Abstract
Indoleamine 2,3-dioxygenase (IDO) has the most important role in modulation of tryptophan-dependent effects in the gastrointestinal tract, including modulation of intestinal immune response. An increased IDO activity maintains immune tolerance and attenuates ongoing inflammation but allows immune escape and uncontrolled growth of gastrointestinal tumors. Accordingly, IDO represents a novel therapeutic target for the treatment of inflammatory and malignant diseases of the gastrointestinal tract. In this review article, we summarize current knowledge about molecular and cellular mechanisms that are involved in IDO-dependent effects. We provide a brief outline of experimental and clinical studies that increased our understanding of how enhanced IDO activity: controls host–microbiota interactions in the gut; regulates detrimental immune response in inflammatory disorders of the gastrointestinal system; and allows immune escape and uncontrolled growth of gastrointestinal tumors. Additionally, we present future perspectives regarding modulation of IDO activity in the gut as possible new therapeutic approaches for the treatment of inflammatory and malignant diseases of the gastrointestinal system.
Keywords: antitumor immunity; gastrointestinal system; indoleamine 2,3-dioxygenase; inflammation; tryptophan
Introduction
Tryptophan (TRP) is an essential amino acid playing several important structural and functional roles in the gastrointestinal tract.1 TRP functions as a biochemical precursor for serotonin (5-hydroxytryptamine, 5-HT), melatonin and niacin. Additionally, since TRP is abundant in transmembrane domains of membrane-bound proteins, it is essential for protein stability/assemblage to the phospholipid bilayer, playing an important ‘anchoring’ role.2 TRP is essential nutrient, mammals are not able to synthesize it, and it has to be obtained from the TRP-reach foods (oats, milk, yogurt, cottage cheese, red meat, eggs, fish, chocolate, etc.).3 After food digestion, TRP is absorbed by the gut epithelium and then transported via the bloodstream to the cells to be used for the synthesis and turn-over of proteins.4 Depending on their function and metabolism, different cells require diverse, but strictly defined amounts of TRP and, accordingly, TRP absorption in the gut is a strictly controlled process. Since, among all amino acids, TRP has the lowest affinity for Na+-dependent transmembrane protein, expressed on the apical membrane of intestinal enterocytes, this carrier molecule regulates TRP absorption in the gut and controls its subsequent transport and biotransformation.4,5
TRP metabolism follows three major pathways: (a) gut microbiota-dependent transformation of TRP into several molecules, including ligands of the aryl hydrocarbon receptor (AhR) that are able to alter function of epithelial barrier and immune homeostasis in the intestine; (b) TRP hydroxylase-1-dependent regulation of 5-HT production in enterochromaffin cells; (c) indoleamine 2,3-dioxygenase (IDO)1-mediated kynurenine (KYN) pathway which plays a critical role in several fundamental biological processes in the gut, including regulation of epithelial cell viability and modulation of immune response.1
In this review article, we summarize current knowledge about molecular and cellular mechanisms that are involved in IDO/KYN-dependent modulation of inflammatory and malignant diseases of the gastrointestinal tract. We provide a brief outline of experimental and clinical studies that increased our understanding of how IDO/KYN pathway: controls host–microbiota interactions in the gut; regulates detrimental immune response in inflammatory disorders of gastrointestinal system; and allows immune escape and uncontrolled growth of gastrointestinal tumors. Additionally, we present future perspectives regarding modulation of IDO activity in the gut as a possible new therapeutic approach for the treatment of inflammatory and malignant diseases of the gastrointestinal system.
The biochemical function and regulation of IDO activity
Since TRP is found at very low concentrations in the body, it plays a rate-limiting role in protein synthesis and intracellular signaling.1 Accordingly, enzymes that regulate TRP metabolism and signaling have a crucial role in regulation of its effects.1 Among them, IDO1 has the most important role in modulation of TRP-dependent effects in the gastrointestinal tract.6
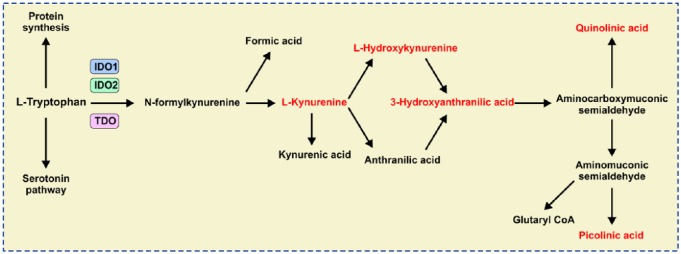
IDO1, a cytosolic and heme-containing enzyme, converts TRP to KYN by cleaving the 2,3-double bond of the indole ring while a molecular oxygen merges into the unsealed molecule.6 The obtained product, N-formylkynurenine, becomes rapidly and spontaneously transformed into KYN. In the next steps, KYN is further converted to other active metabolites, such as 3-hydroxykynurenine (3-HK), anthranilic acid, kynurenic acid (KYNA), 3-hydroxyanthranilic acid (3-HAA), picolinic acid and quinolinic acid (QA), which is a precursor of nicotinamide adenine dinucleotide (NAD+; Figure 1). Since two main end products of the KYN pathway [NAD+ and adenosine triphosphate (ATP)] are energy-carrying molecules that fuel cellular metabolism, the IDO1/KYN pathway has an important role in regulation of cell viability and proliferation.6
Figure 1.
The kynurenine (KYN) pathway of tryptophan (TRP) metabolism.
L-TRP is metabolized in three separate biochemical pathways enabling protein and serotonin synthesis. The KYN-dependent pathway begins when IDO1/IDO2/TDO catalyze L-TRP into N-formylkynurenine. N-formylkynurenine is then transformed into L-KYN and formic acid by kynurenine formamidase. L-KYN is converted to anthranilic acid by kynureninase or L-hydroxykynurenine by kynurenine hydroxylase. Hydroxylation of anthranilic acid results in generation of L-hydroxykynurenine. Kynureninase converts L-hydroxykynurenine to 3-hydroxyanthranilic acid that is further metabolized by hydroxyanthranilate dioxygenase to aminocarboxymuconic semialdehyde. Quinolinic acid and aminomuconic semialdehyde are generated from the semialdehyde. Aminomuconic semialdehyde is then converted to picolinic acid or glutaryl-coenzyme A (CoA) that is metabolized in the tricarbonic acid cycle and terminal oxidation.
IDO1, indoleamine 2,3-dioxygenase; TDO, tryptophan-2,3-dioxygenase.
