Abstract
The ventilation/perfusion lung scan is recommended to exclude chronic thromboembolic pulmonary hypertension in the diagnostic algorithm of pulmonary hypertension, but its role in pulmonary arterial hypertension (PAH) has not been well explored. We characterized the lung perfusion pattern assessed by lung perfusion scintigraphy in idiopathic PAH (IPAH) patients and evaluate the potential prognostic significance of the patchy pattern perfusion defect. A total of 318 patients with IPAH confirmed by right heart catheterization who performed lung perfusion scintigraphy were included. On lung perfusion scintigraphy, 134 patients had normal lung perfusion and 184 patients showed patchy perfusion defects. In comparison to patients with normal lung perfusion, patients with patchy perfusion defects experienced significantly higher mean pulmonary arterial pressure (58.0 ± 15.4 mmHg vs. 54.1 ± 16.2 mmHg, P = 0.027) and total pulmonary resistance (1192.6 ± 533.7 dyn·s·cm−5 vs. 1067.2 ± 549.3 dyn·s·cm−5, P = 0.042). During a median follow-up period of 884.0 days, 53 patients reached the primary endpoint of all-cause mortality. On univariate Cox analysis, the patchy pattern of perfusion defect was significantly associated with the all-cause mortality (hazard ratio [HR] = 2.47, 95% confidence interval [CI] = 1.32–4.63, P = 0.005). Patients with patchy perfusion defects had a worse outcome (log-rank = 8.605, P = 0.003). On multivariate analysis, the patchy pattern remained as a significant independent predictor of the endpoint (HR = 2.30, 95% CI = 1.22–4.31, P = 0.010). IPAH patients presented with heterogeneity in lung perfusion and the patchy pattern of lung perfusion defect commonly existed. Patients with patchy pattern identified by lung perfusion scintigraphy were associated with more severe disease and worse outcome.
Keywords: lung perfusion scintigraphy, idiopathic pulmonary arterial hypertension, lung blood flow, prognosis
Introduction
A lung ventilation/perfusion scan is an established diagnostic test for suspected pulmonary embolism.1 Occlusive thrombi affecting individual pulmonary arteries produces characteristic lobar, segmental or sub-segmental peripheral wedge-shaped defects with the base projecting to the lung periphery, whereas lung ventilation is usually preserved. This pattern of preserved ventilation and absent perfusion, known as ventilation/perfusion mismatch, is the basis for pulmonary embolism diagnosis.2 Accordingly, in the diagnostic algorithm of pulmonary hypertension (PH) from 2015 European Society of Cardiology and European Respiratory Society Guidelines, after exclusion of group 2 (PH due to left heart disease) and group 3 (PH due to lung disease and/or hypoxia), the ventilation/perfusion lung scan is recommended to exclude group 4 (chronic thromboembolic PH [CTEPH]).3 A normal or low-probability ventilation/perfusion scan effectively excludes CTEPH with a sensitivity of 90–100% and a specificity of 94–100%. PH patients present with mismatched lung perfusion defects, suggesting CTEPH is possible. Otherwise, diagnosis of group 1 (pulmonary arterial hypertension [PAH]) should be considered. However, PAH patients may have normal lung perfusion or show non-segmental defects in perfusion. Several reports have described these non-segmental defects as “mottled pattern” or “patchy pattern” in some cases.4–6 Observational results from previous studies are limited by the small number of the study population. Whether the perfusion defect in the lung is associated with the disease severity of PAH is not clear. The clinical significance of this abnormal lung perfusion in patients with PAH has not been well explored. Therefore, the present study aimed to characterize specifically the lung perfusion pattern assessed by lung perfusion scintigraphy in a group of idiopathic PAH (IPAH) patients and evaluate the potential prognostic significance of the perfusion defect.
Methods
Study population
We retrospectively extracted data from the Registry for Pulmonary Hypertension in China (NCT01417338). The clinical database of 1409 consecutive patients with PH who received ventilation/perfusion scans at the National Center for Cardiovascular Diseases and Fuwai Hospital between August 2009 and July 2016 were reviewed. Patients classified into other groups of PH or patients who complicated with lung parenchymal diseases confirmed by chest CT were excluded. A total of 318 patients diagnosed with IPAH were finally included in the current study. The diagnosis of IPAH was made according to the Guidelines on Diagnosis and Treatment of pulmonary hypertension by the European Society of Cardiology and European Respiratory Society.3 Patients with an increase in mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg at rest, with a mean pulmonary capillary wedge pressure (PCWP) < 15 mmHg, and a pulmonary vascular resistance (PVR) > 3 Wood units (WU) measured by right heart catheterization (RHC), without any familiar history of PAH or known triggering factor were diagnosed as IPAH. This study complied with the amended Declaration of Helsinki and was approved by the institutional review board of Fuwai Hospital (ethical approval no. 402). All patients gave informed consent.
