Abstract
Awareness is growing that the clinical course of autosomal dominant polycystic kidney disease (ADPKD) already begins in childhood, with a broad range of both symptomatic and asymptomatic features. Knowing that parenchymal destruction with cyst formation and growth starts early in life, it seems reasonable to assume that early intervention may yield the best chances for preserving renal outcome. Interventions may involve lifestyle modifications, hypertension control and the use of disease-modifying treatments once these become available for the paediatric population with an acceptable risk and side-effect profile. Until then, screening of at-risk children is controversial and not generally recommended since this might cause psychosocial and financial harm. Also, the clinical and research communities are facing important questions as to the nature of potential interventions and their optimal indications and timing. Indeed, challenges include the identification and validation of indicators, both measuring and predicting disease progression from childhood, and the discrimination of slow from rapid progressors in the paediatric population. This discrimination will improve both the cost-effectiveness and benefit-to-risk ratio of therapies. Furthermore, we will need to define outcome measures, and to evaluate the possibility of a potential therapeutic window of opportunity in childhood. The recently established international register ADPedKD will help in elucidating these questions. In this review, we provide an overview of the current knowledge on paediatric ADPKD as a future therapeutic target population and its unmet challenges.
Keywords: ADPKD, childhood, progression marker, treatment
CHILDHOOD ADPKD
Historically, autosomal dominant polycystic kidney disease (ADPKD), the most common hereditary kidney disease, was named adult polycystic kidney disease. Nowadays, awareness is growing that the clinical course of ADPKD already begins in childhood in some patients, although in a variable way. Indeed, the disease comprises a wide phenotypic spectrum, from severely affected neonates to clinically asymptomatic affected adults [1] (Figure 1). Even within families, with patients suffering from the same PKD1 or PKD2 mutation, high phenotypic variability exists, for which the underlying cause(s) are uncertain [2]. Presumably, disease severity correlates with the level of the functional polycystin (PC) complex, which is formed by both PKD gene products, PC1 and PC2, and is modified by other factors [3] such as glucosidase II subunit and DNAJB11, encoded by GANAB and DNAJB11, respectively, and involved in the maturation and trafficking of PC1 [4, 5]. Regarding quantitative defects in PC functionality, two hypotheses have been put forward in the past: first, the somatic second hit model in which the disease, with a dominant inheritance, has the features of a recessive disease on a cellular level [6]; and secondly, the more generally accepted threshold hypothesis, which states that cyst growth can occur if the level of functional PC falls below the cystogenic threshold. These models do not necessarily exclude each other, as the second hit might be one of the factors influencing the functional PC level, together with other ‘third hits’. The latter include low birth weight, urinary tract infections and acute renal injury. Moreover, given the cellular and molecular similarities between renal repair and ADPKD, cystogenesis might be seen as a futile attempt to repair a non-existant renal injury [7].
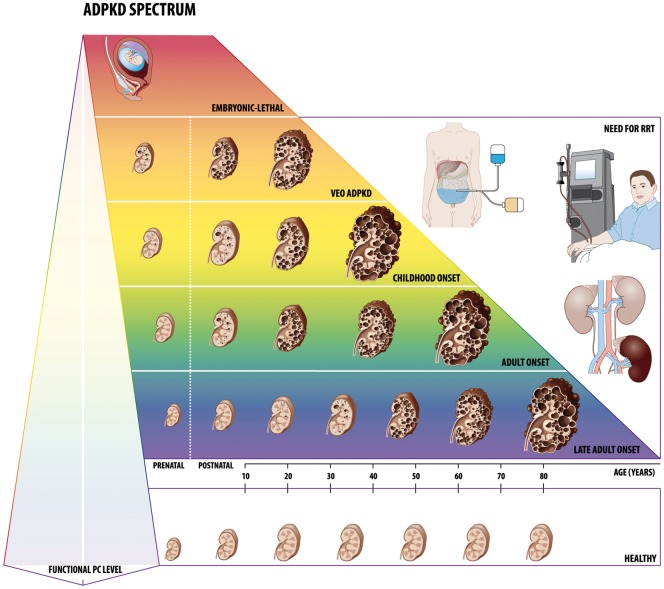
FIGURE 1.
The ADPKD spectrum. There is an enormous interpatient phenotypic variability in ADPKD. Disease severity is presumably related to the functional level of the PC complex, formed by the PKD gene products, PC1 and PC2, and is modified by factors involved in the maturation and trafficking of these proteins, such as glucosidase II subunit and DNAJB11. Also second and third hits, including environmental factors, might be influencing the functional PC level. Homozygous or compound heterozygous mutations of a PKD gene result in an embryonic lethal phenotype or a severe ARPKD-like neonatal presentation. In VEO ADPKD, diagnosis is made prenatally or within the first 18 months of life. Although views regarding the renal outcome of VEO differ, ESKD with a need for renal replacement therapy before age 18 years has been observed in several studies. On the other end of the disease spectrum, adults have a slow progressive disease, hardly evoking any symptoms during childhood and adolescence. RRT, renal replacement therapy.
ADPKD may lead to both renal and extrarenal manifestations, which are also reported in children: urinary concentration defects, hypertension, left ventricular hypertrophy (LVH), microalbuminuria, proteinuria and haematuria [8–13]. The literature on the clinical presentation of childhood ADPKD has recently been summarized [14]. Some of the features may cause symptoms, while others remain silent and undetected unless actively screened for. Related to this, ADPKD can be diagnosed in children following the investigation of symptoms, but the diagnosis may also be the consequence of coincidental findings or active family screening in asymptomatic children (Figure 2). Apart from the phenotypic variability in ADPKD patients per se, this broad diagnostic spectrum is a second factor contributing to the heterogeneity of the data on the paediatric ADPKD population. The diversity of recommendations regarding childhood screening between different expert centres contributes to this diagnostic spectrum, together with the informed opinion of the children’s parents. Also, for individuals <15 years of age, there are no validated diagnostic imaging criteria available [15, 16]. Given the rarity of childhood simple renal cysts [17], children with a family history of ADPKD, having a single cyst, preferably over 1 cm, are assumed to have ADPKD, although this criterion was never fully evaluated [14].
