Abstract
Regression of pulmonary hypertension (PH) is often incomplete after successful left-sided valve replacement (LSVR). Proximal pulmonary arterial (PPA) wall disease can be involved in patients with persistent-PH after LSVR, affecting the right ventricular to pulmonary arterial (RV-PA) coupling. Fifteen patients underwent successful LSVR at least one year ago presenting PH by echo (> 50 mmHg). Prosthesis-patient mismatch and left ventricular dysfunction were discarded. All patients underwent hemodynamic and intravascular ultrasound (IVUS) study. We estimated PPA stiffness (elastic modulus [EM]) and the relative area wall thickness (AWT). Acute vasoreactivity was assessed by inhaled nitric oxide (iNO) testing. RV-PA coupling was estimated by the tricuspid annular plane systolic excursion to systolic pulmonary arterial pressure ratio. Patients were classified as isolated post-capillary PH (Ipc-PH; pulmonary vascular resistance [PVR] ≤ 3 WU and/or diastolic pulmonary gradient [DPG] < 7 mmHg) and combined post- and pre-capillary PH (Cpc-PH; PVR > 3 WU and DPG ≥ 7 mmHg). Both Ipc-PH and Cpc-PH showed a significant increase of EM and AWT. Despite normal PVR and DPG, Ipc-PH had a significant decrease in pulmonary arterial capacitance and RV-PA coupling impairment. Cpc-PH had worse PA stiffness and RV-PA coupling to Ipc-PH (P < 0.05). iNO decreased RV afterload, improving the cardiac index and stroke volume only in Cpc-PH (P < 0.05). Patients with persistent PH after successful LSVR have PPA wall disease and RV-PA coupling impairment beyond the hemodynamic phenotype. Cpc-PH is responsive to iNO, having the worse PA stiffness and RV-PA coupling. The PPA remodeling could be an early event in the natural history of PH associated with left heart disease.
Keywords: pulmonary hypertension, pulmonary arterial wall, left-sided valve replacement, right ventricular to pulmonary arterial coupling, inhaled nitric oxide
Pulmonary hypertension associated with left heart disease (PH-LHD) is the most common type of PH and is independently associated with higher hospitalizations and reduced survival.1 PH affects virtually all patients with severe symptomatic mitral valve disease and up to 65% of those with symptomatic aortic stenosis.2 After successful correction of left-sided valve disease, regression of PH is often incomplete despite normal left ventricular function and absence of prosthetic valve dysfunction. Persistent PH is a risk factor for poor outcomes after left-sided valve replacement (LSVR).2–4
Over the years, different hemodynamic criteria to define the hemodynamic phenotypes of PH-LHD have been used.5 Nowadays, despite the recent guidelines published on the subject, the best combination of hemodynamic parameters to define post-capillary PH phenotypes and its accuracy to predict the severity and reversibility of pulmonary vascular disease (PVD) in patients with PH-LHD continues to be a matter of debate.6 PVD is initially characterized by enlarged and thickened pulmonary veins and pulmonary capillary dilatation, associated with interstitial edema, alveolar hemorrhage and lymphatic vessels enlargement, as a consequence of the upstream transmission of elevated left atrial pressure. In some cases, the pre-capillary circulation may also be involved at the level of distal muscular pulmonary arteries (PAs) (diameter ≤ 500 µm) characterized by different degrees of obstructive remodeling such as medial hypertrophy and intimal fibrosis and proliferation.7,8
We have recently shown that the significant increase of proximal elastic PA (diameter ∼ 2 mm) stiffness and the relative area of wall thickness (AWT) can occur early at a preclinical stage in chronic obstructive pulmonary disease (COPD) and interstitial lung disease candidates for lung transplantation, even when mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR) are normal.9 We hypothesized that proximal PAs are involved in the PVD in patients with persistent PH after LSVR. We aimed to assess the proximal PA stiffness and AWT, and its role on the right ventricular to pulmonary arterial (RV-PA) coupling according to the hemodynamic phenotypes in patients with persistent PH after LSVR and to analyze the acute effects of inhaled nitric oxide (iNO).
Methods
We included 15 consecutive stable adult outpatients diagnosed with persistent PH at least one year after successful mitral valve replacement and normal left ventricular ejection fraction (LVEF; March 2012–October 2014). Persistent PH was screened by follow-up echo (systolic PAP [sPAP] > 50 mmHg) and confirmed by catheterization (mPAP ≥ 25 mmHg). The presence of other forms of PH (PH related to diffuse lung disease and chronic thromboembolic PH) were excluded (lung function tests, multidetector computed tomography [CT], ventilation-perfusion lung scintigraphy, pulmonary angiography). The research protocol was approved by the Institutional Ethics Committee of the Hospital Universitari Vall d’Hebron, Barcelona (EudraCT no. 2009-012005-19). Written informed consent was obtained from patients in accordance with the Declaration of Helsinki.
