether individual neurons showed significant differences in the Δdischarge rates for OO-waived (high-confidence) versus OO-unavailable (mixed confidence) trials as we saw in the averaged data for both monkeys together and individually as shown in Fig. 3. We found that 4/38 (10%) of visuomotor neurons, 6/69 (0.8%) burst neurons and 93/253 (36%) of buildup neurons showed significant differences in Δdischarge rates for OO-waived (high-confidence) versus OO-unavailable (low-confidence) trials. As is apparent from the SDFs in Fig. 3, A and H–K, the buildup activity appears to be driven mostly by the activity of neurons encoding the saccade opposite to the RF (Fig. 3, A and H–K, gray lines). Taken together, these results indicate that the SC contains signals related to decision confidence when confidence and decision accuracy covary. These signals occur primarily in the differential activity of buildup neurons encoding the correct trials to the RF (toRF trials) and the correct trials away from RF (awayRF trials) during the cue and saccade epochs. However, note that monkeys’ decision accuracy is also greater in the OO-waived trials, a point we return to below.
The results described above suggest that buildup neurons in the SC signal decision confidence as the differential activity encoding the toRF and awayRF choices. This is somewhat different from area LIP in which toRF choice neurons signal decision confidence (Kiani and Shadlen 2009). However, a second possible interpretation of our result is that the activity differs between the OO-waived and the OO-unavailable trials, not because of differences in confidence, but because there are different numbers of stimuli on the screen. It is well known that SC neuronal activity is modulated by different numbers of possible targets (Basso and Wurtz 1998; Rizzolatti et al. 1973). To rule out this interpretation, the same monkeys performed an oddball target selection task with either two or four possible isoluminant targets. The monkeys received a reward for making a saccade to the differently colored stimulus (gray among red), one of which always appeared in the RF of the recorded neurons (Fig. 4A; materials and methods). We recorded from 175 SC buildup neurons from 2 monkeys, and 127 from monkey H were also recorded in the Stimulus-Matched task described above. We found that the differential activity of SC neurons for toRF trials minus awayRF trials in the trials with one and three distractors did not differ significantly (Fig. 4B, black lines, Kolmogorov-Smirnov test, ksstat = 0.09, P = 0.45, d = 0.0524). Therefore, we conclude that the differences in activity we find in the different confidence trials of the Stimulus-Matched task do not result from changes in the numbers of possible choice targets (cf. Fig. 4B and Fig. 3A).
We next asked whether SC neurons could better predict stimulus coherence on high-confidence trials. For this we performed a CP analysis. We performed these analyses collapsed over the data for both monkeys and for both the single and multineurons because Figs. 2 and 3 show that the neuronal activity data were similar for single or multineuron recordings and for both monkeys, consistent with their similar behavior shown in Fig. 1. The SDFs in Fig. 5, A and B, show the modulation of SC activity by stimulus coherence for the OO-waived and OO-unavailable conditions respectively. For simplicity of presentation, we grouped the two lowest coherencies for monkey P (0 and 6%) with the two lowest coherence levels for monkey H (0 and 10%) into a low-coherence condition and the two highest coherence levels from each monkey (10 and 20% for monkey P and 20 and 50% for monkey H) into a high-coherence condition. The Δdischarge rate between the toRF and awayRF trials is greater for the high-coherence trials compared with the low-coherence trials. This difference is better shown in the normalized SDFs in Fig. 5, C and D (cf. gray solid and dashed lines versus black solid and dashed lines). We used choice probability to quantify this difference. CP was calculated by comparing the distributions of the discharge rates for each coherence in trials in which the animal chose the toRF target, against the distribution of the discharge rates from all the trials in which the animal chose the awayRF target, collapsed over correct or incorrect trials (see materials and methods; Britten et al. 1992). We calculated two different classes of CPs: one for high-confidence trials (OO waived, Fig. 5E) and another for low-confidence trials (OO unavailable, Fig. 5F). Figure 5, E and F, shows the dynamics of the AUC as a function of time. The mean AUCs were higher for high-confidence trials compared with low-confidence trials in the cue epoch (300–600 ms after cue onset), while monkeys were presumably making their decision and evaluating confidence (Kolmogorov-Smirnov test, ksstat = 0.26, P = 4 × 10−8 d = 0.81) but not at the time of the saccade choice report (Fig. 5, E and F, gray rectangles, Kolmogorov-Smirnov test, ksstat = 0.11, P = 0.06, d = 0.2).
