Abstract
Humans engage in many daily activities that rely on working memory, the ability to hold and sequence information temporarily to accomplish a task. We focus on the process of working memory, based on circuit mechanisms for attending to relevant signals and suppressing irrelevant stimuli. We discuss that connections critically depend on the systematic variation in laminar structure across all cortical systems. Laminar structure is used to group areas into types regardless of their placement in the cortex, ranging from low-type agranular areas that lack layer IV to high-type areas that have six well-delineated layers. Connections vary in laminar distribution and strength based on the difference in type between linked areas, according to the “structural model” (Barbas H, Rempel-Clower N. Cereb Cortex 7: 635–646, 1997). The many possible pathways thus vary systematically by laminar distribution and strength, and they interface with excitatory neurons to select relevant stimuli and with functionally distinct inhibitory neurons that suppress activity at the site of termination. Using prefrontal pathways, we discuss how systematic architectonic variation gives rise to diverse pathways that can be recruited, along with amygdalar and hippocampal pathways that provide sensory, affective, and contextual information. The prefrontal cortex is also connected with thalamic nuclei that receive the output of the basal ganglia and cerebellum, which may facilitate fast sequencing of information. The complement of connections and their interface with distinct inhibitory neurons allows dynamic recruitment of areas and shifts in cortical rhythms to meet rapidly changing demands of sequential components of working memory tasks.
Keywords: inhibitory neurons, memory, oscillations, prefrontal, structural model
OVERVIEW
Working memory refers to the temporary hold and sequencing of information to accomplish a specific task, an ability that is remarkable for its seamless fluidity and versatility as applied for a large variety of everyday tasks. In this review, we focus on the organization of fundamental networks that must be engaged to provide signals to carry out tasks in working memory. We use the extended circuits of the prefrontal cortex in primates, a region that is implicated in working memory. Successful performance of a task within working memory requires selection of relevant stimuli and suppression of irrelevant stimuli. We discuss that cortical circuits are best understood in the context of the fundamental principle of systematic variation in the laminar structure of the mammalian cortex. This discussion is predicated on the fact that cortical connections depend on the systematic variation of the cortex. Connections thus also vary systematically, giving rise to a broad spectrum of laminar patterns of connections, which interface with excitatory and functionally distinct classes of inhibitory neurons. The organization of connections within the context of systematic cortical variation provides a mechanism for dynamic recruitment of areas and layers to accomplish the task at hand.
At the level of circuits, pathways from areas associated with working memory innervate excitatory neurons at the site of termination, which likely select relevant stimuli. Inhibition of irrelevant stimuli is also important for working memory but has received scant attention at the level of circuits. In this review we focus on both pathways for selection of relevant stimuli and suppression of irrelevant stimuli. This analysis is based on the extensive prefrontal network in primates and on the long-standing research in working memory in this region and species. We provide a brief comparison of key cortical inhibitory systems in primates and rodent species to highlight key differences in view of the surge of optogenetic studies that engage or disengage inhibitory systems, especially in mice.
TAPPING INTO DIVERSE SOURCES OF SIGNALS FOR WORKING MEMORY
Discussions of memory processes in the literature have often emphasized differences in memory systems. A classical distinction emerged from the famous study of H.M., who was unable to retain new information after bilateral resection of the hippocampus for relief of intractable seizures. Later studies showed that the hippocampus, most of which was actually spared in H.M. [reviewed in Corkin (2013)], was not the only structure that was damaged, but also included the amygdala and adjacent medial temporal areas (Corkin et al. 1997); the latter are now recognized for their role in long-term memory (Squire and Zola-Morgan 1991; Squire et al. 2004). One of the most striking findings of the early work by Brenda Milner was that H.M. was able to acquire a “procedural” memory task, by following a sketch with a pencil in the mirror, even though he could not remember that he had already practiced and acquired the skill [reviewed in Milner (2005)]. This skill and other motor-related skills, such as riding a bicycle, fall under procedural memory. Remembering to have learned the skill falls under long-term memory. The distinction of procedural memory from long-term memory of autobiographical events is one of the highlights and clearest distinctions in the study of memory. There is strong evidence that these two types of memory are differentially affected in brain diseases. Thus, whereas long-term memory is disrupted in some neurodegenerative diseases, procedural memory is largely spared, although in some apraxias it is aspects of procedural memory that are affected or lost (Packard and Knowlton 2002).
Working memory as classically defined refers to temporarily remembering and sequencing information to accomplish the task at hand, but in a broader sense, and in everyday tasks, individuals frequently tap into long-term memory to select, and then within working memory maintain and sequence information to perform, a task. This information may be obtained from existing cognitive maps, prior knowledge, and long-term memory (Eichenbaum 2017; Manns and Eichenbaum 2009). This cumulative information lays a foundation with experience and acts as a network of road maps, allowing selection of specific routes to take for a specific task to solve novel problems within working memory (Miller and Cohen 2001). Let us consider a task that falls under working memory, the preparation of a meal. It quickly becomes clear that information must be retrieved from past experience and knowledge. To locate the ingredients and the pots and pans, one must engage the long-term memory network. Thus, whereas following steps in a recipe falls under working memory, an amnesic individual or one with neurodegenerative disease would be challenged to initiate the task.
The sources of information that must be tapped for specific tasks are numerous. To understand their organization, we first must describe the laminar structure of the cerebral cortex and how it is related to patterns of connections and, functionally, to the flow of sensory information. We must then describe cortical inhibitory systems, which are essential for suppressing irrelevant information for working memory tasks. Retrieving distributed information requires attention to essential elements and suppression of irrelevant information that impinges constantly from the external world or from internal states of thoughts, drives, or feelings. Finally, we consider the likely contribution of subcortical circuits to working memory, including the thalamus, amygdala, hippocampus, basal ganglia, and cerebellum.
The extended network that contributes information for working memory is thus large, specialized, and complementary. Cortical pathways convey information from sensory and association areas. The hippocampus and amygdala convey signals pertaining to the contextual and affective significance of stimuli and events, which are essential for even simple tasks and decisions in everyday life. Thalamic nuclei associated with the prefrontal cortex receive distilled information from the entire cortex via the output of the basal ganglia and the cerebellum. The selective projection from the output of the basal ganglia to frontal cortex likely facilitates the seamless sequencing of information for working memory tasks, as elaborated below.
RECRUITMENT OF CORTICAL AREAS FOR WORKING MEMORY TASKS
The selection of relevant information for working memory tasks by prefrontal areas proceeds through its widely distributed excitatory connections with a host of other cortical and subcortical structures. These other areas process information pertaining broadly to the sensory environment and to the internal environment of motives, drives, and context. Prefrontal pathways interface with excitatory neurons that likely facilitate selection of relevant stimuli, as well as with diverse inhibitory neurons that help suppress irrelevant signals. We propose that the pattern of cortical connections, which depends on the fundamental structure of the cortex (reviewed below), provides the basis for a dynamic and flexible mechanism for rapid recruitment of diverse pathways to track and sequence signals to accomplish tasks that fall under the rubric of working memory.
The Laminar Structure of Cortical Systems Varies Systematically
To understand patterns of neural connections and their potential recruitment for working memory, it is necessary to discuss the fundamental architecture of the cortex. Contrary to the simplified descriptions in textbooks, not all areas of the cerebral cortex have six layers. Rather, some areas lack a cortical layer IV and thus are called “agranular.” Some areas have a poorly developed layer IV and are called “dysgranular.” Areas that have six layers are called “eulaminate” and also differ in the degree of their laminar elaboration (Fig. 1). We can use the relative distinction among layers and their relative thickness (or density) to differentiate types of cortex. Primary sensory areas (visual, auditory, and somesthetic), for example, have a thick and well-delineated layer IV; they have the most elaborate laminar structure within their respective cortical system. For example, the primary visual area (V1 or area 17) of the cortical visual system (Ungerleider and Mishkin 1982) has the most distinctive and elaborate lamination. At the other extreme of this system, the prostriate area on the medial surface, at the anterior extent of the calcarine fissure, has the simplest lamination. The areas between these two extremes show gradual changes in their laminar structure within the dorsal cortical visual system. The same pattern is seen for the ventral visual system.
Fig. 1.
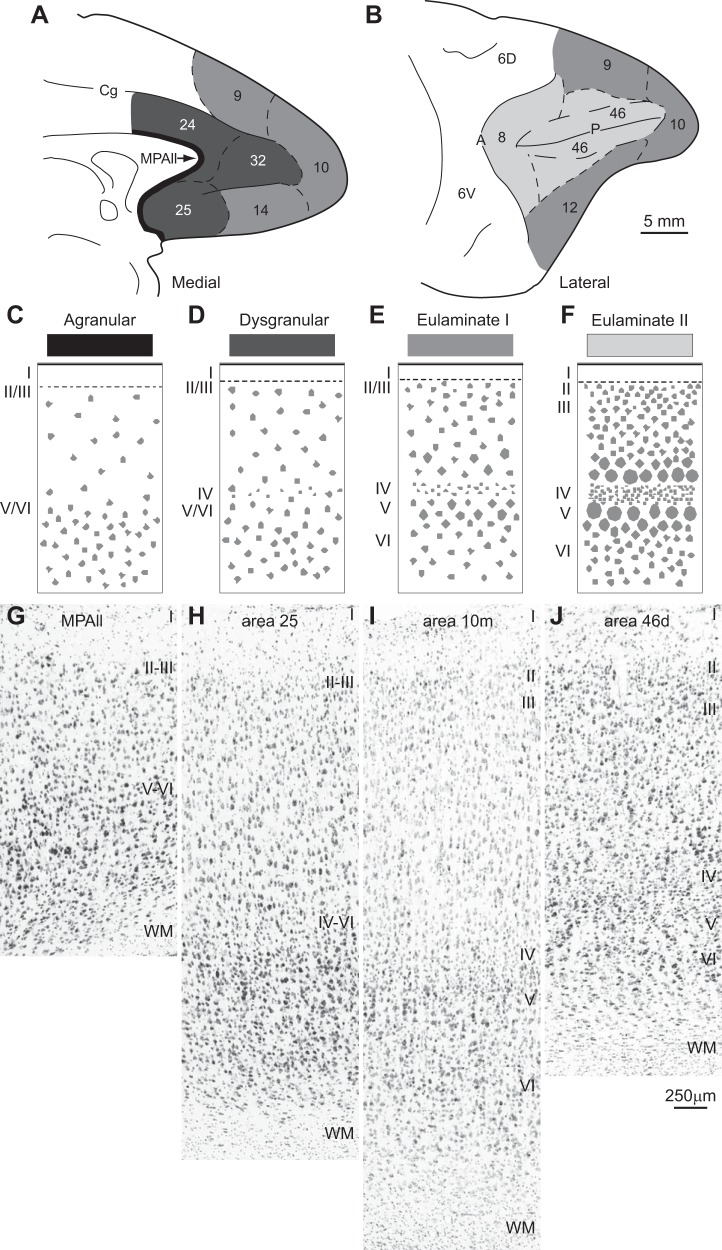
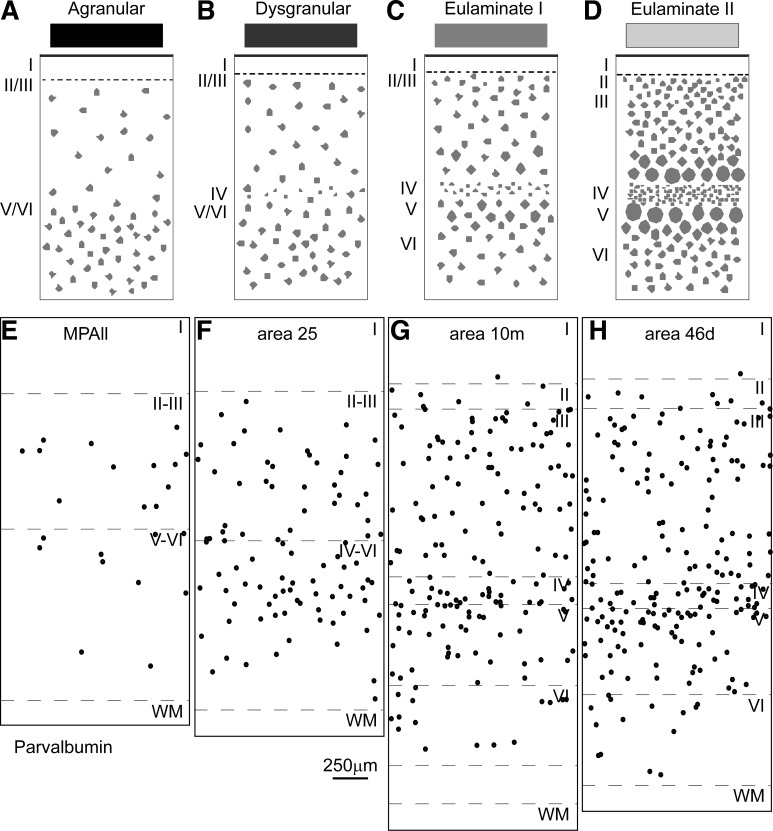
Systematic variation in cortical laminar structure using the prefrontal cortical region as a model system. Laminar structure is used to group areas into types of cortex, indicated by gray shading. The number of types depicted may vary depending on the region (system) and the desired level of resolution. A: medial view of the frontal lobe shows areas with the lowest (agranular; black) through areas with increasing elaboration of laminar structure (shown by lighter shades of gray). B: lateral view of the frontal lobe shows prefrontal areas with the greatest laminar elaboration (lightest gray). C–F: cartoons depict different types of cortex. C: agranular cortex has no layer IV. D: dysgranular cortex has an incipient layer IV. E: eulaminate area with 6 layers and a moderately dense layer IV. F: eulaminate area with a well-developed layer IV and better distinction between other layers. G–J: photomicrographs of coronal sections taken through different prefrontal areas. G: agranular medial prefrontal area MPAll (medial periallocortex). H: the dysgranular part of area 25. I: eulaminate I area 10m. J: eulaminate II area 46d. WM, white matter. Arabic numerals indicate prefrontal areas according to Barbas and Pandya (1989). Roman numerals indicate cortical layers. Calibration bar in J applies to G–J.
