Abstract
Posttraumatic stress disorder is a heterogeneous disorder with disturbances in hyperarousal or avoidance behaviors and intrusive or reexperiencing thoughts. The uncinate fasciculus and cingulum bundle are white matter pathways implicated in stress and trauma pathophysiology, yet their structural integrity related to posttraumatic stress disorder symptom domains is yet to be understood. Forty-four trauma-exposed young adults underwent structural and diffusion-weighted magnetic resonance imaging. Stress and trauma exposure indices and severity of posttraumatic stress disorder symptoms were collected and used to predict current integrity of the uncinate fasciculus and cingulum bundle. Severity of reexperiencing posttraumatic stress disorder symptoms was significantly related to increased fractional anisotropy (r = .469 p < .001) and decreased mean diffusivity (r = −.373, p = .013) of the right posterior cingulum bundle. No other findings emerged with respect to stress exposure or of hyperarousal (p’s > 0.05) or avoidance (p’s > 0.2) posttraumatic stress disorder symptoms. The posterior cingulum connects medial temporal lobe structures with visual areas in the occipital lobe and has been implicated in visual memory and self-referential thought. Increased structural connectivity along this pathway may therefore explain the emergence of reexperiencing posttraumatic stress disorder symptoms. This along with the lack of results with respect to stress exposure suggests that structural aberrations in white matter pathways are more strongly linked with the actual experience of stress-related psychological symptoms than just exposure to stress.
Keywords: posttraumatic stress disorder, white matter, trauma, diffusion tensor imaging, stress
Nearly 90% of people in the United States will experience a traumatic event in their lifetime.1 Although most are resilient in the aftermath of trauma,2–6 a substantial minority go on to develop debilitating psychopathologies.7–10 In particular, stress and traumatic stress lead to increased risk of posttraumatic stress disorder (PTSD), a disorder characterized by symptoms of reexperiencing the event through intrusive thoughts, nightmares, flashbacks, avoidance of trauma-related stimuli, and symptoms of hyperarousal.11–13 Although cumulative stress and trauma exposure increase risk of psychopathology,7 the brain changes that mediate this risk are not well understood.
Stress and traumatic stress can negatively impact the brain, for example, through the integrity of white matter tracts.11,14–16 White matter tracts are the myelinated axons of neurons that connect brain regions to coordinate the transfer of information throughout the brain.17 Estimation of the integrity of white matter tracts can be done with diffusion tensor imaging (DTI), a noninvasive magnetic resonance imaging (MRI) technique that harnesses the diffusion properties of water through brain tissue.18 Studies using DTI have shown that stress and trauma impact white matter integrity through activation of the hypothalamic–pituitary–adrenal (HPA) axis and the release of various stress hormones.19 Excess exposure to stress hormones, particularly in critical windows of brain development, can impair maturation and integrity of white matter pathways20 in circuits supporting memory formation and maintenance as well as emotion regulation, both processes implicated in PTSD.11,15,18,19,21 Therefore, it is important to understand how stress and trauma affect the structural integrity of the brain to understand how structural integrity may underlie altered cognitive and affective processing in PTSD.
One region that has been repeatedly implicated in stress-related psychopathology is the hippocampus as it is especially sensitive to activity of the HPA axis.11,14,19 Specifically, high levels of cortisol and other stress hormones are thought to lead to damage of the hippocampus.11 Some researchers have found that smaller hippocampal volume confers greater risk of developing PTSD if individuals are exposed to trauma.11 As the hippocampus is also important for the encoding and retrieval of memories, particularly emotional or stress-related memories,19,22–24 impaired retention of extinction learning which is consistently observed in PTSD implies hippocampal dysfunction.25,26 Although the effects of stress on the hippocampus are well-documented, the downstream or upstream effects of stress on adjacent white matter pathways that involve the hippocampus are less understood.19,27
The cingulum bundle is a white matter tract that projects from the cingulate gyrus to the hippocampus and medial temporal lobe cortices that allows for the communication of the limbic system to the cortex.28 Integrity of the cingulum has been argued to play an important role in emotion regulation and has been hypothesized to contribute to the dysregulated emotions seen in PTSD.29,30 Decreased integrity of the cingulum has been reported in a variety of populations, including women with high exposure to trauma,31,32 Afghanistan and Iraq war veterans with PTSD,29 adults with major depressive disorder,14 adults with a history of childhood trauma,33 and adolescents with a history of abuse.34 This weaker connectivity in the cingulum has been shown to have functional consequences, including the insufficient top-down emotion appraisal and regulation characteristic of PTSD.15,29,31 Thus, repeated exposure to trauma and subsequent stress may be related to the microstructural degradation of the cingulum.35
By contrast, other researchers have found increased integrity of the cingulum in individuals afflicted by various types of trauma, including an act of terrorism,36 combat,30,37,38 severe mine accident survivors,39 and in a mixed-trauma sample of PTSD patients,40 and that greater cingulum integrity is related to greater symptom severity in these samples.30,37,38,40 Discrepancies in terms of whether trauma is related to decreased versus increased cingulum integrity may be related to the fact that PTSD is a heterogeneous disorder, and the presence of specific symptom clusters of PTSD may be directly related to cingulum integrity. Some researchers have found that increased structural connectivity of the cingulum is related specifically to intrusive reexperiencing symptoms, though some report increased30 while others report decreased symptoms.39 At this time, more research is needed to clarify direction of findings as it relates to the association between cingulum white matter integrity and PTSD symptoms.