The numeric values of the AUCs in Fig. 6, A and C, indicate the probability that, for each trial, SC neuronal activity predicted the trial’s coherence in high- (Fig. 6A) or low-confidence (Fig. 6C) conditions. Figure 6, B and D, shows the distributions of CP values for each coherence (filled bars show the proportion of significant AUCs). We found that coherence was better read out in high-confidence trials from the activity of individual SC neurons. The AUCs for high-confidence trials across all coherences were 0.74, 0.77, 0.77, and 0.79 versus those for low-confidence trials were 0.67, 0.70, 0.71, and 0.71. The mean AUC collapsed over coherences for high-confidence trials was significantly higher than the mean AUC for low-confidence trials (0.76 versus. 0.69, Kolmogorov-Smirnov test, ksstat = 0.26, P = 4 × 10−8, d = 0.81). Figure 6E shows a scatter plot of the AUCs for high-confidence trials (ordinate) versus low-confidence trials (abscissa). Since most all points fall above the line of unity, these results show that the majority of our sample of buildup neurons recorded from the SC can discriminate coherence better in high- than in low-confidence trials in the Stimulus-Matched task.
The results described so far provide evidence that neuronal activity from individual SC neurons contains information reflecting decision confidence. In area LIP, confidence signals appear to be reflected by the same neurons that signal the toRF choice target, indicating that the same neurons signal decisions and confidence, as normative models predict. Although the results shown in Fig. 3 indicate that the toRF neurons of SC do not signal confidence uniquely, these results come from a comparison of trials in which there were high confidence (OO waived) and a mixture of high and low confidence (OO unavailable). To ensure we did not miss possible differences because of this, we performed an additional experiment that compares high- and low-confidence trials directly. While recording from the same neurons described above, monkeys also performed a task in which the OO target appeared in the RF (Fig. 7A). Using only the OO-available trials, we compared the trials in which the monkey chose the OO target (low confidence) to the trials in which they chose correctly (high confidence). We reasoned that if SC neuronal activity provides a signal uniquely related to confidence, a direct comparison of low-confidence trials (chose OO) to high-confidence trials (correct choice) should show differences in neuronal activity, even though the same saccade is made. We found that when the monkeys made a saccade to the RF, the difference in activity between correct and waived trials showed no significant difference (Fig. 7B, Kolmogorov-Smirnov test, ksstat = 0.055, P = 0.8, d = 0.06). This result occurred in both monkeys individually and in the single and multineuron activity (Fig. 7, C–F).
Fig. 7.
Neuronal activity in the superior colliculus (SC) predicts decisions independent of confidence. A. schematic of the spatial arrangement of the task used to assess opt-out (OO) choices (low confidence) and OO-waived choices (high confidence) when the OO choice target was in RF of recorded neurons. The arrangement of the task is the same as that shown in Fig. 1, B and C. OO-available and OO-unavailable trial types and the location of the OO choice target (in the RF or away from the RF) occurred randomly across trials. The choice targets appeared in either the upper or lower hemifield corresponding to either upward or downward motion (see materials and methods). B: comparison of spike density functions (SDFs) (σ = 10 ms) plotted over time for the same 253 neurons recorded singly or in multiples simultaneously as shown in Fig. 2 while monkeys performed the confidence tasks shown in A (A and B for the data shown in Fig. 2). Iconography shows the different trial types. Solid black lines show SDFs from the high-confidence, OO-waived trials (same as shown in Fig. 2C), and the dotted black lines show SDFs for low-confidence trials, when the monkeys chose the OO choice target, placed in the RF of the recorded neurons. If decision neurons of the SC also signal confidence, they should show different activity in high- versus low-confidence trials. Vertical scale bars indicate 10 sp/s and horizontal scale bars indicate 200 ms. The filled gray bars on the abscissas in B indicate the interval of discharge that was quantified (P > 0.05). C–F: same layout as shown in B for the single and multiple neurons shown separately for each monkey. MUA, multiunit activity; SU, single-unit activity.
