Abstract
Premature birth and bronchopulmonary dysplasia (BPD) are risk factors for the development of echocardiographic signs of pulmonary hypertension (PH) and are associated with changes in cardiac structure and function. It is unclear whether this association persists beyond early infancy. The aims of this study are to prospectively investigate the prevalence of PH in children with severe BPD and to investigate the effect of BPD and PH on myocardial structure and function at six months corrected age. Preterm infants (gestational age ≤ 32 weeks) with severe BPD were included. Echocardiography was used to define PH and to measure speckle tracking derived longitudinal and circumferential strain of the left ventricle (LV) and right ventricle (RV). Sixty-nine infants with a median (interquartile range [IQR]) gestational age of 25.6 (24.9–26.4) weeks and a median birthweight of 770 (645–945) gram were included. Eight (12%) infants had signs of PH at six months corrected age. RV fractional area change was lower in infants with severe BPD and PH at six months compared to infants without PH (35% ± 9% vs. 43% ± 9%, P = 0.03). RV mean longitudinal systolic strain was lower in infants with severe BPD and PH compared to infants without PH (17.6% [−19.5%/−16.1%] vs. −20.9% [−25.9%/−17.9%], P = 0.04). RV size and LV longitudinal and circumferential strain in children with BPD with or without PH were similar. Signs of PH were found in 12% of infants with severe BPD at six months corrected age and the presence of PH is associated with reduced RV systolic function.
Keywords: bronchopulmonary dysplasia, pulmonary hypertension, myocardial function
Premature birth is associated with increased right ventricular (RV) mass, reduced RV systolic and diastolic function, and increased pulmonary artery pressures (PAPs) in schoolchildren, adolescents, and young adults.1–3 Bronchopulmonary dysplasia (BPD) is the leading cause of respiratory-disease related pulmonary hypertension (PH) in the neonatal period4 and carries a high risk for mortality and morbidity.5–9 Infants born extremely preterm are at risk of developing BPD, a chronic lung disease that is characterized by a disruption of alveolar and pulmonary vascular growth.6,10 Neonates are now treated at an earlier gestational age (GA), creating a growing population of infants with early disruption of pulmonary and vascular development.11 The incidence of PH in infants and neonates with BPD is in the range of 2–53%, depending on BPD severity, PH definition, type of study design, and age of assessment.9,11–18 The exact pathophysiological pathways that link PH towards unfavorable outcome in infants with BPD are incompletely understood, but changes in the pulmonary vasculature and the increased afterload that is imposed upon the RV might play an important role. Pulmonary vascular disease and increased RV afterload might lead to maladaptation of the RV myocardium and ultimately RV failure.19,20 Cardiac catheterization is the gold standard for assessing the presence or absence of PH, but this invasive procedure is unsuitable for screening.21 Echocardiography is a widely available and commonly used tool to assess the presence of PH. However, there are no published guidelines for the echocardiographic assessment of PH in the pediatric population and correlation with invasively measured PAP is not optimal.22 For this reason other methods should be considered to assess PH and its effect on the RV. A potential useful tool to assess the RV and left ventricular (LV) function includes new echocardiographic methods such as myocardial deformation imaging (speckle tracking echocardiography [STE]).23 Because long-term prospective follow-up studies of children with BPD are scarce, data on the impact of PH on cardiovascular function later in life in children with BPD are limited.8,11,12,15,24
Therefore, the aim of this study is to investigate the prevalence of PH in a prospective non-selected cohort of children with severe BPD. Furthermore, we wanted to investigate the effect of BPD and echocardiographic estimated PH on myocardial structure and function at six months corrected age, using standard echocardiograph imaging and STE. We hypothesize that children with BPD have a high risk of developing PH and changes in RV size and function.
Methods
Patient selection
This is a prospective cohort study. From April 2012 to April 2017, we enrolled preterm neonates born at GA < 32 weeks with severe BPD who were born in the Erasmus Medical Center/Sophia Children Hospital or who were referred to us by other hospitals. Severe BPD was defined as oxygen supplementation for ≥28 days and need for either >30% oxygen, >1 L/min flow, continuous positive airway pressure (CPAP) or ventilator support at 36 weeks post menstrual age (PMA).15 In our hospital, all patients with severe BPD are assessed by a multidisciplinary team (cardiologist, pulmonologist, general pediatrician, psychologist, and physiotherapist) according to a predefined follow-up scheme. A clinical assessment was scheduled at the age of ±6 months corrected age. In addition, we included 12 healthy infants who were born at term at the Leiden University Medical Center who participated in a different study on echocardiographic pediatric normal values (online supplementary table).25
Infants were included in this study if they were diagnosed with severe BPD at 36 weeks PMA and had a cardiac ultrasound at approximately the age of six months (corrected for term date). Exclusion criteria were: (1) hemodynamically significant congenital heart lesion, except patent foramen ovale (<5 mm) and a small patent arterial duct (<1 mm) without volume overload; and (2) overall poor quality of the echocardiograph images. Written informed consent to use these data for research purposes was obtained from both parents of the infants. The study was approved by the medical ethical committee of the Erasmus MC, Rotterdam, The Netherlands (METC-2015-694).