The principle of systematic variation in the cortical landscape emerged from classic studies (Abbie 1940, 1942; Pandya et al. 1988; Sanides 1970; von Economo 2009). This principle can be used to group areas in each system into a few cortical types. The cortex is composed of several regions (or systems), such as the visual, auditory, somatosensory, prefrontal, etc. (Mesulam 1998). Each system, in turn, is composed of several areas that vary systematically in laminar structure. The construct “type” refers to the broad laminar makeup of areas. Areas may belong to the same type whether they are neighbors or far from each other. Cortical type is not based on specific features of areas, as used by Brodmann (1999) or Walker (1940). These classical maps relied on the special features that give areas their identity, such as the size or shape of neurons in different layers, which were used to put borders between areas that differ in morphology. The concept of type was not part of the mapping approach of either Brodmann or Walker. Type transcends individual characteristics by focusing on overall laminar structure that is common across areas, such as the presence or absence of layer IV and its thickness or density when present, as shown diagrammatically in a simple sketch in Fig. 1. For example, agranular areas lack layer IV, a structural feature that binds areas into a common type regardless of their placement within a cortical system or geographic distance. There are other markers that are correlated with the intrinsic structure of the cortex, though not always. One of these is myelin, as shown in Fig. 2, or expression of different proteins, such as the phosphorylated microtubule protein SMI-32 in Fig. 3. Architectonic correlates were identified in classic and modern studies (Barbas and García-Cabezas 2015, 2016; Barbas and Pandya 1989; Campbell and Morrison 1989; Campbell et al. 1991; García-Cabezas et al. 2017; Sanides 1970).
Fig. 2.
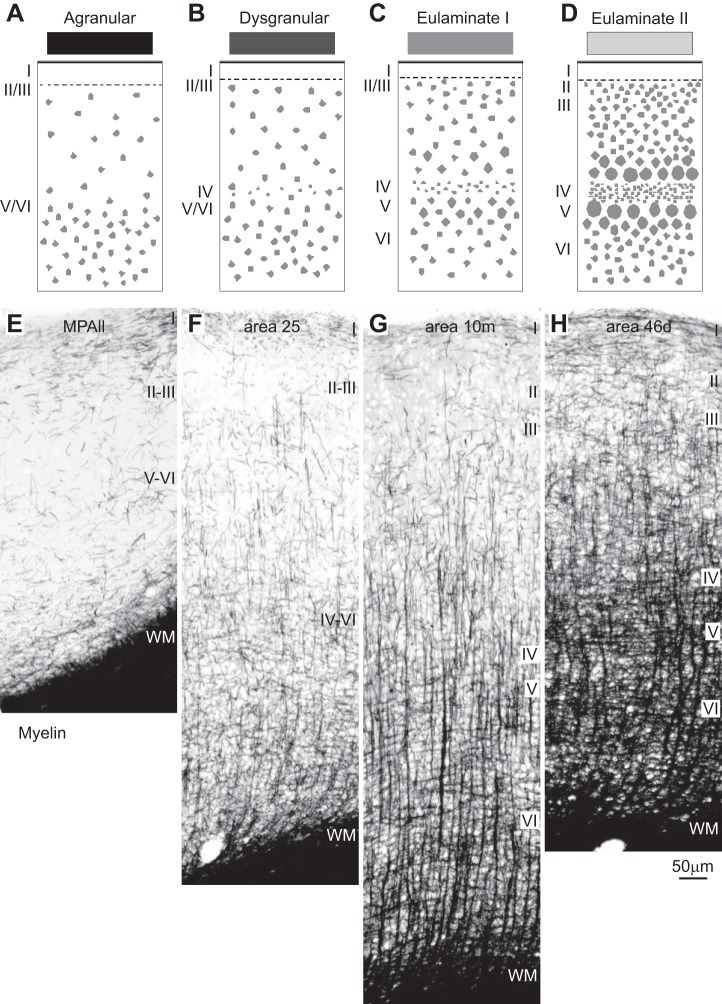
Systematic variation in the laminar structure of the cortex is accompanied by other features. A–D: cartoons show systematic changes in laminar structure that can be used to group areas into types of cortex. Shades of gray (top) show types of cortices from the least (black) to the greatest (lightest gray) elaboration of laminar structure. E–H: coronal sections through the types of prefrontal areas depicted in A–D show that agranular areas have the lowest (left), and the best-delineated eulaminate areas have the highest (right), density of myelinated fibers. Myelin changes often accompany changes in laminar structure, but not always, and thus are a correlate but not a substitute for laminar structure. E: agranular medial prefrontal area MPAll (medial periallocortex). F: the dysgranular part of area 25. G: eulaminate I area 10m. H: eulaminate II area 46d. WM, white matter. Roman numerals indicate cortical layers. Calibration bar in H applies to E–H.
Fig. 3.
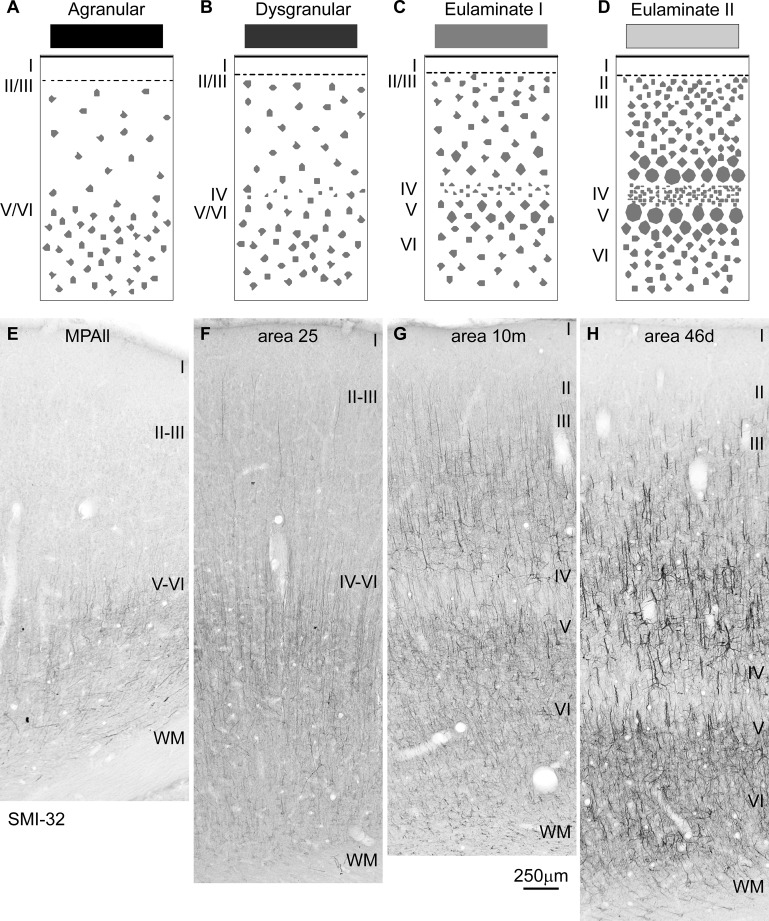
Systematic variation in cortical laminar structure highlighted by SMI32 immunostaining. A–D: cartoons show systematic changes in laminar structure that can be used to group areas into types of cortex. Shades of gray (top) show types of cortices from the least (black) to the greatest (lightest gray) elaboration of laminar structure. E–H: coronal sections through the types of prefrontal areas depicted in A–D show that agranular areas have the lowest (left), and the best-delineated eulaminate areas have the highest (right), density of SMI32-labeled neurons. In eulaminate areas (G and H), layer IV stands out as a band of unstained tissue. E: agranular medial prefrontal area MPAll (medial periallocortex). F: the dysgranular part of area 25. G: eulaminate I area 10m. H: eulaminate II area 46d. WM, white matter. Roman numerals indicate cortical layers. Calibration bar in G applies to E–H.
Systematic laminar variation is a universal principle in the mammalian cortex [reviewed in Barbas (2015)]. In rodents, laminar differentiation is less pronounced than in primates (Goulas et al. 2018; Reep 1984). In rats and mice, even the most differentiated cortical areas have poorly developed layer IV and thin layers II–III, and the prefrontal areas are agranular or dysgranular (Goulas et al. 2018; Wise 2008). In contrast, even though the prefrontal cortex of primates has agranular and dysgranular areas as well, it is dominated by eulaminate areas, which have six layers (Barbas and Pandya 1989). Interspecies differences must be taken into account when experimental data obtained in rodents are extrapolated to the human brain. Figure 1 shows a cartoon of the systematic variation of the prefrontal cortex (C–F) and in samples of stained tissue in macaque monkeys (G–J).
Variation in the Laminar Structure of Cortical Areas Sets the Stage for Their Recruitment
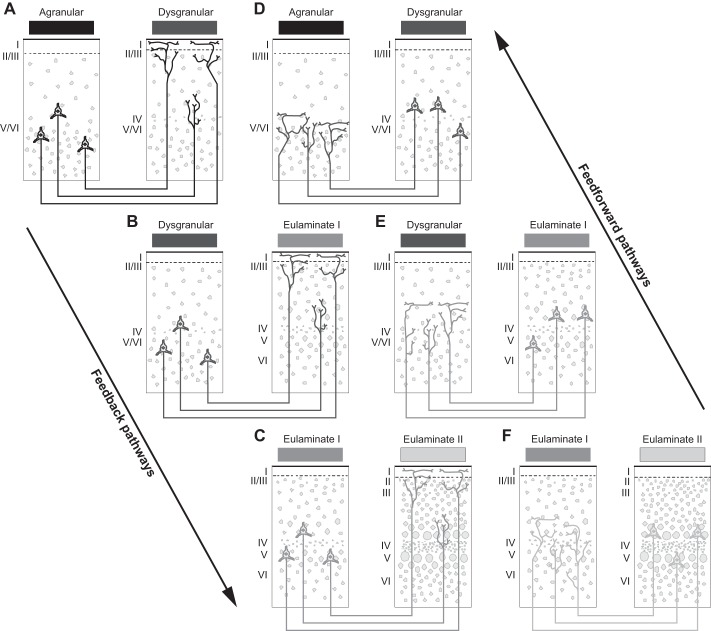
Reducing the dimensionality of the cortex into a few types within each cortical system has important implications for cortical organization, and chiefly for cortical connections because they depend on cortical type. Since the relationship of cortical type and the pattern of connections was discovered (Barbas 1986) and later formalized for bidirectional connections (Barbas and Rempel-Clower 1997), evidence has accumulated that the pattern of connections between areas depends on the type of areas that participate in the connections [reviewed in Barbas (2015)]. Moreover, the critical determinant in connections is the relative type difference between linked areas (Barbas and Rempel-Clower 1997). Thus, when an area of a simple type (e.g., agranular or dysgranular) projects to an area of more elaborate type (e.g., eulaminate), projection neurons originate in the deep layers and their axons terminate in the upper layers, especially layer I, but also layer II and the upper part of layer III. Projections proceeding in the reverse direction, from eulaminate to agranular/dysgranular areas, originate in the upper layers, especially layer III, and their axons terminate in the middle-deep layers of the cortex of destination. The former pattern is often called “feedback” and the latter “feedforward” by analogy with the flow of information in the sensory systems.
