Abstract
The posterior lateral prefrontal cortex—specifically, the inferior frontal junction (IFJ)—is thought to exert a key role in the control of attention. However, the precise nature of that role remains elusive. During the voluntary deployment and maintenance of visuospatial attention, the IFJ is typically coactivated with a core dorsal network consisting of the frontal eye field and superior parietal cortex. During stimulus-driven attention, IFJ instead couples with a ventrolateral network, suggesting that IFJ plays a role in attention distinct from the dorsal network. Because IFJ rapidly switches activation patterns to accommodate conditions of goal-directed and stimulus-driven attention (Asplund CL, Todd JJ, Snyder AP, Marois R. Nat Neurosci 13: 507–512, 2010), we hypothesized that IFJ’s primary role is to dynamically reconfigure attention rather than to maintain attention under steady-state conditions. This hypothesis predicts that in a goal-directed visuospatial cuing paradigm IFJ would transiently deploy attention toward the cued location, whereas the dorsal attention network would maintain attentional weights during the delay between cue and target presentation. Here we tested this hypothesis with functional magnetic resonance imaging while subjects were engaged in a Posner cuing task with variable cue-target delays. Both IFJ and dorsal network regions were involved in transient processes, but sustained activity was far more evident in the dorsal network than in IFJ. These results support the account that IFJ primarily acts to shift attention whereas the dorsal network is the main locus for the maintenance of stable attentional states.
NEW & NOTEWORTHY Goal-directed visuospatial attention is controlled by a dorsal fronto-parietal network and lateral prefrontal cortex. However, the relative roles of these regions in goal-directed attention are unknown. Here we present evidence for their dissociable roles in the transient reconfiguration and sustained maintenance of attentional settings: while maintenance of attentional settings is confined to the dorsal network, the configuration of these settings at the beginning of an attentional episode is a function of lateral prefrontal cortex.
Keywords: functional magnetic resonance imaging, goal-driven attention, inferior frontal junction, lateral prefrontal cortex
INTRODUCTION
Goal-directed “top-down” attention is the set of processes by which we voluntarily select task-relevant information. Much research has indicated that a dorsal cortical network comprised of the frontal eye field (FEF) and parietal cortex [intraparietal sulcus (IPS), superior parietal lobule] is associated with goal-directed attention and is distinct from a ventral, stimulus-driven, “bottom-up” attention network (Corbetta et al. 2008; Corbetta and Shulman 2002; Serences et al. 2005b; Shulman et al. 2009; Yantis et al. 2002). Recent work shows that a posterior lateral prefrontal cortical region, the inferior frontal junction (IFJ), interacts with either the dorsal top-down or ventral bottom-up attention networks depending upon task context (Asplund et al. 2010). This dual role raises the question, What does IFJ contribute to attention in comparison to the dorsal and ventral attention networks? It has been suggested that IFJ may coordinate attentional control, toggling between goal-directed and stimulus-driven attention (Asplund et al. 2010; Brass et al. 2005; Braver et al. 2003; Konishi et al. 1998; Marois and Ivanoff 2005). Such a role is consistent with IFJ’s association with task switching, initiation of attention shifts, and attentional orienting (Asplund et al. 2010; Braver et al. 2003; Chiu and Yantis 2009; Shomstein et al. 2012); cue interpretation (Woldorff et al. 2004); and stimulus-response mapping and response selection (Bhanji et al. 2010; Bunge et al. 2003; Dux et al. 2006, 2009; Ivanoff et al. 2009; Marois and Ivanoff 2005; Tombu et al. 2011) because all of these punctate events involve the transient control of attention. By contrast, FEF and IPS are thought to spatiotopically represent top-down attentional priority, such that attention is deployed to the location of highest priority in a winner-take-all fashion (Koch and Ullman 1985; Ptak 2012; Wolfe 1994, 2007). Such stable deployment may be viewed as maintenance of attention (Kastner et al. 1999), in contrast to the transient processes ascribed above to IFJ. Thus, when examined as a whole, the literature points to potentially distinct roles of the dorsal attention network and IFJ in the control of attention.
Direct evidence for contrasting roles of IFJ and the dorsal network in the control of attention has been difficult to obtain, however, because most studies of attentional control include a complex series of distinct computational processes that make them difficult to tease apart (see Cole and Schneider 2007 for discussion of a similar issue). Specifically, top-down attentional control is generally assessed in a larger task context that combines initial sensory registration of a cue, interpretation of that cue’s meaning to form an attentional template (e.g., Desimone and Duncan 1995), orienting attention consistent with that template (e.g., Posner 1980; Serences and Yantis 2006), maintenance of attentional deployment (e.g., Ikkai and Curtis 2008; Kastner et al. 1999; Yantis et al. 2002), target detection and sometimes also distractor rejection (e.g., Shulman et al. 2001), and, finally, response selection and execution (e.g., Nee et al. 2007). Often, attention tasks conflate these processes because they occur in close temporal proximity. In addition, many studies do not explicitly delineate which processes they have attempted to manipulate, leading to conflation into a more general “attention” process. It is thus unclear which regions of the brain are actually involved in each of the processes that contribute to goal-driven attention. To our knowledge, the distinct functional roles of IFJ and dorsal attention network have not been satisfactorily demonstrated within a single experimental paradigm.
Previous attempts to dissociate the neural correlates of transient attentional reconfiguration from stable maintenance have compared shifting to the maintenance of attentional deployment (i.e., holding), revealing transient processing in the medial superior parietal lobule and in the prefrontal cortex—often in or near IFJ—and sustained processing in the FEF, IPS, and peripheral sensory cortex (Chiu and Yantis 2009; Greenberg et al. 2010; Kelley et al. 2008; Serences et al. 2005a, 2005b; Shulman et al. 2009; Tamber-Rosenau et al. 2011). However, these studies characterized shifting and maintenance with regard to dynamic information sources such as rapid serial visual presentation streams in which new items—distractors and targets—appeared frequently. Another study (Ikkai and Curtis 2008) demonstrated sustained activation in both the dorsal network and IFJ over an extended period of attentional deployment but required subjects to count repeated stimulus dimmings during the delay. In these types of tasks, activity driven by sustained maintenance is difficult to separate from repeated transient processes (e.g., target detection and distractor rejection; see Cole and Schneider 2007). The importance of avoiding this conflation is highlighted by recent work showing that IFJ plays both a transient role in stimulus-evoked orienting and a sustained role in the evaluation of temporally extended, salient, visual “oddball” events (Han and Marois 2014).
Other attempts to separate distinct attentional processes during goal-directed attention tasks also have methodological or interpretational limitations. Specifically, Hopfinger et al. (2000) used a general linear modeling (GLM) approach that did not distinguish sustained delay-period activity from transient target-evoked activity. Corbetta et al. (2000, 2002) reported delay activity in FEF and IPS, as well as precentral sulcus near the left IFJ, following the cue on trials that terminated without targets, but they used short (4.72 s) delays, making it difficult to unambiguously separate delay-related from cue-driven activity. At least one study (Kastner et al. 1999) has demonstrated sustained signals in dorsal attention network brain regions before target presentation. However, this study used identical target locations on every trial of the experiment, thus leaving it unclear to what extent the same cognitive processes were evoked relative to more conventional (randomized location) spatial attention paradigms. In addition, Kastner et al. (1999) did not use a sensitive regions of interest (ROIs) approach to examine delay period signals in IFJ, although they did report activation during the delay period in a more anterior region of the middle frontal gyrus. Finally, Kastner et al. (1999) used a constant-duration delay period, raising the possibility that subjects may not have attended over the entire delay and, consequently, that detected signals could represent memory or vigilance rather than ongoing spatially selective attention. An additional study (Offen et al. 2010) has demonstrated sustained activity in the FEF over a variable-length delay; however, this study also demonstrated a surprising absence of sustained activity in IPS, perhaps because the task required vigilance for a target rather than spatially selective attention. In addition, the study did not examine delay activity in IFJ. Taken together, prior research clearly demonstrates sustained activity in the dorsal network, but aspects of design or analysis prevented strong conclusions about whether or not IFJ activity is transient. In the present study, we expected to replicate sustained dorsal activity, but it is important to evaluate sustained and transient activation patterns of all these brain regions in the same experimental context while learning from past research by avoiding as many design pitfalls as practical.
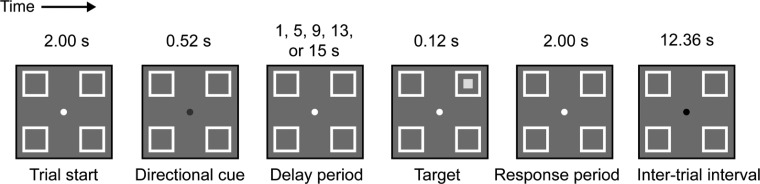
Here we used GLM and event-related averaging (ERA) analyses of functional magnetic resonance imaging (fMRI) data to distinguish between transient and sustained spatial attentional processes in IFJ and the brain regions of the dorsal attention network (FEF, IPS). Using a modified endogenous cuing task (Posner 1980), we required subjects to interpret a centrally presented symbolic cue, voluntarily shift visuospatial attention to one of four static peripheral locations depending upon cue identity, and, critically, maintain attention to this peripheral location for a variable delay before target presentation (see Fig. 1). With such a paradigm, it is possible to distinguish transient processes such as cue interpretation and attentional orienting from sustained processes involved in the maintenance of attention. We characterized fMRI response time courses in IFJ and the dorsal attention network to show that transient processing of cues and targets occurs in both IFJ and the dorsal attention network. However, although for the most part the dorsal network exhibited the predicted sustained activity during attentional maintenance, there was little such activity in IFJ. These results functionally dissociate the roles of IFJ and the dorsal network in the dynamic deployment and maintenance of top-down attention.
Fig. 1.
Task design. After a 2-s warning period indicated by the fixation dot turning white, a central color cue (shown here in black) indicated the cued location. A variable-length delay period followed the cue, which was then followed by target presentation (except in 15-s target-absent trials), a 2-s response period, and trial end. Delay period length was subject to jitter (randomly drawn from a uniform distribution in the range −0.5 to 0.5 s) in addition to the nominal delay duration of each trial. The subsequent intertrial interval was also jittered (−0.5 to 0.5 s).
METHODS
Participants.
Ten right-handed participants (6 men, 4 women; mean age 28.5 ± 3.3 yr) gave written informed consent and were included in this study. All procedures were approved by the Vanderbilt University Institutional Review Board.
Magnetic resonance imaging scanning procedure.
Each subject underwent a single MRI session that included eight functional main task runs, one run of a functional (saccade) localizer task, one run of an unrelated peripheral fixation task (not reported here except for eye tracking control), and a single structural scan. All scanning took place at the Vanderbilt University Institute of Imaging Science with a Philips Intera Achieva 3T MRI scanner.