Data collection
Clinical data were collected using the patients’ medical records on usage of prenatal steroids, pre-eclampsia of the mother during pregnancy, gender, GA, birthweight, Apgar score at 1, 5, and 10 min, medication during infancy, duration of mechanical support and invasive oxygen demand; presence and treatment of patent arterial duct; perinatal infections; use of nitric oxide; and neonatal mortality. General data were collected from the patients’ medical records on height, weight, blood pressure, and heart rate at the time of echocardiographic assessment.
Conventional echocardiographic measurements
An extensive echocardiogram was performed at the age of approximately six months corrected age to exclude congenital or acquired heart disease using an EPIQ 7C (Philips Medical Systems, Best, the Netherlands) or Vivid E9 (GE Healthcare, Wauwatosa, WI, USA) ultrasound system. Apical four-chamber, parasternal long-axis and short-axis views were acquired and stored. M-mode, two-dimensional (2D), color Doppler, and pulsed wave Doppler images were acquired to collect data on tricuspid regurgitant jet, pulmonic valve insufficiency, pulmonary artery acceleration time (PAAT), RV and LV dimensions and mass, shortening fraction of the LV, and fractional area change (FAC) of the RV. Right ventricular systolic pressure (RVSP) was estimated by using the modified Bernoulli equation (tricuspid regurgitant jet2 × 4), with no allowance for the right atrial pressure.16 All measurements were performed off-line using Xcelera (version R4.1, Philips, Best, The Netherlands). Z-scores were calculated for LV and RV dimensions and wall thickness.26 The average of three measurements was used.
The presence of PH was assessed using the following criteria12,18: (1) presence of tricuspid regurgitant jet velocity ≥2.8 m/s in the absence of pulmonary stenosis; and (2) flat or leftward deviated interventricular septal configuration. Children with one or both of these findings were characterized as having PH.
Two-dimensional speckle tracking
Two-dimensional echocardiographic images were stored for off-line analysis of myocardial deformation patterns (circumferential systolic strain and longitudinal systolic strain) of the LV and RV using dedicated speckle tracking software (Cardiac Performance analysis, version 4.6, Tomtec, Unterschleisheim, Germany) as previously described.27 The images were stored as Digital Imaging and Communications in Medicine format with a default setting of 30 frames/s. Longitudinal systolic strain of the LV and RV were measured using apical four-chamber views. Circumferential systolic strain of the LV was measured using short-axis views at the level of the papillary muscles. The endocardial border was manually traced at end-systole (minimal volume). Tracking of the endocardial border was visually inspected and manually adjusted if necessary. After obtaining satisfactory endocardial tracking, the epicardial border was added to incorporate the entire myocardial wall (except for RV strain, were only the endocardial wall was tracked). Tracking was re-inspected throughout the cardiac cycle and adjusted as necessary. The cardiac cycle with the best tracking and visually most credible strain curves was selected for analysis. If a segment was not tracking appropriately, this segment was excluded from the analysis. The software divides the myocardium into six segments (circumferential strain and longitudinal strain of the LV) or one segment (lateral wall of the RV). For LV longitudinal systolic strain, the segments are: basal septum; mid-septum; apical septum; apical lateral; mid-lateral; and basal lateral. For LV circumferential systolic strain, the segments are: mid-anterior; mid-lateral; mid-posterior; mid-inferior; and mid-septal. Mean strain values were calculated from the individual segmental strain values. At least 4/6 segments were necessary to calculate mean strain values; images with >2 missing segments were excluded from analysis.
Statistical analysis
All statistical analyzes were preformed using SPSS software, version 21,0 (IBM SPSS Statistic, IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered statistically significant. Continuous data were tested for normality using the Shapiro Wilk test. Continuous data were described as mean (±SD) if normally distributed or as median (interquartile range [IQR]), if non-normally distributed. Categorical data were described as frequencies and percentages. Baseline differences in categorical variables between infants were analyzed using Chi-square or Fisher’s exact test. Continuous variables were analyzed using independent student-test or ANOVA if normally distributed. Non-normally distributed data were analyzed using Mann–Whitney U test.
Inter- and intra-observer variability
Inter- and intra-observer was tested using a Coefficients of Variation (CoV) and Bland–Altman analysis. To describe intra-observer variability of the strain parameters, 15 patients were analyzed by the same investigator (AB) on two separate occasions, with at least one week of time apart. To describe inter-observer variability, the same 15 patients were analyzed by a second investigator (LK).