Baseline data of the patients were compared with a historical cohort of 10 control individuals who underwent cardiac catheterization for investigation of dyspnea of unknown origin or suspected PH who otherwise had no apparent disease affecting the lungs or the heart following diagnostic assessment.9
Echocardiography study
We used a Vivid 7 system (2.5–3.5 MHz, GE, Spain). We discarded hemodynamically significant residual prosthesis dysfunction or patient-prosthesis mismatch according to current practice guidelines.10 We obtained LVEF (Simpson method, two-dimensional echocardiography in apical four-chamber view) and left atrial area index. sPAP was determined from the peak tricuspid regurgitation jet velocity using the simplified Bernoulli equation and adding this value with an estimate of the right atrial pressure by the diameter and collapsibility of the inferior vena cava.11 We evaluated RV-PA coupling by the ratio of tricuspid annular plane systolic excursion (TAPSE) to sPAP.12
Hemodynamic and intravascular ultrasound measurements
All patients underwent a routine right heart catheterization (RHC; 7 F Swan-Ganz catheter) and simultaneous intravascular ultrasound study (IVUS; Opticross-40 MHz, Boston, MA, USA) after echocardiography. None of the patients received pulmonary vasodilators at the time of the catheterization. Cardiac output (CO) was determined by the Fick method. Pressure measurements were taken at short breath-hold at end-expiration. We used the end-expiratory automated digital mean measurements across the cardiac cycle to estimate pulmonary arterial occlusion pressure (mPAOP).13,14 Transpulmonary gradient (TPG) was calculated as the difference between mPAP and mPAOP. Diastolic pulmonary gradient (DPG) was calculated by subtracting mPAOP from the diastolic PAP (dPAP).
We estimated RV afterload by PVR, total pulmonary resistance (TPR), effective arterial elastance (Ea), and pulmonary arterial compliance (PAC) through the following equations:13,15
| (1) |
| (2) |
| (3) |
| (4) |
where pPAP and SV correspond to pulsatile pulmonary arterial pressure and stroke volume, respectively.
The RC-time was estimated as the product of PVR and PAC, and total systemic vascular resistance was assessed as mean aortic pressure divided by CO.15
Local proximal PA stiffness (Peterson elastic modulus [EM]) and AWT were assessed by the following equations:9,16
| (5) |
| (6) |
Both outer and intimal areas were measured at T-wave onset on the electrocardiogram (diastolic phase) by the external and luminal wall borders, respectively. The use of the relative area wall thickness could allow a more accurate assessment of the anatomical remodeling than the use of the thickness-diameter ratio because of the section of the PA is not necessarily circular. Intra- and inter-observer validation of IVUS measurements in our laboratory have been previously published.17 Post-capillary PH (mPAP ≥ 25 mmHg, mPAOP > 15 mmHg) was classified as either isolated post-capillary PH (Ipc-PH, DPG < 7 mmHg and/or PVR ≤ 3 Wu), or combined post- and pre-capillary PH (Cpc-PH, DPG ≥ 7 mmHg and PVR > 3 Wu).13,18 Both hemodynamic and IVUS measurements were made at baseline breathing air and during breathing 20 ppm NO gas (iNO, INOMAX DSIR system) for 10 min.
Statistical analysis
Continuous data were expressed as the mean ± standard error (SE). Categorical data were expressed as numbers and percentages. We determined the normal distribution of the data by the Kolmogorov–Smirnov test. Independent sample t-tests were used to examine differences between the control and PH groups; paired t-tests were used to compare the effects of iNO within each post-capillary PH group. The Chi-squared test was used for comparing proportions of patients. Intergroup variation was analyzed using one-way analysis of variance (ANOVA). The strength and direction of the association between continuous variables were measured with Pearson’s correlation coefficient. We used a four-quadrant scatter plot to compare the concordance rate of percent variation of mPAP and EM after iNO. The concordance rate was defined as the percentage of the number of data points that are in two of the four quadrants of agreement (upper right and lower left). Although there is no guidance on suitable exclusion zones, we applied an exclusion zone when the percentage of change of data was < 15%.19 The concordance rate is acceptable in the range of 92–100%. A P value (two-tailed) < 0.05 was regarded as significant. Statistical analyses were performed with SPSS Statistics (Version 21.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Clinical and demographic data are shown in Table 1. All patients with persistent PH after LSVR had control rate atrial fibrillation. Six patients (40%) had a concomitant aortic valve replacement, two in the Ipc-PH group (33%) and four in the Cpc-PH group (67%). All patients had > 3 years of their left-sided valve disease. LVEF and left atrial area index were 61 ± 2% and 23.8 ± 1.8 cm2/m2, respectively, without significant differences between the hemodynamic phenotypes.
Table 1.
Demographics and clinical data in patients with persistent PH after left-sided valve replacement (according to the hemodynamic phenotypes) and control individuals.
| Ipc-PH (n = 6) | Cpc-PH (n = 9) | Control (n = 10) | |
|---|---|---|---|
| Baseline clinical data | |||
| Age (years) | 72 ± 3 | 71 ± 5 | 51 ± 4*,† |
| Sex (female/male) | 5/1 | 8/1 | 6/4 |
| Body surface area (m2) | 1.69 ± 0.08 | 1.71 ± 0.05 | 1.8 ± 0.04 |
| WHO FC III–IV (n (%)) | 3 (50) | 7 (78) | 0 (0) |
| MVR (mechanical/biological) | 6/0 | 6/3 | 0 |
| AVR (mechanical/biological) | 2/0 | 3/1 | 0 |
| Atrial fibrillation (n (%)) | 6 (100) | 9 (100) | 0 (0) |
| Medical history (n (%)) | |||
| Hypertension | 2 (33) | 2 (22) | 3 (30) |
| Diabetes | 1 (17) | 2 (22) | 0 (0) |
| Coronary arterial disease | 0 (0) | 0 (0) | 0 (0) |
| Medication (n (%)) | |||
| Oral anticoagulation | 6 (100) | 9 (100) | 0 (0) |
| Loop diuretics | 6 (100) | 9 (100) | 0 (0) |
| ACE inhibitors or ARBs | 5 (83) | 8 (77) | 0 (0) |
| MR antagonists | 2 (33) | 5 (56) | 0 (0) |
Data are presented as mean ± SE unless otherwise specified.