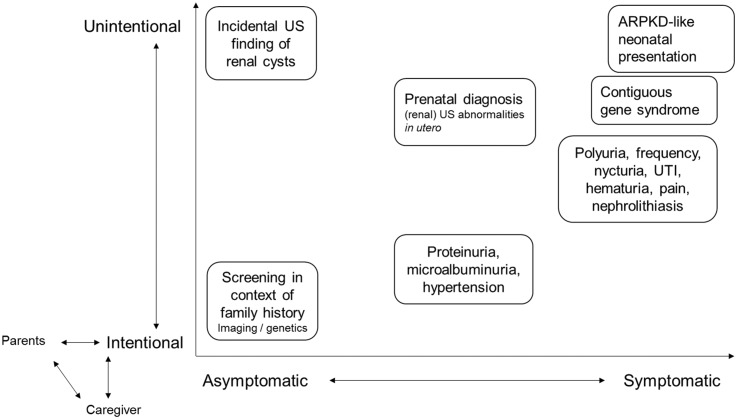
FIGURE 2.
Reasons for diagnosing ADPKD in childhood. Based on the (un)intentionality and the presence or not of symptoms, ADPKD might be diagnosed due to several reasons. The diversity of recommendations regarding childhood screening between different expert centers contributes to this diagnostic spectrum, together with the informed opinion of the children’s parents. US, ultrasound; UTI, urinary tract infection.
RATIONALE FOR EARLY TREATMENT
Fetal cyst formation is an important ADPKD characteristic. Indeed, sonographically detected renal cysts in newborns have had a very high growth rate in utero, under the influence of stochastic, intrinsic and humoral factors. Thereafter, cystic expansion proceeds at much lower growth rates [18]. Also, mathematical models have demonstrated that those cysts that started early in life are the main contributors to the total cyst volume and renal size [19]. Moreover, it is known that ADPKD cysts derive from only 1–3% of the 2 million nephrons in both kidneys [20]. The probability that an epithelial kidney cell will form a cyst is not only dependent on its PC level, but also on its biological context, as taken into account in the so-called ‘cystic probability landscape’ [21]. This small fraction of cyst-forming cells will nonetheless be able to destroy most of the renal parenchyma, leading to end-stage kidney disease (ESKD) in 50% of ADPKD patients in their sixth decade of life, since cysts can block glomerular filtration by upstream nephrons [22]. Additionally, initial cysts are the principal trigger for a snowball effect driving the formation of new cysts, leading to disease progression, including inflammation and fibrosis [23], towards ESKD [24]. Taking together the evidence for early parenchymal destruction and the substantial prevalence of symptoms in children [14, 25], the best chance for preserving long-term renal function may be given by an early therapeutic start [26].
Whereas early intervention is increasingly considered in order to attenuate both long-term renal and cardiovascular complications [27], only two interventional trials have been performed to date in children with ADPKD [28, 29], and another one is currently ongoing [30, 31] (discussed below).
EARLY IDENTIFICATION OF PATIENTS FOR FUTURE THERAPY: WHOM AND WHEN TO TREAT?
In the absence of an effective established treatment for ADPKD children, screening of at-risk children is controversial [32] and not generally recommended [33–35], since this might cause psychosocial and financial harm. Once targeted and safe therapies become available for children, this may clearly change clinician’s attitudes. However, depending on both the efficacy and side-effect profiles of the treatment options, there will be a need for validated methods to stratify patients based on their (predicted rate of) progression. Indeed, correct identification of rapid progressors at childhood age will improve both the cost-effectiveness and benefit-to-risk ratio of therapies. These children will need a more intensive follow-up programme, with an early introduction of supportive treatment. It will be important to identify these patients while avoiding the potential disadvantages of early diagnosis for those children that would not benefit. Indeed, slow progressors should not be over-medicalized and could start lifelong therapy at a later age, but still within the ideal window of opportunity to slow down or halt disease progression maximally. Unfortunately, a validated classification discriminating slow from rapid progressors does not exist in the paediatric population. This is one of the most important challenges in childhood ADPKD for the near future. Ideally, such a classification system is based on a (combination of) biomarker(s), which can be easily determined in all at-risk children at a young age, potentially even without setting the diagnosis. In the adult ADPKD population, different prognostic indicators have been identified from observational studies, and have been shown to correlate with change in renal volume and/or function [2, 36, 37]. Without any doubt, the PKD genotype and total kidney volume (TKV) corrected for height (HtTKV) as measured by magnetic resonance imaging (MRI) are the most extensively validated predictors for disease progression in adults. First, in the Genkyst cohort, renal survival in PKD2 mutation carriers was circa 20 years longer than in PKD1 subjects. Moreover, patients carrying a truncating PKD1 mutation had a worse renal prognosis compared with those with non-truncating PKD1 mutations [38]. Secondly, in the CRISP (The Consortium for Radiologic Imaging Studies in Polycystic Kidney Disease) study, which enrolled relatively young patients (15–46 years of age) with a well-preserved renal function, TKV was identified as a key indicator to select patients with ADPKD at high risk for a progressive decline in renal function, particularly in the early stages of ADPKD where significant renal enlargement occurs prior to any renal function decline [39]. This finding led to the approval of TKV as a prognostic enrichment biomarker by the Food and Drug Administration (FDA) in 2016 [40].
As these parameters enable caregivers to measure ADPKD progression, the next step to take is the prediction of it. Scoring systems, using different combinations of the above-mentioned variables associated with ADPKD progression, have been shown to predict adult patients’ renal outcome [41–43] (Figure 3). The first algorithm, the PRO-PKD score, is based on sex, PKD genotype, the presence of hypertension and/or urologic events such as haemorrhagic events involving gross haematuria or cyst haemorrhages, cyst infections, or flank pain related to cysts, before the age of 35 years. Three risk categories are defined: low risk (0–3 points), intermediate risk (4–6 points) and high risk (7–9 points). These predict corresponding median ages at onset of ESKD of 70.6, 56.9 and 49 years, respectively [41]. As both hypertension and urologic events are well-known symptoms in childhood ADPKD [14], it would be interesting to evaluate the applicability of the PRO-PKD score in children. Another validated tool is the Mayo Imaging Classification, in which patients with typical ADPKD are stratified into subclasses 1A to 1E depending on their HtTKV range for age. Internal validation showed an increased frequency of ESKD onset after 10 years from subclass 1A (2.4%) to 1E (66.9%) while external validation, using the younger CRISP participants, demonstrated increased frequencies from 1C (2.2%) to 1E (22.3%) [42]. Another prediction instrument is the ADPKD Outcomes Model (ADPKD-OM), a fixed-time increment stochastic simulation model based on disease progression equations for HtTKV and estimated glomerular filtration rate (eGFR). The model was internally validated with data from the placebo arm of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3:4 tolvaptan study [43]. Finally, a neural network for eGFR prediction was suggested. This network predicted eGFR within 3 years, based on the input of five consecutive eGFR values, obtained over a 2-year observation period [62]. This method is not validated yet and the current results are not precise enough for clinical trial design as only 33% of the predicted eGFR values were within a 10% range of the of actual eGFR values [63].
