Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a widespread genetic disease that leads to renal failure in the majority of patients. The very first pharmacological treatment, tolvaptan, received Food and Drug Administration approval in 2018 after previous approval in Europe and other countries. However, tolvaptan is moderately effective and may negatively impact a patient’s quality of life due to potentially significant side effects. Additional and improved therapies are still urgently needed, and several clinical trials are underway, which are discussed in the companion paper Müller and Benzing (Management of autosomal-dominant polycystic kidney disease—state-of-the-art) Clin Kidney J 2018; 11: i2–i13. Here, we discuss new therapeutic avenues that are currently being investigated at the preclinical stage. We focus on mammalian target of rapamycin and dual kinase inhibitors, compounds that target inflammation and histone deacetylases, RNA-targeted therapeutic strategies, glucosylceramide synthase inhibitors, compounds that affect the metabolism of renal cysts and dietary restriction. We discuss tissue targeting to renal cysts of small molecules via the folate receptor, and of monoclonal antibodies via the polymeric immunoglobulin receptor. A general problem with potential pharmacological approaches is that the many molecular targets that have been implicated in ADPKD are all widely expressed and carry out important functions in many organs and tissues. Because ADPKD is a slowly progressing, chronic disease, it is likely that any therapy will have to continue over years and decades. Therefore, systemically distributed drugs are likely to lead to potentially prohibitive extra-renal side effects during extended treatment. Tissue targeting to renal cysts of such drugs is one potential way around this problem. The use of dietary, instead of pharmacological, interventions is another.
Keywords: ADPKD, dietary intervention, mTOR, pharmacological intervention, polycystic kidney disease
INTRODUCTION
There is no shortage of ‘promising’ molecular drug targets for the treatment of polycystic kidney disease (PKD). Work in many laboratories over the years has led to the identification of a vast number of signaling pathways, kinases, transcription factors, metabolic pathways, etc. that are aberrantly up- or down-regulated in PKD. Many of these could be targeted with existing or novel drugs, which very often lead to amelioration of renal cyst growth and slowing down of functional deterioration of the kidneys. The caveat is that whereas these drugs are effective in animal models of PKD, mostly mice and rats, the translation of these findings into clinical success is a hurdle that has proved very difficult to overcome.
The main reason for this translation problem is that virtually all of the molecular drug targets that have been implicated in PKD are not unique to polycystic kidneys. In fact, most of them are ubiquitously expressed and serve important functions in many extra-renal tissues and organs. Therefore, drugs that affect PKD targets may be effective in inhibiting renal cyst growth but they also tend to cause adverse effects in both the kidneys and even more importantly extra-renal tissues that would be prohibitive in clinical practice.
A prime example is the mammalian target of rapamycin (mTOR) inhibitors. mTOR is a key signaling kinase that receives inputs from several upstream pathways and regulates fundamental cellular behaviors including cell growth, proliferation, survival and energy metabolism [1]. mTOR is aberrantly activated in cyst-lining cells in human autosomal dominant polycystic kidney disease (ADPKD) and most rodent models of PKD [2, 3]. This was an exciting finding because mTOR inhibitors, such as rapamycin, were already clinically approved as immunosuppressive drugs. Indeed, rapamycin proved to be highly effective in numerous PKD rodent models [3–7] in which it can be used at high doses during the relatively short treatment periods that are required, usually around 2 weeks. The high doses ensure that mTOR in the target organ, the polycystic kidneys, is indeed inhibited. The short treatment period ensures that long-term side effects become irrelevant. For example, immunosuppression as an unwanted effect of mTOR inhibition is generally not a problem for a mouse living for 2 weeks in a nearly pathogen-free animal facility. The problem is that ADPKD in humans is a very slowly progressing, chronic disease that leads to renal deterioration and eventually renal failure during the course of decades. To observe any beneficial effects in clinical trials, a study has to extend for at least a year and ideally longer. All the while, these patients live in environments that are nothing like a mouse cage. In hindsight, it may not have been surprising that clinical trials to test the efficacy of mTOR inhibitors in ADPKD failed to show beneficial effects [8–10]. The long-term tolerable doses of these drugs in humans are much lower than those that can be administered in the short term in rodents. It is likely that these low, tolerable doses have relatively little effect on mTOR in polycystic kidneys, but they still cause significant extra-renal adverse effects in patients. Simply increasing the dose is therefore not an option.
This example illustrates perhaps the most significant hurdle in finding a feasible pharmacological therapy for ADPKD. Due to the chronic, slowly progressive nature of the disease, any drug treatment for ADPKD would likely have to occur continuously over the course of years and decades. Treatment would also likely have to be initiated early in relatively nonsymptomatic patients to avoid permanent renal function loss as much as possible. This puts an extremely high burden on any drug to be used for ADPKD therapy to exhibit an extremely low long-term side effect profile.
Since many of the molecular targets implicated in ADPKD have already been pursued for other indications, primarily cancer, many drugs have already been developed or are even in clinical use for those other indications. The re-purposing of these compounds for ADPKD therapy is a promising avenue. To accomplish the lowest possible side effect profile, a pharmaceutical compound would ideally be highly specific to its intended molecular target. This is often difficult to achieve with small molecule drugs such as tyrosine kinase inhibitors. Biologics such as monoclonal antibodies (mAbs) are a more specific approach, but there have been few attempts, and no success, so far in preclinical PKD studies. The targeting of compounds, both small molecules and antibodies, specifically to polycystic kidneys is a novel approach that has the potential to reduce extra-renal effects and to make the re-purposing of many compounds for long-term therapy in ADPKD potentially feasible. We will discuss tissue targeting further below. First, we will discuss select, molecular targets that have recently emerged in preclinical studies. The names of drugs or drug classes are shown in bold at first mention.
MOLECULAR TARGETS ON THE HORIZON
Catalytic mTOR inhibitors and dual kinase inhibitors
As mentioned above, rapamycin and its analogs (rapalogs) are highly effective in PKD rodent models. These drugs specifically target the activity of mTORC1 (see Figure 1), one of the two complexes in which the kinase mTOR acts. The other complex, mTORC2, has different functions but is also activated in PKD as deduced from the high levels of one of its downstream targets, phospho-Akt (Ser473), compared with wild-type kidneys [4, 11]. Since mTORC2 is not directly targeted by rapamycin, it has been investigated whether combined inhibition of both mTORC1 and mTORC2 may be beneficial. This was achieved in vivo utilizing a catalytic mTORC1/mTORC2 kinase inhibitor, PP242, in the Han:SPRD Cy/+ rat model of PKD [12]. This approach led to inhibition of the progression of renal cystic disease. However, in the absence of a head-to-head comparison with mTORC1-specific inhibition, it is difficult to conclude whether the added inhibition of mTORC2 was beneficial over inhibition of mTORC1 alone. A concern is that combined mTORC1 and -2 inhibitions may lead to increased extra-renal toxicity in the clinic compared with rapalogs, especially during long-term treatment.
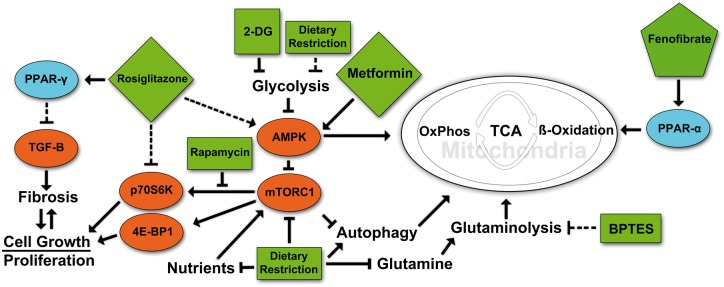
FIGURE 1.
