Abstract
Terrestrial animals increase their walking speed by increasing the activity of the extensor muscles. However, the mechanism underlying how this speed-dependent amplitude modulation is achieved remains obscure. Previous studies have shown that group Ib afferent feedback from Golgi tendon organs that signal force is one of the major regulators of the strength of muscle activity during walking in cats and humans. In contrast, the contribution of group Ia/II afferent feedback from muscle spindle stretch receptors that signal angular displacement of leg joints is unclear. Some studies indicate that group II afferent feedback may be important for amplitude regulation in humans, but the role of muscle spindle feedback in regulation of muscle activity strength in quadrupedal animals is very poorly understood. To examine the role of feedback from muscle spindles, we combined in vivo electrophysiology and motion analysis with mouse genetics and gene delivery with adeno-associated virus. We provide evidence that proprioceptive sensory feedback from muscle spindles is important for the regulation of the muscle activity strength and speed-dependent amplitude modulation. Furthermore, our data suggest that feedback from the muscle spindles of the ankle extensor muscles, the triceps surae, is the main source for this mechanism. In contrast, muscle spindle feedback from the knee extensor muscles, the quadriceps femoris, has no influence on speed-dependent amplitude modulation. We provide evidence that proprioceptive feedback from ankle extensor muscles is critical for regulating muscle activity strength as gait speed increases.
NEW & NOTEWORTHY Animals upregulate the activity of extensor muscles to increase their walking speed, but the mechanism behind this is not known. We show that this speed-dependent amplitude modulation requires proprioceptive sensory feedback from muscle spindles of ankle extensor muscle. In the absence of muscle spindle feedback, animals cannot walk at higher speeds as they can when muscle spindle feedback is present.
Keywords: mice, muscle spindles, proprioception, speed-dependent amplitude modulation, walking
INTRODUCTION
In terrestrial legged animals, the stereotyped and rhythmic leg movements (step cycle) during locomotion can be divided into two phases, swing and stance. The swing phase starts with lifting the foot off the ground and moving it toward the direction of the locomotion in the air and ends with placing the foot back on the ground. Once the foot is placed on the ground, the stance phase starts, in which the foot stays stationary while the body moves in the direction of locomotion. Thus the limb carries the body weight and provides propulsion. In mammals, this leg movement is mainly achieved by the precisely patterned contraction pattern of multiple extensor and flexor muscles that move the hip, knee, and ankle joints (Akay et al. 2014; Engberg and Lundberg 1969; Grillner 1981; Rossignol 1996). In a simplified version, extensor muscles are mostly activated during stance and flexor muscles during the swing phase. This coordinated action of muscles is in turn controlled by the patterned activity of pools of motor neurons (locomotor pattern) in the spinal cord. This locomotor pattern is known to be the result of the integrated function of a network of interconnected interneurons in the spinal cord (central pattern generator, CPG) and the sensory feedback from cutaneous and proprioceptive systems in the periphery (McCrea 2001; Pearson 2004; Rossignol et al. 2006).
Animals can move around their environment at different speeds to fulfill diverse purposes such as hunting, escape, migration, or foraging. When the locomotor speed is increased, the step cycle duration decreases. This is caused by a decrease in the stance phase duration, whereas the swing phase duration stays relatively constant (Grillner 1981). Furthermore, electromyogram (EMG) activity recordings from flexor and extensor muscles during different speeds revealed that with increasing speed, the EMG activity of mainly the extensor muscles increases accordingly (Pierotti et al. 1989; Prilutsky et al. 1994; Roy et al. 1991; Walmsley et al. 1978). However, the circuits that give rise to this speed-dependent regulation of extensor activity are not understood. One possibility is that the speed-dependent amplitude regulation is controlled by proprioceptive sensory feedback. Experiments with walking cats and humans suggest that proprioceptive sensory feedback regulates the activity strength of extensor muscles during stance phase (Donelan and Pearson 2004a, 2004b; Donelan et al. 2009; Grey et al. 2007; Sinkjaer et al. 2000). Experiments with human subjects suggest that extensor activity strength during walking is regulated by proprioceptive sensory feedback from the Golgi tendon organs (GTO) and from the muscle spindles (af Klint et al. 2010; Grey et al. 2004, 2007; Mazzaro et al. 2005; Sinkjaer et al. 2000; Yang et al. 1991). In contrast, experiments with cats suggest that the extensor activity during stance is regulated by the GTO feedback, but no evidence has been found regarding the feedback from muscle spindles (Donelan and Pearson 2004a, 2004b; Donelan et al. 2009). In this article, we present evidence that proprioceptive sensory feedback from the muscle spindles regulates the EMG activity of extensor muscles during stance and that this regulation is necessary for locomotion at higher speeds.
To address the role of proprioceptive sensory feedback from muscle spindles in the regulation of activity strength of extensor muscle during walking at different speeds, we recorded EMG activities of multiple muscles while mice walked on a treadmill at various speeds, as previously described (Akay et al. 2014). We hypothesize that “proprioceptive sensory feedback from muscle spindles is necessary for extensor muscle EMG activity upregulation during waking at different speeds.” To address the global role of muscle spindles in speed-dependent amplitude modulation, we performed the same experiments with the Egr3−/− mice, in which muscle spindles fail to properly develop postnatally (Oliveira Fernandes and Tourtellotte 2015; Tourtellotte and Milbrandt 1998). Furthermore, we developed a viral and mouse genetics-based strategy to acutely eliminated muscle spindles only from quadriceps femoris (QF; knee extensor muscles) or triceps surae muscles (TS; ankle extensor muscles). In these experiments, data from the same animal obtained before and after the removal of the muscle spindles were compared to gain insight into the role of the muscle spindle feedback from specific muscles in the speed-dependent amplitude modulation. Our data suggest that speed-dependent amplitude modulation requires proprioceptive sensory feedback from the muscle spindles specifically from the TS, but not from the QF, muscle groups.
METHODS
The experiments were done on adult mice, ages ranging from 60 to 90 days, of either sex. None of the mice were trained before the experiments. All procedures were in accordance with the Canadian Council on Animal Care and were approved by the University Committee on Laboratory Animals at Dalhousie University.
Removal of muscle spindles.
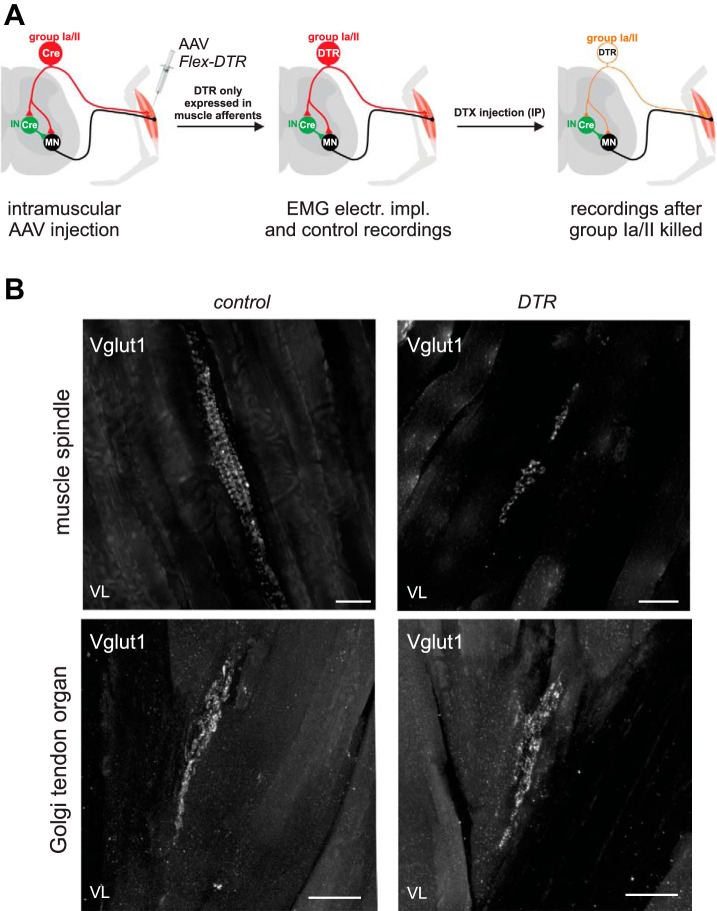
We used two methods to remove proprioceptive feedback from the muscle spindles. The first utilized the Egr3 knockout mice (Egr3−/−) in which all muscle spindles are ablated (Chen et al. 2002; Tourtellotte and Milbrandt 1998). In these mice, the muscle spindles fail to form properly during development, whereas the proprioceptive feedback from the GTOs are left intact. The second method allowed us to acutely eliminate muscle spindle feedback from a subset of muscles (Fig. 1). For this method we used a mouse line that expresses the cre-recombinase under the control of the calcium binding protein parvalbumin (Pv) expression (Pv::cre) (Hippenmeyer et al. 2005). To conditionally and selectively ablate proprioceptors, we generated adeno-associated virus (AAV) serotype 9 encoding diphtheria toxin receptor-green fluorescent protein (DTR-GFP) fusion in a Flex-switch (AAV9-DTR-GFP) (Azim et al. 2014). When injected into the muscle of Pv::cre animals, the AAV infects sensory neurons and expresses DTR-GFP in Pv+ proprioceptors. We injected each one of the QF muscles or the TS muscles when the Pv::cre mice were 7–10 days old (P7–P10). As a control, AAV9 encoding only GFP was injected to the same muscle groups of the contralateral leg. After these AAV injections, Pv::cre mice were kept until adulthood, when they underwent EMG implantation. EMG and kinematic data recordings were performed before and 5–15 days after intraperitoneal injection of diphtheria toxin (DTX; 400 ng dissolved in sterile phosphate buffer).