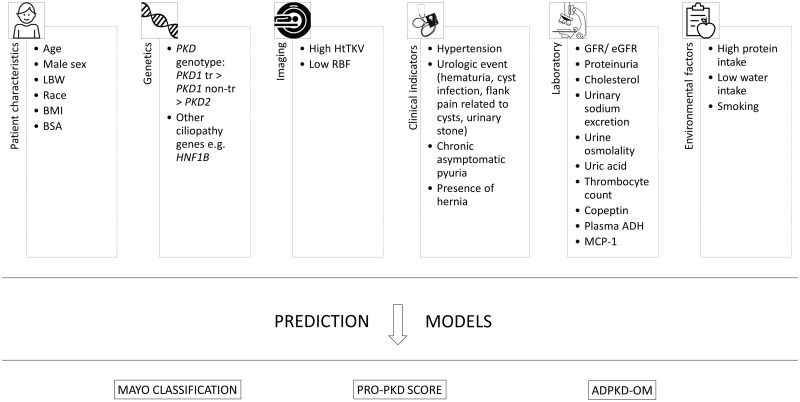
FIGURE 3.
Prognostic indicators for adult patients with ADPKD and prediction models for disease progression. In adult populations, several indicators for disease progression have been described: age (e.g. age at diagnosis) [44], male sex [45], LBW [46], race [47], BMI [48], BSA [49], PKD genotype [38], other ciliopathy genes [50], high HtTKV [51], low RBF [52], hypertension [44], urologic event [44], chronic asymptomatic pyuria [53], hernia [54], (e)GFR [52], proteinuria [55], cholesterol [49], urinary sodium excretion [49], urine osmolality [49], uric acid [56], thrombocyte count [57], copeptin [58], plasma ADH [59], MCP-1 [60], high protein intake [49], low water intake [61] and smoking [54]. These indicators gave rise to the development of different prediction models [41–43]. ADH, antidiuretic hormone; BMI, body mass index; BSA, body surface area; LBW, low birth weight; MCP-1, monocyte chemoattractant protein 1; PKD1 non-tr, PKD1 non-truncating mutation; PKD1 tr, PKD1 truncating mutation; RBF, renal blood flow.
As mentioned before, in children, well-validated measures of and predictors for progression are still missing today. Nevertheless, this is required in order to optimize clinical trial design, treatment and patient care. Different potential paediatric progression markers have been suggested, namely the presence of very-early onset (VEO) ADPKD (this is ADPKD diagnosed before the age of 18 months) [64–67]; hypertension [8–10, 68, 69]; glomerular hyperfiltration [70]; genotype [71, 72]; proteinuria [13]; urine concentration capacity [73]; presentation at diagnosis (screening versus symptoms) [11, 67]; and left ventricular mass index (LVMI) [9] (Table 1). So far, none of them has been validated however. The renal volume is often used as an outcome parameter to evaluate potential predictors, but is also frequently studied as a predictor itself [74]. The comparison of genotype and the clinical modifiers between VEO and non-VEO ADPKD might be helpful in the understanding of both predictors and modifiers of the disease. Until now, only two reports comparing VEO and non-VEO subjects have been published from the same cohort, stating that VEO is a risk factor for disease progression [65, 67]. Important in this context as well is the reason for diagnosis, as one study states that the presence of symptoms at diagnosis is a risk factor for disease progression [67], while another demonstrated that children diagnosed due to symptoms versus those due to screening have similar proportions of nephromegaly, hypertension, microalbuminuria and decreased eGFR [11]. Another issue complicating the interpretation of study results is the fact that differences in definitions of the studied predictors are used. As an example, there are studies comparing hypertensive children (≥95th percentile for age, sex and height [75]) with normotensive children (<95th percentile), but also high blood pressure (≥75th percentile) versus normal blood pressure (<75th percentile). Also, a definition of early severe disease is lacking. One study arbitrarily chose to define severe childhood ADPKD as having ≥10 renal cysts by ultrasound before the age of 12 years [74].
Table 1.