Targeted metabolic regulation in ADPKD. A highly simplified cartoon of some of the major pathways that relate to the pathogenesis of PKD and that are are affected by some of the compounds discussed in this article. Rosiglitazone treatment activates PPAR-γ, causing heterodimeric binding to retinoid x receptor (RXR) followed by translocation to the nucleus, activating gene transcription of PPAR response element-regulated genes. This in turn leads to a decrease in TGF-β signaling and a subsequent reduction in fibrosis. Rosiglitazone also acts independently of PPAR-γ to inhibit p70S6K activation and ribosomal protein S6 phosphorylation. Treatment with 2DG leads to a reduction in glycolysis by inhibition of phosphoglucoisomerase. Decreased glycolytic activity in turn may cause the activation of AMPK and subsequent inhibition of mTORC1, cell growth and proliferation with an increase in fatty acid oxidation. Metformin activates AMPK that directly represses mTORC1 signaling via phosphorylation. Treatment with BPTES inhibits glutaminase (GLS1) disrupting the breakdown of glutamine to glutamate preventing it from being used in the TCA cycle to produce α-ketoglutarate. Fenofibrate treatment activates PPAR-α to bind to PPAR response elements and increase transcription of genes involved in fatty acid utilization, oxidative phosphorylation and mitochondrial biogenesis. Rapamycin inhibits the ability of mTORC1 to activate the S6-Kinase branch of its downstream pathway but has less effect on the 4E-BP1 branch. In contrast, mTOR kinase inhibitors or mTOR ASOs would affect both downstream branches. Dietary restriction simulates the effects of targeted drug therapies by reducing nutrient intake, leading to reductions in key regulatory pathways. Pointed arrowheads indicate activating effects. Blunt arrowheads indicate inhibitory effects. Dashed arrows indicate indirect or multistep effects.
In another study, the effect of dual inhibition of mTORC1/2 and phosphoinositide 3-kinase (PI3K) was tested utilizing NVP-BEZ235 in the Han:SPRD Cy/+ rat model and a mouse Pkd1 model [13]. The authors demonstrated that inhibition of mTORC1 with a rapalog activated both mTORC2 and ERK via two feedback loops. Dual inhibition of mTORC1/2 and PI3K with NVP-BEZ235 was more effective than rapalog-mediated mTORC1 inhibition in terms of reducing cystic disease progression. Although this study highlights the theoretical utility of dual mTOR/PI3K inhibition in PKD, the main concern is the toxicity of this compound, which led to early termination of a Phase I trial [14].
Targeting inflammation
Numerous lines of evidence suggest an important role of inflammation in the progression of PKD. This is supported by the presence of pro-inflammatory markers in ADPKD urine and renal cyst fluid [15], accumulation of inflammatory cells and the role of renal macrophages in PKD progression [16, 17]. The inflammatory cytokine tumor necrosis factor (TNF-α) is present in ADPKD cyst fluid and can promote cyst growth with ex vivo and in vivo administration [18]. TNF-α was reported to disrupt the localization of polycystin-2 to the plasma membrane and primary cilia [18]. A TNF-α inhibitor, etanercept, is a Food and Drug Administration-approved biologic drug used for the treatment of autoimmune disorders. Etanercept acts as a decoy receptor for TNF-α and has been shown to inhibit renal cyst growth in Pkd2+/− mice [18].
The natural polyphenolic compound resveratrol exerts anti-inflammatory, antioxidant and anti-proliferative effects through targeting of mTOR and nuclear factor (NF)-κB [19, 20] and has been shown to slow disease progression through dampening inflammatory pathway activity in animal models of PKD [21]. Treatment of the Han:SPRD Cy/+ rat model with resveratrol led to a decrease in the pro-inflammatory cytokines MCP-1, CFB and TNF-α, and reduction in macrophage infiltration and some amelioration of renal cystic disease [21]. Although there is extensive in vitro and in vivo evidence that resveratrol could be used therapeutically in a wide array of diseases, a major challenge for clinical trials has been its poor bioavailability [22].
Histone deacetylase inhibitors
Histone deacetylases (HDACs) are a family of enzymes that modulate gene expression by removing acetyl groups from histones and regulate a diverse array of intracellular pathways by acting on nonhistone proteins [23]. A chemical modifier screen revealed the positive effect of HDAC inhibition in a Pkd2-deficient zebrafish model. In this study, a pan-HDAC inhibitor, trichostatin A and a Class I HDAC inhibitor, valproic acid (VPA), were shown to affect both body curvature and laterality, two pathological changes associated with cystogenesis in zebrafish [24]. It was further demonstrated that VPA treatment decreased renal cyst progression and preserved kidney function in an orthologous mouse model (Pkd1flox/flox; Pkhd1-Cre) [24].
The histone deacetylase, HDAC6, has heightened expression and activity in Pkd1 mutant renal epithelial cells [25]. HDAC6 is predominantly localized to the cytoplasm and uniquely interacts with nonhistone proteins [26]. In ADPKD models, blocking HDAC6 with specific inhibitors, ACY-1215 and tubacin, slows cyst growth in vivo and prevents cyst formation in vitro [27, 28]. Both ACY-1215 and tubacin are thought to inhibit proliferation of cyst-lining epithelia by preventing deacetylation of α-tubulin, where deacetylation would typically promote cell cycle progression. In addition, both ACY-1215 and tubacin were found to downregulate cyclic adenosine monophosphate (cAMP); however, the exact mechanism underlying this finding requires further investigation [27, 28]. HDAC6 inhibition may also function by blocking EGF-mediated β-catenin nuclear localization, resulting in suppression of epithelial proliferation and an increase in EGF receptor (EGFR) degradation [29].
With the promising results of HDAC inhibition in animal models of ADPKD, there has been a focus on the role of histone acetylation, which by modifying chromatin structure can recruit DNA-binding factors such as bromodomain and extra-terminal proteins (BET) [30, 31]. It has been shown that an inhibitor of the Brd4 BET protein, JQ1, was effective in suppressing cyst growth and kidney size, and maintaining kidney function in two Pkd1-mutant mouse models [32].
RNA-targeted therapeutic strategies
The two primary pharmacological approaches to target RNA that have emerged over the past decades are antisense oligonucleotides (ASOs) and RNA-mediated interference, including micro-RNA (miRNA) [33], which we will discuss in the context of PKD.
Antisense oligonucleotides
ASOs are short oligonucleotides with complementary sequence to a specific mRNA designed to reduce the expression of a target mRNA and its protein product. Antisense strategies against certain targets have been exploited in a number of diseases [34], leading to the approval of four ASO-based therapies as of 2017 [35].
As has already been noted above, the mTOR pathway is highly activated in PKD. To determine whether downregulation of mTOR is an effective treatment strategy, an ASO targeting mTOR expression has been investigated in the Pkd2WS25/− mouse model [36]. The mTOR ASO effectively reduced mTOR levels and reduced the activity of mTORC1 and mTORC2, as assessed by surrogate markers. This resulted in a significant improvement of various aspects of the renal cystic phenotype [36].
The renin–angiotensin system has been shown to be upregulated in PKD [37, 38], and clinical trials utilizing angiotensin-converting enzyme (ACE) inhibitors have been conducted in ADPKD patients [39, 40]. Although ACE inhibitors did not affect the decline in renal function in these clinical studies [41], the feasibility of targeting angiotensinogen (AGT) levels directly with ASOs has been investigated in preclinical studies [42–44]. In all three studies, the AGT ASO was shown to accumulate in the kidney and reduce AGT mRNA and protein in kidney, serum and urine. In terms of therapeutic efficacy, AGT ASOs, but not scrambled ASOs, significantly ameliorate several aspects of the renal cystic disease phenotype in both the PKD2WS25 model [42] and a global, tamoxifen-inducible knockout model of PKD (Pkd1flox/flox: CAGG-CreER) [43, 44].
Taken together, these studies suggest that targeting AGT with ASOs may be superior to ACE inhibitors. These studies also suggest that the utilized second-generation ASOs accumulate in kidneys and/or cysts, but this is unlikely to be a specific effect. Nevertheless, such ASOs could be considered to target the expression of other proteins implicated in PKD. For example, AZD9150, an ASO that targets STAT3, has shown promising initial activity in lymphoma patients in a Phase 1 clinical trial [45]. Given that STAT3 is activated in ADPKD and multiple preclinical models of PKD [46–49], the potential efficacy of ASOs targeting STAT3 may be of interest.