P < 0.05 vs. Cpc-PH
P < 0.05 vs. Ipc-PH.
AVR/MVR, aortic/mitral valve replacement; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; Cpc-PH/Ipc-PH, combined pre- and post-capillary/isolated post-capillary pulmonary hypertension; MR, mineralocorticoid receptor; WHO FC, World Health Organization functional class.
Both Ipc-PH and Cpc-PH patients were older than the control group (P < 0.05), but with similar proportion of women. The proportion of patients in World Health Organization (WHO) functional class (FC) III–IV did not show significant differences between both groups.
Pulmonary hemodynamics and pulmonary arterial wall disease
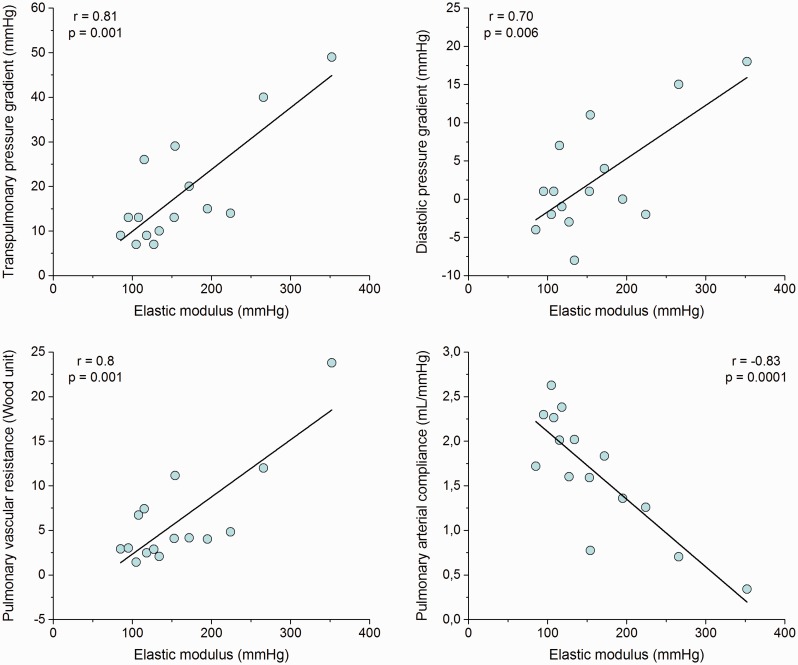
Both Ipc-PH and Cpc-PH showed a significant increase of EM and AWT. Cpc-PH had worse PA stiffness with respect to Ipc-PH (P < 0.05). By contrast, there were no significant differences in AWT between Ipc-PH and Cpc-PH patients (Table 2). All hemodynamic criteria used to identify a relevant pre-capillary component (DPG, TPG, PAC, and PVR) were significantly associated with EM (Fig. 1). RC-time was significantly shorter in both PH groups compared to control individuals; Ipc-PH patients had the shortest values (P < 0.05) (Table 2). Moreover, RC-time decreases with increasing occlusion pressure in Ipc-PH and Cpc-PH (r = –0.71, P < 0.05).
Table 2.
Hemodynamic and IVUS data in patients with persistent PH after left-sided valve replacement (according to the hemodynamic phenotypes) and control individuals.
| All (n = 15) | Ipc-PH (n = 6) | Cpc-PH (n = 9) | Control (n = 10) | P 1–2 * | P 2–3 † | P 1–3 ‡ | P § | |
|---|---|---|---|---|---|---|---|---|
| mPAP (mmHg) | 39 ± 3 | 32 ± 2 | 44 ± 4 | 15 ± 2 | 0.016 | 0.000 | 0.000 | 0.000 |
| pPAP (mmHg) | 40 ± 5 | 30 ± 2 | 46 ± 8 | 11 ± 3 | 0.076 | 0.002 | 0.000 | 0.000 |
| CI (L/min/m2) | 2.1 ± 0.1 | 2.3 ± 0.2 | 2.0 ± 0.1 | 2.6 ± 0.1 | 0.3 | 0.006 | 0.18 | 0.01 |
| SV (mL) | 55 ± 3 | 62 ± 3 | 50 ± 4 | 65 ± 1 | 0.04 | 0.004 | 0.33 | 0.002 |
| HR (bpm) | 66 ± 3 | 62 ± 6 | 68 ± 4 | 73 ± 1 | 0.39 | 0.24 | 0.14 | 0.153 |
| RAP (mmHg) | 12 ± 2 | 12 ± 2 | 11 ± 2 | 5 ± 1 | 0.79 | 0.000 | 0.000 | 0.000 |
| mPAOP (mmHg) | 21 ± 0.8 | 23 ± 2 | 19 ± 0.5 | 8.3 ± 0.6 | 0.09 | 0.000 | 0.000 | 0.000 |
| DPG (mmHg) | 3.9 ± 2.2 | −2.8 ± 1.2 | 9.1 ± 2.9 | 2.7 ± 1 | 0.004 | 0.054 | 0.003 | 0.003 |
| TPG (mmHg) | 20 ± 3 | 9 ± 1 | 27 ± 4 | 14 ± 2 | 0.003 | 0.001 | 0.11 | 0.000 |
| TPR (WU) | 12 ± 2 | 8.7 ± 1.1 | 14.2 ± 2.4 | 3.3 ± 0.3 | 0.048 | 0.002 | 0.003 | 0.000 |
| PVR (WU) | 6.2 ± 1.5 | 2.5 ± 0.3 | 9.0 ± 2.2 | 2.8 ± 0.3 | 0.018 | 0.022 | 0.528 | 0.004 |
| PAC (mL/mmHg) | 1.7 ± 0.2 | 2.1 ± 0.2 | 1.4 ± 0.2 | 6.2 ± 0.4 | 0.024 | 0.000 | 0.000 | 0.000 |
| Ea (mmHg/mL) | 0.77 ± 0.11 | 0.52 ± 0.03 | 0.95 ± 0.15 | 0.23 ± 0.05 | 0.024 | 0.002 | 0.000 | 0.000 |
| RC-time (s) | 0.45 ± 0.06 | 0.3 ± 0.03 | 0.56 ± 0.07 | 1.1 ± 0.1 | 0.009 | 0.003 | 0.000 | 0.000 |
| EM (mmHg) | 165 ± 19 | 114 ± 8 | 193 ± 26 | 21 ± 6 | 0.014 | 0.000 | 0.000 | 0.000 |
| AWT (%) | 22 ± 1 | 22 ± 0.9 | 22 ± 1.7 | 1.4 ± 1 | 0.96 | 0.000 | 0.000 | 0.000 |
Data are presented as mean ± SE.