Structural scans used standard procedures to acquire 1-mm isovoxel resolution whole brain coverage. Functional echo-planar imaging scans varied in duration (125–161 volumes; see Endogenous cuing task) and used an 80 × 80 matrix (reconstructed at 128 × 128) to cover 33 axial slices (3.0-mm thickness, 0.5-mm gap) with a 240 × 240 mm field of view, leading to a reconstructed voxel size of 1.875 × 1.875 × 3.5 mm. Functional volumes were acquired with a repetition time (TR) of 2 s, a 35-ms echo time, and a 79° flip angle.
During functional runs, task stimuli were projected with an LCD projector onto a screen at the head of the bore of the magnet. Stimuli were viewed via a mirror mounted to the head coil. Responses were collected via magnet-safe manual response button boxes (Rowland Institute of Science, Cambridge, MA). Eye position was monitored via an ASL remote eye tracking system (Applied Science Laboratories, Bedford, MA) that viewed the eye via the same mirror used for stimulus display. Stimulus display and response collection were controlled with custom MATLAB (The MathWorks, Natick, MA) procedures and the Psychophysics Toolbox (Brainard 1997; Kleiner et al. 2007; Pelli 1997).
Saccade localizer task and region of interest definition.
Subjects performed a single run of the saccade localizer task. In the localizer, eight 20-s blocks of fixation alternated with seven 20-s blocks of a saccade task. In the saccade task, a single dot subtending ~0.25° of visual angle changed positions randomly, twice per second. Subjects tracked the dot as it jumped from location to location. Such a localizer task has previously been used to identify FEF and regions in the vicinity of the IFJ (e.g., Kastner et al. 2007) as well as IPS (e.g., Connolly et al. 2000; Curtis and Connolly 2008). Localizer runs were preprocessed identically to the main task (see below) and analyzed with separate fixed-effects GLMs in each subject. Saccade task blocks were contrasted with fixation blocks to yield localizer-defined ROIs in FEF, IPS, and IFJ.
Each ROI localized via the saccade task was defined separately in each hemisphere as the peak-activated voxel near appropriate anatomical landmarks (FEF: intersection of the superior frontal sulcus and the precentral sulcus; IPS: mid- to anterior intraparietal sulcus; IFJ: in the depth of the intersection of the inferior frontal sulcus and precentral sulcus) plus the surrounding voxels in a 2-voxel-radius sphere, leading to a total ROI volume of 33 resampled functional voxels. Peak voxels in the localizer contrast exceeded a false discovery rate (FDR)-corrected significance threshold (Benjamini and Hochberg 1995) of q < 0.05. In the rare (6 of 60 subject × region combinations) cases in which no activated voxel could be identified in a given subject-region combination, the mean coordinates across the remaining subjects for that region were used as the locus of that ROI in that subject (left IFJ, 2 subjects; left FEF, 2 subjects; right IFJ, 1 subject; right IPS, 1 subject). Our results were qualitatively similar when considering only participants for whom all ROIs could be defined based on individual localizers (n = 7). All ROI details are presented in Table 1.
Table 1.
Regions of interest (ROIs) and main task ROI GLM results
| Talairach Coordinates |
Cue |
Delay |
Target/Trial End |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | X | Y | Z | Identified in n Subjects | Beta | t | P | Beta | t | P | Beta | t | P |
| L IFJ | −37.75 (0.94) | 2.88 (1.96) | 24.75 (1.77) | 8 | 2.7563 | 3.0365 | 0.014098 | 0.1885 | 1.7480 | 0.114406 | 28.9393 | 6.3866 | 0.000127 |
| R IFJ | 40.67 (1.83) | 1.22 (1.54) | 25.00 (1.50) | 9 | 2.0007 | 1.9744 | 0.079771 | 0.1913 | 2.4810 | 0.034936 | 38.2085 | 7.2417 | 0.000049 |
| L FEF | −23.50 (1.13) | −9.88 (1.13) | 45.75 (0.75) | 8 | 6.6371 | 6.0333 | 0.000194 | 0.2156 | 3.2087 | 0.010680 | 32.7605 | 8.6127 | 0.000012 |
| R FEF | 25.10 (1.27) | −9.80 (1.56) | 45.20 (1.33) | 10 | 4.6798 | 4.8940 | 0.000855 | 0.4118 | 5.7588 | 0.000273 | 28.5575 | 7.3160 | 0.000045 |
| L IPS | −22.60 (0.98) | −57.20 (1.36) | 48.30 (1.22) | 10 | 5.8286 | 5.1154 | 0.000632 | 0.2581 | 3.2113 | 0.010637 | 40.0633 | 8.3021 | 0.000016 |
| R IPS | 20.00 (0.50) | −54.33 (1.59) | 44.00 (0.87) | 9 | 4.2487 | 4.3976 | 0.001726 | 0.4471 | 5.2125 | 0.000555 | 39.6873 | 8.1008 | 0.000020 |
ROIs were identified via the saccade localizer. Reported coordinates are mean Talairach coordinates with SE in parentheses. Where group coordinates were used because of the absence of a subject-defined region (see methods), SEs were calculated before addition of group-defined regions. Identified in n Subjects represents the number of participants in whom we identified a subject-specific ROI via the functional localizer; we resorted to group average coordinates for the remaining 1 or 2 subject(s) when we failed to identify an ROI via the localizer. Mean general linear model (GLM) parameter (beta) weight across the 9-, 13-, and 15-s delay trials for each trial phase (cue, delay, target/trial end) is also shown. FEF, frontal eye field; IFJ, inferior frontal junction; IPS, intraparietal sulcus; L, left; R, right.
It should be noted that IFJ lies immediately adjacent to a region generally referred to as inferior FEF (Silver and Kastner 2009), and at least one study has argued that IFJ does not activate for saccades, whereas inferior FEF does (Derrfuss et al. 2012), leading to the recommendation to isolate IFJ with a Stroop paradigm. However, Derrfuss et al. (2012) used an unusual saccade paradigm (cued by auditory stimuli and in darkness). Furthermore, other research has shown that Stroop paradigms may activate regions adjacent to IFJ instead of IFJ proper (Muhle-Karbe et al. 2016, their Supplemental Figs. 1 and 2), although this evidence is limited because it stems from reverse inference from the BrainMap database (i.e., examining the probability of a Stroop task given activation across studies in a large database). Nevertheless, we suggest that this evidence eliminates Stroop as an unambiguously superior IFJ localizer. Perhaps most importantly, the IFJ regions localized with the present saccade paradigm were in the depth of each individual’s precentral sulcus, consistent with IFJ but not inferior FEF (Derrfuss et al. 2012).
Endogenous cuing task.
During functional runs, participants performed a variant of the endogenous-attention Posner cuing task (Fig. 1). Participants viewed a gray screen with a central (0.25°) fixation dot and four peripheral target-location placeholders (subtending 1° and presented at 5.2° eccentricity), one per quadrant. After a 2-s warning cue (fixation dot change from black to white), the fixation dot changed to one of four colors, each corresponding to one of the four peripheral target locations. This location cue was displayed for ~520 ms and subsequently replaced by the white fixation dot. Subjects were asked to covertly attend the cued target location for the duration of the trial (up to 15 more seconds; see below). The target—a light gray square subtending 0.33°—was presented for ~120 ms within one of the four placeholders and appeared in the cued location on 80% of target-present trials, termed valid trials. On the remaining target-present trials, termed invalid trials, the target appeared in a different location that was randomly drawn from a uniform distribution on each invalid trial. Participants made a speeded detection response to target appearance by pressing a button with the right index finger during the 2-s window following target offset. After this window, the fixation dot returned to black and an ~11.86- to 12.86-s intertrial interval elapsed before the beginning of the next trial. It should be noted that all timings above represent approximate average timings, as trial-specific timings were quantized at the level of the display refresh rate (60 Hz); individual trial timings were recorded and modeled.
Cue-target interstimulus intervals, i.e., the delay periods, for each trial were drawn from a distribution intended to lead to a constant hazard function. For any time bin (see below) in which a target could be presented, there was an approximately equal probability of target appearance, thus avoiding a buildup of anticipation and corresponding neural activity over time (Los and Agter 2005; Nobre et al. 2007; Trillenberg et al. 2000). To implement these features, we drew discrete interstimulus intervals from an exponential distribution such that the target was as likely to appear at each potential position in time as at the previous position, given that it had not already occurred at the previous position. This led to 32 trials with a 1-s delay, 21 trials with a 5-s delay, 14 trials with a 9-s delay, and 10 trials with a 13-s delay. An additional 19 trials (termed target-absent trials) ended after 15 s without the presentation of the target. On these trials, the 2-s response window was presented without the visual presentation of any target, and then the trial’s end was signaled by the fixation point turning black (after a total of 17 s of elapsed time from the cue). We refer to these trials as 15-s delay trials so that the delay referenced in each trial type corresponds to the same portion of the trial—beginning with the cue onset and ending with the beginning of the response period. Each delay (1, 5, 9, 13, or 15 s) was further jittered by randomly drawing a jitter value from a uniform distribution in the range (−500, 500) ms from its nominal time in order to avoid subjects using periodic scanner sounds as a temporal cue for target detection and responding; this led to 1-s total duration time bins centered on each nominal delay duration. For each of these 96 trials, target location was assigned randomly with the constraint that the cue was valid ~80% of the time in every delay condition (except for the target-absent 15-s delay condition). Each functional run included 12 trials drawn randomly without replacement from the full set of 96 trials such that functional run duration varied from 250 to 322 s (125–161 2-s imaging volume acquisitions).
Preprocessing of functional magnetic resonance imaging data.
Data analysis was performed with BrainVoyager QX (versions 1.10.2–2.8; Brain Innovation, Maastricht, The Netherlands) and custom MATLAB procedures as well as the BVQXtools and NeuroElf MATLAB toolboxes (NeuroElf.net; Jochen Weber, New York, NY). Each participant’s functional scans were aligned to his or her structural scan and then preprocessed with three-dimensional motion correction, temporal linear trend removal, slice acquisition time correction, 6-mm Gaussian spatial smoothing, and temporal high-pass filtering (3 cycles per run). Functional scans were then warped to Talairach space (Talairach and Tournoux 1988) and resampled to 3-mm isotropic resolution.
Whole brain GLM.
We conducted an initial whole brain GLM in which subject was treated as a random effect (Friston et al. 1995). This and all other GLMs included separate hemodynamic response function-convolved (Boynton et al. 1996) cue (boxcar of cue presentation duration), delay (boxcar of delay period), and target/trial end (boxcar of target duration) regressors for each delay duration condition, with two exceptions. First, 1-s delay trials were modeled as a single boxcar lasting the duration of the entire trial, as all events occurred within the space of approximately one TR. Second, rather than a target regressor, 15-s trials included a trial end regressor after the end of the response period to signify the end of the trial, i.e., locked to the fixation point signal that the trial had ended without a target. All whole brain analyses were evaluated via open contrasts of one or more regressors greater than unmodeled baseline and thresholded at FDR-corrected (Benjamini and Hochberg 1995) q ≤ 0.05. To avoid interpreting extremely small-volume activations, we further applied an arbitrary cluster size threshold of 3 resampled functional voxels (81 mm3). We do not present results from 1- and 5-s delay duration trials in this or any other fMRI analysis, as trial phases (cue, delay, target) occurred in too close temporal proximity to be confidently resolvable with fMRI. However, these trials were modeled to remove their evoked signals from the implicit baseline of the open contrasts.