Another white matter tract that is implicated in the pathophysiology of PTSD is the uncinate fasciculus (UF), a tract which connects the temporal cortices involved in memory formation and the frontal cortices involved in emotion regulation.16,41,42 Decreased UF integrity has been linked to impaired extinction learning41,43,44 as well as misevaluation of social-emotional stimuli that may be linked to avoidance behaviors seen in PTSD.41,43,44 A review of the development of the UF indicates that early life abuse or stress may be associated with decreased structural integrity due to the sensitivity of limbic regions to stress hormones.43 Decreased integrity of the UF has been reported in children with a history of maltreatment,33,45 police officers with PTSD,44 and in mixed-trauma community samples.46,47 In addition, reduced UF integrity is predictive of psychological vulnerability to future stress or trauma.45 Therefore, impaired structural integrity of the UF may be another potential indicator of stress history and PTSD symptom severity.
Although structural changes have been identified in various populations with adverse backgrounds of stress and trauma, very few of these studies have included samples with emerging adults.48,49 Longitudinal studies investigating developmental changes in major white matter pathways suggest that the cingulum and UF stop maturing around the mid-20 s, which means white matter in emerging adults may still be developing.20,50 Understanding how stress and trauma affect white matter pathways while they are still maturing may help inform the trajectory of mental health outcomes later in life. Moreover, very few studies have compared the effects of both stress exposure and severity of stress-related symptoms across the domains of reexperiencing, avoidance, and hyperarousal symptoms on white matter integrity.38,51 Therefore, one of the primary purposes of the current study is to investigate how stress exposure and symptoms of PTSD may impact the structural integrity of white matter pathways, particularly the cingulum bundle and the UF in emerging adults. Previous research has shown that the direction of integrity changes in the cingulum may explain different stress-related symptoms.15,29–31 Owing to this hetereogeneity of findings, we predicted that altered cingulum integrity would be associated with greater life stress and increased severity of PTSD symptoms. In addition, we also predicted that decreased integrity of the UF would be related to greater life stress as well as increased severity of PTSD symptoms.
Method
Participants
Sixty-seven trauma-exposed young adults (23 males and 44 females; Mage = 22.32, standard deviation (SD) = 3.75) originally participated in this study. Inclusion criteria included being right handed, over 18 years old, and reporting experience of at least one past trauma as measured by the Life Events Checklist (LEC).52 Exclusion criteria included the presence of neurological disorders, mania, psychosis, history of head trauma, or any contraindications for a MR scan including metal in the body, pregnancy, or claustrophobia. To ensure normality, seven participants were excluded from analysis for outlying ( ± 2.5 SD) stress and PTSD symptom severity scores (n = 4 females, n = 1 male) and outlying diffusion measures (n = 2 females). In addition, due to the uneven distribution of males and females, and to account for potential gender49,53–55 and age differences20,54 that have been well documented in the white matter pathways of interest, the final sample included in analysis was matched in terms of gender and age distribution. In addition, the final sample was also matched on total PTSD severity scores from the PTSD Checklist Civilian (PCL-C).56 The final sample thus had 44 participants (22 males and 22 females; Mage = 22.93, SD = 4.13). The study was approved by the University of Wisconsin—Milwaukee Institutional Review Board. According to the Declaration of Helsinki, participants provided written informed consent and were paid for their participation in the study.