Lung perfusion scan and imaging interpretation
All patients in the supine position were intravenously injected with 111–185 MBq of 99mTc-macroaggregated albumin (2–7 × 105 MAA particles). Lung perfusion imaging was performed using a dual-head gamma camera equipped with low-energy, high-resolution, parallel-hole collimators (e.cam, Siemens, Germany; Discovery NM630, GE, USA) as reported previously.7 Images were acquired with a 128 × 128 matrix in eight views (anterior, posterior, left anterior oblique, left lateral, left posterior oblique, right anterior oblique, right lateral, and right posterior oblique). A total of 500 kilocounts per projection was collected.
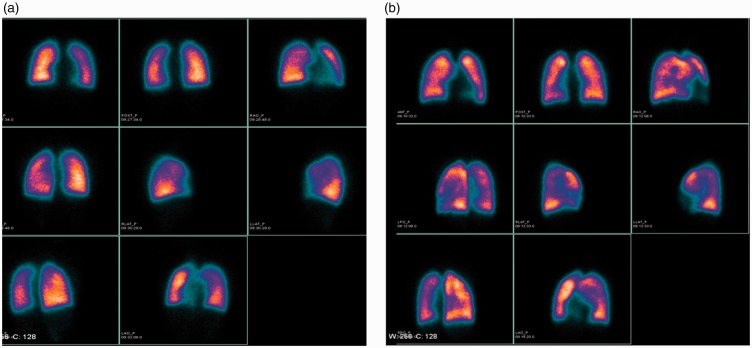
Lung perfusion images were interpreted by two independent experienced nuclear physicians. The third expert participated when the two physicians could not reach agreement. Lung perfusion images are categorized as normal or patchy pattern. The patchy pattern is defined as non-uniform perfusion or non-segmental perfusion defects rather than the pulmonary embolism-type.5 Representative images of different lung perfusion patterns are shown in Fig. 1.
Fig. 1.
Representative images of lung perfusion scintigraphy of two patients demonstrate different lung perfusion patterns. (a) A 17-year-old female patient who had normal lung perfusion (mean PAH = 31 mmHg; mRAP = 2 mmHg; cardiac index = 3.07 L/min/m2; TPR = 559.81 dyn·s·cm−5; right ventricular anteroposterior dimension = 18 mm; NYHA FC I) is event-free during follow-up. (b) A 15-year-old male patient who showed patchy pattern of lung perfusion (mean PAH = 75 mmHg; mRAP = 22 mmHg; cardiac index = 1.5 L/min/m2; TPR = 1184.8 dyn·s·cm−5; right ventricular anteroposterior dimension = 32 mm; NYHA FC III) died due to right heart failure (survival time = 529 days).
Pulmonary hemodynamic measurements
Hemodynamic parameters including mean right atrial pressure (mRAP), mPAP, and PCWP were recorded for RHC examination. Cardiac output (CO) was measured by the thermodilution method (the mean value of three-time measurement). PVR was calculated using the following equation: PVR = (mPAP − PCWP)/CO. Total pulmonary resistance (TPR) was calculated using the following equation: TPR = mPAP /CO. RHC was performed within one week after the lung perfusion scan.
Follow-up
All patients were regularly contacted every six months by telephone interview and medical records were reviewed over the follow-up period. The primary endpoint was defined as all-cause mortality. Follow-ups of patients who died were calculated from the date of lung perfusion imaging examination to the date of death. Patients who survived or were lost to follow-up were censored from the date of lung perfusion imaging examination to 15 January 2017.