P value between Ipc-PH and Cpc-PH.
P value between Cpc-PH and control.
P value between Ipc-PH and control.
P value analyzed by one-way ANOVA.
AWT, relative area wall thickness; CI, cardiac index; Cpc-PH/Ipc-PH, combined pre- and post-capillary/isolated post-capillary pulmonary hypertension; DPG, diastolic pressure gradient; Ea, effective arterial elastance; EM, elastic modulus; HR, heart rate; mPAP/pPAP, mean/pulsatile pulmonary arterial pressure; PAC, pulmonary arterial compliance; mPAOP, mean pulmonary arterial occlusion pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SV, stroke volume; TPG, transpulmonary pressure gradient; TPR, total pulmonary resistance.
Fig. 1.
Scatter plot of transpulmonary pressure gradient, diastolic pulmonary gradient, pulmonary vascular resistance, and pulmonary arterial compliance with elastic modulus.
We obtained negative DPG values in 6/15 (40%) patients (one Cpc-PH and five Ipc-PH patients).
Right ventricular to pulmonary arterial coupling
Of the patients with persistent PH after LSVR, 87% showed severe impairment of RV-PA coupling (≤0.36). The RV-PA coupling was worse in Cpc-PH than Ipc-PH patients (0.21 ± 0.02 vs. 0.32 ± 0.03; P < 0.05). Although all Ipc-PH patients had a PVR ≤ 3 WU and a DPG < 7 mmHg, the RV-PA coupling was impaired in association with an increase of proximal PA wall stiffness and AWT, and a decrease of PAC (P < 0.05). TAPSE/sPAP ratio was significantly correlated with PAC (r = 0.62), PVR (r = –0.76), DPG (r = –0.8), and EM (r = –0.6) (Suppl. Fig. 1). Patients with TAPSE/sPAP values lower than the median (≤0.23 mm/mmHg) had a higher RC-time than those who presented a more preserved TAPSE/sPAP ratio (0.63 ± 0.09 s vs. 0.34 ± 0.02 s, P < 0.01).
Response to iNO
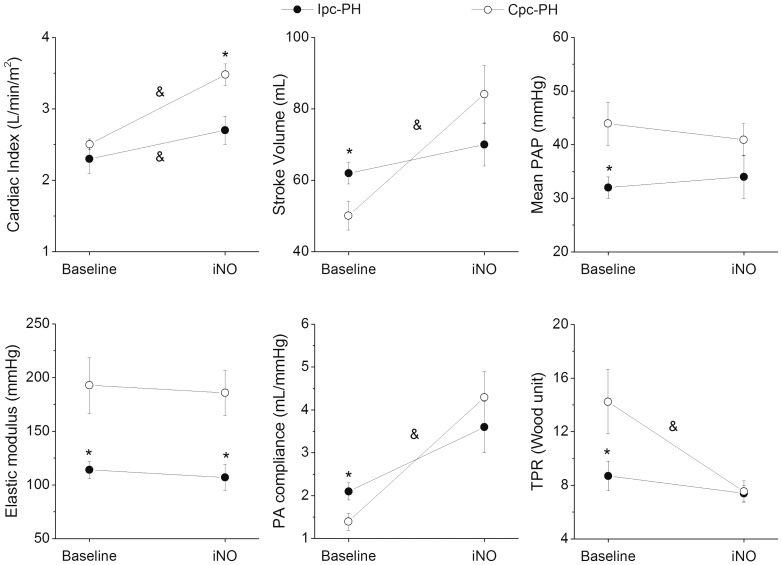
Patients with Cpc-PH showed a significant decrease of RV afterload (Ea decrease of 38 ± 10 %) during inhalation of NO (Table 3). Moreover, they showed significant improvements in cardiac index, SV, PAC, and TPR (Fig. 2, Table 3). One Cpc-PH patient (11%) fulfilled the classic “hemodynamic responder” criteria (mPAP dropped by ≥ 10 mmHg and ≤ 40 mmHg without decrease of CO) and another Cpc-PH patient had a fall in mPAP of ≥ 10 mmHg but not ≤ 40 mmHg (non-classical response).13 The reduction of total systemic vascular resistance (P < 0.05) despite mild mean aortic pressure elevation is associated with the significant increase of cardiac index (Table 3). In contrast, only an isolated mild increase in cardiac index occurred in Ipc-PH patients (P < 0.05) (Fig. 2).