Region of interest GLMs.
We also extracted ROI-specific GLM parameter (beta) weights from the localizer-defined ROIs (FEF, IPS, and IFJ, separately in each hemisphere) to evaluate the presence of cue, delay-period, and target/trial end fMRI signal in each ROI. (We treat homologous ROIs from the two hemispheres separately because preliminary analysis of ERAs from our data suggested differences across hemispheres.) Beta weights were calculated by fitting a GLM (model identical to that used for the whole brain GLM) to the signal averaged over each localizer-defined ROI. As with the whole brain GLM, we considered only betas from the 9-, 13-, and 15-s trials because of the impossibility of separating signals from distinct trial stages in the 1- and 5-s trials. Beta weights for 9-, 13-, and 15-s trials were first pooled within subjects by taking their mean, leading to a single average beta weight for each subject for each trial phase. Next, we evaluated the significance of activation for each trial phase (cue, delay, target/trial end) in each region with one-sample t-tests. As the primary goal of these t-tests was to determine whether the activity during the delay phase of the task returned to baseline levels of signal (i.e., was statistically indistinguishable from zero), the most conservative approach is to adopt a liberal α that results in the least likelihood of claiming a return to baseline. Thus we do not correct these t-tests for multiple comparisons.
Event-related averages.
In each ROI identified via the localizer (FEF, IPS, and IFJ, separately in each hemisphere), we extracted ERAs time-locked to the TR of cue presentation separately for all trial types over a window from −2 s until 17 s after the end of the nominal cue-target delay. To create ERAs, we transformed raw cue-locked time courses into percent signal change, baselined to the mean of signal over the window −2 to 0 s, pooled across delay conditions 5–15 s but specific to each run. Time courses were then pooled across runs to form subject-level time courses. Finally, subject-level time courses were averaged to form a group-level mean time course for each delay condition (9, 13, or 15 s) in each region. Because preliminary analyses revealed differences between nominally homologous regions across the two brain hemispheres, we treat all ROIs in a lateralized fashion.
ERAs were compared to baseline by calculating 95% confidence intervals across subjects for all TRs, equivalent to performing a one-sample t-test. Just as for the ROI GLM analyses, the primary goal of the ERAs was to determine whether any delay-phase time points returned to baseline levels of signal (i.e., were statistically indistinguishable from zero); the most conservative approach is to adopt a liberal α, which results in the narrowest possible confidence intervals. Thus we do not correct these ERA confidence intervals for multiple comparisons. This initial confidence interval analysis of ERAs is subject to three important limitations that necessitate further analysis: First, this confidence interval analysis compares the signal in each region to its respective pretrial baseline. Because this analysis only statistically compares each data point to pretrial baseline and never statistically compares between regions or trial phases, it does not directly allow conclusions to be drawn about differences between regions and/or trial phases. Second, the confidence intervals were computed across subjects at each time point locked to cue onset (trial start) and thus did not take into account individual differences in blood oxygenation level-dependent (BOLD) response shape and latency. In other words, they could reveal whether or not a particular region at a particular time point had a significant ERA response, but they could not reveal whether the peak (or trough) of the ERA response was above baseline because each subject’s ERA could have reached its maximum peak (or minimum trough) at a different time point, which would lead the group ERA to underestimate the true peak (and overestimate the true trough). Third, this initial analysis considered each delay condition separately in order to preserve the shape and timing information, rather than pooling across delay conditions to reduce noise. To address these limitations, we performed further analyses of ERA responses to each trial phase in each brain region in each hemisphere, as detailed below.
To compare the role of dorsal network regions to the role of IFJ during each of the three trial phases (cue, delay, and target/trial end), we compared peaks and troughs of ERAs across regions. Specifically, we ascertained 1) whether each region exhibited sustained delay-period activity or if the trough of its time course instead returned to baseline during the delay, which would indicate a transient-only response in that region; 2) whether the dorsal network exhibited significantly more delay-period activity than IFJ, which would indicate that, compared with the dorsal network, IFJ plays less of a role in maintaining attention over the delay; and 3) whether the magnitude of ERA responses to cues and targets/trial end predicted the magnitude of delay activity in IFJ, which could indicate that low delay activity was actually driven by overall low activation of IFJ by the task. In the context of these three analyses, we wish to highlight our definition of sustained delay-period activity—activity that never dips below baseline. This definition is critical because delay-period activity that dips below baseline but subsequently rises again is not consistent with sustained deployment of attention by that brain region. Instead, it would be consistent with either anticipatory target/response activity and/or a reinstantiated deployment of attention, perhaps due to input from another brain region. Thus, for these analyses, we only consider a region to be sustained if the trough of its signal remains significantly above baseline throughout the delay period.
To accomplish these three additional analyses, we extracted ERA peaks and troughs in each region as follows: We took the maximum ERA response for each subject during the time window from 4 to 8 s after cue onset as the peak cue response. This time period was chosen because it included both the typical BOLD hemodynamic response peak (Boynton et al. 1996) and the observed group mean ERA peak in each region (which always occurred at either 4 or 6 s after cue onset). We took the minimum ERA response for each subject during the time window from 8 to 12 s as the trough of the delay response. This time period was chosen based on the observed group mean ERA delay minima, which varied across regions and delay conditions but never occurred earlier than 8 s or later than 12 s after cue onset. Finally, we took the maximum ERA response for each subject during the time window from 3 to 9 s after the end of the delay period as the peak target/trial end response. This time period included the expected time of the peak based on the typical BOLD hemodynamic response function, and also the observed group mean ERA maximum in each region and condition (which ranged from delay length + 5 s to delay length + 9 s).
RESULTS
Behavior.
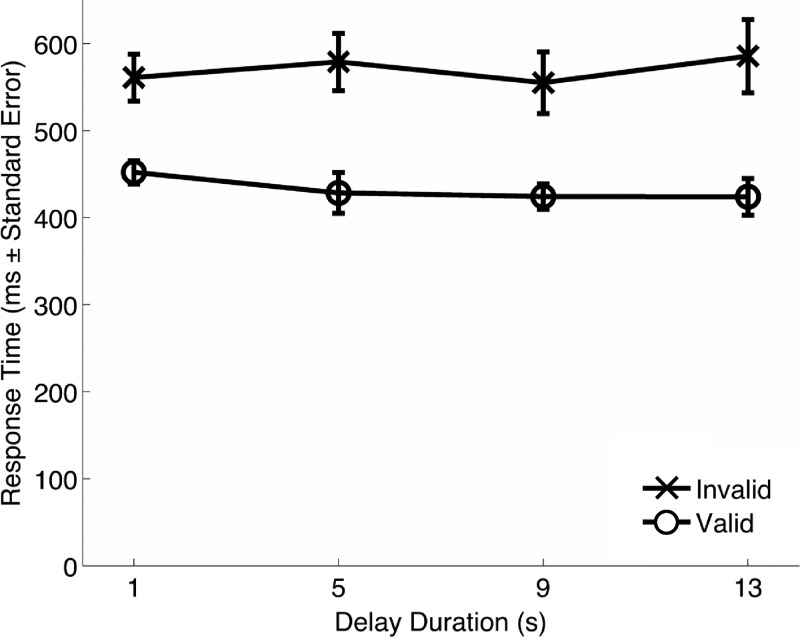
Overall accuracy was 99.0% with a standard deviation (SD) of 2.0% across participants. Accuracy data for target-present trials (1-, 5-, 9-, and 13-s delays) were subjected to a two-way ANOVA (cue validity × delay duration). As expected for this simple detection task, no main effects were significant, nor was the interaction significant (all F < 1.5, all P > 0.24). Response times revealed a large (~140 ms) cue validity effect for all delay conditions (Fig. 2). When response times were subjected to an ANOVA identical to that for accuracy data, there was a main effect of cue validity (F1,9 = 42.97, P = 0.0001), but, importantly, there was no main effect of delay duration and no interaction (all F < 1, all P > 0.49). Thus, despite the sometimes long, highly variable delay periods, subjects sustained visuospatial attention to the cued location throughout the delay period. This result suggests that our manipulation of trial number at each delay successfully yielded a flat hazard function over the target-present trials. As such, no neural effect of target anticipation was expected.
Fig. 2.
Behavioral response times. Error bars represent SE.
Eye tracking resulted in consistent pupil lock and collection of useful eye movement data for 6 of the 10 participants. For these participants, fixation (>150 ms within 1°) was detected inside of a 2° radius around the central fixation point on 88.67 (SD = 7.69) of the 96 trials’ cue-target delay periods, representing more consistent fixation than during the cue period, when all events occurred at fixation (78.17 of 96, SD = 13.42). No subject fixated within a 2° radius of any peripheral target location on any trial. It should be noted that had subjects fixated in the periphery we would have had the sensitivity to detect it, because eye tracking during a peripheral-fixation task (not otherwise reported here) acquired in a separate run of the same MRI sessions successfully tracked fixations and saccades in the periphery. Thus our results were not driven by eye movements or shifts of overt attention.
Whole brain GLM.
All whole brain activations were assessed with open contrasts of the appropriate regressor(s). Whole brain cue-evoked fMRI activity (pooled across 9-, 13-, and 15-s delay duration trials) is presented for completeness in Table 2 but is not evaluated further as it was not the primary focus of this research. Similarly, whole brain target/trial end activity is not analyzed further as it is not pertinent to the main goal of the study and as it led to extensive cortical activation in the occipital, temporal, parietal, and frontal lobes, as well as to subcortical activity.
Table 2.