Results
Patient selection
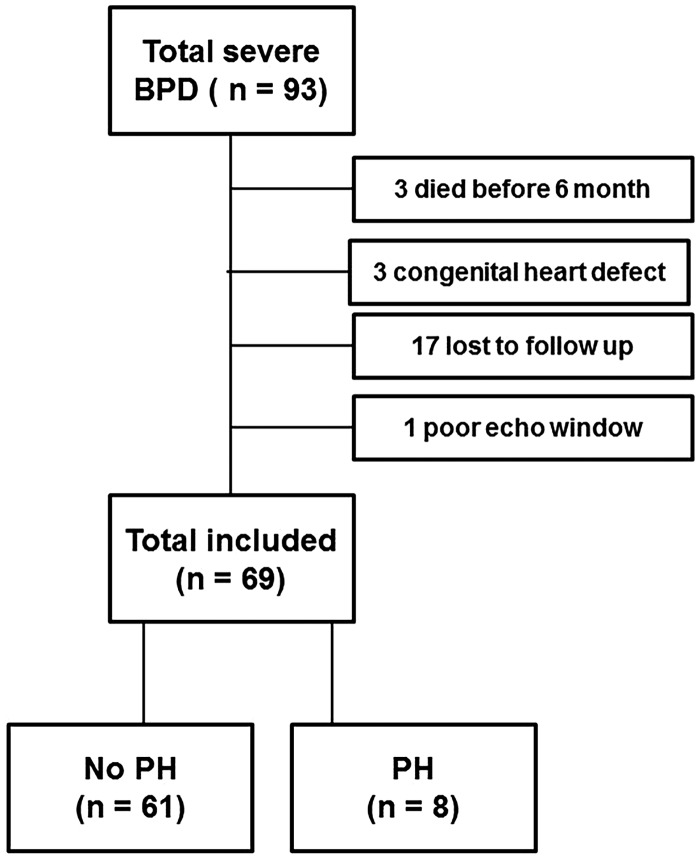
In the study period, a total of 93 patients were diagnosed with severe BPD. We excluded 24 patients (Fig. 1). Three died before the age of six months: one died of respiratory failure caused by BPD; one died of therapy refractory PH; and one died as the consequences of a complex congenital syndrome. Of the remaining 21 excluded infants, three had a hemodynamically significant congenital heart defect, 17 were unable to attend the follow-up clinic at six months of age, and one had an echocardiogram with poor quality. All general characteristics were similar in excluded versus included children except for GA (26.3 [IQR = 25.3–27.2] vs. 25.6 [IQR = 24.6–26.5 weeks], P = 0.02), prevalence of maternal pre-eclampsia (42% vs. 15%, P = 0.01), 5-min Apgar score (8 [IQR = 7–9] vs. 7 [IQR = 6–8], P = 0.04) and prevalence of prenatal steroid treatment (78% vs. 96%, P = 0.03).
Fig. 1.
Flow charts of patient selection.
Of the 69 included patients the median (IQR) corrected age at echocardiographic follow-up was six months (5–7 months).
Patient characteristics
In Table 1, the general patient characteristics are shown. Eight infants (12%) had echocardiographic signs of PH at the age of six months. The majority of children (n = 63) with severe BPD were born at a GA < 28 weeks. Only six children were born at 28–32 weeks. All children underwent invasive mechanical ventilation during hospital admission, except one child who received CPAP only and one child who received non-invasive mechanical ventilatory support only. Duration of mechanical ventilation at 36 weeks of age was statistically significantly longer in infants with PH compared to infants without PH at six months (40 ± 16 vs. 24 ± 16 days, P = 0.02). PH was based on TI-jet ≥2.8 in five cases and flat intraventricular septum in seven cases (overlap of inclusion criteria in four children). Detailed information of the children with PH is provided in Table 2. One child was treated with sildenafil for 2.5 years, one child was treated with sildenafil (4 mg/kg/day) and nitric oxide, and one child was treated with nitric oxide only (during the neonatal phase). Five out of eight children were still on oxygen therapy at six months of corrected age at the echocardiographic assessment.
Table 1.
General patient characteristics.
| Total BPD (n = 69) | No PH 6 months (n = 61) | PH 6 months (n = 8) | P value* | |
|---|---|---|---|---|
| Sex | 0.27 | |||
| – Male | 40 (58) | 37 (60.7) | 3 (37.5) | |
| – Female | 29 (42) | 24 (39.3) | 5 (62.5) | |
| Gestational age (weeks) (median (range)) | 26 (25–26) | 26 (25–26) | 25 (24–26) | 0.15 |
| Birth weight (g) (median (range)) | 770 (645–945) | 780 (650–965) | 645 (492–778) | 0.05 |
| Prenatal steroid | 0.43 | |||
| – No | 3 (4.5) | 3 (5.2) | 0 (0) | |
| – Yes | 63 (95.5) | 55 (94.2) | 8 (100) | |
| Surfactant | 0.28 | |||
| – No | 9 (13.0) | 9 (14.8) | 0 (0) | |
| – Yes, 1 dose | 28 (40.6) | 26 (42.6) | 2 (25.0) | |
| – Yes > 1 dose | 32 (46.4) | 26 (42.6) | 6 (75.0) | |
| Maternal pre-eclampsia | 0.43 | |||
| – No | 56 (84.8) | 51 (86.4) | 5 (71.4) | |
| – Yes | 10 (15.2) | 8 (13.6) | 2 (28.6) | |
| PDA in neonatal phase | 0.67 | |||
| – No | 10 (14.5) | 9 (14.8) | 1 (12.5) | |
| – Yes | 59 (85.5) | 52 (85.2) | 7 (87.5) | |
| Nitric oxide | ||||
| – No | 53 (79.1) | 47 (79.7) | 6 (75) | 0.77 |
| – Yes | 14 (20.9) | 12 (20.3) | 2 (25) | |
| Apgar score 5 min (median (range)) | 7 (6–8) | 7 (6–8) | 6.5 (6–8) | 0.24 |
| Mechanical ventilation until week 36 (days) (mean ± SD) | 25 ± 17 | 23 ± 16 | 40 ± 16 | 0.02 |
| Total days O2 use until week 36 (days) (median (range)) | 59 (39–70) | 55 (37–70) | 71 (57–79) | 0.06 |
Values are presented as n (%) unless otherwise specified.