In humans, IDO1 has an evolutionary paralog (indolamine-2,3-dioxygenase 2; IDO2) and a functional ortholog (tryptophan-2,3-dioxygenase; TDO). Both IDO2 and TDO catalyze the same biochemical reaction as IDO1 (Figure 1), but these two enzymes have strict tissue specificity: TDO is expressed only in the liver, where it controls and regulates blood concentration of TRP, while IDO2 is expressed at low levels in the placenta and liver.6–8 On the contrary, IDO1 is expressed in a broad number of peripheral tissues, including the gastrointestinal tract. Within the gastrointestinal system, IDO1 activity has been observed in epithelial cells, endothelial cells, fibroblasts, mesenchymal stem cells (MSCs), as well as immune cells, including professional antigen-presenting cells [dendritic cells (DCs), macrophages, B lymphocytes], natural killer (NK) cells, activated monocytes and granulocytes, while lymphoid cells rarely express IDO1.9
In addition to TRP catabolic activity, IDO1 protein is an important signal-transducing molecule.10 IDO1 has two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) which, after phosphorylation, act as docking sites for different molecules, which either activate positive (transcriptional) or induce negative (post-translational) modulation of IDO1 protein.10 Molecular patterns that prolong IDO1’s half-life maintain IDO1-mediated immunosuppression while molecules that shorten IDO1’s half-life, reduce IDO1-dependent immunoregulatory effects and promote inflammation.10
The first discovery of IDO-dependent immunoregulatory effects was made in 1984 when Pfefferkorn found that recombinant interferon gamma (IFN-γ) successfully inhibited growth of Toxoplasma gondii in fibroblasts by inducing the host cells to degrade tryptophan.11 Consequent accumulation of toxic KYN metabolites (3-HK, QA, 3-HAA) restricted the growth of this obligate intracellular parasite, suggesting the importance of IFN-γ for activation of the IDO1/KYN pathway.11 Binding of IFN-γ to its receptor activate Janus kinases (Jak1 and 2) resulting in phosphorylation and dimerization of signal transducer and activator of transcription 1 (STAT1) that enters the nucleus to induce transcription of IFN-γ-stimulated genes. Mammalian IDO1 gene promoters possess IFN-γ-stimulated-response elements and IFN-γ-activated sites, enabling IFN-γ-mediated induction of IDO1 expression.12,13 The transcriptional factor DAP12 regulates IFN-γ-induced IDO1 transcription, while suppressor of cytokine signaling (SOCS)-3 targets IDO1 protein for proteasomal degradation.13–15 A broad number of in vitro and in vivo studies confirmed that IFN-γ is the most potent activator of IDO1 activity, although IFN types I [IFN alpha/beta (IFN-α/β)], tumor necrosis factor alpha (TNF-α), lipopolysaccharide (LPS), toll-like receptor 7 (TLR7) and TLR9 ligands or even anti-inflammatory cytokines [interleukin (IL)-10 and transforming growth factor beta (TGF-β)] may induce enhanced IDO1 expression.16–19
IDO1/KYN-dependent modulation of immune cells
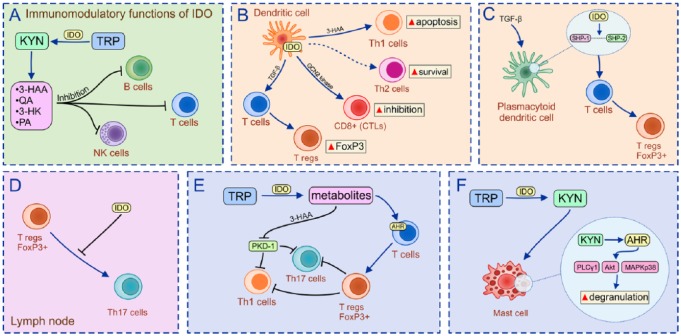
Initially, increased IDO1 activity and consequent accumulation of KYN metabolites were considered only an important mechanism for the regulation of cellular metabolism due to their effect on generation of NAD+ and ATP.20 Nevertheless, results obtained in a large number of preclinical studies demonstrated that IDO1-dependent TRP starvation and accumulation of 3-HAA, KYNA, QA and 3-HK directly inhibit proliferation of activated T and B lymphocytes, contributing to attenuation of the adaptive immune response [Figure 2(a)].21–23 Interestingly, IFN-γ-producing Th1 cells were more susceptible to IDO1-induced apoptosis compared with IL-4-producing Th2 cells.24 This, KYN-dependent selective apoptosis of Th1 cells involves a Fas-independent mechanism: activation of caspase-8 and the release of cytochrome c from mitochondria.24 Since IFN-γ is the main activator of IDO1, IDO1/KYN-induced selective apoptosis of IFN-γ-producing Th1 cells could represent a compensatory mechanism responsible for the maintenance of Th1/Th2 balance and peripheral lymphocyte homeostasis.
Figure 2.
IDO1/KYN-dependent modulation of immune cells.
(a) IDO1-dependent TRP starvation and accumulation of 3-HAA, KYNA, QA and 3-HK directly inhibits proliferation of activated T and B lymphocytes; (b) through the increased IDO1 activity, DCs promote generation and expansion of Tregs, enabling induction and maintenance of immune tolerance; (c) an increased IDO1 activity in TGF-β-stimulated murine pDCs results in formation of an intracellular scaffold that binds SHP-1 and SHP-2 enabling conversion of CD4+T cells into immunosuppressive Tregs; (d) IDO1 prevents conversion of FoxP3+Tregs in the inflammatory process and Th17 cells in the lymph nodes; (e) Tregs suppress IFN-γ-producing Th1 and IL-17-producing Th17 cells, and attenuate inflammation; and (f) through the activation of AhR, IDO1-derived KYN activates Akt and MAPK p38 signaling pathways in mast cells resulting in massive degranulation and release of leukotrienes and prostaglandins.
IDO1, indoleamine 2,3-dioxygenase; KYN, kynurenine; TRP, tryptophan; 3-HAA, 3-hydroxyanthranilic acid; KYNA, kynurenic acid; GCN2, general control nonderepressible 2; QA, quinolinic acid; PA, ; 3-HK, 3-hydroxykynurenine; DCs, dendritic cells; Tregs, T-regulatory cells; TGF-β, transforming growth factor beta; pDC, plasmacytoid DC; SHP, Src-homology-region-2-domain-containing phosphatase; CD4+, cluster of differentiation 4+; FoxP3, forkhead box P3; Th17, T-helper cell 17; IFN-γ, interferon gamma; IL, interleukin; AhR, aryl hydrocarbon receptor; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; PKD-1, ; PLCγ1, ; CTLs, cytotoxic T cells.