Targeting miRNAs
miRNAs are a class of endogenous small (∼22 nt) noncoding RNAs that regulate the expression of target mRNAs and their protein product [50]. In the context of PKD, several miRNAs have been shown to be expressed and modulated in the disease state [51–53]. Kidney-specific, collecting duct knockout of key miRNA pathway genes has been shown to result in epithelial-to-mesenchymal transition and fibrosis [54], suggesting that these miRNAs may also play a role in fibrosis in PKD. A number of studies have demonstrated that the miR-17–92 cluster [55, 56], which encodes six miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92a-1), is upregulated in several models of PKD [57–59], and Pkd1 has been shown to be a direct target of miR-20 [60]. Overexpression or knockdown of the miR-17–92 cluster influences the development of PKD [58]. Utilizing the slow-progressing Pkd1RC/RC mouse model, a kidney-targeted anti-miR-17 oligonucleotide proved to be effective in reducing kidney growth, decreasing proliferation and fibrosis and extending animal survival [57].
Outside of the miR-17–92 cluster, several other miRNAs have been associated with PKD. These include miR-21 [61], miR-20b-5p and miR-106a-5p [62], miR-199a-5p [63] and miR-501-5p [64] and suggest that targeting miRNAs pharmacologically may represent a new paradigm in PKD treatment [50].
Glucosylceramide synthase inhibitors
Glycosphingolipids (GSLs) play fundamental roles in a variety of cellular processes [65, 66], and their dysregulation leads to a number of diseases, including Gaucher, Fabry [67, 68] and renal diseases [69]. A potential role for GSLs in PKD has been suggested based on upregulation of certain GSLs in preclinical models and ADPKD samples [70–73]. The glucosylceramide synthase (GCS) inhibitor Genz-123346 was tested in multiple animal models of PKD and showed remarkable efficacy in terms of reducing kidney size, cystic index and improving renal function [71]. Genz-123346 treatment led to inhibition of glucosylceramide (GlcCer), lactosylceramide (LacCer) and GM3 production, and was shown to inhibit key cellular pathways known to be activated in PKD, including Akt-mTOR and various cell cycle proteins. Genetic ablation experiments revealed that deletion of GM3 synthase or sphingokinase 1 either ameliorated or exacerbated the renal cystic phenotype in jck mice, respectively, suggesting that specific GSLs, or combinations of multiple GSLs, are important modifiers of renal cyst growth [74]. The GCS inhibitor, venglustat, has recently entered a Phase 3 clinical trial in which its efficacy will be tested in ADPKD patients with rapidly progressing disease (see ClinicalTrials.gov Identifier: NCT03523728). In future work, it will be important to better understand the biological mechanisms underlying the upregulation of GSLs in PKD and the consequences this may have on signaling pathways [75], membrane properties and trafficking [76–78].
Targeting metabolism
A series of recent findings [79–82] have suggested that cysts in PKD exhibit an altered energy metabolism characterized by a high rate of glycolysis and a low rate of mitochondrial oxidative phosphorylation similar to the Warburg effect in many cancer cells. A concrete mechanistic explanation for the Warburg effect in cancer is still lacking and, not surprisingly, it is lacking for PKD as well. Similar to many cancer cells, PKD cells exhibit an increased need for glycolysis [79], a defect in fatty acid oxidation [83] and increased mitochondrial damage [80, 84]. Persistent mTORC1 activity in PKD (see above) could play a role in mitochondrial damage because it would antagonize autophagy [85], which would thwart the removal and replacement of defective mitochondria [86]. Even though the metabolic changes in PKD have been described as ‘aerobic’ glycolysis, renal cystic tissue has been found to be hypoxic [87, 88]. Therefore, these metabolic changes may be a survival adaptation of cyst-lining cells to the hypoxic environment. Just as in cancer, increased glycolysis with reduced oxidative phosphorylation is also expected to lead to increased generation of glycolytic intermediates that form the building blocks for anabolic pathways needed to sustain cell growth and proliferation.
AMP-activated protein kinase (AMPK), a metabolic sensor that negatively regulates mTORC1 and affects many other pathways, has been reported to be less active in some PKD models [79, 81, 89], but not in all [90], and may play a role in mediating the metabolic changes in PKD [91]. Metformin, a widely used diabetes medication and a known AMPK activator, was shown to inhibit disease progression in PKD mouse [92] and zebrafish models [90] and is currently being tested in a Phase II clinical trial in ADPKD patients [93]. Numerous other compounds exist—including widely used ones—that function at least partly as AMPK activators, and it could be contemplated to investigate their efficacy in PKD [91].
Due to the presumed glucose dependency of cystic cells, targeting glucose utilization has been attempted in PKD models with promising results. 2-deoxy glucose (2DG) is a potent inhibitor of glycolysis [94] by inhibiting conversion of glucose-6-phosphate by phosphoglucoisomerase in the second step of glycolysis [94] with resultant inhibition of hexokinase [95]. Mouse embryonic fibroblasts (MEFs) lacking functional PC1 display increased levels of ATP, ERK and mTORC1 signaling [79]. Increased levels of ATP and lactate have also been observed in human ADPKD cell cultures and are reduced following treatment with 2DG [89]. Upon glucose withdrawal, Pkd1−/−MEFs were unable to activate autophagy and displayed increased apoptosis [79]. Treatment of a Pkd1 mouse [79, 96] and the Han:SPRD Cy/+ rat models [89] of PKD with 2DG led to reduced cystic burden and kidney volume [89, 96]. Timing of the knockout of Pkd1 in mice at either postnatal day 12 or 25 using a tamoxifen-inducible transgene leads to differences in the rate of disease progression, and in both rapidly and slowly progressing PKD, 2DG treatment was effective in retarding disease progression [96]. 2DG is reasonably well tolerated based on clinical trials in the cancer setting [97]. Whether long-term therapy in ADPKD is feasible remains an open question.
Besides increased reliance on glycolysis, cystic cells appear to have altered metabolic pathways that may be due to mitochondrial defects. Cells from the cpk mouse model have an altered tricarboxylic acid cycle (TCA) cycle and overproduce citrate and the oncometabolite 2-hydroxyglutarate, most likely due to increased utilization of glutamine that requires the activity of glutaminase for entry into the TCA cycle [98]. The tumor suppressor Lkb1 activates AMPK and has been shown to have decreased activity in PKD [79]. Concomitant ablation of Lkb1 and the mTORC1 regulator TSC1 leads to PKD and glutamine dependence [99]. Utilizing a primary human ADPKD cell line, inhibition of glutaminolysis with Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) and CB-839 prevented forskolin-induced cystogenesis [100]. It has been suggested that these cells are glutamine addicted and that glutaminase inhibitors may be effective in PKD [98, 100].
Fatty acid beta oxidation, which also occurs in mitochondria, has been found to be impaired in cystic cells [83, 101]. The mitochondria in these cystic cells may no longer function primarily as energy centers but instead primarily produce metabolic intermediates to supply anabolic pathways. Structural and functional abnormalities of mitochondria as well as reduced mitochondrial numbers have been found in human ADPKD and animal models [80]. These mitochondrial defects may involve dysregulation of the peroxisome proliferator-activated receptor (PPAR) family of transcription factors that control a suite of genes involved in fatty acid utilization, inflammation, lipid uptake and lipogenesis [102]. Cells lacking Pkd1 also show altered levels of the PPARs with either decreased PPAR-α [101] or increased PPAR-γ expression [103].