Statistics
Continuous data were expressed as mean ± standard deviation (SD) and categorical data were expressed as frequency with percentage (%). Differences between the two groups were analyzed by the unpaired Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. A univariate Cox proportional hazards model was used to test the association between the primary endpoint and baseline covariates, with results presented as hazard ratio (HR) with 95% confidence interval (CI). To determine whether the patchy pattern found on lung perfusion scans was an independent predictor associated with outcome, forward stepwise multivariable Cox regression analysis was performed. Variables reaching P < 0.05 on univariate analysis were considered for entry in the multivariable models. Survival curves of the endpoint were estimated by the Kaplan–Meier method and compared by the log-rank test.
All statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a two-tailed P value < 0.05.
Results
Baseline and clinical characteristics
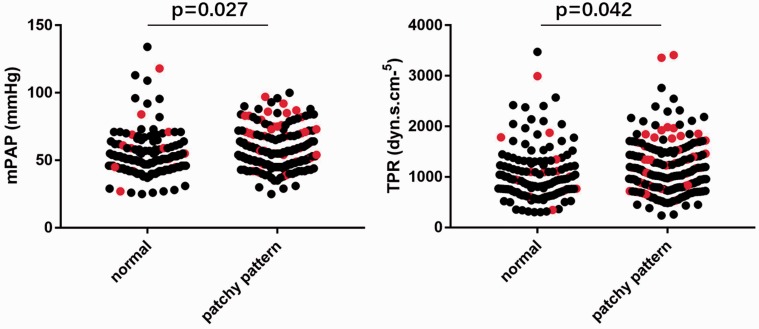
Baseline characteristics of 318 IPAH patients were summarized in Table 1. Two groups of patients with different patterns of lung perfusion were compared. A total of 184 patients (57.9%) showed patchy perfusion defects and the other 134 patients (42.1%) had normal lung perfusion. Patients with patchy pattern were relatively younger (32.4 ± 12.3 years vs. 35.9 ± 12.1 years, P = 0.011) and lower body mass index (22.2 ± 3.2 kg/m2 vs. 23.8 ± 3.5 kg/m2, P < 0.001). Notably, differences in mPAP and TPR between the two groups reached statistical significance (mPAP = 58.0 ± 15.4 mmHg vs. 54.1 ± 16.2 mmHg, P = 0.027; TPR = 1192.6 ± 533.7 dyn·s·cm−5 vs. 1067.2 ± 549.3 dyn·s·cm−5, P = 0.042), suggesting patients with a patchy pattern tended to have relatively more severe disease in terms of worse pulmonary hemodynamics (Fig. 2). Other hemodynamic parameters between the two groups were not statistically different. Moreover, patients with a patchy pattern were more likely to receive treatment with PAH-targeted agents, while patients with normal lung perfusion were more likely to receive calcium channel blocker. There was no statistically significant difference in other treatments given to these patients.
Table 1.
Baseline characteristics.
| Characteristics | All patients (n = 318) | Normal perfusion group (n = 134) | Patchy pattern group (n = 184) | P value |
|---|---|---|---|---|
| Age (years) | 33.9 ± 12.3 | 35.9 ± 12.1 | 32.4 ± 12.3 | 0.011 |
| Sex, male (%) | 73 (23.0) | 32 (23.9) | 41 (22.3) | 0.788 |
| BMI | 22.9 ± 3.4 | 23.8 ± 3.5 | 22.2 ± 3.2 | 0.000 |
| NYHA FC (n (%)) | 0.268 | |||
| I | 27 (8.5) | 12 (9.0) | 15 (8.2) | |
| II | 146 (45.9) | 66 (49.3) | 80 (43.5) | |
| III | 133 (41.8) | 54 (40.3) | 79 (42.9) | |
| IV | 12 (3.8) | 2 (1.5) | 10 (5.4) | |
| Heart rate (bpm) | 79.3 ± 13.6 | 79.1 ± 13.1 | 79.5 ± 14.0 | 0.777 |
| NT-proBNP (pg/mL) | 1435.6 ± 1384.4 | 1324.4 ± 1129.2 | 1516.6 ± 1542.0 | 0.222 |
| Pericardial effusion (n (%)) | 86 (27.0) | 38 (28.4) | 48 (26.1) | 0.702 |