IDO1 is crucially important for the crosstalk between DCs and immunosuppressive T-regulatory cells (Tregs).25,26 Through the increased IDO1 activity, DCs promote generation and expansion of Tregs, enabling induction and maintainance of immune tolerance [Figure 2(b)].24,26 During the interaction with naïve T cells, DCs, in an IDO1-dependent manner, generate KYN, which promotes expression of Treg lineage-defining transcription factor (forkhead box P3, FoxP3) in cluster of differentiation 4+ (CD4+)T cells, enabling generation of immunosuppressive CD4+FoxP3+Tregs.13
During initial TCR-mediated activation of resting Tregs, signals via the protein kinase B (PKB/Akt) and mammalian target of rapamycin (mTOR) pathways can potentially destabilize the immunoregulatory phenotype of Tregs and cause their reprogramming into a pro-inflammatory helper-like phenotype (‘ex-Tregs’), characterized by enhanced production of inflammatory cytokines. A low level of TRP in the local microenvironment activates stress-response pathways, including activation of general control nonderepressible 2 (GCN2) kinase and suppression of Akt/mTOR2 signaling.27,28 Accordingly, in order to prevent transdifferention of Tregs in inflammatory CD4+T cells in the inflammed gut, intestinal regulatory DCs produce IDO1 that induces low TRP levels, enabling activation of GCN2 kinase and consequent inhibition of Akt/mTORC2 signaling in Tregs.13 In a similar manner, an increased IDO1 activity and activation of GCN2 kinase is responsible for the downregulation of the TCR zeta-chain in activated CD8+ cytotoxic T cells (CTLs) resulting in inappropriate antigen recognition and reduced cytotoxicity of CTLs.23
In addition to direct immunosuppressive effects on activated T cells, IDO1 was involved in intracellular signaling events responsible for the self-amplification and maintenance of a stably regulatory phenotype in plasmacytoid DCs (pDCs). These pDCs are an important cellular source of IFN type I that are able to induce enhanced expression of IDO1 and promote expansion of Tregs.29 This process is, at least partially, regulated through the activation of the AhR.10,30 IDO1-mediated degradation of TRP yields a series of KYN catabolites that act as ligands for AhR.10,30 Binding of KYN catabolites to AhR induces conformational changes of AhR that promotes its nuclear translocation.10 In the nucleus, AhR induces enhanced transcription of target genes, including FoxP3.31 Accordingly, IDO1/KYN-dependent activation of AhR results in increased generation of FoxP3+Tregs, contributing to creation of the immunosuppressive microenvironment.10,30–32 Additionally, an increased IDO1 activity in TGF-β-stimulated murine pDCs resulted in formation of an intracellular scaffold that binds Src-homology-region-2-domain-containing phosphatase (SHP)-1 and SHP-2, enabling conversion of CD4+T cells into immunosuppressive Tregs [Figure 2(c)].33 In the lymph nodes, IDO1 prevents conversion of FoxP3+Tregs in the inflammatory process, IL-17-producing Th17 cells [Figure 2(d)], resulting in the increased accumulation of immunosuppressive Tregs in peripheral tissues.30 In the inflammed tissues, Tregs, through the production of immunosuppressive IL-10 and TGF-β, suppress IFN-γ-producing Th1 and IL-17-producing Th17 cells and resolve ongoing inflammation [(Figure 2(e)].25
In addition to the modulation of adaptive immunity, IDO1/KYN pathways regulate function of innate immune cells. IDO1 promoted conversion of inflammatory M1 macrophages into alternatively activated IL-10 and TGF-β-producing M2 macrophages resulting in creation of the immunosuppressive microenvironment.34 Through the activation of AhR, IDO1-derived KYN activates Akt and mitogen-activated protein kinase (MAPK) p38 signaling pathways in mast cells, resulting in massive degranulation and release of leukotrienes and prostaglandins (PG) [Figure 2(f)].35 Among them, PGE2 was particularly important for IDO1-based suppression of cytotoxic NK cells.36
IDO1-dependent modulation of inflammatory diseases of the gastrointestinal tract
Since the IDO1/KYN pathway regulates immune response, its role in the pathogenesis of inflammatory diseases of the gastrointestinal tract has been explored in a large number of experimental and clinical studies.
Several research groups demonstrated IDO-mediated attenuation of inflammation in the oral cavity.37–42 Increased IDO1 expression was detected in gingival fibroblasts, gingiva-derived MSCs (G-MSCs), dental-pulp-derived MSCs (DP-MSCs), periodontal-ligament stem cells (PDL-SCs) and DCs that infiltrated inflammatory lesions in the oral cavity. IDO1 is constitutively expressed in human gingiva, and its expression was upregulated in chronic periodontitis.43 As first indicated by Nisapakultorn and coworkers43 and later confirmed by several other groups,37–40 bacterial products and inflammatory cytokines were mainly responsible for an increased IDO1 expression in periodontitis lesions. LPS and IFN-γ-activated gingival fibroblasts, G-MSCs, DP-MSCs, PDL-SCs and DCs produce IDO1 that, in a KYN-dependent manner, suppressed expansion of inflammatory CD4+T-bet+IFN-γ-producing Th1 and CD4+ RORyT+IL-17-producing Th17 cells by promoting their conversion to Tregs, creating an immunosuppressive microenvironment in the oral cavity that resulted in attenuation of ongoing inflammation.37–40 Similarly, in an IDO1-dependent manner, MSCs obtained from periapical lesions (PL-MSCs) were able to induce a generation of tolerogenic phenotype in DCs which, due to the poor allostimulatory activity, induced anergy of effector Th1 cells and promoted generation of Tregs.41 In line with these findings are results obtained by Lewkowicz and colleagues, who investigated molecular mechanisms responsible for Treg-based attenuation of recurrent aphthous stomatitis (RAS) and concluded that IDO was crucially important for the maintenance of immune tolerance in this chronic T-cell-driven inflammatory disease.42 Decreased constitutive expression of IDO in oral mucosa of RAS patients resulted in impaired generation and function of Tregs. The total number of immunosuppressive Tregs in peripheral blood of RAS patients was significantly lower compared with healthy subjects. Additionally, Tregs from RAS patients were not able to optimally suppress production of inflammatory, pro-Th1 cytokines (IFN-γ, TNF-α, IL-2) in effector T cells. Thus, reduced IDO activity resulted in the loss of Treg-dependent immune tolerance, enabling T cell-mediated damage of the epithelium and the development of oral ulcers in RAS patients.42
Results recently obtained by Larussa and colleagues,44 suggest an important role of the IDO1/KYN signaling pathway in the pathogenesis of Helicobacter pylori infection and H. pylori-associated gastritis. The analysis of gastric biopsy samples obtained from 42 patients who underwent upper gastrointestinal endoscopy revealed significantly enhanced IDO1 expression in H. pylori-infected patients compared with uninfected subjects. It is well known that activation of IFN-γ-producing Th1 and IL-17-producing Th17 cells contribute to the efficient eradication of H. pylori infection.45,46 Accordingly, H. pylori enhances its own survival in human gastric mucosa by downregulating expression of T-bet, resulting in attenuated Th1 immune response.44,45 Since IDO1 inhibition notably increases the expression of T-bet, IFN-γ and IL-17 messenger ribonucleic acid (mRNA) in gastric samples of H. pylori-infected patients, Larussa and colleagues concluded that immunological escape implemented by H. pylori involves the increased IDO1 activity that downregulates Th1/Th17 immune response and induces immune tolerance, enabling long-term colonization of H. pylori in gastric mucosa and consecutive development of H. pylori-associated gastritis.44
IDO1-dependent modulation of inflammatory bowel diseases
IDO1 is expressed in the normal colon and is upregulated in the setting of colitis.47 An increased IDO1 expression has been observed in inflamed colons of experimental animals and in patients suffering from inflammatory bowel diseases (IBDs).25,47–49 Pharmacological inhibition or genetic deletion of IDO1 resulted in increased mortality of 2,4,6-trinitrobenzene sulfonic acid (TNBS)- or dextran sodium sulfate (DSS)-treated experimental animals.25,47,48 Conversely, increased IDO1 activity and elevated serum levels of KYN were accompanied with increased presence of immunosuppressive Tregs in the injured gut, resulting in the attenuation of colon inflammation.50 Enhanced IDO1 activity was noticed in colon-infiltrating immune cells and in colon epithelial cells of IBD patients and significantly decreased after treatment with steroids and salicylates.51 Wolf and colleagues were first to show increased production of IDO1 in CD123(+) mononuclear cells infiltrating the submucosal areas of the inflamed lesions.52 Furuzawa-Carballeda and coworkers further analyzed IDO1 expression in colonic biopsies of IBD patients and confirmed that the main producer of IDO1 in inflamed gut was CD123+pDCs that counterbalance the tissue-damaging effects of activated T cells.9 Increased IDO1 activity was also observed in regulatory CD8α-positive pDCs. Additionally, several other subpopulations of CD16+/CD56+/CD80+IDO1-producing regulatory DCs were noticed in the colons of IBD patients,9 while CD103+DCs were considered as the main cellular source of IDO in mice.25 Human CD123+/IDO+ pDCs constitute only 0.2–0.8% of peripheral blood cells and are recruited from the peripheral blood in the inflamed gut in order to induce tolerance.9 IDO1-producing DCs possess an exclusive TLR repertoire, characterized by high expression of TLR7 and TLR9. TLR7 agonist simultaneously activates IDO1/KYN signaling pathway in colon-infiltrating DCs,53 while induction of IDO1 by a TLR-9 agonist, immunostimulatory DNA, led to the attenuation of experimental colitis in mice.54 Importantly, IFN-γ/STAT-1 signaling was crucially important for enhanced IDO1 activity in DCs since IDO1 could not be induced in colon-infiltrating DCs of STAT-1 deficient mice.54 Enhanced Th1 immune response and elevated concentration of IFN-γ in the gut promoted IDO1 expression in CD103+DCs, while enhanced Th2 immune response and high concentration of IL-4 inhibited IDO1 activity in gut DCs.55 Human CD123+/IDO+ pDCs and murine CD103+DCs produce large amounts of IDO1 which promote conversion of effector Th1 and Th17 cells in Tregs, enabling creation of the immunosuppressive microenvironment in the gut.25,56 Tregs, in a CTLA-4 and IL-10-dependent manner, suppress activation of gut-infiltrated Th1 and Th17 cells, contributing to the attenuation of colitis.57 We50 and others49,58 noticed significantly higher serum and fecal levels of KYN and higher presence of IDO-producing DCs and Tregs in the lamina propria of IBD patients compared with healthy subjects that might represent a compensatory mechanism for functional induction of tolerance in active IBD, due to the increase in absolute number of colon-infiltrating, IFN-γ-producing Th1 and IL-17-producing Th17 inflammatory cells.
