Abstract
Background:
Single-tablet regimens are preferred prescription choices for HIV treatment, but there are limited outcomes data comparing single-tablet regimens to multiple-tablet regimens.
Methods:
We retrospectively assessed treatment-naïve patients at a single urban HIV clinic in the United States for viral load suppression at 6 and 12 months after initiating either single-tablet or multiple-tablet regimens. Multivariate regression was performed to obtain relative risks and adjust for potential confounders.
Results:
Of 218 patients, 47% were on single-tablet regimens and 53% on multiple-tablet regimens; 77% of single-tablet regimen patients had undetectable viral load at 6 months compared to 61% of multiple-tablet regimen patients (p = 0.012). At 12 months, 82% on single-tablet regimens and 66% on multiple-tablet regimens (p = 0.019) had undetectable viral load. Relative risk of any detectable viral load was 1.6 (95% confidence interval: 1.1–2.5) for patients on multiple-tablet regimens compared to single-tablet regimens at 6 months, and 2.2 (95% confidence interval: 1.2–4.0) at 12 months.
Conclusion:
Single-tablet regimens may provide better virologic control than multiple-tablet regimens in urban HIV-infected persons.
Keywords: HIV, antiretroviral therapy, sexually transmitted infections, fixed-dosed combinations, single-tablet regimens
Background
Since the advent of antiretroviral (ARV) therapy for human immunodeficiency virus (HIV), treatment regimens have changed from complex, burdensome regimens to one pill once daily. Fixed-dose combination (FDC) pills for HIV are readily available and frequently used. Of the recommended initial regimens in the US Department of Health and Human Services’ (DHHS) treatment guidelines from October 2018, five are available as single-tablet regimens (STR).1
The importance of adherence to ARV therapy has been well-described, with poor adherence being associated with virologic failure and subsequent disease progression.2–6 Multiple studies have shown that adherence to ARV therapy is improved by switching to a regimen with lower pill burden.7–12 A meta-analysis by Nachega et al.13 of twice-daily versus once-daily dosing across all regimens showed an improvement of both adherence and virologic suppression with decrease in pill burden, but this was modest at best, and most commonly seen in treatment-naïve patients. While the above studies have investigated the effects of once-daily dosing and pill burden, none have directly assessed the use of STR.
Several observational studies have evaluated the effect of STR on factors such as adherence, patient satisfaction, hospitalization risks, and costs with mixed results.9,14–19 Both Sax et al.18 and Cohen et al.16 showed improvement in hospitalization rates among patients using STR compared to multiple-tablet regimens (MTR). Bangsberg et al.14 showed improvements in both adherence and virologic suppression in a population of marginally housed and homeless persons in San Francisco. Altogether, however, relatively few studies have assessed the impact of STR specifically compared to MTR for the outcome of virologic failure in a clinic population.
Understanding the impact of STR is especially important today, as we stand on the verge of multiple effective agents being available as generic formulations with the potential to decrease the cost of ARV therapy, and affect significant cost-savings nationally, as shown by Walensky et al.20 in 2013. These cost-savings may result in a “desimplification” of treatment as patients on currently branded STR may be asked to change to a less costly multiple-pill alternative. The impact of such a proposed switch toward higher pill burden on adherence and outcome, especially in vulnerable populations, needs to be assessed.
We assessed virologic suppression at 6 and 12 months among urban ARV therapy-naïve patients starting their first regimen and compared virologic and immunologic outcomes of patients on STR with those on MTR.
Methods
Study design and setting
This is a retrospective cohort study. Patients were enrolled in the Infectious Disease Practice (IDP) at the New Jersey Medical School in Newark, NJ. Of the patients seen at this practice from 2007–2013, 46% were enrolled in Medicaid, 25% self-pay or in hospital-based charity care, 22% in Medicare, and 7% with commercial insurance.