| RV anteroposterior dimension (mm) | 31.9 ± 6.9 | 31.1 ± 6.8 | 32.5 ± 7.0 | 0.090 |
| Hemodynamics | ||||
| mRAP (mmHg) | 6.2 ± 5.4 | 6.3 ± 5.4 | 6.1 ± 5.5 | 0.813 |
| mPAP (mmHg) | 56.4 ± 15.8 | 54.1 ± 16.2 | 58.0 ± 15.4 | 0.027 |
| CI (L/min/m2) | 2.8 ± 0.9 | 2.8 ± 0.9 | 2..7 ± 0.9 | 0.425 |
| TPR (dyn·s·cm–5) | 1139.7 ± 543.0 | 1067.2 ± 549.3 | 1192.6 ± 533.7 | 0.042 |
| Medication history (n (%)) | ||||
| PAH targeted therapy | 254 (79.9) | 100 (74.6) | 154 (83.7) | 0.049 |
| Anticoagulants | 199 (62.6) | 88 (65.7) | 111 (60.3) | 0.350 |
| Calcium channel blocker | 55 (17.3) | 32 (23.9) | 23 (12.5) | 0.010 |
| Diuretics | 302 (95.0) | 126 (94.0) | 176 (95.7) | 0.606 |
| Digoxin | 222 (69.8) | 96 (71.6) | 126 (68.5) | 0.621 |
| TSH (uIU/mL) | 3.0 ± 2.2 | 2.7 ± 1.7 | 3.2 ± 2.4 | 0.070 |
| Endothelin (pmol/L) | 0.72 ± 0.78 | 0.64 ± 0.58 | 0.77 ± 0.90 | 0.156 |
| Blood urea nitrogen (mmol/L) | 5.5 ± 1.8 | 5.5 ± 1.6 | 5.5 ± 1.9 | 0.902 |
| Creatinine (umol/L) | 70.9 ± 22.2 | 71.7 ± 17.0 | 70.3 ± 25.3 | 0.567 |
| Alkaline phosphatase (IU/L) | 74.5 ± 41.8 | 71.1 ± 35.2 | 76.9 ± 46.0 | 0.227 |
| D-dimer (ng/mL) | 12.8 ± 69.2 | 12.9 ± 70.4 | 12.8 ± 68.5 | 0.994 |
BMI, body mass index; NYHA, New York Heart Association; NT-proBNP, serum N-terminal pro B-type natriuretic peptide; RV, right ventricular; mRAP, mean right atrial pressure; mPAP, mean pulmonary arterial pressure; CI, cardiac index; TPR, total pulmonary resistance; TSH, thyroid-stimulating hormone.
Fig. 2.
Distribution of mPAP and TPR values among patients with normal or patchy pattern. Differences in mPAP and TPR between two groups reached statistically significance (patchy pattern vs. normal, mPAP = 58.0 ± 15.4 mmHg vs. 54.1 ± 16.2 mmHg, P = 0.027; TPR = 1192.6 ± 533.7 dyn·s·cm–5 vs. 1067.2 ± 549.3 dyn·s·cm–5, P = 0.042). Red dots show patients who reached the primary endpoint of all-cause mortality.
Univariable/multivariable Cox proportional hazard analysis
Patients were followed up for a median duration of 884.0 days (interquartile range = 454.5–1357.5 days). During follow-up, 14 patients were lost to follow-up. Fifty-three patients reached the primary endpoint of all-cause mortality (46 deaths due to right heart failure, two due to respiratory failure, two due to sudden cardiac arrest, two due to severe gastrointestinal bleeding, one with unknown reason).
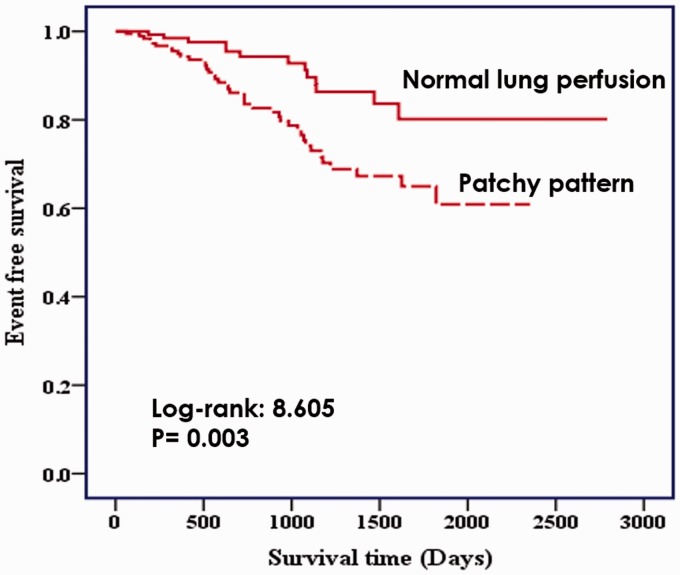
The patchy perfusion defect was entered into Cox proportional hazards analysis as a potential risk factor. Table 2 showed the result of unadjusted HR from univariate Cox proportional hazard analysis. The patchy pattern of perfusion defect was revealed to be a significant predictor of time to all-cause mortality (HR = 2.47, 95% CI = 1.32–4.63, P = 0.005). Patients with patchy perfusion defects had a significantly higher rate of all-cause mortality than those with normal perfusion in Kaplan–Meier survival analysis (log-rank = 8.605, P = 0.003) (Fig. 3). Among 184 patients with patchy perfusion defects, 40 (21.7%) reached primary endpoint in comparison with only 13/134 (9.7%) patients with normal perfusion.