Fig. 1.
Acute elimination of proprioceptive afferents from selected muscles. Adeno-associated virus (AAV) was used to deliver a cre conditional (flexed) gene encoding the receptor for diphtheria toxin (DTX) into specific muscle of a Pv::cre mouse, where parvalbumin (Pv) is selectively expressed in proprioceptive afferents and some interneurons. A: Pv::cre mice were injected at less than 2 wk of age, infecting proprioceptive afferents and motor neurons innervating that muscle. Because motor neurons do not express Pv, the diphtheria toxin receptor (DTR) gene was only expressed in proprioceptive afferents. Once the mice were older than 50 days, electromyogram electrodes were implanted into their muscles and control recordings were performed. Their locomotor pattern was recorded again 5–15 days after intraperitoneal (IP) DTX injection. The locomotor patterns recorded during pre- and post-DTX sessions were compared. IN, interneurons; MN, motor neurons. B: confocal images of proprioceptive afferents innervating muscle spindle (top) and a Golgi tendon organ (GTO; bottom) from a vastus lateralis (VL) muscle. Afferent fibers were labeled with antibody staining against VGluT1. Control muscle is from the left leg, which did not receive an AAV injection. Experimental muscle shown is from the right leg after AAV and DTR injection. Notice that the typical annulospiral structure is degraded to some punctuated structures in the experimental leg after DTX injection, but the GTOs appear normal. Scale bars, 50 µm.
Electrode implantation surgeries.
Each wild type, Egr3−/−, or Pv::cre mouse injected with AAV9-DTR-GFP received an electrode implantation surgery as previously described (Akay et al. 2014; Mayer and Akay 2018). Briefly, the animals were anesthetized with isoflurane, ophthalmic eye ointment was applied to the eyes, and the skin of the mice was sterilized with three-part skin scrub using Hibitane, alcohol, and povidone-iodine. A set of six bipolar EMG electrodes were implanted in all experimental mice (Akay et al. 2006; Pearson et al. 2005) as follows: The neck region and the right hind leg were shaved. Small incisions were made to the neck region and in the leg above muscles. The electrodes were drawn subcutaneously from the neck incision to the leg incisions, and the headpiece connector was stitched to the skin around the neck incision. The EMG recording electrodes were implanted into hip flexor (iliopsoas, Ip) and extensor (anterior biceps femoris, BF), knee flexor (semitendinosus, St) and extensor (vastus lateralis, VL), and ankle flexor (tibialis anterior, TA) and extensor (gastrocnemius, Gs). The incisions were closed, and buprenorphine (0.03 mg/kg) and ketoprofen (5 mg/kg) were injected subcutaneously as analgesics. Additional buprenorphine injections were performed at 12-h intervals for 48 h. The anesthetic was discontinued, and mice were placed in a heated cage for 3 days and then finally returned to their regular mouse rack. Food mash and hydrogel were provided for the first 3 days after the surgery. Handling of the mice was avoided until they were fully recovered. The first recording session was started no earlier than 10 days after electrode implantation surgeries.
Behavioral recording sessions.
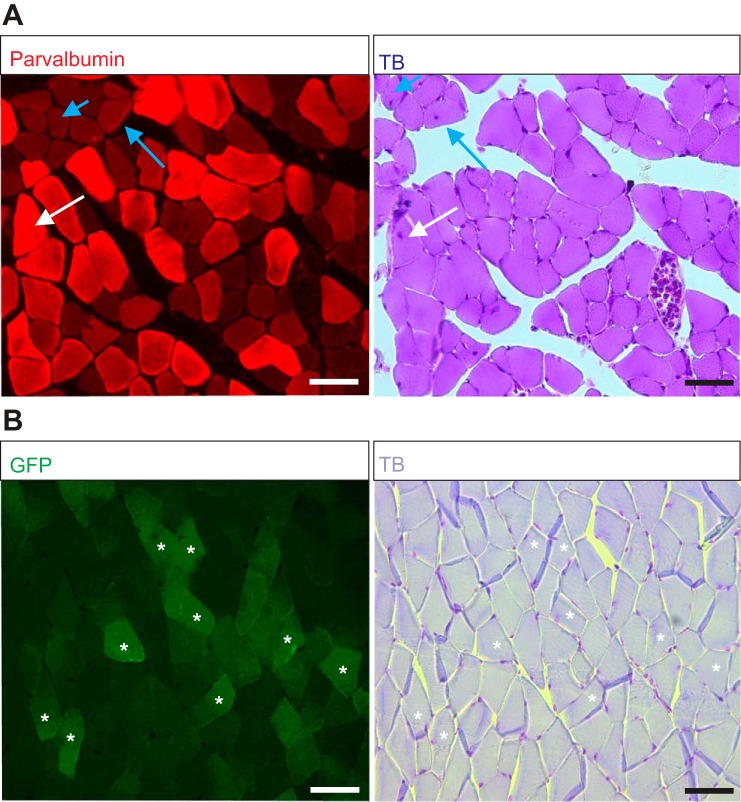
Following the full recovery from electrode implantation surgeries, the behavioral recordings were performed as previously described (Akay et al. 2006; Pearson et al. 2005). Under brief anesthesia with isoflurane, custom-made cone-shaped reflective markers (1–2 mm in diameter) were attached to the skin at the level of anterior tip of the iliac crest, hip, knee, ankle, and metatarsal phalangeal joint (MTP) and the tip of the fourth digit (toe). The anesthesia was discontinued and the mouse placed on the mouse treadmill (model 802; custom built in the workshop of the Zoological Institute, University of Cologne, Germany). The electrodes were connected to the amplifier (model 102; custom built in the workshop of the Zoological Institute, University of Cologne, Germany). We waited at least 5 minutes before the session started to allow the mouse to fully recover from anesthesia. The mouse started walking when the treadmill was turned on. The speed of the treadmill was changed, starting from 0.2 m/s and increasing to 0.6 m/s, a speed at which all wild-type mice could walk. The walking mouse was filmed from the sagittal plane with a high-speed video camera (IL3; Fastec Imaging) at 250 frames/s, and video files were stored on the computer for later motion analysis. The EMG data were stored separately on the computer by using the Digitizer (Power 1401, Cambridge Electronic Design, Cambridge, UK) combined with Spike2 software (version 8; Cambridge Electronic Design). Only walking sequences where the mouse would walk stationary without drifting forward or backward, indicating equal walking speed and treadmill speed, were considered for data analysis. These recordings were performed once with each wild-type and Egr3−/− mouse. In most of the experiments with the Pv::cre mice that received AAV9 injection, three sets of recordings on different days were performed before the DTX injection, and three sets of recordings were performed after DTX injection. The three recordings before and after DTX injections were used to ensure stable EMG recordings over multiple days. Because Pv is also expressed in some extrafusal muscle fibers (Celio and Heizmann 1982) and AAV9 is known to also infect extrafusal muscle fibers (Katwal et al. 2013), we did histological assessments to show that in our experiments, DTX injection did not affect extrafusal muscle fibers (Fig. 2; see discussion).
Fig. 2.
No sign of muscle fiber degeneration in Pv::cre mice previously injected with adeno-associated virus serotype 9 (AAV9) to deliver gene encoding diphtheria toxin receptor (DTR) after diphtheria toxin (DTX) injection. A: fluorescence microscope images of a cross section through a gastrocnemius (Gs) muscle previously injected with AAV9 to deliver DTR-GFP gene and after DTX injection. Parvalbumin (Pv)-expressing fibers (red; left) have a very healthy appearance in toluidine blue (TB) staining (right). Occasional fibers with centralized nuclei were observed in either Pv+ (white arrow) and Pv− (blue arrows) muscle fibers, indicating that this feature was independent of DTX effect. B: fluorescence images of muscle previously injected with AAV9 to deliver DTR-GFP gene and after DTX injection. The fibers that are expressing GFP are Pv+ fibers that were infected with AAV9 (asterisks; left). The same fiber successfully infected with AAV9 and expressing the delivered DTR-GFP gene shows no sign of any degeneration (asterisks; right). Scale bars, 50 µm.
Immunohistology.
After each experiment where muscle spindle afferents were removed acutely by AAV9 and DTX injections in Pv::cre mice, the efficiency of muscle spindle removal was assessed with immunohistology. After the last recording sessions following DTX injection, mice were euthanized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg). After thoracotomy, the animals were perfused with 20 ml of saline solution followed by 10 ml of 4% paraformaldehyde (PFA) through the left cardiac ventricle. The Gs or the VL were dissected and then cryoprotected by immersion in 30% sucrose-PBS solution overnight at 4°C. The following day, the muscles were embedded in optimal cutting temperature compound (OCT) mounting medium, flash frozen on dry ice, and stored at −80°C. Muscle tissue was sectioned longitudinally at 80 µm by using a cryostat (Leica CM3050 S), and the sections were placed on microscope slides. For immunofluorescence staining, the sections were washed in PBS to remove the OCT, incubated in blocking solution (PBS-1% BSA-0.3% Triton) for 1 h, and later incubated overnight with primary rabbit antibody against vesicular glutamate transporter 1 (anti-VGluT1, 1:8,000) (de Nooij et al. 2015). The next day, tissues received multiple washes in blocking solution and were incubated overnight at room temperature with secondary antibody (goat anti-rabbit conjugated to Alexa Fluor 488, 1:500; Life Technologies) in the blocking solution. The sections were washed with blocking solution, followed by a wash with PBS to remove the BSA. Finally, the microscope slides with the sections were coverslipped using mounting medium (Permafluor).