Of interest is the PPAR-α activator fenofibrate and the PPAR-γ activators rosiglitazone and pioglitazone. PPAR-α levels have been reported to be reduced in human ADPKD cells and aggressive models of mouse PKD [57]. Reduced fatty acid oxidation is observed in the Pkd1RC/RCmouse model of PKD along with decreased levels of PPAR-α mRNA [101]. Use of fenofibrate restored fatty acid oxidation and decreased the cystic phenotype following 10 days of administration [101]. Fenofibrate-treated mice also showed increased expression of pyruvate dehydrogenase kinase 4—a negative regulator of glycolysis—and an increase in the mitochondrial biogenic protein PPARGC1A and fatty acid β-oxidation [101]. Additionally, a reduction in cell proliferation and inflammation was observed following fenofibrate treatment [101]. PPAR-γ activators have been used in the treatment of diabetes to promote glucose uptake via insulin sensitization [104]. Use of the PPAR-γ agonists rosiglitazone and pioglitazone have each shown efficacy in treating animal models of PKD [105, 106]. Rosiglitazone and pioglitazone treatment reduced levels of the profibrogenic cytokine TGF-β [107–109], in turn decreasing fibrogenesis [108]. Rosiglitazone and pioglitazone also affect the activity of several overactive pathways in PKD including p70S6K [109, 110], phospho-S6 [109] and phospho-ERK [108]. The reduction in p70S6k by rosiglitazone is not directly mediated through mTOR as concomitant treatment with rapamycin further reduced levels of p70S6K in rodents [110]. The non-PPAR-mediated effects of rosiglitazone have been observed previously in nonsmall-cell lung carcinoma and shown to activate AMPK [111]. This supports an additional mechanism by which rosiglitazone may provide therapeutic potential. Additionally, treatment with rosiglitazone was also able to inhibit proliferation in ADPKD cyst-lining cells by increasing levels of the cell cycle regulators p21 and p27, decreasing levels of cyclin D1 and Cdk4, causing G1-arrest [103]. This was coupled with an increase in apoptosis by a reduction in Bcl-2 and increased levels of Bax [103]. Taken together these studies provide strong evidence for the use of PPAR agonists in the treatment of PKD, with a clinical trial currently testing the efficacy of pioglitazone (see ClinicalTrials.gov Identifier: NCT02697617). However, it is important to keep in mind that the safety of rosiglitazone has been controversial due to conflicting reports of cardiovascular risks that led to suspension of this drug from European markets [112].
Dietary restriction
As detailed above, mTORC1 is typically activated in PKD cysts and is a driver of cyst growth. Given that mTORC1 is not only regulated by growth factor signaling but also by nutrient supply and the energy status of a cell, it could be that these latter factors contribute to mTORC1 activation in PKD. Two groups tested this independently by subjecting three slowly progressive mouse models (Pkd1RC/RC, Pkd2WS25/− and Pkd1flox/flox: Nestin-Cre) to food restriction, without malnutrition, and found this treatment to be surprisingly effective [113, 114]. Mild to moderate reduction of food intake—even a reduction as low as 10% below that of controls—resulted in very significant inhibition of renal cyst growth, proliferation, fibrosis and markers of inflammation. In these studies, food intake was reduced overall meaning that all macro- and micronutrients were proportionally reduced. Food reduction by 40% not only inhibited the rate of cyst growth but also actually led to a decrease of existing cystic burden and therefore reversed the disease. Such a reversal has previously only been seen from mTORC1 inhibition with a high dose of rapamycin [4].
Food restriction led to inhibition of not only the S6 branch downstream of mTORC1 [113, 114], but also even more substantial inhibition of the 4E-BP1 branch [114] (see Figure 1). Although previous work mainly evaluated the S6 branch, this new finding suggests that 4E-BP1 may be a more important driver of renal cyst growth. Rapamycin effectively inhibits the S6 branch, but is a less effective inhibitor of the 4E-BP1 branch [115]. This consideration may possibly help to explain why high-dose rapamycin is effective in rodent models of PKD but low-dose rapamycin failed in clinical trials (see above).
A mechanistic explanation for the surprisingly high efficacy of food restriction in PKD mouse models still remains outstanding. However, the translational potential of these findings is very high. It seems possible that ADPKD may prove to be treatable by dietary interventions in many patients. If so, this would provide a safe and inexpensive therapy but may encounter resistance and potential conflicts of interest [116, 117] because it would also undermine significant investments by academic investigators, patient advocacy organizations and the biotech and pharmaceutical industry to develop new drugs for ADPKD therapy.
Tissue targeting approaches
Many of the compounds discussed above would likely be too toxic to be used for long-term therapy in ADPKD patients. A possible way to circumvent unwanted extra-renal effects is to target these compounds specifically, or at least preferentially, to polycystic kidneys. Work in our laboratory has led to two successful strategies, one for the targeting of small molecules to PKD kidneys and the other for the targeting of antibodies to cyst lumens in PKD kidneys (Figure 2). Because small molecule drugs and biologics (such as mAbs) differ dramatically in their pharmacological properties, very different approaches are needed to direct them to a specific target tissue.
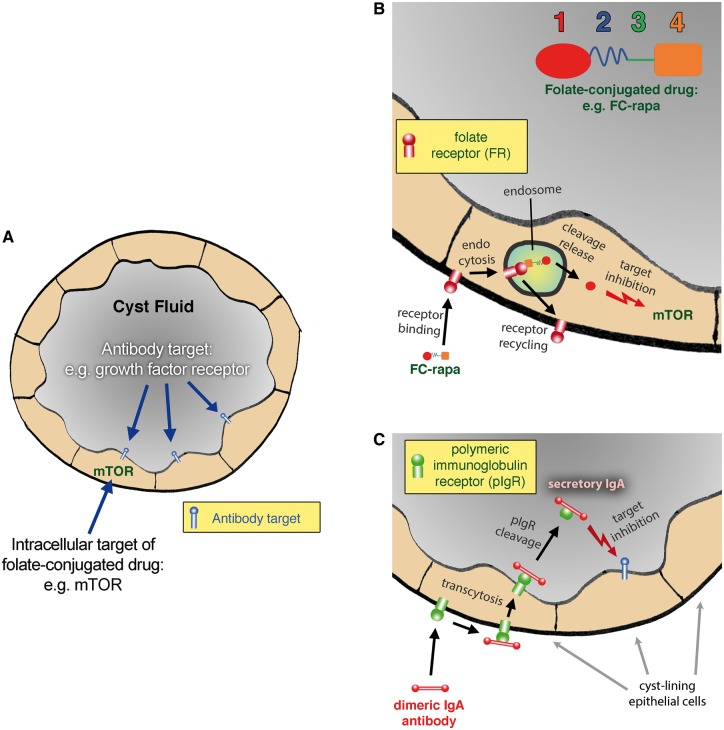
FIGURE 2.
Drug targeting to renal cyst lumens in PKD. (A) Cartoon of a cross-section of a renal cyst in PKD that is lined by a single-layer epithelium forming an enclosed space. The kinase mTOR is depicted as an example of an intracellular pharmacological target that can be reached via folate-conjugated small molecule drugs as shown in (B). The cyst lumen contains growth factors and cytokines that activate receptors on the apical plasma membranes of the cyst-lining cells. These growth factors and their receptors receptors are the intended targets of antagonistic mAbs in dIgA format as shown in (C). (B) Magnified cartoon. A depiction of a folate-conjugated small molecule drug consisting of the ligand folate (1), a hydrophilic spacer (2), a cleavable bond (3) and the payload (4), which could be rapamycin in the case of FC-rapa. FC-rapa binds to the FR on the plasma membrane of cyst-lining cells and is internalized via receptor-mediated endocytosis, followed by cleavage and release of the drug in the endosome. The free, activated drug is subsequently released into the cytoplasm where it can inhibit its intended target mTOR. The FR is subsequently recycled back to the plasma membrane for additional rounds of drug uptake. Whereas cysts in PKD express the FR, most other cells instead utilize the reduced folate carrier (RFC) for folate uptake. Because the RFC cannot transport folate-conjugated drugs, these cells will be unaffected. (C) An antagonistic antibody in dIgA format (red) binds to the pIgR (green) on the basolateral surface of cyst-lining cells and is transcytosed to the apical surface. After arrival at the apical surface, the pIgR is cleaved which leads to the release of the complex between dIgA and the ectodomain of the pIgR (secretory IgA) into the cyst fluid. The dIgA antibody can then inhibit its intended target such as a growth factor or growth factor receptor (blue). Because renal cysts have enclosed spaces, the dIgA antibody will accumulate in the cyst fluid. In contrast, pIgR-mediated transcytosis will lead to excretion and loss of the dIgA antibody in other epithelial tissues such as the intestinal epithelium.