The principle of systematic variation is thus at the crux of connections and applies to all connections regardless of their placement in the cortex or membership to a cortical system. As the laminar type of areas varies systematically across a region (e.g., the cortical visual system), so does the laminar pattern of connections. The graded pattern of connections reflects the structural (type) relationship of the linked areas. Feedback pathways describe those that project from an area with a simpler type to an area of a more elaborate type. Feedforward pathways describe those that project from an area with a more elaborate type to an area of a simpler type. Connection patterns predicted by the structural model for connections are depicted in a diagram in Fig. 4. The diagram is simplified and does not depict the entire spectrum of possible connections. When the differences between linked areas are large, feedforward or feedback patterns are extreme; that is, they involve only a few layers. We see these extreme patterns when a pathway from a well-delineated eulaminate area projects to an agranular (limbic) area (feedforward) or in the reverse direction (feedback). Connections between areas with diametrically different laminar structure are not common. There are many more connections between areas that have comparable type, and the more similar they are, the more layers are involved in the connections. In these cases, cortical projection neurons originate equally in the upper layers (II–III) and deep layers (V and VI), and their axons terminate in a columnar pattern in all layers of the area of termination. Systematic cortical variation in all cortical systems thus explains both the laminar distribution of connections and their relative strength.
Fig. 4.
The structural model links laminar patterns of connections to the cortical type difference between linked areas. A–C: sequence of predicted patterns of connections that link areas with lower (left) to areas with higher (right) laminar elaboration. These connections have been called “feedback.” D–F: sequence of predicted patterns of connections that link areas with higher (right) to areas with lower (left) laminar elaboration. These connections are called “feedforward.” The diagram is oversimplified and does not depict all possible combinations of connections, such as those between areas with the lowest and highest laminar structure, which are in the minority among actual connections. The diagram also does not depict connections between areas of similar type, which have been called “lateral” and are ubiquitous in the cortex, or other combinations between areas of different type. Roman numerals indicate cortical layers.
In the sensory systems, knowledge about the flow of information from the periphery to the thalamus to primary areas and beyond was used to construct a hierarchical model. This model was based on the predominant laminar pattern of connections between areas to infer whether a projection is feedforward, feedback, or “lateral,” with the latter connecting areas thought to be at a similar hierarchical level (Felleman and Van Essen 1991). The model is based on a useful synthesis of data from the literature on connections but is not predictive, is largely unconstrained (Hilgetag et al. 1996), and was later characterized by one of the original authors as simplistic (Hegdé and Felleman 2007). By contrast, the structural model excellently predicts the laminar patterns of connections within the cortical visual system without reference to hierarchies (Hilgetag et al. 2016). These connections simply reflect the systematic variation seen in all cortical systems. The study of sensory areas, especially the visual, has been useful because one has a handle on the input. The lessons learned by study of the visual cortical system can be used to interpret findings in high-order association areas or between subcortical areas and the cortex [e.g., Höistad and Barbas (2008)].
Classifying cortical areas by type helps explain why nearby areas, which are of similar type, are often strongly connected. This principle also explains why distant areas are often strongly connected, as well. These connections occur between distant areas when they are of a similar type (Zikopoulos et al. 2018). In this context, the connections of the prefrontal cortex with temporal, parietal, and occipital areas are both distant and strong.
The power of the structural model for connections lies in its relational nature; it predicts many different laminar patterns of connections, whether the differences in the types of cortex between linked areas are large or small. The structural model thus predicts the variability in the laminar distribution of connections and their strength across the entire cortex. The predictions of the structural model for connections have been supported by actual connections across cortical systems, hemispheres and in different mammalian species [e.g., Barbas (1986); Barbas and Rempel-Clower (1997); Barbas et al. (1999); Beul et al. (2017); García-Cabezas and Barbas (2017); Grant and Hilgetag (2004, 2005); Hilgetag and Grant (2010); Hilgetag et al. (2016); Joyce and Barbas (2018); Medalla and Barbas (2006)].
The Structural Model for Connections and Working Memory
What does the structural model tell us about working memory? Let us consider two cortical regions that have been associated with working memory, the lateral prefrontal cortex and the lateral intraparietal cortex. Studies in the prefrontal cortex have focused on area 46 and parts of the adjacent area 8, and studies in the parietal region have focused on the lateral bank of the intraparietal sulcus and the adjacent gyral parietal cortex (Barbas and Mesulam 1981; Cavada and Goldman-Rakic 1989a, 1989b). Classical studies have associated each of these areas, which are found at a considerable distance from each other, with attention and eye movements during working memory tasks [reviewed in Bisley and Goldberg (2010); Colby and Goldberg (1999); Fuster (1984); Hyvärinen (1982); Lynch (1980); Tehovnik et al. (2000)].
Lateral prefrontal and intraparietal areas are strongly interconnected, although it is not clear where activity is initiated in working memory tasks. The pattern of their interconnections helps advance hypotheses in this regard. Both of these regions are eulaminate, as shown by their well-developed six-layer laminar architecture. In most sensory and association cortices, the density of neurons can be used to approximate laminar structure quantitatively [e.g., Dombrowski et al. (2001); Hilgetag et al. (2016)]. The best delineated sensory and association areas (high type) have a higher density of neurons than lesser delineated areas (low type). Even though neuronal density is not the only or universal proxy for cortical type, it is a good approximation to help compare association areas with subtle differences in laminar structure [see also García-Cabezas and Barbas (2017)]. We used neuronal density at the actual injection site in prefrontal areas 8 and 46 and at the sites of connections in the intraparietal cortex and examined the pattern of connections between them. We found that even a small difference in the density of neurons between linked areas accurately predicted the laminar pattern of their interconnections (Medalla and Barbas 2006). Thus the comparatively lower density area 8 projects to the upper layers of the ventral lateral intraparietal area (LIPv), which has a higher density of neurons; this pattern suggests a feedback relationship. On the other hand, the comparatively denser area 8 innervates the middle layers of the comparatively less dense dorsal lateral intraparietal area (LIPd) and the adjacent parietal area PGa, in a feedforward pattern.
The above discussion pertains directly to the question about initiation of activity in a working memory task: does activity originate in the parietal cortex and then get transmitted to lateral prefrontal cortex, or does activity proceed in the reverse direction? Based on the structural model for connections, we suggest that it depends on the specific areas recruited for a particular task. Variation in the laminar architecture of areas suggests that the laminar pattern of connections may also predict the site of initiation of activity, a hypothesis that can be tested. Laminar information of recorded neural activity is sorely lacking in most physiological studies.
THE PREFRONTAL CORTEX AND INHIBITORY CONTROL
Attending to relevant signals to perform a task requires access to relevant signals and suppression of irrelevant signals. The prefrontal cortex has classically been associated with inhibitory control, manifested at many levels (Aron 2007; Macmillan 1992). In simple attentional tasks to sensory stimuli, classical findings revealed that frontal lobe damage, or even mild cognitive decline with age, reduces the ability of individuals to suppress distracting auditory stimuli in humans, manifested as higher activity in auditory cortices (Chao and Knight 1997).
Inhibitory control extends beyond influence of sensory stimuli. In psychology, the term inhibition has been variously used to include cognitive control and behavioral control (Harnishfeger 1995; Miller and Cohen 2001). Inhibitory control also includes the influence of internal states, often inferred from behavioral responses in individuals with brain damage. Clinicians have long suspected lack of inhibitory control in humans who display incongruent behavior following prefrontal cortex damage. Such behavior ranges from an inability to appreciate the severity of a brain injury and even to joke about it, to inappropriate social behavior, as described in the famous Phineas Gage case (Damasio et al. 1994; Macmillan 2000). These types of behavior have been attributed to injury of lateral prefrontal cortex or orbitofrontal/ventromedial prefrontal cortex, respectively.
Early studies had promoted the idea that the orbitofrontal cortex (OFC) was the site for inhibitory control, based on lesion studies or injury and perseverative behavior to nonrewarded behavior in nonhuman primates (Fuster 1980; Mishkin 1964) and humans (Hornak et al. 1996). Subsequent studies refuted the idea that the OFC is the exclusive domain for inhibitory control, by discovering that other prefrontal areas also contribute to social, emotional, cognitive, and executive control in nonhuman primates and humans (Gläscher et al. 2012; Roberts and Wallis 2000).
In agreement with the latter studies, we discuss that diverse pathways contribute critical components for working memory tasks. The ubiquitous presence of inhibitory neurons in the primate cortex and subcortical structures, and the widespread influence of prefrontal pathways on other systems, argues against localization of inhibitory control. Rather, the association of the prefrontal cortex with inhibitory control depends on its circuits. Here we use the term inhibition at the level of the circuit, the influence of one neuron on another neuron. The discussion is based on studies that address this issue from the system to the synapse. Pathways that connect cortices are excitatory. How does the prefrontal cortex then engage in inhibitory control across cortical systems, and how is inhibition engaged in working memory?
To exercise inhibitory control, prefrontal pathways must innervate at least some inhibitory neurons at the site of termination. The process for inhibitory control at the level of neural circuits had remained remarkably unaddressed for the cortex, in general, and the prefrontal cortex, in particular. In the past 15 years we have addressed this issue at the level of circuits and synapses (Germuska et al. 2006) for a variety of prefrontal pathways to other cortical and subcortical structures [reviewed in Anderson et al. (2015); Barbas (2015); Barbas and García-Cabezas (2016); Barbas and Zikopoulos (2007); Medalla and Barbas (2014)]. To address this issue, we must first review the types of neurons that prefrontal pathways innervate and determine whether they are excitatory or inhibitory. We explore this issue below, starting with some essential features of cortical inhibitory neurons.
THE NATURE OF INHIBITORY NEURONS
In primates, cortical inhibitory neurons are local, meaning that they do not extend long axons across cortical areas [see also Tomioka and Rockland (2007)]. The observation that some cortical neurons have short axons dates back to Ramón y Cajal. The short axon neurons of Cajal are local; that is, their axons do not enter the white matter as cortical excitatory projection neurons do. Cajal recognized the important role of short axon neurons in the cortex, even as the concept of inhibition was being introduced by Sherrington using physiological methods [(Sherrington 1892); reviewed in Sherrington (1906)]. Some cortical excitatory neurons have short axons, as well, such as those in layer IV. However, Cajal described in exquisite detail short-axon neurons that we now know are inhibitory and which he found to be more abundant in the human cerebral cortex than in the cortex of mice, cats, and dogs (DeFelipe and Jones 1988; Ramón y Cajal 2002). Cajal’s observations have been confirmed with modern stains, which show that in rodents, cortical inhibitory neurons account for ~15% of all cortical neurons, as opposed to ~25% in primates [reviewed in DeFelipe (2011)]. Cortical short-axon neurons are also simpler in rodents and other mammals, exemplified by the double bouquet interneurons in primates, which are unsurpassed in morphological detail among mammalian species (DeFelipe and Jones 1988; Ramón y Cajal 2002). Cajal’s observations were confirmed with modern labeling with the calcium binding protein calbindin (Yáñez et al. 2005). In monkeys and humans, double bouquet cell bundles are organized in a pattern that is roughly complementary to the radial fasciculi of pyramidal neuron axons (Bacon et al. 1996; DeFelipe et al. 1990, 1999, 2002; del Rio and DeFelipe 1997; Fonseca and Soriano 1995; Gabbott and Bacon 1996a; Gabbott et al. 1997b; Jones and Rakic 2010; Peters and Sethares 1997).
The origin of cortical inhibitory neurons in rodents and primates appears to differ, as well, even though controversy surrounds this issue. In rodents and ferrets, inhibitory neurons of the cerebral cortex originate subcortically (Reillo et al. 2017). Cortical GABAergic neurons that express parvalbumin (PV) or somatostatin (SOM) originate in the medial ganglionic eminence (MGE), and those that express calbindin (CB) and the serotonin receptor 5-HT3a originate in the caudal ganglionic eminence (CGE) (Clowry 2015; Marín and Rubenstein 2003). On the other hand, in primates, neurogenesis is thought to originate from progenitors in the cortical matrix for a significant proportion of cortical GABAergic neurons (Al-Jaberi et al. 2015; Fertuzinhos et al. 2009; Jakovcevski et al. 2011; Letinic et al. 2002; Rakic and Zecevic 2003; Zecevic et al. 2011). Others, however, have shown that most cortical GABAergic neurons originate subcortically across the species studied [Hansen et al. (2013); Ma et al. (2013); for discussion see Clowry (2015)].
Known by their shape before their attributed function, inhibitory neurons in primates constitute a powerful minority at ~20–30% of all cortical neurons and exercise a critical role in cortical function. Before we address the issue of inhibitory control at the level of circuits, we describe the varied kinds of inhibitory neurons that initiate inhibition once activated by excitatory corticocortical pathways. We focus this description on the cortex, with emphasis on the prefrontal cortex, which must be recruited, along with an extended network for even simple working memory tasks.