In inflamed mucosa of IBD patients, increased IDO1 activity was observed in colon epithelial cells, particularly at borders of crypt abscesses or at sites where epithelial cells flanked ulcers, suggesting involvement of the IDO1/KYN pathway in the repair process of mucosal healing.59 In line with these observations are our recently published results50 that indicate the importance of IDO1-dependent expansion of endogenous Tregs as a possible new therapeutic approach for the induction of mucosal healing. We demonstrated that colon-infiltrating DCs, through the production of KYN, induced expansion of Tregs that promote mucosal healing in the injured colons.50 Both IDO1-producing DCs and immunosuppressive Tregs were crucially important for the maintenance of mucosal healing and recovery from DSS-induced colitis, since depletion of each cell population led to the significant aggravation of disease.50 Keeping in mind that clinical application of Tregs in IBD patients is not easy to perform given their rarity in peripheral blood,60 we propose that IDO1-dependent expansion of endogenous Tregs should be further explored as a potentially new approach for the induction and maintenance of mucosal healing in IBD patients.
In addition to the potential therapeutic application, measurement of IDO1 activity can be used for the monitoring of mucosal healing in IBD patients. Currently, measurement of fecal calprotectin is the most commonly used stool-based test for assessing progression of ulcerative colitis (UC),61 and reduction in concentration of fecal calprotectin represents the most reliable predictor of mucosal healing in UC patients.62 Nevertheless, the fecal calprotectin test lacks a validated cutoff, optimal specificity and accuracy, indicating the need for other stool-based biomarkers to complement fecal calprotectin in monitoring mucosal healing.61 Our recently obtained results50 demonstrated increased serum and fecal levels of KYN in UC patients with mucosal healing compared with UC patients who had chronic, persistent disease. Both serum and fecal levels of KYN negatively correlated with disease severity and concentration of fecal calprotectin, indicating that measurement of KYN in serum and fecal samples of UC patients could be considered as a useful diagnostic tool that can complement fecal calprotectin in monitoring or predicting mucosal healing.50
In addition to its effect on DCs and Tregs’ crosstalk, IDO1 exerts antimicrobial effects in colon epithelium, as well. Since increased IDO1 expression was mainly observed in the vicinity of interruptions of the epithelial barrier where bacterial invasion is more pronounced, IDO1-mediated depletion of TRP might be an important mechanism for the elimination of TRP-dependent microorganisms.59 Additionally, IDO1 regulates immune response to the gut commensal microbiota63 and plays an important role in the interaction between probiotics and the intestinal immune system.64 Zhao and colleagues recently demonstrated that Bifidobacteria induced enhanced IDO1 expression in colon-infiltrating DCs resulting in expansion of Tregs and attenuation of TNBS-induced colitis.64
It is well known that MSCs are, due to their immunomodulatory characteristics, considered as new therapeutic agents in the cell-based therapy of IBD.65,66 Several recently published studies emphasized the crucial importance of MSC-derived IDO1 for the expansion of colon-infiltrating Tregs and the attenuation of colon inflammation,38,67 further indicating therapeutic potential of IDO1 in the therapy of IBD.
The role of IDO1 in the development and progression of gastrointestinal tumors
Since increased IDO1 activity in tumor-infiltrating immune cells reduces availability of essential amino acid TRP for tumor cells, IDO1 has been originally considered an enzyme with strong anticancer potential.6 Nevertheless, with the discovery of IDO1-based immunosuppression, the procancer activity of this enzyme has been documented in a large number of preclinical and clinical studies and nowadays, IDO1 is considered one of the main therapeutic targets in cancer diagnostics and therapy.6,68
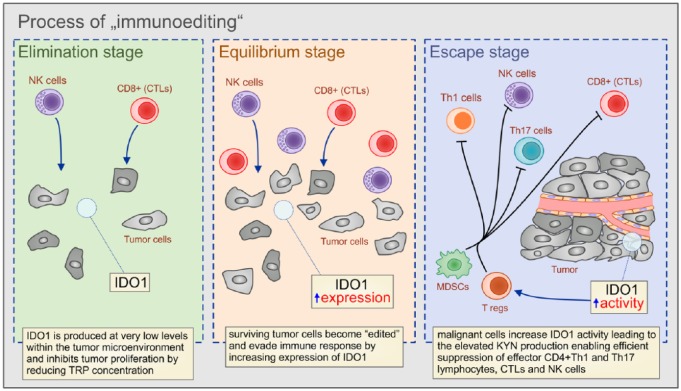
IDO1 is overexpressed in primary and metastatic gastrointestinal tumors that use IDO1-dependent immunosuppression to attenuate antitumor immunity and to promote tumor growth and progression.69 Paradoxically, the activity of the antitumor immune response in the gut, which is elicited to eleminate malignant cells and to prevent tumor growth and progression, promotes the formation of the highly aggressive IDO1-expressing gastrointestinal tumors.70 The immunosuppressive properties of the developing tumor are established during the process of ‘immunoediting’ that involves changes in the genetic background of malignant cells during the three consecutive phases called the ‘elimination,’ ‘equilibrium,’ and ‘escape’ stages (Figure 3).6 During the first phase of tumor surveillance (‘elimination stage’), when most malignant cells are efficiently recognized and destroyed by the cytotoxic effects of NK and CD8+T cells, IDO1 is produced at very low levels within the tumor microenvironment and inhibits tumor proliferation by reducing TRP concentration. During the ‘equilibrium stage,’ surviving tumor cells become ‘edited’ by the continuous attack of the immune cells, accumulate mutations and enhance their capacity to evade immune response, mainly by increasing expression and activity of immunosuppressive enzymes, including IDO1.71 Finally, during the last ‘escape stage’ of ‘immunoediting’, malignant cells increase IDO1 activity, leading to the elevated KYN production, enabling efficient suppression of effector CD4+Th1 and Th17 lymphocytes, CTLs and NK cells. Additionally, the IDO1/KYN pathway induces expansion of Tregs and myeloid-derived suppressor cells, creating an immunosuppressive tumor microenvironment that supports tumor growth and progression.6,71 Accordingly, IDO1 positivity is strongly associated with multidrug resistance of gastrointestinal tumors and inversely correlates with patient survival.72–81
Figure 3.
The role of IDO1 in the ‘immunoediting’ of tumor cells.