Study participants
After institutional internal review board approval, electronic medical records of new patients presenting for HIV care at the Infectious Diseases Practice between 2006 and 2013 were evaluated. Patients selected for study inclusion were ⩾18 years at initial visit, diagnosed as HIV-1 seropositive with a positive secondary laboratory conformation in the form of an Enzyme-Linked Immunosorbent Assay (ELISA)/Western Blot, or an HIV-1 Ribonucleic Acid (RNA) Polymerase Chain Reaction (PCR) (“viral load”), previously never on ARV therapy, had a CD4+ T-lymphocyte count and HIV RNA viral load in their medical record before initiation of ARV therapy (“baseline”), and at least one additional CD4 count and viral load at least 6 months after initiation of ARV therapy. Furthermore, eligible patients were subsequently started on STR or MTR as chosen by their provider, and continued on an uninterrupted and unchanged (as noted by the physician in the medical record) ARV regimen for a minimum of 6 months; patients switching therapy after 6 months were included in the study; however, 12-month data were censored.
Patient data were excluded if the patient did not complete 6 months of uninterrupted or unchanged ARV regimen. We defined an “interrupted” ARV regimen as a physician directive to stop taking medication. Patients were also excluded if baseline or 6-months post–ARV initiation CD4 count and viral load laboratory values were not present in the medical record.
ARV regimens
STR acceptable for inclusion consisted of the following three FDC pills: (1) emtricitabine, rilpivirine, and tenofovir disoproxil fumarate (TDF) (brand name Complera®); (2) efavirenz, emtricitabine, and TDF (brand name Atripla®), or (3) elvitegravir, cobicistat, emtricitabine, and TDF (brand name Stribild®), taken as one tablet once daily.
MTR acceptable for inclusion consisted of a nucleoside/nucleotide reverse transcriptase inhibitor, non-nucleoside/nucleotide reverse transcriptase inhibitor, protease inhibitor (PI), entry inhibitor, integrase inhibitor, or chemokine receptor 5 antagonist taken in any formulation of two or more tablets or capsules daily.
Data collection
Two study staff reviewed the medical record of every HIV-infected patient newly presenting to the IDP between 2006 and 2013. Data collected were age, gender, ethnicity, specific ARV medication regimen, date of ARV initiation, as well as baseline, 6 month, 12 month, and most recent (at time of data collection) HIV viral load and CD4 count. The nearest value, in time, to the 6- or 12-month point was recorded for each patient. Current or past history of any treated or untreated mental illness, including depression, schizophrenia, bipolar disorder, and anxiety, as evidenced by either a formally listed diagnosis with ICD-9 (International Classification of Diseases) coding or a mention in the narrative of a physician’s note was recorded. These same criteria were used to determine inclusion of current or past substance use in study data; substances included were cocaine, heroin, methamphetamine, phencyclidine, MDMA, lysergic acid diethylamide, marijuana, and excessive alcohol use. Moderate or minimal alcohol use without dependence (as determined at the discretion of study staff because of variable phrasing in physicians’ notes) was excluded.
Outcomes
Primary outcome was defined as HIV viral load below the limit of quantification (“undetectable”) of the specific sensitivity of the PCR utilized in analyzing each unique blood sample 6 months after initiation of first ARV regimen. For the majority of patients seen prior to 2012, the lower limit of quantification was <48 copies/mL. For the majority of patients seen after 2012, the lower limit of quantification was <20 copies/mL. Secondary outcomes were viral load 12 months after initiation of first ARV regimen, and CD4 counts 6 and 12 months after initiation.
Data analysis
Statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Baseline characteristics were considered at the medical visit at which ARV therapy was started. The Wilcoxon rank-sum test or the t-test were used to analyze differences in continuous variables. Pearson chi-square analysis was used for categorical variables. Differences between treatment groups in primary and secondary outcome measures at 6 and 12 months were performed using Pearson chi-square tests for viral load detectability (yes vs no) and the Wilcoxon rank-sum test for median CD4 count and CD4 count change. Multivariate log binomial regression analysis was performed to determine relative risks (RRs) for two models: (1) all patients, controlling for age, gender, mental illness, substance use disorder, and baseline viral load, and (2) for Black and Hispanic patients only, controlling for age, gender, mental illness, substance use disorder, baseline viral load, and race/ethnicity. The second model was performed to allow adjustment for race/ethnicity, which was not included in the first model because of low numbers of other-race patients.