P value based on Student’s t-test or Mann–Whitney test as appropriate.
PDA, patent ductus arteriosus.
Table 2.
Patient characteristics of the infants with PH at 6 months.
| Patient | GA (weeks) | Birth weight (g) | Allocation PH | Cardiac catherization | O2 at 6 months CA | Duration of O2 (weeks) | PH medication | Remarks, follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 3/7 | 365 | TR 2.9 m/s, IVS | Yes, 7 months CA | No | 25 | – | At 3 months CA TR-jet 3.8 m/s. At catheterization PVRi 4 WU, MPAP = 20 mm Hg. Echo at 1 year CA; no signs of PH |
| 2 | 23 6/7 | 480 | TR 2.8 m/s, IVS | No | Yes | 125 | Sildenafil | Sildenafil stopped after 120 weeks, now 5 years old and no signs of PH |
| 3 | 25 1/7 | 770 | TR 2.8 m/s, IVS | No | No | 34 | NO, sildenafil | Only PH medication during early neonatal phase. No echo follow-up after 1 year; clinical follow-up at 1 year thriving well |
| 4 | 27 4/7 | 660 | TR 2.8 m/s, IVS | No | Yes | 44 | – | No longer follow-up yet |
| 5 | 25 4/7 | 976 | TR 3.1 m/s, IVS | Yes, 14 months CA | Yes | 80 | – | Persistent echocardiographic signs of PH, at catheterization PVRi 2.9 WU, mPAP 18 mmHg. Echo at 16 and 26 months CA; no signs of PH |
| 6 | 24 3/7 | 780 | TR 2.8 m/s | No | No | 30 | NO | NO during neonatal phase. Echo at 9 and 12 months CA; no signs of PH |
| 7 | 25 6/7 | 530 | IVS | No | Yes | 52 | – | Echo at 24 months CA; no signs of PH |
| 8 | 24 2/7 | 630 | IVS | No | Yes | 42 | – | Echo at 12 months CA; no signs of PH |
CA, corrected age; GA, gestational age; IVS, flat or leftward deviated interventricular septal; mPAP, mean pulmonary arterial pressure; NO, nitric oxygen; PVRi, indexed pulmonary vascular resistance; TR, tricuspid regurgitation; WU, Woods unit.
Echocardiographic measurements
Table 3 summarizes the anthropometric and standard echocardiographic measurements at the age of six months corrected for GA. Anthropometric parameters were similar in infants with and without PH. The presence of tricuspid regurgitation was higher in infants with severe BPD and PH compared to infants with severe BPD without PH (63% vs. 20%, P = 0.02). RV FAC was statistically significantly lower in infants with PH compared to infants without PH (35.4 ± 9.2% vs. 43.1 ± 9.4%, P = 0.03). No differences between the groups were observed in RV dimensions and PAAT. The results of the myocardial strain parameters are presented in Table 4. RV global longitudinal strain was statistically significantly lower (less negative) in BPD infants with PH compared to BPD infants without PH (−17.6% [−19.5/−16.1] vs. −20.9% [−25.9/−17.9], P = 0.04). No differences were observed in LV strain values between infants with severe BPD with or without signs of PH. Comparisons of echocardiographic parameters with age-matched at-term born-healthy controls are shown in the online supplementary table. No differences in echocardiographic parameters were seen between term-born healthy children and infants with severe BPD without PH.
Table 3.
Patient characteristics and standard echocardiographic measurements at approximately 6 months of corrected age.