During the first phase of tumor surveillance (‘elimination stage’), when most malignant cells are efficiently recognized and destroyed by the cytotoxic effects of NK and CD8+T cells, IDO1 is produced at very low levels within the tumor microenvironment and inhibits tumor proliferation by reducing TRP concentration. During the ‘equilibrium stage’, surviving tumor cells become ‘edited’ by the continuous attack of the immune cells, accumulate mutations, and enhance their capacity to evade immune response, mainly by increasing expression of IDO1. Finally, during the last ‘escape stage’ of ‘immunoediting’, malignant cells increase IDO1 activity, leading to the elevated KYN production, enabling efficient suppression of effector CD4+Th1 and Th17 lymphocytes, CTLs and NK cells.
NK, natural killer cells; CD, cluster of differentiation; IDO1, indoleamine 2,3-dioxygenase; TRP, tryptophan; KYN, kynurenine; CTLs, cytotoxic T cells; Th, T-helper cells.
An increased IDO1 expression was detected in malignant tumors of the oral cavity.72–74 IDO1 expression positively correlated with progression of oral squamous cell carcinoma (OSCC) and negatively correlated with overall survival rate of patients who suffered from OSCC.72 Seppälä and coworkers showed that IDO1 promotes growth of tongue squamous cell carcinoma (TSCC) cells by enabling their escape from the immune surveillance.73 Similar conclusions were made by Kuales and colleagues who showed that DCs, located in the border between the squamous cell carcinoma of the lower lip (SCC-LL) and the inflammatory infiltrate, through the production of IDO1, create immunosuppressive microenvironment enabling immune escape and uncontrolled growth of malignant cells.74 An increased IDO1 activity correlated with poor survival of patients with malignant diseases of the oral cavity73 suggesting IDO1 as potential therapeutic target for enhancement of antitumor immune response in the oral cavity.
An incresed IDO1 expression was observed in esophageal tumors, as well.75–77 Sakurai and colleagues75 have reported increased expression of IDO1 at the mRNA level in the peripheral blood and in tumor samples of patients suffering from esophageal squamous cell carcinoma (ESCC). In line with these findings, Jia and associates found a negative correlation between IDO1 expression and clinical outcome of ESCC patients.76 Most recently, by analyzing tumor samples obtained from 50 ESCC patients, Cui and coworkers noticed enhanced IDO1 activity in tumor-associated fibroblasts and endothelial cells,77 supporting the hypothesis that in addition to tumor cells, stromal and endothelial cells are also able, in an IDO1-dependant manner, to create an immunosuppressive environment in esophageal tumors, contributing to the poor prognosis of IDO1-positive ESCC patients.
IDO1 plays an importnat role in suppression of CTLs in gastric cancer and, accordingly, it was recently proposed as a new, negative prognostic biomarker for overall survival of gastric cancer patients.78–82 Cytotoxicity and capacity for proliferation of CTLs, obtained from gastric cancer patients, were significantly attenuated when these cells were cocultured with IDO1 overexpressing human gastric cancer BGC-823 cells.78 An addition of 1-methyl-tryptophan, a competitive inhibitor of IDO1, to the CTLs (BGC-823 culture) completely restored antitumor properties of CTLs, confirming the importance of IDO1 for suppression of CTLs.78 Additionally, several lines of evidence indicate that gastric-cell-derived IDO1 reduces the capacity of DCs to promote generation of memory CD4+ and CD8+T cells. Significantly lower number of activated DCs, CD4+ and CD8+ memory T cells were noticed in tumor tissues obtained from gastric cancer patients with increased IDO1 activity.79,80 This phenomenon was associated with deeper tumor invasion, massive lymph-node metastasis and poor clinical prognosis.79,80 In order to analyze the prognostic value of IDO1 expression in gastric cancer, Nishi and colleagues analyzed IDO1 expression in 60 patients who underwent curative gastrectomy for stage III gastric cancer and confirmed that overall survival rate was significantly lower in the IDO1 positive group of patients. This finding correlated with massive infiltration of immunosuppressive Tregs within the tumors, confirming that enhanced IDO1 expression resulted with the creation of an immunosuppressive microenvironment, leading to poor prognosis.82 In line with these findings are results obtained by Liu and coworkers who examined correlation between overall survival rate and intratumoral IDO1 expression in 357 patients with gastric adenocarcinoma who underwent gastrectomy.81 An increased expression of IDO1 was associated with poor postoperative clinical outcome, confirming that IDO1 could be considered as a new, valuable, negative prognostic biomarker for estimation of overall survival of patients with gastric cancer who underwent gastrectomy.81
Several lines of evidence demonstrated the crucial importance of IDO1/KYN signaling for the development of colon cancer.83–89 IDO1 regulates viability and proliferation of colon cancer cells, promotes progression of preneoplastic cells in neoplastic lesions and plays an important immunomodulatory role in antitumor immunity in the colon.83–85 Inhibition of IDO1 activity in colon cancer cells decreased the transcription of CDC20, resulting in G2/M cycle arrest83 and reduced nuclear, activated β-catenin and transcription of its target genes (cyclin D1 and Axin2).84 Importantly, mitosis of IDO1-suppressed colon cancer cells was completely restored by KYN, suggesting IDO1-derived KYN as a crucially important mediator for the proliferation of colon cancer cells.83,84 In addition to mitotic cell death, long-term exposure to IDO1 inhibitor induced mitochondrial injury and caspase-dependent apoptosis of colon cancer cells, confirming the significance of IDO1 for viability of colon cancer cells.83 These data were confirmed in vivo, as well.83–85 Upregulation of IDO1 activity was observed in the azoxymethane (AOM)-induced colonic preneoplastic lesions, while use of IDO1 inhibitor significantly decreased the total number of aberrant crypt foci and β-catenin-accumulated crypts that overexpressed IDO1.85 By using IDO1-deficient mice and an animal model of AOM/DSS-induced colon cancer, Liu and colleagues83 and Thaker and coworkers84 confirmed the crucial importance of IDO1 in colon carcinogenesis. IDO1-deficient mice harbored fewer tumors that had a higher number of tumor-infiltrating CTLs and a lower number of immunosuppressive Tregs in comparison with wild-type tumor-bearing animals. Interestingly, IDO1 inhibitor managed to prevent the development of colon cancer in Rag1-deficient mice (who lacked T cells), indicating that IDO1 inhibition could suppress cancer development by inducing cell-cycle arrest of colon cancer cells, independent of affecting T-cell-based antitumor immunity.83 In addition to these findings, Takamatsu and colleagues investigated the effects of genetic deletion of IDO1 on adaptive immune response to colon cancer and noticed that IDO1 deficiency significantly altered cellular make up and cytokine network in the colon tumor microenvironment in a similar manner as in all other gastrointestinal tumors.86 A remarkable decrease in the total number of tumor-infiltrating Tregs and significantly increased mRNA expression of pro-inflammatory cytokines (IFN-γ and TNF-α) was observed in the colon tumor microenvironment of IDO1-deficient tumor-bearing mice,86 leading to the conclusion that IDO1 activation in colon cancer has two complementary functions: acceleration of colon cancer cell proliferation and induction of Treg-mediated immune tolerance. In line with these conclusions are findings recently reported by Ito and colleagues, who demonstrated that inhibition of IDO1 activity could enhance the therapeutic efficacy of TLR7 agonist, which has already been approved for clinical use. Intratumoral injection of TLR7 agonist and simultaneous inhibition of IDO1 activity significantly increased presence of tumor-infiltrating activated DCs, CTLs and enhanced mRNA expression of pro-Th1 cytokines (IL-12, IFN-γ, IL-2) in colon cancers, leading to the suppression of established colon cancer growth in vivo.53 Crucial importance of DC-derived IDO1 for attenuation of antitumor immune response against colon cancer cells was also confirmed in a study conducted by Yen and colleagues, who silenced IDO1 expression in the skin DCs of tumor-bearing mice and elicited an effective CD4+ and CD8+ T-cell-based antitumor immunity, resulting in inhibited colon cancer growth and prolonged survival of experimental animals.87 Additionally, Brandacher and coworkers showed significant correlation between increased IDO1 expression in colon cancer cells and poor prognosis of patients suffering from this disease.88 In order to evaluate whether monitoring of IDO1 activity in colon cancer patients could be used in diagnosis and therapy, Cavia-Saiz and coworkers measured the plasma concentration of L-KYN in 78 colon cancer patients (stage I–IV) and compared it with that in a control group of 70 healthy subjects.89 Overall survival analysis after 45 months of follow up revealed an increased survival rate of colon cancer patients who had low plasma levels of L-KYN,89 suggesting that elevated concentration of L-KYN in plasma could be used as a biomarker to differentiate individuals with colorectal cancer from healthy individuals.
