Abstract
Inflammatory cytokines, including tumor necrosis factor-α (TNFα), were elevated in patients with cardiovascular diseases and are also considered as crucial factors in the pathogenesis of preeclampsia; however, the underlying pathogenic mechanism has not been clearly elucidated. This study provides novel evidence that TNFα leads to endothelial dysfunction associated with hypertension and vascular remodeling in preeclampsia through down-regulation of endothelial nitric-oxide synthase (eNOS) by NF-κB–dependent biogenesis of microRNA (miR)-31-5p, which targets eNOS mRNA. In this study, we found that miR-31-5p was up-regulated in sera from patients with preeclampsia and in human endothelial cells treated with TNFα. TNFα-mediated induction of miR-31-5p was blocked by an NF-κB inhibitor and NF-κB p65 knockdown but not by mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase inhibitors, indicating that NF-κB is essential for biogenesis of miR-31-5p. The treatment of human endothelial cells with TNFα or miR-31-5p mimics decreased endothelial nitric-oxide synthase (eNOS) mRNA stability without affecting eNOS promoter activity, resulting in inhibition of eNOS expression and NO/cGMP production through blocking of the functional activity of the eNOS mRNA 3′-UTR. Moreover, TNFα and miR-31-5p mimic evoked endothelial dysfunction associated with defects in angiogenesis, trophoblastic invasion, and vasorelaxation in an ex vivo cultured model of human placental arterial vessels, which are typical features of preeclampsia. These results suggest that NF-κB–responsive miR-31-5p elicits endothelial dysfunction, hypertension, and vascular remodeling via post-transcriptional down-regulation of eNOS and is a molecular risk factor in the pathogenesis and development of preeclampsia.
Keywords: microRNA (miRNA), endothelial dysfunction, tumor necrosis factor (TNF), inflammation, nitric-oxide synthase, eNOS, miRNA-31-5p, preeclampsia
Introduction
The microRNAs (miRNAs)2 are small noncoding RNAs of ∼22 nucleotides, and their biosynthesis is controlled at multiple levels, such as transcription and nuclear and cytoplasmic processing. Mature miRNAs induce post-transcriptional silencing of target genes by binding to their 3′-untranslated regions (3′-UTR) (1). Like gene expression, miRNA biogenesis is also regulated at the transcriptional level under pathophysiological conditions, including inflammation, and contributes to the pathogenesis of vascular diseases.
The inflammatory response is finely controlled by transcription factors, including NF-κB (NF-κB), to prevent tissue or organ injury; however, prolonged and persistent activation of NF-κB evokes elevated production of cytokines, such as tumor necrosis factor-α (TNFα) and the interleukin (IL) family, which are important risk factors for several human diseases, including cardiovascular disorders and preeclampsia (2, 3), although anti-angiogenic factors, hypoxia, and reactive oxygen species are also involved in the pathogenesis of these diseases (4). Chronic inflammation elicits functional alterations in vascular endothelial cells, which play a key role in regulating vascular relaxation and homeostasis (3, 5). Studies have demonstrated that miRNA biogenesis is stimulated under inflammatory conditions and causes endothelial cell dysfunction, indicating that some miRNAs are risk factors in the pathogenesis of vascular diseases (2, 3, 5).
Preeclampsia is a unique disease occurring after 20 weeks of gestation and is characterized by hypertension with proteinuria. In patients with preeclampsia, circulating levels of proinflammatory cytokines, including TNFα and ILs, are elevated in maternal and cord blood (6) and are associated with endothelial dysfunction (6, 7), resulting in vascular dysfunction associated with hypertension (8, 9). In addition, recent studies have shown that expression levels of some miRNAs were increased in placentas or maternal blood in patients with preeclampsia (3, 10). In fact, miR-155-5p is up-regulated in such patients and endothelial cells treated with TNFα, which impairs vasorelaxation through down-regulation of endothelial nitric-oxide synthase (eNOS) (3). This suggests that miRNAs regulated under inflammatory states are involved in the development of vascular dysfunction.
Nitric oxide (NO) produced by eNOS is a potent vasodilator that contributes to the maintenance of vascular tone and blood pressure. Thus, eNOS-deficient mice spontaneously develop hypertension and defects in vascular remodeling (11, 12). The pathogenic role of NO in vascular abnormalities and a preeclampsia-like phenotype have been demonstrated in mice deficient in eNOS or administered a NOS inhibitor (12, 13). Recent studies showed that eNOS is down-regulated by several miRNAs, which target the 3′-UTR of eNOS mRNA, leading to inhibition of endothelial function and vasorelaxation (7, 10). This suggests that miRNAs targeting the eNOS transcript induce endothelial dysfunction associated with preeclampsia.
In this study, we found that miR-31-5p was up-regulated in an NF-κB–dependent manner in human umbilical vein endothelial cells (HUVECs) stimulated with TNFα and in sera from patients with preeclampsia. This miRNA suppressed eNOS expression by targeting its transcript and subsequently inhibited endothelial function and behavior, which are typical characteristics of preeclampsia. These results indicate that NF-κB–responsive miR-31-5p contributes importantly, but along with other miRNAs including miR-155-5p (3), to preeclamptic hypertension through negative regulation of the eNOS/NO pathway.
Results
miR-31-5p predictively targeting eNOS is up-regulated in TNFα-stimulated HUVECs
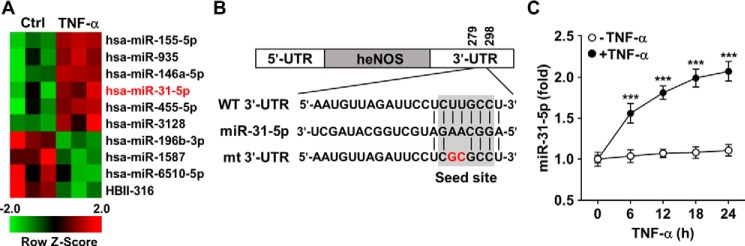
TNFα is up-regulated in patients with atherosclerosis and preeclampsia and induces endothelial dysfunction through biogenesis of miRNAs (3, 10). To identify new miRNAs up-regulated in human endothelial cells under inflammatory conditions, we stimulated HUVECs with 10 ng/ml TNFα for 24 h and analyzed the HUVEC miRNA expression profile using Affymetrix microarrays. Several mRNAs were identified as significantly up- or down-regulated compared with their levels of untreated control cells (Fig. 1A). As shown previously (3, 7, 14), miR-155-5p and miR-146a-5p were up-regulated in response to TNFα. Among the up-regulated miRNAs, miR-31-5p was newly identified as potentially targeting the 3′-UTR of human eNOS mRNA, but not its mutant generated in the seed sequences of the eNOS mRNA 3′-UTR (Fig. 1, A and B). The expression level of miR-31-5p was continuously elevated up to 24 h after stimulation with TNFα (Fig. 1C).