Results
Baseline characteristics
A total of 218 patients met the inclusion criteria. Of these, 103 (47%) were initiated on STR and 115 (53%) on MTR. Table 1 shows the differences in the baseline characteristics between the two groups. Patients in the STR group were less likely to be female (25% vs 44%, p < 0.005) and less likely to be Black (54% vs 70%, p = 0.025). A higher proportion of patients in the MTR group had a reported history of substance abuse: 53% compared to 39% in the STR group (p = 0.051).
Table 1.
Baseline characteristics of study population by treatment regimen.
| Characteristic | All patients (n = 218) | Single-tablet regimens (n = 103) | Multiple-tablet regimens (n = 115) | P-valuea |
|---|---|---|---|---|
| Mean age, years (SD) | 43.58 (11.50) | 42.11 (12.25) | 44.90 (10.66) | 0.074 |
| Female (%) | 77 (35.32%) | 26 (25.24%) | 51 (44.35%) | 0.003 |
| Race/ethnicity | 0.025 | |||
| # Black (%) | 136 (62.39%) | 56 (54.37%) | 80 (69.57%) | |
| # Hispanic (%) | 45 (20.64%) | 29 (28.16%) | 16 (13.91%) | |
| # Other (%) | 37 (16.97%) | 18 (17.48%) | 19 (16.52%) | |
| History of substance abuse | 102 (46.79%) | 41 (39.81%) | 51 (53.04%) | 0.051 |
| History of mental illness | 61 (27.98%) | 25 (24.27%) | 36 (31.30%) | 0.248 |
| Median HIV-1 viral load, copies/mL (IQR) | 42,705.50 (147,500) | 45,679.00 (120,232) | 35,900.00 (151,529) | 0.714 |
| Median CD4 count, mm3 (IQR) | 233.50 (261) | 281.00 (290) | 226.00 (249) | 0.069 |
SD, standard deviation; IQR, interquartile range.
The Wilcoxon rank-sum test or the t-test (for age) assessed group differences in continuous variable. The Pearson chi-square test assessed group differences in categorical variables.
Bold value represents an alpha of 0.05.
Baseline virologic characteristics were similar between the two groups. Median HIV viral load for each group was comparable. The MTR group had a lower median CD4 count of 226 cells/mm3 as compared to 281 cells/mm3 in the STR group, but the difference was not statistically significant (p = 0.069).
Of the 103 patients on STR, 93 (91%) were on a FDC of efavirenz, TDF, and emtricitabine. Three patients (3%) were on a FDC of elvitegravir, cobicistat, TDF, and emtricitabine; and 7 (6%) on a FDC of rilpivirine, TDF, and emtricitabine.
The MTR regimens were more diverse: All patients were treated with a dual-nucleoside backbone. Forty-five patients (39%) were on boosted atazanavir, 34 (29%) on boosted darunavir, 22 patients (19%) on raltegravir, 9 (8%) on boosted fosamprenavir, 4 (3%) on boosted lopinavir, and 1 (<1%) on neviripine. Of the nucleoside backbones, 105 (91%) were TDF with emtricitabine, 7 (6%) were abacavir with lamivudine, and 3 (3%) were zidovudine with lamivudine (Figure 1).
Figure 1.
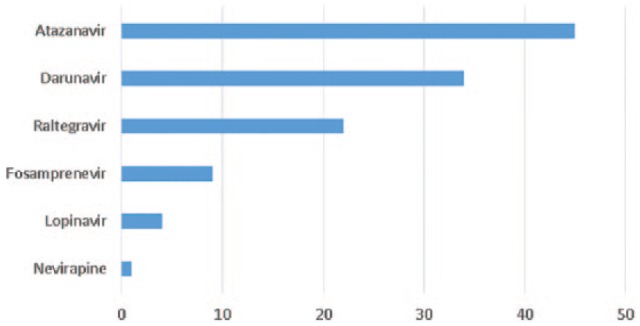
Multiple-tablet regimen components.