Table 3.
Multivariate Cox proportional hazard analysis.
| Adjusted HR (95% CI) | P value | |
|---|---|---|
| Patchy pattern of lung perfusion | 2.30 (1.22–4.31) | 0.010 |
| mPAP | 1.02 (1.00–1.03) | 0.036 |
| NT-proBNP (per 50 pg/mL) | 1.02 (1.01–1.02) | 0.000 |
| NYHA functional class ≥ III | 2.19 (1.17–4.10) | 0.015 |
HR, hazard ratio; CI, confidence interval; mPAP, mean pulmonary arterial pressure; NT-proBNP, serum N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
Table 2.
Unadjusted hazard ratios (HR) for all-cause mortality in univariable analyses.
| Unadjusted HR (95% CI) | P value | |
|---|---|---|
| Age | 1.00 (0.98–1.03) | 0.644 |
| Male sex | 0.50 (0.29–0.88) | 0.016 |
| BMI | 1.01 (0.93–1.09) | 0.843 |
| NYHA functional class ≥ III | 3.12 (1.73–5.62) | 0.000 |
| Heart rate | 1.02 (1.0–1.04) | 0.083 |
| NT-proBNP (per 50 pg/mL) | 1.02 (1.01–1.02) | 0.000 |
| Pericardial effusion | 1.98 (1.14–3.44) | 0.016 |
| Patchy pattern of lung perfusion | 2.47 (1.32–4.63) | 0.005 |
| mRAP | 1.07 (1.02–1.11) | 0.004 |
| mPAP | 1.03 (1.01–1.04) | 0.001 |
| CI | 0.55 (0.38–0.80) | 0.002 |
| TPR | 1.00 (1.00–1.00) | 0.001 |
| PAH-targeted drug | 1.40 (0.70–2.79) | 0.339 |
| Anticoagulants | 0.62 (0.36–1.07) | 0.087 |
| Calcium channel blockers | 0.78 (0.38–1.60) | 0.492 |
| Diuretics | 21.21 (0.02–27013.42) | 0.402 |
| Digoxin | 1.19 (0.58–2.46) | 0.642 |
| RV anteroposterior dimension | 1.06 (1.02–1.09) | 0.003 |
| TSH | 1.13 (1.02–1.24) | 0.016 |
| Endothelin | 1.44 (1.14–1.82) | 0.002 |
| Blood urea nitrogen | 1.32 (1.16–1.51) | 0.000 |
| Creatinine | 1.0 (1.0–1.01) | 0.510 |
| Alkaline phosphatase | 1.0 (1.0–1.01) | 0.180 |
| D-dimer | 1.00 (1.0–1.01) | 0.247 |
CI, confidence interval; BMI, body mass index; NYHA, New York Heart Association; NTproBNP, serum N-terminal pro B-type natriuretic peptide; mRAP, right atrial pressure; mPAP, mean pulmonary arterial pressure; RV: right ventricular; CI, cardiac index; TPR, total pulmonary resistance; TSH, thyroid-stimulating hormone.
Fig. 3.
Kaplan–Meier estimates of the time to all-cause mortality. Patients with patchy perfusion defects had a significantly higher rate of all-cause mortality than those with normal perfusion.