Taken together, the results support the hypothesis that the relative activity of SC buildup neurons signals confidence. However, this type of activity is also linked to decision accuracy (Kim and Basso 2010; Mazurek et al. 2003; Ratcliff et al. 2003). Indeed, in the Stimulus-Matched task, monkeys perform better on OO-waived trials (Fig. 1), indicating that decision accuracy and decision confidence covary. Therefore, we cannot exclude the interpretation the differences we obtained are related to decision accuracy rather than to confidence. In what follows, we test this hypothesis directly, through an experiment in which we manipulate confidence while holding decision accuracy constant.
SC Activity Reflects the Accuracy of Perceptual Decisions
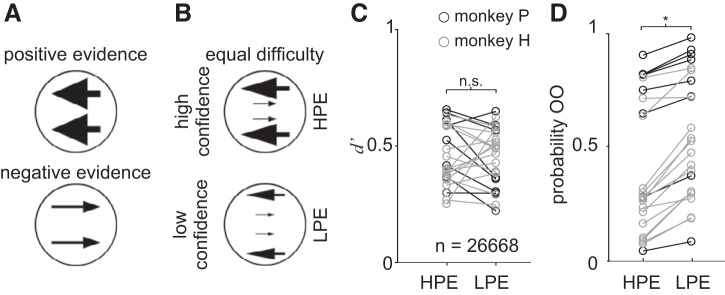
Figure 1, C and E, shows that when monkeys are confident, they are also performing better. This raises the very critical possibility that the signals we measured in SC reflect monkeys’ perceptual sensitivity and thus, better performance, rather than confidence per se. To determine whether the relationship between neuronal activity and confidence we observed in the Stimulus-Matched task reflects confidence or accuracy, we designed a novel psychophysical task. We developed a d′ or Sensitivity-Matched stimulus display adapted from theoretical and experimental work performed in humans showing that people base their decisions on the relative amount of motion evidence in favor or against a decision, whereas they base their assessment of confidence mainly on the amount of evidence in favor of their decision (Koizumi et al. 2015; Zylberberg et al. 2012). We divided the motion stimulus into three components, motion rightward, motion leftward, and noise. We defined these components as motion congruent with the direction of the correct choice (referred to as PE) or motion opposite to the correct choice (referred to as NE) and noise, which was motion in all other directions. We created two stimulus conditions by manipulating the amount of PE and NE for individual monkeys and individual sessions empirically to obtain two stimulus conditions in which monkeys showed matched d′ and thus matched sensitivity (accuracy) but different levels of confidence; high confidence with HPE and low confidence with LPE (Fig. 8, A and B). The noise component ensured an equal number of dots for the motion cues in the different conditions. With this display we could assess whether neuronal activity was more correlated with decision accuracy (defined as d′) or decision confidence (defined by proportion of OO trials). The sequence of the events and the possible choice targets were identical to those described above for the Stimulus-Matched task. The only difference between the Stimulus-Matched and the Sensitivity-Matched tasks was the motion cue.
Fig. 8.
Dissociation of information for decisions and information for confidence. A: schematic representation of the task used to dissociate the information that will lead to a decision from the information leading to a report of confidence. Positive evidence (PE) is the motion direction corresponding to the correct decision (response congruent evidence) whereas negative evidence (NE) corresponds to the opposite motion direction (response incongruent evidence). B: using isoluminant displays, the relative amount of positive and negative motion evidence can remain constant while the PE varies. We refer to these stimuli as d′ or Sensitivity-Matched stimuli. C: d′ is plotted for low-positive evidence (LPE) and high-positive evidence (HPE) for all data sets from both monkeys. Dark circles show trials from monkey P, and light gray circles show trials from monkey H. Total trial n = 26,668, 23 sessions. There were no significant differences in d′ between the HPE and LPE conditions (P > 0.05). D: proportion of trials in which monkeys chose the opt-out (OO) for the LPE and HPE conditions for the same data set shown in C. *P < 0.0001. Bars in C and D show SE.
Figures 8, C and D, shows the behavior of the two monkeys separately in the Sensitivity-Matched task, over 23 sessions and 26,668 trials. Whereas d′ did not differ significantly in the HPE and LPE conditions (signed rank test, z = 1.651, P = 0.0987, d = 0.3954), the probability of opting out was significantly higher in LPE than in HPE conditions (Fig. 8D, signed rank test, z = −4.01, P < 0.0001, d = 0.6375). This shows that monkeys behave in this task similar to humans (Koizumi et al. 2015; Zylberberg et al. 2012); that is, they base their decisions on the relative amount of PE and NE but report their confidence based mainly on the amount of PE. This means that confidence and decision accuracy (d′) can be dissociated.