Summary of observational studies, suggesting possible prognostic indicators for childhood ADPKD (chronologically ordered)
| Publication | Objective(s) | Study details | Result(s) and conclusion relevant in this context |
|---|---|---|---|
| Sedman et al. [66] | To define the natural history. | A prospective, long-term observational study of 154 children at-risk for ADPKD (family history), receiving a renal US on age (range) 22 weeks GA – 18 years, with a FU period of 7.6 ± 1.4 yearsa in 68 children of which 19 were affected. | Two children progressed to ESKD <18 years (at age 3.5 and 15 years); both were diagnosed <1 year. |
| Conclusion: Children diagnosed <1 year may have an early deterioration in renal function. | |||
| Fick et al. [64] | To define VEO ADPKD. | A prospective, long-term observational study of 11 children with VEO ADPKD (6 diagnosed in utero and 5 <1 year; from 8 families); with FU period of 6.8 years (3–15 years)b. |
|
| Seeman et al. [69] | To perform 24 h ABPM. | A cross-sectional observational study of 32 children, aged 12.3 ± 4.7 yearsa. | Conclusion: Blood pressure correlated with renal size, but not with GFR, concentrating capacity, PU and plasma renin activity. |
| Sharp et al. [13] | To evaluate PU and microalbuminuria. | A prospective, cross-sectional observational study of children at-risk for ADPKD (family history), of which 103 affected, aged 11.2 ± 0.4 yearsa. They were classified as ‘non-ADPKD’ if no renal cysts on US, regardless of GLA result, as ADPKD if ≥1 renal cyst, and then subdivided in ‘moderate ADPKD’ (MADPKD) if 1-10 and ‘severe ADPKD’ (SADPKD) if >10 and VEO ADPKD in case of diagnosis <1 year. |
|
| Fick-Brosnahan et al. [74] | To define risk factors for more rapid progression. | An observational study of 185 children aged 8.2 ± 0.4 yearsa, with a FU period of 5.7 yearsa in 108 patients. |
|
Progression was assessed by the rate of increase in US renal volume; with 2 arbitrarily chosen definitions of ‘early severe disease’:
| |||
| Seeman et al. [68] | To evaluate the relation between BP and renal size. | A cross-sectional observational study of 62 children, aged 12.3 ± 4.3 yearsa. |
|
| Seeman et al. [73] | To evaluate the relation between renal concentrating capacity and BP. | A cross-sectional observational study of 53 children, aged 11.8 ± 4.4 yearsa. | Significantly higher prevalence of AHT in children with decreased renal concentrating capacity (35%) than in children with normal renal concentrating capacity (5%). |
| Conclusion: Decreased renal concentrating capacity should be considered as an early marker of functional impairment in ADPKD and a further risk factor for hypertension. | |||
| Shamshirsaz et al. [67] | To evaluate outcome in VEO ADPKD. | An observational study of 46 VEO children (5.5 ± 5.4 yearsa) versus 153 non-VEO ADPKD children (10.4 ± 4.5 yearsa), with a FU period of 4.1 yeara. |
|
| Cadnapaphornchai et al. [9] | To evaluate LVMI, renal volume, renal function and microalbuminuria in relation to systolic and diastolic BP. | A cross-sectional observational study of 85 children and young adults, aged 12.8 ± 1 yearsa. |
|
| Fencl et al. [72] | To compare phenotypes between children with mutations in the PKD1/PKD2 genes. | A retrospective study on 50 PKD1 children aged 8.6 ± 5.4 yearsa versus 10 PKD2 children aged 8.9 ± 5.6 yearsa. |
|
| Mekahli et al. [11] | To compare disease manifestations in children diagnosed by postnatal US screening versus those presenting with symptoms. | A retrospective study on 47 children aged 7.2 ± 4.4 yearsa with a FU period of 5.7± 3.6 yearsa. |
|
| Helal et al. [70] | To evaluate GH as indicator of more rapid disease progression. | An observational study of 180 children, aged 10.9 yearsa (4–18 years)b, with a FU period of 5 years. | Patients with GH at baseline (18%) demonstrated an increased rate of total renal volume growth and a faster decline in creatinine clearance compared with those without GH at baseline (82%). Conclusion: GH may be used as an early marker for a more severe progression. |
| Cadnapaphornchai et al. [8] | To evaluate the utility of MRI for serial assessment of kidney and cyst volume. | A prospective, long-term observational study of 77 children and young adults, aged 13 ± 4 yearsa, with a FU period of 5 years. | Hypertensive subjects demonstrated a greater increase in fractional cyst volume over time versus normotensive subjects. Cyst number increased more rapidly in hypertensive children. Conclusion: MRI is an acceptable means to follow kidney and cyst volume as well as cyst number. |
| Audrézet et al. [71] | To assess the frequency of additional variations in PKD1, PKD2, HNF1B, and PKHD1 associated with the familial PKD mutation. | A retrospective study on 42 children prenatally diagnosed with ADPKD at GA of 24 weeksa and a FU period of 4.2 yearsa. |
|
| Nowak et al. [65] | To assess the association between VEO status and adverse clinical outcomes. | A longitudinal retrospective study on 70 VEO patients and 70 non-VEO patients diagnosed at 10 years (6–14 years)c with a FU period until the age of 16 years (12–21 years)c. |
|
| Massella et al. [10] | To evaluate ABPM, kidney function, BP treatment, and kidney US. | A retrospective cross-sectional study on 310 children aged 11.5 ± 4.1 yearsa. |
|
Mean; or mean ± SD.
Median (range); median; or (range).
Median (interquartile range).
AHT, arterial hypertension; BP, blood pressure; FU, follow-up; GA, gestational age; GH, glomerular hyperfiltration; GLA, gene linkage analysis; PU, proteinuria; US, ultrasound.
Finally, there is a definite need to uniformly define which outcome parameters are of interest in the paediatric population. This is necessary to avoid the use of different primary endpoints in different studies, which dramatically compromises their comparability. In the two paediatric interventional trials, outcome parameters were renal volume, LVMI or microalbuminuria in the first [29], whereas it was ≥20% change of the same parameters in the second [28]. In adult clinical trials assessing treatment to slow down or halt progression, outcome parameters most frequently are measured by (over time change in) TKV and renal function, evaluated by eGFR or serum creatinine, as primary endpoints [37]. However, the highly variable kidney growth curves [39] and the fact that GFR evolution does not always follow a linear decline but can comprise prolonged stable intervals [76], complicates this approach. It is obvious that these biases may even be more relevant in a paediatric population. Other endpoints used are change in liver volume, change in total cyst volume (renal), blood pressure and change in urinary fatty acid-binding protein [37].
A clear definition of parameters of progression and outcome might also help to define the optimal therapeutic window of opportunity, assuming its existence.
(FUTURE) TREATMENT OPTIONS IN CHILDHOOD ADPKD
Disease-specific therapeutic options
Over the last decades, the PC complex, its aberrant downstream signalling pathways in ADPKD and possible approaches to target them have been studied extensively. Still, cystogenesis is not completely understood [77]. A curative treatment is not available so far.
In adults, the only currently available targeted disease-modifying option, the selective vasopressin V2 receptor antagonist tolvaptan, is not curative but only slows down disease progression [78]. The product’s adverse effects are mostly related to increased aquaresis: thirst, polyuria, nocturia and polydipsia, as a result of the excretion of electrolyte-free water. Furthermore, although rare, significant elevations of liver enzyme levels have been described in ADPKD patients on tolvaptan. Based on the results of the TEMPO 3:4 trial [78] and REPRISE (Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD) study [79], the European Medicines Agency (EMA) and the FDA approved tolvaptan in May 2015 and April 2018, respectively. The drug is recommended for adult patients who have documented rapid disease progression or are likely to have rapid disease progression [36]. Currently, a phase IIIb double-blind, placebo-controlled clinical trial, focusing on the safety, tolerability, pharmacokinetics and dynamics and efficacy of tolvaptan in ADPKD children, aged 4–17 years, is ongoing [31].
