Abstract
Background
Alcohol consumption is considered to be a major health problem among people living with HIV/AIDS. Our previous reports have shown that ethanol reduced intracellular concentrations of antiretroviral drugs elvitegravir and darunavir in the HIV-1-infected U1 cell line. Ethanol also increased HIV-1 replication despite the presence of elvitegravir. Our previous finding has also shown that the levels of cytochrome P450 enzyme 2E1 (CYP2E1) and oxidative stress in blood monocytes were induced, while the concentration of alcohol in the plasma was reduced in HIV-1-infected alcohol users compared to uninfected alcohol users. However, the role of CYP2E1 in ethanol-enhanced oxidative stress and HIV-1 replication is still unclear.
Methods
This study examined the chronic effects (14 days) of ethanol on HIV viral load, oxidative DNA damage, expression of CYP2E1, expression of antioxidant enzymes (AOEs), expression of reactive oxygen species (ROS) in human monocyte-derived macrophages (MDM). Further, to evaluate the role of CYP2E1 in mediating ethanol-induced viral replication, CYP2E1 siRNA and CYP2E1 selective inhibitor were used in the HIV-1-infected U1 cell line following ethanol treatment.
Results
Chronic ethanol exposure demonstrated an increase in oxidative DNA damage and CYP2E1 expression in both non-infected and HIV-1-infected MDM. Our results showed that ethanol chronic exposure increased HIV-1 replication by ~3-fold in HIV-1-infected MDM. This ethanol-enhanced HIV-1 replication was associated with an increased oxidative DNA damage, an increased expression of CYP2E1, and a decreased expression of antioxidant enzyme PRDX6. In HIV-1-infected U1 cell line, we observed a decreased viral replication (~30%) and a decreased DNA damage (~100%) after repression of CYP2E1 by siRNA, upon ethanol exposure. We also observed a decreased viral replication (~25%) after inhibition of CYP2E1 by using selective CYP2E1 inhibitor.
Conclusions
The data suggest that chronic ethanol exposure increases HIV-1 replication in MDM, at least in part, through CYP2E1-mediated oxidative stress. These results are clinically relevant to potentially find effective treatment strategies for HIV-1-infected alcohol users.
Abbreviations: MDM, monocyte-derived macrophages; CYP2E1, cytochrome P450 enzyme 2E1; AOEs, antioxidant enzymes; ROS, reactive oxygen species; PLWHA, people living with HIV/AIDS; DE, diethyl ether
Keywords: Ethanol, Cytochrome P450 2E1, HIV-1, Oxidative stress, Monocytes-derived macrophages
Highlights
-
•
Chronic EtOH exposure increased HIV-1 replication and oxidative DNA damage in MDM.
-
•
Chronic EtOH exposure increased CYP2E1 expression in MDM.
-
•
EtOH-enhanced HIV replication and DNA damage were prevented by CYP2E1 siRNA.
-
•
Selective CYP2E1 inhibitor decreased HIV-1 replication upon ethanol exposure.
1. Introduction
Mild-to-moderate drinking is prevalent in people living with HIV/AIDS (PLWHA) [1]. Alcohol consumption is considered to be a major health problem among PLWHA [2]. Alcohol consumption is known to increase the risk of acquisition of HIV-1 infection and the rate of HIV-1 disease progression [3], [4]. Furthermore, chronic alcohol consumption in HIV-1-infected patients has been reported to impact HIV-1 replication and response to antiretroviral therapy [5], [6], [7]. Studies with HIV-1-infected macaques have shown increased viral load in the blood and cerebral compartments of chronic-drinking animals [8]. Moreover, previous reports from our lab suggest that ethanol reduced intracellular concentrations of antiretroviral drugs (elvitegravir, darunavir) in the HIV-1-infected U1 cell line [9], [10]. Ethanol also increased HIV-1 replication despite the presence of elvitegravir [10]. Despite this, little is currently known about the cellular pathways involved with alcohol consumption and HIV-1 replication.
It is well known that monocyte/macrophage lineage cells perform an important role in initial HIV-1 infection and serve as a major viral reservoir for HIV-1 [11], [12]. Additionally, HIV-1-infected monocytes/macrophages can infiltrate into the brain and spread the virus into the central nervous system, causing neurocognitive disorders [13]. Thus, the aim of this study was to examine the cellular pathway associated with alcohol metabolism and HIV-1 replication in monocyte-derived macrophages (MDM).
Cytochrome P450 2E1 (CYP2E1), an important isozyme of CYP superfamily, can metabolize alcohol and generate acetaldehyde and reactive oxygen species (ROS), which can cause oxidative stress [14]. Alcohol is known to induce liver CYP2E1 in chronic drinkers [15]. Previous studies have shown that MDM express CYP2E1 [16], which plays a central role in ethanol metabolism in MDM [17]. Furthermore, a two-day acute ethanol exposure up-regulated CYP2E1 activity in both uninfected and HIV-1-infected MDM, and these effects are assumed to be associated with increased production of ROS [18]. Moreover, our group has shown that alcohol exposure: 1) causes cellular toxicity through the induction of CYP2E1 followed by CYP2E1-mediated oxidative stress in U937 monocytic and SVGA astrocytic cells [19], [20] and 2) induces the expression level of CYP2E1 and oxidative stress in blood monocytes and decreases the level of alcohol in the plasma in HIV-1 positive drinkers compared to HIV-1 negative drinkers [21]. We now hypothesize that chronic ethanol exposure will enhance oxidative stress and HIV-1 replication in human primary MDM, and CYP2E1 plays a critical role on ethanol-enhanced oxidative stress and HIV-1 replication in MDM. The data in this study suggest that chronic ethanol exposure increase HIV-1 replication in MDM, at least in part, through CYP2E1-mediated oxidative stress. These results are clinically relevant to potentially find effective treatment strategies for HIV-1-infected alcohol users.