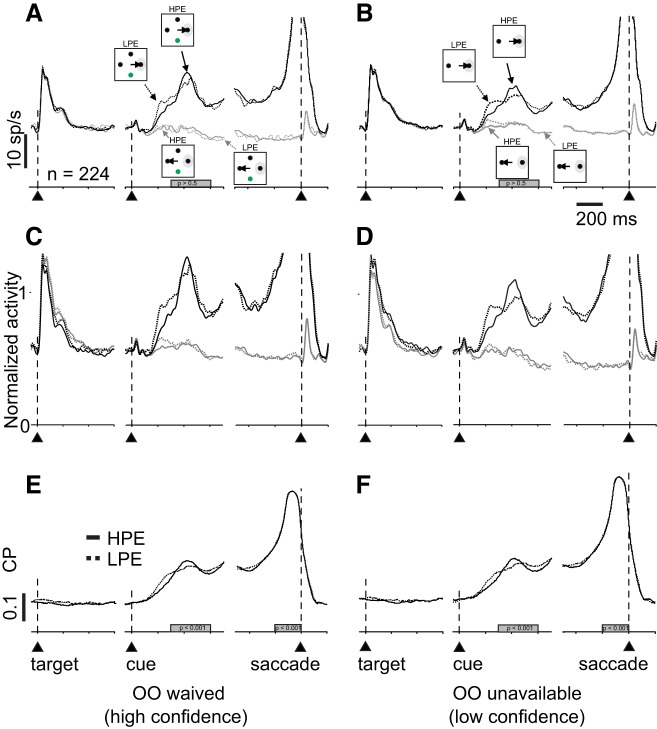
Figure 9 shows the activity of 224 single and multibuildup neurons recorded from both monkeys in the Sensitivity-Matched experiment, arranged in the same format at Fig. 5. Note that we separated out the OO-available and OO-unavailable trials in this figure simply for consistency with Fig. 5 and the previous experiment. The relevant comparison in the Sensitivity-Matched task is between HPE (high-confidence trials) and LPE (low-confidence trials). In principle, the OO-waived and the OO-unavailable conditions should be similar. Figure 9A shows the trials in which d′ was matched but contained either HPE or LPE and monkeys chose toRF or awayRF (solid and dashed black lines and solid and dashed gray lines, respectively). There was no significant difference in the activity of SC buildup neurons between the HPE and LPE conditions for either the toRF or awayRF choices in OO-waived trials (Fig. 9A, Kolmogorov-Smirnov test, ksstat = 0.064, P = 0.76, d = 0.009). We obtained similar findings for the OO-unavailable trials (Fig. 9B; Kolmogorov-Smirnov test, ksstat = 0.045, P = 0.97, d = 0.009). The same result is evident in the normalized SDFs shown in Fig. 9, C and D. We also calculated the Δdischarge rate for toRF and awayRF activities of buildup neurons as we did for the Stimulus-Matched task data and found no differences between HPE (high-confidence) and LPE (low-confidence) conditions for either the OO-waived or the OO-unavailable trials (Fig. 9A, Kolmogorov-Smirnov test, ksstat = 0.045, P = 0.97, d = 0.05; Fig. 9B, Kolmogorov-Smirnov test, ksstat = 0.078, P = 0.5, d = 0.002).
Fig. 9.
Neuronal activity in the superior colliculus (SC) fails to distinguish decision confidence. A and B: average spike density functions (SDFs) (σ = 10 ms) plotted over time for 224 single and multineurons while a monkey performed the Stimulus-Matched task in trials with opt-out (OO) unavailable (A) or waived (B). Solid lines show high-positive evidence (HPE) trials and dotted lines indicate low-positive evidence (LPE) trials. The activity in HPE and LP trials is not significantly different. C and D: normalized SDF (σ = 10 ms) of the same neurons shown in A and B. The activity was normalized to a 300-ms time window starting 300 ms after the random moving dots appeared. E: choice probability (CP) as a function of time sorted by each coherence in OO-waived trials. Solid lines indicate HPE trials, and dotted lines indicate LPE trials. Trial alignment is indicated by the vertical dashed lines and the upward arrowheads. F: same as in E for OO-unavailable trials. Gray rectangles indicate the epoch used for quantification; 200 ms timescale is for E and F (P > 0.05).