Atazanavir, darunavir, fosamprenavir, and lopinavir regimens included ritonavir for pharmacologic boosting. All regimens included dual nucleoside reverse transcriptase inhibitor backbone.
Virologic and immunologic outcomes
Data were available for all 218 patients at the 6-month follow-up. The median time to follow-up was 182 days. Data were available for 169 patients at the 12-month follow-up. The median time to follow-up for this group was 364 days.
Outcomes are shown in Table 2. Of patients on STR, 77% had an undetectable HIV viral load at 6 months compared to 61% of patients on MTR (p = 0.012). These numbers improved at 12 months to 82% on STR arm and 66% on MTR arm (p = 0.019), but the STR continued to show statistically significant higher rates of HIV virologic suppression compared to the MTR arm. At 6 months, the STR group had a significantly greater CD4 cell increase; however, CD4 count increases at 12 months were similar.
Table 2.
Outcomes at 6 and 12 months by treatment group.
| 6-month outcome |
12-month outcome |
|||||
|---|---|---|---|---|---|---|
| Single-tablet regimens (n = 103) | Multiple-tablet regimens (n = 115) | P-valuea | Single-tablet regimens (n = 74) | Multiple-tablet regimens (n = 95) | P-valuea | |
| # undetectable VL (95% CI) | 79 (77%) | 70 (61%) | 0.012 | 61 (82%) | 63 (66%) | 0.019 |
| Median CD4 count, mm3 (IQR) | 407 (332) | 326 (319) | 0.045 | 477 (339) | 389 (309) | 0.166 |
| Median change in CD4, mm3 (IQR) | +121 (124) | +121 (172) | 0.39 | +180 (219) | +165 (224) | 0.52 |
VL, viral load; CI, confidence interval; IQR, interquartile range.
Using the Pearson chi-square test for undetectable VL and Wilcoxon rank-sum for median CD4 and change in CD4.
Bold value represents an alpha of 0.05.
We also looked for differences in HIV virologic outcomes among specific subpopulations (Table 3). Both men and women in the STR group had higher rates of virologic suppression compared to their counterparts in the MTR group; however, statistical significance was only seen for men at the 6-month time point, perhaps because of lower numbers of women at both time points and men at the 12-month time point.
Table 3.
Patients with undetectable VL at 6 and 12 months.
| 6-month outcome |
12-month outcome |
|||||
|---|---|---|---|---|---|---|
| Single-tablet regimens (n = 103) |
Multiple-tablet regimens (n = 115) |
P-valuea | Single-tablet regimens (n = 74) |
Multiple-tablet regimens (n = 95) |
P-valuea | |
| All patients (%) | 79/103 (77%) | 70/115 (61%) | 0.012 | 61/74 (82%) | 63/95 (66%) | 0.019 |
| Gender | ||||||
| Men | 60/77 (78%) | 39/64 (61%) | 0.028 | 45/56 (80%) | 36/54 (67%) | 0.104 |
| Women | 19/26 (73%) | 31/51 (61%) | 0.286 | 16/18 (89%) | 27/41 (66%) | 0.06 |
| Race/ethnicity | ||||||
| Black | 46/56 (82%) | 49/80 (61%) | 0.015 | 34/42 (81%) | 44/65 (68%) | 0.199 |
| Hispanic | 21/29 (72%) | 10/16 (63%) | 0.262 | 18/21 (86%) | 10/15 (67%) | 0.171 |
| Other | 12/18 (67%) | 11/19 (58%) | 0.842 | 9/11 (82%) | 9/15 (60%) | 0.226 |
| Baseline CD4 (mm3) | ||||||
| ⩽200 mm3 | 26/35 (74%) | 28/49 (57%) | 0.106 | 20/26 (77%) | 23/39 (59%) | 0.135 |
| >200 mm3 | 53/68 (78%) | 42/66 (64%) | 0.068 | 41/48 (85%) | 40/56 (71%) | 0.086 |
| Baseline VL (copies/mL) | ||||||
| ⩽100,000 copies/mL | 57/70 (81%) | 46/71 (65%) | 0.026 | 39/47 (83%) | 43/63 (68%) | 0.079 |
| >100,000 copies/mL | 22/33 (67%) | 24/44 (55%) | 0.284 | 22/27 (81%) | 20/32 (63%) | 0.109 |
VL, viral load.