Figure 1.
eNOS mRNA-targeting miR-31-5p is up-regulated in TNFα-stimulated endothelial cells. A, HUVECs were treated with TNFα (10 ng/ml) for 24 h, followed by analysis of miRNA expression profiles using Affymetrix miRNA microarrays (n = 3). Ctrl, control. B, computational target prediction using TargetScan showed complementarity between miR-31-5p and the 3′-UTR of human eNOS and generation of its 3′-UTR mutant (mt). Wildtype (WT) and mutant eNOS mRNA 3′-UTRs were cloned into the psiCHECK-2 vector. C, time-dependent expression of miR-31-5p in HUVECs stimulated with TNFα was determined at the indicated time periods by qRT-PCR (n = 5). ***, p < 0.001.
miR-31-5p is elevated in patients with preeclampsia and is induced by TNFα-mediated NF-κB activation
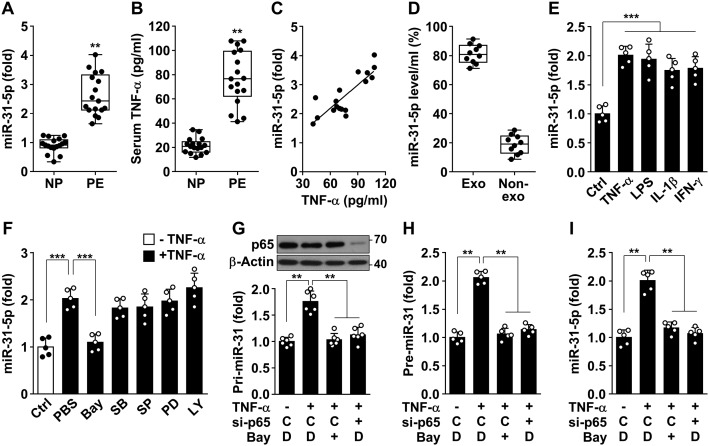
We examined biogenic regulation of miR-31-5p in patients with preeclampsia and endothelial cells under inflammatory conditions. miR-31-5p levels increased by 2.7-fold in the sera of patients with preeclampsia compared with those in the sera of healthy pregnant women (Fig. 2A), whose clinical characteristics are summarized in Table S1. Serum levels of TNFα were also elevated in the patients compared with those in normal pregnant women (76.9 ± 5.7 versus 21.5 ± 1.6 pg/ml) (Fig. 2B), and the TNFα levels were highly correlated with miR-31-5p levels in the sera of the patients (Fig. 2C). Approximately 80% of total miR-31-5p in sera from preeclamptic patients was found in the exosomes, and the rest was present in the nonexosomal fraction (Fig. 2D), which is consistent with a previous report (15). Serum levels of miR-31-5p were also elevated in patients with atherosclerosis (Fig. S1). We next examined whether TNFα regulates miR-31-5p biogenesis and eNOS expression in endothelial cells. Stimulation of HUVECs with 0.05–10 ng/ml TNFα increased miR-31-5p levels and down-regulated eNOS protein levels in a dose-dependent manner at concentrations of TNFα greater than 0.05 ng/ml (Fig. S2, A and B), which are comparable with serum TNFα levels (41.39–107.94 pg/ml) in preeclamptic patients. Moreover, direct treatment with HUVECs with the sera from patients with preeclampsia resulted in elevated miR-31-5p levels and down-regulated eNOS levels, as compared with the cells exposed to the sera from healthy pregnancies (Fig. S2, C–E). The miRNA levels also increased in HUVECs subjected to other inflammatory stimuli, such as lipopolysaccharide (LPS), IL-1β, and interferon (IFN)-γ (Fig. 2E), which have previously been shown to down-regulate eNOS expression in HUVECs (7). Because miRNA biogenesis is regulated at the transcriptional and post-transcriptional levels (1), we examined whether miR-31-5p biogenesis is transcriptionally regulated by various signal transduction inhibitors. TNFα-induced biogenesis of miR-31-5p was blocked by the NF-κB inhibitor Bay 11-7082 but not by the p38 MAPK inhibitor SB203580, the JNK inhibitor SP600125, the extracellular signal-regulated kinase inhibitor PD98059, or the phosphatidylinositol 3-kinase inhibitor LY294002 (Fig. 2F), suggesting that TNFα stimulates miR-31-5p expression through activation of NF-κB. As mature miRNA is generated by a sequential two-step process involving cleavage of primary miRNA (pri-miRNA) into precursor miRNA (pre-miRNA) in the nucleus and its subsequent maturation in the cytoplasm (1), we further examined which step of miR-31-5p biogenesis was regulated by NF-κB inhibition. TNFα-induced increases in the levels of pri-, pre-, and mature miR-31-5p were blocked by treatment with Bay 11-7082 or knockdown of NF-κB p65 (Fig. 2, G–I). These results suggest that TNFα increases the transcriptional biogenesis of miR-31-5p via NF-κB activation under inflammatory conditions.