Table 3.
Relative changes in hemodynamic and IVUS data after vasodilator challenge in patients with persistent PH after left valve replacement according to the hemodynamic phenotypes.
| Ipc-PH (n = 6) | Cpc-PH (n = 9) | |
|---|---|---|
| ΔmPAo | 14.6 ± 4.8 | 12 ± 4.2 |
| ΔTSVR | –2.9 ± 9.1* | –29.3 ± 8.7 |
| ΔmPAP | 5.8 ± 9.9 | –5.3 ± 6.3 |
| ΔpPAP | –15.9 ± 8.1 | 7.9 ± 11.1 |
| ΔCI | 26.7 ± 9.7* | 77.7 ± 16 |
| ΔSV | 14.4 ± 8.9* | 76.1 ± 21 |
| ΔHR | 7.7 ± 7.4 | 1.3 ± 3.6 |
| ΔTPR | –10 ± 11.7* | –39.7 ± 8.4 |
| ΔPAC | 79 ± 29* | 390 ± 164 |
| ΔEa | –0.02 ± 0.11* | –0.38 ± 0.1 |
| ΔTPR/TSVR | –8.3 ± 5.7 | –15.7 ± 4.1 |
| ΔEM | –4.1 ± 8.6 | 3.8 ± 13 |
Data are presented as mean ± SE of percent variation after vasodilator challenge.
P < 0.05 vs. Cpc-PH.
CI, cardiac index; Cpc-PH/Ipc-PH, combined pre and post-capillary/isolated post-capillary pulmonary hypertension; DPG, diastolic pressure gradient; Ea, effective arterial elastance; EM, elastic modulus; HR, heart rate; mPAP/pPAP, mean/pulsatile pulmonary arterial pressure; mPAo, mean aortic pressure; PAC, pulmonary arterial compliance; SV, stroke volume; TPR, total pulmonary resistance; TSVR, total systemic vascular resistance.
Fig. 2.
Changes in hemodynamic parameters and elastic modulus after inhaled nitric oxide (iNO) in combined post-capillary and pre-capillary pulmonary hypertension (Cpc-PH) and isolated post-capillary pulmonary hypertension (Ipc-PH) patients. mPAP, mean pulmonary arterial pressure; PA compliance, pulmonary arterial compliance; TPR, total pulmonary resistance. *P < 0.05 Ipc-PH vs. Cpc-PH within the same state; &P < 0.05 baseline vs. iNO within the same group.
Mean values of EM did not change during inhalation of NO, either in Cpc-PH or Ipc-PH patients (Fig. 2).
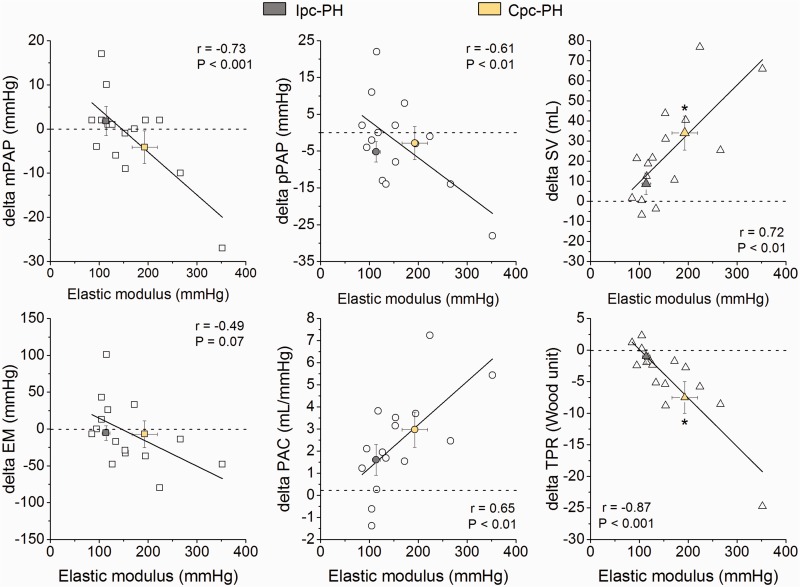
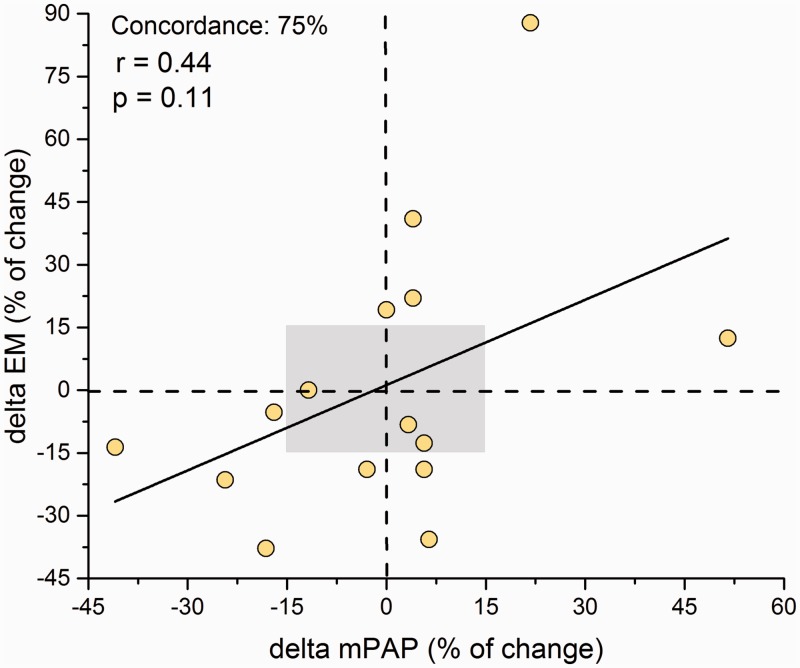
The absolute change in mPAP, pPAP, SV, PAC, and TPR after iNO administration correlated significantly with baseline EM (Fig. 3). To analyze the influence of the distending pressure (mPAP) on PA stiffness, we plotted the percentage of change of mPAP and the percentage of change of EM after iNO administration. A poor concordance rate was obtained (75%) without a significant correlation (r = 0.44; P = 0.11) (Fig. 4).