2. Materials and methods
2.1. Cell culture and treatment
Primary human MDM were isolated from peripheral blood mononuclear cells (PBMCs). The protocol diagram for collecting HIV-1-infected MDM and studying the chronic exposure of ethanol in MDM are described in Supplementary Fig. 1. Briefly, buffy coats were isolated from whole blood obtained from de-identified healthy individuals (Interstate Blood Bank Inc.; Memphis, TN). Buffy coats were incubated with the RosetteSep enrichment cocktail (StemCell Technologies, Vancouver, Canada) for 20 min to enrich the yield of PBMCs. The buffy coats were then layered over the lymphocyte separation medium (Corning Life Sciences, Tewksbury, MA), and centrifuged for 20 min at 1200 g. PBMCs were then collected from buffy coats and cultured in Roswell Park Memorial Institute 1640 (RPMI, Corning Inc.; Tewksbury, MA) media containing 10% human serum, 1% L-glutamine (Corning Inc.), and 5% penicillin-streptomycin solution (Corning Inc.). Human macrophage colony stimulating factor (M-CSF) (50 ng/mL) (PeproTech; Rocky Hill, NJ) was added to the media for macrophage differentiation. Our pervious study confirmed that primary MDM obtained through this protocol yield 95% purity in CD14 + monocytic cells with less than 5% contamination from B and T cells [22]. MDM were collected after 7–10 days of differentiation, then incubated with hexadimethrine bromide (polybrene) (Sigma-Aldrich; St. Louis, MO) and HIV-1 Ada strain (NIH AIDS Reagent Program; Germantown, MD) at 20 ng/million for HIV infection in a 6-well plate with 0.8 million cells/well. After overnight incubation, MDM were washed with PBS twice to remove the polybrene and HIV-1 Ada strain. The cells attached on the bottom of the well were considered as healthy primary MDM with initial HIV-1 infection. The recombinant Human IL-2 (10 ng/mL) (PeproTech) and M-CSF (50 ng/mL) (PeproTech) were added to the culture media of MDM as necessary supplementary for HIV-1 infected MDM. After 7–10 days of the initial HIV-1 infection, cell culture media samples were collected to assess HIV-1 p24 level using a p24 ELISA kit (ZeptoMetrix Corp.; Buffalo, NY). To determine the chronic effects of ethanol, both non-infected and HIV-1 infected primary macrophage were treated either with a non-significant toxic concentration of ethanol (20 mM) or equivalent volume of media (control) for 14 days. The concentration of ethanol (20 mM) was selected to mimic the human blood alcohol concentration (BAC) levels to 0.10% (weight/volume), which is a standard for drunkenness in most of the places [23]. The chronic ethanol treatment pattern was selected based on our existing study [24]. Briefly, the cells were treated every 24 h with 20 mM of ethanol added with 0.5 mL fresh media. Ethanol treatments were done in an incubator humidified with 20 mM ethanol to prevent loss due to ethanol evaporation. DNA, RNA, and protein were isolated from the total cell pellets by using Allprep DNA/RNA/Protein kit (Qiagen; Valencia, CA). DNA and RNA concentrations were measured using a Nanodrop 2000c UV–Vis Spectrophotometer (Thermo Fischer Scientific; Rockford, IL). Protein quantifications were performed with a BCA protein assay kit (Thermo Fischer Scientific).
The HIV-1-infected U1 monocytic cell line was obtained from the NIH AIDS Reagent Program. U1 cell line is an HIV-1-infected U937 cell line, which is a commonly utilized model cell line for study of monocytes and macrophages. It also has been shown to correlate very well to primary cells [25]. U1 cells were grown in RPMI media supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 1% L-glutamine, and penicillin-streptomycin solution, and plated at 0.8 million/wells in a 6-well plate. The cells were differentiated into macrophages using 100 nM phorbol-12-myristate-13 acetate [26] (Sigma-Aldrich) for 72 h. Two treatment protocols were set up in differentiated U1 cells to evaluate the role of CYP2E1 in HIV-1 replication after ethanol exposure: 1) U1 cells were transfected with scramble siRNA or CYP2E1 siRNA (Hs00559368_m1) (Life Technologies; Carlsbad, CA) for 24 h following the manufacturer's protocol. The cells were then washed and treated with control (media only) or ethanol (20 mM) every day for 3 days. 2) U1 cells were treated with 20 mM ethanol then treated with CYP2E1 inhibitor diethyl ether (DE) (Sigma-Aldrich) every day for 3 days. Cell culture media was collected to measure HIV p24 level.