Anticancer therapy with IDO1 inhibitors: opportunity or additional risk for patients with gastrointestinal tumors?
Several pharmacological IDO1 inhibitors (epacadostat, indoximod, navoximod) are currently being explored as anticancer agents in the therapy of solid tumors with the aim of reducing IDO1-dependent immunosuppression in the tumor microenvironment and enabling improved tumor surveillance and enhanced antitumor immune response (Table 1).90–94 Antineoplastic effects of IDO1 inhibitors were based on inhibition of IDO1 transcription and translation and on suppression of TRP transport across the cell membrane.90–93 As expected, IDO1 inhibitors significantly decreased the serum level of KYN, followed by alterations of immune response.91 Accordingly, preliminary results obtained in several clinical trials indicated severe side effects of indoximod treatment, such as immunosuppression, accompanied by an increased risk for recurrent infections, gastrointestinal hemorrhage, decreased appetite, nausea, vomiting, cough, anemia, fatigue and hyperglycemia.91–93 Similar adverse effects were noticed in patients receiving navoximod or epacadostat.94,95 Importantly, the only observed beneficial effect of single-agent IDO1 inhibition was prolonged stable disease.94,95 Accordingly, many clinical trials have been initiated with the aim of exploring whether combined therapy of IDO1 inhibitors and chemotherapeutic agents, checkpoint inhibitors or immunostimulatory drugs will manage to reduce growth and progression of solid tumors (Table 1).
Table 1.
Pharmacological IDO1 inhibitors currently explored as anticancer agents in the therapy of solid tumors.
| Type of tumor | Therapy |
ClinicalTrials.gov identifier/status of the study |
Beneficial effects | Side effects |
|---|---|---|---|---|
| Recurrent or advanced solid tumors | IDO1 inhibitor navoximod (GDC-0919, NLG-919) | NCT 02048709/ completed |
Prolonged stable disease93 | Gastrointestinal hemorrhage; decreased appetite; nausea; vomiting; cough; pruritus; fatigue |
| Treatment-refractory advanced solid tumors | Epacadostat | NCT 01195311/ completed |
Prolonged stable disease94 | Nausea, fatigue; decreased appetite; vomiting; constipation, abdominal pain; diarrhea, dyspnea; cough |
| Metastatic or locally advanced sarcoma | Combination of epacadostat, and anti-PD1 monoclonal antibody (pembrolizumab) | NCT 03414229/ recruiting |
Not applicable | Not applicable |
| Pediatric brain tumors; glioblastoma multiforme; glioma; gliosarcoma; ependymoma; medulloblastoma |
Combination of indoximod and temozolomide | NCT 02502708/ recruiting |
Not applicable | Not applicable |
| Solid tumors; lung cancer; urothelial cancer; head and neck cancer |
Combination of epacadostat and pembrolizumab with standard chemotherapy | NCT 03085914/ recruiting |
Not applicable | Not applicable |
| Advanced or metastatic solid tumors | Combination of epacadostat and nivolumab with ipilimumab, or lirilumab | NCT 03347123/ recruiting |
Not applicable | Not applicable |
| Solid tumors; non-small cell lung cancer; squamous cell carcinoma of the head and neck; urothelial carcinoma; brain metastasis |
Anti-IDO1 agent (LY3381916) alone or in combination with anti-PD-L1 checkpoint antibody (LY3300054) | NCT 03343613/ recruiting |
Not applicable | Not applicable |
| Unresectable stage III or stage IV melanoma | Combination of indoximod with immune checkpoint inhibitors | NCT 02073123/ active, not recruiting |
Not applicable | Not applicable |
| Glioblastoma multiforme; glioma; gliosarcoma |
Combination of indoximod and temozolomide | NCT 02052648/ active, not recruiting |
Not applicable | Not applicable |
| Metastatic pancreatic cancer | Combination of indoximod and the standard of care chemotherapy gemcitabine and nab-paclitaxel | NCT 02077881/ active, not recruiting |
Not applicable | Not applicable |
IDO1, indoleamine 2,3-dioxygenase; PD1, programmed cell-death 1; PD-L1, programmed cell-death ligand 1.
It should be kept in mind that no IDO1 inhibitors have been tested in patients with gastrointestinal tumors. IDO1 is crucially important for the regulation of immune tolerance in the gut. Accordingly, pharmacologic inhibition of IDO1 may affect intestinal immune response and it is highly expected that suppression of IDO1 activity in the gut will increase sensitivity of cancer patients to colitis.96 These safety concerns must be explored in detail in experimental studies before IDO1 inhibitors can be approved in the therapy of gastrointestinal tumors.
Conclusion
IDO1-dependent control of TRP metabolism is a highly versatile regulator of innate and adaptive immune responses, playing an important role in the pathogenesis of inflammatory and malignant diseases of the gastrointestinal tract. The IDO1/KYN pathway has an important immunoregulatory role in the gut, providing homeostatic balance between immunity and tolerance. An increased IDO1 activity maintains immune tolerance and attenuates ongoing inflammation but allows immune escape and uncontrolled growth of gastrointestinal tumors. Accordingly, the IDO1/KYN pathway represents a novel therapeutic target for the treatment of inflammatory and malignant diseases of the gastrointestinal tract, and consequences of its activation and inhibition should be further explored in future experimental and clinical studies.
Footnotes
Contributor Information
Aleksandar Acovic, Center for Molecular Medicine and Stem Cell Research, Department of Microbiology and Immunology, Faculty of Medical Sciences University of Kragujevac, Kragujevac, Serbia.
Marina Gazdic, Center for Molecular Medicine and Stem Cell Research, Department of Microbiology and Immunology, Faculty of Medical Sciences University of Kragujevac, Kragujevac, Serbia.
Nemanja Jovicic, Center for Molecular Medicine and Stem Cell Research, Department of Microbiology and Immunology, Faculty of Medical Sciences University of Kragujevac, Kragujevac, Serbia.
C. Randall Harrell, Regenerative Processing Plant-RPP, LLC, Palm Harbor, Florida, USA.
Crissy Fellabaum, Regenerative Processing Plant-RPP, LLC, Palm Harbor, Florida, USA.
Nebojsa Arsenijevic, Center for Molecular Medicine and Stem Cell Research, Department of Microbiology and Immunology, Faculty of Medical Sciences University of Kragujevac, Kragujevac, Serbia.
Vladislav Volarevic, Center for Molecular Medicine and Stem Cell Research, Department of Microbiology and Immunology, Faculty of Medical Sciences University of Kragujevac, 69 Svetozar Markovic Street, 34000 Kragujevac, Serbia.