Figure 2.
miR-31-5p levels are elevated in patients with preeclampsia and in HUVECs stimulated with TNFα in an NF-κB–dependent manner. A, miR-31-5p levels were determined in maternal sera from women with normal (NP) and preeclamptic (PE) pregnancies. B, TNFα levels were quantified in human sera by ELISA. C, levels of miR-31-5p and TNFα were determined in sera from preeclamptic patients. D, exosome (Exo) and nonexosomal (Non-exo) fractions were prepared from sera of preeclamptic patients by serial centrifugation. Level of miR-31-5p was determined by qRT-PCR. E, HUVECs were stimulated with TNFα (10 ng/ml), LPS (1 μg/ml), IL-1β (20 ng/ml), and IFN-γ (20 ng/ml) for 24 h. miR-31-5p levels were determined by qRT-PCR. F, cells were stimulated with TNFα in the presence or absence of Bay 11-7082 (Bay, 5 μm), SB203580 (SB, 10 μm), SP600125 (SP, 10 μm), PD98059 (PD, 10 μm), and LY294002 (LY, 10 μm). miR-31-5p levels of were analyzed. Ctrl, control. G–I, cells were transfected with 80 nm control (C) or NF-κB p65 siRNA (si-p65), followed by stimulation with TNFα in the presence or absence of Bay 11-7082 (Bay) or dimethyl sulfoxide (DMSO, D) as a vehicle for 24 h. Pri-, pre-, and mature miR-31-5p levels were analyzed. The levels of p65 were determined by Western blotting. **, p < 0.01, and ***, p < 0.001.
miR-31-5p down-regulates human eNOS, but not mouse eNOS, by targeting its mRNA 3′-UTR
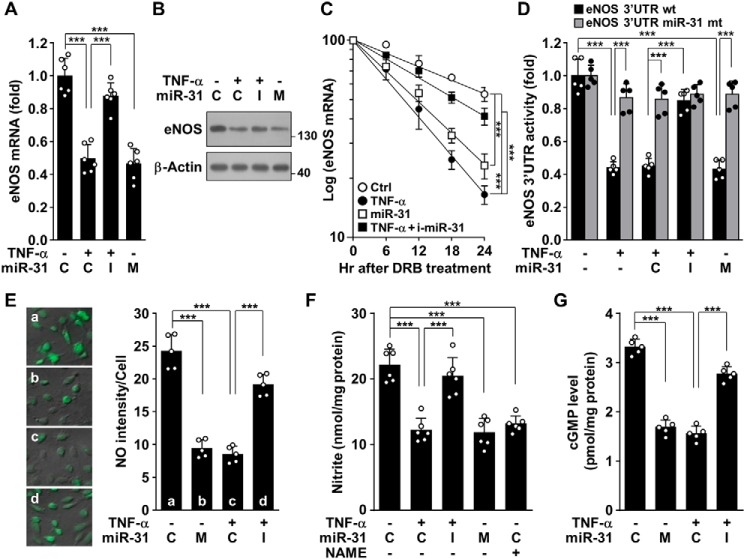
Because miR-31-5p was predicted to target the 3′-UTR of eNOS mRNA (Fig. 1B), we examined the expression levels of eNOS in HUVECs treated with TNFα or transfected with miR-31-5p mimic and inhibitor. Treatment with TNFα or miR-31-5p mimic decreased eNOS mRNA and protein levels, and the effects of TNFα were reversed by transfection with an miR-31-5p inhibitor (Fig. 3, A and B). In addition, treatment of primary human aortic endothelial cells (HAECs) with TNFα resulted in increased miR-31-5p biogenesis and down-regulated eNOS, whose effects were blocked by Bay 11-7082 and an miR-31-5p inhibitor (Fig. S3, A and B), suggesting that the TNFα/NF-κB pathway is also essential for miR-31-5p–dependent down-regulation of eNOS in HAECs. However, the promoter activity of eNOS was not affected by either treatment with TNFα or transfection with miR-31-5p mimic and inhibitor (Fig. S4). Notably, treatment with TNFα and miR-31-5p mimic resulted in significant decreases in the half-life of eNOS mRNA, from 27.2 to 9.4 h and 11.5 h, respectively, and the TNFα-mediated decrease was recovered to 20.1 h by an miR-31-5p inhibitor (Fig. 3C). This suggests that TNFα inhibits eNOS expression by biogenesis of miR-31-5p, which targets the eNOS transcript. To verify whether eNOS is a bona fide target of miR-31-5p, we explored the ability of miR-31-5p to target the 3′-UTR of eNOS mRNA. TNFα treatment inhibited the reporter activity of the eNOS mRNA 3′-UTR, but not of its mutant, and the inhibitory effect of TNFα was attenuated by an miR-31-5p inhibitor (Fig. 3D). In addition, transfection with miR-31-5p mimic inhibited the WT 3′-UTR activity, but not its mutant activity as did TNFα (Fig. 3D). As expected, treatment with TNFα or miR-31-5p mimic decreased NO production and cGMP synthesis, and the effects of TNFα were abolished by an miR-31-5p inhibitor (Fig. 3, E–G). Notably, computational analysis showed that miR-31-5p could also target the eNOS mRNA 3′-UTR of nonhuman primates, but not other species, such as mice and rats (Fig. S5A). Treatment with TNFα inhibited eNOS expression in mouse endothelial cells, and this inhibition was not reversed by a mouse miR-31-5p inhibitor. In addition, transfection with a mouse miR-31-5p mimic did not affect eNOS expression in mouse endothelial cells (Fig. S5B). Taken together, our data suggest that NF-κB–responsive miR-31-5p is essential for TNFα-mediated down-regulation of eNOS in humans, but not in rodents, leading to impairment of the human NO/cGMP signaling pathway.
Figure 3.
miR-31-5p decreases eNOS expression and NO/cGMP production. A and B, HUVECs were transfected with 80 nm control miRNA (C), miR-31-5p mimic (M), or miR-31-5p inhibitor (I), followed by stimulation with TNFα for 24 h. eNOS mRNA and protein levels were determined by qRT-PCR and Western blotting. C, cells transfected with miR-31-5p mimic or miR-31-5p inhibitor (mi-miR-31) were stimulated with TNFα for 10 h, followed by incubation with DRB for the indicated time periods. eNOS mRNA levels were analyzed (n = 5). D, cells were transfected with or without psiCHECK-2–eNOS 3′-UTR–reporter constructs (WT or mutant) alone or in combination with control miRNA (C), miR-31-5p mimic (M), or miR-31-5p inhibitor (I), followed by stimulation with TNFα for 24 h. eNOS 3′-UTR–reporter activity was determined by luciferase activity assay. E–G, cells were transfected with miR-31-5p analogues, followed by treatment with TNFα for 24 h. Intracellular NO, total nitrite, and cGMP levels were determined by confocal microscopy, Griess reaction, and ELISA, respectively. ***, p < 0.001.