Data analysis.
The kinematic parameters of walking were obtained from the video files using custom-made software written by Dr. Nicolas Stifani with ImageJ (KinemaJ) and R (KinemaR) (Bui et al. 2016; Fiander et al. 2017). The coordinates and the angular joint movements were then imported into the Spike2 files containing the EMG data by using a custom-written Spike2 script to analyze the kinematic and EMG data. All plots were created with Excel 2016 software and statistical analysis with the data analysis package for Excel (statistiXL; version 1.8). Student’s t-test was used to compare data between wild-type and Egr3−/− mice and for the walking at 0.2 and 0.4 m/s in wild-type mice. Moreover, a t-test for paired data was used to compare data before and after DTX injection in Pv::cre mice injected with AAV. The changes were considered statistically significant if P < 0.05.
RESULTS
Speed-dependent amplitude modulation in wild-type mice.
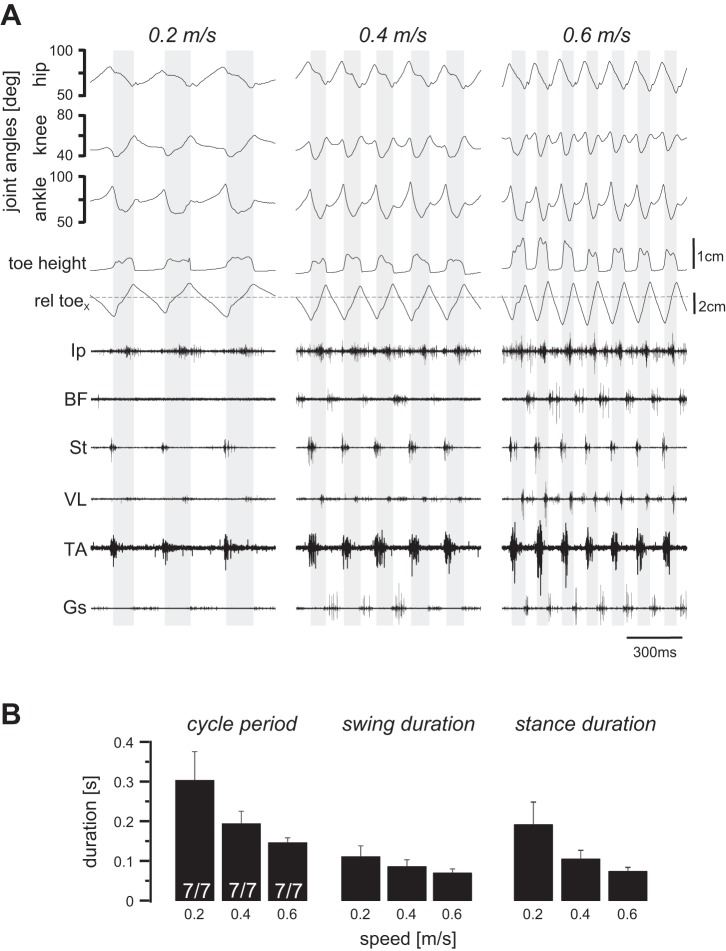
To provide insights into the mechanisms of amplitude modulation, we first examined the muscle EMG activities of different muscles in wild-type mice. None of the seven recorded wild-type mice had any difficulty walking on the treadmill at speeds up to 0.6 m/s (Fig. 3). In Fig. 3A, kinematic and EMG recording data during three episodes of walking at 0.2, 0.4, and 0.6 m/s speed are illustrated including three, five, and seven swing phases (shaded backgrounds), respectively. As the walking speed increased, the step duration decreased (Fig. 3B). The decrease in the step duration was largely the result of a decrease in the stance phase and, to a lesser extent, a change in the swing duration (Fig. 3B). Note also that the EMG activity recorded from the BF (hip extensor), VL (knee extensor), and the Gs (ankle extensor) increased with increased walking speed. The increase in the extensor muscle activity was also shown in pooled average traces of the rectified EMG traces from all muscle in Fig. 4, showing minor increase in the flexor muscles (left traces) but major upregulation in the extensor muscles (right traces). These data suggest that during walking, the EMG activity of the extensor muscles are upregulated in a speed-dependent manner.
Fig. 3.
Locomotor pattern during walking at different speeds in wild-type mice. All wild-type mice (n = 7) were able to walk on the treadmill up to the 0.6 m/s speed. A: angular movement of hip, knee, and ankle joints, toe coordinates represented as toe height and horizontal toe position relative to hip position (rel toex; dashed horizontal line), and electromyogram activities of the 6 recorded flexor and extensor muscles at 3 different speeds are shown. Shaded background indicates swing phase. Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius. B: bar graphs illustrating means and SD of step cycle period, swing duration, and stance duration at 3 different walking speeds. Notice that all parameters decrease with the increasing walking speed, with a stronger effect in cycle period and stance duration than in swing phase.
Fig. 4.
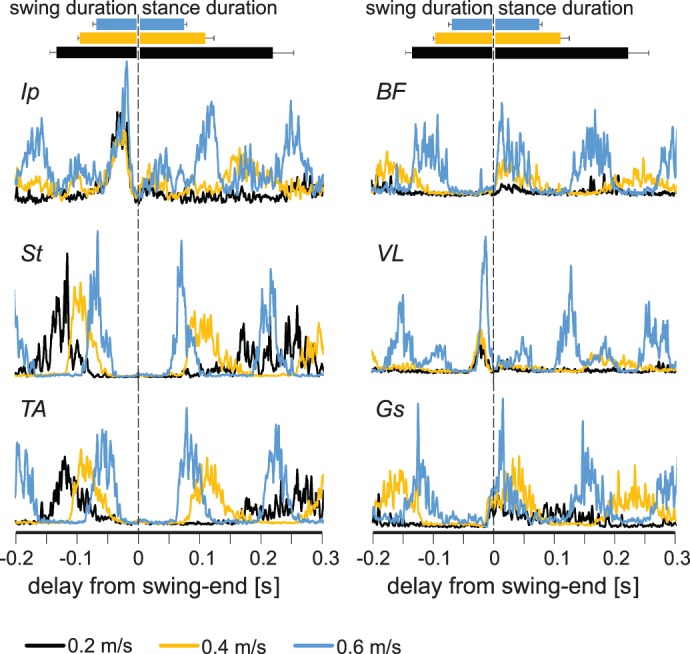
Electromyogram (EMG) activity in leg muscles increases at higher walking speeds. Average EMG activities (rectified and smoothed) from all flexor (left) and extensor (right) muscles at different speeds (black, 0.2 m/s; yellow, 0.4 m/s; blue, 0.6 m/s) recorded in this study indicate that the EMG activity of extensor muscles increases at higher speeds. Durations of stance and swing are shown by horizontal bars (top) indicating averages ± SD. Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius.
The maximal EMG activities normalized to the EMG amplitude at 0.2 m/s walking speed in individual animals are plotted against walking speed to more easily visualize the speed-dependent amplitude modulation (Fig. 5). The EMG amplitude of all extensor muscles was upregulated depending on the speed of locomotion (Gs and VL, P < 0.05; BF, P < 0.01; after ANOVA). In contrast, the activity amplitude of the other flexor muscles was not dependent on the walking speed, except for that of the TA muscle (P < 0.05; after ANOVA).
Fig. 5.
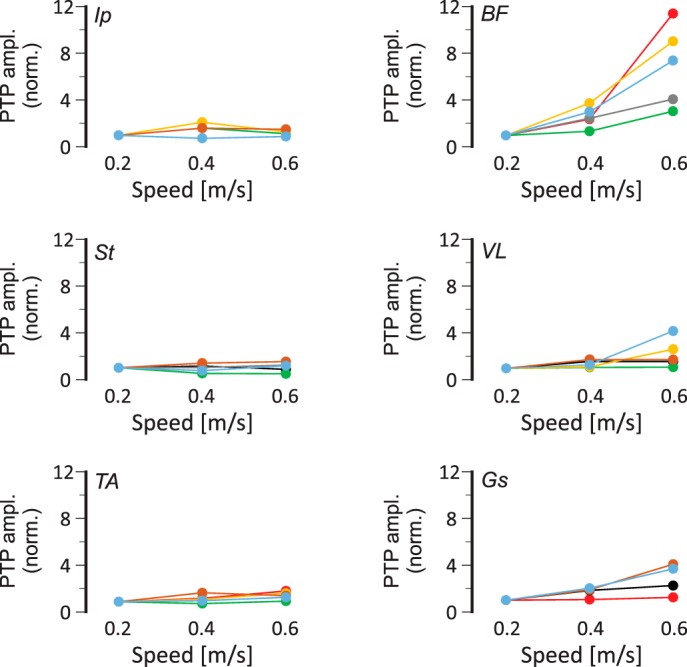
Speed-dependent amplitude modulation of extensor electromyogram (EMG) activities in wild-type mice. Maximal EMG activities (peak-to-peak amplitude, PTP ampl.) in all recorded flexor (left) and extensor (right) muscles, normalized (norm.) to the maximal activity at 0.2 m/s as a function of walking speed. Note that activity in extensor muscles increases with increasing speed, but flexor muscle activities remain relatively unchanged. Colors indicate individual animals (n = 7). Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius.