Fig. 3.
Correlations between absolute changes in hemodynamic parameters and elastic modulus (EM) after iNO and baseline EM. Cpc-PH/Ipc-PH, combined pre and post-capillary/isolated post-capillary pulmonary hypertension; mPAP/pPAP, mean/pulsatile pulmonary arterial pressure; PAC, pulmonary arterial compliance; SV, stroke volume; TPR, total pulmonary resistance. Delta is computed as after NO minus before NO administration (baseline) values. *P < 0.05 Ipc-PH vs. Cpc-PH.
Fig. 4.
Correlation and concordance rate between the percentage of change after iNO of mean pulmonary arterial pressure (mPAP) and elastic modulus (EM). Gray area: area of data exclusion.
Discussion
Persistent PH after successful LSVR shows different hemodynamic phenotypes which cannot be identified by echo-Doppler follow-up. All patients with persistent PH after successful LSVR showed significant proximal elastic PA wall disease regardless of the hemodynamic phenotypes. Cpc-PH patients showed the lowest PAC, and the highest PVR, RC-time, and PA stiffness which was associated with the worst RV-PA coupling. The proximal PAs remodeling already present in Ipc-PH patients would be related to the decrease in PAC leading to the impairment of the RV-PA coupling.
PVD in PH-LHD in patients with heart failure is associated with a prevalence of pulmonary veins and capillary remodeling.20 In addition, histological data support that distal muscular PAs may also be involved.7 Accordingly, Gerges et al. using the pulmonary artery occlusion technique to analyze the PVR partitioning pattern in heart failure, have reported that upstream resistance is significantly lower in Cpc-PH than in Ipc-PH patients, consistent with the presence of pre-capillary PVD in Cpc-PH.21 The development of intravascular imaging modalities provides a real-time in vivo characterization of proximal elastic PA wall (∼2–3 mm in diameter) in the PH workup.22 We have shown in COPD and interstitial lung disease candidates for lung transplantation that patients with normal mPAP have a significant increase of EM.9 Jorge et al. reported a high thickness–diameter ratio (13 ± 1.6%) in patients with severe mitral valve disease and with a predominantly Ipc-PH phenotype.23 In the present study, we showed that even Ipc-PH patients with PVR and DPG within normal limits have a significant anatomical (AWT) and functional (EM) proximal PA wall disease, suggesting the presence of early PVD and questioning the definition of “passive” PH-LHD. Local arterial stiffness reflects the increase in arterial blood pressure per relative increase in arterial area (strain). Although EM (ratio between pressure to strain) is not equivalent to the incremental (Young) elastic modulus (ratio between stress to strain and the gold standard for the assessment of the elastic properties of a vessel wall) since it lacks thickness data, it is a valid approximation.16 Because the arterial wall exhibits a non-linear, viscoelastic behavior, PA stiffening is often viewed as a consequence of elevated mPAP (distended arterial pressure) and thus, of the artery operating on a steeper part of its pressure–volume relationship. However, chronic stiffening due to wall thickening and vascular remodeling of the extracellular matrix, with loss of elastin and increase in collagen content may be involved.24 The significant increase of AWT and the absence of correlation plus the poor concordance rate between relative changes of mPAP and EM after iNO administration may explain the proximal PA stiffening for intrinsic wall remodeling.