miR-31-5p is a more potent inhibitor of eNOS expression than miR-155-5p
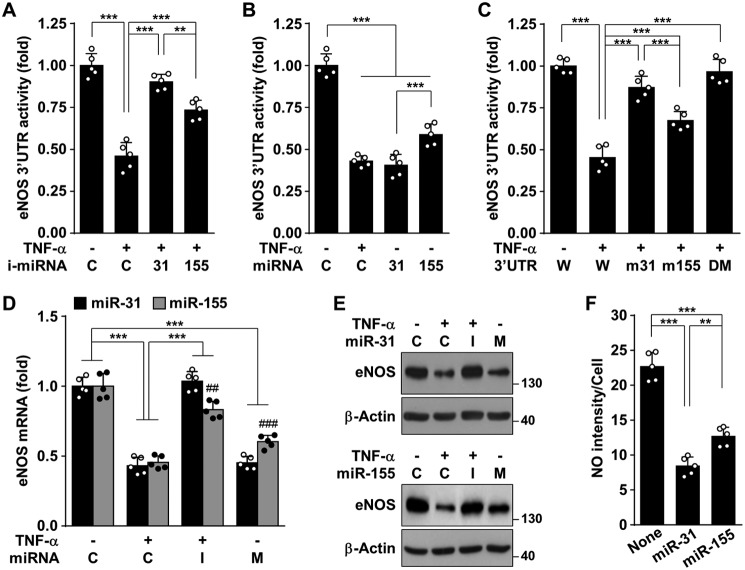
As TNFα-responsive miR-155-5p is known to target the eNOS transcript (3, 7), we compared the expression levels of miR-31-5p and miR-155-5p and their inhibitory effects on eNOS expression. Treatment of HUVECs with TNFα increased the levels of both miRNAs, with a higher increase in the levels of miR-155-5p than of miR-31-5p, and their induction was completely blocked by Bay 11-7082 (Fig. S6). The TNFα-induced decrease in eNOS 3′-UTR–reporter activity was rescued by transfection with an inhibitor of miR-31-5p or miR-155-5p; however, the effect of miR-31-5p inhibitor was stronger than that of miR-155-5p inhibitor (Fig. 4A). Moreover, miR-31-5p mimic more potently inhibited eNOS 3′-UTR–reporter activity than did miR-155-5p mimic (Fig. 4B). The inhibitory effect of TNFα on eNOS 3′-UTR activity was more effectively reversed by mutation of its complementary sequence for miR-31-5p than for miR-155-5p, as well as completely blocked by mutation of both miRNA-binding sites (Fig. 4C). Consistently, the suppressive effects of TNFα on eNOS expression and NO production were more potently blocked by an miR-31-5p inhibitor than an miR-155-5p inhibitor, and transfection with miR-31-5p mimic more effectively suppressed eNOS expression and NO production than did transfection with miR-155-5p mimic (Fig. 4, D–F). These data suggest that miR-31-5p is a more potential negative regulator of eNOS expression than miR-155-5p.
Figure 4.
miR-31-5p is more potent than miR-155-5p in silencing eNOS. A and B, HUVECs were transfected with 2 μg/ml psiCHECK-2 vectors containing eNOS mRNA 3′-UTR in combination with 80 nm control (C), inhibitor (i-mRNA), or mimic (miRNA) for miR-31-5p or miR-155-5p, followed by stimulation with TNFα for 24 h, and luciferase activity was assayed. C, cells were transfected with psiCHECK-2 vectors containing WT eNOS mRNA 3′-UTR (W), mutant for miR-31-5p (m31), mutant for miR-155-5p (m155), or double-mutant (DM). The cells were stimulated with TNFα for 24 h, and luciferase activity was assayed. D–F, cells were transfected with control (C), inhibitor (I), or mimic (M) to miR-31-5p and miR-155-5p, followed by stimulation with TNFα for 24 h. eNOS mRNA and protein levels and intracellular NO were determined by qRT-PCR, Western blotting, and confocal microscopy, respectively. **, p < 0.01, and ***, p < 0.001; ##, p < 0.01, and ###, p < 0.001 versus miR-31-5p mimic or inhibitor.
miR-31-5p inhibits in vitro eNOS-dependent angiogenesis
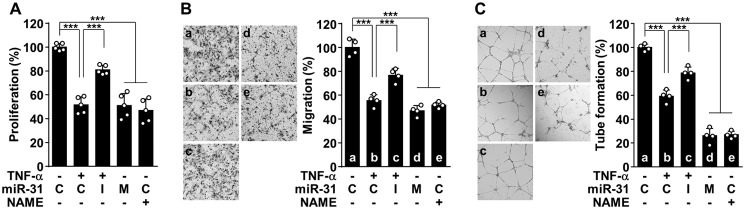
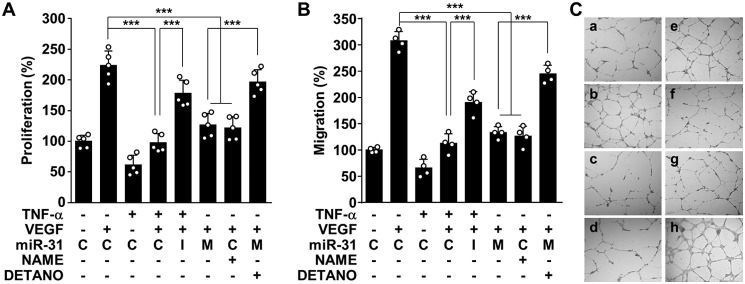
Decreased eNOS expression and activity result in endothelial dysfunction associated with impaired angiogenesis and vascular remodeling, which are currently accepted as characteristics of the pathogenesis of preeclampsia (12, 13, 16). Thus, we examined the effect of TNFα and miR-31-5p on the in vitro angiogenic properties of endothelial cells. TNFα inhibited endothelial cell proliferation, a key characteristic of angiogenesis, and this effect was reversed by transfection with an miR-31-5p inhibitor; however, treatment with miR-31-5p mimic or the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) suppressed endothelial cell proliferation (Fig. 5A). Similar regulatory effects on cell migration and tube formation, which are other important processes in angiogenesis (3), were also observed in HUVECs treated with TNFα, miR-31-5p mimic or inhibitor, and l-NAME (Fig. 5, B and C). Because vascular endothelial growth factor (VEGF) is important for angiogenesis, we further examined the effect of TNFα-induced miR-31-5p on VEGF-induced angiogenesis. Treatment with TNFα, miR-31-5p mimic, or l-NAME effectively inhibited VEGF-induced proliferation, migration, and tube formation in HUVECs, and the anti-angiogenic effects of TNFα and miR-31-5p were blocked by treatment with an miR-31-5p inhibitor and the chemical NO donor DETA NONOate (DETANO), respectively (Fig. 6, A–C, and Fig. S7). These results suggest that TNFα-induced miR-31-5p mediates endothelial dysfunction by inhibiting the eNOS/NO pathway.