Speed-dependent amplitude modulation is compromised in Egr3−/− mice.
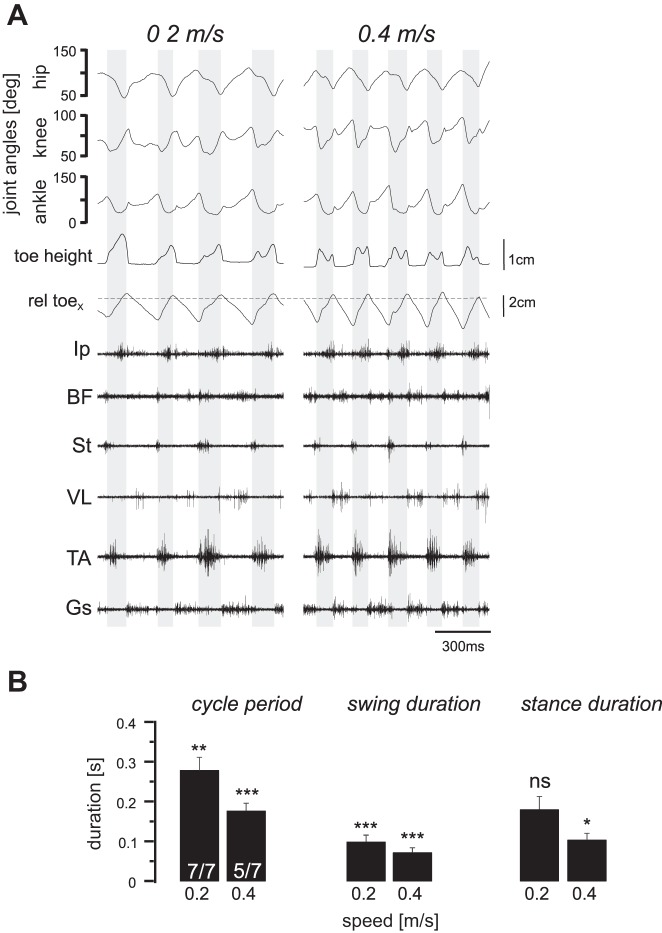
To address the role of proprioceptive sensory feedback from muscle spindles in the speed-dependent modulation of muscle activity, we measured EMG signals from leg muscles during walking at different treadmill speeds in Egr3−/− mice. In contrast to wild-type mice, none of the seven Egr3−/− mice could walk at speeds higher than 0.4 m/s, and only five of seven mice could reach the 0.4 m/s speed. The kinematic and EMG recording data during two episodes of walking at 0.2 and 0.4 m/s speeds, shown in Fig. 6A, that include four and five swing phases (shaded backgrounds), respectively. As in wild-type mice, the step duration decreased as the walking speed increased from 0.2 to 0.4 m/s (Fig. 6B). Furthermore, in the Egr3−/− mice, the decrease in the step duration was the result of the decreasing stance duration with lesser contribution of a change in the swing duration, similar to that in wild-type animals (Fig. 6B). Comparing the duration from Egr3−/− mice and wild-type mice revealed that the cycle periods and the swing durations were significantly shorter in Egr3−/− mice than in wild-type mice. Stance duration, however, was not significantly different at 0.2 m/s but was significantly shorter at 0.4 m/s in Egr3−/− mice compared with wild-type mice (P < 0.05). One possible explanation for the altered swing phase with minor change in stance phase is that the lack of muscle spindle feedback may be compensated by GTO signaling during stance (Akay et al. 2014). These data suggest that proprioceptive sensory feedback from the muscle spindles is important for regulating the temporal characteristics of leg movement during walking at different speeds.
Fig. 6.
Locomotor pattern during walking at different speeds in Egr3−/− mice. None of the Egr3−/− mice would walk at 0.6 m/s, and only 5 of 7 could walk at 0.4 m/s. A: hip, knee, and ankle joint movements, along with toe height and horizontal toe position relative to hip position (rel toex; dashed horizontal line) and electromyogram activities of the 6 recorded flexor and extensor muscles at 3 different speeds are shown. Shaded background indicates swing phase. Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius. B: all means and SD of step cycle period, swing duration, and stance duration at 3 different walking speeds in the Egr3−/− mice were smaller than the same parameters in wild-type mice. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (after Student’s t-test to detect statistical significance in differences between these parameters with the wild-type parameters shown in Fig. 2).
Further examination of extensor muscle activity revealed that the strength of the EMG signal of each extensor muscle did not change considerably when Egr3−/− mice increased walking speed from 0.2 to 0.4 m/s (Fig. 6A). This missing upregulation of the extensor muscles is illustrated in Fig. 7, where the pooled averages of the rectified EMG activities from flexor (left) and extensor muscles (right) during walking at 0.2 and 0.4 m/s are shown. Our data therefore suggest that in the absence of muscle spindles, mice are unable to reach walking speeds >0.4 ms. Furthermore, when they do increase their walking speed, there is no speed-dependent amplitude modulation of extensor muscles.
Fig. 7.
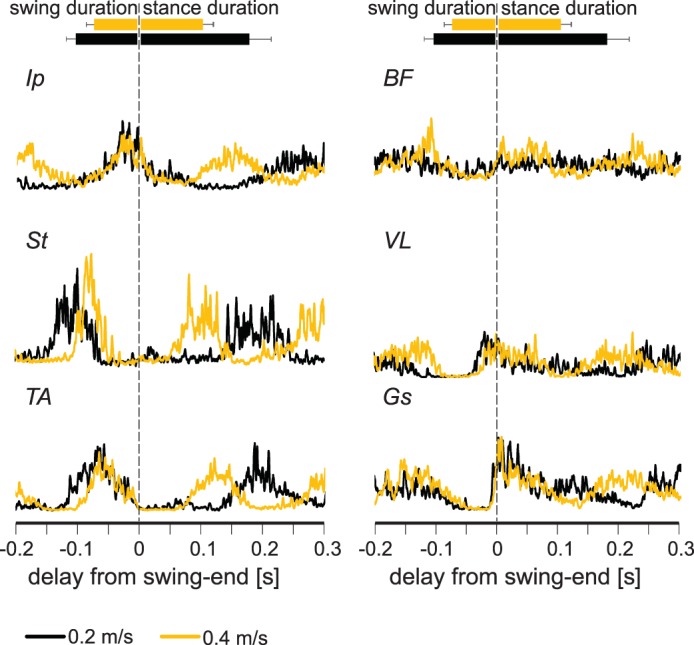
Electromyograph (EMG) activity in extensor muscles does not increase as the walking speed increases from 0.2 to 0.4 m/s in Egr3−/− mice. Average EMG activities (rectified and smoothed) from all flexor (left) and extensor (right) muscles recorded at different speeds (black, 0.2 m/s; yellow, 0.4 m/s) indicate that the EMG activity of extensor muscles increases at higher speeds. Durations of stance and swing are shown by horizontal bars (top) indicating averages ± SD. Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius.
Comparison of maximal EMG activities in wild-type mice and Egr3−/− mice during walking at 0.2 and 0.4 m/s revealed that the amplitude modulation in hip extensor muscle was reduced (Fig. 8). When the overall increases in the amplitudes at 0.4 m/s were compared between Egr3−/− and wild-type mice, only the amplitude modulation in BF activity was significantly different. When wild-type mice walked at 0.4 m/s, the BF EMG amplitude increased, on average, 260 ± 88% (SD). In Egr3−/− mice, the EMG amplitude increase was 142 ± 42%, which was statistically smaller (P = 0.045, Student’s t-test; Fig. 8). There were no significant differences in VL (wild type: 138 ± 29%; Egr3−/−: 168 ± 73%; P = 0.43) or Gs (wild type: 170 ± 44%; Egr3−/−: 131 ± 68%; P = 0.34). We could not detect any significant change in EMG amplitudes at 0.4 m/s in wild-type vs. Egr3−/− mice in any of the recorded flexor muscles (Ip: P = 0.24; St: P = 0.13; TA: P = 0.44). These data suggest that in the five of seven Egr3−/− mice that could walk at 0.4 m/s, the increase in extensor EMG activity was compromised but strong and consistent enough to be statistically significant only in the hip extensor muscle.
Fig. 8.