Another therapy tested in paediatric ADPKD is the use of HMG-CoA reductase inhibitors. In a 3-year randomized, double-blind, placebo-controlled phase III clinical trial, including patients with ADPKD aged 8–22 years, pravastatin was shown to slow down the progression of structural kidney disease, as fewer patients in the intervention group [treated with angiotensin-converting enzyme inhibitor (ACEi) and pravastatin] achieved ≥20% increase in HtTKV compared with the placebo group (only treated with ACEi) [28]. Importantly, therapy was well tolerated with no adverse effects on serum liver or muscle enzymes. Currently, pravastatin is approved for use in children from the age of 8 years with heterozygous familial hypercholesterolaemia by the FDA [80], but not by the EMA. The underlying mechanism of how pravastatin leads to potential positive effects in ADPKD remains to be fully elucidated, but statins have been shown to enhance renal blood flow and therefore glomerular filtration, to attenuate inflammation through vascular and glomerular nitric oxide production and may lower cAMP through downregulation of Gαs protein, leading to decreased cell proliferation [27]. Also, in in vitro experiments on proliferating murine tubular cells, simvastatin induced apoptosis in a time- and dose-dependent manner via down-regulation of Bcl-xL expression [81]. Moreover, in the paediatric pravastatin trial, the intervention group had reduced plasma concentrations of cyclooxygenase- and lipoxygenase-derived plasma lipid mediators as compared with the placebo group. These metabolites are known to enhance the pro-fibrotic effects of angiotensin II [82]. In a post hoc analysis of the HALT-PKD (HALT Progression of Polycystic Kidney Disease) trials, statin therapy was not shown to be beneficial in adult ADPKD patients, although the study had several limitations such as a small number of statin users and the use of different statin drugs and doses [83].
Apart from tolvaptan, largely studied therapy classes in adult patients are the mammalian target of rapamycin (mTOR) inhibitors and somatostatin analogues. First, the mTOR inhibitors most probably have no place in paediatric treatment due to the many side effects and the disappointing effects on outcome in adults [84]. The same might apply to somatostatin analogues, which seem to have a role in managing ADPKD-associated polycystic liver disease [85, 86] but until now, data are not suggestive that these agents have the potential to preserve renal function. In the recently presented results of the DIPAK (Developing intervention strategies to halt progression of autosomal dominant polycystic kidney disease) trial, the use of lanreotide in adult patients with advanced disease (eGFR 30–60 mL/min/1.73 m2), was associated with less renal volume growth, but not with an improved rate of eGFR loss. Moreover, the lanreotide group had an increased incidence of adverse events compared with the control group receiving standard care only [87]. In the ALADIN (A Long-Acting somatostatin on DIsease progression in Nephropathy due to autosomal dominant polycystic kidney disease) study, patients with eGFR >40 mL/min/1.73 m2 were randomized to a 3-year treatment with octreotide or placebo. After the first year, the average increase in TKV was significantly lower in the intervention group compared to placebo; however, in the third year, this outcome measure did not reach statistical significance. Over the 3 years, the annual reduction in GFR was not significantly different between both groups [88]. However, the full results of the DIPAK trial, ALADIN2 trial and LIPS (Lanreotide In Polycystic kidney disease Study) should be awaited.
The following drugs, currently tested in adult patients, might be tested in paediatric trials in the future, depending on their efficacy and safety in adults: the selective vasopressin V2 receptor antagonist lixivaptan (NCT03487913); AMPK activator metformin (NCT02656017); aldosterone antagonist spironolactone (NCT01853553); and tesevatinib, an inhibitor of several tyrosine receptor kinases. The latter compound is also studied in paediatric autosomal recessive polycystic kidney disease (ARPKD) (NCT03203642).
Promising curative approaches are cell-based and gene therapies, as demonstrated in animal models using bone marrow-derived mesenchymal stromal cells or induced pluripotent stem cells [89, 90]. The use of autologous bone marrow mesenchymal stromal cell infusion in ADPKD patients was shown to be safe and well tolerated in a single-arm phase I clinical trial with a 12-month follow-up. However, as the study included only six patients [91], no definite conclusions on outcome parameters can be drawn [92].
In general, starting treatment in ADPKD children, implying a life-long compliance for a slowly progressing disease that does not necessarily lead to apparent clinical symptoms over a long time, will only be feasible with agents inducing an absolute minimum of adverse effects.
Supportive measures
Counselling and promoting a healthy life style with regular exercise, abstinence from smoking, a healthy diet with appropriate amounts of calories, salt, protein and fat, increased fluid intake throughout the day and treatment of hypertension should be offered from an early age onwards both to patients with clinical disease manifestations and to those at risk for developing ADPKD [26, 93]. As overweight and, particularly, obesity were recently shown to strongly and independently associate with the rate of progression in early-stage ADPKD, a normal body mass index should be pursued from childhood [48].
Hypertension is highly prevalent in children with ADPKD, occurring in one-fifth to one-third of the population [10, 94]. From the HALT-PKD trial, it is known that in young adult ADPKD patients, strict blood pressure control compared with standard control was associated with 14.2% slower annual increase in TKV, reduced urinary albumin excretion and a greater reduction in LVMI, but no overall change in eGFR [95]. Therefore, it was suggested to prescribe ACEi to children with (borderline) hypertension (>75th percentile for age, sex and height [75]), and to achieve a goal blood pressure ≤50th percentile [25]. However, this is not generally implemented in clinical practice, as reflected by the absence of a corresponding recommendation in the currently available position statements [33–35]. Importantly, from a large population-based study, the superiority of 24-h ambulatory blood pressure measurement (ABPM) was shown over clinic or home measurements as a predictor of all-cause and cardiovascular mortality, which was highest in case of masked hypertension [96]. A recent study on ABPM in ADPKD children not only confirmed the prevalence of hypertension, but also showed high rates of nocturnal non-dipping and isolated nocturnal hypertension [10]. However, it is still unclear whether these findings should prompt antihypertensive treatment or not.