| Total BPD (n = 69) | No PH 6 months (n = 61) | PH 6 months (n = 8) | P value* | |
|---|---|---|---|---|
| Corrected age at echo (months) (median (range)) | 6.0 (5.0–6.5) | 6.0 (5.0–6.5) | 5.9 (5.3–6.8) | 0.87 |
| Length (cm) | 65.4 ± 4.3 | 65.8 ± 4.0 | 63.6 ± 6.1 | 0.36 |
| Weight (kg) | 6.8 ± 1.0 | 6.9 ± 1.0 | 6.5 ± 1.1 | 0.35 |
| Heartrate (bpm) | 122 ± 16 | 121 ± 16 | 129 ± 16 | 0.22 |
| RVEDD Z-score | 1.1 ± 0.7 | 1.0 ± 0.6 | 1.4 ± 0.7 | 0.13 |
| IVSD Z-score | −0.6 ± 1.0 | −0.6 ± 1.0 | −0.2 ± 0.9 | 0.30 |
| LVEDD Z-score | −0.7 ± 0.9 | −0.7 ± 0.9 | −1.2 ± 0.6 | 0.11 |
| LVEDS Z-score | −1.3 ± 1.1 | −1.3 ± 1.1 | −1.8 ± 1.2 | 0.29 |
| LVPDW Z-score | −0.5 ± 1.1 | −0.5 ± 1.0 | −0.2 ± 1.2 | 0.44 |
| LV fractional shorting (%) | 42 ± 5 | 42 ± 5 | 42 ± 6 | 0.81 |
| Left ventricle mass (g) (median (range)) | 18.8 (15.3–21.3) | 19 (15–21) | 19 (14–20) | 0.53 |
| Left ventricle mass Z- score | −1.0 ± 1.0 | −1.0 ± 1.0 | −1.0 ± 0.9 | 0.95 |
| PI (n (%)) | 0.68 | |||
| - No | 50 (72.5) | 45 (74) | 5 (63) | |
| - Yes | 19 (27.5) | 16 (26) | 3 (37) | |
| PAAT (ms) (median (range)) | 80 (70–90) | 80 (70–90) | 70 (63–90) | 0.73 |
| TR (n (%)) | 0.02 | |||
| - No | 61 (88) | 49 (80) | 3 (37) | |
| - Yes | 8 (12) | 12 (20) | 5 (63) | |
| RVSP (mmHg) if TR is present | 22.5 ± 7.8 | 18.1 ± 3.6 | 33.0 ± 3.7 | <0.001 |
| TV annulus Z-score | −0.6 ± 1.0 | −0.7 ± 0.9 | −0.02 ± 1.09 | 0.06 |
| RVED (cm2) (median (range)) | 6.6 (5.6–7.3) | 6.6 (5.6–7.3) | 6.5 (5.5–7.7) | 0.89 |
| RVES (cm2) (median (range)) | 3.7 (3.2–4.2) | 3.7 (3.1–4.2) | 3.9 (3.8–4.4) | 0.10 |
| RV FAC (%) | 42 ± 10 | 43 ± 9 | 35 ± 9 | 0.03 |
Values are presented as mean ± SD unless otherwise specified.
P value based on Student’s t-test or Mann–Whitney test as appropriate.
bpm, beats per minute; IVDS, interventricular septum dimension in diastole; LVEDD, left ventricle dimension end-diastole; LVEDS, left ventricle dimension in systole; LVPWD, left ventricle posterior wall dimension in diastole; NA, not assessed; PAAT, pulmonary artery acceleration time; PI, pulmonary valve insufficiency; RVEDD, right ventricle end-diastole diameter parasternal long axis; RVED, right ventricle end-diastolic surface area; RVES, right ventricle end-systolic surface area; RV FAC, right ventricle fractional area change; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Table 4.
Myocardial strain in infants with severe BPD.
| Total BPD (n = 69) | No PH 6 months (n = 61) | PH 6 months (n = 8) | P value* | |
|---|---|---|---|---|
| Left ventricle | ||||
| Global circumferential strain (%) | −24.8 ± 4.6 | −24.4 ± 4.6 | −27.6 ± 3.8 | 0.07 |
| Global longitudinal strain (%) | −20.3 ± 4.8 | −20.4 ± 4.9 | −20.0 ± 4.3 | 0.84 |
| Right ventricle | ||||
| Global longitudinal strain free wall (%) (median (range)) | −20.6 (−25.9 – −17.8) | −20.9 (−25.9 – −17.9) | −17.6 (−19.5 – −16.1) | 0.04 |
Values are presented as mean ± SD unless otherwise specified.
P value based on Student’s t-test or Mann–Whitney test as appropriate.
Feasibility and inter- and intra-observer variability
The majority of the echocardiographic images were of sufficient quality for analysis. Feasibility for RV global longitudinal strain was 93%. Feasibility for measuring LV global longitudinal strain was 96%, whereas the feasibility for the LV global circumferential strain was 86%. The reproducibility for myocardial strain measurements are shown in the online supplement.
Discussion
We found in a prospective cohort of children with severe BPD that approximately 12% of the infants had echocardiographic sign of PH at the age of six months. We found echocardiographic signs of RV systolic dysfunction in children with PH compared to children without PH as indicated by lower RV global longitudinal strain and RV FAC in the absence of changes in RV size. We also found that preterm infants with severe BPD and PH at the age of six months required a longer period of mechanical ventilation than infants with severe BPD who did not develop PH.