Figure 5.
miR-31-5p inhibits angiogenic activity of endothelial cells. A–C, HUVECs were transfected with 80 nm control miRNA (C), miR-31-5p mimic (M), or miR-31-5p inhibitor (I), followed by stimulation with TNFα in the presence or absence of l-NAME (1 mm). A, endothelial cell proliferation was determined by [3H]thymidine incorporation assay. B, cell migration was determined by the Boyden chamber assay. C, endothelial cell tube formation was determined on a layer of growth factor-reduced Matrigel. ***, p < 0.001.
Figure 6.
miR-31-5p inhibits angiogenic activity. A–C, HUVECs were transfected with control miRNA (C), miR-31-5p mimic (M), miR-31-5p inhibitor (I), or DETANO (100 μm), followed by stimulation with TNFα in the presence or absence of l-NAME (1 mm), and cells were then incubated with or without VEGF (10 ng/ml). A, endothelial cell proliferation was determined by [3H]thymidine incorporation assay. B, cell migration was determined by the Boyden chamber assay. C, endothelial cell tube formation was determined on a layer of growth factor-reduced Matrigel. ***, p < 0.001.
miR-31-5p suppresses endothelial cell-mediated trophoblast invasion
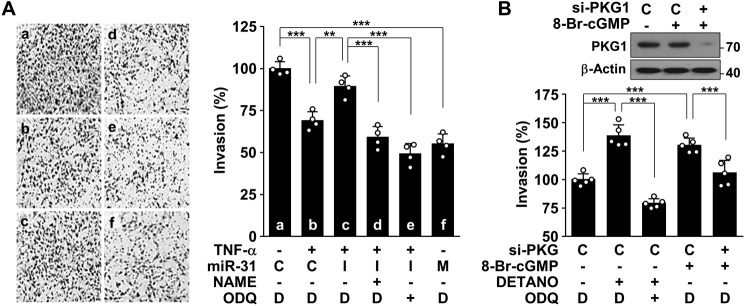
Interaction between endothelial cells and endovascular trophoblasts is critical for successful trophoblast invasion (17), resulting in encouragement of uterine spiral artery remodeling, which is crucial in decreasing maternal vascular resistance and increasing placental blood flow. We examined the effect of TNFα-induced miR-31-5p on endothelial cell–mediated trophoblast invasion in a co-culture system. HUVECs treated with TNFα or miR-31-5p mimic suppressed invasion of co-cultured trophoblastic HTR-8/SVneo cells compared with untreated controls, and the suppressive effect of TNFα was reversed by transfection with an miR-31-5p inhibitor (Fig. 7A). Moreover, the reversal effect of miR-31-5p inhibitor was abolished by l-NAME or the soluble guanylyl cyclase (sGC) inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (Fig. 7A). These data suggest that TNFα-induced endothelial miR-31-5p inhibits trophoblast invasion by impairing the eNOS-derived NO/cGMP pathway. Thus, we sought to identify the role of the NO/cGMP axis in trophoblast invasion. Direct treatment of HTR-8/SVneo cells with the NO donor DETANO and the membrane-permeable cGMP analogue 8-Br-cGMP increased trophoblast invasion, and these effects were blocked by co-treatment with ODQ and knockdown of protein kinase G, a downstream mediator of cGMP (Fig. 7B). These results suggest that TNFα-induced endothelial miR-31-5p inhibits trophoblast invasion associated with arterial remodeling during pregnancy by decreasing eNOS expression.
Figure 7.
miR-31-5p inhibits trophoblast invasion by suppressing the eNOS/NO/cGMP pathway. A, HUVECs were transfected with control miRNA (C), miR-31-5p mimic (M), or miR-31-5p inhibitor (I), followed by stimulation with TNFα (10 ng/ml), l-NAME (1 mm), ODQ (50 μm), or DMSO (D) as a vehicle for 24 h. The cells were co-cultured with trophoblastic HTR-8/SVneo cells in Transwell plates for 5 h. Trophoblast invasion was determined and quantified using the ImageJ software. B, HTR-8/SVneo cells were transfected with 80 nm control (C) or PKG1 siRNA (si-PKG), followed by treatment with 8-Br-cGMP (300 μm), DETANO, ODQ, or DMSO (D) as a vehicle in Transwell plates for 5 h. Trophoblast invasion was quantified, and PKG1 levels were determined by Western blotting. **, p < 0.01, and ***, p < 0.001.