As done for the Stimulus-Matched task data shown in Figs. 5 and 6, we next calculated the CP to determine whether SC buildup neuron activity better predicted the choice location in conditions of high confidence (HPE) compared with low confidence (LPE). We did this for trials in which the OO was waived and for trials in which the OO was unavailable, again for completeness. Figures 9, E and F, shows the AUC as a function of time during the Sensitivity-Matched task for the OO-waived (Fig. 9E) and the OO-unavailable trials (Fig. 9F) for high-confidence (Fig. 9, E and F, solid black lines) and low-confidence trials (Fig. 9, E and F, dashed black lines). The mean AUCs were not significantly different in the cue period between HPE and LPE conditions in OO-waived conditions (Fig. 10, A and B, Kolmogorov-Smirnov test, ksstat = 0.18, P = 0.14, d = 0.135, α = 0.05) or in OO-unavailable conditions (Fig. 10, C and D, Kolmogorov-Smirnov test, ksstat = 0.04, P = 0.99, d = 0.014). The same was true during the saccade period (OO waived, Kolmogorov-Smirnov test, ksstat = 0.045, P = 0.94, d = 0.026; OO unavailable, ksstat = 0.06, P = 0.76, d = 0.031). Figure 10E shows a scatter plot of the AUCs for high-confidence trials (ordinate) versus low-confidence trials (abscissa). Since most all points fall along the line of unity, these results show that on average, our sample of buildup neurons recorded from the SC cannot discriminate coherence better in high- than in low-confidence trials in the Sensitivity-Matched task. We repeated the same analysis in burst neurons and none of the comparisons turned out significant during the cue period (OO waived, HPE versus LPE, Kolmogorov-Smirnov test, ksstat = 0.14, P = 0.15, d = 0.33; OO unavailable, HPE versus LPE, Kolmogorov-Smirnov test, ksstat = 0.12, P = 0.34, d = 0.19) or the saccade period (OO waived, HPE versus LPE, Kolmogorov-Smirnov test, ksstat = 0.17, P = 0.52, d = 0.028; OO-unavailable trials, ksstat = 0.08, P = 0.76, d = 0.22).
Consistent with the finding that only a few neurons in the SC show a relationship to confidence in the Sensitivity-Matched task, CP analysis revealed that, comparing the Δdischarge rate toRF-awayRF, 48/224 (20%) of the buildup neurons and 12/199 (12%) of the burst neurons significantly predicted HPE versus LPE trials when we collapsed OO-waived and OO-unavailable trials. Specifically, 49/224 (21%) buildup neurons were significant in OO-waived trials and 29/224 (13%) in the OO-unavailable trials; 31/119 (26%) burst neurons were significant in OO-waived trials and 8/119 (6.7%) in the OO-unavailable trials.
Finally, to compare directly whether SC neuronal activity correlated best with accuracy or confidence, we plotted the differential activity (toRF-awayRF; Δdischarge rate) as a function of coherence (Fig. 11, A and C) and as a function of d′ (Fig. 11, B and D). We found that the discharge rate of SC buildup neurons was significantly modulated, at least in OO-unavailable trials as a function of both coherence and d′ in the Stimulus-Matched task [Fig. 11, A and C; ANOVA, F(3,998) = 3.61, P = 0.01, η2 = 0.0107]. On the other hand, in the Sensitivity-Matched experiment, the discharge rates were not significantly different in HPE versus LPE conditions [Fig. 11B; ANOVA, F(1,434) = 0.0001, P = 0.99, η2 = 10−7; Fig. 11D ANOVA, F(1,434) = 3.38, P = 0.06, η2 = 0.0077]. Performance of the same sets of comparisons on SC burst neuronal activity revealed similar findings (toRF correct OO-waived HPE versus toRF correct OO-waived LPE, Kolmogorov-Smirnov test, ksstat = 0.0613, P = 0.979, d = 0.0029; toRF correct OO-unavailable HPE versus toRF correct OO-unavailable LPE, Kolmogorov-Smirnov test, ksstat = 0.05, P = 0.99, d = 0.001; Δdischarge rate OO-waived HPE versus Δdischarge rate OO-waived LPE, Kolmogorov-Smirnov test, ksstat = 0.10, P = 0.52, d = 0.0347; correct Δdischarge rate OO-unavailable HPE versus correct Δdischarge rate OO-unavailable LPE, Kolmogorov-Smirnov test, ksstat = 0.07, P = 0.88, d = 0.09; correct awayRF OO-waived HPE versus correct awayRF OO-unavailable LPE, Kolmogorov-Smirnov test, ksstat = 0.05, P = 0.98, d = 0.011; correct awayRF OO-waived HPE versus correct awayRF OO-unavailable LPE, Kolmogorov-Smirnov test, ksstat = 0.04, P = 0.99, d = 0.0078, Bonferroni correction, α = 0.0083).