Stress and Trauma Measures
Life stress and trauma were assessed using the LEC,52 the Life Events Scale (LES),57 and the Daily Hassles Scale (DHS).58 LEC has good internal (Kappa = .61) and test–retest reliability (r = .82)52 and assesses occurrence of major life events that a person has experienced firsthand, witnessed, or heard about happening to someone close to them (e.g. natural disaster, assault, combat, life-threatening illness, or injury). The LES assesses the occurrence and severity of major life events that have happened in the last 12 months (e.g. marriage, unwed pregnancy, death of parent, failure of a grade in school, and serious illness). The DHS has demonstrated good test–retest reliability (r = .79)58 and assesses the degree to which an individual is bothered by daily pressures, or difficulties (e.g. financial security, preparing meals, not getting enough sleep, job dissatisfaction, and traffic).
Based on Vinkers et al.,59 in addition to examining these three self-report measures individually, a cumulative stress index (CSI) was calculated by z-transforming all three scales (LEC, LES, and DHS) and summing the three z scores. Although it is unlikely these stress measures should be weighted equally when computing the cumulative index, it would be difficult to determine appropriate weights of these various measures, and this method allows for at least a preliminary investigation of an index of cumulative stress.
PTSD symptoms were assessed using the PCL-C.56 The PCL-C is a 17-item self-report measure that assesses each of the symptom domains of PTSD including reexperiencing, avoidance, and arousal symptoms. Participants rate their degree on a scale of 1 to 5 (1 = not at all, 5 = extremely) for which they have been bothered by a given problem. Example items include “repeated, disturbing dreams of a stressful experience”, “having physical reactions (e.g., heart pounding, trouble breathing, sweating) when something reminded you of a stressful experience,” and “being ‘super-alert’ or watchful or on guard.” Responses can be summed to attain a total PTSD symptom severity score or can be summed by symptom domain.
MRI Acquisition
MRI was collected on a 3 T short bore GE Signa Excite system. High-resolution spoiled gradient recalled anatomical images were acquired in a sagittal orientation (repetition time (TR) = 8.2 ms; echo time (TE) = 3.2 ms; field of view (FOV) = 24 cm; flip angle = 12°; voxel size = 1 × 0.9375 × 0.9375 mm). Diffusion-weighted images were collected using an echoplanar pulse sequence with 70 contiguous 2-mm-thick axial slices and 38 noncollinear diffusion gradients (TR = 10 s; TE = 77.99 ms; b value = 800 s/mm2; FOV = 25.6 cm; flip angle = 90°; voxel size = 2 × 2 × 2 mm).
Image Analysis
Diffusion weighted images (DWIs) were processed using a standard pipeline in FreeSurfer’s TRActs Constrained by UnderLying Anatomy (TRACULA).60 First, DWIs were preprocessed to correct for image and eddy current distortions. Then, individual participant’s DWIs were registered to their anatomical T1 images using FreeSurfer’s affine transformation bbregister based on its surface reconstruction to optimize registration. The anatomical T1 images were then registered to a standard Montreal Neurological Institute (MNI152) template for group comparison. Head motion was accounted for using the translation and rotation parameters calculated in TRACULA (Table 1).
Table 1.
Sample characteristics of trauma-exposed individuals (LEC > 0).
| Full sample (no outliers) (n = 60) |
Matched sample (n = 44) |
|
|---|---|---|
| M (SD) | M (SD) | |
| Demographics | ||
| Age | 22.46 (3.82) | 22.9 (4.13) |
| Gender (% male) | 22 (36) | 22 (50) |
| LEC | 2.96 (1.56) | 3.13 (1.70) |
| LES | 520.90 (364.37) | 506.04 (327.15) |
| DHS | 37.43 (28.58) | 35.68 (28.34) |
| Cumulative stress index | 0 (2.09) | 0 (2.06) |
| PCL-C total | 28.65 (10.89) | 26.61 (9.81) |
| PCL-C reexperiencing | 7.95 (3.43) | 7.09 (2.71) |
| PCL-C avoidance | 11.50 (4.85) | 10.95 (4.95) |
| PCL-C arousal | 9.20 (3.73) | 8.56 (3.23) |
| Head motion parameters | ||
| Average translation | 0.63 (0.11) | 0.63 (0.10) |
| Average rotation | 0.005 (0.001) | 0.005 (0.0009) |
| TMI | 0.643 (0.117) | 0.638 (0.108) |
M: mean; SD: standard deviation; LEC: Life Events Checklist; LES: Life Events Scale; DHS: Daily Hassles Scale; PCL-C: PTSD Checklist Civilian. TMI: total motion index.