NF-κB–responsive miR-31-5p impairs relaxation of human placental arteries
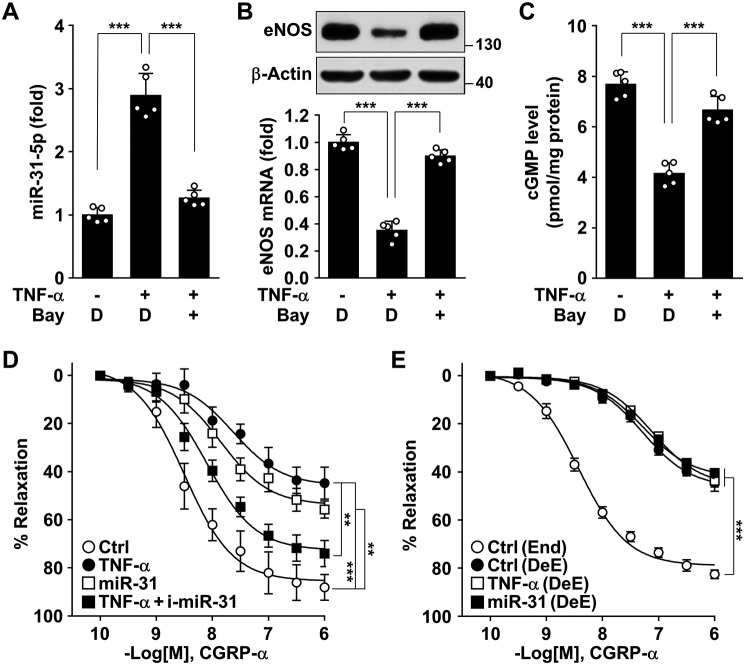
Because endothelium-derived NO stimulates vascular relaxation by activation of the sGC/cGMP pathway (18), we examined whether TNFα-induced miR-31-5p regulates vasorelaxation in an ex vivo cultured model of placental arterial vessels from healthy pregnant women. Treatment of the arterial rings with TNFα increased miR-31-5p biogenesis (Fig. 8A), resulting in decreased eNOS levels (Fig. 8B), and these events were reversed by co-treatment with Bay 11-7082 (Fig. 8, A and B). Consistent with these findings, TNFα decreased cGMP production in cultured arterial rings, and this effect was reversed by Bay 11-7082 (Fig. 8C). Moreover, treatment of human placental arterial rings with TNFα or miR-31-5p mimic inhibited the vasorelaxant response to the vasodilator calcitonin gene-related peptide (CGRP)-α, and the vasoconstrictor effect of TNFα was hampered by an miR-31-5p inhibitor (Fig. 8D). However, de-endothelialized arterial rings failed to generate a vasorelaxant response to CGRP-α, and this unresponsiveness was not altered by pretreatment with either TNFα or miR-31-5p mimic (Fig. 8E), indicating that TNFα-induced miR-31-5p induces vasoconstriction in an endothelium-dependent manner. Collectively, these results suggest that NF-κB–responsive miR-31-5p attenuates vascular relaxation by impairing the eNOS-dependent NO/cGMP pathway.
Figure 8.
miR-31-5p impairs relaxation of human placental arteries. A–C, arterial ring segments isolated from placentas of healthy pregnant women were treated with TNFα alone or in combination with Bay 11-7082 (Bay) or DMSO (D) as a vehicle for 24 h. A, miR-31-5p levels were analyzed (n = 5). B, eNOS mRNA and protein levels were analyzed. C, cGMP levels were measured using a cGMP assay kit. D and E, arterial rings were transfected with or without miR-31-5p mimic or miR-31-5p inhibitor (i-miR-31), followed by stimulation with TNFα for 24 h. D, concentration-dependent vasorelaxant responses to CGRP-α were measured by strain-gauge plethysmography (n = 6). E, endothelialized (End) or de-endothelialized arterial rings (DeE) were treated as above, and the vasorelaxant responses to CGRP-α were measured (n = 5). **, p < 0.01, and ***, p < 0.001.
Discussion
The inflammatory response is tightly regulated by activation of NF-κB in various vascular diseases, such as atherosclerosis and preeclampsia (19, 20), which are highly associated with endothelial dysfunction. Among many inflammatory cytokines, TNFα is the most potent activator of NF-κB and is considered an important mediator of endothelial dysfunction in the pathogenesis of preeclampsia. This strongly suggests that endothelial dysfunction is linked to transcriptional expression of NF-κB–responsive genes in endothelial cells. Although NF-κB–responsive adhesion molecules, such as vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin, are known biomarkers of endothelial dysfunction, they cause vascular inflammation by interacting with immune cells, but they do not directly alter endothelial cell function. Therefore, how NF-κB induces endothelial dysfunction is still unclear.
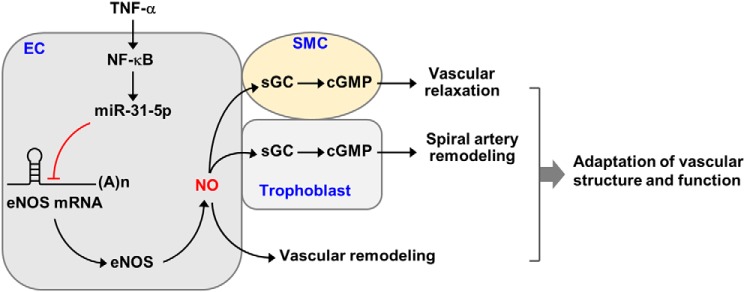
Several miRNAs are reportedly involved in the pathogenesis of cardiovascular diseases (21), such as miR-155-5p, which targets BCL6 or high-mobility group box-transcription protein in atherosclerosis (22, 23), as well as miR-31-5p, which targets cardiac troponin-T or large tumor suppressor homologue 2 in myocardial infarction and neointimal growth (24, 25). Our previous report demonstrated that TNFα stimulated NF-κB–dependent impairment of endothelial function and vasorelaxation via biogenesis of miR-155-5p, which targets the eNOS transcript (3). This study also found that NF-κB–responsive miR-31-5p caused endothelial dysfunction via destabilization of eNOS mRNA. Consequently, both miRNAs were involved in dysregulation of the eNOS/cGMP pathway, leading to impairment of angiogenesis, trophoblast invasion, and vasorelaxation, which are known pathological characteristics of preeclampsia. However, miR-31-5p inhibited the eNOS/NO pathway more potently than miR-155-5p. These results suggest that NF-κB–dependent miR-31-5p, in addition to miR-155-5p, is crucial for endothelial dysfunction and vasorelaxation through down-regulation of eNOS expression under inflammatory conditions (Fig. 9).
Figure 9.
Schematic diagram showing that NF-κB–responsive miR-31-5p contributes to the pathogenesis of preeclampsia. miR-31-5p up-regulated by activation of NF-κB under inflammatory conditions inhibits post-translational regulation of eNOS expression, leading to the decreased NO production. The decreased NO bioavailability impairs endothelial cell (EC) function associated with angiogenesis (vascular remodeling), vascular smooth muscle cell (SMC) activity responsible for vascular relaxation via dysregulation of the sGC/cGMP pathway, and trophoblast invasion (spiral artery remodeling) by decreasing cGMP production. These events contribute to the pathogenesis of preeclampsia.