Whole brain activations by task phase
| Peaks |
Center of Mass |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | Region | Volume (Functional Voxels) | X | Y | Z | t | P | X | Y | Z |
| Cue period | ||||||||||
| R | Putamen | 51 | 21 | 14 | 10 | 6.40 | 0.00013 | 21 | 7 | 3 |
| R | Inferior frontal junction | 4 | 36 | 8 | 22 | 4.13 | 0.00255 | 34 | 6 | 21 |
| R | Frontal eye field | 115 | 19 | −1 | 56 | 5.29 | 0.00050 | 25 | −2 | 49 |
| R | Thalamus | 13 | 5 | −25 | 21 | 4.17 | 0.00240 | 7 | −26 | 20 |
| R | Thalamus | 35 | 7 | −28 | 8 | 5.89 | 0.00023 | 6 | −28 | 10 |
| R | Intraparietal sulcus/precuneus/medial superior parietal lobule | 111 | 19 | −63 | 41 | 6.11 | 0.00018 | 16 | −62 | 42 |
| R | Occipital | 22 | 33 | −89 | −4 | 5.50 | 0.00038 | 31 | −87 | −3 |
| L | Middle frontal gyrus | 6 | −36 | 38 | 34 | 4.00 | 0.00310 | −36 | 38 | 35 |
| L | Anterior cingulate | 5 | −12 | 15 | 35 | 3.58 | 0.00594 | −10 | 16 | 33 |
| L | Anterior cingulate | 61 | −3 | 9 | 43 | 5.73 | 0.00029 | −4 | 6 | 41 |
| L | Putamen | 103 | −26 | 8 | 5 | 7.03 | 0.00006 | −24 | 11 | 4 |
| L | Caudate | 4 | −3 | 8 | 8 | 4.53 | 0.00143 | −3 | 7 | 9 |
| L | Thalamus | 3 | −2 | −4 | 1 | 3.72 | 0.00476 | −2 | −5 | 1 |
| L | Frontal eye field/inferior frontal junction | 236 | −26 | −6 | 49 | 8.38 | 0.00002 | −30 | −4 | 44 |
| L | Cerebral peduncle | 6 | −9 | −16 | −13 | 4.21 | 0.00229 | −11 | −17 | −14 |
| L | Thalamus | 7 | −9 | −25 | 19 | 6.79 | 0.00008 | −12 | −28 | 17 |
| L | Thalamus | 7 | −11 | −31 | 7 | 7.56 | 0.00004 | −11 | −33 | 6 |
| L | Superior colliculus | 4 | −6 | −31 | 1 | 4.36 | 0.00183 | −6 | −34 | 0 |
| L | Occipital | 49 | −32 | −67 | 17 | 8.25 | 0.00002 | −31 | −69 | 21 |
| L | Intraparietal sulcus/precuneus/medial superior parietal lobule | 443 | −17 | −73 | 45 | 8.90 | 0.00001 | −20 | −63 | 44 |
| L | Occipital | 21 | −33 | −85 | −10 | 4.43 | 0.00166 | −33 | −87 | −7 |
| Delay period | ||||||||||
| R | Anterior insula | 23 | 30 | 17 | 4 | 4.07 | 0.00279 | 29 | 19 | 4 |
| R | Precentral gyrus | 9 | 60 | 9 | 28 | 4.14 | 0.00253 | 59 | 8 | 25 |
| R | Superior frontal gyrus | 6 | 15 | 8 | 54 | 3.90 | 0.00361 | 16 | 6 | 55 |
| R | Frontal eye field | 25 | 39 | −4 | 46 | 5.36 | 0.00045 | 35 | −7 | 44 |
| R | Intraparietal sulcus/precuneus/medial superior parietal lobule | 67 | 13 | −54 | 40 | 7.33 | 0.00004 | 19 | −56 | 42 |
| R | Occipital | 4 | 28 | −70 | 19 | 3.93 | 0.00347 | 29 | −70 | 18 |
| L | Frontal eye field | 17 | −24 | −10 | 43 | 4.41 | 0.00170 | −26 | −9 | 38 |
| L | Intraparietal sulcus | 11 | −24 | −48 | 41 | 3.89 | 0.00370 | −24 | −52 | 44 |
Each task phase is tested for activation as described in results. In brief, 9-, 13-, and 15-s regressors were tested via a pooled open contrast for the cue and delay task phases. The target/trial end phase is not presented, as it led to extensive activation throughout cortex. Volume is in 3-mm isotropic (i.e., 27 mm3) functional voxels. L, left; R, right.
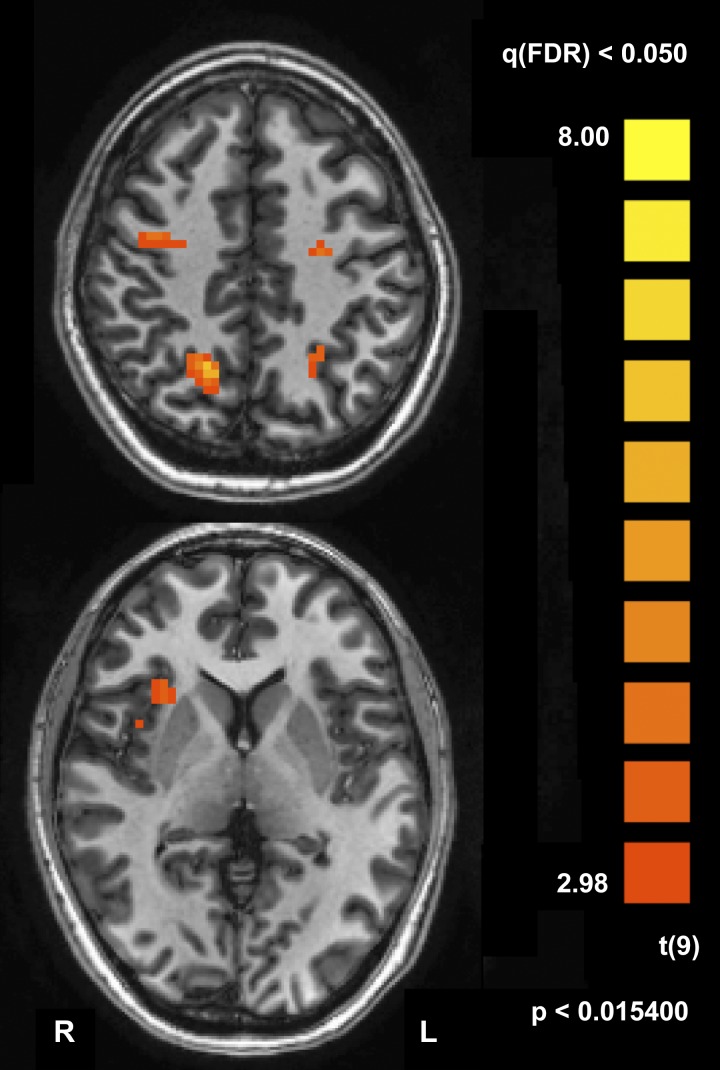
Unlike cue- or target-evoked activity, there were only a few regions that displayed delay activity (analyzed pooled across 9-, 13-, and 15-s trials; see Table 2 and Fig. 3). These regions were primarily situated in the dorsal attention network, namely, the bilateral FEF (right inferior FEF; see Silver and Kastner 2009) and IPS (compare Table 2 and Fig. 3 to localizer-defined ROIs in Table 1). A full description of regions activated during the delay is presented in Table 2.
Fig. 3.
Delay-period activity. Group statistical map of the open contrast of delay activity, i.e., delay > baseline, pooled for 9-, 13-, and 15-s delay trials. We observed delay-period activity in the bilateral frontal eye field and intraparietal sulcus as well as the right anterior insula. Map is thresholded at false discovery rate-corrected q < 0.05. Bottom: Talairach Z coordinate = 6. Top: Z = 42.
Another region in which we observed delay activity in the whole brain GLM was the right anterior insula (AI). AI delay activity either could reflect non-spatially specific arousal or vigilance related to sustaining attention over the delay period or could reflect spatially specific attentional deployments—a process we hypothesize to be primarily embodied in the dorsal network. Alternatively, our results are also consistent with the recent hypothesis that the AI may serve an alerting function to the (potential) presence of a behaviorally meaningful event (e.g., Han and Marois 2014; Han et al. 2018). We do not discuss these AI results further because they deviate from the primary goal of this paper—to dissociate IFJ from the dorsal attention network.
Region of interest GLMs.
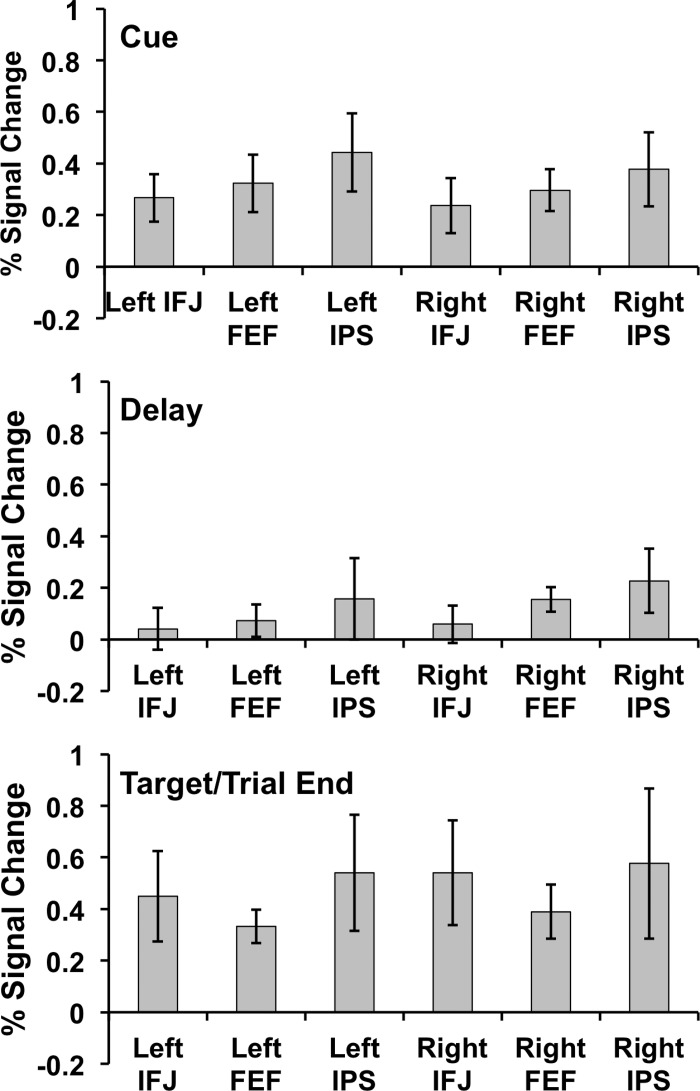
We also examined GLM parameter (beta) weights in the localizer-defined IPS, FEF, and IFJ ROIs (see Table 1) by fitting GLMs (with regressors identical to the whole brain GLM) to signal extracted from each ROI. In each region, we observed significant cue- and target/trial end-related activation [all t(9) > 3.036, all P < 0.014, except right IFJ cue-related activity, t = 1.974, P = 0.080]. In contrast, delay activity was more variable across regions: In the dorsal network, delay activity was significant in each region [all t(9) > 3.208, all P < 0.011], but in IFJ significant delay activity was observed only in the right hemisphere (right IFJ, t = 2.481, P = 0.035; left IFJ, t = 1.748, P = 0.114). These delay-period GLM results must be interpreted with caution, however, because they effectively collapse across delay time points and ignore systematic time point-to-time point variability within the delay period. Thus they may indicate above-baseline delay-period activity when, in fact, activity returns to baseline at some point during the delay. In addition, these delay-period beta estimates may be inflated by the gradually building activity we observed in anticipation of the end of the trial (see below). For these and other reasons, we next report ERA analyses that can circumvent these issues.