Inhibitory Neurons in the Cortex of Primates
Inhibitory neurons in the cortex of primates are GABAergic. However, labeling neurons for GABA does not provide information about the variety of inhibitory neurons, which are distinguished by morphology, expression of markers, innervation targets, and functional attributes. In macaque monkeys and humans, a convenient way to classify cortical inhibitory neurons is by their expression of one of three calcium binding proteins (CBPs): PV, CB, and calretinin (CR). The CBPs reflect calcium dynamics but do not confer inhibitory activity on neurons. The CBPs are an effective way to label cortical inhibitory neurons for study of synaptic interactions. One of the advantages of CBPs is their labeling of not only the cell body (such as GABA) but also a good part of the extensive dendritic tree of neurons, where most synapses are formed. The CBPs also label axons, especially when viewed at high resolution in the electron microscope. Another advantage of the three CBPs is their labeling of largely nonoverlapping neurochemical classes of inhibitory neurons, which together account for the vast majority of cortical inhibitory neurons in primates.
None of the three CBP labels exclusively one morphological class of inhibitory neurons. PV, for example, labels cortical chandelier and basket cells of varied size, and nearly all of the labeled neurons are GABAergic in both primates and rats (DeFelipe 1997; Kawaguchi and Kubota 1997). PV neurons are also the most readily distinguished functional group, identified by their fast-spiking activity (Zaitsev et al. 2009). In the prefrontal cortex, PV neurons show a specific distribution that varies by cortical type (Fig. 5). The other two classes include CR neurons and CB neurons, which label several types of bitufted inhibitory neurons, including double bouquet neurons. Neurons that are positive for CR and CB are also mostly GABAergic, although some are excitatory pyramidal neurons, and are found in proportions that differ depending on the cortical area (Barbas et al. 2005; DeFelipe 1997; Kondo et al. 1999).
Fig. 5.
Systematic variation in the laminar structure of the cortex is accompanied by other features. A–D: cartoons show systematic changes in laminar structure that can be used to group areas into types of cortex. Shades of gray (top) show types of cortices from the least (black) to the greatest (lightest gray) elaboration of laminar structure. E–H: the laminar structure of the cortex is accompanied by other cellular and molecular features. In the prefrontal cortex, the trend is seen in the distribution of the neurochemical class of parvalbumin (PV) inhibitory neurons, which show the lowest density in agranular areas (E) and increasing density through sequential types of areas with increasing elaboration of laminar structure (F–H). E: agranular medial prefrontal area MPAll (medial periallocortex). F: the dysgranular part of area 25. G: eulaminate I area 10m. H: eulaminate II area 46d. WM, white matter. Roman numerals indicate cortical layers. Calibration bar in F applies to E–H.
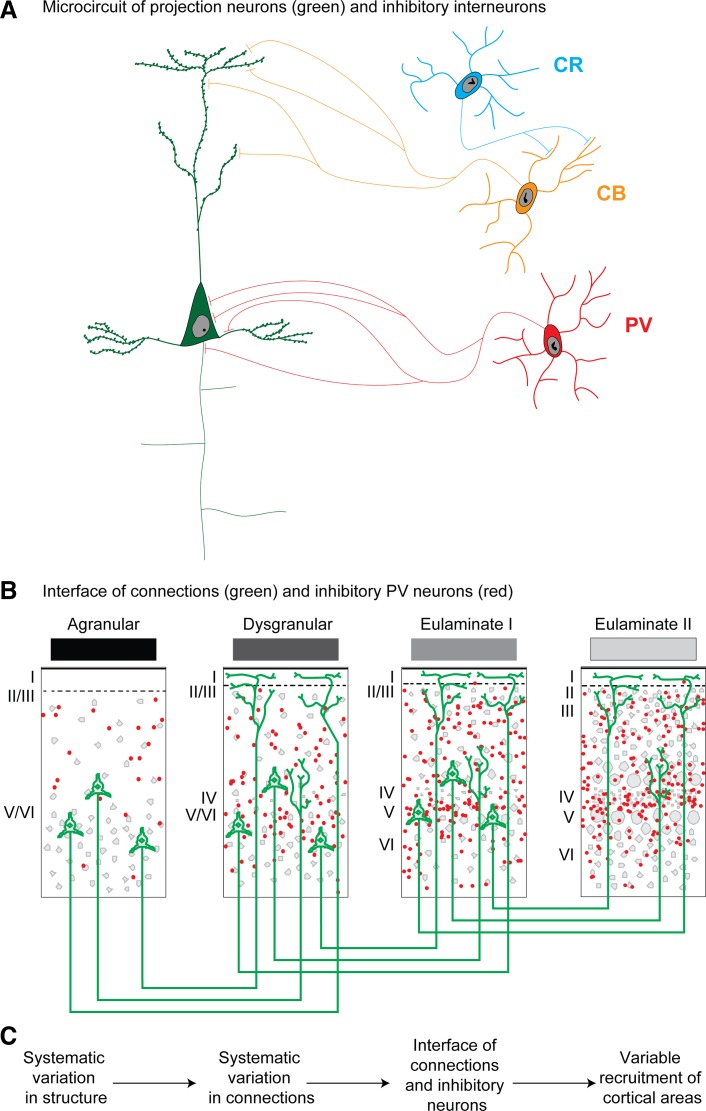
One of the most useful features of inhibitory neurons labeled by one of the three CBPs is their distinct innervation of neighboring neurons; another is their preferential laminar distribution. Thus PV neurons innervate perisomatic sites of pyramidal neurons (Fig. 6A). PV inhibitory neurons are preferentially found in the middle-deep cortical layers, which receive robust pathways from the thalamus. CR neurons are found in layers I and II, but also in the deep parts of layers V and VI (DeFelipe 1997; Dombrowski et al. 2001; Gabbott and Bacon 1996a, 1996b; Zaitsev et al. 2005, 2009). CR neurons innervate other inhibitory neurons in the upper layers, at least in the visual cortex of monkeys, and thus are thought to have a disinhibitory role (Meskenaite 1997), as also seen in mice and rats [reviewed in Tremblay et al. (2016)]. On the other hand, CR neurons innervate pyramidal neurons in the deep layers, at least in the primate visual cortex (Meskenaite 1997). More work is needed to investigate if this interesting class of inhibitory neurons shows a similar pattern of innervation in other cortical areas. CB neurons in primates innervate intermediate and distal dendrites of pyramidal neurons, suggesting a modulatory role (DeFelipe 1997; Wang et al. 2004). They are most densely distributed in layer II and upper layer III, and to a lesser extent in the deep layers in monkeys or humans (DeFelipe, 1997; Dombrowski et al. 2001; Gabbott and Bacon 1996a, 1996b; Zaitsev et al. 2005, 2009). Figure 6A shows the pattern of innervation of each of the three classes of CBP neurons in the cortical microcircuit, drawn according to their preferential placement in layers, from layer I (CR) to the middle-deep layers (PV), with CB neurons in between.
Fig. 6.
Systematic laminar variation and functionally distinct inhibitory neurons. A: relationship of pyramidal projection neurons (green) with functionally distinct inhibitory neurons labeled for parvalbumin (PV; red), calbindin (CB; orange), and calretinin (CR; blue). B: summary of cortical pathways as they interface with inhibitory neurons (e.g., PV) at the site of termination. C: summary of systematic cortical variation. Variation of cortical types, laminar connections, and interface of pathways with distinct classes of inhibitory neurons suggest a possible circuit mechanism for recruitment of areas and layers with functional consequences. Roman numerals indicate cortical layers.
Comparison of Cortical Inhibitory Neurons in Primates and Rodents
Classifying cortical inhibitory neurons in rats and mice, two widely used rodent model species, has proved to be more challenging than in primates. The most striking difference in cortical inhibitory neurons between primates and rodents is their higher proportion in primates, a fact recognized by Cajal and confirmed in subsequent studies (Condé et al. 1994; Gabbott and Bacon 1996a, 1996b; Gabbott et al. 1997b). Classification of inhibitory neurons in rodents into largely nonoverlapping classes has been stymied by the choice of several other markers that also label GABAergic neurons, which have been variably used by investigators. For example, in rats and mice, CR neurons also express vasoactive intestinal peptide (VIP). The CB class overlaps with neurons that express somatostatin (SOM), neuropeptide Y (NPY) and nitric oxide synthase (NOS) (Riedemann et al. 2016; Rogers 1992; Tremblay et al. 2016; Xu et al. 2010). The overlap in these neuropeptides and CBP may be conserved in the primate cortex, though this issue has not been studied systematically. In the rodent cortex, the segregation by function and immunoreactivity is less clear than in primates [for discussion see Gabbott et al. (1997a)]. In a detailed study, Kubota et al. (1994) showed that inhibitory neurons in the rodent frontal cortex can be divided into three groups based on CBP expression, as in primates. Recent studies in rodents indicate a partial shift in strategy from the CBP to a different complement of three neurochemical classes, which include PV, SOM, and the serotonin receptor 5-HT3a (Lee et al. 2010). Together, these markers appear to label nearly all GABAergic neurons in the cortex of rats and mice [reviewed in Kubota et al. (2016); Tremblay et al. (2016)].
INTERFACE OF CORTICAL PATHWAYS WITH DISTINCT INHIBITORY MICROENVIRONMENTS
Corticocortical connections depend critically on the relationship of the type difference between the linked areas and thus are variously distributed within cortical layers. Based on this principle, pathways that terminate primarily in the upper layers (feedback-like) come in contact with excitatory neurons as well as populations of CB inhibitory neurons, which are thought to have a modulatory role (DeFelipe et al. 1989a; Wang et al. 2004). In addition, feedback pathways come in contact with CR neurons, which are thought to have a disinhibitory role (Meskenaite 1997). Feedback pathways thus may have a predominant, though mild, excitatory effect in the cortex. On the other hand, pathways that originate in areas with more elaborate structure than the site of termination (feedforward) terminate preferentially in the middle-deep layers. Feedforward pathways come in contact with PV inhibitory neurons, known for their perisomatic innervation of neighboring pyramidal neurons and strong inhibition (DeFelipe et al. 1989b; Mikkonen et al. 1997).
Figure 6 summarizes patterns of corticocortical pathways and how they may encounter distinct inhibitory microenvironments at the site of termination (Fig. 6B). To illustrate the interface of prefrontal cortical pathways with different layers and within varied inhibitory microenvironments, let us use some examples. Pathways from the orbitofrontal cortex project mostly to the upper layers of inferior temporal visual areas, in a feedback pattern, but innervate the middle-deep layers of agranular entorhinal area 28, in a feedforward pattern (Rempel-Clower and Barbas 2000). We recently found that whereas dysgranular area 25 innervates preferentially the upper layers of temporal auditory areas, it innervates the middle-to-deep layers of entorhinal cortex (Joyce and Barbas 2018). These patterns are consistent with the structural model for connections and have distinct functional implications. Pathways that innervate the upper layers of visual and auditory association areas may help select relevant information for working memory tasks. In the entorhinal cortex, the upper layers receive high-order sensory and multimodal signals and project to the hippocampus (Insausti et al. 1987; Van Hoesen and Pandya 1975a, 1975b; Van Hoesen et al. 1975; Witter and Amaral 1991). On the other hand, the deep layers of area 28 receive hippocampal output destined for the cortex and may have a role in long-term memory processes (Anderson et al. 2015; Barbas et al. 2013a; Bunce and Barbas 2011; Canto et al. 2008). Theta oscillations in the deep layers of human entorhinal area 28 are associated with autobiographical memory retrieval (Steinvorth et al. 2010), processing of internal mental context (von Stein et al. 2000), and transfer of salient signals from hippocampus to cortex (Kirk and Mackay 2003). The contact of prefrontal pathways with neurons that receive the output of the hippocampus may thus provide a link to long-term memory processes summoned for working memory tasks. In our earlier example, these pathways may help retrieve signals associated with remembering the place of items needed to prepare a meal.
Other examples have emerged from the connections of the anterior cingulate cortex (ACC), a region associated with attention. ACC area 32 is distinguished for having strong connections with most other prefrontal areas (Barbas et al. 1999). Most of the ACC areas are dysgranular and thus project from their deep layers to lateral (eulaminate) prefrontal cortex. These ACC pathways terminate in the upper layers of lateral prefrontal cortex and innervate preferentially CB inhibitory neurons (Medalla and Barbas 2009, 2010). As shown in a combination of physiologic and computational studies, CB neurons are well suited to decrease noise and enhance signal in working memory tasks (Constantinidis et al. 2002; Wang et al. 2004). The pathway from ACC to lateral prefrontal areas may thus facilitate working memory tasks. Interestingly, this pathway appears to be weakened in schizophrenia and may contribute to distractibility in working memory tasks, as described for the disease [reviewed in Barbas et al. (2013a)]. Information on the laminar pattern of connections, their strength and interface with functionally distinct classes of inhibitory neurons, is thus essential for understanding circuit disruption in psychiatric diseases that affect working memory.