Using the Pearson chi-square test for undetectable VL and Wilcoxon rank-sum for median CD4 and change in CD4.
Bold value represents an alpha of 0.05.
Regimen switching
Eleven patients of the 218 studied switched regimens between the 6-month and 12-month measurements. At the time of switch, 8 patients had a detectable viral load and 3 had an undetectable viral load. Four patients switched from STR to MTR, of which 3 had a detectable viral load at the time of switch. Two patients switched from one STR formulation to another, both with an undetectable viral load at the time of switch. Four patients switched from one MTR to another, and 1 from MTR to STR and all of these had detectable viral loads at the time of switch.
Multivariate regression
Multivariate log binomial regression analysis to obtain RRs was performed controlling for demographic variables, baseline viral load, and history of substance abuse or mental illness as documented at the start of the study (Model A in Table 4). RR of virologic failure defined as any detectable viral load at 6 months was 1.6 (95% confidence interval (CI): 1.1–2.5) for patients on MTR compared to STR. At 12 months, RR of failure (MTR compared with STR) was 2.2 (95% CI: 1.2–4.0). Substance abuse, mental illness, gender, and age at enrollment were not found to be significant variables.
Table 4.
Relative risk of virologic failure, all patients.
| Variable | 6 months | 6 months controlling for baseline VL | 12 months | 12 months controlling for baseline VL |
|---|---|---|---|---|
| Model A: All patients | ||||
| MTR vs STR | 1.7 [1.1, 2.6] | 1.6 [1.1, 2.5] | 2.2 [1.2, 4.0] | 2.2 [1.2, 4.0] |
| Substance abuse | 1.2 [0.8, 1.8] | 1.1 [0.7, 1.9] | ||
| Mental illness | 1.2 [0.7, 1.9] | 0.8 [0.5, 1.3] | ||
| Female vs male | 1.2 [0.8, 0.7] | 1.0 [0.6, 1.7] | ||
| Age groups | 0.8 [0.5, 1.5] | 1.0 [0.5, 2.3] | ||
| Model B: Black and Hispanic patients only | ||||
| MTR vs STR | 1.8 [1.1, 2.6] | 1.8 [1.1, 2.9] | 2.1 [1.1, 4.0] | 2.2 [1.2, 4.3] |
VL, viral load; STR, single-tablet regimens; MTR, multiple-tablet regimens.
Regression analysis showed a similar effect when assessing only Black and Hispanic patients, who accounted for most of the study population, controlling for above variables (Model B in Table 4). RR of failure on MTR compared to STR was 1.8 (95% CI: 1.1–2.9) at 6 months and 2.2 (95% CI: 1.2–4.3) at 12 months.
Discussion
In this retrospective analysis of 218 treatment-naïve HIV infected persons in an urban US population, we found that initiation and maintenance of STR were associated with a significantly increased probability of undetectable HIV viral load at both 6 months and 12 months after initiation of therapy. This effect remained significant even when controlling for baseline viral load as well as age, gender, self-identified race and ethnicity, and the presence of comorbid psychiatric illness or substance use disorder. In fact, at 12 months after controlling for the above variables, the RR of virologic failure was over twice as high for patients on an MTR compared to those on an STR.
Rates of virologic suppression in the STR arm of our study were 82% at one year. This success rate is comparable to 48-week virologic suppression rates in clinical trials of efavirenz combined with two nucleosides, which ranged from 78% to 94%.21–29 In contrast, the rate of virologic suppression in the MTR arm, 66% at 12 months, are lower than that observed in clinical trials of raltegravir and ritonavir-boosted darunavir, atazanavir, and lopinavir, which range from 76% for lopinavir to 86% for raltegravir.26–34 The discrepancy between our results and clinical trial results, however, is likely related to different follow-up practices and monitoring in clinical trial populations, as well as our patient selection.