The other significant univariate predictors were male, New York Heart Association (NYHA) functional class (FC) ≥ III, the presence of pericardial effusion, right ventricular anteroposterior dimension, blood urea nitrogen, serum N-terminal pro B-type natriuretic peptide (NT-proBNP), endothelin, thyroid stimulating hormone, mRAP, mPAP, heart rate, cardiac index, and TPR.
The multivariate analysis showed that the presence of patchy perfusion defect remained a significant independent predictor to all-cause mortality in IPAH patients (HR = 2.30, 95% CI = 1.22–4.31, P = 0.010). The other significant independent covariates were mPAP, NT-proBNP, and NYHA FC ≥ III.
Discussion
To our knowledge, the present study is the largest study to characterize the lung perfusion pattern and investigate its prognostic value in patients with IPAH. IPAH patients presented with heterogeneity in lung perfusion and the patchy pattern of lung perfusion defect was found commonly existed in the study population. More importantly, patients with a patchy pattern were associated with poorer prognosis.
In the current cohort, patchy pattern defects in perfusion were observed in more than half of the IPAH patients (57.9%). IPAH is a disease characterized with pulmonary vascular remodeling driven by endothelial dysfunction, vascular cell proliferation, inflammation, and thrombosis leading to the occlusion of small pulmonary arteries, raised pulmonary arterial pressure, and right ventricular hypertrophy.8 The perfusion abnormalities found in patients with IPAH may be due to the in situ thrombosis in pulmonary arteries. It has been shown there is a high prevalence of vascular thrombotic lesions at postmortem examination in IPAH.9,10 Abnormalities in coagulation and fibrinolytic pathways have been reported. The thrombin activity is also found increased in patients with IPAH.11 With the in situ thrombosis, proliferative and vasoconstrictive pulmonary arteries would affect the blood flow, which may lead to the perfusion defect observed in our study.
This observation is in accordance with previous studies. Early in 1985, Lisbona et al. described non-segmental, patchy defects of perfusion on lung scan in four patients with IPAH.5 In 1993, Ogawa et al. reported that 8/15 cases of IPAH showed multiple, small, ill-defined defects.12 Fukuchi et al. quantitatively assessed lung perfusion in 22 patients with IPAH and found various degrees of diffuse patchy defects.13 Similarly, results from CT studies showed similar observation. In a recent study by Giordano et al., perfusion imaging by dual-energy CT was rated as abnormal in around 53.6% of PAH patients.14 Kim et al. also reported that diffuse heterogeneously decreased lung perfusion on dual-energy pulmonary CT angiography was a common pattern for patients with IPAH, which accounted for 50% in their study population.15 Taken together, it suggested that IPAH patients have heterogeneous lung perfusion and the patchy pattern as a common feature in IPAH may be of great clinical value.
To further characterize this group of patients, the clinical, hemodynamic, and functional characteristics were compared to those having normal lung perfusion. Results suggested that although patients with patchy pattern perfusion defects were younger, they tended to have worse disease condition in comparison to those with normal perfusion. Moreover, univariate and multivariate Cox analysis revealed that the presence of patchy pattern was an independent predictor of worse outcome in patients with IPAH. It seems that lung perfusion scintigraphy identified a subgroup of patients with altered lung blood flow who were at risk of experiencing higher mortality irrespective of therapies.
The therapy for IPAH patients has evolved progressively in the past decade; however, the prognosis of IPAH remains poor.16 Although specific drug therapy is the mainstay of treatment for IPAH, the oral anticoagulation is one of the most important supportive therapies. However, registry data appear to be heterogeneous and whether oral anticoagulants are beneficial for IPAH patients remains inconclusive.17 In 2014, the COMPERA study suggested that the use of anticoagulation is associated with a survival benefit in patients with IPAH.18 In 2015, the other large registry study, the REVEAL analysis, found no significant survival advantage was observed in IPAH patients who started warfarin, in contradistinction from COMPERA.19 In our study, receiving oral anticoagulants was not found to be associated with the prognosis of IPAH patients. Consequently, considering that the rationale for oral anticoagulation in PAH is based on a high prevalence of vascular thrombotic lesions at postmortem examination in patients with IPAH and abnormalities in coagulation and fibrinolytic pathways, patients with altered blood flow in the lung seem to be potential candidates to receive oral anticoagulants: the certain subset of IPAH patients in whom thrombosis plays a greater role and for whom oral anticoagulants may be more likely to be useful. Supportive results from an early prospective, non-randomized study, in which the decision to use warfarin was based on abnormal perfusion lung scan, have shown that the use of warfarin was associated with improved survival.20 Therefore, as we observed in the present study that IPAH patients presented with heterogeneous lung perfusion and the patchy perfusion defect associated with worse outcome, lung perfusion scintigraphy might be used as a non-invasive tool stratifying IPAH population for optimal treatment, in particular, oral anticoagulants. However, whether patients with lung perfusion defects are more likely to benefit from oral anticoagulants requires further prospective studies.