References
- 1. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018; 23: 716–724. [DOI] [PubMed] [Google Scholar]
- 2. De Jesus AJ, Allen TW. The role of tryptophan side chains in membrane protein anchoring and hydrophobic mismatch. Biochim Biophys Acta 2013; 1828: 864–876. [DOI] [PubMed] [Google Scholar]
- 3. Clarke G, Stilling RM, Kennedy PJ, et al. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 2014; 28: 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palego L, Betti L, Rossi A, et al. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids 2016; 2016: 8952520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil 2009; 21: 1239–1249. [DOI] [PubMed] [Google Scholar]
- 6. Hornyák L, Dobos N, Koncz G, et al. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol 2018; 9: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci 2017; 3: 52–56. [Google Scholar]
- 8. Van Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Front Immunol 2015; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuzawa-Carballeda J, Fonseca-Camarillo G, Lima G, et al. Indoleamine 2,3-dioxygenase: expressing cells in inflammatory bowel disease-a cross-sectional study. Clin Dev Immunol 2013; 2013: 278035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pallotta MT, Fallarino F, Matino D, et al. AhR-mediated, non-genomic modulation of IDO1 function. Front Immunol 2014; 5: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A 1984; 81: 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horvath CM. The Jak-STAT pathway stimulated by interferon gamma. Sci STKE 2004; 2004: tr8. [DOI] [PubMed] [Google Scholar]
- 13. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 2016; 37: 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orabona C, Pallotta MT, Volpi C, et al. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc Natl Acad Sci U S A 2008; 105: 20828–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orabona C, Puccetti P, Vacca C, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood 2006; 107: 2846–2854. [DOI] [PubMed] [Google Scholar]
- 16. Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res 2009; 2: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida R, Imanishi J, Oku T, et al. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A 1981; 78: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujigaki S, Saito K, Takemura M, et al. Species differences in L-tryptophan-kynurenine pathway metabolism: quantification of anthranilic acid and its related enzymes. Arch Biochem Biophys 1998; 358: 329–335. [DOI] [PubMed] [Google Scholar]
- 19. Takikawa O, Yoshida R, Kido R, et al. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem 1986; 261: 3648–3653. [PubMed] [Google Scholar]
- 20. Selvan SR, Dowling JP, Kelly WK, et al. Indoleamine 2,3-dioxygenase (IDO): biology and target in cancer immunotherapies. Curr Cancer Drug Targets 2016; 16: 755–764. [DOI] [PubMed] [Google Scholar]
- 21. Terness P, Bauer TM, Röse L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002; 196: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SM, Lee YS, Choi JH, et al. Tryptophan metabolite 3-hydroxyanthranilic acid selectively induces activated T cell death via intracellular GSH depletion. Immunol Lett 2010; 132: 53–60. [DOI] [PubMed] [Google Scholar]
- 23. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006; 176: 6752–6761. [DOI] [PubMed] [Google Scholar]
- 24. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ 2002; 9: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 25. Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010; 59: 595–604. [DOI] [PubMed] [Google Scholar]
- 26. Luu M, Steinhoff U, Visekruna A. Functional heterogeneity of gut-resident regulatory T cells. Clin Transl Immunology 2017; 6: e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fougeray S, Mami I, Bertho G, et al. Tryptophan depletion and the kinase GCN2 mediate IFN-γ-induced autophagy. J Immunol 2012; 189: 2954–2964. [DOI] [PubMed] [Google Scholar]
- 28. Metz R, Rust S, Duhadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012; 1: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol 2012; 42: 1932–1937. [DOI] [PubMed] [Google Scholar]
- 30. Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011; 12: 870–878. [DOI] [PubMed] [Google Scholar]
- 31. Mezrich JD, Fechner JH, Zhang X, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010; 185: 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014; 511: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009; 113: 6102–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. François M, Romieu-Mourez R, Li M, et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 2012; 20: 187–195. [DOI] [PubMed] [Google Scholar]
- 35. Kawasaki H, Chang HW, Tseng HC, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 2014; 69: 445–452. [DOI] [PubMed] [Google Scholar]
- 36. Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008; 111: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 37. Moon JS, Cheong NR, Yang SY, et al. Lipopolysaccharide-induced indoleamine 2,3-dioxygenase expression in the periodontal ligament. J Periodontal Res 2013; 48: 733–739. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 2009; 183: 7787–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Özdemir AT ÖzgülÖzdemir RB Kırmaz C et al. . The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol 2016; 310: 108–115. [DOI] [PubMed] [Google Scholar]
- 40. Lee S, Zhang QZ, Karabucak B, et al. DPSCs from inflamed pulp modulate macrophage function via the TNF-α/IDO axis. J Dent Res 2016; 95: 1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dokić J, Tomić S, Marković M, et al. Mesenchymal stem cells from periapical lesions modulate differentiation and functional properties of monocyte-derived dendritic cells. Eur J Immunol 2013; 43: 1862–1872. [DOI] [PubMed] [Google Scholar]
- 42. Lewkowicz N, Lewkowicz P, Dzitko K, et al. Dysfunction of CD4+CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med 2008; 37: 454–461. [DOI] [PubMed] [Google Scholar]
- 43. Nisapakultorn K, Makrudthong J, Sa-Ard-Iam N, et al. Indoleamine 2,3-dioxygenase expression and regulation in chronic periodontitis. J Periodontol 2009; 80: 114–121. [DOI] [PubMed] [Google Scholar]
- 44. Larussa T, Leone I, Suraci E, et al. Enhanced expression of indoleamine 2,3-dioxygenase in Helicobacter pylori-infected human gastric mucosa modulates Th1/Th2 pathway and interleukin 17 production. Helicobacter 2015; 20: 41–48. [DOI] [PubMed] [Google Scholar]
- 45. Bagheri N, Salimzadeh L, Shirzad H. The role of T helper 1-cell response in Helicobacter pylori-infection. Microb Pathog 2018; 123: 1–8. [DOI] [PubMed] [Google Scholar]
- 46. Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter 2011; 16: 1–8. [DOI] [PubMed] [Google Scholar]
- 47. Takamatsu M, Hirata A, Ohtaki H, et al. IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol 2013; 191: 3057–3064. [DOI] [PubMed] [Google Scholar]
- 48. Hoshino S, Kurishima A, Inaba M, et al. Amelioration of 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice by immunoregulatory dendritic cells. J Gastroenterol 2011; 46: 1368–1381. [DOI] [PubMed] [Google Scholar]
- 49. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017; 153: 1504. e2–1516e2. [DOI] [PubMed] [Google Scholar]
- 50. Acovic A, Simovic Markovic B, Gazdic M, et al. Indoleamine 2,3-dioxygenase dependent expansion of T regulatory cells is crucially important for maintenance of mucosal healing in ulcerative colitis. Therap Adv Gastroenterol 2018; 11: 1756284818793558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou L, Chen H, Wen Q, et al. Indoleamine 2,3-dioxygenase expression in human inflammatory bowel disease. Eur J Gastroenterol Hepatol 2012; 24: 695–701. [DOI] [PubMed] [Google Scholar]
- 52. Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol 2004; 113: 47–55. [DOI] [PubMed] [Google Scholar]