Meta-analyses by Nachega et al.13 and Clay et al.35 found consistent results of improved adherence and reduced risk of virologic failure in patients with STR. A meta-analysis by Van Galen et al.36 also suggested improved adherence though it did not assess the effect on virologic outcomes. Each of these analyses cited lack of randomized clinical trials addressing the issue.
An observational study in a Veterans’ Administration cohort conducted by Sutton et al.37 found improvements in adherence rates resulted in a statistically significant odds ratio of 1.21 for undetectable viral load on follow-up analysis. Our study supports these findings in a younger population with high rates of poor insurance status. While our study was limited in the number of women enrolled, our results are consistent with an analysis in the WIHS cohort conducted by Hanna et al.17 which found a modest improvement in virologic outcomes in a time period associated with increased STR use.
Our results are most consistent with a prospective study by Bangsberg et al. in a cohort of homeless and marginally housed individuals in San Francisco that compared adherence and viral suppression between patients receiving an FDC of efavirenz, TDF, and emtricitabine to those receiving MTR including non-nucleoside reverse transcriptase inhibitor and boosted PI containing regimens. They found that adherence was improved in the STR group compared to either of the MTR groups. Furthermore, the proportion of individuals with HIV viral loads <50 at 6 months was higher in the STR group than the MTR group (69% compared to 46%, p = 0.02).14
Conversely, Engsig et al. analyzed results of the Danish HIV Cohort Study during a period where all patients previously on an STR were instead switched to an MTR for cost-saving purposes. This study found no significant difference in viral suppression. Notably, this study excluded patients with anticipated problems with adherence as chosen by their physician. While adherence was not formally measured in the study, the exclusion of patients at high risk of poor adherence may have blunted the potential benefit of a coformulated regimen.38
It is known that adherence to therapy can be an important marker of clinical indicators, including hospitalization, disease progression, virologic failure, and death.2–6 In prior studies, adherence has been shown to be improved in patients initiated on once-daily regimens and those with less pill-burden, although not necessarily STR.9–19,35,36 Our study was not able to directly measure adherence to therapy, but we suspect the increase in adherence associated with a lower pill burden may have helped drive the outcomes found here, specifically virologic suppression. In addition, our study was limited to patients who tolerated their regimens for 6 months. Since intolerance is a common reason for switching from efavirenz, the primary STR used in this study, we were able to highlight the potential adherence effect of the pill formulation used, rather than adverse events related to the component drug.39
The need for information regarding the value of STR in HIV therapy is partially due to the anticipated arrival of entirely generic ARV regimens. The prospect of a generic regimen has important implications for cost-savings. An US-based estimate of cost savings calculated that a switch from branded therapy to generic therapy across the country would result in a $920 million dollar cost savings in the first year alone, or $42,500 per patient lifetime. When the same study considered decreased effectiveness of generic regimens, the analysis suggested cost savings persisted; however, a potential loss of life expectancy was considered as a possible concomitant result.20 The overall threshold for cost-effectiveness, however, is not likely to be interpreted equally among payer sources. One area for concern is that certain payers, including those that serve the most vulnerable populations, would be more inclined to accept cost-savings for a modest sacrifice in effectiveness. This is especially relevant to our practice population, as over two-thirds of patients seen in the practice during this study period were either enrolled in Medicaid, uninsured, or using hospital-based charity care. Over the past few months, we have heard anecdotes of several patients forced by their insurance providers to switch from a STR regimen to a payer-preferred MTR regimen, despite viral suppression on the STR. The next few years may tell us if these switches will become more common and result in inferior virologic outcomes.