Until now, one trial studying the effect of ACEi in patients with ADPKD aged 4–21 years is published. Over a 5-year follow-up period, the authors were unable to demonstrate a significant effect of ACEi on renal growth [29]. However, it is unclear whether this study was sufficiently powered, with 85 patients included in six different subgroups, depending on their baseline blood pressure and treatment allocation, and a dropout rate of 27%. Moreover, while ACEi monotherapy was effective in controlling blood pressure in children with borderline hypertension, multiple antihypertensive medications were necessary in hypertensive subjects.
FUTURE PERSPECTIVES
Given the limited data on clinical courses of paediatric ADPKD, more clinical research is needed to describe the development of patients during childhood and adolescence and the potential effects of, for example, early antihypertensive treatment. International collaborative research approaches have shown an enormous potential to identify and characterize specific aspects of paediatric kidney disease and to generate an observational evidence base for clinical recommendations. This includes studies on estimating times to ESKD in children with chronic kidney disease [97], cardiovascular consequences of chronic kidney disease in childhood [98, 99] and courses of paediatric dialysis [100]. A more recent study, ARegPKD, is dedicated to describe the details of clinical courses of patients suffering from ARPKD [101, 102]. This register study has collected the largest cohort of ARPKD subjects, as a basis to identify, for example, clinical risk markers of disease progression [103]. For ADPKD, the characterization of a similar cohort study, describing the courses of children diagnosed with ADPKD, is currently initiated as ADPedKD (https://www.adpedkd.org). In this international, web-based database, prospective and retrospective data on multiple clinical and genetic aspects are collected in basic data questionnaires and yearly follow-up visits to understand long-term clinical courses. In this way, ADPedKD aims to establish the first large and clinically deeply phenotyped paediatric ADPKD cohort. Obviously, such a work needs to be complemented by detailed information on genotype and family history to establish cohorts that fulfil unified disease criteria. Moreover, both paediatric and adult nephrologists involved in clinical ADPKD research should be stimulated to cooperate on the future scientific transition of childhood cohorts to the adult setting. This will allow investigators to study long-term follow-up, even after subjects have reached adulthood, which would be of great value to correlate long-term outcome parameters with early markers of progression.
In translational approaches, the collection of corresponding biosamples will be helpful and required to benefit from the power of modern high-throughput technologies with the potential to identify novel and complimentary disease-specific biomarkers. Non-invasive collection of biosamples such as patient cells from urine may help to study the effects of specific mutations.
In summary, the clinical and research communities on ADPKD are faced with important questions related to the nature of potential interventions, and their optimal indications and timing. From a paediatric perspective, challenges include the identification and validation of indicators for disease progression from childhood in order to discriminate slow from rapid progressors, and to define outcome measures. The ADPedKD initiative will help in elucidating these questions.
ACKNOWLEDGEMENTS
This paper is part of a Supplement on ADPKD supported by an educational grant from Otsuka Pharmaceutical Europe Ltd.
FUNDING
D.M. is supported by the Clinical Research Fund of UZ Leuven and by the Fund for Scientific Research, Flanders G0B1313N. S.D.R. is supported by the Fund for Scientific Research, Flanders 11M5214N. M.C.L. is supported by the Marga and Walter Boll-Foundation, and the German Federal Ministry of Research and Education (BMBF grant 01GM1515).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Koratala A, Malpartida FR, Kazory A.. An 88-year-old patient with ADPKD: underscoring the importance of risk factor modification. Clin Case Rep 2017; 5: 2146–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ong AC, Devuyst O, Knebelmann B. et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015; 385: 1993–2002 [DOI] [PubMed] [Google Scholar]
- 3. Ong AC, Harris PC.. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int 2015; 88: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornec-Le Gall E, Olson RJ, Besse W.. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet 2018; 102: 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porath B, Gainullin VG, Cornec-Le Gall E. et al. Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 2016; 98: 1193–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaimori JY, Germino GG.. ARPKD and ADPKD: first cousins or more distant relatives? J Am Soc Nephrol 2008; 19: 416–418 [DOI] [PubMed] [Google Scholar]
- 7. Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 2007; 293: F1423–F1432 [DOI] [PubMed] [Google Scholar]
- 8. Cadnapaphornchai MA, Masoumi A, Strain JD. et al. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 2011; 6: 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cadnapaphornchai MA, McFann K, Strain JD. et al. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 2008; 74: 1192–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massella L, Mekahli D, Paripović D. et al. Prevalence of hypertension in children with early-stage ADPKD. Clin J Am Soc Nephrol 2018; 13: 874–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mekahli D, Woolf AS, Bockenhauer D.. Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol 2010; 25: 2275–2282 [DOI] [PubMed] [Google Scholar]
- 12. Selistre L, de Souza V, Ranchin B. et al. Early renal abnormalities in children with postnatally diagnosed autosomal dominant polycystic kidney disease. Pediatr Nephrol 2012; 27: 1589–1593 [DOI] [PubMed] [Google Scholar]
- 13. Sharp C, Johnson A, Gabow P.. Factors relating to urinary protein excretion in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1998; 9: 1908–1914 [DOI] [PubMed] [Google Scholar]
- 14. De Rechter S, Breysem L, Mekahli D.. Is autosomal dominant polycystic kidney disease becoming a pediatric disorder? Front Pediatr 2017; 5: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gabow PA, Kimberling WJ, Strain JD. et al. Utility of ultrasonography in the diagnosis of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 1997; 8: 105–110 [DOI] [PubMed] [Google Scholar]
- 16. Reed B, Nobakht E, Dadgar S. et al. Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am J Kidney Dis 2010; 56: 50–56 [DOI] [PubMed] [Google Scholar]
- 17. Ravine D, Gibson RN, Donlan J. et al. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis 1993; 22: 803–807 [DOI] [PubMed] [Google Scholar]
- 18. Grantham JJ, Cook LT, Wetzel LH. et al. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5: 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grantham JJ, Cook LT, Torres VE. et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 2008; 73: 108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terryn S, Ho A, Beauwens R. et al. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812: 1314–1321 [DOI] [PubMed] [Google Scholar]
- 21. Leonhard WN, Happe H, Peters DJ.. Variable cyst development in autosomal dominant polycystic kidney disease: the biologic context. J Am Soc Nephrol 2016; 27: 3530–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grantham JJ, Torres VE.. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 2016; 12: 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mun H, Park JH.. Inflammation and Fibrosis in ADPKD. Adv Exp Med Biol 2016; 933: 35–44 [DOI] [PubMed] [Google Scholar]
- 24. Leonhard WN, Zandbergen M, Veraar K. et al. Scattered deletion of PKD1 in kidneys causes a cystic snowball effect and recapitulates polycystic kidney disease. J Am Soc Nephrol 2015; 26: 1322–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reddy BV, Chapman AB.. The spectrum of autosomal dominant polycystic kidney disease in children and adolescents. Pediatr Nephrol 2016; 32: 31–42 [DOI] [PubMed] [Google Scholar]
- 26. Grantham JJ. Rationale for early treatment of polycystic kidney disease. Pediatr Nephrol 2014; 30: 1053–1062 [DOI] [PubMed] [Google Scholar]
- 27. Cadnapaphornchai MA. Clinical trials in pediatric autosomal dominant polycystic kidney disease. Front Pediatr 2017; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cadnapaphornchai MA, George DM, McFann K. et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9: 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cadnapaphornchai MA, McFann K, Strain JD. et al. Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 2009; 4: 820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janssens P, Weydert C, De Rechter S. et al. Expanding the role of vasopressin antagonism in polycystic kidney diseases: From adults to children? Pediatr Nephrol 2018; 33: 395–408 [DOI] [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov; Safety, Pharmacokinetics, Tolerability and Efficacy of Tolvaptan in Children and Adolescents With ADPKD (Autosomal Dominant Polycystic Kidney Disease). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02964273: 2017
- 32. De Rechter S, Kringen J, Janssens P. et al. Clinicians' attitude towards family planning and timing of diagnosis in autosomal dominant polycystic kidney disease. PLoS One 2017; 12: e0185779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chapman AB, Devuyst O, Eckardt KU. et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2015; 88: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European ADPKD Forum. Translating science into policy to improve ADPKD care. 2015. http://www.senefro.org/modules/noticias/images/eaf_report_jan2015final_english1.pdf.