Targeting of small molecules to renal cysts via the folate receptor
In this approach, drug payloads are chemically conjugated to the B vitamin, folic acid. The resulting folate-conjugated drugs bind to the high-affinity folate receptor (FR) and are internalized into cells by endocytosis (Figure 2B). Tissue specificity of these drugs is due to the fact that most cells in the body do not express the FR, but instead import folate via the reduced folate carrier, which does not allow the import of folate-conjugated drugs [118, 119]. The FRα isoform has a restricted pattern of expression in normal tissues [120] but is expressed at high levels in renal proximal tubule epithelial cells [120, 121]. In addition, FRα is highly expressed in several epithelial cancers, which is why folate-conjugated drugs preferentially target to cancer cells and have been developed for tumor therapy [122, 123].
FRα expression was also found to be high in renal cysts in mouse models of PKD and human ADPKD samples [11, 124], which suggested that PKD could be amenable to FR-targeting strategies. A folate-targeted form of the mTORC1 inhibitor (see above) rapamycin (FC-rapa or EC0371) was developed that included a hydrophilic spacer and a self-immolative linker designed to be cleaved intracellularly to reconstitute the active drug [11]. In the nonorthologous, early-onset bpk model of PKD, FC-rapa administered by intraperitoneal injection proved highly effective in inhibiting mTOR activity in the kidneys—but not in other organs—and led to strong attenuation of cyst growth and proliferation and preserved renal function [11]. In a follow-up study, FC-rapa was tested at lower doses in an orthologous Pkd1flox/−: Nestin-Cre mouse model and compared head-to-head to unconjugated rapamycin. Both, FC-rapa and unconjugated rapamycin were similarly effective in inhibiting PKD disease progression. However, FC-rapa exhibited much reduced extra-renal effects, including effects on the immune system and reduced systemic toxicity as assessed by body weight gain over time [124]. In the same study, it could also be directly demonstrated that renal cysts are accessible to folate-conjugated compounds as assessed using a novel fluorescent folate-conjugated reporter [124]. This reporter was efficiently taken up by the collecting duct-derived cysts that are prevalent in the bpk mouse model [124], and FC-rapa was effective in inhibiting the growth of these cysts [11]. These results indicate that the FR is expressed on collecting duct-derived cysts and not restricted to cysts that originate from proximal tubules.
Although the preclinical data to date suggest that targeting drugs to PKD kidneys via FR is a feasible approach [125], it has yet to be tested in a clinical trial in ADPKD patients. In addition to targeting FR with folate-conjugated small molecules, other FR-targeting approaches have been investigated that may also have application in PKD. The most advanced is mirvetuximab soravtansine, an FR-targeted antibody–drug conjugate, which consists of an anti-FR antibody linked to maytansoid DM4, a potent tubulin-disrupting agent. Preclinical and clinical studies suggest that the overall efficacy of mirvetuximab soravtansine is linked to the relative expression of FR [126–128], and its relative efficacy is currently being tested in patients with platinum-resistant ovarian cancer [129] including a pivotal Phase 3 clinical trial in this patient population [130].
Targeting of mAbs to renal cysts via the polymeric immunoglobulin receptor
As opposed to small molecule drugs, a major advantage of mAbs is their typically higher target specificity that greatly reduces or eliminates off-target adverse effects [131, 132]. The disadvantage due to lack of oral bioavailability is partially overcome by much longer half-lives of mAbs compared with small molecules, which means that dosing is required much less frequently.
Many potential targets for PKD therapy are cytokines, growth factors and their receptors. For many of these targets, very effective antagonistic mAbs have already been developed—and are even in clinical use, primarily for cancer therapy. A prime example is the EGFR, which is over-expressed in PKD. Small molecule tyrosine kinase inhibitors against the EGFR effectively inhibit renal cyst growth in PKD mouse models [133]. However, the known toxicity of small molecule EGFR inhibitors would seem to make them poor candidates as long-term therapeutics for ADPKD. Antagonistic EGFR mAbs would seem much more promising at first glance. However, to our knowledge, no publication reporting efficacy of any mAb in rodent models of PKD has yet appeared. Our own efforts to use existing mAbs against several targets in PKD mouse models have led to failure. It seems likely that other labs have found similar results, but such failed studies are rarely published.
A likely explanation for these failures is that many of the growth factors/receptors implicated in PKD are localized to the luminal compartment of renal cysts. For example, the EGFR has been found to be activated and apically localized in cysts [134], and EGFR ligands have been found in cyst fluid [135]. Since cysts are enclosed, epithelial-lined spaces [136], any growth factors secreted into cyst fluid should be able to stimulate their cognate receptors that are present on apical membranes of cyst-lining cells. This would lead to continuous auto- and paracrine activation of cyst cells and may lead to an inescapable, permanent state of activation once cysts are formed.
The biotech industry exclusively uses IgG isotypes to develop mAb therapeutics. However, IgG antibodies are not capable of crossing the epithelial barrier of renal cysts and should therefore never gain access to this compartment. Considering this problem, it is not surprising that mAbs in IgG format should be ineffective in PKD if the intended target resides in cyst lumens.
To overcome this problem, we have utilized antibodies of a different isotype, IgA, specifically dimeric IgA (dIgA). The purpose of dIgA in nature is to cross epithelial barriers so that it can be excreted into external secretions as a first line of defense against pathogens. This is accomplished by transcytosis via the polymeric immunoglobulin receptor (pIgR) that binds dIgA at the basolateral side of epithelial cells and releases it into the apical space in complex with the ecto-domain of the pIgR [137, 138]. This final ‘secretory IgA’ (sIgA) consists of the dIgA molecule tightly bound to the pIgR ecto-domain, which also provides the antibody increased stability and protection from proteases.
We found that the pIgR is highly expressed on cyst-lining cells in human ADPKD and mouse models, and that its expression is driven by the aberrant activation of the STAT6 pathway [139] that is one of the driving forces of renal cyst growth [140, 141]. Importantly, we found that dIgA administered by intraperitoneal injection in PKD mouse models is indeed targeted to polycystic kidneys and accumulates in renal cyst fluid [139]. In contrast, injected IgG does not measurably reach renal cyst lumens [139]. Because pIgR is a sacrificial transporter, it can only transcytose dIgA unidirectionally into cyst lumens but not back out. Because the majority of renal cysts have lost their connection to the tubular system, once dIgA has reached cyst lumens, it will be trapped there. Therefore, administered dIgA can accumulate in renal cyst lumens (Figure 2C). In contrast, due to the relatively short serum half-life of dIgA, the remainder of injected dIgA would be rapidly cleared systemically by secretion, primarily via the intestinal epithelium and via the bile [142, 143]. The net effect is that parenterally administered dIgA is specifically targeted to renal cyst lumens and would be expected to have minimal systemic effects.
Using this approach, we estimated that concentrations of dIgA in the microgram per milliliter range can be achieved in renal cyst lumens in mice, which far exceeds the 50% effective concentrations of any IgG therapeutic mAb currently in use. These findings suggest that it is feasible to utilize antagonistic mAbs against any number of growth factors/receptors implicated in PKD, provided that the mAbs are in dIgA format. This approach would allow the re-purposing of numerous existing mAbs, after re-formatting to dIgA—including those mAbs that are already in clinical use such as mAbs against the EGFR. Since the pIgR can also transcytose pentameric IgM antibodies, this isotype could potentially also be utilized. However, the large size of pentameric IgM is likely to create additional challenges with regard to manufacturing and pharmacokinetics.