Fig. 11.
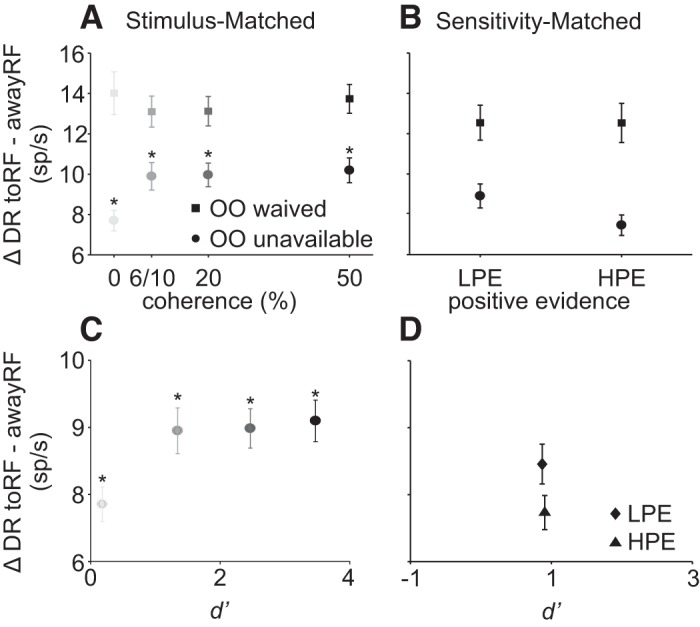
Neuronal activity in the superior colliculus (SC) correlates with decisions but not confidence. A: differential activity for target in the response field (toRF) choices − target opposite the RF (awayRF) trials as a function of coherence. The activity of SC neurons correlates with coherence in opt-out (OO)-unavailable trials [circles; ANOVA, F(3,998) = 3.61, P = 0.01] but not in OO-waived trials [squares; ANOVA, F(3,977) = 0.29, P = 0.83]. Each point is the average of 253 neurons in 2 monkeys. B: same as in A for the Sensitivity-Matched experiment. SC activity is not significantly different for high-positive evidence (HPE) versus low-positive evidence (LPE) trials both in OO waived [squares; ANOVA, F(1,434) = 0.0001, P = 0.99, η2 = 10−7] and OO unavailable [circles; ANOVA, F(1,434) = 3.38, P = 0.06, η2 = 0.0077]. C: differential activity in OO-unavailable trials for toRF trials − awayRF plotted as a function of d′. Grayscale indicates the different coherencies. The differential discharge rate of SC neurons is significantly modulated as a function of d′ [ANOVA, F(3,998) = 3.61, P = 0.01]. D: same as C for the Sensitivity-Matched experiment. SC activity is not significantly different in HPE and LPE conditions [ANOVA, F(1,434) = 3.38, P = 0.06, η2 = 0.0077]. *P < 0.05 in A and C.
Taken together, these results indicate that whereas some individual SC buildup neurons may signal decision confidence independent of decision accuracy in the Sensitivity-Matched task, the majority do not. Moreover, whether they signal confidence appears unreliable between the OO-waived trials and the OO-unavailable trials, something that should not occur if the signal was a bona fide confidence signal. Therefore, we conclude that neither buildup neurons nor burst neurons in the SC contain a signal uniquely related to decision confidence. Neuronal activity modulations we see in these tasks is best explained as signaling decision accuracy.