- 35. Harris T, Sandford R, de Coninck B. et al. European ADPKD Forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care: European ADPKD Forum and Multispecialist Roundtable participants. Nephrol Dial Transplant 2018; 33: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gansevoort RT, Arici M, Benzing T. et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 2016; 31: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woon C, Bielinski-Bradbury A, O’Reilly K. et al. A systematic review of the predictors of disease progression in patients with autosomal dominant polycystic kidney disease. BMC Nephrol 2015; 16: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cornec-Le Gall E, Audrézet M-P, Chen J-M. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 2013; 24: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grantham JJ, Torres VE, Chapman AB. et al. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354: 2122–2130 [DOI] [PubMed] [Google Scholar]
- 40. FDA (2016). http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm458483.pdf (accessed 8 June 2018)
- 41. Cornec-Le Gall E, Audrezet M-P, Rousseau A. et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2015; 27: 942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Irazabal MV, Rangel LJ, Bergstralh EJ. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2015; 26: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McEwan P, Bennett Wilton H, Ong ACM. et al. A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): the ADPKD outcomes model. BMC Nephrol 2018; 19: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schrier RW, Brosnahan G, Cadnapaphornchai MA. et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol 2014; 25: 2399–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gabow PA, Johnson AM, Kaehny WD. et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 1992; 41: 1311–1319 [DOI] [PubMed] [Google Scholar]
- 46. Orskov B, Christensen KB, Feldt-Rasmussen B. et al. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int 2012; 81: 919–924 [DOI] [PubMed] [Google Scholar]
- 47. Yium J, Gabow P, Johnson A. et al. Autosomal dominant polycystic kidney disease in blacks: clinical course and effects of sickle-cell hemoglobin. J Am Soc Nephrol 1994; 4: 1670–1674 [DOI] [PubMed] [Google Scholar]
- 48. Nowak KL, You Z, Gitomer B. et al. Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2018; 29: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torres VE, Grantham JJ, Chapman AB. et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6: 640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bergmann C, von Bothmer J, Ortiz Brüchle N. et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 2011; 22: 2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perrone RD, Mouksassi MS, Romero K. et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep 2017; 2: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torres VE, King BF, Chapman AB. et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2007; 2: 112–120 [DOI] [PubMed] [Google Scholar]
- 53. Hwang JH, Park HC, Jeong JC. et al. Chronic asymptomatic pyuria precedes overt urinary tract infection and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol 2013; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ozkok A, Akpinar TS, Tufan F. et al. Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol 2013; 17: 345–351 [DOI] [PubMed] [Google Scholar]
- 55. Chapman AB, Johnson AM, Gabow PA. et al. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1994; 5: 1349–1354 [DOI] [PubMed] [Google Scholar]
- 56. Helal I, McFann K, Reed B. et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant 2013; 28: 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen D, Ma Y, Wang X. et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS One 2014; 9: e92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tasneem M, Mannix C, Wong A. et al. Is serum copeptin a modifiable biomarker in autosomal dominant polycystic kidney disease? World J Nephrol 2018; 7: 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Devuyst O, Torres VE.. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 459–470 [DOI] [PubMed] [Google Scholar]
- 60. Messchendorp AL, Meijer E, Boertien WE. et al. Urinary biomarkers to identify autosomal dominant polycystic kidney disease patients with a high likelihood of disease progression. Kidney Int Rep 2018; 3: 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amro OW, Paulus JK, Noubary F. et al. Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: a pilot randomized controlled trial. Am J Kidney Dis 2016; 68: 882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Niel O, Boussard C, Bastard P.. Artificial intelligence can predict GFR decline during the course of ADPKD. Am J Kidney Dis 2018; 71: 911–912 [DOI] [PubMed] [Google Scholar]
- 63. Brosnahan GM, Moore CG, Abebe KZ.. In reply to ‘Artificial intelligence can predict GFR decline during the course of ADPKD’ and ‘Linear and nonlinear estimated GFR slopes in ADPKD patients reaching ESRD’. Am J Kidney Dis 2018; 71: 913. [DOI] [PubMed] [Google Scholar]
- 64. Fick GM, Johnson AM, Strain JD. et al. Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1993; 3: 1863–1870 [DOI] [PubMed] [Google Scholar]
- 65. Nowak KL, Cadnapaphornchai MA, Chonchol MB. et al. Long-term outcomes in patients with very-early onset autosomal dominant polycystic kidney disease. Am J Nephrol 2016; 44: 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sedman A, Bell P, Manco-Johnson M. et al. Autosomal dominant polycystic kidney disease in childhood: a longitudinal study. Kidney Int 1987; 31: 1000–1005 [DOI] [PubMed] [Google Scholar]
- 67. Shamshirsaz A, Bekheirnia RM, Kamgar M. et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int 2005; 68: 2218–2224 [DOI] [PubMed] [Google Scholar]
- 68. Seeman T, Dušek J, Vondřichová H. et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit 2003; 8: 107–110 [DOI] [PubMed] [Google Scholar]