2.2. Quantitation of oxidative DNA damage
The concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a common marker for DNA damage. The amount of 8-OHdG in each sample was determined using the manufacturer's instructions as described previously [27]. The Oxiselect oxidative DNA damage ELISA kit (Cell Biolabs; San Diego, CA) was used for MDM samples, which had a lower DNA yield (<300 ng/sample), while the EpiQuik 8-OHdG DNA Damage kit (Colorimetric) (Epigentek; Farmingdale, NY) was used for U1 cell line samples, which had a higher DNA yield (≥300 ng/sample). The absorbance was measured at 450 nm using a microplate reader. The amount of 8-OHdG was calculated using the standards provided with the kit.
2.3. Reactive oxygen species quantitation
The concentration of reactive oxygen species (ROS) was measured as previously described [20]. In brief, MDM from chronic ethanol treatment were collected and washed with PBS. Cells were incubated with the 5 µM H2DCFDA dye (Life Technologies) in PBS for 30 min at room temperature. The cells were washed with PBS and the median fluorescent intensity (MFI) was measured at 525 ± 20 nm using flow cytometer (BD Biosciences; San Jose, CA). The data were analyzed by using the BD FACS software version 8.
2.4. Western blot analysis
Equal amount of proteins (20 µg) from 14-days treated experiments of MDM were loaded on 10% acrylamide gel. After SDS-PAGE, the proteins were transferred to a PVDF membrane. The membrane was blocked in Li-Cor blocking buffer (LI-COR Biosciences; Lincon, NE) for 1 h and incubated with primary antibodies against CYP2E1 (1:500, Millipore; Billerica, MA), catalase (1:1000, Proteintech; Rosemont, IL), PRDX6 (1:400, Proteintech), SOD1 (1:500, Santa Cruz; Dallas, Texas), SOD2 (1:500, Santa Cruz), and β-actin (1:4000, Cell Signaling; Danvers, MA) at 4 °C overnight. The membrane was then washed and incubated with secondary antibodies: goat anti-mouse Mab (1:10,000, LI-COR Biosciences) and goat anti-rabbit Mab (1:10,000, LI-COR Biosciences). The blots were scanned with a Li-Cor Scanner (LI-COR Biosciences).
2.5. Reverse transcription polymerase chain reaction
Real-time reverse transcription polymerase chain reaction (rt-PCR) was performed to measure the relative mRNA fold expression of CYP2E1 (Hs00559367_m1) and beta-actin (Hs9999903_m1) (Life Technologies, Grand Island, NY) in MDM after ethanol exposure. TaqMan Gene Expression kit (Applied Biosystems, Foster City, CA), SimpliAmp Thermal Cycler (Applied Biosystems, Foster City, CA), and Step-One Plus Real-Time PCR system (Applied Biosystems, Foster City, CA) were used to perform the PCR reaction. The relative fold expression was calculated by using 2-∆∆Ct method by using beta-actin as the housekeeping gene.
2.6. HIV-1 p24 ELISA
The level of HIV-1 viral load was determined from cell culture media by using HIV-1 p24 antigen ELISA kit following the manufacturer's protocol. The p24 was detected by measuring optical density at 450 nm using a microplate reader (Citation 5 from Life Technologies, Grand Island, NY). The concentration of HIV-1 p24 antigen was calculated using standard calibration curve. Since there is a significant variations between experiments and donors, p24 level were normalized to the control group (non-ethanol treated group), and reported as a percentage of the control group.
2.7. Safety precautions
All the HIV-1 related experiments were performed in the Regional Biocontainment Laboratory (RBL) of UTHSC. All the HIV-1 related work was conducted according to biosafety level BSL-3 practices under the protection of biosafety cabinets, personal protective equipment (PPE), and N95 respirators. All the liquid waste was chemically disinfected using 10% (v/v) bleach and all the solid waste was double bagged and autoclaved before removal from containment areas.
2.8. Statistical analysis
All graphs were performed using GraphPad Prism 6 (GraphPad Software; La Jolla, CA). All statistical analysis were performed using RStudio (Version 1.0.153, RStudio, Inc.; Boston, MA). Student's t-test analysis was used for comparisons between two groups. The statistical significance among treatments were determined by using one-way ANOVA post-hoc Tukey HSD Test. A p-value ≤ 0.05 among the treatment groups was considered significant.