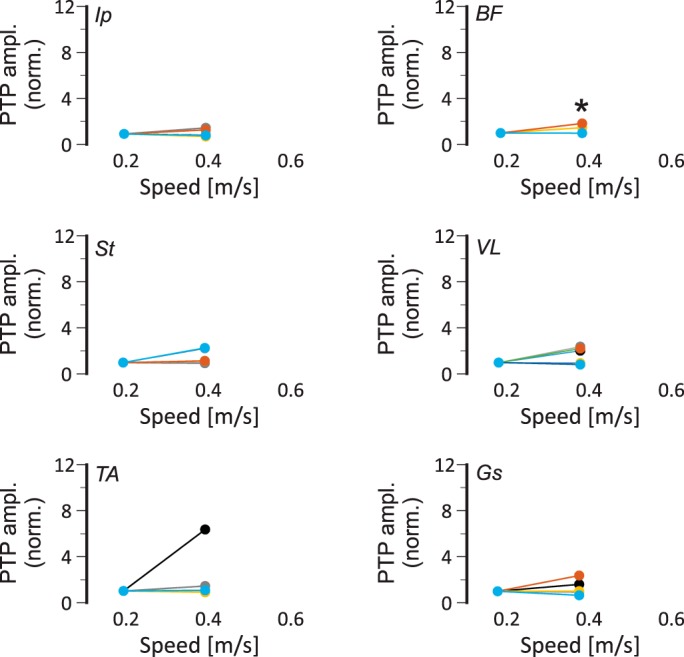
Speed-dependent amplitude modulation of extensor electromyograph (EMG) activities is compromised in Egr3−/− mice. Maximal EMG activities (peak-to-peak amplitude, PTP ampl.) in all recorded flexor (left) and extensor (right) muscles, normalized (norm.) to the maximal activity at 0.2 m/s at 0.2 and 0.4 m/s walking speeds in individual (color coded) Egr3−/− mice (n = 7). Each color represents data from one animal. Based on the Student’s t-test, only in biceps femoris (BF) muscle was the amplitude modulation at 0.4 m/s sufficiently weaker in Egr3−/− mice compared with wild-type mice to reach statistical significance (*P < 0.05). Ip, iliopsoas; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius.
Our data suggest that the speed-dependent increase of the hip extensor EMG activity requires proprioceptive sensory feedback from the muscle spindles. In the absence of feedback from muscle spindles, the hip extensor amplitude modulation is compromised, preventing the animal from reaching higher walking speeds.
Muscle spindle feedback specifically from the TS muscle group is particularly important in speed-dependent amplitude modulation.
How is the information from the muscle spindle processed by the central nervous system to achieve the speed-dependent amplitude modulation of the extensor muscle activities? We reasoned that one possibility might be that information signaling individual joint proprioception could affect the amplitude modulation of the extensor muscles of the same joints (local processing). Alternatively, the information from muscle spindles of multiple joints could be collectively processed in the central nervous system to regulate all or a larger group of the extensor muscles (global processing). To differentiate between these two possibilities, we selectively removed feedback from either the ankle extensor TS muscles (including Gs muscles) or the knee extensor QF muscles (including VL muscle) and measured the amplitude modulation as described above.
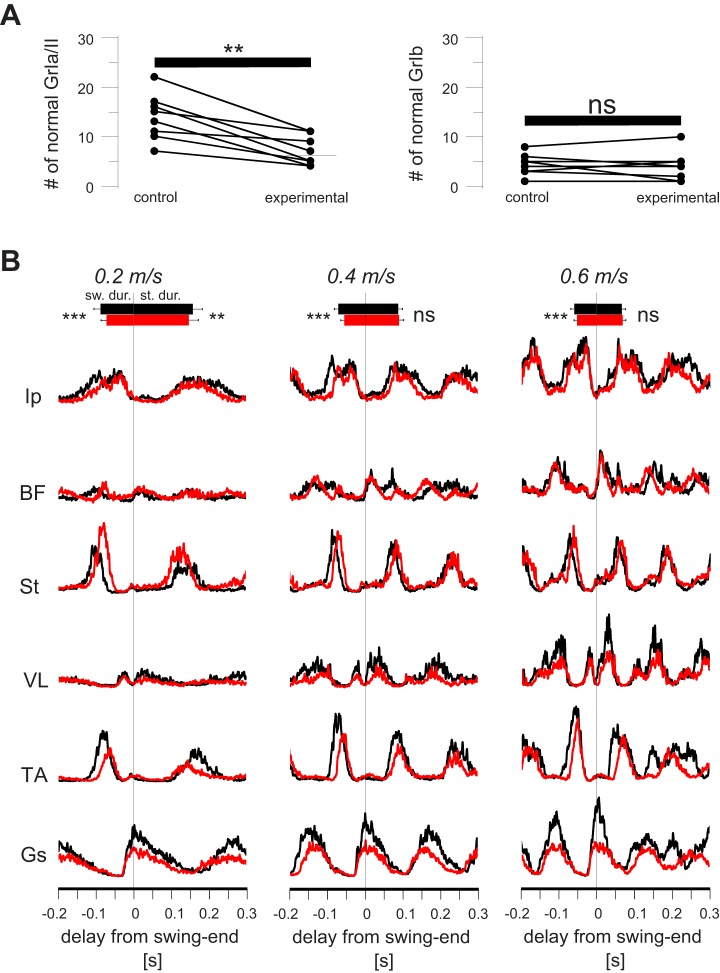
If the information from the muscle spindles is processed locally, elimination of the muscle spindles only from the TS muscles should selectively affect speed-dependent amplitude modulation only in Gs muscle, which is one of the three TS muscles. To ensure that with this method we were effectively removing muscle spindle innervation, we counted all the afferent endings at the muscle spindles in the Gs muscle of the right leg and the left leg (Fig. 9A). The left leg received AAV injections that only express GFP and did not envelop the gene that encodes the DTR (control leg), and therefore no ablation of nerve endings after the DTX infection. The right leg was injected with AAV enveloping the gene that encodes the DTR (experimental leg), which would affect the nerve endings after the DTX infection. After the final post-DTX recording session was performed, we counted the VGluT1-positive afferent endings at muscle spindles and the GTOs in the left (control) and right (experimental) legs. The results suggested that, on average, 55 ± 23% of the muscle spindle afferents in the Gs muscle were eliminated after DTX injection, whereas GTOs were not affected (Fig. 9A).
Fig. 9.
Acute elimination of the muscle spindle afferents only from triceps surae (TS) muscle group affects speed-dependent amplitude modulation in distal extensor muscles. A: graphs show that, on average, 55 ± 23% (mean ± SD) of afferent endings at muscle spindles (GrIa/II; left) were degenerated in the gastrocnemius (Gs) muscle (**P < 0.01 after paired t-test), whereas no difference (ns) was found in the number of afferents to Golgi tendon organs (GrIb; right). Comparisons were made between the right legs (control) and the left legs (experimental). B: average electromyogram (EMG) activities (rectified and smoothed) from all recorded muscles at different speeds recorded before (black) and after (red) the elimination of muscle spindles in TS muscle group through diphtheria toxin (DTX) injection. Note that the Gs EMG traces after removal of muscle spindles (red trace) in TS are much lower than before removal (black trace) at 0.4 and 0.6 m/s. In vastus lateralis (VL), there is a milder change that is more obvious only at 0.6 m/s. Durations of swing and stance are shown by horizontal bars (top) indicating averages ± SD. **P < 0.01; ***P < 0.001; ns, not significant (after paired t-test). Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; TA, tibialis anterior.
The averaged EMG traces from all recorded muscles before and after DTX injection to acute elimination of proprioceptive feedback from muscle spindles from only the TS muscles are illustrated in Fig. 9B. Note that in all muscles and at all speeds, pre-DTX and post-DTX EMG traces overlapped, except for the Gs and, to a lesser extent, the VL and TA muscles. These three muscles showed reduced activity already at 0.2 m/s speed, and the difference increased with increasing locomotor speed in Gs and VL, whereas the difference diminished in TA at 0.6 m/s. These observations were consistent when EMG traces from individual animals were investigated. That is, in seven of nine Gs recordings and in five of nine TA recordings, the speed-dependent amplitude modulation was absent. Moreover, in VL muscle the amplitude modulation was present but less prominent in three of four recordings and absent in the one remaining recording. Our data suggest that acute elimination of muscle spindles from only TS muscles reduced amplitude modulation in two distal extensor muscles, the VL and Gs, and partly in one of the distal flexor muscles, the TA.
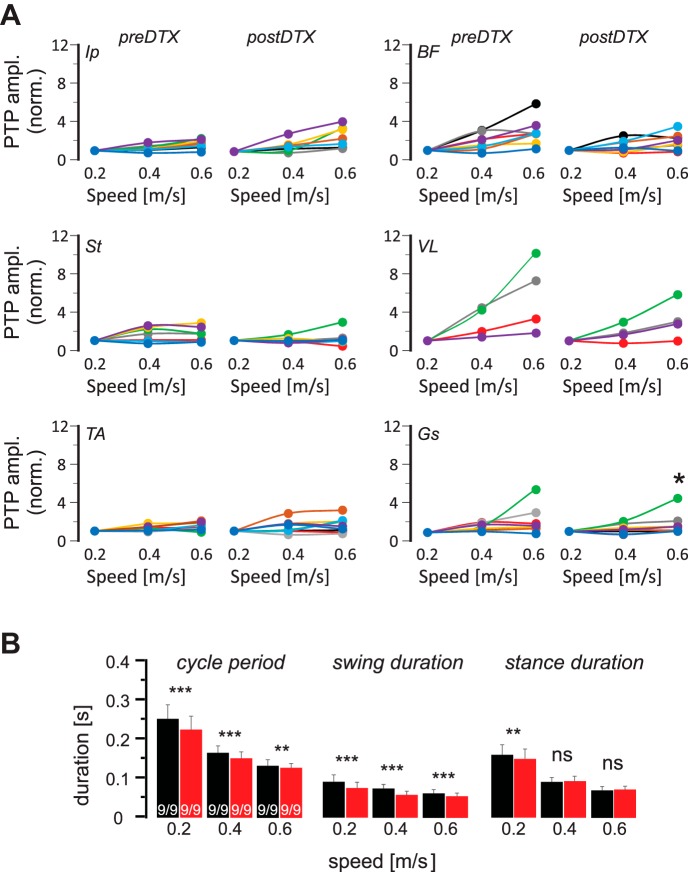
As in Figs. 5 and 8, the maximum of rectified EMG activity in each step was averaged and normalized to the average amplitude of that value at 0.2 m/s and is plotted as a function of treadmill speed before and after the DTX injection (Fig. 10A). The values at each speed were then compared before and after the DTX injection using a paired t-test. Only the amplitude increase at Gs muscle at 0.6 m/s was significantly compromised after the removal of muscle spindle feedback from the TS muscles by DTX injection (pre-DTX: 229 ± 156%, post-DTX: 175 ± 125%, P = 0.018). There was no difference in the amplitude modulation of all other recorded muscles. Analysis of the cycle period, swing duration, and stance duration revealed that cycle period and swing duration consistently decreased after removal of muscle spindle feedback from TS muscles, but changes in stance duration were limited to 0.2 m/s only (Fig. 10B). This result suggests that muscle spindle feedback from the TS muscles mainly regulate the amplitude modulation selectively in the one TS muscle that was recorded in this experiment: the Gs muscle.