One intriguing and largely understudied aspect of systematic cortical variation is the change in the density gradient of some inhibitory neuron classes. A clear and relatively well-studied example is the progressive increase in PV neuron density with ascending cortical type such that progressively more laminated areas have denser populations of PV neurons (Dombrowski et al. 2001; García-Cabezas et al. 2017). Evidence in rodents suggests a similar pattern (Kim et al. 2017). Figure 5 shows the distribution of PV neurons in representative areas of different types in the prefrontal region. The gradient of PV density likely contributes to differential dynamics that can be initiated by cortical pathways that terminate in different layers.
There is considerable controversy on the relationship of rhythms in the cortex with respect to feedforward and feedback connections. Some investigators have suggested that feedforward pathways are associated with gamma oscillations, and feedback pathways with alpha/beta oscillations [e.g., Bastos et al. (2015); Buffalo et al. (2011); Michalareas et al. (2016); Spaak et al. (2012); van Kerkoerle et al. (2014)]. However, other studies suggest more complex patterns in cortical rhythms (Csercsa et al. 2010; Haegens et al. 2015).
Oscillations depend on populations of inhibitory neurons [e.g., Le Van Quyen et al. (2016); Teleńczuk et al. (2017)]. The structural model suggests a large variety in the pattern of connections, and thus varied interface with functionally distinct classes of inhibitory neurons with different dynamics; Fig. 6C summarizes these relationships. We suggest that the systematic variation of the cortex and laminar patterns of connections provides the framework for variability in activation and shifts in cortical rhythms when specific pathways are recruited in time to meet task demands. The dynamic nature of cortical oscillations (Cannon et al. 2014; Kopell et al. 2014) thus may be understood in the context of the graded variability of connections and cortical inhibitory microenvironments (Barbas 2015). This hypothesis is consistent with the dependence of cortical rhythms on task demands (Cannon et al. 2014; Schroeder and Lakatos 2009; Wimmer et al. 2016), which must shift dynamically during distinct phases of sequential tasks, as seen for working memory.
Moreover, preliminary observations suggest that the density of the chandelier cell subclass of PV neurons varies across areas (DeFelipe et al. 1999; Inda et al. 2007). Whereas the distribution of PV neurons increases with increase in cortical type, the distribution of chandelier neurons decreases. In the mouse, chandelier cells can hyperpolarize pyramidal neurons that are in an “up state” of excitability or depolarize them when they are in a down state (Woodruff et al. 2010). The higher prevalence of chandelier neurons in limbic areas may bestow greater flexibility in excitatory and inhibitory dynamics in these areas. As more complex aspects of the cortical inhibitory microenvironment are revealed, it becomes clear that the dynamic nature of cortical oscillations may be part of a more predictable framework than currently thought.
SUBCORTICAL STRUCTURES AND WORKING MEMORY
The pathway interactions between prefrontal and other areas that are engaged in working memory tasks are not restricted to the cortex, but extend to subcortical structures, including the thalamus. In our specific comparison between rodents and primates, the thalamus differs in one important way: The primate thalamus has inhibitory neurons, but the rat thalamus has only sparsely distributed inhibitory neurons in the visual thalamic nucleus (Jones 2007). The nature of inhibitory neurons in the dorsal thalamus is largely unknown. Neurons that are positive for PV, CB, or CR in the thalamus are not inhibitory, but represent projection classes directed to the cortex (Jones 1998; Rausell and Jones 1991a, 1991b; Rausell et al. 1992; Timbie and Barbas 2014).
The mediodorsal (MD) thalamic nucleus, which is associated with the prefrontal cortex, has a major influence on lateral prefrontal areas while primates perform working memory tasks. Specifically, this association was shown for the lateral (multiform) sector of MD (MDmf), which is connected with the frontal eye fields (area 8), and for the parvicellular (MDpc) sector, which is connected with area 46 (Barbas et al. 1991; Giguere and Goldman-Rakic 1988; Siwek 1989). Neurons in MDmf and intralaminar nuclei fire in association with eye movements and orientation to stimuli in the environment (Schlag-Rey and Schlag 1984; Schlag and Schlag-Rey 1984), consistent with a role in working memory tasks. Moreover, the thalamic MD nucleus and lateral prefrontal cortex show collaborative activity in working memory tasks. Classic findings have shown that cooling lateral prefrontal areas disrupts neuronal activity during working memory tasks in monkeys (Alexander and Fuster 1973).
Like other thalamic nuclei, connections of MD and the prefrontal cortex are bidirectional. Pathways from the thalamus to lateral prefrontal cortex terminate in the middle cortical layers (layer IV, the lower part of layer III, and the upper part of layer V), akin to feedforward corticocortical pathways. Returning pathways from the prefrontal cortex to MD originate mostly in layer VI, and to a lesser extent in layer V, akin to corticothalamic feedback pathways. However, some pathways from cortical layer V to the thalamus have features that suggest feedforward communication (Zikopoulos and Barbas 2007b), as suggested also in the corticothalamic system in mice (Sherman 2012). In addition, feedback-like pathways from the thalamus reach the upper layers of cortex (Jones 1985); these important pathways have been largely forgotten or ignored in many accounts of thalamocortical interactions. The pathways that link the cortex with the thalamus thus can be similarly described as the corticocortical. A similar pattern is seen in rodents (Galazo et al. 2008; Rubio-Garrido et al. 2009; Rodriguez-Moreno et al. 2018).
Moreover, the thalamic pathways also depend on the types of cortex they innervate. In the prefrontal cortex, areas with the most elaborate laminar structure in the dorsolateral prefrontal region receive most of their thalamic projections from MD, which terminate in the middle layers (Giguere and Goldman-Rakic 1988), and receive projections from only a few neurons found in other thalamic nuclei (Barbas et al. 1991; Dermon and Barbas 1994). On the other hand, posterior orbitofrontal areas and ACC areas, which are either agranular or dysgranular (limbic), have widespread connections with many thalamic nuclei (Barbas 2000; Barbas et al. 2011). The limbic cortices of the posterior orbitofrontal cortex (pOFC) and medial (ACC) areas are connected with another part of MD, the medial, or magnocellular, sector (MDmc). The MDmc has particularly strong connections with pOFC, a region distinguished for its multimodal connections with late-processing sensory association areas (Barbas 1993). MDmc is a strong recipient of projections from the amygdala (Russchen et al. 1987; Timbie and Barbas 2015), known for its key role in processing the affective aspect of stimuli and events. The strong connections of MDmc with pOFC may signal the affective import of stimuli to lateral prefrontal cortex (Wallis and Miller 2003).
On the other hand, the ACC stands out for robust thalamic connections with midline thalamic nuclei, including the reuniens, as well as anterior thalamic nuclei, which are connected with the hippocampus in both rats and primates [Aggleton et al. (1986); Vogt et al. (1979, 1987); reviewed in Vertes et al. (2015)]. These strong connections may convey information about the context of events to the ACC [reviewed in Barbas et al. (2011)].
Are these MDmc and midline thalamic connections to pOFC and ACC areas associated with working memory? We suggest that they are, because working memory tasks are often inextricably embedded in an emotional context, reflecting the choices that individuals make and their personal points of view. The lateral prefrontal areas that are associated with working memory tasks do not receive significant projections from the amygdala or the hippocampus. How do stimuli associated with emotional context in a variety of everyday tasks reach lateral prefrontal cortex? The pathways from the amygdala to pOFC innervate all layers, including the middle layers, which resemble feedforward pathways (Ghashghaei et al. 2007). The pOFC is distinguished for receiving information from each and every sensory modality through late-processing sensory areas [reviewed in Barbas (2000); Barbas and Zikopoulos (2006)]. We have shown that sensory-related pathways to the amygdala fit a feedforward pattern (Höistad and Barbas 2008). This evidence suggests that sensory input reaches the amygdala and is then conveyed to all layers of pOFC. Based on the relational structural model for connections, information from pOFC reaches higher type areas via feedback pathways, through anterior orbitofrontal areas, anterior lateral areas, and eventually caudal area 46 and the frontal eye fields (area 8), the areas associated with working memory. These sequential pathways follow a feedback pattern and thus proceed in the opposite direction than the sequence of sensory signals from the periphery to the primary sensory and association areas (Barbas and García-Cabezas 2017).
The connections of pOFC with the amygdala are strong and bidirectional (Ghashghaei et al. 2007; Porrino et al. 1981; Timbie and Barbas 2014); The pOFC is distinguished for issuing a uniquely strong pathway to the entirely inhibitory intercalated masses (IM) of the amygdala (Ghashghaei et al. 2007). The IM have a key role in processes within the amygdala [reviewed in Paré et al. (2003); García-Cabezas and Barbas (2017)]. On the other hand, whereas the ACC has strong bidirectional connections with the amygdala, as well, it innervates most strongly the basolateral nucleus. Based on the distinct classes of inhibitory neurons found in the striatal-related IM, and their interconnections (Zikopoulos et al. 2016), we have suggested that the pOFC and ACC pathways to the amygdala have distinct functions. The pathway from pOFC to IM likely modulates autonomic activity under normal levels of dopamine, by activating inhibitory neurons in IM that inhibit amygdalar output to autonomic structures. On the other hand, the ACC pathway innervates neurons in the basolateral amygdala, which activate the amygdalar output to autonomic structures and increase autonomic drive, as seen in fear-conditioning tasks (Zikopoulos et al. 2017). However, when dopamine levels are very high, in conditions of high anxiety, the largest class of amygdalar IM neurons become hyperpolarized and ineffective in modulating the output of the amygdala to autonomic centers, resulting in high levels of anxiety. High dopamine levels are also known to disrupt working memory tasks in dorsolateral prefrontal cortex (Arnsten et al. 2012; Wang et al. 2007).
The hippocampus targets the ACC, including area 32 (Barbas and Blatt 1995; Insausti and Muñoz 2001), which is distinguished for its strong connections with lateral prefrontal areas (Barbas et al. 1999). These prefrontal connections include the frontal pole (area 10), a region associated with complex aspects of working memory, as well as area 46, associated with ordinary working memory tasks [reviewed in Mansouri et al. (2017); Medalla and Barbas (2014); Teffer and Semendeferi (2012)]. This network shows how areas associated with working memory may obtain information about the affective significance of stimuli within context, functions associated with the amygdala and hippocampus. As is the case for corticocortical connections, pathways from the amygdala and the hippocampus also interface with distinct excitatory and inhibitory microenvironments at the site of termination in prefrontal cortex (Timbie and Barbas 2014).
The prefrontal cortex and the amygdala also have a distinct relationship with another system in the thalamus, the entirely inhibitory thalamic reticular nucleus (TRN). Thus, although the entire cortex projects topographically to TRN, pathways from some prefrontal areas, including area 46, the pOFC, and the amygdala, reach not only the frontal sector of TRN, where they are densest, but also the posterior TRN, the sites innervated by sensory cortices (Zikopoulos and Barbas 2006, 2007a, 2012). The TRN has a role in filtering signals between thalamus and cortex. The privileged extent of some lateral prefrontal and amygdalar pathways on sensory TRN sectors suggests selection of relevant signals within an affective context for the task at hand and suppression of distracting signals. The filtering of stimuli thus occurs at a very early stage through the thalamus, suggesting that sensory or other stimuli may be selected or suppressed by the prefrontal cortex, perhaps according to what is needed for the task at hand (John et al. 2016; Zikopoulos and Barbas 2007a).