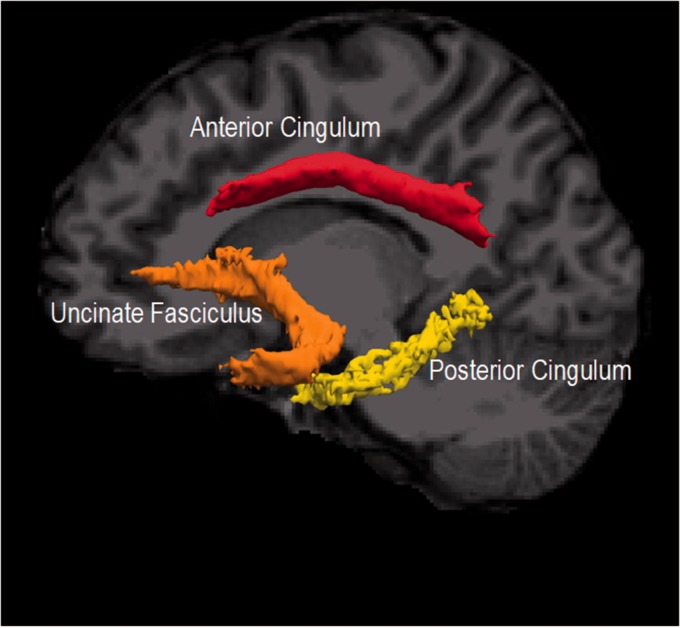
Next, FMRIB Software Library's (FSL’s) bedpost was used to fit the ball-and-stick model of diffusion at every voxel. Two anisotropic compartments were fit at every voxel to account for crossing fibers. We then used TRACULA to conduct global probabilistic tractography to reconstruct white matter pathways. This reconstruction references an atlas of manually segmented white matter pathways of 33 healthy individuals. TRACULA segments the cingulum into anterior and posterior components. The anterior cingulum spans the cingulate gyrus from the anterior cingulate cortex until reaching the turning point of the cingulum bundle at the posterior cingulate cortex (PCC). The posterior cingulum runs the rest of the cingulum bundle from the posterior cingulate gyrus to the entorhinal and parahippocampal cortices. Fractional anisotropy (FA) and mean diffusivity (MD) values were extracted for the UF, and anterior cingulum (CCG, cingulum cingulate gyrus) and posterior cingulum (CAB, cingulum angular bundle) (See Figure 1 for pathways from a representative subject). FA is a measure of the degree of anisotropic diffusion and estimates the overall coherence of tissue such that higher values indicate greater white matter integrity. MD approximates the overall rate of diffusion with higher values indicating poorer white matter integrity. FA and MD values were weighted on a voxel-wise basis according to the probability of each voxel being in the tract and then averaged over all voxels in that tract.
Figure 1.
White matter pathways of interest rendered by TRACULA for a representative subject.
Results
To better characterize this study’s sample, demographic, stress, and PTSD symptom measures for the full sample (n = 60 excluding outliers) and matched sample (n = 44) are reported in Table 1. All subsequent results reported utilize the matched sample (n = 44).
Since previous research has found significant gender differences in diffusion measures in the tracts of interest to this study, we first examined gender differences by tract. The only tract that showed gender differences was the left UF where males had higher FA, F(1, 43) = 5.571, p = .023, and lower MD, F(1, 43) = 7.837, p = .008), than females. However, there were no gender effects for relations between tract integrity and PTSD or trauma exposure, and because the sample was matched for gender, all subsequent analyses do not include gender as a factor. In addition, following the example of Olson et al.,46 a total motion index was calculated and entered into the regressions as a nuisance covariate with and without gender; however, none of the subsequent results changed, so it was removed from all regressions. To correct for multiple comparisons, the Holm–Bonferroni method (target alpha = .05) was applied to the three sets of stress and PTSD symptom regressions for each tract of interest.61 Mean FA and MD values for each tract are reported in Table 2.
Table 2.
White matter tract characteristics (n = 44).
| Left |
Right |
||
|---|---|---|---|
| M (SD) | M (SD) | ||
| UF | FA | .400 (.034) | .403 (.024) |
| MD | .764 (.034) | .771 (.031) | |
| CAB | FA | .356 (.033) | .389 (.035) |
| MD | .800 (.047) | .773 (.045) | |
| CCG | FA | .607 (.035) | .536 (.042) |
| MD | .682 (.033) | .666 (.036) |
M: mean; SD: standard deviation; FA: fractional anisotropy; MD: mean diffusivity; UF: uncinate fasciculus; CCG: cingulum cingulate gyrus (anterior cingulum); CAB: cingulum angular bundle (posterior cingulum).