Event-related averages.
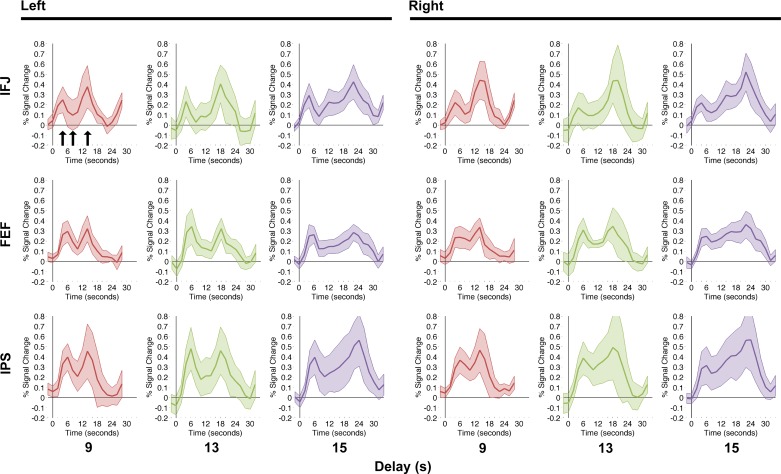
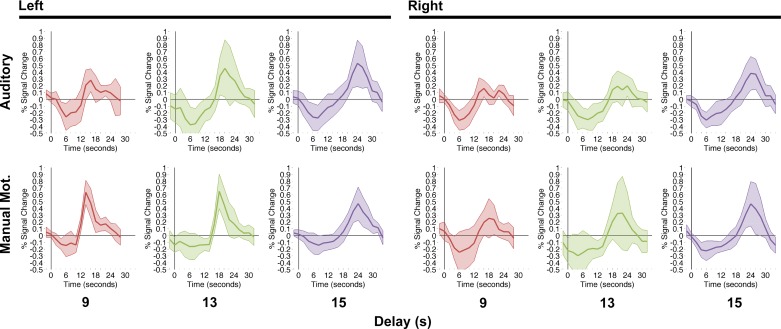
ERAs were extracted from each ROI identified via the functional localizer, separately for each delay duration (9, 13, and 15 s) in each region (IFJ, FEF, IPS) in each hemisphere (Fig. 4). Visual inspection of these plots suggests that all regions exhibited significant cue and target/trial end responses but, critically, delay responses varied across regions. Specifically, IFJ responses during the delay period approached baseline at their troughs, i.e., they had only nonsignificant or marginal differences from baseline. In FEF and IPS, we instead observed that in most delay conditions delay responses were significantly above baseline.
Fig. 4.
Event-related averages for each region of interest and delay condition, 9, 13, and 15 s. Error bands represent 95% confidence intervals against 0. The 3 arrows in top left panel mark the group peak cue response, group trough delay response, and group peak target/trial end response for illustrative purposes. Peaks and troughs for analysis were identified separately in each subject in each condition. IFJ, inferior frontal junction; FEF, frontal eye field; IPS, intraparietal sulcus.
To determine whether these results hold under greater statistical scrutiny, we performed more systematic analyses of ERA responses to each trial phase in each brain region in each hemisphere (see methods). These analyses sought to answer three questions: First, does each region follow a transient activation pattern (i.e., response peaks for cue and target/trial end coupled with a return to baseline during the delay period) or does it also show sustained activity (i.e., significantly above-baseline ERAs at the subject-specific trough of the delay period activity)? Second, do regions significantly differ in amplitude of delay activity, i.e., is IFJ activation significantly less sustained than dorsal network activation? Third, can low delay activity in an ROI (e.g., IFJ) be explained by overall low activation of this ROI by the task; i.e., do cue and target/trial end peak amplitude predict delay trough amplitude? Each of these additional analyses was run on the subject-specific ERA peaks and troughs corresponding to cue, delay, and target/trial end trial phases (Fig. 5),1 as detailed in methods. Finally, after these three analyses, we examined the timing of delay-period troughs to ask whether delay-period and target/trial end activity could reflect processes other than sustained spatial attentional maintenance and target detection, respectively.
Fig. 5.
Event-related average signal maxima and minima at subject-specific time points in each trial phase. Bars represent mean across subjects at the subject-specific maximum or minimum in each region for each trial phase, collapsed over the 9-, 13-, and 15-s delay conditions. See text for details. Error bars represent 95% confidence intervals against 0. IFJ, inferior frontal junction; FEF, frontal eye field; IPS, intraparietal sulcus.
ERAs: Transient vs. sustained regional activation patterns.
To determine whether each region follows a transient or sustained activation pattern, we performed a series of 18 t-tests, one for each combination of region (IFJ, FEF, IPS), hemisphere (right, left), and task phase (cue, delay, target/trial end). We evaluated these tests against a Bonferroni-corrected α of 0.05 ÷ 18 = 0.002778. For this analysis, we considered a region to show a transient pattern of responses if it had significant cue and target/trial end activity. If a region showed delay activity as well as transient activity, we considered it to show sustained activity as well as transient activity. No region tested showed sustained activity without transient activity. Both right and left IFJ followed a transient-only pattern of activation: cue and target/trial end peaks were significantly elevated from baseline [all t(9) > 5.03, all P < 0.00071], and delay troughs were not significantly above baseline [all t(9)s < 1.83, all P > 0.1019]. To more directly evaluate whether we should accept the null hypothesis (no sustained delay activity) in IFJ given the nonsignificant P values, we additionally applied a Bayesian t-test approach that produces Bayes factors instead of P values. Such Bayes factors can be thought of as odds ratios in favor of either the null (no transient or no sustained delay activity) or alternative (transient or sustained delay activity) hypothesis. Applying this analysis (Rouder et al. 2009; all calculation performed with the tool at http://pcl.missouri.edu/bf-one-sample) revealed high odds in favor of transient cue and target/trial end responses in IFJ (all Bayes factors > 54.5 in favor of alternative) but little evidence for above-baseline delay activity in IFJ [right hemisphere, Bayes factor = 1.04, suggesting even odds; left hemisphere, Bayes factor = 0.523, suggesting that it was twice as likely that there was no delay activity (null) than that there was delay activity].
In contrast, right hemisphere dorsal network regions (right FEF and right IPS) exhibited a clear sustained activation pattern: Cue and target/trial end peaks were significantly elevated from baseline [all t(9) > 4.47, all P < 0.001534; Bayes factors > 28.63], but so were delay responses [all t(9) > 4.12, all P < 0.002562; Bayes factors > 18.74]. In the left hemisphere, dorsal network results were more ambiguous: Despite significant cue and target/trial end responses [all t(9) > 5.43, all P < 0.0004122; Bayes factors > 85.89], delay responses did not survive correction for multiple comparisons [left FEF: t(9) = 2.6038, P = 0.028558; left IPS: t(9) = 2.2655, P = 0.049724] and the Bayesian analysis revealed that sustained activity was only approximately two to three times as likely as not (Bayes factors: FEF, 2.71; IPS, 1.77). Thus each hemisphere’s IFJ followed a transient activation pattern, right FEF and IPS followed a clear sustained activation pattern, and left FEF and IPS likely followed a sustained pattern as well, albeit more weakly. Such dorsal network hemispheric asymmetries have been observed previously by neuroimaging (e.g., Corbetta et al. 2002) as well as in a recent transcranial magnetic stimulation study (Esterman et al. 2015).
ERAs: Regional differences in delay-period activity.
Because the goals of this study were not merely to characterize the transient and sustained activation patterns in each attention region but also to determine the relative strength of the sustained activation components in each brain region, we sought to directly compare activation patterns between IFJ, FEF, and IPS. Separately for each hemisphere, we compared delay-period trough activity between IFJ, which showed a transient activation pattern in the previous analysis, and FEF and IPS, which showed sustained delay activation. Each hemisphere-based ANOVA considered only delay-period activity, resulting in one-way repeated-measures ANOVAs with a single factor (region) with three levels (IFJ, FEF, or IPS). The effect of region was significant in the right hemisphere [F(2,9) = 5.2568, P = 0.01592] but not in the left hemisphere [F(2,9) = 2.0038, P = 0.1638]. We elaborated these results using three contrasts in the context of these ANOVAs: We first compared delay activity in IFJ to delay activity pooled across the dorsal network to maximize power for this comparison. Then, we compared IFJ to FEF and IPS individually to add specificity to our results. We evaluated these contrasts against a Bonferroni-corrected α of 0.05 ÷ 3 = 0.01667 because we performed three contrasts in the context of each ANOVA.
In the right hemisphere, IFJ exhibited significantly less delay activity than the dorsal network [F(1,18) = 8.5847, P = 0.003305], bolstering the claim that right IFJ activation is more transient than dorsal network activity. In a direct comparison to IPS, IFJ exhibited significantly less delay activity [F(1,18) = 10.4449, P = 0.001576]. IFJ also exhibited less delay activity than FEF [F(1,18) = 3.3966, P = 0.04132], although this difference did not survive correction for multiple comparisons. In the left hemisphere, the comparison of delay activity from IFJ to pooled delay signal from the dorsal network was not significant [F(1,18) = 1.9928, P = 0.1028], nor was the direct comparison of IFJ and FEF (F < 1). Left IFJ did exhibit less delay activity than left IPS [F(1,18) = 3.7336, P = 0.03398], although this difference did not survive multiple-comparisons correction. Critically, these nonsignificant results in the left hemisphere do not imply sustained delay activity in left IFJ, as shown by the absence of left IFJ delay activity vs. baseline reported above [t(9) = 1.1442, P = 0.2821]. Instead, they indicate that left hemisphere dorsal network activity is not significantly more sustained than left IFJ activity. The absence of significantly greater delay activity in the dorsal network compared with IFJ may be driven by the weak sustained activity in left FEF and IPS compared with pretrial baseline (see above).
We also addressed regional differences in delay-period activity with Bayesian t-test analysis (Rouder et al. 2009; see above). Specifically, we compared evidence for delay activity across regions by taking the ratio of the Bayes factors in favor of delay activity in each region. This analysis revealed that in the left hemisphere FEF is 5.18 times as likely to have sustained activity as IFJ and IPS is 3.38 times as likely to have sustained activity as IFJ. In the right hemisphere FEF is 546.62 times as likely to have sustained activity as IFJ, and IPS is 18.01 times as likely to have sustained activity as IFJ.