SMOOTH AND SPEEDY RECRUITMENT OF AREAS FOR WORKING MEMORY
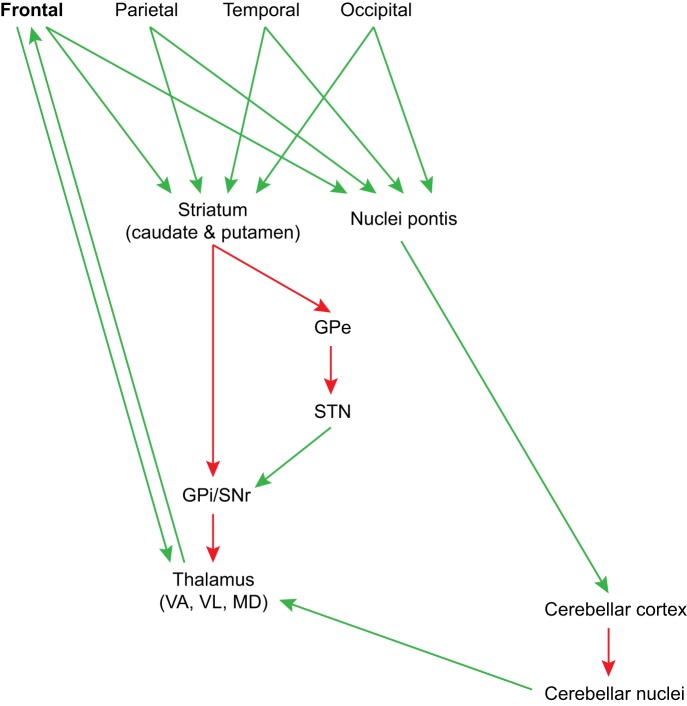
One of the most remarkable aspects of working memory is the seemingly automatic sequencing of items to accomplish a task. For example, most humans can sequence words and sentences in effortless streams. Clues about this type of sequencing may be traced to the circuit organization of two major structures, the basal ganglia and the cerebellum, and their unique relationship with the frontal cortex. The entire cortex projects topographically to the caudate or putamen [Yeterian and Pandya (1991, 1993, 1995); reviewed in Haber (2003); Middleton and Strick (2000)]. Similarly, the cortex projects to the pontine nuclei, which project to the cerebellum [reviewed in Stoodley and Schmahmann (2010)]. A common element in these pathways is their nonreciprocity with the cortex: they do not send projections back to the cortex directly. The caudate and putamen project to the globus pallidus, which then directly or indirectly innervates the thalamus, as shown in Fig. 7. Similarly, the output of the cerebellum is directed to the deep cerebellar nuclei, which project to the thalamus. What is remarkable about these circuits is their preferential, though not exclusive, innervation of some thalamic nuclei. Both structures innervate mostly ventral thalamic nuclei (ventral lateral, ventral anterior), which are connected with motor and premotor areas, and ventral anterior, mediodorsal (MD), and anterior medial nuclei, which are connected with prefrontal cortices [(Xiao and Barbas 2002a, 2002b); reviewed in Barbas et al. (2013b); Zikopoulos and Barbas (2007b)]. This circuit shows that the lion’s share of the output of the basal ganglia and cerebellum through the thalamus is biased toward the frontal lobe.
Fig. 7.
Proposed circuit mechanism for smooth recruitment of sequential items for working memory tasks. The entire cortex projects to the basal ganglia and the cerebellum; the output of these large structures projects preferentially to thalamic nuclei (VL, VA, and MD), which are connected with the frontal cortex, including motor/premotor and prefrontal areas. The basal ganglia are associated with habit formation, and the cerebellum with smooth sequencing of information. The preferential output of these structures to thalamic nuclei that project to prefrontal cortex may facilitate quick sampling of signals from the cortex for working memory tasks. GPe, globus pallidus external part; GPi, globus pallidus internal part; MD, mediodorsal thalamic nucleus; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; VA, ventral anterior thalamic nucleus; VL, ventral lateral thalamic nucleus.
The specific information conveyed from the basal ganglia and cerebellum to the thalamus is unknown. However, evidence from experimental studies using a variety of approaches suggests that through topographic and overlapping connections with the cerebral cortex, the basal ganglia are associated with a variety of cognitive and affective behaviors, reinforcement learning, and formation of habits (Graybiel 2008; Haber and Behrens 2014). The cerebellum was classically associated with smooth operation of movements, based on the clinical literature, after its damage. Research in the last decades has shown, however, that the cerebellum is associated with processing sensory, motor, cognitive, and affective signals, consistent with its unidirectional input from the entire cerebral cortex [reviewed in Middleton and Strick (2000); Stoodley and Schmahmann (2010)]. Limited in the number of layers it has, the cerebellum seems to be excellent for sequencing information, and likely does so in all domains, be it sensory, cognitive, affective, or motor (Leiner 2010). The output of each of these major structures to ventral and medial thalamic nuclei may provide “packaged” information to the prefrontal cortex, facilitating the smooth sequencing of cognitive, affective, and motor operations for working memory, as summarized in Fig. 7.
Classically considered to be separate in their connections with the thalamic nuclei, there is now evidence that the output of the cerebellum and the basal ganglia overlaps in the thalamus [reviewed in Barbas et al. (2013b)]. Moreover, through multisynaptic subcortical pathways, the basal ganglia and the cerebellum appear to be interconnected, setting the stage for broad network interactions of these major structures for normal function and for disruption in diseases that classically were thought to reflect dysfunction in one of the two large structures, but not in both (Bostan and Strick 2018). Such interconnections include caudate regions of the basal ganglia and lateral cerebellum that receive projections from dorsolateral prefrontal areas associated with working memory as well as verbal fluency (Bostan and Strick 2018).
Many questions with regard to each of these systems remain unanswered. The output of the basal ganglia is from inhibitory neurons. Through the direct pathway, this output disinhibits thalamic neurons and allows initiation of movement, or is inhibitory to them via the indirect pathway and prevents movement (Fig. 7). On the other hand, the output of the cerebellum through the deep cerebellar nuclei to the thalamus is excitatory (Mason et al. 1996). The limited direct overlap of the output of the basal ganglia and the cerebellum within the thalamic nuclei is associated with motor, premotor, and prefrontal cortices, suggesting functional specificity [Rouiller et al. (1994); Sakai et al. (1996); reviewed in Barbas et al. (2013b)]. These structures may have collaborative and complementary roles in facilitating smooth operations for working memory. The symptomatology patterns in diseases that affect preferentially, though not exclusively, the cerebellum or basal ganglia may shed light on the unique contribution of each of these major structures to working memory.
CONCLUSION
Let us now consider the complement of connections that give the prefrontal cortex a panoramic view of the entire external world and the internal environment of motives and drives. The widespread connections of different sectors of the prefrontal cortex are excellently suited to recruit relevant information and suppress distracting signals. The fundamental principle of systematic variation of the cortex applies to the entire cortex. Connections vary in strength and laminar distribution and are dependent on the systematic variation of the cortex, and thus are also graded. One of the major targets of the amygdala is the medial (magnocellular) part of MD, which has strong bidirectional connections with agranular/dysgranular pOFC and ACC areas. However, the amygdala also projects directly to all layers of these areas, suggesting multifaceted impact in the processing of stimuli with affective significance. The pathways from the thalamus, and particularly MD, to prefrontal cortex convey signals from other subcortical structures and output from the basal ganglia and cerebellum, which collect information about the status of the entire cortex.
The pattern of connections between the thalamus and the cortex is also variable: some nuclei project primarily to the middle cortical layers, but others, including the midline, intralaminar, and anterior, project preferentially to the upper layers or have a mixed pattern (Jones 2007). As the corticocortical pathways are variable in their strength and laminar distribution, so are the thalamic pathways to cortex. The entire complement of connections thus comes in contact with variable laminar microenvironments where the excitatory and especially laminar-specific inhibitory microenvironments vary, as well. This scheme provides great flexibility for recruiting stimuli for working memory tasks. The connections place the prefrontal cortex, in particular, in an ideal position to select relevant stimuli and suppress distracting stimuli, and to shift activity across layers efficiently and in time to meet the specific aspects of sequential components of a task.
On the basis of the graded pattern of connections, we have predicted that the variable architecture of the cortex, which gives rise to variable cortical types, has its roots in development (Dombrowski et al. 2001). This prediction has been substantiated to the extent of available data (Barbas and García-Cabezas 2016; Rakic 2002). Moreover, we have suggested that elaboration of the cortex and emergence of high cortical types is based on differences in the progenitor pool below areas of different cortical types, which also has been supported with preliminary data in humans (Barbas and García-Cabezas 2016). According to our hypothesis, areas that have low type (limbic) appear early in development, and areas with more elaborate structure appear increasingly later (Dombrowski et al. 2001). Connections may similarly follow a sequential pattern of establishment. This hypothesis may help explain the exquisite regularity of corticocortical connections, which depend on cortical systematic variation.
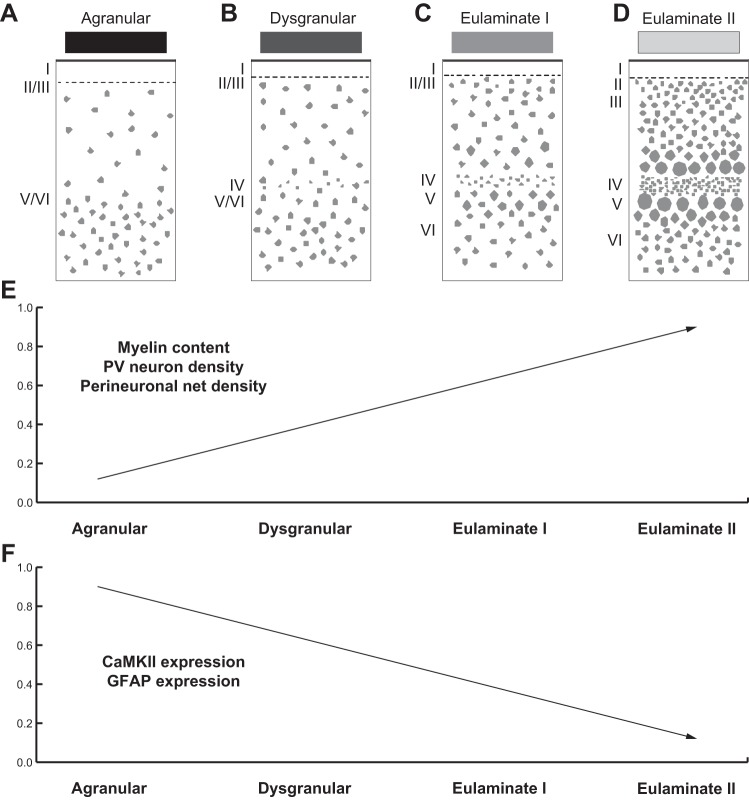
We have previously suggested that limbic areas, which complete their development earlier than eulaminate areas, retain some developmental features into adulthood (Barbas 1995). The complement of these markers may help explain the great plasticity of limbic areas and involvement in learning and memory, but also their relative lack of stability and preferential vulnerability to disruption in disease. On the other hand, eulaminate areas are endowed with markers associated with high stability but comparatively lower plasticity (García-Cabezas et al. 2017). Figure 8 summarizes cortical systematic variation and expression of markers associated with stability/plasticity along the spectrum of cortical types.
Fig. 8.
Expression of markers of plasticity across cortical areas parallels laminar differentiation. A–D: cartoons show systematic changes in laminar structure that can be used to group areas into types of cortex. Shades of gray (top) show types of cortices from the least (black) to the greatest (lightest gray) elaboration of laminar structure. E: expression of factors that limit synaptic plasticity, such as myelin content, parvalbumin (PV) neuron density, and perineuronal net density, is higher in eulaminate than in limbic areas. F: expression of factors that enhance synaptic plasticity, such as calcium/calmodulin-dependent protein kinase II (CaMKII), is higher in limbic areas; expression of glial fibrillary acidic protein (GFAP), a marker of activated astrocytes, is also higher in limbic areas than in eulaminate areas. Roman numerals indicate cortical layers. [Adapted from García-Cabezas et al. (2017)].
This review provides the theoretical framework to understand the organization of connections based on classic findings of the systematic variation of the cortex. Our theoretical framework allows us to make predictions about circuits, their interface with functionally distinct inhibitory neurons, and recruitment of areas in behavior, which can be tested using functional approaches. A lot more information is needed to fill the gaps in our knowledge of the specific distribution of classes of inhibitory neurons in distinct areas, their interface with pathways, and engagement during specific aspects of working memory tasks. More work needs to be done to determine how connections are established in development and how function is affected by expression of different markers in development or imposed later by epigenetic modifications (García-Cabezas et al. 2018). This information may help explain why some regions, such as the prefrontal cortex, have a panoramic view of the status of the entire cortex and can participate in seemingly automatic cognitive operations flexibly and efficiently in working memory tasks, a process that is impaired in some psychiatric diseases.
GRANTS
This work was supported by National Institutes of Health Grants R01MH057414 and R01NS024760. M. Á. García-Cabezas was the recipient of Brain and Behavior Research Foundation 2014 National Alliance for Research on Schizophrenia & Depression Young Investigator Grant 22777 (P&S Fund Investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., M.K.P.J., and M.A.G.-C. prepared figures; H.B. drafted manuscript; H.B., J.W., M.K.P.J., and M.A.G.-C. edited and revised manuscript; H.B., J.W., M.K.P.J., and M.A.G.-C. approved final version of manuscript.