DISCUSSION
We combined a new psychophysical task with single and multiple neuron recordings to determine whether neurons of the SC of monkeys contain signals associated with decision confidence in a perceptual decision task and used analytical tools based in signal detection theory. We recorded SC activity in two different decision tasks; one we refer to as Stimulus-Matched and in which decision accuracy and confidence reports covary. Using this task, we found that SC neurons contained a robust signal of decision confidence, similar to that seen in area LIP of cerebral cortex (Kiani and Shadlen 2009). However, in the second task, we refer to as Sensitivity-Matched task and in which decision accuracy and decision confidence are dissociated, we found no evidence for a unique signal of decision confidence in discharge rates. Based on these results, we conclude that the SC does not contain a unique signal associated with decision confidence. Our results, together with those of others, call into question normative models of decision confidence that propose that decision-confidence is an optimal readout of decision signals (Hangya et al. 2016; Mainen et al. 2016; Pouget et al. 2016). Below, we discuss our results in the light of other work exploring the neurophysiology of decision confidence and then briefly highlight the implications of these results for future work on decision-making confidence.
Relationship to Previous Work
Neurophysiological results support the hypothesis that decision confidence is an optimal readout of decision signals by showing that neuronal activity in cerebral cortical areas involved in decision-making such as area the SC, LIP, and SEF of monkeys (Horwitz and Newsome 1999, 2001; Ray and Heinen 2015; Roitman and Shadlen 2002; Shadlen and Newsome 2001; Yang and Heinen 2014) correlates with monkeys’ reports of decision confidence (Kiani and Shadlen 2009; Middlebrooks and Sommer 2012). Because signals associated with decision confidence are also found in neurons encoding information about the decision, this observation is consistent with normative models of decision confidence in which confidence is considered an optimal readout of decision accuracy (Hangya et al. 2016; Mainen et al. 2016; Pouget et al. 2016). However, a number of recent experiments in humans (Koizumi et al. 2015; Komura et al. 2013; Zylberberg et al. 2012) and monkeys (Komura et al. 2013) reveal that confidence can be dissociated from decision accuracy indicating that decision confidence is not always optimal.
These recent results, together with our results from the SC in the Sensitivity-Matched experiments, call into question the definition of confidence and suggest that there is another type of confidence that we refer to as subjective confidence. Subjective confidence is the sense of confidence that is dissociable from sensitivity, may not be optimal, and can be measured by the probability of opting out in conditions in which the information leading to a decision remains constant while the information leading to a confidence report varies.
Implications for Understanding Decision Confidence
In our Sensitivity-Matched task, we developed a novel visual stimulus display in which the information leading to decisions was matched across two conditions but contained information leading to different confidence reports. The novelty of this task revealed that monkeys report different levels of confidence in the two trial types even when the information for the decision was the same, providing strong evidence that confidence reports can be suboptimal. If confidence was an optimal read out of decision-making signals, neither monkeys nor humans should report different levels of confidence with this display, yet they do. Most studies of the neurophysiology of confidence do not make this distinction; therefore, it is not surprising that neuronal signals recorded in decision areas of the brain also correlate with measures of confidence. Our results show that recordings from a decision-making area that receives input from cortical areas associated with confidence, SEF and LIP, fails to distinguish between trials with different reports of confidence and indistinguishable decision-related activity. The results reported here corroborate and extend our other findings that a multivariate decoder also failed to correctly predict confidence reports made by monkeys in this task. Instead the decoder predicted only decision accuracy (Odegaard et al. 2018). Taken together, these findings call into question the role of sensorimotor areas related to decision-making in reporting confidence and provide support for the hypothesis that nondecision-related areas of the brain such as the pulvinar, may play a role in confidence (Komura et al. 2013). Our results also lend support to the hypotheses that there are dedicated regions of the brain that process signals related to the sense of confidence such as prefrontal cortex (Cortese et al. 2016; Lak et al. 2014; Lau and Passingham 2006; Rounis et al. 2010) rather than confidence signals being distributed throughout multiple brain areas. Whether there are signals encoding subjective confidence as defined here are found in other regions of the brain or whether the signals encoding subjective confidence interact downstream of decision-making areas remain as critical questions for future investigation.