Finally, we considered whether the differences between IFJ and FEF/IPS delay activity could be due to non-task-related factors such as intrinsic differences in hemodynamic response properties. If that were the case, such differences should still be present at other time points of the task, namely during the cuing and response stages. We therefore assessed whether IFJ activity in each trial phase differed from FEF/IPS activity (as it did during delay activity) by performing two-way ANOVAs (1 per hemisphere) with factors region (IFJ, FEF, IPS) and trial phase (cue, delay, target/trial end) on the peaks/trough of the ERA time courses. In the right hemisphere, we observed a significant main effect of trial phase [F(2,9) = 12.7929, P = 0.0003494], a nonsignificant effect of region [F(2,9) = 2.4485, P = 0.1147], and a significant interaction between these factors [F(4,18) = 6.5411, P = 0.0004602]. In the left hemisphere, we observed a significant main effect of region [F(2,9) = 21.1531, P = 1.8802 × 10−5], a marginal main effect of trial phase [F(2,9) = 3.0772, P = 0.07087], and a marginal interaction [F(4,18) = 2.5635, P = 0.05481]. Follow-up contrasts indicated that the modulation of signal by trial phase was greater in right IFJ than in the dorsal network of FEF and IPS [F(1,36) = 7.9607, P = 0.003486]. We therefore conclude that the delay activity differences between IFJ and the dorsal regions do not reflect only general, non-task-specific processes, as these would drive a main effect of region but not an interaction of region × trial phase. Instead, we surmise that the delay activity likely reflects genuine differences of task involvement between regions.
ERAs: Does low task-related IFJ activation explain low IFJ delay activity?
It is plausible that we found an absence of delay activity in IFJ simply because IFJ is only weakly recruited by the present task. On this account, the amplitude of the entire time course of IFJ signal should be feeble, and the absence of delay activity would reflect insufficient power to detect a small effect at that stage. The region × trial phase ANOVA described in the immediately preceding section does not support this argument, but here we addressed this concern more directly. We reasoned that if low IFJ delay activity actually reflected overall low IFJ responsiveness to the task, the ERA magnitude at the trough of the delay period should be correlated with the ERA magnitudes at the peak of the cue and/or target/trial end response. We expect this correlation because, on such an account, the subjects with the smallest IFJ signal during any one phase of the task should also show reduced IFJ signal during other task phases. Thus we correlated IFJ signal at the peaks of the cue and target/trial end periods with the delay trough. To accomplish this, we pooled across delay conditions by taking the mean of the 9-, 13-, and 15-s delay peaks and troughs. This analysis revealed that IFJ delay trough magnitudes were neither significantly correlated with the cue peak magnitude (left IFJ, r = 0.50309, P = 0.13828; right IFJ, r = 0.3294, P = 0.35266) nor significantly correlated with the target/trial end peak magnitude (left IFJ, r = 0.034373, P = 0.9249; right IFJ, r = −0.018075, P = 0.96047). Thus the fact that IFJ delay troughs were statistically indistinguishable from pretrial baseline is not attributable to overall low task-evoked activation of IFJ.
ERAs: What do delay and target/trial end activations signify?
Inspection of ERAs in Fig. 4 makes clear that the delay-period ERA response trough in each region occurred early during the delay period regardless of its duration, with the signal beginning to increase above the trough before that signal could have been driven by target onset or trial end. If the time of group troughs is averaged over regions and hemispheres separately for each delay condition, the mean trough times for 9-, 13-, and 15-s delay trials are 9.33, 9.67, and 8.33 s, respectively. That the trough latency does not increase with increasing delay durations runs contrary to the expectation that delay-period ERAs should have sustained positive activation (for sustained attention regions, i.e., the dorsal network) or (for transient regions, i.e., IFJ) activation that decreases toward baseline over the length of each delay period until the expected trough of the cue-evoked signal (~16 s after cue onset; Boynton et al. 1996; also see SPM software canonical fMRI hemodynamic response function, https://www.fil.ion.ucl.ac.uk/spm/). Instead, we observed ERAs increasing for the remainder of each trial after these early troughs. It is possible that these increasing ERAs reflected anticipation of the target or trial end (akin to top-down activity due to anticipation of event boundaries in cortex near IFJ; see Zacks et al. 2001) or transitions between high- and low-demand task states (Konishi et al. 2001; Shulman et al. 2002). Although Shulman et al. (2002) argued that fMRI activation locked to the end of attention-task trials reflected “termination of a sustained state of readiness” (p. 598), they used short delay periods that could not discern whether the rise of activity toward the target/trial end peak began at a time locked to cue presentation rather than trial end. Here, based on clear evidence of signal rising before target/trial end, we speculate that this late ERA peak could reflect preparation for the end of the trial, not just a response triggered directly by the end of a trial. Our observation of potentially anticipatory activity in the brain is surprising given that we manipulated the hazard rate (i.e., the probability that a target would occur during the next possible delay duration time bin) to be constant across trials and even more surprising given that we observed no behavioral correlate of anticipation—recall that the cue validity effect size did not change over delay period durations (see Fig. 2). Thus the timing of the ERA troughs combined with the absence of an effect of delay period duration on cue validity response time effects suggests that this rising BOLD activity may be unrelated to behavioral anticipation. To determine whether this brain-behavior dissociation is specific to brain regions underlying attention or reflects a more general phenomenon (e.g., arousal), we examined ERAs in two additional control ROIs derived from coordinates reported in Dux et al. (2009) (see Table 3): auditory cortex (AudC) and manual motor cortex (MMC). We reasoned that AudC should not be modulated by the present task because it lacked an auditory component and MMC would be expected to show task modulation only in the left hemisphere, as the present task required only right-handed button presses. In both hemispheres, AudC activity dropped below pretrial baseline consequent to cue presentation but eventually rose to a peak whose timing was consistent with the target/trial end phase of the task (Fig. 6). Although left MMC revealed the expected pattern for trials requiring a button press—baseline activity until a peak consistent with motor response execution—we unexpectedly observed a similar peak in left MMC during the 15-s delay (no target and, critically, no response) trials. In addition, right MMC revealed a late peak in all conditions (although this peak did not consistently exceed baseline), even though no left-handed button presses were required. Thus widespread regions of cortex reflect rising activity before the target or trial end, leading us to report a brain-behavior dissociation that is consistent with generalized arousal building over the course of a trial before its end. However, this rising activity does not correspond to task performance, as cue validity effects on response times were of constant magnitude across delay durations (Fig. 2). We note that there is no evidence to suggest that this rising activity reflects conscious expectation or preparedness for a target; instead, the presence of this effect in brain regions that are seemingly unrelated to the task (e.g., AudC) suggests a general arousal origin, perhaps to counteract task fatigue. It is also possible that this signal buildup reflects increased difficulty in maintaining the task set over an extended period of time, but that is unlikely to account for the entire effect because we observed increasing activation outside of brain regions likely to be involved in maintaining the present task set.
Table 3.
Control ROIs
| Talairach Coordinates |
|||
|---|---|---|---|
| ROI | X | Y | Z |
| L auditory cortex | −51 | −20 | 10 |
| R auditory cortex | 54 | −20 | 10 |
| L manual motor cortex | −34 | −20 | 53 |
| R manual motor cortex | 34 | −20 | 53 |
These were 33 resampled functional voxel spheres centered on Talairach coordinates derived from Dux et al. (2009). Because Dux et al. did not report coordinates for right manual motor cortex, we mirrored the left hemisphere coordinates to obtain a right manual motor cortex region of interest (ROI). L, left; R, right.
Fig. 6.
Event-related averages in auditory and manual motor cortex for delay condition, 9, 13, and 15 s. Error bands represent 95% confidence intervals against 0.
DISCUSSION
The main goal of the present study was to tease apart the distinct roles in top-down attention played by IFJ on the one hand and the classic dorsal attention network of FEF and parietal cortex on the other. In recent years, a variety of functions for IFJ have been proposed, including the coordination of the dorsal top-down and ventral bottom-up attention networks (Asplund et al. 2010); attention to and evaluation of salient stimuli (Han and Marois 2014); coordination of the flow of information among brain networks according to task goals (Cole et al. 2013); integration of, and switching between, information representations in support of task sets (Brass et al. 2005; Vergauwe et al. 2015); rule updating (Montojo and Courtney 2008); and response selection and stimulus-response mapping (Dux et al. 2006, 2009; Ivanoff et al. 2009; Marois and Ivanoff 2005; Tamber-Rosenau et al. 2013; Tombu et al. 2011). Several of these processes ascribed to IFJ are not independent of one another and may relate to a more fundamental computation that has yet to be fully described. Thus the specific computation(s) of IFJ remains ambiguous. Our direct comparison of IFJ to the dorsal attention network—in the context of a visuospatial attention task whose neural correlates can unambiguously be ascribed to cue, delay, or target/trial end processes—represents a significant step toward resolving this ambiguity. We found that IFJ and the dorsal network both exhibit activity during the cue and target/trial end task phases but that IFJ activates far less than the dorsal network for attentional maintenance during the delay period. Thus our results clarify these regions’ differential involvement in top-down attention.
IFJ primarily supports transient, not sustained, attentional processes.
Two results suggest that IFJ does not support sustained spatial attentional processes: First, IFJ delay-period activity is statistically indistinguishable from baseline levels. Second, IFJ is less active than the dorsal network during the delay period. The finding that IFJ is less active than the dorsal network is important because the former finding, that IFJ delay activation is statistically indistinguishable from pretrial baseline, relies on a null effect (although this null is supported by a Bayesian analysis as well). In addition, even if the absence of sustained IFJ delay-period activity were merely due to low statistical power instead of a true return of activation to baseline, it would not necessarily mean that IFJ is involved in the sustained maintenance of attention because delay-period activity also included activation in anticipation of trial end (see results), not just activation due to sustained maintenance of spatial attention. From these results we conclude that IFJ does not readily support sustained spatial attentional processes (although we note that the presence of rising activity across the brain over the long trial durations complicates the interpretation that IFJ is restricted to exclusively transient activity). This conclusion may at first appear to conflict with previous studies (e.g., Chiu and Yantis 2009; Greenberg et al. 2010; Han and Marois 2014; Ikkai and Curtis 2008; Serences et al. 2005b; Shulman et al. 2009), but apparent sustained activity in IFJ in these attention studies may have instead been driven by repeated perceptual transients or task-relevant events (see introduction). Our conclusion may also seem at odds with working memory (WM) studies suggesting a role of IFJ in the maintenance of information during a WM delay period (Courtney et al. 1998; Kastner et al. 2007; Roth et al. 2006; Srimal and Curtis 2008; Todd et al. 2011). However, sustained WM activity in IFJ and/or neighboring areas is not a universal finding (Cole and Schneider 2007; Courtney et al. 1998; Sreenivasan et al. 2014; Srimal and Curtis 2008), and it has been argued that lateral prefrontal activation does not represent sustained item information in WM (Mackey et al. 2016; Sreenivasan et al. 2014). Some of the studies showing sustained IFJ involvement with WM maintenance contained perceptual transients and/or task-related items, which could potentially explain IFJ WM delay activity. It is also possible that information manipulation, updating, or other transient WM processes known to activate IFJ (Barber et al. 2013; Mohr et al. 2006; Vergauwe et al. 2015) have been confounded with sustained WM activity. Perhaps most importantly, even the studies that showed sustained IFJ activity in the absence of any confounds from WM maintenance (e.g., Todd et al. 2011) are not inconsistent with the present results. In accord with models proposing that multiple stimuli are maintained in WM via repeated cycling of transient attentional deployment (e.g., Barrouillet et al. 2004; Johnson et al. 2005; Vergauwe et al. 2015), it is possible that IFJ is repeatedly activated during WM delays. In that context, the lack of sustained IFJ activity in the present attention paradigm would arise from the lack of any necessity to engage an attention-refreshing process when there are no stimuli to maintain in WM.