- 69. Seeman T, Sikut M, Konrad M. et al. Blood pressure and renal function in autosomal dominant polycystic kidney disease. Pediatr Nephrol 1997; 11: 592–596 [DOI] [PubMed] [Google Scholar]
- 70. Helal I, Reed B, McFann K. et al. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6: 2439–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Audrézet MP, Corbiere C, Lebbah S. et al. Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2016; 27: 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fencl F, Janda J, Bláhová K. et al. Genotype-phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 2009; 24: 983–989 [DOI] [PubMed] [Google Scholar]
- 73. Seeman T, Dušek J, Vondrak K.. Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 2004; 53: 629–634 [PubMed] [Google Scholar]
- 74. Fick-Brosnahan GM, Tran ZV, Johnson AM. et al. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 2001; 59: 1654–1662 [DOI] [PubMed] [Google Scholar]
- 75. Flynn JT, Kaelber DC, Baker-Smith CM. et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140 [DOI] [PubMed] [Google Scholar]
- 76. Brosnahan GM, Abebe KZ, Moore CG.. Patterns of kidney function decline in autosomal dominant polycystic kidney disease: a post hoc analysis from the HALT-PKD trials. Am J Kidney Dis 2018; 71: 666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ferreira FM, Watanabe EH, Onuchic LF.. Polycystins and molecular basis of autosomal dominant polycystic kidney disease In: Li XS. (ed) Polycystic Kidney Disease. Brisbane, Australia: Codon Publications, 2015 [PubMed] [Google Scholar]
- 78. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 2017; 377: 1930–1942 [DOI] [PubMed] [Google Scholar]
- 80. FDA (2011). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/019898Orig1s061.pdf (accessed 8 June 2018)
- 81. Blanco-Colio LM, Justo P, Daehn I. et al. Bcl-xL overexpression protects from apoptosis induced by HMG-CoA reductase inhibitors in murine tubular cells. Kidney Int 2003; 64: 181–191 [DOI] [PubMed] [Google Scholar]
- 82. Klawitter J, McFann K, Pennington AT. et al. Pravastatin therapy and biomarker changes in children and young adults with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2015; 10: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brosnahan G, Abebe KZ, Rahbari-Oskoui FF. et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease. A secondary analysis of the HALT PKD trials. Curr Hypertens Rev 2017; 13: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bolignano D, Palmer SC, Ruospo M. et al. Interventions for preventing the progression of autosomal dominant polycystic kidney disease. Cochrane Database Syst Rev 2015: Cd010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gevers TJ, Hol JC, Monshouwer R. et al. Effect of lanreotide on polycystic liver and kidneys in autosomal dominant polycystic kidney disease: an observational trial. Liver Int 2015; 35: 1607–1614 [DOI] [PubMed] [Google Scholar]
- 86. Pisani A, Sabbatini M, Imbriaco M. et al. Long-term effects of octreotide on liver volume in patients with polycystic kidney and liver disease. Clin Gastroenterol Hepatol 2016; 14: 1022–1030.e4 [DOI] [PubMed] [Google Scholar]
- 87. Gansevoort RDJ, de Fijter J, Meijer E. et al. on behalf of the DIPAK Consortium; Renoprotective efficacy and safety of the somatostatin analogue lanreotide in later stage ADPKD. ERA-EDTA Congress. Copenhagen, Denmark, 2018
- 88. Caroli A, Perico N, Perna A. et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 2013; 382: 1485–1495 [DOI] [PubMed] [Google Scholar]
- 89. Cheng L, Nagata S, Hirano K. et al. Cure of ADPKD by selection for spontaneous genetic repair events in Pkd1-mutated iPS cells. PLoS One 2012; 7: e32018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Franchi F, Peterson KM, Xu R. et al. Mesenchymal stromal cells improve renovascular function in polycystic kidney disease. Cell Transplant 2015; 24: 1687–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Makhlough A, Shekarchian S, Moghadasali R. et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther 2017; 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xu Y, Li A, Wu G. et al. Perspectives of gene therapies in autosomal dominant polycystic kidney disease. Curr Gene Ther 2017; 17: 43–49 [DOI] [PubMed] [Google Scholar]
- 93. PKD International tEAFE. ADPKD Patient Route Map. https://pkdinternational.org/adpkd-route-map (accessed 8 June 2018)
- 94. Marlais M, Cuthell O, Langan D. et al. Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child 2016; 101: 1142–1147 [DOI] [PubMed] [Google Scholar]
- 95. Schrier RW, Abebe KZ, Perrone RD. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Banegas JR, Ruilope LM, de la Sierra A. et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018; 378: 1509–1520 [DOI] [PubMed] [Google Scholar]
- 97. Furth SL, Pierce C, Hui WF. et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis 2018; 71: 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Querfeld U, Anarat A, Bayazit SK. et al. The cardiovascular comorbidity in children with chronic kidney disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol 2010; 5: 1642–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schaefer F, Doyon A, Azukaitis K. et al. Cardiovascular phenotypes in children with CKD: The 4C Study. Clin J Am Soc Nephrol 2017; 12: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ha IS, Yap HK, Munarriz RL. et al. Risk factors for loss of residual renal function in children treated with chronic peritoneal dialysis. Kidney Int 2015; 88: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ebner K, Feldkoetter M, Ariceta G. et al. Rationale, design and objectives of ARegPKD, a European ARPKD registry study. BMC Nephrol 2015; 16: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ebner K, Schaefer F, Liebau MC.. Recent progress of the ARegPKD registry study on autosomal recessive polycystic kidney disease. Front Pediatr 2017; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burgmaier K, Kunzmann K, Ariceta G. et al. Risk factors for early dialysis dependency in autosomal recessive polycystic kidney disease. J Pediatr 2018; 199: 22–28 e6 [DOI] [PubMed] [Google Scholar]