- 53. Ito H, Ando T, Arioka Y, et al. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a toll-like receptor 7 agonist in an established cancer model. Immunology 2015; 144: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol 2010; 184: 3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tu L, Chen J, Zhang H, et al. Interleukin-4 inhibits regulatory T cell differentiation through regulating CD103+ dendritic cells. Front Immunol 2017; 8: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen W, Liang X, Peterson AJ, et al. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 2008; 181: 5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coquerelle C, Oldenhove G, Acolty V, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut 2009; 58: 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sznurkowska K, Żawrocki A, Sznurkowski J, et al. Indoleamine 2,3-dioxygenase and regulatory T cells in intestinal mucosa in children with inflammatory bowel disease. J Biol Regul Homeost Agents 2017; 31: 125–131. [PubMed] [Google Scholar]
- 59. Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol 2008; 21: 289–295. [DOI] [PubMed] [Google Scholar]
- 60. Lord JD. Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World J Gastroenterol 2015; 21: 11236–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Di Ruscio M, Vernia F, Ciccone A, et al. Surrogate fecal biomarkers in inflammatory bowel disease: rivals or complementary tools of fecal calprotectin? Inflamm Bowel Dis 2017; 24: 78–92. [DOI] [PubMed] [Google Scholar]
- 62. Kristensen V, Røseth A, Ahmad T, et al. Fecal calprotectin: a reliable predictor of mucosal healing after treatment for active ulcerative colitis. Gastroenterol Res Pract 2017; 2017: 2098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harrington L, Srikanth CV, Antony R, et al. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect Immun 2008; 76: 3045–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao L, Suolang Y, Zhou D, et al. Bifidobacteria alleviate experimentally induced colitis by upregulating indoleamine 2, 3-dioxygenase expression. Microbiol Immunol 2018; 62: 71–79. [DOI] [PubMed] [Google Scholar]
- 65. Nikolic A, Simovic Markovic B, Gazdic M, et al. Intraperitoneal administration of mesenchymal stem cells ameliorates acute dextran sulfate sodium-induced colitis by suppressing dendritic cells. Biomed Pharmacother 2018; 100: 426–432. [DOI] [PubMed] [Google Scholar]
- 66. Markovic BS, Kanjevac T, Harrell CR, et al. Molecular and cellular mechanisms involved in mesenchymal stem cell-based therapy of inflammatory bowel diseases. Stem Cell Rev 2018; 14: 153–165. [DOI] [PubMed] [Google Scholar]
- 67. Ryu DB, Lim JY, Lee SE, et al. Induction of indoleamine 2,3-dioxygenase by pre-treatment with poly(I:C) may enhance the efficacy of MSC treatment in DSS-induced colitis. Immune Netw 2016; 16: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Amobi A, Qian F, Lugade AA, et al. Tryptophan catabolism and cancer immunotherapy targeting IDO mediated immune suppression. Adv Exp Med Biol 2017; 1036: 129–144. [DOI] [PubMed] [Google Scholar]
- 69. Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 2014; 63: 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Iversen TZ, Andersen MH, Svane IM. The targeting of indoleamine 2,3 dioxygenase-mediated immune escape in cancer. Basic Clin Pharmacol Toxicol 2015; 116: 19–24. [DOI] [PubMed] [Google Scholar]
- 71. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22: 329–360. [DOI] [PubMed] [Google Scholar]
- 72. Laimer K, Troester B, Kloss F, et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol 2011; 47: 352–357. [DOI] [PubMed] [Google Scholar]
- 73. Seppälä M, Halme E, Tiilikainen L, et al. The expression and prognostic relevance of indoleamine 2,3-dioxygenase in tongue squamous cell carcinoma. Acta Otolaryngol 2016; 136: 729–735. [DOI] [PubMed] [Google Scholar]
- 74. Kuales MA, Wenzel J, Schmid-Wendtner MH, et al. Myeloid CD11c+ S100+ dendritic cells express indoleamine 2,3-dioxygenase at the inflammatory border to invasive lower lip squamous cell carcinoma. Histol Histopathol 2011; 26: 997–1006. [DOI] [PubMed] [Google Scholar]
- 75. Sakurai K, Enomoto K, Amano S, et al. Study of indoleamine 2,3-dioxygenase expression in patients of esophageal squamous cell carcinoma. Gan To Kagaku Ryoho 2004; 31: 1780–1782. [PubMed] [Google Scholar]
- 76. Jia Y, Wang H, Wang Y, et al. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer 2015; 137: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 77. Cui G, Li C, Xu G, et al. Tumor-associated fibroblasts and microvessels contribute to the expression of immunosuppressive factor indoleamine 2, 3-dioxygenase in human esophageal cancers. Pathol Oncol Res 2018; 24: 269–275. [DOI] [PubMed] [Google Scholar]
- 78. Zhang R, Li H, Yu J, et al. Immunoactivative role of indoleamine 2,3-dioxygenase in gastric cancer cells in vitro. Mol Med Rep 2011; 4: 169–173. [DOI] [PubMed] [Google Scholar]
- 79. Zhang R, Liu H, Li F, et al. The correlation between the subsets of tumor infiltrating memory T cells and the expression of indoleamine 2,3-dioxygenase in gastric cancer. Dig Dis Sci 2013; 58: 3494–3502. [DOI] [PubMed] [Google Scholar]
- 80. Li F, Huang J, Li S, et al. The subsets of dendritic cells and memory T cells correspond to indoleamine 2,3-dioxygenase in stomach tumor microenvironment. Tumour Biol 2014; 35: 8691–8698. [DOI] [PubMed] [Google Scholar]
- 81. Liu H, Shen Z, Wang Z, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep 2016; 6: 21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nishi M, Yoshikawa K, Higashijima J, et al. The impact of indoleamine 2,3-dioxygenase (IDO) expression on stage III gastric cancer. Anticancer Res 2018; 38: 3387–3392. [DOI] [PubMed] [Google Scholar]
- 83. Liu X, Zhou W, Zhang X, et al. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. Int J Cancer. Epub ahead of print 1 April 2018. DOI: 10.1002/ijc.31417. [DOI] [PubMed] [Google Scholar]
- 84. Thaker AI, Rao MS, Bishnupuri KS, et al. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology 2013; 145: 416–425.e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ogawa K, Hara T, Shimizu M, et al. Suppression of azoxymethane-induced colonic preneoplastic lesions in rats by 1-methyltryptophan, an inhibitor of indoleamine 2,3-dioxygenase. Cancer Sci 2012; 103: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Takamatsu M, Hirata A, Ohtaki H, et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci 2015; 106: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yen MC, Lin CC, Chen YL, et al. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res 2009; 15: 641–649. [DOI] [PubMed] [Google Scholar]
- 88. Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 2006; 12: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 89. Cavia-Saiz M, Muñiz Rodríguez P, Llorente Ayala B, et al. The role of plasma IDO activity as a diagnostic marker of patients with colorectal cancer. Mol Biol Rep 2014; 41: 2275–2279. [DOI] [PubMed] [Google Scholar]
- 90. Kozłowska A, Mackiewicz J, Mackiewicz A. Therapeutic gene modified cell based cancer vaccines. Gene 2013; 525: 200–207. [DOI] [PubMed] [Google Scholar]
- 91. Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer 2017; 76: 167–182. [DOI] [PubMed] [Google Scholar]
- 92. Soliman HH, Jackson E, Neuger T, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 2014; 5: 8136–8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014; 3: e957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nayak-Kapoor A, Hao Z, Sadek R, et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J Immunother Cancer 2018; 6: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beatty GL, O’Dwyer PJ, Clark J, et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 2017; 23: 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang MY, Smith C, DuHadaway JB, et al. Cardiac and gastrointestinal liabilities caused by deficiency in the immune modulatory enzyme indoleamine 2,3-dioxygenase. Cancer Biol Ther 2011; 12: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]