Tract Integrity and Stress Exposure
To examine the relationship between white matter integrity and stress, FA and MD measures were extracted in each hemisphere for the UF, anterior (CCG) and posterior cingulum (CAB). LEC, LES, and DHS were z transformed and summed to generate a CSI. DTI measures of each tract in each hemisphere were entered as predictors in separate linear regressions with each of the stress exposure measures as outcome variables. Results showed no significant associations with the UF and any of the stress measures (all p’s > .06). In addition, there were no significant associations between stress measures and either the anterior (CCG) (all p’s > .13) or posterior cingulum (CAB) (all p’s > .20).
Tract Integrity and PTSD Symptom Severity
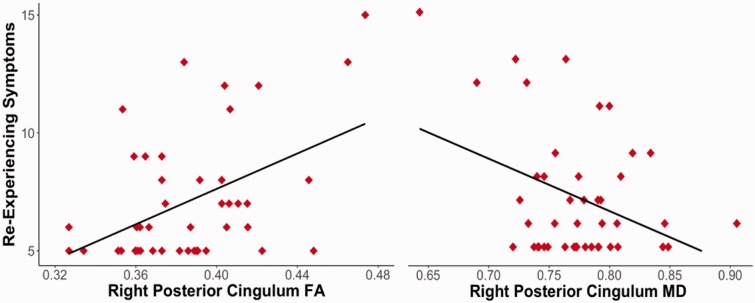
To examine the relationship between white matter integrity and PTSD symptom severity, again FA and MD measures in each hemisphere for the UF, anterior (CCG) and posterior (CAB) cingulum, were entered into four separate linear regressions predicting total PCL-C symptom severity, reexperiencing, avoidance, and hyperarousal symptoms. Severity of reexperiencing symptoms was positively correlated with FA in the right posterior cingulum (r = .469, p < .001). Although it did not survive the Holm–Bonferroni correction, reexperiencing symptoms were also negatively correlated with MD (r = −.373, p = .013) of the right posterior cingulum bundle (CAB) (Figure 2). These results suggest that greater integrity of the right posterior cingulum bundle (CAB) is related to greater reexperiencing symptoms. PCL-C total and subscale scores were not associated with FA or MD values in the anterior cingulum (all p’s > .15) or UF (all p’s > .11).
Figure 2.
Greater integrity of the right posterior cingulum is related to reexperiencing symptoms. FA: fractional anisotropy; MD: mean diffusivity.
Discussion
The current study used DTI measures of FA and MD to investigate how stress exposure and PTSD symptoms predicted alterations in the integrity of key white matter tracts, the cingulum bundle and the UF, in an emerging adult sample. We expected that stress exposure and PTSD symptoms would be related to altered cingulum integrity and decreased integrity in the UF and found partial support for our hypotheses. Lifetime stress exposure was not related to UF or cingulum bundle integrity; however, there was evidence that reexperiencing symptoms of PTSD were related to cingulum, but not UF, integrity. Therefore, greater structural connectivity of this pathway may be a biomarker for increased reexperiencing symptoms.
In accordance with our findings, others have also found that increased cingulum integrity is related to PTSD symptom severity.30,36 Montag et al.49 also found a positive correlation between posterior cingulum (CAB) integrity and trait anxiety in healthy adults. Together these results indicate that internalizing symptoms whether in PTSD or anxiety may be related to increased cingulum integrity.
Reexperiencing symptoms in PTSD are intrusive memories of a traumatic event.13 These intrusive memories are largely involuntary and may manifest as flashbacks or nightmares involving vivid imagery or other strong sensory elements.24 The increased integrity of the posterior cingulum we observed may explain reexperiencing symptoms due to its connectivity between the hippocampus and PCC.28 As stated previously, the hippocampus is involved in the encoding and retrieval of memories particularly stress-related memories.19,22–24 Involuntary retrieval of memories involves the hippocampus along with the structures associated with the original perception of the event; however, all in the absence of prefrontal cortex recruitment.23,62,63 The absence of prefrontal activation suggests the lack of top-down control in the involuntary memory retrieval process.63 Therefore, involuntary retrieval of salient memories, in this case, intrusive memories from a stressful or traumatic event, are mediated by hippocampal processes. In addition, the PCC, a key structure in the default mode network, has been implicated in internally focused thought.64 Functional connectivity studies have shown that the PCC plays an important role in directing attention internally and retrieving episodic and semantic memories.64,65 Due to its structural and functional connections, the PCC is sometimes viewed as a “hub” for which information from the cortex and subcortical structures can be integrated.65 Thus, hyperconnectivity of the posterior cingulum (CAB) from the hippocampus to the PCC could be reflective of greater internally directed attention and memory retrieval66 which may lead to the increase in reexperiencing symptoms in PTSD.