Although IFJ delay activity minima were statistically indistinguishable from pretrial baseline, IFJ was involved in both cue and target/trial end task phases, driving the conclusion that IFJ supports transient attentional processes. Cue-evoked activation should have included at least two such transient processes: cue interpretation and a spatial attention shift from central fixation to the cued peripheral location. The target/trial end activation should include the transient processes of target detection and response execution, followed by the transition from a state of readiness during the task to a less vigilant state during the intertrial interval (Konishi et al. 2001; Shulman et al. 2002). Coupled with previous findings that IFJ coordinates with either the dorsal or ventrolateral attention network depending upon task context (Asplund et al. 2010), these results are consistent with IFJ playing a general role in transient attentional processes such as cue interpretation, orienting, target detection, or response selection/execution. Thus it seems clear that IFJ’s primary role in attention is one of transient shifting and reconfiguration, not sustained maintenance.
Because a good deal of prior research has examined the role of lateral prefrontal cortex in nonhuman primates—including evidence that portions of lateral prefrontal cortex exhibit sustained activity during task delay periods (e.g., Fuster and Alexander 1971; Goldman-Rakic 1987, 1995; Lara and Wallis 2014)—it would be advantageous to be able to relate the present results to nonhuman primate research on the function of IFJ. Unfortunately, putative monkey homologs of IFJ are in close proximity to FEF and dorsolateral prefrontal cortex (Diamond 2006; Nakahara et al. 2002; Neubert et al. 2014), two regions that are functionally distinct from IFJ, and nonhuman primate experiments generally do not target the IFJ separately from these regions. One important sequence of studies avoided this pitfall, however, by relating monkey lesions directly to human brain imaging: Consistent with our conclusion that IFJ supports transient attentional reconfiguration, Rossi et al. (2007) demonstrated that unilateral lesions of macaque lateral prefrontal cortex led to hemifield-specific deficits in reconfigurations of attentional set but not simply in the maintenance of such set. Rossi et al. (2009) went on to use the same task with fMRI in healthy humans to demonstrate elevated activation in IFJ for trials demanding such attentional reconfiguration, suggesting that the present results may be applicable to nonhuman primate as well as human models of attentional control.
Additional prior research has examined the role of lateral prefrontal cortex in humans in patient populations with localized brain lesions. Such studies have an advantage over functional neuroimaging in that they are more likely to reflect causal mechanisms. However, we know of no human lesion studies in which effects can be uniquely attributed to IFJ rather than surrounding cortex. That said, extant human lesion studies are broadly consistent with our results: Card sorting and Stroop-like tasks have demonstrated that the left lateral prefrontal cortex plays a role in stimulus-response mapping, task setting, and control (Alexander et al. 2007; Shallice et al. 2008a, 2008b; Stuss 2006, 2011; Stuss et al. 2000), consistent with neuroimaging suggesting a key role for left IFJ in response selection (Dux et al. 2006, 2009). The same lesion studies suggest that lateral prefrontal cortex is associated with monitoring task demands and sustaining attention (Alexander et al. 2007; Shallice et al. 2008a, 2008b; Stuss 2006, 2011; Stuss et al. 2000) or with inhibiting outdated response mappings and task sets (Aron et al. 2004a, 2004b), both of which are somewhat consistent with a role for right IFJ in transiently reconfiguring attentional deployment when task demands change, as we observed in the present study. In contrast to these studies, we did not observe sustained right IFJ activity, suggesting that sustained attention may be a property of portions of the right lateral prefrontal cortex other than IFJ. We should also note that these lesion patient studies inferred sustained attention deficits from prolonged response times (Alexander et al. 2007) or increased errors (Shallice et al. 2008b; Stuss et al. 2000) during the performance of dynamic tasks. Given the dynamic nature of the tasks used, it is difficult to unambiguously attribute the performance changes to sustained attention.
Dorsal attention network supports sustained spatial attention.
Our finding of sustained FEF and IPS activity over delay periods during attention tasks is consistent with many previous studies (Chiu and Yantis 2009; Corbetta et al. 2000, 2002; Greenberg et al. 2010; Hopfinger et al. 2000; Ikkai and Curtis 2008; Kastner et al. 1999; Kelley et al. 2008; Offen et al. 2010; Serences et al. 2005a, 2005b; Shulman et al. 2009, 2010; Tamber-Rosenau et al. 2011; Yantis et al. 2002). However, as detailed in introduction, these previous studies used paradigms that leave open plausible accounts of sustained dorsal network activity other than, or in addition to, the maintenance of attention in the visual periphery. In brief, these accounts include 1) reliance on WM rather than attention, a possibility made more plausible in studies that did not demonstrate behavioral correlates of sustained attention, and 2) repeated transient target- or distractor-evoked activations. In contrast to these previous experiments, in the present experiment sustained signals in the dorsal network 1) cannot be explained by WM (or by general arousal) separate from a concurrent spatial attentional deployment, because we observed faster responses at validly cued locations for all delay period durations, and 2) cannot be attributed to target or distractor processing because there were no targets or distractors during the delay. Thus we do not merely present evidence of sustained signals in FEF and IPS, particularly in the right hemisphere, throughout the delay period; we present this evidence in a context where we can unambiguously link sustained signals to visuospatial attention. We note that our finding of sustained dorsal network activity is not novel, but it is reassuring that we observed such sustained activity in the same experiment in which we observed only transient IFJ activity.
Unlike IFJ (see above), both FEF and IPS have clear nonhuman primate homologs (Courtney et al. 1998; Culham and Kanwisher 2001; Petit et al. 1993, 1996) that have been extensively investigated. Our dorsal network sustained spatial attention results accord with this nonhuman primate research. In particular, attentional priority maps—whose existence was predicted by cognitive and neural models (e.g., Koch and Ullman 1985; Wolfe 1994)—have been described in nonhuman primates in IPS area LIP (Bisley and Goldberg 2003, 2010) and in FEF (Bisley 2011; Thompson et al. 2005a, 2005b; Thompson and Bichot 2005) as well as in humans (e.g., Jerde et al. 2012; Silver and Kastner 2009). Such priority maps could plausibly support temporally extended attentional deployments, like those we observed in human FEF and IPS, as well as initial orienting to locations. This nonhuman primate research, together with previous human investigations (reviewed above) and the present work, suggests that in addition to its involvement in transient cue and target/trial end processes, the classic dorsal network of FEF and IPS is associated with the sustained maintenance of visuospatial attention.
Relationship between IFJ and dorsal network.
The present results echo previous findings of IFJ coactivation with the dorsal network when top-down attentional control dominates behavior (Asplund et al. 2010). Adding to this account, we found that even under conditions of top-down attention IFJ activity resembles activity in the dorsal network only during periods of transient attentional reconfiguration, not during periods of sustained spatial attentional deployment. This is consistent with the idea that IFJ initiates attentional configurations and mediates interactions between two sources of attentional control—the dorsal and ventral attention networks (Asplund et al. 2010; Chica et al. 2013; Corbetta et al. 2008; Fox et al. 2006; He et al. 2007; Vossel et al. 2014). Network analyses also support the idea that IFJ is a hub that mediates between sources of task control, with IFJ and/or nearby cortex classified as part of a frontoparietal control network (Cole et al. 2013; Dosenbach et al. 2007, 2008) that can flexibly connect with dorsal or ventral attention networks (Spreng et al. 2010, 2013). In further accord with the idea that IFJ mediates between sources of control to initiate attentional deployments, a number of functional and structural connectivity studies have recently observed that multiple brain networks converge in IFJ (Cole et al. 2013; Power et al. 2011, 2013; Power and Petersen 2013; Yeo et al. 2011, 2014), making it an ideal location for flexible resolution of control signals. Finally, at least one directed-connectivity study has shown that IFJ transiently influences the dorsal network, at least under conditions of WM encoding (Sneve et al. 2013).
Conclusions.
The present study disambiguates previously conflated neural processes in goal-directed attention. Specifically, we suggest that the role of IFJ is mainly one of transient, dynamic changes to attentional deployments, whereas the classic dorsal attention network of FEF and IPS is involved in the maintenance of such deployments over time. However, this dissociation is not absolute, as the dorsal regions were involved in both transient and sustained processes, and we cannot exclude the possibility of weak delay-related activity in IFJ. Such a partial dissociation should not come as a surprise: While each cortical region is likely to carry out somewhat distinct computations, these regions must also share their information with other brain regions with which they are connected. Hence, the functional properties of a given region may be echoed in other regions with which it is closely associated. It is presumably via such communication between components of a neural network that coherent attention-based behavior emerges.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 MH-70776 to R. Marois and by NIH Grants 5T32 EY-007135 and P30 EY-008126 to the Vanderbilt Vision Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.A. and R.M. conceived and designed research; C.L.A. performed experiments; B.J.T.-R. and C.L.A. analyzed data; B.J.T.-R. and R.M. interpreted results of experiments; B.J.T.-R. prepared figures; B.J.T.-R. drafted manuscript; B.J.T.-R., C.L.A., and R.M. edited and revised manuscript; B.J.T.-R., C.L.A., and R.M. approved final version of manuscript.
Footnotes
We also analyzed ERAs by fitting GLMs to the ERAs and evaluating the presence of sustained vs. transient activity. These ERA-based GLMs yielded results comparable to those from the main ERA analyses presented here except that they did not take into account individual latency differences. Thus we do not further discuss these GLMs.