CONCLUSIONS
In conclusion, numerous pharmacological agents targeting a multitude of pathways and molecules have shown promise in preclinical studies. Although renal and extra-renal side effects are a concern for the long duration of therapy needed in ADPKD, novel approaches for targeting of drugs to renal cysts may overcome this problem. New findings suggest that ADPKD therapy may even be possible without drugs but instead using dietary intervention.
ACKNOWLEDGEMENTS
This article is part of a Supplement on ADPKD supported by an educational grant from Otsuka Pharmaceutical Europe Ltd.
FUNDING
This work was supported by a grant from the National Institutes of Health (R01DK109563) to T.W. and gifts from the Lillian Goldman Charitable Trust and the Amy P. Goldman Foundation to University of California Santa Barbara to support the work of T.W.
CONFLICT OF INTEREST STATEMENT
In the last three years, T.W. has received research funding from Endocyte, Inc. and eFFECTOR Therapeutics, Inc. J.M.S. has previously been an employee of Endocyte, Inc.
REFERENCES
- 1. Saxton RA, Sabatini DM.. mTOR ignaling in growth, metabolism, and disease. Cell 2017; 168: 960–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim H-J, Edelstein CL.. Mammalian target of rapamycin inhibition in polycystic kidney disease: from bench to bedside. Kidney Res Clin Pract 2012; 31: 132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shillingford JM, Murcia NS, Larson CH. et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 2006; 103: 5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shillingford JM, Piontek KB, Germino GG. et al. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 2010; 21: 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zafar I, Ravichandran K, Belibi FA. et al. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int 2010; 78: 754–761 [DOI] [PubMed] [Google Scholar]
- 6. Tao Y, Kim J, Schrier RW. et al. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 2005; 16: 46–51 [DOI] [PubMed] [Google Scholar]
- 7. Wahl PR, Serra AL, Le Hir M.. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 2006; 21: 598–604 [DOI] [PubMed] [Google Scholar]
- 8. Walz G, Budde K, Mannaa M. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363: 830–840 [DOI] [PubMed] [Google Scholar]
- 9. Serra AL, Poster D, Kistler AD. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363: 820–829 [DOI] [PubMed] [Google Scholar]
- 10. Watnick T, Germino GG.. mTOR inhibitors in polycystic kidney disease. N Engl J Med 2010; 363: 879–881 [DOI] [PubMed] [Google Scholar]
- 11. Shillingford JM, Leamon CP, Vlahov IR. et al. Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol 2012; 23: 1674–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravichandran K, Zafar I, Ozkok A. et al. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant 2015; 30: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Pejchinovski M, Wang X. et al. Dual mTOR/PI3K inhibition limits PI3K-dependent pathways activated upon mTOR inhibition in autosomal dominant polycystic kidney disease. Sci Rep 2018; 8: 5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pongas G, Fojo T.. BEZ235: when promising science meets clinical reality. Oncologist 2016; 21: 1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meijer E, Boertien WE, Nauta FL. et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis 2010; 56: 883–895 [DOI] [PubMed] [Google Scholar]
- 16. Chen Li, Xia Z, Lucy XF. et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 2015; 125: 2399–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karihaloo A, Koraishy F, Huen SC. et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22: 1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Magenheimer BS, Xia S. et al. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med 2008; 14: 863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitada M, Koya D.. Renal protective effects of resveratrol. Oxid Med Cell Longev 2013; 2013: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saldanha JF, de OLeal V, Stenvinkel P. et al. Resveratrol: why is it a promising therapy for chronic kidney disease patients? Oxid Med Cell Longev 2013; 2013: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu M, Gu J, Mei S. et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol Dial Transplant 2016; 31: 1826–1834 [DOI] [PubMed] [Google Scholar]
- 22. Berman AY, Motechin RA, Wiesenfeld MY. et al. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 2017; 1: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu X, Xiao X, Li N. et al. Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog Neuropsychopharmacol Biol Psychiatry 2017; 72: 60–72 [DOI] [PubMed] [Google Scholar]
- 24. Cao Y, Semanchik N, Lee SH. et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci USA 2009; 106: 21819–21824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu W, Fan LX, Zhou X. et al. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PLoS One 2012; 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seidel C, Schnekenburger M, Dicato M. et al. Histone deacetylase 6 in health and disease. Epigenomics 2015; 7: 103–118 [DOI] [PubMed] [Google Scholar]
- 27. Yanda MK, Liu Q, Cebotaru L.. An inhibitor of histone deacetylase 6 activity, ACY-1215, reduces cAMP and cyst growth in polycystic kidney disease. Am J Physiol Renal Physiol 2017; 313: F997–F1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cebotaru L, Liu Q, Yanda MK. et al. Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int 2016; 90: 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Zhang X, Polakiewicz RD. et al. HDAC6 is required for epidermal growth factor-induced beta-catenin nuclear localization. J Biol Chem 2008; 283: 12686–12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LeRoy G, Rickards B, Flint SJ.. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell 2008; 30: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kouzarides T. Chromatin modifications and their function. Cell 2007; 128: 693–705 [DOI] [PubMed] [Google Scholar]
- 32. Zhou X, Fan LX, Peters DJM. et al. Therapeutic targeting of BET bromodomain protein, Brd4, delays cyst growth in ADPKD. Hum Mol Genet 2015; 24: 3982–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lieberman J. Tapping the RNA world for therapeutics. Nat Struct Mol Biol 2018; 25: 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moreno PMD, Pêgo AP. Therapeutic antisense oligonucleotides against cancer: hurdling to the clinic. Front Chem 2014; 2: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein CA, Castanotto D.. FDA-approved oligonucleotide therapies in 2017. Mol Ther J Am Soc Gene Ther 2017; 25: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravichandran K, Zafar I, He A. et al. An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2. Hum Mol Genet 2014; 23: 4919–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saigusa T, Dang Y, Bunni MA. et al. Activation of the intrarenal renin–angiotensin-system in murine polycystic kidney disease. Physiol Rep 2015; 3: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tkachenko O, Helal I, Shchekochikhin D. et al. Renin-angiotensin-aldosterone system in autosomal dominant polycystic kidney disease. Curr Hypertens Rev 2013; 9: 12–20 [DOI] [PubMed] [Google Scholar]
- 39. Torres VE, Abebe KZ, Chapman AB. et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schrier RW, Abebe KZ, Perrone RD. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellison DH, Ingelfinger JR.. A quest–halting the progression of autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2329–2331 [DOI] [PubMed] [Google Scholar]
- 42. Ravichandran K, Ozkok A, Wang Q. et al. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am J Physiol Renal Physiol 2015; 308: F349–F357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saigusa T, Dang Y, Mullick AE. et al. Suppressing angiotensinogen synthesis attenuates kidney cyst formation in a Pkd1 mouse model. FASEB J 2016; 30: 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fitzgibbon WR, Dang Y, Bunni MA. et al. Attenuation of accelerated renal cystogenesis in Pkd1 mice by renin-angiotensin system blockade. Am J Physiol Renal Physiol 2018; 314: F210–F218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hong D, Kurzrock R, Kim Y. et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med 2015; 7: 314ra185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talbot JJ, Shillingford JM, Vasanth S. et al. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci USA 2011; 108: 7985–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weimbs T, Talbot JJ.. STAT3 signaling in polycystic kidney disease. Drug Discov Today Dis Mech 2013; 10: e113–e118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takakura A, Nelson EA, Haque N. et al. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 2011; 20: 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leonhard WN, van der Wal A, Novalic Z. et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 2011; 300: F1193–F1202 [DOI] [PubMed] [Google Scholar]