Multiple hemodynamic factors with the underlying PVD are involved in the RV-PA uncoupling in PH-LHD. It is well known that PH is associated with an early and progressive decrease in PAC and that the impairment of PAC occurs even when PVR is normal and is an independent predictor of mortality of patients with heart failure with preserved ejection fraction.25,26 In the natural history of PH-LHD, we can speculate that the presence of proximal PA wall disease in addition with the abnormal pulsatile loading component due to upstream transmission of elevated left atrial pressure, may explain the significant lower PAC and impairment of RV-PA coupling with normal PVR and DPG in Ipc-PH patients. The elevation of PVR and DPG would occur later with dramatic reductions in PAC associated with distal PVD, as seen in Cpc-PH patients. Current dogma suggests that distal changes in pulmonary vasculature usually precede those associated with stiffening in the proximal vessels, so decreased PAC in PH was thought to be a consequence of small distal vessel proliferative vasculopathy leading to increased PVR and mPAP. However, recent evidence suggests a loss of capacitance in the proximal PAs may actually initiate and promote PH, causing the distal proliferative vasculopathy by increasing flow pulsatility and leading to intimal inflammation and fibrosis, medial hypertrophy, muscularization of arterioles, and adventitial thickening.24,27 The distal and proximal PAs “communicate” with one another in a cycle of positive feedback leading to remodeling progression and stiffening the arterial wall.28 It could be suggested that both the proximal and distal involvement worsens from the Ipc-PH to the Cpc-PH phenotype. Interestingly, the PA wall disease and the hemodynamic profile of Cpc-PH patients are very similar compared to historical patients with PAH.22 Although the cellular and molecular mechanisms that drive the interactions of distal and proximal arterial remodeling in PH remains unclear, the correlation between the changes of the pulmonary hemodynamic parameters after iNO and the baseline proximal PA stiffness could illustrate the close cross-talk between the distal and proximal PAs.27
Finally, the early proximal PA wall remodeling associated with the impairment of RV-PA coupling, together with the parallel effect of the left ventricle to RV through the interventricular septum function, could explain the absence of association of the distal pulmonary vascular remodeling with RV dysfunction in PH-LHD. Bosch et al. reported that RV dysfunction is worse in heart failure with reduced ejection fraction than heart failure with preserved ejection fraction regardless of the degree of PH.29 Based on the helical ventricular myocardial band model proposed by Torrent-Guasp, they suggested a parallel effect of LV and RV dysfunction through the loss of the oblique orientation of the fibers of the apical loop of the helix that constitutes the interventricular septum.30
Despite the successful valve surgery, the patients persisted with high values of mPAOP, which can be explained by the persistent venous and small vessel intimal thickening. This is in accordance with a very recently comprehensive quantitative histomorphometry of pulmonary vessels from patients with diagnosed heart failure.20 The authors proposed a preferential remodeling of pulmonary veins with a pattern similar to patients with pulmonary veno-occlusive disease which was associated with the severity of PH and the presence of RV dysfunction.20
The hemodynamic response of an acute vasodilator challenge in heart failure has often been studied using systemic infusions of nitrates.31,32 The major advantages of iNO to assess acute vasoreactivity are rapid onset, pulmonary specificity, and short duration of action. We documented that only Cpc-PH patients are responsive to iNO, showing significant improvements in RV afterload (Ea, PAC, and TPR) with a significant increase of SV and cardiac index. These findings are in agreement with those of Gerges et al., who showed that patients with Cpc-PH patients improved RV afterload with a concomitant 8% of upstream PVR increase, indicating a decrease of distal vascular resistance.21 It is well known that the vasodilator effects of iNO are strictly limited to small arterioles into which alveolar gas diffuses. Although mean values of EM did not show changes after iNO regardless the hemodynamic phenotype, in the scatter plot some individual patients showed changes in EM as large as 101 and –80 mmHg. This apparent paradox can be explained by the short-term arterial adaptation linked to the vasomotor tone response to changes in transmural pressure (myogenic response) and/or flow (shear stress) modified by iNO.33
This study has several limitations. One limitation relates to the small sample size and, consequently, the reduced study power, which increases the chance of incurring Type II error in comparisons of Cpc-PH and Ipc-PH. However, we only included valve heart disease patients. Those correlations which seem to be driven by extreme data points should be interpreted more conservatively. Assessment of mPAOP is critical to define the hemodynamic phenotype in PH-LHD patients. We used the end-expiratory automated digital mean measurements across the cardiac cycle of mPAOP that may better estimate the pressure to which the pulmonary circulation is submitted.6 Despite the absence of mitral regurgitation, the presence of atrial fibrillation in all patients could explain the negative values of DPG in 40% of patients, which is in accordance with other studies.12,26 Although we used a historical not age-matched control group, the effect of aging in PA stiffness begins to be significant from the age of ∼50 years.34 We did not obtain the mPAOP during iNO; therefore, we could not assess TPG, DPG, and PVR. However, based on previous data, we can speculate an elevation of mPAOP after iNO.21,35,36 Like Gerges et al. mPAP increased in Ipc-PH and decreased in Cpc-PH patients, so that we could raise a decrease of PVR in Cpc-PH during inhalation of NO with a non-significant change in Ipc-PH.21
In summary, patients with persistent PH after successful LSVR have proximal elastic PA wall disease and RV-PA coupling impairment beyond the hemodynamic phenotype. Increased RV afterload is driven not only by the upstream transmission of elevated left atrial pressure but also by a significant increase of proximal PA stiffness and area of wall thickness in Ipc-PH patients with normal PVR and DPG. Cpc-PH represents patients with the worst PVR, PAC, and EM, aggravating the RV-PA coupling. Substantial hemodynamic improvements were observed in Cpc-PH patients after iNO administration. This response is significantly correlated with proximal PA stiffness.
Supplemental Material
Supplemental Material for Proximal pulmonary arterial wall disease in patients with persistent pulmonary hypertension after successful left-sided valve replacement according to the hemodynamic phenotype by Enric Domingo, Juan C. Grignola, Pedro Trujillo, Rio Aguilar and Antonio Roman in Pulmonary Circulation
Acknowledgments
The preliminary data of this study were presented in part in the European Society of Cardiology Congress of Heart Failure and World Congress on Acute Heart Failure Vienna, Austria. Eur J Heart Fail 2018; 20(Suppl. S1): 203, 866; https://doi.org/10.1002/ejhf.1197.
Juan C. Grignola is a researcher of PEDECIBA (Programa para el Desarrollo de las Ciencias Básicas) and of SNI-ANII (Sistema Nacional de Investigadores-Agencia Nacional de Investigación e Innovación).
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research was partially funded by Pfizer, Spain.