Others have found results contradictory to ours when investigating the relationship between symptom severity of PTSD and tract integrity in adults. For example, Zhang et al.39 found that increased FA and thus increased integrity of the posterior cingulum was related to decreased reexperiencing symptoms in a sample of adult males in their 30 s with PTSD. Similarly, increased cingulum FA was shown to relate to decreased reexperiencing symptoms in samples of adult females in their 30 s and 40 s with PTSD.31,32 This indicates that structural changes in the brain may be differentially affected for adults versus emerging adults due to different neurodevelopmental stages of brain maturation. As white matter architecture is still maturing in emerging adults,20,50 symptoms from stress or trauma exposure may alter structural integrity of the cingulum, resulting in hyper connectivity of this pathway in those who have PTSD symptoms. However, in adults, the maturation process has largely ceased, and cingulum integrity slowly degrades over time.20,50 Thus, symptoms from stress and trauma experiences may only be reflected through increased degradation of this tract in more mature samples. This differential pattern in the way stressful experiences may be integrated into cingulum integrity could explain the discrepancies in white matter integrity findings in the literature.
Interestingly in our sample, stress exposure measures did not correlate with integrity of the cingulum. This suggests that stress or trauma exposure alone may not be sufficient to contribute to structural brain changes, but that such changes are more strongly linked with the actual experience of stress-related psychological symptoms, although this finding warrants replication. The CSI created for this study was created by combining multiple existing measures as a way to examine white matter integrity in relation to cumulative stress. Although this index did not yield any significant results, it would be useful for the field to develop a validated measure of composite stress, including both major stressors and smaller hassles, to assess this and other relationships. Although others have established the effects of stress exposure on brain structure in depression14,19,45,67 and anxiety,45,68 no one, to our knowledge, has investigated correlations of stress and trauma exposure effects alone on brain structure within the context of PTSD.
We did not find any significant differences in UF integrity related to stress exposure or PTSD symptoms. This result is inconsistent with the literature as stress and trauma have been shown to have negative effects on UF integrity,33,43 increasing an individual’s risk for development of psychopathology symptoms.45 Fani et al.32 and Averill et al.38 also reported a null relationship with UF integrity and PTSD symptoms, arguing that the UF may not be contributing to PTSD symptoms. Although impaired integrity of the UF has been implicated in extinction learning, a common model of PTSD symptomology,41,43,44 it is possible that the UF does not directly contribute to stress-related psychopathology. As stated before, given the current study’s sample, the discrepancy in UF integrity findings may be a result of differential effects of stress at this unique neurodevelopmental stage compared to adulthood. Alternatively, the discrepancy in UF integrity findings may be a result of methodological differences in MRI acquisition and analysis.
There were a few limitations in the current study. First, our sample of emerging adults, though all exposed to trauma, did not necessarily meet criteria for PTSD diagnosis. A score of 30 on the PCL-C has been shown to be a significant clinical cutoff for diagnosis of PTSD.69 Our sample had a mean PCL-C score of 27.32 indicating a subthreshold sample, so interpretation of findings with regard to PTSD diagnosis should be done with caution. In addition, this study was not a prospective one, so we cannot interpret the direct outcomes of brain changes and how they vary with symptoms, nor can we establish whether brain changes precede stress-related symptoms or vice versa.
Results of the current study indicate the importance of understanding how stress and its related symptoms correspond to the structural organization of the brain in emerging trauma-exposed adults. First, identification of neural markers of stress exposure as well as specific symptoms of PTSD, such as reexperiencing symptoms, could lead to more precise interventions and treatments in trauma-exposed populations. Second, discrepancies between findings reported here and prior papers underscore the necessity of examining the relationship between stress, symptoms, and white matter integrity across the lifespan. Therefore, future research should use a longitudinal experimental design to better characterize how structural changes over time relate to changes in symptoms.