Prevalence of pulmonary hypertension
The prevalence of PH in infants and neonates with BPD described in the literature is in the range of <1% to 53%.11–18,24 The differences in PH prevalence described in various studies probably reflect differences in study design (retrospective versus prospective), timing of echocardiographic assessment, study population (differences in BPD severity), neonatal ventilation, and other treatment strategies and, to some extent, differences in PH definition. The majority of studies that describe PH in children with BPD perform the echocardiographic assessment at or before two months of corrected age.11–13,15–18,24 At this young age, neonates are within or close to the time window in which the physiological transition from high pulmonary vascular resistance (PVR) intra-uterine to low vascular PVR after birth takes place. In addition, the arterial duct is patent in many neonates close to birth, which potentially interferes with the diagnosis of PH. We therefore chose to assess PH at six months of corrected age and found a prevalence of 12%. The only prospective study in older preterm children we are aware of is the multicenter study by Levy et al.14 The study population consisted of 239 children born at <29 weeks of gestation, of which 164 were assessed at 36 weeks PMA and 81 were assessed at one year of corrected age.14 Of these children, 52% developed BPD in various degrees of severity. Echocardiographic defined PH was found in 10% of children with moderate to severe BPD at 36 weeks PMA and in 1% at the age of one year.14 However, recent studies have shown that school-aged children and adolescents who were born prematurely (and especially those children with BPD) have a greater peak tricuspid regurgitation velocity assessed by echocardiography and higher mean PAP assessed by cardiac catheterization compared to term controls.2,3 These findings, together with the findings of ours and other studies, suggest that PH prevalence in prematurely born children decreases over time but that these children remain at risk for the development and persistence of pulmonary vascular disease, especially those children with severe BPD. The determination of the true prevalence of PH is hampered by the limitations of echocardiography, since the velocity of the tricuspid regurgitation jet and the position of the intraventricular septum are at best surrogate markers of increased PAPs.22 Recently, the PAAT has been suggested as a reliable estimation of PAP and PVR in children.28 Pediatric reference values for PAAT have been published;29 a recent study by Levy et al. found a decreased PAAT in preterm born children compared to term-born controls and lower values in children with BPD compared to children without BPD at the age of one year.30 We found a median PAAT of 80 ms in our total population, compared to 73 ms in preterm-born infants in the study by Levy et al. at the age of one year.30 We found similar PAAT in children with and without PH, which suggests that PAAT is not a sensitive marker to detect PH at the age of six months of corrected age. We believe that the results published by Levy et al. should be interpreted with caution, since most of the children in their study had a PAAT within the normal range (64–116 ms for girls and 62–114 ms for boys).29 In addition, PAAT has been validated against invasively assessed pulmonary hemodynamics in children with a median age of 6–8 years and no children aged <1 year were included in this validation study.28
We found that infants with severe BPD and PH at the age of six months had a longer duration of mechanical ventilation at 36 weeks PMA compared to infants with severe BPD without PH. In a large retrospective study by An et al., it was found that severity of BPD, long-term ventilator care, oxygen supplementation, and a high ventilator setting, were associated with PH beyond two months of age based on univariate analysis.11 Ali et al. performed a study in preterm neonates on predictors of BPD and PH and found that the duration of invasive oxygen use was significantly longer in neonates with BPD and PH at 36 weeks PMA.31 Our results are in accordance with these studies.
RV function and adaptation
We found that in children with severe BPD the RV FAC and RV global longitudinal strain values were lower in infants with PH compared to infants without PH at six months of corrected age. Levy et al. investigated the maturational (age- and weight-related) changes of RV FAC in preterm and term children.32 They found that preterm infants, independent of the presence of PH, with moderate to severe BPD had a lower RV FAC at 36 weeks PMA compared to preterm infants without BPD. Levy et al. performed another prospective multicenter cohort study on changes in systolic ventricular strain mechanics and found that preterm infants (mean GA = 27 weeks) with PH as assessed at the age of 36 weeks PMA had significant lower RV free wall longitudinal strain at the age of one year compared to infants who did not have PH at 36 weeks PMA.14 In addition, they found that at the age of 32 weeks PMA, 36 weeks PMA, and one year, children with BPD had lower RV global longitudinal strain values compared to children without BPD.14,30 Even after adjusting for the presence of BPD, the RV free wall strain at the age of one year remained lower in infants with PH compared to infants without PH defined at the age of 36 weeks PMA.14
RV FAC is reduced in older children and adults with idiopathic pulmonary arterial hypertension (IPAH) and is a strong predictor of mortality.33 In addition, the RV is dilated in adult patients with IPAH and increased RV volume strongly predicts mortality in these patients.34 In contrast, increased afterload of the RV in the setting of congenital pulmonary valve stenosis is associated with normal systolic function as assessed by RV FAC, possibly because in early infancy the RV is used to the physiological high PVR and high PAP and can therefore maintain adequate RV function.35,36 Interestingly, in our study, RV size as assessed by tricuspid valve annulus size or RV dimension in diastole were similar between children with and without PH. Our data, together with the data by Levy et al.,14,32 suggest that adaptation of the RV in extremely premature-born children with BPD and PH is fundamentally different as compared to older children or adults with IPAH (decreased RV function and RV dilatation) as well as infants and children with pulmonary valve stenosis (relatively well-preserved RV function). Levy et al. also found that a small group of premature infants without BPD can develop echocardiographic signs of PH at the age of 36 weeks and that these infants also show lower RV global longitudinal strain values.14 These observations suggest that a primary pulmonary vascular injury coupled with RV dysfunction may occur in some preterm-born infants irrespective of the underlying lung disease.14 Lower RV global longitudinal strain values are also found in other studies comparing children with BPD to children without BPD.37–39 However, these studies were performed earlier in life, mostly around birth, and included children with BPD ranging from mild to severe. In our study, we found no differences in RV global longitudinal strain between infants with severe BPD without PH and healthy infants. This suggests that later in life the influence of BPD on RV function is less important and the presence of PH becomes more important. Further studies are needed to provide more insight in these time-related and disease-related differences in RV adaptation, preferably in a larger multicenter prospective cohort where all prematurely born children are routinely screened according to a standard protocol. These data are important to design prevention and treatment strategies for infants born extremely premature.