Fig. 10.
Decline of speed-dependent amplitude modulation is only statistically significant in gastrocnemius (Gs) muscle after acute removal of muscle spindle afferents from the triceps surae (TS) muscles. A: maximal electromyogram activities (peak-to-peak amplitude, PTP ampl.) in all recorded flexor (left) and extensor (right) muscles, normalized (norm.) to the maximal activity at all 3 walking speeds in individual (color coded) Pv::cre mice in which TS muscle groups were infected with adeno-associated virus before and after diphtheria toxin (DTX) injection. Each color represents data from one animal. Based on the paired t-test, the decrease in amplitude modulation at 0.6 m/s was only statistically significant (*P < 0.05) for Gs muscle. B: means and SD of step cycle period, swing duration, and stance duration at 3 different walking speeds before (black) and after (red) DTX injection indicate that after DTX injection, cycle period and swing duration decreased at all speeds, whereas stance duration only decreased at 0.2 m/s. **P < 0.01; ***P < 0.001; ns, not significant (after paired t-test). Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior.
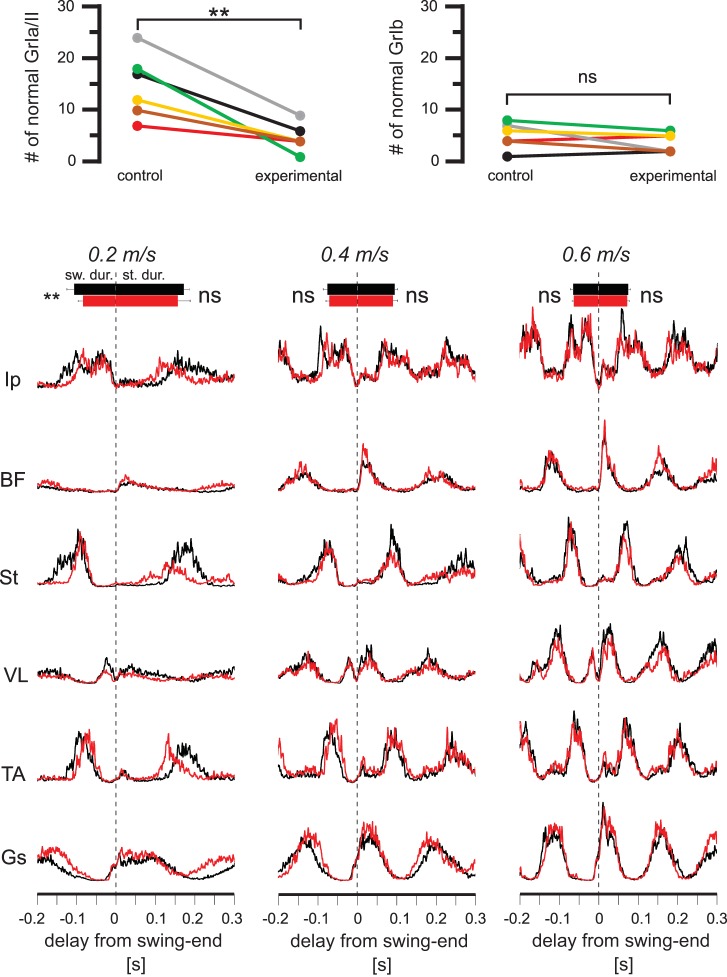
To address the question of whether the amplitude modulation was achieved by muscle spindles only from the TS group, we performed another set of experiments where muscle spindle afferents only to the QF muscles were removed. As for the Gs muscle presented in Fig. 9A, we counted the afferent endings at muscle spindles and the GTOs. Similar to the Gs muscles, we found a 73 ± 10% reduction of muscle spindle afferents in the VL muscles after DTX injection, whereas the GTOs were not affected (Fig. 11A).
Fig. 11.
Acute elimination of the muscle spindle afferents only from the quadriceps femoris (QF) muscle group does not cause a systematic change in speed-dependent amplitude modulation. A: graphs show that, on average, 73 ± 10% (mean ± SD) of afferent endings at muscle spindles (GrIa/II; left) were degenerated in the vastus lateralis (VL) muscle (**P < 0.01 after paired t-test), whereas no differences were found in the afferents to the Golgi tendon organs (GrIb; right). Comparisons were made between the right legs (control) and the left legs (experimental). B: average electromyogram (EMG) activities (rectified and smoothed) from all recorded muscles at different speeds recorded before (black) and after (red) the elimination of muscle spindles in the QF muscle group through diphtheria toxin injection. Notice that all black and red EMG traces overlap at all speeds. Durations of stance and swing are indicated by horizontal bars (top) indicating averages ± SD. **P < 0.01; ns, not significant (after paired t-test). Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; TA, tibialis anterior.
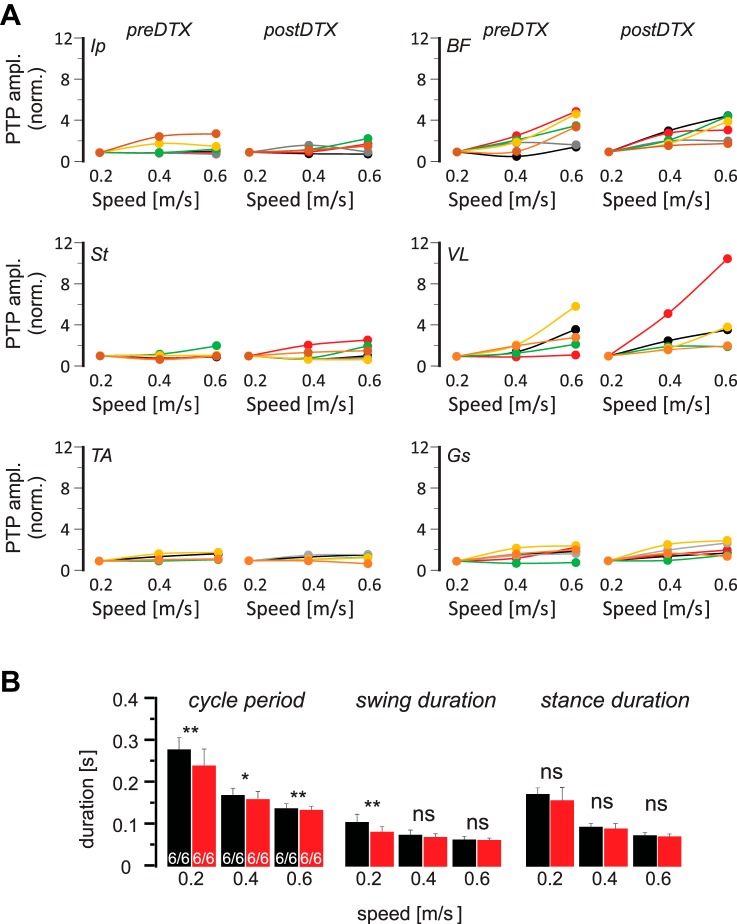
The average EMG traces from the recorded muscles before and after DTX injection to remove muscle spindles only from QF muscles are illustrated in Fig. 11B. After elimination of the proprioceptive sensory feedback from muscle spindles of the QF muscles, no visual effect could be detected on the speed-dependent amplitude in any of the recorded muscles. As for the Gs recordings, these observations were consistent when EMG traces from individual animals were investigated. That is, the speed-dependent amplitude modulation was present in all recorded extensor muscles. This finding suggests that proprioceptive sensory feedback from the QF muscle spindles are not used to regulate the activity strength in any of the recorded muscles. To quantify this observation, we analyzed the maximum EMG activity in all muscles at different speeds before and after DTX injection (Fig. 12). Note that the changes in amplitude modulation were not statistically significant in any of the recorded muscles (Fig. 12A). Following the removal of muscle spindle feedback from the QF muscles, a consistent decrease was only detected in cycle period, whereas only a minor change in swing duration limited to 0.2 m/s was observed (Fig. 12B). No change could be detected in stance duration following DTX injection (Fig. 12B). These data confirm that muscle spindle feedback from QF muscles is not used in speed-dependent amplitude regulation.