3. Results
3.1. Chronic ethanol exposure in non-infected MDM
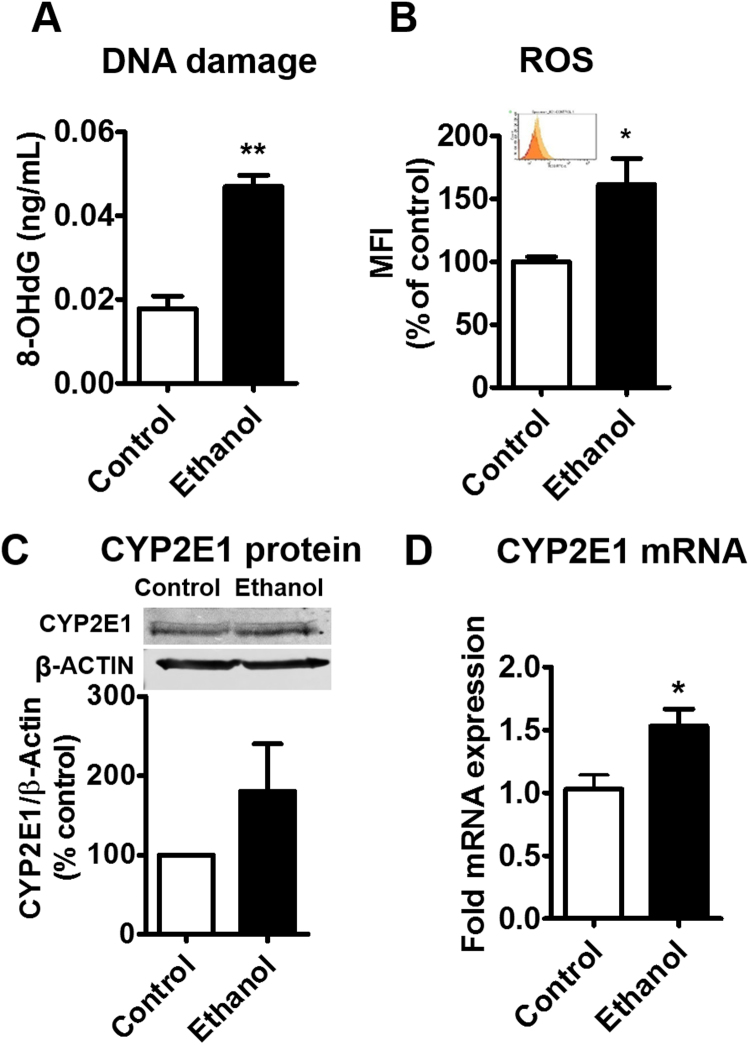
Our previous study has shown that acute ethanol exposure causes cellular toxicity through induction of CYP2E1 followed by CYP2E1-mediated oxidative stress in U937 monocytic cells [19], [20]. The next logical step is to study the effect of chronic ethanol exposure to macrophages. Therefore, we performed a 14-day ethanol treatment (chronic) in human primary MDM to examine the effect of chronic ethanol exposure on oxidative DNA damage, ROS production, and CYP2E1 expression. We observed a ~1.5-fold increase on oxidative DNA damage after 14-day ethanol treatment in non-infected MDM (Fig. 1A, **p < 0.01). Similarly, an increased ROS production was observed in ethanol-treated cells (Fig. 1B, *p < 0.05). We observed a pattern of increased CYP2E1 protein expression in the ethanol-treated non-infected MDM (Fig. 1C). To confirm the CYP2E1 induction, we examined the mRNA level of CYP2E1 in non-infected MDM after chronic ethanol exposure. An increased CYP2E1 mRNA expression was observed in ethanol-treated MDM (Fig. 1D, *p < 0.05).
Fig. 1.
Effect of chronic exposure of ethanol on DNA damage, ROS production, and CYP2E1 expression in non-infected primary monocyte-derived macrophages (MDM). A. The concentration of 8-OHdG was measured using Oxiselect Oxidative DNA Damage ELISA kit. B. Reactive Oxygen Species (ROS) level was measured using flow cytometry and reported as a percentage of the control MDM. The inset indicates absolute MFI between control (orange) and ethanol-treated (yellow) groups C. The CYP2E1 protein relative expression were quantified by western blot and reported as a percentage of the control group (control MDM). ᵦ-actin was used as an endogenous control. D. The CYP 2E1 mRNA fold expression was calculated using qRT-PCR and normalized with control group. ᵦ-actin was used as an endogenous control. All assays were performed on at least triplicate samples with MDM derived from the same donor. Mean ± SEM values were graphed. * and ** indicate p < 0.05 and p < 0.01, respectively, compared to control MDM. Student's t-test. MFI – Median fluorescence intensity.
3.2. Effect of chronic ethanol exposure on oxidative DNA damage and HIV-1 replication in HIV-1-infected MDM
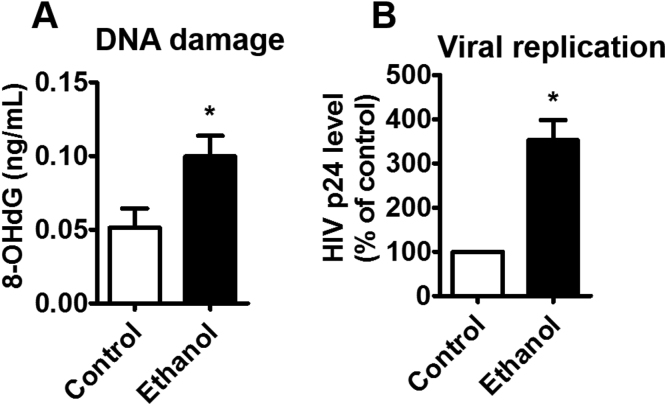
To determine effect of chronic ethanol exposure on HIV-1 replication in human MDM, primary MDM were infected by HIV-1 prior to ethanol treatment, as described in the Method section. HIV-1 infected MDM, isolated from 3 different donors, was treated with 20 mM ethanol for 14 days. A significant increase (~2-fold) in DNA damage level was observed in ethanol-treated MDM compared to control MDM (Fig. 2A, *p < 0.05). Moreover, the increased oxidative DNA damage level was associated with enhanced viral replication. Chronic ethanol exposure demonstrated a significant (~2.5-fold) increase in viral load (Fig. 2B, *p < 0.05).