There are a number of limitations to this study. As a retrospective study, unmeasured confounders may exist that impact results: including insurance status, adherence, and provider beliefs about patients’ likely adherence. Sample size did not allow for between-regimen comparisons in subgroups, so this study cannot compare effectiveness among individual regimens. Nonetheless, clinical trial data and meta-analysis have demonstrated similar rates of viral load suppression for raltegravir, atazanavir, and darunavir—the main regimens in the MTR group—when compared to efavirenz.40 As a consequence of the study period, a majority of the patients on STR were placed on efavirenz/TDF/emtricitabine, and newer STR including dolutegravir/abacavir/lamivudine were not available for this study population. Perhaps because of the high use of efavirenz as an STR and the risk of teratogenic effects associated with this drug, fewer women are represented in the STR group overall. This likely no longer represents the trend, as STR without this drawback are readily available. Finally, information for patients with incomplete treatment before 6 months or loss-to-follow-up before that time was not available, and this may impact interpretation of the results.
Providers’ perception of a patient’s potential adherence may have affected regimen choice. Emergent ARV resistance is very uncommon with current boosted PI containing regimens, and with the integrase inhibitor dolutegravir, in contrast to the emergent resistance described among individuals failing efavirenz-containing regimens.29,41,42 Therefore, clinicians may preferentially choose boosted-PI or integrase inhibitor containing regimens for patients determined to be high risk for medication nonadherence. While factors leading to treatment decision making could not be addressed directly in this study, we attempted to mitigate this by assessing impact of age, substance use disorder, and mental illness (risk factors for non-adherence) on virologic suppression. After controlling for these factors, the favorable effect on virologic suppression persisted in patients receiving STR. Additional unmeasured factors such as intended pregnancy, or drug interactions, may have impacted provider choice and are not accounted for in this analysis.
In summary, we found that daily STR had higher rates of undetectable viral load at 6 months and 12 months of therapy than MTR in a population of patients largely dependent on public assistance for ARV therapy. Generic MTR are expected to become available within the next few years, allowing a less costly treatment option for patients and payers. While the choice between cost and convenience remains complex, this study helps support the continued use of STR, especially in our most vulnerable populations.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.S. has received grant support from Gilead Sciences and consulting fees from Viiv and Gilead Sciences. S.H. has received consulting fees from Bristol-Meyers Squibb, Gilead Sciences, Janssen Pharmaceuticals, and Viiv. Her spouse has received consulting fees from Johnson and Johnson and Inovia, and retains stock options from Merck.
Ethical approval: Ethical approval for this study was obtained from Rutgers New Jersey Medical School Institutional Review Board (Protocol Pro20120028).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not sought for the present study because it was a retrospective study of existing data and consent would not have been feasible.
ORCID iD: Shashi N Kapadia  https://orcid.org/0000-0001-6363-2839
https://orcid.org/0000-0001-6363-2839
References
- 1. Panel on Antiretroviral Guidelines for Adults Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services, 2018, https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
- 2. Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15(9): 1181–1183. [DOI] [PubMed] [Google Scholar]
- 3. Fielden SJ, Rusch ML, Yip B, et al. Nonadherence increases the risk of hospitalization among HIV-infected antiretroviral naive patients started on HAART. J Int Assoc Physicians AIDS Care 2008; 7(5): 238–244. [DOI] [PubMed] [Google Scholar]
- 4. Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS 2004; 15(12): 803–810. [DOI] [PubMed] [Google Scholar]
- 5. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133(1): 21–30. [DOI] [PubMed] [Google Scholar]
- 6. Wood E, Hogg RS, Yip B, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 x 10(9) cells/L. Ann Intern Med 2003; 139(10): 810–816. [DOI] [PubMed] [Google Scholar]
- 7. Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 2010; 4: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dejesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr 2009; 51(2): 163–174. [DOI] [PubMed] [Google Scholar]
- 9. Hodder SL, Mounzer K, Dejesus E, et al. Patient-reported outcomes in virologically suppressed, HIV-1-infected subjects after switching to a simplified, single-tablet regimen of efavirenz, emtricitabine, and tenofovir DF. AIDS Patient Care STDS 2010; 24(2): 87–96. [DOI] [PubMed] [Google Scholar]