Fig. 12.
Decline of speed-dependent amplitude modulation is not statistically significant in any muscle after acute removal of muscle spindle afferents from the quadriceps femoris (QF muscles. A: maximal electromyogram activities (peak-to-peak amplitude, PTP ampl.) in all recorded flexor (left) and extensor (right) muscles, normalized to the maximal activity at all 3 walking speeds in individual (color coded) Pv::cre mice in which QF muscles were infected with adeno-associated virus before and after diphtheria toxin (DTX) injection. Based on the paired t-test, amplitude modulation did not change significantly change after DTX injection. B: means and SD of step cycle period, swing duration, and stance duration at 3 different walking speeds before (black) and after (red) DTX injection indicates that after DTX injection, cycle period decreased at all speeds whereas swing duration only decreased at 0.2 m/s. No change could be detected in stance duration at any speed. *P < 0.05; **P < 0.01; ns, not significant (after paired t-test). Ip, iliopsoas; BF, anterior biceps femoris; St, semitendinosus; VL, vastus lateralis; TA, tibialis anterior; Gs, gastrocnemius.
DISCUSSION
Proprioceptive feedback has been known to play a major role in the regulation of muscle activity strength during walking in humans and cats. However, the type of proprioceptive sensory feedback used for this amplitude control is somewhat controversial, and how this proprioceptive information is processed is not known. We have shown that proprioceptive feedback from muscle spindles is important in regulating the muscle activity strength during locomotion in mice. Furthermore, the amplitude modulation required muscle spindle feedback to regulate the extensor activity increase at higher speeds. Finally, we have shown that muscle spindle feedback from knee extensor muscles did not have an effect on amplitude modulation, whereas muscle spindle feedback from the ankle was important for the regulation of activity mainly in ankle extensor muscles.
Speed-dependent amplitude modulation of extensor muscle activity requires feedback from muscle spindles.
Proprioceptive feedback is known to be important for regulating muscle activity strength during walking (Donelan and Pearson 2004a; Hiebert and Pearson 1999; Pearson 2004). In humans, proprioceptive sensory feedback from the group Ib afferents that innervate GTOs as well as the afferent fibers that innervate muscle spindles are both important for regulating muscle activity strength during walking (af Klint et al. 2010; Grey et al. 2004, 2007; Mazzaro et al. 2005; Sinkjaer et al. 2000; Yang et al. 1991). In contrast, in a quadrupedal animal, the cat, group Ib afferent feedback from the GTOs has been shown to be important for amplitude modulation (Donelan and Pearson 2004a, 2004b; Donelan et al. 2009), but the contribution from muscle spindles has not been appreciated.
Our data provide evidence that muscle spindle feedback is important for regulating muscle contraction strength during walking in a quadrupedal animal. When wild-type mice walk at different speeds, an increasing activity is observed in extensor muscles (Figs. 3–5) as has been described previously in cat (Kaya et al. 2003; Prilutsky et al. 1994; Smith et al. 1993; Walmsley et al. 1978). However, in a mutant mouse model in which muscle spindles do not properly form, the Egr3 knockout mice (Egr3−/−), the mice no longer walk at speeds higher than 0.4 m/s (Fig. 6) and the extensor amplitude modulation is compromised (Figs. 7–9). Furthermore, the swing duration is significantly shorter at the measured speeds in Egr3−/− mice with minor changes in the stance duration leading to decreased cycle period (Fig. 6B). These data suggest that muscle spindle feedback is necessary for both the increased speed of walking and the speed-dependent amplitude modulation.
Because the Egr3−/− mice could not walk at 0.6 m/s, we measured muscle activity at different walking speeds in another model, where muscle spindles were removed only from subset of muscles in an acute way, which resulted in a milder phenotype. Using this method that combines Pv::cre mice and gene delivery with AAV9 and later DTX injection, we could successfully acutely eliminate muscle spindle afferents only in a subset of muscles while leaving the GTO afferents intact (Fig. 10). This is an interesting observation given that the group Ib proprioceptive afferent fibers from the GTOs have also been shown to express Pv (de Nooij et al. 2015). Why then did we not observe significantly lower number of GTOs after DTX injection, even though the number of MS afferents was consistently decreased? One possible explanation is that the AAV9 was injected approximately in the center of the belly of the muscles. This could possibly avoid the infection of the group Ib afferents because the GTOs are typically located at the myotendinous junctions, farther away from the injection site. Nevertheless, regardless of the explanation as to why GTOs were not eliminated by the AAV9/DTX method, our data clearly suggest the number of GTOs after DTX injection remained unaffected.
Using the AAV9/DTX method to acutely eliminate MS feedback, we have shown that acute elimination of muscle spindle feedback from only a subset of muscles does not affect the animals’ ability to walk at higher speed but compromises the amplitude modulation in the extensor muscles (Figs. 9 and 10). Our data provide evidence that proprioceptive feedback from the muscle spindles is important for the regulation of muscle activity strength during walking. Previous studies with humans (Sinkjaer et al. 2000) concluded that afferent feedback from the group II afferents from the muscle spindles and group Ib afferents from the GTOs are important for the regulation of muscle activity strength during walking. Our results provide evidence that muscle spindle feedback-dependent amplitude modulation is necessary for the animals to walk at higher speeds.
One concern with elimination of muscle spindle afferents with AAV9 injection into Pv::cre mice is the possibility of DTX also killing extrafusal muscle fiber that also expresses Pv (Celio and Heizmann 1982). It is known that AAV9 can infect extrafusal muscle fibers (Katwal et al. 2013). Therefore, it is conceivable to expect that in our experiments, DTX injections would have killed Pv-expressing muscle fibers that could also compromise amplitude modulation measured in this study. To exclude this possibility, we performed histological assessment to provide proof that extrafusal muscle fibers were not affected (Fig. 2). We have shown that Pv-expressing extrafusal muscle fibers do not present any sign of damage. Therefore, we are confident that the effect measured in the AAV9/DTX experiments are due to elimination of proprioceptive feedback from the muscle spindles.
Could the reduced activity modulation at higher speeds be an indirect effect produced by inability to achieve the faster speeds because of the ataxia previously observed in Egr3−/− mice (Akay et al. 2014; Takeoka et al. 2014) rather than a direct influence of spindle feedback on muscle activity? Our data suggest that compromised amplitude modulation in the absence of muscle spindle feedback is due to the elimination of direct influence of spindle feedback on muscle activity. Our observation is that amplitude modulation is compromised when muscle spindles are degenerated in only a small group of muscles with the AAV9/DTX approach. None of these animals showed any sign of ataxia or had any difficulty walking on the treadmill at higher speeds. The only change we could detect after DTX injection was that the speed-dependent amplitude modulation, mostly in the Gs muscle after muscle spindle feedback from the TS, was significantly attenuated.
Sensory feedback from muscle spindles of only TS muscles regulate the strength of distal extensor muscle activity.
The results from the experiments in which muscle spindles were removed acutely and selectively from the TS or the QF muscle groups strongly suggest that proprioceptive feedback from only TS muscles is necessary for speed-dependent amplitude modulation. Speed-dependent amplitude modulation of extensor muscle activity was described in the past in rat (Hutchison et al. 1989; Roy et al. 1991) and cat (Kaya et al. 2003; Prilutsky et al. 1994; Smith et al. 1993; Walmsley et al. 1978) model systems, but the mechanism for this modulation was not understood. We have presented evidence that proprioceptive sensory feedback from muscle spindles in the ankle extensors, the TS muscle group, is necessary for the speed-dependent amplitude modulation of distal extensor muscles. Interestingly, our data also suggest that the muscle spindles of the QF group, the knee extensor muscles, are not necessary for the speed-dependent amplitude modulation. These data, however, do not suggest that this is the exclusive picture of amplitude control. Muscle spindles from other muscles that were not infected with AAV in this project could have additional function, and also, QF muscle spindles might have influence on other muscles not recorded in this study. Nevertheless, the data presented suggest distinctive roles of muscle spindles in specific muscles such that TS, but not QF, controls the amplitude modulation in muscles recorded. This finding is in accordance with the previous finding that activation of muscle afferents from the ankle extensor muscles, but not from the knee extensor muscles, strongly enhances ipsilateral extensor activity during fictive locomotion elicited by electrical stimulation of the mesencephalic locomotor region (MLR) in decerebrated cats (Guertin et al. 1995; McCrea 2001). Our data provide evidence that proprioceptive muscle spindle feedback, selectively from the ankle extensor muscles, regulates the speed-dependent amplitude modulation of the distal extensor muscle during walking.
In addition, when the muscle spindles were removed from the TS, the changes in the swing and stance durations and the cycle period during walking at different speeds mimicked the results observed in Egr3−/− mice (Fig. 6B). That is, the swing durations significantly decreased following muscle spindle removal from the TS with s significant decrease in the stance duration only at 0.2 m/s, leading to a significant decrease in the cycle period (Fig. 10B). Interestingly, reduction of the muscle spindle afferents from the QF resulted in a much milder effect on swing duration and the cycle period and no effect on the stance duration (Fig. 12B). This suggests that muscle spindles from the TS have a more prominent effect on step duration parameters than the muscle spindles from the QF muscles.