Fig. 2.
Effect of chronic exposure of ethanol on DNA damage and HIV-1 replication in HIV-1-infected primary MDM. A. The concentration of 8-OHdG was measured using Oxiselect Oxidative DNA Damage ELISA kit. B. Viral load was measured from culture media using a p24 ELISA kit. The p24 levels, obtained from the same donor and for each experiment, were normalized to the respective control MDM, and reported as a percentage of the control group. As expected, the absolute level of p24 varied significantly from donor-to-donor. All assays were performed on triplicate samples with MDM derived from three different donors. * indicates p < 0.05 compared to control MDM, student's t-test.
3.3. Effect of chronic ethanol exposure on the expression of CYP2E1 and antioxidant enzymes in HIV-1-infected MDM
CYP2E1 and antioxidant enzymes (AOEs) both play a major role in alcohol-induced oxidative stress and tissue/organ damage [28]. Therefore, we determined the level of ethanol-metabolizing CYP2E1 enzyme, and important AOEs; Catalase, SOD1, SOD2, and PRDX6, which are known to play important role in detoxifying ROS and maintaining cellular oxidative homeostasis [29], [30]. Compared with the non-ethanol treated group, we observed a significant increase (~20%) in the protein expression of CYP2E1 (Fig. 3A, *p < 0.05) and a pattern of increase in the mRNA expression of CYP2E1 (Fig. 3B) in chronic ethanol-treated HIV-1-infected MDM among 3 different donors. A significant decrease (~20%) of PRDX6 protein expression was observed and there was no significant change in the expression of other AOEs upon ethanol treatment (Fig. 3C, *p < 0.05).
Fig. 3.
Effect of chronic exposure of ethanol on CYP2E1 and antioxidant enzymes (AOEs) protein expressions in HIV-1-infected primary MDM. A. The CYP2E1 protein relative expression was examined using Western blots. B. The CYP 2E1 mRNA fold expression was calculated using qRT-PCR. C. AOEs protein relative expressions were examined using Western blots. Protein/mRNA relative expressions were quantified reported as a percentage/fold change of the control group (control MDM). ᵦ-actin was used as an endogenous control. Western blot and qRT-PCR results, obtained from the same donor and for each experiment, were normalized to the respective control MDM and reported as a percentage of the control group. As expected, the absolute intensity of Western blots and qRT-PCR varied significantly from donor-to-donor. All assays were performed with at least duplicate samples with MDM derived from three different donors. * indicates p < 0.05 compared to control MDM, student's t-test.
3.4. Role of CYP2E1 on ethanol-mediated oxidative DNA damage and HIV-1 replication in U1 cells
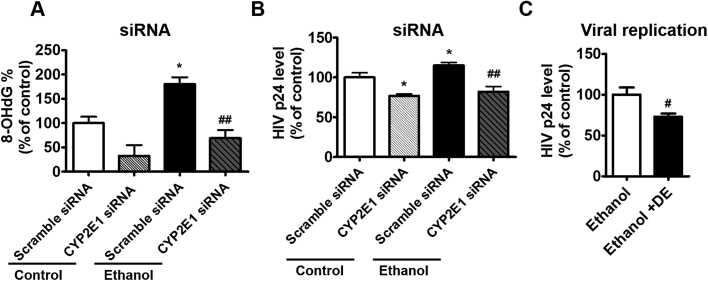
Overall our finding showed that ethanol induces oxidative DNA damage, HIV-1 replication, and CYP2E1 expression in HIV-1-infected human primary MDM. To further determine the role of CYP2E1 in ethanol-enhanced HIV-1 replication, CYP2E1 siRNA was transfected into HIV-1-infected U1 monocytic cell lines followed by ethanol treatment. We selected to perform the CYP2E1 siRNA experiment in the HIV-1-infected U937 cell line (U1 cells), because it is an important and established in vitro model system to study HIV-1 pathogenesis [25]. After ethanol exposure, a significantly increased oxidative DNA damage (~80%) was observed in ethanol-treated group compared to control (non-ethanol treated) group. This increased oxidative DNA damage in ethanol-treated cells was rescued by CYP2E1 siRNA, which significantly decreased the DNA damage to the control level (~100%) compared with the treatment of scrambled siRNA (Fig. 4A, *p < 0.05 compared to control scramble siRNA, ##p < 0.01 compared to ethanol scramble siRNA). We also examined HIV-1 replication after CYP2E1 siRNA transfection. As expected, an increased HIV-1 replication was observed in the ethanol-treated scramble group compared to control scramble group. CYP2E1 siRNA was able to decrease the viral replication in both control and ethanol-treated groups. However, the effect of CYP2E1 siRNA appears to be more pronounced in the ethanol-treated group, which showed a significant decrease (~30%) in HIV-1 replication compared to the ethanol-treated control (Fig. 4B, *p < 0.05 compared to control scramble siRNA, ##p < 0.001 compared to ethanol scramble siRNA). Furthermore, we treated U1 cells with a novel CYP2E1 inhibitor diethyl ether (DE), which has been identified by our group [31], to further examine the role of CYP2E1 in ethanol-enhanced HIV-1 replication. As expected, selective CYP2E1 inhibitor DE significantly decreased the level of HIV-1 replication (~25%) upon ethanol exposure (Fig. 4C, #p < 0.05 compared to ethanol control).