Supplemental Material
Supplemental material for Structural Connectivity of the Posterior Cingulum Is Related to Reexperiencing Symptoms in Posttraumatic Stress Disorder by Carissa N. Weis, Emily L. Belleau, Walker S. Pedersen, Tara A. Miskovich and Christine L. Larson in Chronic Stress
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant K01 MH086809 (PI: Larson).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 2013; 26(5): 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson NJ, Fiorillo D, Rothbaum BO, Ressler KJ, Michopoulos V. Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. J Affect Disord 2018; 225: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf EJ, Miller MW, Sullivan DR, Amstadter AB, Mitchell KS, Goldberg J, et al. A classical twin study of PTSD symptoms and resilience: Evidence for a single spectrum of vulnerability to traumatic stress. Depress Anxiety 2017; 35: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Ahn YS, Jeong KS, Chae JH, Choi KS. Resilience buffers the impact of traumatic events on the development of PTSD symptoms in firefighters. J Affect Disord 2014; 162: 128–133. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, et al. Understanding Resilience. Front Behav Neurosci 2013; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol 2004; 59(1): 20–28. [DOI] [PubMed] [Google Scholar]
- 7.Karam EG, Friedman MJ, Hill ED, Kessler RC, McLaughlin KA, Petukhova M, et al. Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depress Anxiety 2014; 31(2): 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers MB, Warren AM, Rosenfield D, Roden-Foreman K, Bennett M, Reynolds MC, et al. Predictors of PTSD symptoms in adults admitted to a Level I trauma center: a prospective analysis. J Anxiety Disord 2014; 28(3): 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foa EB, Riggs DS. Posttraumatic stress disorder following assault: theoretical considerations and empirical findings. Curr Dir Psychol Sci 1995; 4(2): 61–65. [Google Scholar]
- 10.Riggs DS, Rothbaum BO, Foa EB. A prospective examination of symptoms of posttraumatic stress disorder in victims of nonsexual assault. J Interpers Violence 1995; 10(2): 201–214. [Google Scholar]
- 11.Lucassen PJ, Pruessner J, Sousa N, Almeida OFX, Van Dam AM, Rajkowska G, et al. Neuropathology of stress. Acta Neuropathol 2014; 127: 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annu Rev Clin Psychol 2008; 4: 33–52. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 14.Sara P, Aggio V, Brioschi S, Bollettini I, Falini A, Colombo C, et al. Impact of early and recent stress on white matter microstructure in major depressive disorder. J Affect Disord 2018; 225: 289–297. [DOI] [PubMed] [Google Scholar]
- 15.Ayling E, Aghajani M, Fouche J, van der Wee N. Diffusion tensor imaging in anxiety disorders. Curr Psychiatry Rep 2012; 14: 197–202. [DOI] [PubMed] [Google Scholar]
- 16.Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry 2009; 66(9): 814–823. [DOI] [PubMed] [Google Scholar]
- 17.Blumenfeld H. Neuroanatomy through clinical cases. 2nd ed. Sunderland, MA: Sinauer Associates, Inc. Publishers; 2010.
- 18.Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front Neurosci 2013; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 2013; 52: 24–37. [DOI] [PubMed] [Google Scholar]
- 20.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 2011; 31(30): 10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbison CE, Allen K, Robinson M, Newnham J, Pennell C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev Psychopathol 2017; 29(4): 1443–1454. [DOI] [PubMed] [Google Scholar]
- 22.Huijgen J, Samson S. The hippocampus: A central node in a large-scale brain network for memory. Revue Neurologique 2015; 171: 204–216. [DOI] [PubMed] [Google Scholar]
- 23.Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev 2010; 117(1): 210–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks EH, Franklin AR, Zoellner LA. Can't get it out of my mind: A systematic review of predictors of intrusive memories of distressing events. Psychol Bull 2018; 144: 584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 2010; 167(6): 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 2008; 42(7): 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006; 30(7): 1004–1031. [DOI] [PubMed] [Google Scholar]
- 28.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007; 130(Pt 3): 630–653. [DOI] [PubMed] [Google Scholar]
- 29.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res 2013; 214(3): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bierer LM, Ivanov I, Carpenter DM, Wong EW, Golier JA, Tang CY, et al. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: A pilot study. Psychoneuroendocrinology 2015; 51: 567–576. [DOI] [PubMed] [Google Scholar]
- 31.Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, et al. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 2015; 64: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, et al. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 2012; 37: 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depress Anxiety 2013; 30(3): 207–216. [DOI] [PubMed] [Google Scholar]