The LV strain parameters did not differ between infants with or without PH. This is in line with current literature.14,37,39 In young adults born premature, it has been shown that LV mass is increased, and systolic and diastolic LV function are decreased compared to healthy controls born at term, although in this study the presence of BPD is not mentioned.40
Strengths and limitations
The main strengths of this study are the prospective study design, the echocardiographic assessment at an older age compared to previously published studies, and the use of new echocardiographic techniques.
To determine PH, we used echocardiography. Although this is the safest and most commonly used method to assess PH in preterm infants, the gold standard is right heart catheterization (RHC). There are no standardized methods to detect PH in infants using echocardiography and agreement with PH assessed by catheterization is, at most, moderate.22 It is therefore possible that by using ultrasound instead of RHC we may have missed or over-diagnosed infants with PH. However, RHC is an invasive procedure and, due to ethical reasons, not suitable for screening.
Although we included a relatively large number of patients compared to other studies on this topic, the sample size was too small to perform multiple logistic regression analysis in a meaningful way. Larger studies are needed to investigate the association between PH and RV function and its potential confounders.
In the initial study design, we did not include a term control group. Instead, we used echocardiographic studies of children who had participated in a different study on normal values for myocardial deformation. We acknowledge that this approach is sub-optimal; however, since the main echocardiographic parameters were similar between the infants with BPD without PH and the term control group, we believe that our findings in infants with BPD and PH are of considerable value.
All echocardiographic images were stored as Digital Imaging and Communications in Medicine format with a default setting of 30 frames/s. We decided not to include strain rate parameters in our study, since higher frame rates are needed to quantify strain rate reliably. For this reason, no data on diastolic cardiac function were obtained.
Lastly, we only included infants with severe BPD. As we only screen infants with severe BPD, we cannot make any statements regarding infants with mild to moderate BPD and preterm infants without BPD. More research on PH and the cardiac consequences later in life for prematurely born children with and without BPD and healthy controls born at term is needed to determine prevention and treatment strategies.
In conclusion, the prevalence of echocardiographic signs of PH in preterm infants with severe BPD was 12% at the median corrected age of six months. Although the clinical and echocardiographic features in these infants with PH seems relatively mild, they show signs of decreased RV function assessed by RV fractional area change and RV global longitudinal strain compared to infants with severe BPD without PH and compared to term healthy controls. Our study suggests that BPD-associated PH might leave a negative impact on the RV function, and that the mechanisms of RV adaptation in these premature born infants are different from RV adaptation in children and adults with increased RV afterload caused by IPAH or pulmonary valve stenosis.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental Material
Supplemental Material for Right ventricular function in infants with bronchopulmonary dysplasia and pulmonary hypertension: a pilot study by Arabella J. Blanca, Liesbeth Duijts, Esther van Mastrigt, Marielle W. Pijnenburg, Derk-Jan D. Ten Harkel, Willem A. Helbing, Beatrijs Bartelds, Irwin Reis and Laurens P. Koopman in Pulmonary Circulation
References
- 1.Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013; 128(7): 713–720. [DOI] [PubMed] [Google Scholar]
- 2.Zivanovic S, Pushparajah K, Calvert S, et al. Pulmonary artery pressures in school-age children born prematurely. J Pediatr 2017; 191: 42–49. [DOI] [PubMed] [Google Scholar]
- 3.Goss KN, Beshish AG, Barton GP, et al. Early pulmonary vascular disease in young adults born preterm. Am J Respir Crit Care Med 2018. doi: 10.1164/rccm.201710-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379(9815): 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grégoire M-C, Lefebvre F, Glorieux J. Health and developmental outcomes at 18 months in very preterm infants with bronchopulmonary dysplasia. Pediatrics 1998; 101(5): 856–860. [DOI] [PubMed] [Google Scholar]
- 6.Soudee S, Vuillemin L, Alberti C, et al. Fetal growth restriction is worse than extreme prematurity for the developing lung. Neonatology 2014; 106(4): 304–310. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126(3): 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007; 120(6): 1260–1269. [DOI] [PubMed] [Google Scholar]
- 9.Kim GB. Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean J Pediatr 2010; 53(6): 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276(7): 357–368. [DOI] [PubMed] [Google Scholar]