Fig. 4.
Effect of ethanol exposure on HIV-1 replication and DNA damage by using CYP2E1 siRNA and CYP2E1 inhibitors in U1 cells. A. CYP2E1 siRNA experiment: The percentage of 8-OHdG in siRNA-transfected cells was determined using EpiQuik 8-OHdG DNA Damage Quantification Direct kit and reported as a percentage of the control scramble group. Mean ± SEM values were graphed from 3 independent experiments. B. CYP2E1 siRNA experiment: Viral load was measured from culture media of siRNA-transfected cells using a p24 ELISA kit and reported as percentage of the control scramble group. Mean ± SEM values were graphed from 6 independent experiments. C. CYP2E1 inhibition experiment: Viral load was measured from culture media using a p24 ELISA kit and reported as percentage of ethanol control group (20 mM ethanol only). Mean ± SEM values were graphed from 3 independent experiments. * indicates p < 0.05 compared to control. # and ##indicate p < 0.05, p < 0.01 compared to ethanol treated cells, respectively. Fig. A and B were analyzed by one-way ANOVA post-hoc Tukey HSD Test. Fig. C was analyzed by student t-test. DE - diethyl ether.
4. Discussion
Ethanol-induced oxidative stress is known to cause alcoholic liver disease [32] and other conditions such as blood-brain barrier dysfunction in brain endothelial cells [33]. Oxidative DNA damage has been widely used as a biomarker for oxidative stress [34], and oxidative stress has been identified as a potent inducer of HIV-1 replication [15]. In this study, we observed increased oxidative DNA damage in both non-infected and HIV-1-infected human primary MDM after chronic ethanol exposure, which clearly suggests increased oxidative stress associated with chronic ethanol exposure. Moreover, it has been reported that ROS plays a physiological role in activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which induces HIV-1 replication [35]. The increased accumulation of ROS in ethanol-treated primary macrophages is likely to activate NF-κB and subsequently cause HIV-1 replication [35].
The majority of xenobiotics, including alcohol, are metabolized through CYP pathways [36]. Our previous reports have shown that several CYP enzymes, including CYP2E1, are expressed in the U937 monocytic cell line and primary monocytes, and are associated with ethanol-induced oxidative stress in those cells [20], [21]. In this study, our goal is to investigate whether CYP2E1 is associated with ethanol-induced oxidative stress and HIV-1 replication in MDM. Our observations show that chronic ethanol treatment significantly increases protein expression of CYP2E1 in HIV-1-infected primary MDM. Further, a subsequent study with CYP2E1 siRNA and CYP2E1 selected inhibitors suggested the role of CYP2E1 in ethanol-mediated HIV-1 replication. These results are consistent with our earlier observations in U937 monocytic cells [24]. Our previous studies have also shown that acute ethanol treatment caused significant upregulation of CYP2E1 protein in U937 monocytic cell, as well as in SVGA astrocyte, which is also known to be infected by HIV-1 [19], [20]. More importantly, those findings were consistent with our ex vivo data, which showed higher expression level of CYP2E1 in blood monocytes of HIV-1-infected alcohol users compared to uninfected alcohol users, while the concentration of alcohol in the plasma was reduced in HIV-1-infected drinkers compared to uninfected drinkers [21].
It is well known that antioxidant systems of the cell reduce oxidative stress [37]. Oxidative stress occurs when ROS generation increases to an extent that the cellular antioxidants cannot counteract [29]. It has been previously suggested that alcohol consumption can decrease the expression of AOEs and further attenuate cellular antioxidant capacity [38], [39]. Moreover, the literature shows that PLWHA are under chronic oxidative stress due to the changes in AOE expression during HIV-1 infection [40]. In this study we have shown decreased expression of PRDX6 after chronic ethanol exposure, while the expression of other major AOEs did not change. This result is consistent with our previous study that reported significant decrease in the expression of PRDX6 after ethanol exposure in U937 cells [24]. PRDX6 is well known as an antioxidant enzyme that can reduce hydrogen peroxide, fatty-acid hydroperoxides, and phospholipid hydroperoxides [41]. Our result is consistent with the literature report that suggests that PRDX6 plays an important role in ethanol-induced oxidative stress [30]. Thus, a decrease in PRDX6 expression and no change in other AOEs, with an increase in CYP2E1 expression suggest an increase in oxidative stress resulting into an increase in HIV-1 replication.