REFERENCES
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68: 1515–1523, 2007. doi: 10.1212/01.wnl.0000261482.99569.fb. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain 127: 1561–1573, 2004a. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177, 2004b. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci 13: 507–512, 2010. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Effects of working memory demand on neural mechanisms of motor response selection and control. J Cogn Neurosci 25: 1235–1248, 2013. doi: 10.1162/jocn_a_00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrouillet P, Bernardin S, Camos V. Time constraints and resource sharing in adults’ working memory spans. J Exp Psychol Gen 133: 83–100, 2004. doi: 10.1037/0096-3445.133.1.83. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Series B 57: 289–300, 1995. [Google Scholar]
- Bhanji JP, Beer JS, Bunge SA. Taking a gamble or playing by the rules: dissociable prefrontal systems implicated in probabilistic versus deterministic rule-based decisions. Neuroimage 49: 1810–1819, 2010. doi: 10.1016/j.neuroimage.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW. The neural basis of visual attention. J Physiol 589: 49–57, 2011. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science 299: 81–86, 2003. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221, 1996. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9: 314–316, 2005. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726, 2003. doi: 10.1016/S0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90: 3419–3428, 2003. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Chica AB, Paz-Alonso PM, Valero-Cabré A, Bartolomeo P. Neural bases of the interactions between spatial attention and conscious perception. Cereb Cortex 23: 1269–1279, 2013. doi: 10.1093/cercor/bhs087. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Yantis S. A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J Neurosci 29: 3930–3938, 2009. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16: 1348–1355, 2013. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37: 343–360, 2007. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, DeSouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol 84: 1645–1655, 2000. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297, 2000. [Erratum in Nat Neurosci 3: 521, 2000.] doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci 14: 508–523, 2002. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324, 2008. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science 279: 1347–1351, 1998. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11: 157–163, 2001. doi: 10.1016/S0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol 99: 133–145, 2008. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Vogt VL, Fiebach CJ, von Cramon DY, Tittgemeyer M. Functional organization of the left inferior precentral sulcus: dissociating the inferior frontal eye field and the inferior frontal junction. Neuroimage 59: 3829–3837, 2012. doi: 10.1016/j.neuroimage.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222, 1995. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diamond A. Bootstrapping conceptual deduction using physical connection: rethinking frontal cortex. Trends Cogn Sci 10: 212–218, 2006. doi: 10.1016/j.tics.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci 12: 99–105, 2008. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078, 2007. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron 52: 1109–1120, 2006. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron 63: 127–138, 2009. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Liu G, Okabe H, Reagan A, Thai M, DeGutis J. Frontal eye field involvement in sustaining visual attention: evidence from transcranial magnetic stimulation. Neuroimage 111: 542–548, 2015. doi: 10.1016/j.neuroimage.2015.01.044. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051, 2006. [Erratum in Proc Natl Acad Sci USA 103: 13560, 2006.] doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage 2: 157–165, 1995. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook of Physiology, The Nervous System, Higher Functions of the Brain, edited by Plum F, Mountcastle V. Bethesda, MD: American Physiological Society, 1987, p. 373–417. doi: 10.1002/cphy.cp010509. [DOI] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 14: 477–485, 1995. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci 30: 14330–14339, 2010. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Marois R. Functional fractionation of the stimulus-driven attention network. J Neurosci 34: 6958–6969, 2014. doi: 10.1523/JNEUROSCI.4975-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Eaton HP, Marois R.. Functional fractionation of the cingulo-opercular network: alerting insula and updating cingulate. Cerebr Cortex. 2018. doi: 10.1093/cercor/bhy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53: 905–918, 2007. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci 3: 284–291, 2000. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time locked to the shift and maintenance of spatial attention. Cereb Cortex 18: 1384–1394, 2008. doi: 10.1093/cercor/bhm171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff J, Branning P, Marois R. Mapping the pathways of information processing from sensation to action in four distinct sensorimotor tasks. Hum Brain Mapp 30: 4167–4186, 2009. doi: 10.1002/hbm.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE. Prioritized maps of space in human frontoparietal cortex. J Neurosci 32: 17382–17390, 2012. doi: 10.1523/JNEUROSCI.3810-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci 5: 339–361, 2005. doi: 10.3758/CABN.5.3.339. [DOI] [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol 97: 3494–3507, 2007. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761, 1999. doi: 10.1016/S0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex 18: 114–125, 2008. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3? Perception 36: 1–16, 2007. [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol 4: 219–227, 1985. [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage 13: 364–374, 2001. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci 1: 80–84, 1998. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Lara AH, Wallis JD. Executive control processes underlying multi-item working memory. Nat Neurosci 17: 876–883, 2014. doi: 10.1038/nn.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los SA, Agter F. Reweighting sequential effects across different distributions of foreperiods: segregating elementary contributions to nonspecific preparation. Percept Psychophys 67: 1161–1170, 2005. doi: 10.3758/BF03193549. [DOI] [PubMed] [Google Scholar]
- Mackey WE, Devinsky O, Doyle WK, Meager MR, Curtis CE. Human dorsolateral prefrontal cortex is not necessary for spatial working memory. J Neurosci 36: 2847–2856, 2016. doi: 10.1523/JNEUROSCI.3618-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci 9: 296–305, 2005. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Mohr HM, Goebel R, Linden DE. Content- and task-specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J Neurosci 26: 4465–4471, 2006. doi: 10.1523/JNEUROSCI.5232-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron 59: 173–182, 2008. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB. Co-activation-based parcellation of the lateral prefrontal cortex delineates the inferior frontal junction area. Cereb Cortex 26: 2225–2241, 2016. doi: 10.1093/cercor/bhv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295: 1532–1536, 2002. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7: 1–17, 2007. doi: 10.3758/CABN.7.1.1. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MF. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron 81: 700–713, 2014. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Nobre A, Correa A, Coull J. The hazards of time. Curr Opin Neurobiol 17: 465–470, 2007. doi: 10.1016/j.conb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Offen S, Gardner JL, Schluppeck D, Heeger DJ. Differential roles for frontal eye fields (FEFs) and intraparietal sulcus (IPS) in visual working memory and visual attention. J Vis 10: 28, 2010. doi: 10.1167/10.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Crivello F, Berthoz A, Mazoyer B. Functional anatomy of a prelearned sequence of horizontal saccades in humans. J Neurosci 16: 3714–3726, 1996. doi: 10.1523/JNEUROSCI.16-11-03714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Salamon G, Mazoyer B, Berthoz A. PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulate cortex involvement. J Neurophysiol 69: 1009–1017, 1993. doi: 10.1152/jn.1993.69.4.1009. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol 32: 3–25, 1980. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron 72: 665–678, 2011. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol 23: 223–228, 2013. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron 79: 798–813, 2013. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist 18: 502–515, 2012. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci 27: 11306–11314, 2007. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res 192: 489–497, 2009. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cereb Cortex 16: 1595–1603, 2006. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16: 225–237, 2009. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Serences JT, Liu T, Yantis S. Parietal mechanisms of attentional control: locations, features, and objects. In: Neurobiology of Attention, edited by Itti L, Rees G, Tsotsos JK. New York: Academic, 2005a, p. 35–41. doi: 10.1016/B978-012375731-9/50011-2 [DOI] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci 16: 114–122, 2005b. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci 10: 38–45, 2006. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Front Neurosci 2: 79–85, 2008a. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Multiple effects of prefrontal lesions on task-switching. Front Hum Neurosci 1: 2, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Kravitz DJ, Behrmann M. Attentional control: temporal relationships within the fronto-parietal network. Neuropsychologia 50: 1202–1210, 2012. doi: 10.1016/j.neuropsychologia.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci 29: 4392–4407, 2009. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Linenweber M, Petersen SE, Corbetta M. Multiple neural correlates of detection in the human brain. Proc Natl Acad Sci USA 98: 313–318, 2001. doi: 10.1073/pnas.98.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30: 3640–3651, 2010. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Tansy AP, Kincade M, Petersen SE, McAvoy MP, Corbetta M. Reactivation of networks involved in preparatory states. Cereb Cortex 12: 590–600, 2002. doi: 10.1093/cercor/12.6.590. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci 13: 488–495, 2009. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneve MH, Magnussen S, Alnæs D, Endestad T, D’Esposito M. Top-down modulation from inferior frontal junction to FEFs and intraparietal sulcus during short-term memory for visual features. J Cogn Neurosci 25: 1944–1956, 2013. doi: 10.1162/jocn_a_00426. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25: 74–86, 2013. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53: 303–317, 2010. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, D’Esposito M. Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci 18: 82–89, 2014. doi: 10.1016/j.tics.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. Neuroimage 39: 455–468, 2008. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Frontal lobes and attention: processes and networks, fractionation and integration. J Int Neuropsychol Soc 12: 261–271, 2006. doi: 10.1017/S1355617706060358. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc 17: 759–765, 2011. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38: 388–402, 2000. doi: 10.1016/S0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical, 1988. [Google Scholar]
- Tamber-Rosenau BJ, Dux PE, Tombu MN, Asplund CL, Marois R. Amodal processing in human prefrontal cortex. J Neurosci 33: 11573–11587, 2013. doi: 10.1523/JNEUROSCI.4601-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamber-Rosenau BJ, Esterman M, Chiu YC, Yantis S. Cortical mechanisms of cognitive control for shifting attention in vision and working memory. J Cogn Neurosci 23: 2905–2919, 2011. doi: 10.1162/jocn.2011.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res 147: 251–262, 2005. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol 93: 337–351, 2005a. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci 25: 9479–9487, 2005b. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Han SW, Harrison S, Marois R. The neural correlates of visual working memory encoding: a time-resolved fMRI study. Neuropsychologia 49: 1527–1536, 2011. doi: 10.1016/j.neuropsychologia.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A unified attentional bottleneck in the human brain. Proc Natl Acad Sci USA 108: 13426–13431, 2011. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillenberg P, Verleger R, Wascher E, Wauschkuhn B, Wessel K. CNV and temporal uncertainty with “ageing” and “non-ageing” S1–S2 intervals. Clin Neurophysiol 111: 1216–1226, 2000. doi: 10.1016/S1388-2457(00)00274-1. [DOI] [PubMed] [Google Scholar]
- Vergauwe E, Hartstra E, Barrouillet P, Brass M. Domain-general involvement of the posterior frontolateral cortex in time-based resource-sharing in working memory: an fMRI study. Neuroimage 115: 104–116, 2015. doi: 10.1016/j.neuroimage.2015.04.059. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20: 150–159, 2014. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hazlett CJ, Fichtenholtz HM, Weissman DH, Dale AM, Song AW. Functional parcellation of attentional control regions of the brain. J Cogn Neurosci 16: 149–165, 2004. doi: 10.1162/089892904322755638. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided Search 2.0 A revised model of visual search. Psychon Bull Rev 1: 202–238, 1994. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided Search 4.0: current progress with a model of visual search. In: Integrated Models of Cognitive Systems, edited by Gray W. New York: Oxford, 2007, p. 99–119. doi: 10.1093/acprof:oso/9780195189193.003.0008 [DOI] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002, 2002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage 88: 212–227, 2014. doi: 10.1016/j.neuroimage.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165, 2011. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Braver TS, Sheridan MA, Donaldson DI, Snyder AZ, Ollinger JM, Buckner RL, Raichle ME. Human brain activity time-locked to perceptual event boundaries. Nat Neurosci 4: 651–655, 2001. doi: 10.1038/88486. [DOI] [PubMed] [Google Scholar]