- 10. Parienti JJ, Bangsberg DR, Verdon R, et al. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis 2009; 48(4): 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juday T, Grimm K, Zoe-Powers A, et al. A retrospective study of HIV antiretroviral treatment persistence in a commercially insured population in the United States. AIDS Care 2011; 23(9): 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 2005; 40(1): 158–163. [DOI] [PubMed] [Google Scholar]
- 13. Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58(9): 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bangsberg DR, Ragland K, Monk A, et al. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS 2010; 24(18): 2835–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buscher A, Hartman C, Kallen MA, et al. Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. Int J STD AIDS 2012; 23(5): 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open 2013; 3(8): e003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr 2014; 65(5): 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS ONE 2012; 7(2): e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Chen K, Kalichman SC. Barriers to HIV medication adherence as a function of regimen simplification. Ann Behav Med 2017; 51: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158(2): 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379(9835): 2439–2448. [DOI] [PubMed] [Google Scholar]
- 22. Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 2011; 378(9787): 238–246. [DOI] [PubMed] [Google Scholar]
- 23. Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 2011; 378(9787): 229–237. [DOI] [PubMed] [Google Scholar]
- 24. Cohen C, Wohl D, Arribas JR, et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. AIDS 2014; 28(7): 989–997. [DOI] [PubMed] [Google Scholar]
- 25. ENCORE1 Study Group. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet 2014; 383(9927): 1474–1482. [DOI] [PubMed] [Google Scholar]
- 26. Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374(9692): 796–806. [DOI] [PubMed] [Google Scholar]
- 27. Andersson LM, Vesterbacka J, Blaxhult A, et al. Lopinavir/ritonavir, atazanavir/ritonavir, and efavirenz in antiretroviral-naive HIV-1-infected individuals over 144 weeks: an open-label randomized controlled trial. Scand J Infect Dis 2013; 45(7): 543–551. [DOI] [PubMed] [Google Scholar]
- 28. Honda M, Ishisaka M, Ishizuka N, et al. Open-label randomized multicenter selection study of once daily antiretroviral treatment regimen comparing ritonavir-boosted atazanavir to efavirenz with fixed-dose abacavir and lamivudine. Intern Med 2011; 50(7): 699–705. [DOI] [PubMed] [Google Scholar]
- 29. Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154(7): 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molina JM, Clotet B, vanLunzen J, et al. Once-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naive HIV-1-positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc 2014; 17(4 Suppl. 3): 190490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lennox JL, Dejesus E, Berger DS, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet 2008; 372(9639): 646–655. [DOI] [PubMed] [Google Scholar]
- 33. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 2008; 22(12): 1389–1397. [DOI] [PubMed] [Google Scholar]
- 34. Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381(9868): 735–743. [DOI] [PubMed] [Google Scholar]
- 35. Clay PG, Nag S, Graham CM, et al. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine 2015; 94(42): e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. vanGalen KA, Nellen JF, Nieuwkerk PT. The effect on treatment adherence of administering drugs as fixed-dose combinations versus as separate pills: systematic review and meta-analysis. AIDS Res Treat 2014; 2014: 967073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sutton SS, Hardin JW, Bramley TJ, et al. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care 2016; 22(4): 242–248. [PubMed] [Google Scholar]
- 38. Engsig FN, Gerstoft J, Helleberg M, et al. Effectiveness of antiretroviral therapy in individuals who for economic reasons were switched from a once-daily single-tablet regimen to a triple-tablet regimen. J Acquir Immune Defic Syndr 2014; 66(4): 407–413. [DOI] [PubMed] [Google Scholar]
- 39. Leutscher PD, Stecher C, Storgaard M, et al. Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis 2013; 45(8): 645–651. [DOI] [PubMed] [Google Scholar]
- 40. Kryst J, Kawalec P, Pilc A. Efavirenz-based regimens in antiretroviral-naive HIV-infected patients: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2015; 10(5): e0124279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008; 358(20): 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Llibre JM, Pulido F, Garcia F, et al. Genetic barrier to resistance for dolutegravir. AIDS Rev 2015; 17(1): 56–64. [PubMed] [Google Scholar]