Why are the changes in amplitude modulation statistically significant only in BF when all muscle spindles are removed systemically as in Egr3−/− mice, but not different when muscle spindles are removed acutely only from a subset of muscles? There could be multiple reasons for this observation. First, when muscle spindles improperly form during development (Oliveira Fernandes and Tourtellotte 2015; Tourtellotte and Milbrandt 1998; Tourtellotte et al. 2001), there may be resultant compensatory changes in BF. Therefore, the acute elimination of muscle spindle feedback from a subset of muscles would have no effect on BF. Second, BF modulation might be controlled by muscle spindle feedback from different muscles that did not receive AAV injection in these studies. Third, all extensor muscles could contribute to the speed-dependent modulation of BF activity, and therefore elimination of feedback from only a small number of muscles does not cause a detectable effect. Our current data do not clearly differentiate between these possibilities.
Negative feedback from muscle spindles controls amplitude modulation.
Previous studies suggest that positive force feedback signals from the group Ib afferents from the GTOs are the major source of amplitude regulation during walking (Duysens et al. 2000; Gossard et al. 1994; McCrea et al. 1995; Pearson and Collins 1993). In addition, cutaneous afferents have also been shown to be important of the regulation of extensor muscle activity (Duysens and Stein 1978; Duysens et al. 1996). In contrast, how feedback from the muscle spindles might contribute to amplitude modulation has been unclear. It has been shown that muscle spindle muscle afferents (group Ia and II) from the triceps surae muscles and from vasti muscles (only known for group Ia; group II not known) are active during the early part of stance (Prochazka et al. 1989; Prochazka and Gorassini 1998) when ankle and knee joints flex. In addition, there is an excitatory influence from the muscle spindle afferents from the TS and VL muscle groups on the extensor muscles (Guertin et al. 1995; McCrea et al. 1995), indicating the existence of a negative feedback pathway (Pearson 2004). Our data provide functional evidence of this negative angular displacement feedback during walking behavior.
Role of muscle spindle feedback during walking.
Locomotion is controlled by a network of interconnected spinal premotor interneurons and sensory feedback from the periphery (McCrea 2001; Pearson 2004; Rossignol et al. 2006). Previous data and our present data suggest that proprioceptive sensory feedback from the muscle spindles from leg muscle regulates three aspects of a step cycle. First, it has an important role in the phase transitions. That is, muscle spindle feedback signaling leg extension at the end of stance phase is an important signal to initiate swing phase (Grillner and Rossignol 1978; Hiebert et al. 1996). Second, muscle spindle feedback is important in the regulation of the precise timing of the activity offset of flexor muscles and therefore important for the precise foot placement at the end of swing phase (Akay et al. 2014). Third, muscle spindle feedback from ankle extensors is important for the regulation of muscle activity strength during stance phase, as our current data suggest. It is well established that ankle joint exerts a brief flexion movement at the beginning of the stance phase that causes the ankle extensor muscles to stretch, activating the stretch-sensitive muscle spindles (Engberg and Lundberg 1969). It has also been shown that the speed of this yield and the ankle muscle stretch increase with the walking speed (Prilutsky et al. 1994), which would result in higher muscle spindle signaling. The higher muscle spindle signaling would then in turn provide stronger excitatory drive to the distal extensor motor neurons to accommodate the speed-dependent amplitude modulation of muscle activity.
GRANTS
W.P. Mayer is supported by Brazilian National Council for Scientific and Technological Development (CNPq) Science Without Borders Fellowship 202749/2015-0. This work was funded by the Dalhousie Medical Research Foundation and Natural Sciences and Engineering Research Council of Canada Grant RGPIN-2015-03871 (awarded to T. Akay).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.P.M. and T.A. conceived and designed research; W.P.M., A.J.M., and S.B.-M. performed experiments; W.P.M. and T.A. analyzed data; W.P.M. and T.A. interpreted results of experiments; W.P.M. and T.A. prepared figures; W.P.M. and T.A. drafted manuscript; W.P.M., A.J.M., S.B.-M., T.M.J., W.G.T., and T.A. edited and revised manuscript; W.P.M., A.J.M., S.B.-M., T.M.J., W.G.T., and T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brenda Ross for technical assistance during this research. We thank Dr. Joriene de Nooij for suggestions on the visualization of afferents innervating muscle spindles and Golgi tendon organs with VGluT1 antibody staining. We also thank Dr. Jacques Duysens and Dr. Robert Brownstone for comments on the manuscript.
REFERENCES
- af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- Akay T, Acharya HJ, Fouad K, Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96: 642–651, 2006. doi: 10.1152/jn.00174.2006. [DOI] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 111: 16877–16882, 2014. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508: 357–363, 2014. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Stifani N, Akay T, Brownstone RM. Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. eLife 5: e21715, 2016. doi: 10.7554/eLife.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Heizmann CW. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature 297: 504–506, 1982. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Chen H-H, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci 22: 3512–3519, 2002. doi: 10.1523/JNEUROSCI.22-09-03512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Simon CM, Simon A, Doobar S, Steel KP, Banks RW, Mentis GZ, Bewick GS, Jessell TM. The PDZ-domain protein Whirlin facilitates mechanosensory signaling in mammalian proprioceptors. J Neurosci 35: 3073–3084, 2015. doi: 10.1523/JNEUROSCI.3699-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, McVea DA, Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol 101: 360–371, 2009. doi: 10.1152/jn.90918.2008. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol 82: 589–598, 2004a. doi: 10.1139/y04-043. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92: 2093–2104, 2004b. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, Stein RB. Reflexes induced by nerve stimulation in walking cats with implanted cuff electrodes. Exp Brain Res 32: 213–224, 1978. doi: 10.1007/BF00239728. [DOI] [PubMed] [Google Scholar]
- Duysens J, van Wezel BM, Prokop T, Berger W. Medial gastrocnemius is more activated than lateral gastrocnemius in sural nerve induced reflexes during human gait. Brain Res 727: 230–232, 1996. doi: 10.1016/0006-8993(96)00525-2. [DOI] [PubMed] [Google Scholar]
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand 75: 614–630, 1969. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Fiander MD, Stifani N, Nichols M, Akay T, Robertson GS. Kinematic gait parameters are highly sensitive measures of motor deficits and spinal cord injury in mice subjected to experimental autoimmune encephalomyelitis. Behav Brain Res 317: 95–108, 2017. doi: 10.1016/j.bbr.2016.09.034. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Mazzaro N, Nielsen JB, Sinkjaer T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol 82: 610–616, 2004. doi: 10.1139/y04-077. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol 581: 99–105, 2007. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Brooks V. Bethesda, MD: American Physiological Society, 1981, p. 1176–1236. doi: 10.1002/cphy.cp010226. [DOI] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146: 269–277, 1978. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487: 197–209, 1995. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol 81: 758–770, 1999. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75: 1126–1137, 1996. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3: e159, 2005. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison DL, Roy RR, Hodgson JA, Edgerton VR. EMG amplitude relationships between the rat soleus and medial gastrocnemius during various motor tasks. Brain Res 502: 233–244, 1989. doi: 10.1016/0006-8993(89)90618-5. [DOI] [PubMed] [Google Scholar]
- Katwal AB, Konkalmatt PR, Piras BA, Hazarika S, Li SS, John Lye R, Sanders JM, Ferrante EA, Yan Z, Annex BH, French BA. Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene Ther 20: 930–938, 2013. doi: 10.1038/gt.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M, Leonard T, Herzog W. Coordination of medial gastrocnemius and soleus forces during cat locomotion. J Exp Biol 206: 3645–3655, 2003. doi: 10.1242/jeb.00544. [DOI] [PubMed] [Google Scholar]
- Mayer WP, Akay T. Stumbling corrective reaction elicited by mechanical and electrical stimulation of the saphenous nerve in walking mice. J Exp Biol 221: jeb178095, 2018. doi: 10.1242/jeb.178095. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol 93: 167–177, 2005. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol 533: 41–50, 2001. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487: 527–539, 1995. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Fernandes M, Tourtellotte WG. Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis. J Neurosci 35: 5566–5578, 2015. doi: 10.1523/JNEUROSCI.0241-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Acharya H, Fouad K. A new electrode configuration for recording electromyographic activity in behaving mice. J Neurosci Methods 148: 36–42, 2005. doi: 10.1016/j.jneumeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Gregor RJ, Edgerton VR. Electromyographic activity of cat hindlimb flexors and extensors during locomotion at varying speeds and inclines. Brain Res 481: 57–66, 1989. doi: 10.1016/0006-8993(89)90485-X. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Herzog W, Allinger TL. Force-sharing between cat soleus and gastrocnemius muscles during walking: explanations based on electrical activity, properties, and kinematics. J Biomech 27: 1223–1235, 1994. doi: 10.1016/0021-9290(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol 507: 293–304, 1998. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Trend P, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80: 61–74, 1989. doi: 10.1016/S0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movement. In: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. Bethesda, MD: American Physiological Society, 1996, p. 173–216. doi: 10.1002/cphy.cp120105. [DOI] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol (1985) 70: 2522–2529, 1991. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523: 817–827, 2000. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Chung SH, Zernicke RF. Gait-related motor patterns and hindlimb kinetics for the cat trot and gallop. Exp Brain Res 94: 308–322, 1993. doi: 10.1007/BF00230301. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159: 1626–1639, 2014. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J. The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol 232: 388–399, 2001. doi: 10.1006/dbio.2001.0202. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 20: 87–91, 1998. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41: 1203–1216, 1978. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 87: 679–687, 1991. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]