The majority of CYPs are located in the liver, which is the main site of CYP-mediated drug metabolism and drug-drug interaction [42]. However, we have earlier shown that CYPs are also expressed in extra-hepatic cells, including monocytes and astrocytes [20], [43]. It is generally considered that the level of CYPs in monocytes or astrocytes, is not sufficient to cause ethanol-mediated oxidative stress. However, our study has clearly suggested the role of CYP2E1 in ethanol-mediated oxidative stress and HIV-1 replication in MDM. Furthermore, our laboratory has been studying the effect of alcohol consumption on antiretroviral drugs in the past few years. We have shown that the metabolism of darunavir, an important ARV drug, is influenced by ethanol, and has faster hepatic intrinsic clearance in the presence of a ritonavir-boosted darunavir combination [9]. Our previous studies have also shown that ethanol has the ability to interact with microsomal CYP3A4 and alter elvitegravir metabolism and HIV-1 replication in monocytic cells [44]. These studies suggest a potential role of ethanol in causing a reduced response to antiretroviral drugs, which subsequently increases HIV-1 replication. Taken together, ethanol has the capability of increasing HIV-1 replication, not only directly through CYP2E1-mediated oxidative stress, but also through decreased efficacy of ARV drugs. Our results represent a step forward in potentially finding improved treatment strategies for HIV-1 positive alcohol users.
In conclusion, the results from this study suggest that ethanol increases HIV-1 replication and oxidative stress in human primary MDM. The enhanced oxidative stress is accompanied by an induction of CYP2E1 and a failure of cellular antioxidant mechanisms to maintain redox homeostasis. This is the first study that demonstrates enhanced HIV-1 replication in human primary MDM following chronic ethanol treatment. Further, the study demonstrated the role of CYP2E1 in ethanol-induced oxidative stress and viral replication. This was done by using CYP2E1 siRNA and a CYP2E1 novel inhibitor to eliminate CYP2E1 expression and activity, respectively. Overall, this study suggests the involvement of CYP2E1 and oxidative stress in ethanol-induced HIV-1 replication in MDM. These results are clinically relevant to potentially find effective treatment strategies for HIV-1-infected alcohol users.
Acknowledgements
We acknowledge the Flow Cytometry Core Service at UTHSC for the use of flow cytometry and Regional Biocontainment Laboratory for pursuing our experiment with HIV-1-infected cells.
Acknowledgments
Funding
This work was supported by National Institutes of Health grants to Santosh Kumar (AA022063).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.11.008.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.11.008.
Appendix A. Transparency document
Supplementary material
Appendix A. Supplementary material
Supplementary material
References
- 1.Galvan F.H., Bing E.G., Fleishman J.A., London A.S., Caetano R., Burnam M.A., Longshore D., Morton S.C., Orlando M., Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV cost and services utilization study. J. Stud. Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 2.Chander G., Josephs J., Fleishman J.A., Korthuis P.T., Gaist P., Hellinger J., Gebo K., H.I.V.R.N. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Med. 2008;9:196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samet J.H., Horton N.J., Traphagen E.T., Lyon S.M., Freedberg K.A. Alcohol consumption and HIV disease progression: are they related? Alcohol.: Clin. Exp. Res. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 4.Hutton H.E., McCaul M.E., Chander G., Jenckes M.W., Nollen C., Sharp V.L., Erbelding E.J. Alcohol use, anal sex, and other risky sexual behaviors among HIV-infected women and men. AIDS Behav. 2013;17:1694–1704. doi: 10.1007/s10461-012-0191-4. [DOI] [PubMed] [Google Scholar]
- 5.Neblett R.C., Hutton H.E., Lau B., McCaul M.E., Moore R.D., Chander G. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J. Women's Health. 2011;20:279–286. doi: 10.1089/jwh.2010.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Jin M., Ande A., Sinha N., Silverstein P.S., Kumar A. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis. Expert Opin. Drug Metab. Toxicol. 2012;8:1363–1375. doi: 10.1517/17425255.2012.714366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miguez M.J., Shor-Posner G., Morales G., Rodriguez A., Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict. Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R., Perez-Casanova A.E., Tirado G., Noel R.J., Torres C., Rodriguez I., Martinez M., Staprans S., Kraiselburd E., Yamamura Y., Higley J.D., Kumar A. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. JAIDS J. Acquir. Immune Defic. Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- 9.Midde N.M., Gong Y., Cory T.J., Li J., Meibohm B., Li W., Kumar S. Influence of ethanol on darunavir hepatic clearance and intracellular PK/PD in HIV-infected monocytes, and CYP3A4-darunavir interactions using inhibition and in silico binding studies. Pharm. Res. 2017;34:1925–1933. doi: 10.1007/s11095-017-2203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Midde N.M., Sinha N., Lukka P.B., Meibohm B., Kumar S. Alterations in cellular pharmacokinetics and pharmacodynamics of elvitegravir in response to ethanol exposure in HIV-1 infected monocytic (U1) cells. PLoS One. 2017;12:e0172628. doi: 10.1371/journal.pone.0172628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kedzierska K., Crowe S.M. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr. Med. Chem. 2002;9:1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- 12.Le Douce V., Herbein G., Rohr O., Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7 doi: 10.1186/1742-4690-7-32. (32-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scutari R., Alteri C., Perno C., Svicher V., Aquaro S. The role of HIV infection in neurologic injury , Brain Sci. 2017;7:38. doi: 10.3390/brainsci7040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res. Health. 2006 (245+) [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutson J.L., Wickramasinghe S.N. Expression of CYP2E1 by human monocyte-derived macrophages. J. Pathol. 1999;188:197–200. doi: 10.1002/(SICI)1096-9896(199906)188:2<197::AID-PATH295>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Wickramasinghe S.N. Observations on the biochemical basis of ethanol metabolism by human macrophages. Alcohol Alcohol. 1986;21:57–63. [PubMed] [Google Scholar]