- 50. Yheskel M, Patel V.. Therapeutic micrornas in polycystic kidney disease. Curr Opin Nephrol Hypertens 2017; 26: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hajarnis S, Lakhia R, Patel V.. MicroRNAs and polycystic kidney disease In: Li X. (ed). Polycystic Kidney Disease. Vol. 1. Brisbane: Codon Publications, 2015, 313–334 [PubMed] [Google Scholar]
- 52. Pandey P, Qin S, Ho J. et al. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst Biol 2011; 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pandey P, Brors B, Srivastava PK. et al. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics 2008; 9: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hajarnis S, Yheskel M, Williams D. et al. Suppression of microRNA factivity in kidney collecting ducts induces partial loss of epithelial phenotype and renal Fibrosis. J Am Soc Nephrol 2018; 29: 518–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mogilyansky E, Rigoutsos I.. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 2013; 20: 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Noureddine L, Hajarnis S, Patel V.. MicroRNAs and polycystic kidney disease. Drug Discov Today Dis Models 2013; 10: e137–e1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hajarnis S, Lakhia R, Yheskel M. et al . microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Commun 2017; 8: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel V, Williams D, Hajarnis S. et al. miR-17∼92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 2013; 110: 10765–10770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tran U, Zakin L, Schweickert A. et al. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 2010; 137: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bartram MP, Höhne M, Dafinger C. et al. Conditional loss of kidney microRNAs results in congenital anomalies of the kidney and urinary tract (CAKUT). J Mol Med 2013; 91: 739–748 [DOI] [PubMed] [Google Scholar]
- 61. Lakhia R, Hajarnis S, Williams D. et al. MicroRNA-21 aggravates cyst growth in a model of polycystic kidney disease. J Am Soc Nephrol 2016; 27: 2319–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shin Y, Kim DY, Ko JY. Regulation of KLF12 by microRNA-20b and microRNA-106a in cystogenesis. FASEB J 2018; 32: 3574–3582 [DOI] [PubMed] [Google Scholar]
- 63. Sun L, Zhu J, Wu M. Inhibition of MiR-199a-5p reduced cell proliferation in autosomal dominant polycystic kidney disease through targeting CDKN1C. Med Sci Monit Int Med J Exp Clin Res 2015; 21: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Stephanis L, Mangolini A, Servello M. et al. MicroRNA501-5p induces p53 proteasome degradation through the activation of the mTOR/MDM2 pathway in ADPKD cells. J Cell Physiol 2018; 233: 6911–6924 [DOI] [PubMed] [Google Scholar]
- 65. Shayman JA. The design and clinical development of inhibitors of glycosphingolipid synthesis: will invention be the mother of necessity? Trans Am Clin Climatol Assoc 2013; 124: 46–60 [PMC free article] [PubMed] [Google Scholar]
- 66. Schnaar RL, Kinoshita T.. Glycosphingolipids In: Varki A, Cummings RD, Esko JD. et al. (eds). Essentials of Glycobiology. 3rd edn. Cold Spring Harbor (NY: ): Cold Spring Harbor Laboratory Press, 2015, 3e.011 [PubMed] [Google Scholar]
- 67. Ferraz MJ, Kallemeijn WW, Mirzaian M. et al. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim Biophys Acta 2014; 1841: 811–825 [DOI] [PubMed] [Google Scholar]
- 68. Grabowski GA. Gaucher disease and other storage disorders. Hematol Am Soc Hematol Educ Program 2012; 2012: 13–18 [DOI] [PubMed] [Google Scholar]
- 69. Shayman JA. Targeting glucosylceramide synthesis in the treatment of rare and common renal disease. Semin Nephrol 2018; 38: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chatterjee S, Shi WY, Wilson P. et al. Role of lactosylceramide and MAP kinase in the proliferation of proximal tubular cells in human polycystic kidney disease. J Lipid Res 1996; 37: 1334–1344 [PubMed] [Google Scholar]
- 71. Natoli TA, Smith LA, Rogers KA. et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med 2010; 16: 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ruh H, Sandhoff R, Meyer B. et al. Quantitative characterization of tissue globotetraosylceramides in a rat model of polycystic kidney disease by PrimaDrop sample preparation and indirect high-performance thin layer chromatography-matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry with automated data acquisition. Anal Chem 2013; 85: 6233–6240 [DOI] [PubMed] [Google Scholar]
- 73. Rogers KA, Moreno SE, Smith LA. et al. Differences in the timing and magnitude of Pkd1 gene deletion determine the severity of polycystic kidney disease in an orthologous mouse model of ADPKD. Physiol Rep 2016; 4: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Natoli TA, Husson H, Rogers KA. et al. Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease. Hum Mol Genet 2012; 21: 3397–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Russo D, Parashuraman S, D’Angelo G.. Glycosphingolipid-protein interaction in signal transduction. Int J Mol Sci 2016; 17: 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sillence DJ, Platt FM.. Glycosphingolipids in endocytic membrane transport. Semin Cell Dev Biol 2004; 15: 409–416 [DOI] [PubMed] [Google Scholar]
- 77. Batta G, Soltész L, Kovács T. et al. Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease. Sci Rep 2018; 8: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuech E-M, Brogden G, Naim HY.. Alterations in membrane trafficking and pathophysiological implications in lysosomal storage disorders. Biochimie 2016; 130: 152–62 [DOI] [PubMed] [Google Scholar]
- 79. Rowe I, Chiaravalli M, Mannella V. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 2013; 19: 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ishimoto Y, Inagi Y, Yoshihara D. et al. Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease. Mol Cell Biol 2017; 37: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lian X, Zhao J, Wu X. et al. The changes in glucose metabolism and cell proliferation in the kidneys of polycystic kidney disease mini-pig models. Biochem Biophys Res Commun 2017; 488: 374–381 [DOI] [PubMed] [Google Scholar]
- 82. Padovano V, Kuo VI, Stavola LK. et al. The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol Biol Cell 2017; 28: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Menezes LF, Lin C, Zhou F. et al. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 2016; 5: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin C, Kurashige M, Liu Y. et al. A cleavage product of Polycystin-1 is a mitochondrial matrix protein that affects mitochondria morphology and function when heterologously expressed. Sci Rep 2018; 8: 2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jung CH, Ro S-H, Cao J. et al. mTOR regulation of autophagy. FEBS Lett 2010; 584: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ding W-X, Yin X-M.. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012; 393: 547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Belibi F, Zafar I, Ravichandran K. et al. Hypoxia-inducible factor-1α (HIF-1α) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol 2011; 300: F1235–F1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bernhardt WM, Wiesener MS, Weidemann A. et al. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol 2007; 170: 830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Riwanto M, Kapoor S, Rodriguez D. et al. Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 2016; 11: e0146654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chang M-Y, Ma T-L, Hung C-C. et al. Metformin inhibits cyst formation in a zebrafish model of polycystin-2 deficiency. Sci Rep 2017; 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rajani R, Pastor-Soler NM, Hallows KR.. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr Opin Nephrol Hypertens 2017; 26: 375–383 [DOI] [PubMed] [Google Scholar]
- 92. Takiar V, Nishio S, Seo-Mayer P. et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 2011; 108: 2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Seliger SL, Abebe KZ, Hallows KR. et al. A randomized clinical trial of metformin to treat autosomal dominant polycystic kidney disease. Am J Nephrol 2018; 47: 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wick AN, Drury DR, Nakada HI. et al. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 1957; 224: 963–969 [PubMed] [Google Scholar]
- 95. Pelicano H, Martin DS, Xu R-H. et al. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25: 4633–4646 [DOI] [PubMed] [Google Scholar]