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. [DOI] [PubMed] [Google Scholar]
- 2.Magne J, Pibarot P, Sengupta PP, et al. Pulmonary hypertension in valvular disease: A comprehensive review on pathophysiology to therapy from the HAVEC group. JACC Cardiovasc Imaging 2015; 8: 83–99. [DOI] [PubMed] [Google Scholar]
- 3.Martinez C, Bernard A, Dulgheru R, et al. Pulmonary hypertension in aortic stenosis and mitral regurgitation: Rest and exercise echocardiography significance. Prog Cardiovasc Dis 2016; 59: 59–70. [DOI] [PubMed] [Google Scholar]
- 4.Murashita T, Okada Y, Kanemitsu H, et al. The impact of preoperative and postoperative pulmonary hypertension on long-term surgical outcome after mitral valve repair for degenerative mitral regurgitation. Ann Thorac Cardiovasc Surg 2015; 21: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: Pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017; 69: 1718–1734. [DOI] [PubMed] [Google Scholar]
- 6.Naeije R, Gerges M, Vachiery JL, et al. Hemodynamic phenotyping of pulmonary hypertension in left heart failure. Circ Heart Fail 2017; 10: e004082. [DOI] [PubMed] [Google Scholar]
- 7.Delgado JF, Conde E, Sanchez V, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 2005; 7: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 8.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: A predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 9.Domingo E, Grignola JC, Aguilar R, et al. Pulmonary arterial wall disease in COPD and interstitial lung diseases candidates for lung transplantation. Respir Res 2017; 18: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 589–590. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713; quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: Stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017; 10: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 14.Houston BA, Tedford RJ. What we talk about when we talk about the wedge pressure. Circ Heart Fail 2017; 10: e004450. [DOI] [PubMed] [Google Scholar]
- 15.Morimont P, Lambermont B, Ghuysen A, et al. Effective arterial elastance as an index of pulmonary vascular load. Am J Physiol Heart Circ Physiol 2008; 294: H2736–2742. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 17.Grignola JC, Domingo E, Aguilar R, et al. Acute absolute vasodilatation is associated with a lower vascular wall stiffness in pulmonary arterial hypertension. Int J Cardiol 2013; 164: 227–231. [DOI] [PubMed] [Google Scholar]
- 18.Gerges M, Gerges C, Lang IM. How to define pulmonary hypertension due to left heart disease. Eur Respir J 2016; 48: 553–555. [DOI] [PubMed] [Google Scholar]
- 19.Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 2010; 111: 1180–1192. [DOI] [PubMed] [Google Scholar]
- 20.Fayyaz AU, Edwards WD, Maleszewski JJ, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 2018; 137: 1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerges C, Gerges M, Fesler P, et al. In-depth haemodynamic phenotyping of pulmonary hypertension due to left heart disease. Eur Respir J 2018; 51: 1800067. [DOI] [PubMed] [Google Scholar]
- 22.Grignola JC, Domingo E, Aguilar R, et al. Assessment of structural and functional pulmonary vascular disease in patients with pulmonary hypertension. In: Preston I, Sulica R. (ed). Pulmonary hypertension - from bench research to clinical challenges, Rijeka, Croatia: InTech Open Access Publisher, 2011, pp. 169–190. [Google Scholar]
- 23.Jorge E, Baptista R, Calisto J, et al. Pulmonary vascular remodeling in mitral valve disease: An optical coherence tomography study. Int J Cardiol 2016; 203: 576–578. [DOI] [PubMed] [Google Scholar]
- 24.Schafer M, Myers C, Brown RD, et al. Pulmonary arterial stiffness: Toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 2016; 18: 4. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Labate V. Pulmonary hypertension in heart failure patients: Pathophysiology and prognostic implications. Curr Heart Fail Rep 2016; 13: 281–294. [DOI] [PubMed] [Google Scholar]
- 26.Al-Naamani N, Preston IR, Paulus JK, et al. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015; 3: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan W, Madhavan K, Hunter KS, et al. Vascular stiffening in pulmonary hypertension: Cause or consequence? (2013 Grover Conference Series). Pulm Circ 2014; 4: 560–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloodworth NC, West JD, Merryman WD. Microvessel mechanobiology in pulmonary arterial hypertension: Cause and effect. Hypertension 2015; 65: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch L, Lam CSP, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017; 19: 1664–1671. [DOI] [PubMed] [Google Scholar]
- 30.Torrent-Guasp F, Kocica MJ, Corno AF, et al. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg 2005; 27: 191–201. [DOI] [PubMed] [Google Scholar]
- 31.Guglin M, Mehra S, Mason TJ. Comparison of drugs for pulmonary hypertension reversibility testing: A meta-analysis. Pulm Circ 2013; 3: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghio S, Crimi G, Temporelli PL, et al. Haemodynamic effects of an acute vasodilator challenge in heart failure patients with reduced ejection fraction and different forms of post-capillary pulmonary hypertension. Eur J Heart Fail 2018; 20: 725–734. [DOI] [PubMed] [Google Scholar]
- 33.Westerhof N, Stergiopulos N and Noble MIM. Mechanotransduction and vascular remodeling. In: Snapshots of hemodynamics: An aid for clinical research. London: Springer, 2010: 197–205.
- 34.Oliveira RK, Agarwal M, Tracy JA, et al. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J 2016; 47: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 35.Preston IR, Sagliani KD, Roberts KE, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide and inhaled epoprostenol in patients with pulmonary hypertension. Pulm Circ 2013; 3: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haraldsson A, Kieler-Jensen N, Nathorst-Westfelt U, et al. Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest 1998; 114: 780–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Proximal pulmonary arterial wall disease in patients with persistent pulmonary hypertension after successful left-sided valve replacement according to the hemodynamic phenotype by Enric Domingo, Juan C. Grignola, Pedro Trujillo, Rio Aguilar and Antonio Roman in Pulmonary Circulation