Abnormal implantation of the placenta causes poor placental perfusion and leads to placental and fetal hypoxia, causing systemic inflammation. Therefore, serum levels of pro-inflammatory cytokines, such as TNFα and IL-6, are significantly increased in patients with preeclampsia compared with those in normal pregnant women (3, 8), indicating that preeclampsia is a systemic inflammatory disorder. Although the pathological link between inflammation and preeclampsia is unclear, recombinant TNFα infusion in animal models induces proteinuric hypertension, which is known to be the clinical symptom of human preeclampsia (9, 26). TNFα activates NF-κB in placentas and in circulating immune cells of preeclamptic patients (27, 28), indicating that placental and maternal NF-κB activations are involved in the pathogenesis of preeclampsia. Accumulating evidence has shown that inflammatory NF-κB activation accelerates endothelial dysfunction, which is an early determinant of hypertension and impaired vascular remodeling in the progression to preeclampsia (3, 7). In fact, inhibition of NF-κB activation or TNFα activity using a pharmacological inhibitor or a neutralizing antibody prevents TNFα-mediated endothelial dysfunction and high blood pressure in hypertensive patients (3, 29). Therefore, reciprocal cross-talk between inflammation and NF-κB activation is an important factor in endothelial dysfunction, which is a key event in the pathogenesis of preeclampsia.
Among biomarkers associated with vascular function, the reduced bioavailability of eNOS-derived NO is regarded as a representative parameter of endothelial dysfunction (12). Although eNOS is a constitutively expressed enzyme, its expression can be up-regulated by growth factors and hormones, such as VEGF and estrogen (30). However, inflammatory stimuli, such as TNFα and LPS, down-regulate the expression of this enzyme without transcriptional alteration through activation of NF-κB (7). In addition, it has been shown that inhibition of the NF-κB pathway can rescue decreased eNOS expression by preventing destabilization of eNOS mRNA under inflammatory conditions (3–7). This suggests that eNOS is negatively regulated in an NF-κB–dependent manner at the post-transcriptional level. Our data demonstrated that NF-κB activation is crucially involved in TNFα-induced eNOS down-regulation and endothelial dysfunction via biogenesis of miR-31-5p, which destabilizes eNOS mRNA. We also found that serum levels of miR-31-5p were significantly increased and mostly associated with circulating exosomes in patients with preeclampsia. The levels of exosomes in maternal circulation were markedly increased in preeclampsia (31, 32). Their levels were highly correlated with quantitative concentrations of exosome-associated placenta-specific alkaline phosphatase, a membrane-bound protein in syncytiotrophoblasts (31). The exosomes originated from syncytiotrophoblasts can mediate intercellular communication with neighboring cells or endothelial cells via delivery of their bioactive components, including miRNAs (33). Thus, it is possible that placenta-derived exosomes impair endothelial dysfunction in patients with preeclampsia by delivering miR-31-5p. However, TNFα, but not miR-31-5p, decreased mouse eNOS expression, indicating that the targeting activity of miR-31-5p against eNOS mRNA has species specificity. These results suggest the possible involvement of TNFα-induced unidentified miRNAs in the down-regulation of mouse eNOS expression and in the pathogenesis of a murine preeclampsia-like syndrome (8, 9), which is different from the naturally occurring human disease (34).
Our previous data demonstrated that eNOS levels were reduced in HUVECs freshly isolated from women with preeclampsia and that the levels were restored after 60 h of culture in fresh medium to those of normal HUVECs (3). This suggests that the decreased eNOS levels in the patients may be due to elevated levels of cytokines, including TNFα (3), and are rapidly recovered by deprivation of cytokines during in vitro culture. This phenomenon is similar to the resolution of preeclampsia symptoms, probably due to reduction of cytokine levels within a few weeks after delivery (35). These observations provide evidence that inflammatory eNOS down-regulation is crucially involved in the pathogenesis of preeclampsia. Thus, our findings strongly suggest that the NF-κB/miR-31-5p axis is essential to vascular dysfunction in the development of preeclampsia by inhibiting the eNOS/NO pathway.
Noncoding miRNAs inhibit global post-translation gene expression via destabilization of target gene transcripts and contribute to the pathogenesis of various human diseases, including preeclampsia. A number of miRNAs, including miR-155-5p, miR-335, and miR-854, reportedly decrease eNOS mRNA stability and its translation (7, 10). Particularly, miR-155-5p is up-regulated through activation of NF-κB in response to TNFα and LPS (7). Thus, the eNOS/NO pathway is negatively regulated by activation of NF-κB during inflammation that resembles the pathogenic conditions of preeclampsia (3, 13, 34). miR-31-5p is also up-regulated by NF-κB signaling in keratinocytes stimulated with inflammatory cytokines, including TNFα (36). Consistent with this, we also found that miR-31-5p is NF-κB–responsive and inhibits eNOS expression. This suggests that NF-κB–induced miR-31-5p, in addition to miR-155-5p, causes endothelial dysfunction by inhibiting the eNOS/NO pathway and is a molecular risk factor for preeclampsia.
Endothelial NO, produced from l-arginine by the catalytic reaction of eNOS, plays a crucial role in regulating vascular relaxation and homeostasis via sGC-dependent cGMP production. Indeed, eNOS-deficient mice develop spontaneous hypertension and sFlt-1–induced proteinuria (11, 13). Chronic inhibition of NOS activity in rats with l-NAME induces preeclampsia-like syndromes, such as systemic hypertension, glomerular sclerotic injury, and proteinuria (16). Therefore, decreased eNOS-derived NO synthesis is thought to be an important risk factor for endothelial dysfunction linked to the development of preeclampsia; however, some studies have shown conflicting results, such as increases, decreases, or no changes in plasma NO levels of patients (37–39). Our previous and present studies clearly demonstrated that TNFα was elevated in maternal plasma and stimulated endothelial dysfunction by decreasing eNOS expression, subsequently inducing characteristic features of preeclampsia in vitro (3). A preeclampsia-like syndrome can be induced in pregnant baboons by infusion with TNFα (26), probably via biogenesis of miR-31-5p and miR-155-5p. These observations suggest that TNFα increases the risk of preeclampsia by impairing the eNOS/NO pathway. Our results showed that NF-κB–responsive biogenesis of miR-31-5p induced by TNFα is essential for impairment of human endothelial function associated with vascular relaxation and remodeling.