- 34.Rinne-Albers MA, van der Werff SJ, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. Eur Child Adolesc Psychiatry 2016; 25(8): 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Lei D, Li L, Huang X, Suo X, Xiao F, et al. White matter abnormalities in post-traumatic stress disorder following a specific traumatic event. EBioMedicine 2016; 4: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res 2006; 146(3): 231–242. [DOI] [PubMed] [Google Scholar]
- 37.Kennis M, van Rooij SJ, Tromp do PM, Fox AS, Rademaker AR, Kahn RS, et al. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology 2015; 40(10): 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Averill CL, Averill LA, Wrocklage KM, Scott JC, Akiki TJ, Shweinsburg B, et al. Altered white matter diffusivity of the cingulum angular bundle in posttraumatic stress disorder. Mol Neuropsychiatry 2018; 4: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Li W, Shu N, Zheng H, Zhang Z, Zhang Y, et al. Increased white matter integrity of posterior cingulate gyrus in the evolution of post-traumatic stress disorder. Acta Neuropsychiatr 2012; 24(1): 34–42. [DOI] [PubMed] [Google Scholar]
- 40.Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, et al. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum Brain Mapp 2016; 37(2): 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 2013; 136(Pt 6): 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 2009; 29(37): 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci 2015; 14: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J Psychiatry Neurosci 2017; 42(5): 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson JL, Knodt AR, Brigidi BD, Hariri AR. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol 2015; 27(4 Pt 2): 1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson EA, Cui J, Fukunaga R, Nickerson LD, Rauch SL, Rosso IM. Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: a TBSS and tractography study. Depress Anxiety 2017; 34: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fani N, Clifford Z, Stevens J, van Rooij S, Jovanovic T, Rothbaum B, et al. White matter biomarkers of risk for posttraumatic anhedonia: a prospective examination. Neuropsychopharmacology. 2017; 42: S484.
- 48.Li H, Li W, Wei D, Chen Q, Jackson T, Zhang Q, et al. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage 2014; 92: 1–7. [DOI] [PubMed] [Google Scholar]
- 49.Montag C, Reuter M, Weber B, Markett S, Schoene-Bake JC. Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience 2012; 217: 77–83. [DOI] [PubMed] [Google Scholar]
- 50.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 2012; 60(1): 340–352. [DOI] [PubMed] [Google Scholar]
- 51.Sun YW, Hu H, Wang Y, Ding WN, Chen X, Wan JQ, et al. Inter-hemispheric functional and anatomical connectivity abnormalities in traffic accident-induced PTSD: a study combining fMRI and DTI. J Affect Disord 2015; 188: 80–88. [DOI] [PubMed] [Google Scholar]
- 52.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the Life Events Checklist. Assessment 2004; 11(4): 330–341. [DOI] [PubMed] [Google Scholar]
- 53.Kranz GS, Hahn A, Kaufmann U, Kublbock M, Hummer A, Ganger S, et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci 2014; 34(46): 15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, et al. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 2008; 39(2): 566–577. [DOI] [PubMed] [Google Scholar]
- 55.Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, et al. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 2011; 54(4): 2557–2562. [DOI] [PubMed] [Google Scholar]
- 56.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996; 34(8): 669–673. [DOI] [PubMed] [Google Scholar]
- 57.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res 1967; 11(2): 213–218. [DOI] [PubMed] [Google Scholar]
- 58.Kanner AD, Coyne JC, Schaefer C, Lazarus RS. Comparison of two modes of stress measurement: daily hassles and uplifts versus major life events. J Behav Med 1981; 4(1): 1–39. [DOI] [PubMed] [Google Scholar]
- 59.Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 2014; 31(9): 737–745. [DOI] [PubMed] [Google Scholar]
- 60.Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 2011; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6(2): 65–70. [Google Scholar]
- 62.Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ. Brain regions supporting intentional and incidental memory: a PET study. Neuroreport 1997; 8(5): 1283–1287. [DOI] [PubMed] [Google Scholar]
- 63.Hall NM, Gjedde A, Kupers R. Neural mechanisms of voluntary and involuntary recall: a PET study. Behav Brain Res 2008; 186(2): 261–272. [DOI] [PubMed] [Google Scholar]
- 64.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137(Pt 1): 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry 2015; 78(4): 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 2011; 77(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 2010; 44(13): 799–807. [DOI] [PubMed] [Google Scholar]
- 68.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 2012; 72(1): 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.U.S. Department of Veterans Affairs. Using the PTSD Checklist for DSM-IV (PCL). Washington DC: National Center for PTSD; 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Structural Connectivity of the Posterior Cingulum Is Related to Reexperiencing Symptoms in Posttraumatic Stress Disorder by Carissa N. Weis, Emily L. Belleau, Walker S. Pedersen, Tara A. Miskovich and Christine L. Larson in Chronic Stress