REFERENCES
- Abbie AA. Cortical lamination in the monotremata. J Comp Neurol 72: 429–467, 1940. doi: 10.1002/cne.900720302. [DOI] [Google Scholar]
- Abbie AA. Cortical lamination in a polyprotodont marsupial, Perameles nasuta. J Comp Neurol 76: 509–536, 1942. doi: 10.1002/cne.900760310. [DOI] [Google Scholar]
- Aggleton JP, Desimone R, Mishkin M. The origin, course, and termination of the hippocampothalamic projections in the macaque. J Comp Neurol 243: 409–421, 1986. doi: 10.1002/cne.902430310. [DOI] [PubMed] [Google Scholar]
- Al-Jaberi N, Lindsay S, Sarma S, Bayatti N, Clowry GJ. The early fetal development of human neocortical GABAergic interneurons. Cereb Cortex 25: 631–645, 2015. doi: 10.1093/cercor/bht254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Fuster JM. Effects of cooling prefrontal cortex on cell firing in the nucleus medialis dorsalis. Brain Res 61: 93–105, 1973. doi: 10.1016/0006-8993(73)90518-0. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bunce JG, Barbas H. Prefrontal-hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Mem 134: 145–161, 2015. doi: 10.1016/j.nlm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76: 223–239, 2012. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist 13: 214–228, 2007. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res 720: 211–219, 1996. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252: 415–422, 1986. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience 56: 841–864, 1993. doi: 10.1016/0306-4522(93)90132-Y. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev 19: 499–510, 1995. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barbas H. Complementary roles of prefrontal cortical regions in cognition, memory, and emotion in primates. Adv Neurol 84: 87–110, 2000. [PubMed] [Google Scholar]
- Barbas H. General cortical and special prefrontal connections: principles from structure to function. Annu Rev Neurosci 38: 269–289, 2015. doi: 10.1146/annurev-neuro-071714-033936. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5: 511–533, 1995. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Barbas H, Bunce JG, Medalla M. Prefrontal pathways that control attention. In: Principles of Frontal Lobe Functions, edited by Stuss DT, Knight RT. New York: Oxford University Press, 2013a, p. 31–48. [Google Scholar]
- Barbas H, García-Cabezas MA. Motor cortex layer 4: less is more. Trends Neurosci 38: 259–261, 2015. doi: 10.1016/j.tins.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MA. How the prefrontal executive got its stripes. Curr Opin Neurobiol 40: 125–134, 2016. doi: 10.1016/j.conb.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MA. Prefrontal cortex integration of emotions and cognition. In: The Prefrontal Cortex as an Executive, Emotional and Social Brain, edited by Watanabe M. Tokyo: Springer, 2017, p. 51–76. doi: 10.1007/978-4-431-56508-6_4. [DOI] [Google Scholar]
- Barbas H, García-Cabezas MA, Zikopoulos B. Frontal-thalamic circuits associated with language. Brain Lang 126: 49–61, 2013b. doi: 10.1016/j.bandl.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol 410: 343–367, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol 313: 65–94, 1991. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex 15: 1356–1370, 2005. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol 200: 407–431, 1981. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286: 353–375, 1989. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex 7: 635–646, 1997. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. Sequential and parallel circuits for emotional processing in primate orbitofrontal cortex. In: The Orbitofrontal Cortex, edited by David Z, Scott R. Oxford: Oxford University Press, 2006, p. 57–91. doi: 10.1093/acprof:oso/9780198565741.003.0004. [DOI] [Google Scholar]
- Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist 13: 532–545, 2007. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry 69: 1133–1139, 2011. doi: 10.1016/j.biopsych.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85: 390–401, 2015. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Beul SF, Barbas H, Hilgetag CC. A predictive structural model of the primate connectome. Sci Rep 7: 43176, 2017. doi: 10.1038/srep43176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19: 338–350, 2018. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Brodmann’s Localisation in the Cerebral Cortex, translated and edited by Garey LJ. London: Imperial College Press, 1999. (Original work published 1909). [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA 108: 11262–11267, 2011. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JG, Barbas H. Prefrontal pathways target excitatory and inhibitory systems in memory-related medial temporal cortices. Neuroimage 55: 1461–1474, 2011. doi: 10.1016/j.neuroimage.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Hof PR, Morrison JH. A subpopulation of primate corticocortical neurons is distinguished by somatodendritic distribution of neurofilament protein. Brain Res 539: 133–136, 1991. doi: 10.1016/0006-8993(91)90695-R. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Morrison JH. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol 282: 191–205, 1989. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- Cannon J, McCarthy MM, Lee S, Lee J, Börgers C, Whittington MA, Kopell N. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci 39: 705–719, 2014. doi: 10.1111/ejn.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast 2008: 381243, 2008. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 287: 393–421, 1989a. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445, 1989b. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Age-related prefrontal alterations during auditory memory. Neurobiol Aging 18: 87–95, 1997. doi: 10.1016/S0197-4580(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Clowry GJ. An enhanced role and expanded developmental origins for gamma-aminobutyric acidergic interneurons in the human cerebral cortex. J Anat 227: 384–393, 2015. doi: 10.1111/joa.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349, 1999. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 341: 95–116, 1994. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci 5: 175–180, 2002. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Corkin S. Permanent Present Tense: the Unforgettable Life of the Amnesic Patient, H.M. New York: Basic Books, 2013. [Google Scholar]
- Corkin S, Amaral DG, González RG, Johnson KA, Hyman BT. H. M.’s medial temporal lobe lesion: findings from magnetic resonance imaging. J Neurosci 17: 3964–3979, 1997. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csercsa R, Dombovári B, Fabó D, Wittner L, Eross L, Entz L, Sólyom A, Rásonyi G, Szucs A, Kelemen A, Jakus R, Juhos V, Grand L, Magony A, Halász P, Freund TF, Maglóczky Z, Cash SS, Papp L, Karmos G, Halgren E, Ulbert I. Laminar analysis of slow wave activity in humans. Brain 133: 2814–2829, 2010. doi: 10.1093/brain/awq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264: 1102–1105, 1994. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14: 1–19, 1997. doi: 10.1016/S0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat 5: 29, 2011. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol 31: 299–316, 2002. doi: 10.1023/A:1024130211265. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, González-Albo MC, Del Río MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol 412: 515–526, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Hashikawa T, Molinari M, Jones EG. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience 37: 655–673, 1990. doi: 10.1016/0306-4522(90)90097-N. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res 503: 49–54, 1989a. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc Natl Acad Sci USA 86: 2093–2097, 1989b. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex: an Annotated Translation of the Complete Writings. New York: Oxford University Press, 1988. [Google Scholar]
- del Rio MR, DeFelipe J. Double bouquet cell axons in the human temporal neocortex: relationship to bundles of myelinated axons and colocalization of calretinin and calbindin D-28k immunoreactivities. J Chem Neuroanat 13: 243–251, 1997. doi: 10.1016/S0891-0618(97)00050-1. [DOI] [PubMed] [Google Scholar]
- Dermon CR, Barbas H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. J Comp Neurol 344: 508–531, 1994. doi: 10.1002/cne.903440403. [DOI] [PubMed] [Google Scholar]
- Dombrowski SM, Hilgetag CC, Barbas H. Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex 11: 975–988, 2001. doi: 10.1093/cercor/11.10.975. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci 18: 547–558, 2017. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex 19: 2196–2207, 2009. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M, Soriano E. Calretinin-immunoreactive neurons in the normal human temporal cortex and in Alzheimer’s disease. Brain Res 691: 83–91, 1995. doi: 10.1016/0006-8993(95)00622-W. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. New York: Raven, 1980. [Google Scholar]
- Fuster JM. Behavioral electrophysiology of the prefrontal cortex. Trends Neurosci 7: 408–414, 1984. doi: 10.1016/S0166-2236(84)80144-7. [DOI] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol 364: 567–608, 1996a. doi:. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol 364: 609–636, 1996b. doi:. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol 377: 465–499, 1997a. doi:. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Jays PRL, Bacon SJ. Calretinin neurons in human medial prefrontal cortex (areas 24a,b,c, 32′, and 25). J Comp Neurol 381: 389–410, 1997b. doi:. [DOI] [PubMed] [Google Scholar]
- Galazo MJ, Martinez-Cerdeño V, Porrero C, Clascá F. Embryonic and postnatal development of the layer I-directed (“matrix”) thalamocortical system in the rat. Cereb Cortex 18: 344–363, 2008. doi: 10.1093/cercor/bhm059. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MA, Barbas H. Anterior cingulate pathways may affect emotions through orbitofrontal cortex. Cereb Cortex 27: 4891–4910, 2017. doi: 10.1093/cercor/bhw284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MA, Barbas H, Zikopoulos B. Parallel development of chromatin patterns, neuron morphology, and connections: Potential for disruption in autism. Front Neuroanat 12: 70, 2018. doi: 10.3389/fnana.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MA, Joyce MK, John YJ, Zikopoulos B, Barbas H. Mirror trends of plasticity and stability indicators in primate prefrontal cortex. Eur J Neurosci 46: 2392–2405, 2017. doi: 10.1111/ejn.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuska M, Saha S, Fiala J, Barbas H. Synaptic distinction of laminar-specific prefrontal-temporal pathways in primates. Cereb Cortex 16: 865–875, 2006. doi: 10.1093/cercor/bhj030. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34: 905–923, 2007. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277: 195–213, 1988. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA 109: 14681–14686, 2012. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas A, Zilles K, Hilgetag CC. Cortical gradients and laminar projections in mammals. Trends Neurosci 41: 775–788, 2018. doi: 10.1016/j.tins.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Grant S, Hilgetag CC. Structural model explains laminar origins of projections in cat visual cortex (Abstract). Soc Neurosci Abstr 30: 300.309, 2004. [Google Scholar]
- Grant S, Hilgetag CC. Graded classes of cortical connections: quantitative analyses of laminar projections to motion areas of cat extrastriate cortex. Eur J Neurosci 22: 681–696, 2005. doi: 10.1111/j.1460-9568.2005.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31: 359–387, 2008. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26: 317–330, 2003. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Behrens TE. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron 83: 1019–1039, 2014. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Barczak A, Musacchia G, Lipton ML, Mehta AD, Lakatos P, Schroeder CE. Laminar profile and physiology of the α rhythm in primary visual, auditory, and somatosensory regions of neocortex. J Neurosci 35: 14341–14352, 2015. doi: 10.1523/JNEUROSCI.0600-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci 16: 1576–1587, 2013. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnishfeger K. Development of cognitive inhibition. In: Interference and Inhibition in Cognition, edited by Dempster F, Brainerd C. San Diego, CA: Academic, 1995, p. 175–204. doi: 10.1016/B978-012208930-5/50007-6. [DOI] [Google Scholar]
- Hegdé J, Felleman DJ. Reappraising the functional implications of the primate visual anatomical hierarchy. Neuroscientist 13: 416–421, 2007. doi: 10.1177/1073858407305201. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Grant S. Cytoarchitectural differences are a key determinant of laminar projection origins in the visual cortex. Neuroimage 51: 1006–1017, 2010. doi: 10.1016/j.neuroimage.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Medalla M, Beul SF, Barbas H. The primate connectome in context: Principles of connections of the cortical visual system. Neuroimage 134: 685–702, 2016. doi: 10.1016/j.neuroimage.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, O’Neill MA, Young MP. Indeterminate organization of the visual system. Science 271: 776–777, 1996. doi: 10.1126/science.271.5250.776. [DOI] [PubMed] [Google Scholar]
- Höistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage 40: 1016–1033, 2008. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 34: 247–261, 1996. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J. Posterior parietal lobe of the primate brain. Physiol Rev 62: 1060–1129, 1982. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Muñoz A. The distribution of chandelier cell axon terminals that express the GABA plasma membrane transporter GAT-1 in the human neocortex. Cereb Cortex 17: 2060–2071, 2007. doi: 10.1093/cercor/bhl114. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol 264: 356–395, 1987. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R, Muñoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis). Eur J Neurosci 14: 435–451, 2001. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex 21: 1771–1782, 2011. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John YJ, Zikopoulos B, Bullock D, Barbas H. The emotional gatekeeper: a computational model of attentional selection and suppression through the pathway from the amygdala to the thalamic reticular nucleus. PLoS Comput Biol 12: e1004722, 2016. doi: 10.1371/journal.pcbi.1004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum, 1985. doi: 10.1007/978-1-4615-1749-8. [DOI] [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience 85: 331–345, 1998. doi: 10.1016/S0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus (2nd ed). New York: Cambridge University Press, 2007. [Google Scholar]
- Jones EG, Rakic P. Radial columns in cortical architecture: it is the composition that counts. Cereb Cortex 20: 2261–2264, 2010. doi: 10.1093/cercor/bhq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MK, Barbas H. Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J Neurosci 38: 1677–1698, 2018. doi: 10.1523/JNEUROSCI.2363-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486, 1997. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, García Del Molino LC, Fitzgerald G, Ram K, He M, Levine JM, Mitra P, Huang ZJ, Wang XJ, Osten P. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell 171: 456–469.e22, 2017. doi: 10.1016/j.cell.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex 39: 993–1008, 2003. doi: 10.1016/S0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Kondo H, Tanaka K, Hashikawa T, Jones EG. Neurochemical gradients along monkey sensory cortical pathways: calbindin-immunoreactive pyramidal neurons in layers II and III. Eur J Neurosci 11: 4197–4203, 1999. doi: 10.1046/j.1460-9568.1999.00844.x. [DOI] [PubMed] [Google Scholar]
- Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the connectome: the dynome. Neuron 83: 1319–1328, 2014. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649: 159–173, 1994. doi: 10.1016/0006-8993(94)91060-X. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Karube F, Nomura M, Kawaguchi Y. The diversity of cortical inhibitory synapses. Front Neural Circuits 10: 27, 2016. doi: 10.3389/fncir.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Muller LE 2nd, Telenczuk B, Halgren E, Cash S, Hatsopoulos NG, Dehghani N, Destexhe A. High-frequency oscillations in human and monkey neocortex during the wake-sleep cycle. Proc Natl Acad Sci USA 113: 9363–9368, 2016. doi: 10.1073/pnas.1523583113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30: 16796–16808, 2010. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner HC. Solving the mystery of the human cerebellum. Neuropsychol Rev 20: 229–235, 2010. doi: 10.1007/s11065-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature 417: 645–649, 2002. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lynch JC. The functional organization of posterior parietal association cortex. Behav Brain Sci 3: 485–534, 1980. doi: 10.1017/S0140525X00006324. [DOI] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci 16: 1588–1597, 2013. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Macmillan M. Inhibition and the control of behavior. From Gall to Freud via Phineas Gage and the frontal lobes. Brain Cogn 19: 72–104, 1992. doi: 10.1016/0278-2626(92)90038-N. [DOI] [PubMed] [Google Scholar]
- Macmillan M. Restoring Phineas Gage: a 150th retrospective. J Hist Neurosci 9: 46–66, 2000. doi: 10.1076/0964-704X(200004)9:1;1-2;FT046. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem 16: 616–624, 2009. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Koechlin E, Rosa MGP, Buckley MJ. Managing competing goals–a key role for the frontopolar cortex. Nat Rev Neurosci 18: 645–657, 2017. doi: 10.1038/nrn.2017.111. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci 26: 441–483, 2003. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Mason A, Ilinsky IA, Beck S, Kultas-Ilinsky K. Reevaluation of synaptic relationships of cerebellar terminals in the ventral lateral nucleus of the rhesus monkey thalamus based on serial section analysis and three-dimensional reconstruction. Exp Brain Res 109: 219–239, 1996. doi: 10.1007/BF00231783. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci 23: 161–179, 2006. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron 61: 609–620, 2009. doi: 10.1016/j.neuron.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. J Neurosci 30: 16068–16081, 2010. doi: 10.1523/JNEUROSCI.1773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Specialized prefrontal “auditory fields”: organization of primate prefrontal-temporal pathways. Front Neurosci 8: 77, 2014. doi: 10.3389/fnins.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskenaite V. Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol 379: 113–132, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain 121: 1013–1052, 1998. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, Kennedy H, Fries P. Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89: 384–397, 2016. doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31: 236–250, 2000. doi: 10.1016/S0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Pitkänen A. Distribution of parvalbumin-, calretinin-, and calbindin-D28k-immunoreactive neurons and fibers in the human entorhinal cortex. J Comp Neurol 388: 64–88, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. The medial temporal-lobe amnesic syndrome. Psychiatr Clin North Am 28: 599–611, 2005. doi: 10.1016/j.psc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Preservation of central sets after frontal lesions in monkeys. In: The Frontal Granular Cortex, edited by Warren GW, Akert K. New York: McGraw-Hill, 1964, p. 219–241. [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25: 563–593, 2002. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B, Barbas H. Input-output organization of the primate cerebral cortex. In: Comparative Primate Biology. Neurosciences, edited by Steklis HD, Erwin J. New York: Alan R. Liss, 1988, vol. 4, p. 39–80. [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci 985: 78–91, 2003. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J Neurocytol 26: 779–797, 1997. doi: 10.1023/A:1018518515982. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 198: 121–136, 1981. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3: 65–71, 2002. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex 13: 1072–1083, 2003. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Textura del Sistema Nervioso del Hombre y de los Vertebrados. Zaragoza, Spain: Gobierno de Aragón. Departamento de Cultura y Turismo, 2002, vol. II, part 2 (Original work published 1904). [Google Scholar]
- Rausell E, Bae CS, Viñuela A, Huntley GW, Jones EG. Calbindin and parvalbumin cells in monkey VPL thalamic nucleus: distribution, laminar cortical projections, and relations to spinothalamic terminations. J Neurosci 12: 4088–4111, 1992. doi: 10.1523/JNEUROSCI.12-10-04088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones EG. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci 11: 226–237, 1991a. doi: 10.1523/JNEUROSCI.11-01-00226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones EG. Histochemical and immunocytochemical compartments of the thalamic VPM nucleus in monkeys and their relationship to the representational map. J Neurosci 11: 210–225, 1991b. doi: 10.1523/JNEUROSCI.11-01-00210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep R. Relationship between prefrontal and limbic cortex: a comparative anatomical review. Brain Behav Evol 25: 5–24, 1984. doi: 10.1159/000118849. [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, Cárdenas A, Clascá F, Martínez-Martinez MA, Borrell V. A complex code of extrinsic influences on cortical progenitor cells of higher mammals. Cereb Cortex 27: 4586–4606, 2017. doi: 10.1093/cercor/bhx171. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex 10: 851–865, 2000. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Riedemann T, Schmitz C, Sutor B. Immunocytochemical heterogeneity of somatostatin-expressing GABAergic interneurons in layers II and III of the mouse cingulate cortex: a combined immunofluorescence/design-based stereologic study. J Comp Neurol 524: 2281–2299, 2016. doi: 10.1002/cne.23948. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex 10: 252–262, 2000. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno J, Rollenhagen A, Arlandis J, Santuy A, Merchan-Perez A, DeFelipe J, Lubke JH, Clasca F. Quantitative 3D ultrastructure of thalamocortical synapses from the “lemniscal” ventral posteromedial nucleus in mouse barrel cortex. Cereb Cortex 28: 3159–3175, 2018. doi: 10.1093/cercor/bhx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JH. Immunohistochemical markers in rat cortex: co-localization of calretinin and calbindin-D28k with neuropeptides and GABA. Brain Res 587: 147–157, 1992. doi: 10.1016/0006-8993(92)91439-L. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesendanger M. Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: a multiple tracing study in macaque monkeys. J Comp Neurol 345: 185–213, 1994. doi: 10.1002/cne.903450204. [DOI] [PubMed] [Google Scholar]
- Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ, Clascá F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex 19: 2380–2395, 2009. doi: 10.1093/cercor/bhn259. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol 256: 175–210, 1987. doi: 10.1002/cne.902560202. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol 368: 215–228, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: The Primate Brain: Advances in Primatology, edited by Noback CR, Montagna W. New York: Appleton-Century-Crofts Educational Division/Meredith Corporation, 1970, p. 137–208. [Google Scholar]
- Schlag J, Schlag-Rey M. Visuomotor functions of central thalamus in monkey. II. Unit activity related to visual events, targeting, and fixation. J Neurophysiol 51: 1175–1195, 1984. doi: 10.1152/jn.1984.51.6.1175. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Schlag J. Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. J Neurophysiol 51: 1149–1174, 1984. doi: 10.1152/jn.1984.51.6.1149. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32: 9–18, 2009. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Thalamocortical interactions. Curr Opin Neurobiol 22: 575–579, 2012. doi: 10.1016/j.conb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Notes on the arrangement of some motor fibres in the lumbo-sacral plexus. J Physiol 13: 621–772.17, 1892. doi: 10.1113/jphysiol.1892.sp000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner’s, 1906. [Google Scholar]
- Siwek DF. The Topographic Organization of the Connections Between the Mediodorsal Nucleus and the Prefrontal Cortex in the Rhesus Monkey (Dissertation). Boston, MA: Boston University, 1989. [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol 22: 2313–2318, 2012. doi: 10.1016/j.cub.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 27: 279–306, 2004. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 253: 1380–1386, 1991. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Wang C, Ulbert I, Schomer D, Halgren E. Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus 20: 166–173, 2010. doi: 10.1002/hipo.20597. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46: 831–844, 2010. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res 195: 191–218, 2012. doi: 10.1016/B978-0-444-53860-4.00009-X. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev 32: 413–448, 2000. doi: 10.1016/S0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Teleńczuk B, Dehghani N, Le Van Quyen M, Cash SS, Halgren E, Hatsopoulos NG, Destexhe A. Local field potentials primarily reflect inhibitory neuron activity in human and monkey cortex. Sci Rep 7: 40211, 2017. doi: 10.1038/srep40211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbie C, Barbas H. Specialized pathways from the primate amygdala to posterior orbitofrontal cortex. J Neurosci 34: 8106–8118, 2014. doi: 10.1523/JNEUROSCI.5014-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbie C, Barbas H. Pathways for emotions: Specializations in the amygdalar, mediodorsal thalamic, and posterior orbitofrontal network. J Neurosci 35: 11976–11987, 2015. doi: 10.1523/JNEUROSCI.2157-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol 505: 526–538, 2007. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91: 260–292, 2016. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider L, Mishkin M. Two cortical visual systems. In: Analysis of Visual Behavior, edited by Ingle DJ, Goodale MA, Mansfield RJ. Cambridge, MA: MIT Press, 1982, p. 549–586. [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res 95: 1–24, 1975a. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res 95: 25–38, 1975. doi: 10.1016/0006-8993(75)90205-X. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res 95: 39–59, 1975b. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA 111: 14332–14341, 2014. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev 54: 89–107, 2015. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262: 256–270, 1987. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 204: 205–207, 1979. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- von Economo C. Cellular Structure of the Human Cerebral Cortex, translated and edited by Triarhou LC. Basel, Switzerland: Karger, 2009. (Original work published 1927). [Google Scholar]
- von Stein A, Chiang C, König P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci USA 97: 14748–14753, 2000. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73: 59–86, 1940. doi: 10.1002/cne.900730106. [DOI] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci 18: 2069–2081, 2003. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129: 397–410, 2007. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegnér J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer K, Ramon M, Pasternak T, Compte A. Transitions between multiband oscillatory patterns characterize memory-guided perceptual decisions in prefrontal circuits. J Neurosci 36: 489–505, 2016. doi: 10.1523/JNEUROSCI.3678-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31: 599–608, 2008. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol 307: 437–459, 1991. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci 4: 201, 2010. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory. I. input and output zones linking the anterior thalamic nuclei with prefrontal cortices in the rhesus monkey. Thalamus Relat Syst 2: 21–32, 2002a. doi: 10.1016/S1472-9288(02)00031-6. [DOI] [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory. II. afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic areas and the basal ganglia in the rhesus monkey. Thalamus Relat Syst 2: 33–48, 2002b. doi: 10.1016/S1472-9288(02)00030-4. [DOI] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518: 389–404, 2010. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez IB, Muñoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol 486: 344–360, 2005. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol 312: 43–67, 1991. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. J Comp Neurol 332: 175–197, 1993. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticostriatal connections of extrastriate visual areas in rhesus monkeys. J Comp Neurol 352: 436–457, 1995. doi: 10.1002/cne.903520309. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kröner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15: 1178–1186, 2005. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2-3 of monkey prefrontal cortex. Cereb Cortex 19: 1597–1615, 2009. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol 71: 18–33, 2011. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 26: 7348–7361, 2006. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci 18: 417–438, 2007a. doi: 10.1515/REVNEURO.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One 2: e848, 2007b. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci 32: 5338–5350, 2012. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, García-Cabezas MA, Barbas H. Parallel trends in cortical gray and white matter architecture and connections in primates allow fine study of pathways in humans and reveal network disruptions in autism. PLoS Biol 16: e2004559, 2018. doi: 10.1371/journal.pbio.2004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Höistad M, John Y, Barbas H. Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J Neurosci 37: 5051–5064, 2017. doi: 10.1523/JNEUROSCI.3940-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, John YJ, García-Cabezas MA, Bunce JG, Barbas H. The intercalated nuclear complex of the primate amygdala. Neuroscience 330: 267–290, 2016. doi: 10.1016/j.neuroscience.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]