- 96. Chiaravalli M, Rowe I, Mannella V. et al. 2-Deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol 2016; 27: 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xi H, Kurtoglu M, Lampidis TJ.. The wonders of 2‐deoxy‐d‐glucose. IUBMB Life 2014; 66: 110–121 [DOI] [PubMed] [Google Scholar]
- 98. Hwang VJ, Kim J, Rand A. et al. The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am J Physiol Renal Physiol 2015; 309: F492–F498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Flowers EM, Sudderth J, Zacharias L. et al. Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nat Commun 2018; 9: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Soomro I, Sun Y, Li Z. et al. Glutamine metabolism via glutaminase 1 in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant 2018; 33: 1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lakhia R, Yheskel M, Flaten A. et al. PPARα agonist fenofibrate enhances fatty acid β-oxidation and attenuates polycystic kidney and liver disease in mice. Am J Physiol Renal Physiol 2018; 314: F122–F131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahmed W, Ziouzenkova O, Brown J. et al. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med 2007; 262: 184–198 [DOI] [PubMed] [Google Scholar]
- 103. Liu Y, Dai B, Fu L. et al. Rosiglitazone inhibits cell proliferation by inducing G1 cell cycle arrest and apoptosis in ADPKD cyst-lining epithelia cells. Basic Clin Pharmacol Toxicol 2010; 106: 523–530 [DOI] [PubMed] [Google Scholar]
- 104. Jones AB. Peroxisome proliferator-activated receptor (PPAR) modulators: diabetes and beyond. Med Res Rev 2001; 21: 540–552 [DOI] [PubMed] [Google Scholar]
- 105. Dai B, Liu Y, Mei C. et al. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci 2010; 119: 323–333 [DOI] [PubMed] [Google Scholar]
- 106. Flaig SM, Gattone VH, Blazer-Yost BL.. Inhibition of cyst growth in PCK and Wpk rat models of polycystic kidney disease with low doses of peroxisome proliferator-activated receptor γ agonists. J Transl Intern Med 2016; 4: 118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu Y, Dai B, Xu C. et al. Rosiglitazone inhibits transforming growth factor-β1 mediated fibrogenesis in ADPKD cyst-lining epithelial cells. PLoS One 2011; 6: e28915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yoshihara D, Kurahashi H, Morita M. et al. PPAR-γ agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 2011; 300: F465–F474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu C, Zhang Y, Yuan L. et al. Rosiglitazone inhibits insulin-like growth factor 1-induced polycystic kidney disease cell growth and p70S6 kinase activation. Mol Med Rep 2013; 8: 861–864 [DOI] [PubMed] [Google Scholar]
- 110. Liu C, Li H, Gao X. et al. Concomitant use of rapamycin and rosiglitazone delays the progression of polycystic kidney disease in Han:SPRD rats: a study of the mechanism of action. Am J Physiol Renal Physiol 2018; 314: F844–F854 [DOI] [PubMed] [Google Scholar]
- 111. Han S, Roman J.. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol Cancer Ther 2006; 5: 430–437 [DOI] [PubMed] [Google Scholar]
- 112. Hoogwerf BJ, Manner DH, Fu H. et al. Perspectives on some controversies in cardiovascular disease risk assessment in the pharmaceutical development of glucose-lowering medications. Diabetes Care 2016; 39: S219–S227 [DOI] [PubMed] [Google Scholar]
- 113. Warner G, Hein KZ, Nin V. et al. Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol 2016; 27: 1437–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kipp KR, Rezaei M, Lin L. et al. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 2016; 310: F726–F731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thoreen CC, Sabatini DM.. Rapamycin inhibits mTORC1, but not completely. Autophagy 2009; 5: 725–726 [DOI] [PubMed] [Google Scholar]
- 116. Rose SL, Highland J, Karafa MT. et al. Patient advocacy organizations, industry funding, and conflicts of interest. JAMA Intern Med 2017; 177: 344–350 [DOI] [PubMed] [Google Scholar]
- 117. McCoy MS, Carniol M, Chockley K. et al. Conflicts of interest for patient-advocacy organizations. N Engl J Med 2017; 376: 880–885 [DOI] [PubMed] [Google Scholar]
- 118. Carron PM, Crowley A, O’Shea D. et al. Targeting the folate receptor: improving efficacy in inorganic medicinal chemistry. Curr Med Chem 2018; 25: 1–34 [DOI] [PubMed] [Google Scholar]
- 119. Assaraf YG, Leamon CP, Reddy JA.. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat 2014; 17: 89–95 [DOI] [PubMed] [Google Scholar]
- 120. Weitman SD, Lark RH, Coney LR. et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 1992; 52: 3396–3401 [PubMed] [Google Scholar]
- 121. Sandoval RM, Kennedy MD, Low PS. et al. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol 2004; 287: C517–C526 [DOI] [PubMed] [Google Scholar]
- 122. Vergote I, Leamon CP.. Vintafolide: a novel targeted therapy for the treatment of folate receptor expressing tumors. Ther Adv Med Oncol 2015; 7: 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fernández M, Javaid F, Chudasama V.. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci 2018; 9: 790–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kipp KR, Kruger SL, Schimmel MF.. Comparison of folate-conjugated rapamycin versus unconjugated rapamycin in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 2018; 315: F395–F405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chung EJ, Hallows KR.. First do no harm: kidney drug targeting to avoid toxicity in ADPKD. Am J Physiol Renal Physiol 2018; 315: F535–F536 [DOI] [PubMed] [Google Scholar]
- 126. Ab O, Whiteman KR, Bartle LM. et al. IMGN853, a folate receptor-α (FRα)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol Cancer Ther 2015; 14: 1605–1613 [DOI] [PubMed] [Google Scholar]
- 127. Martin LP, Konner JA, Moore KN. et al. Characterization of folate receptor alpha (FRα) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: a phase I expansion study of the FRα-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol Oncol 2017; 147: 402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Moore KN, Martin LP, O'Malley DM. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol 2017; 35: 1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Moore KN, Martin LP, O'Malley DM. et al. A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol 2018; 14: 123–136 [DOI] [PubMed] [Google Scholar]
- 130. Moore KN, Vergote I, Oaknin A. et al. FORWARD I: a Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol 2018; 14: 1669–1678 [DOI] [PubMed] [Google Scholar]
- 131. Sliwkowski MX, Mellman I.. Antibody therapeutics in cancer. Science 2013; 341: 1192–1198 [DOI] [PubMed] [Google Scholar]
- 132. Dalziel M, Beers SA, Cragg MS. et al. Through the barricades: overcoming the barriers to effective antibody-based cancer therapeutics. Glycobiology 2018; 28: 697–712 [DOI] [PubMed] [Google Scholar]
- 133. Sweeney WE, Chen Y, Nakanishi K. et al. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int 2000; 57: 33–40 [DOI] [PubMed] [Google Scholar]
- 134. Orellana SA, Sweeney WE, Neff CD. et al. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int 1995; 47: 490–499 [DOI] [PubMed] [Google Scholar]
- 135. Du J, Wilson PD.. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 1995; 269: C487–C495 [DOI] [PubMed] [Google Scholar]
- 136. Grantham JJ, Geiser JL, Evan AP.. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int 1987; 31: 1145–1152 [DOI] [PubMed] [Google Scholar]
- 137. Mostov KE, Altschuler Y, Chapin SJ. et al. , Regulation of protein traffic in polarized epithelial cells: the polymeric immunoglobulin receptor model. Cold Spring Harb Symp Quant Biol 1995; 60: 775–781 [DOI] [PubMed] [Google Scholar]
- 138. Turula H, Wobus CE.. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses 2018; 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Olsan EE, Matsushita T, Rezaei M. et al. Exploitation of the polymeric immunoglobulin receptor for antibody targeting to renal cyst lumens in polycystic kidney disease. J Biol Chem 2015; 290: 15679–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Olsan EE, Mukherjee S, Wulkersdorfer B. et al. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 2011; 108: 18067–18072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Olsan EE, West JD, Torres JA. et al. Identification of targets of interleukin-13 and signal transducer and activator of transcription-6 (STAT6) signaling in polycystic kidney disease. Am J Physiol Renal Physiol 2018; 315: F86–F96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Brown WR, Kloppel TM.. The liver and IgA: immunological, cell biological and clinical implications. Hepatol Baltim Md 1989; 9: 763–784 [DOI] [PubMed] [Google Scholar]
- 143. Moldoveanu Z, Moro I, Radl J. et al. Site of catabolism of autologous and heterologous IgA in non-human primates. Scand J Immunol 1990; 32: 577–583 [DOI] [PubMed] [Google Scholar]