Study limitations
There are limitations to the data presented. This is a retrospective study. The prognostic value of patch pattern on lung perfusion scintigraphy should be interpreted cautiously and validated in prospective studies. The present study merely qualitatively described the patchy pattern on the planar lung perfusion scintigraphy. In future studies, quantitative assessment of lung perfusion measured by SPECT/CT should be considered, which may facilitate characterization in a more detailed and precise method. Critical determinants of prognosis to PAH patients such as 6-min walking distance and right heart function evaluated by cardiac magnetic resonance should also be collected in a more comprehensive risk assessment. Further studies are required to investigate the role of lung perfusion imaging stratifying patients for certain therapies.
Conclusions
The value of lung perfusion scintigraphy in PH may not be limited from screening CTEPH. IPAH patients with patchy pattern of lung perfusion identified by lung perfusion scintigraphy may experience more severe disease and may need to be paid on more attention in the clinics. Whether they are more likely to benefit from anticoagulants requires further prospective studies in the future.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research was supported by grants from National Natural Science Foundation of China (no. 81371586) and National Key Technology R&D Program of China (project no. 2011BAI11B15).
References
- 1.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3069, 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- 2.Bajc M, Neilly JB, Miniati M, et al. EANM guidelines for ventilation/perfusion scintigraphy: Part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. Eur J Nucl Med Mol Imaging 2009; 36: 1356–1370. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 4.Fishman AJ, Moser KM, Fedullo PF. Perfusion lung scans vs pulmonary angiography in evaluation of suspected primary pulmonary hypertension. Chest 1983; 84: 679–683. [DOI] [PubMed] [Google Scholar]
- 5.Lisbona R, Kreisman H, Novales-Diaz J, et al. Perfusion lung scanning: differentiation of primary from thromboembolic pulmonary hypertension. AJR Am J Roentgenol 1985; 144: 27–30. [DOI] [PubMed] [Google Scholar]
- 6.Rich S, Pietra GG, Kieras K, et al. Primary pulmonary hypertension: radiographic and scintigraphic patterns of histologic subtypes. Ann Intern Med 1986; 105: 499–502. [DOI] [PubMed] [Google Scholar]
- 7.He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun 2012; 33: 459–463. [DOI] [PubMed] [Google Scholar]
- 8.Schermuly RT, Ghofrani HA, Wilkins MR, et al. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster V, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984; 70: 580–587. [DOI] [PubMed] [Google Scholar]
- 10.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 1989; 80: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg PR, Lucore C, Kaufman L, et al. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation 1990; 82: 841–847. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa Y, Nishimura T, Hayashida K, et al. Perfusion lung scintigraphy in primary pulmonary hypertension. Br J Radiol 1993; 66: 677–680. [DOI] [PubMed] [Google Scholar]
- 13.Fukuchi K, Hayashida K, Nakanishi N, et al. Quantitative analysis of lung perfusion in patients with primary pulmonary hypertension. J Nucl Med 2002; 43: 757–761. [PubMed] [Google Scholar]
- 14.Giordano J, Khung S, Duhamel A, et al. Lung perfusion characteristics in pulmonary arterial hypertension (PAH) and peripheral forms of chronic thromboembolic pulmonary hypertension (pCTEPH): Dual-energy CT experience in 31 patients. Eur Radiol 2017; 27: 1631–1639. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Seo JB, Oh SY, et al. Assessment of perfusion pattern and extent of perfusion defect on dual-energy CT angiography: correlations between the causes of pulmonary hypertension and vascular parameters. Korean J Radiol 2014; 15: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 17.Frantz RP. Whither anticoagulation in pulmonary arterial hypertension? Conflicting evidence REVEALed. Circulation 2015; 132: 2360–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 19.Preston IR, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL). Circulation 2015; 132: 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327: 76–81. [DOI] [PubMed] [Google Scholar]