GRANTS
This work was supported by National Eye Institute Grant EY-013692 (to M. A. Basso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.G., S.H.C., H.L., and M.A.B. conceived and designed research; P.G. and S.H.C. performed experiments; P.G. and S.H.C. analyzed data; P.G., S.H.C., H.L., and M.A.B. interpreted results of experiments; P.G. prepared figures; P.G. drafted manuscript; P.G., S.H.C., H.L., and M.A.B. edited and revised manuscript; P.G., S.H.C., H.L., and M.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Xueqi Cheng for programming support and Adam Myers for excellent animal care.
REFERENCES
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature 389: 66–69, 1997. doi: 10.1038/37975. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Moversushon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese A, Amano K, Koizumi A, Kawato M, Lau H. Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance. Nat Commun 7: 13669, 2016. doi: 10.1038/ncomms13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988. . [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966. [Google Scholar]
- Grimaldi P, Lau H, Basso MA. There are things that we know that we know, and there are things that we do not know we do not know: confidence in decision-making. Neurosci Biobehav Rev 55: 88–97, 2015. doi: 10.1016/j.neubiorev.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Sanders JI, Kepecs A. A mathematical framework for statistical decision confidence. Neural Comput 28: 1840–1858, 2016. doi: 10.1162/NECO_a_00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science 284: 1158–1161, 1999. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86: 2543–2558, 2001. doi: 10.1152/jn.2001.86.5.2543. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: a Researchers Handbook (3rd ed.). Englewood Cliffs, NJ: Prentice Hall, 1991. [Google Scholar]
- Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324: 759–764, 2009. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci 30: 2340–2355, 2010. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Maniscalco B, Lau H. Does perceptual confidence facilitate cognitive control? Atten Percept Psychophys 77: 1295–1306, 2015. doi: 10.3758/s13414-015-0843-3. [DOI] [PubMed] [Google Scholar]
- Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A. Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nat Neurosci 16: 749–755, 2013. doi: 10.1038/nn.3393. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Hays AV Jr, Richmond BJ, Optican LM. Unix-Based Multiple-Process System, for Real-Time Data Acquisition and Control (Online). https://www.osti.gov/scitech/biblio/5213621. 1982. [24 March 2017].
- Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron 84: 190–201, 2014. doi: 10.1016/j.neuron.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA 103: 18763–18768, 2006. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci 25: 11357–11373, 2005. doi: 10.1523/JNEUROSCI.3825-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell AB, Pettya RE, Cunningham W, Díazd D. Metacognitive confidence: a neuroscience approach. Int J Soc Psychol 28: 317–332, 2013. doi: 10.1174/021347413807719148. [DOI] [Google Scholar]
- Mainen ZF, Häusser M, Pouget A. A better way to crack the brain. Nature 539: 159–161, 2016. doi: 10.1038/539159a. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex 13: 1257–1269, 2003. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Middlebrooks PG, Sommer MA. Neuronal correlates of metacognition in primate frontal cortex. Neuron 75: 517–530, 2012. doi: 10.1016/j.neuron.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333, 1995. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- Odegaard B, Grimaldi P, Cho SH, Peters MA, Lau H, Basso MA. Superior colliculus neuronal ensemble activity signals optimal rather than subjective confidence. Proc Natl Acad Sci USA 115: E1588–E1597, 2018. doi: 10.1073/pnas.1711628115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Drugowitsch J, Kepecs A. Confidence and certainty: distinct probabilistic quantities for different goals. Nat Neurosci 19: 366–374, 2016. doi: 10.1038/nn.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol 90: 1392–1407, 2003. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- Ray S, Heinen SJ. A mechanism for decision rule discrimination by supplementary eye field neurons. Exp Brain Res 233: 459–476, 2015. doi: 10.1007/s00221-014-4127-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Grupp LA, Pisa M. Inhibition of visual responses of single units in the cat superior colliculus by the introduction of a second visual stimulus. Brain Res 61: 390–394, 1973. doi: 10.1016/0006-8993(73)90544-1. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounis E, Maniscalco B, Rothwell JC, Passingham RE, Lau H. Theta-burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cogn Neurosci 1: 165–175, 2010. doi: 10.1080/17588921003632529. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Yang SN, Heinen S. Contrasting the roles of the supplementary and frontal eye fields in ocular decision making. J Neurophysiol 111: 2644–2655, 2014. doi: 10.1152/jn.00543.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberberg A, Barttfeld P, Sigman M. The construction of confidence in a perceptual decision. Front Integr Nuerosci 6: 79, 2012. doi: 10.3389/fnint.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]