- 18.Haorah J., Heilman D., Diekmann C., Osna N., Donohue T.M., Ghorpade A., Persidsky Y. Alcohol and HIV decrease proteasome and immunoproteasome function in macrophages: implications for impaired immune function during disease. Cell. Immunol. 2004;229:139–148. doi: 10.1016/j.cellimm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Jin M., Ande A., Kumar A., Kumar S. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated PKC/JNK/SP1 pathway. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin M., Arya P., Patel K., Singh B., Silverstein P.S., Bhat H.K., Kumar A., Kumar S. Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin. Exp. Res. 2011;35:132–139. doi: 10.1111/j.1530-0277.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ande A., Sinha N., Rao P.S.S., McArthur C.P., Ayuk L., Achu P.N., Njinda A., Kumar A., Kumar S. Enhanced oxidative stress by alcohol use in HIV+ patients: possible involvement of cytochrome P450 2E1 and antioxidant enzymes. AIDS Res. Ther. 2015;12:29. doi: 10.1186/s12981-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory T.J., He H., Winchester L.C., Kumar S., Fletcher C.V. Alterations in P-glycoprotein expression and function between macrophage subsets. Pharm. Res. 2016;33:2713–2721. doi: 10.1007/s11095-016-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modig F., Fransson P.-A., Magnusson M., Patel M. Blood alcohol concentration at 0.06 and 0.10% causes a complex multifaceted deterioration of body movement control. Alcohol. 2012;46:75–88. doi: 10.1016/j.alcohol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Rao P.S., Kumar S. Chronic effects of ethanol and/or Darunavir/Ritonavir on U937 monocytic cells: regulation of cytochrome P450 and antioxidant enzymes, oxidative stress, and cytotoxicity. Alcohol Clin. Exp. Res. 2016;40:73–82. doi: 10.1111/acer.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassol E., Alfano M., Biswas P., Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J. Leukoc. Biol. 2006;80:1018–1030. doi: 10.1189/jlb.0306150. [DOI] [PubMed] [Google Scholar]
- 26.Holder B., Jones T., Sancho Shimizu V., Rice T.F., Donaldson B., Bouqueau M., Forbes K., Kampmann B. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic. 2016;17:168–178. doi: 10.1111/tra.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ande A., McArthur C., Ayuk L., Awasom C., Achu P.N., Njinda A., Sinha N., Rao P.S., Agudelo M., Nookala A.R., Simon S., Kumar A., Kumar S. Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals. PLoS One. 2015;10:e0122402. doi: 10.1371/journal.pone.0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallikarjuna K., Shanmugam K.R., Nishanth K., Wu M.-C., Hou C.-W., Kuo C.-H., Reddy K.S. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol. 2010;44:523–529. doi: 10.1016/j.alcohol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Matés J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 30.Adachi J., Matsushita S., Yoshioka N., Funae R., Fujita T., Higuchi S., Ueno Y. Plasma phosphatidylcholine hydroperoxide as a new marker of oxidative stress in alcoholic patients. J. Lipid Res. 2004;45:967–971. doi: 10.1194/jlr.M400008-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Rahman M.A., Midde N.M., Wu X., Li W., Kumar S. Kinetic characterizations of diallyl sulfide analogs for their novel role as CYP2E1 enzyme inhibitors. Pharmacol. Res. Perspect. 2017;5 doi: 10.1002/prp2.362. (e00362-n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambade A., Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int. J. Hepatol. 2012;2012 doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haorah J., Knipe B., Leibhart J., Ghorpade A., Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 34.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 35.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anzenbacher P., Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 2001 doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aitken R.J., Roman S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polavarapu R., Spitz D.R., Sim J.E., Follansbee M.H., Oberley L.W., Rahemtulla A., Nanji A.A. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 39.Jaruga P., Jaruga B., Gackowski D., Olczak A., Halota W., Pawlowska M., Olinski R. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic. Biol. Med. 2002;32:414–420. doi: 10.1016/s0891-5849(01)00821-8. [DOI] [PubMed] [Google Scholar]
- 40.Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 41.Manevich Y., Fisher A.B. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic. Biol. Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Ogu C.C., Maxa J.L. Drug interactions due to cytochrome P450. Proc. (Bayl. Univ. Med. Cent.) 2000;13:421–423. doi: 10.1080/08998280.2000.11927719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavek P., Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 2008;9:129–143. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- 44.Midde N.M., Rahman M.A., Rathi C., Li J., Meibohm B., Li W., Kumar S. Effect of ethanol on the metabolic characteristics of HIV-1 integrase inhibitor Elvitegravir and Elvitegravir/Cobicistat with CYP3A: an analysis using a newly developed LC-MS/MS method. PLoS One. 2016;11:e0149225. doi: 10.1371/journal.pone.0149225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material