- 11.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010; 40(3): 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat R, Salas AA, Foster C, et al. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012; 129(3): e682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Check J, Gotteiner N, Liu X, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol 2013; 33(7): 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy PT, El-Khuffash A, Patel MD, et al. Maturational patterns of systolic ventricular deformation mechanics by two-dimensional speckle-tracking echocardiography in preterm infants over the first year of age. J Am Soc Echocardiogr 2017; 30(7): 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirza H, Ziegler J, Ford S, et al. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr 2014; 165(5): 909–914. [DOI] [PubMed] [Google Scholar]
- 16.Mourani PM, Sontag MK, Younoszai A, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015; 191(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaughter JL, Pakrashi T, Jones DE, et al. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011; 31(10): 635–640. [DOI] [PubMed] [Google Scholar]
- 18.Stuart BD, Sekar P, Coulson JD, et al. Health-care utilization and respiratory morbidities in preterm infants with pulmonary hypertension. J Perinatol 2013; 33(7): 543–547. [DOI] [PubMed] [Google Scholar]
- 19.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr 2013; 25(3): 329–337. [DOI] [PubMed] [Google Scholar]
- 20.Collaco JM, Romer LH, Stuart BD, et al. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol 2012; 47(11): 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30(20): 2493–2537. [DOI] [PubMed] [Google Scholar]
- 22.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008; 121(2): 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens LL, Friedberg MK. Imaging the right ventricle–current state of the art. Nat Rev Cardiol 2010; 7(10): 551–563. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Kim HS, Choi CW, et al. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology 2012; 101(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 25.Klitsie LM, Roest AA, van der Hulst AE, et al. Assessment of intraventricular time differences in healthy children using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2013; 26(6): 629–639. [DOI] [PubMed] [Google Scholar]
- 26.Colan SD. Normal echocardiographic values for cardiovascular structures. In: Lai WW, Mertens LL, Cohen MS, et al. (eds) Echocardiography in Pediatric and Congenital Heart Disease. Chichester: Wiley-Blackwell, 2009.
- 27.Koopman LP, Slorach C, Manlhiot C, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr 2011; 24(1): 37–44. [DOI] [PubMed] [Google Scholar]
- 28.Levy PT, Patel MD, Groh G, et al. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J Am Soc Echocardiogr 2016; 29(11): 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koestenberger M, Grangl G, Avian A, et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ Cardiovasc Imaging 2017; 10(1): e005336. [DOI] [PubMed] [Google Scholar]
- 30.Levy PT, Patel MD, Choudhry S, et al. Evidence of echocardiographic markers of pulmonary vascular disease in asymptomatic infants born preterm at one year of age. J Pediatr 2018; 197: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali Z, Schmidt P, Dodd J, et al. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan Med J 2013; 60(8): A4688. [PubMed] [Google Scholar]
- 32.Levy PT, Dioneda B, Holland MR, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr 2015; 28(5): 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driessen MM, Hui W, Bijnens BH, et al. Adverse ventricular-ventricular interactions in right ventricular pressure load: Insights from pediatric pulmonary hypertension versus pulmonary stenosis. Physiol Rep 2016; 4(11): e12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28(10): 1250–1257. [DOI] [PubMed] [Google Scholar]
- 35.Jurcut R, Giusca S, Ticulescu R, et al. Different patterns of adaptation of the right ventricle to pressure overload: a comparison between pulmonary hypertension and pulmonary stenosis. J Am Soc Echocardiogr 2011; 24(10): 1109–1117. [DOI] [PubMed] [Google Scholar]
- 36.Mahfouz RA, Moustafa TM, Gouda M, et al. Longitudinal function and ventricular dyssynchrony are restored in children with pulmonary stenosis after percutaneous balloon pulmonary valvuloplasty. Int J Cardiovasc Imaging 2017; 33(4): 533–538. [DOI] [PubMed] [Google Scholar]
- 37.Czernik C, Rhode S, Helfer S, et al. Development of left ventricular longitudinal speckle tracking echocardiography in very low birth weight infants with and without bronchopulmonary dysplasia during the neonatal period. PLoS One 2014; 9(9): e106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helfer S, Schmitz L, Buhrer C, et al. Tissue Doppler-derived strain and strain rate during the first 28 days of life in very low birth weight infants. Echocardiography 2014; 31(6): 765–772. [DOI] [PubMed] [Google Scholar]
- 39.James AT, Corcoran JD, Breatnach CR, et al. Longitudinal assessment of left and right myocardial function in preterm infants using strain and strain rate imaging. Neonatology 2016; 109(1): 69–75. [DOI] [PubMed] [Google Scholar]
- 40.Lewandowski AJ, Augustine D, Lamata P, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013; 127(2): 197–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Right ventricular function in infants with bronchopulmonary dysplasia and pulmonary hypertension: a pilot study by Arabella J. Blanca, Liesbeth Duijts, Esther van Mastrigt, Marielle W. Pijnenburg, Derk-Jan D. Ten Harkel, Willem A. Helbing, Beatrijs Bartelds, Irwin Reis and Laurens P. Koopman in Pulmonary Circulation