A typical miRNA is capable of silencing hundreds of genes because of its complementary base-pairing with sequence-specific sites on the 3′-UTR of RNA transcripts, and thus a single gene transcript can be targeted by more than one miRNA. Accordingly, eNOS expression has been shown to be regulated by multiple miRNAs, including miR-155-5p, miR-335, miR-543, and miR-584 (3, 10, 40). Notably, among these miRNAs, miR-155-5p has been shown to increase in sera of patients with preeclampsia and to contribute to TNFα-mediated endothelial dysfunction and vasoconstriction in vitro (3). The effects of TNFα were reversed by the NF-κB inhibitor aspirin and the anti-inflammatory molecule carbon monoxide (3, 5), which can reduce the risk of preeclampsia (41, 42). This indicates that TNFα-induced endothelial dysfunction may be associated with NF-κB–responsive miRNAs. We also found that TNFα-induced miR-31-5p was elevated in preeclamptic patients and down-regulated eNOS expression, as well as the impaired angiogenic and vasorelaxant activity of endothelial cells. However, our data showed that miR-31-5p was more potent than miR-155-5p in suppressing eNOS expression. This difference in inhibition can be explained by the different target sites on the eNOS transcript, such as 8-mer for miR-31-5p and 7-mer–m8 for miR-155-5p (43). Thus, miR-31-5p is more important to endothelial dysfunction caused by reduced eNOS expression under inflammatory conditions, which results in impairment of vascular relaxation and remodeling. However, we suggest that both miRNAs may be synergistically involved in the pathogenesis of preeclampsia.
Taken together, our findings demonstrated that NF-κB–responsive miR-31-5p decreases eNOS expression by down-regulating eNOS mRNA stability, which decreases the NO/cGMP axis activity. This event elicits endothelial dysfunction associated with preeclamptic characteristics of hypertension and defects in vascular remodeling. These findings also suggest a possible mechanistic link between NF-κB–induced miR-31-5p and vascular dysfunction through down-regulation of eNOS expression during the development of preeclampsia. Furthermore, our data provide evidence that NF-κB–responsive miR-31-5p may be a novel predictive risk factor of endothelial dysfunction and synergistically contribute to miR-155-5p to the pathogenesis of preeclampsia.
Experimental procedures
Materials and methods
Cell culture media and supplements, Lipofectamine RNAiMAX, and Lipofectamine 3000 were purchased from Invitrogen, Life Technologies, Inc. Control siRNA (sc-37007), NF-κB p65 siRNA (sc-29410), and PKG1 siRNA (mixture containing equal amounts of 1121990, 1121993, and 1121997) were obtained from Santa Cruz Biotechnology or Bioneer (Daejeon, Korea). Human miR-31-5p mimic (MSY0000089), mouse miR-31-5p mimic (MSY0000538), human miR-155-5p mimic (MSY0000646), control miRNA (1027281), human miR-31-5p inhibitor (MIN0000089), mouse miR-31-5p inhibitor (MIN0000538), human miR-155-5p inhibitor (MIN0000646), miRNA inhibitor control (1027271), miR-31-5p PCR primers (MS00003290), miScript SYBR Green PCR kit, and miRNeasy mini kit were purchased from Qiagen (Hilden, Germany). Dual-luciferase reporter assay kits were obtained from Promega (Madison, WI). VEGF, TNFα, interferon-γ, IL-1β, and cGMP assay kits were purchased from R&D Systems (Minneapolis, MN). Antibodies against eNOS (610297, 1:1000) and β-actin (A5441, 1:5000) were purchased from BD Biosciences and Sigma, respectively. 4-Amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate was obtained from Molecular Probes (Eugene, OR). CGRP-α was purchased from AnyGen (Kwangju, South Korea). DETANO, l-NAME, ODQ, Bay 11-7082, LY294002, and PD98059 were purchased from Cayman Chemical (Ann Arbor, MI). LPS, 8-Br-cGMP, SB203580, and SP600125 were obtained from Sigma.
Animals
6–8-Week-old male C57BL/6 mice were purchased from Orient Bio Inc. (Sungnam, South Korea). All mice were maintained on a 12:12-h dark/light cycle in a pathogen-free animal facility. Mouse lung tissues and aortas were obtained following intraperitoneal injection of AvertinTM (250 mg/kg, Sigma) and used for isolation of lung endothelial cells and measurement of vascular tone as described below. Animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Ethics Committee of Kangwon National University (KW-171228-1). Moreover, this investigation conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (2011), 8th Ed.). Both mouse tissues were used.
Human specimens
Human specimens were obtained from 11 healthy male adults and 11 male patients with atherosclerosis as well as 17 normal pregnant women and 17 patients with preeclampsia after full-term normal deliveries according to the protocols approved by the Institutional Review Board at Kangwon National University Hospital (KNUH-2017-01-010-004), and informed consent was obtained from women with normal and preeclamptic pregnancies. This study conformed to the principles outlined in the Declaration of Helsinki. Blood samples were centrifuged at 12,000 × g for 8 min at 4 °C, and plasma/serum was obtained and kept at −80 °C until use. Preeclampsia was defined by clinical diagnosis with systolic blood pressure >140 mm Hg or diastolic blood pressure ≥90 mm Hg and proteinuria ≥0.3 g/24 h.
Endothelial cell culture and treatment
HAECs were purchased from ScienCell Research Laboratories (Carlsbad, CA). HUVECs were cultured, as described previously (3), and used at 2–6 passages, because they preserve their specific characteristics, which are quite similar to those of freshly isolated normal cells (44). The cells were grown in M199 media supplemented with 20% fetal bovine serum (FBS), 100 units/ml penicillin, 100 ng/ml streptomycin, 3 ng/ml basic fibroblast growth factor, and 5 units/ml heparin at 37 °C in a humidified CO2 incubator. Cells were cultured in serum-free media for 2 h and transfected with 80 nm siRNAs or miRNAs using Lipofectamine RNAiMAX as described previously (5). After recovery in fresh medium for 24 h, cells were pretreated with or without Bay 11-7082 (5 μm), SB203580 (10 μm), SP600125 (10 μm), PD98059 (10 μm), LY294002 (10 μm), and l-NAME (1 mm) for 30 min, followed by stimulation with TNFα (10 ng/ml), LPS (1 μg/ml), IL-1β (20 ng/ml), and IFN-γ (20 ng/ml) for the indicated time periods or 24 h. In contrast, mouse endothelial cells were isolated from lungs of male C57BL/6 mice using Dynabeads® magnetic beads (Invitrogen), as described previously (45), and according to the manufacturer's instructions. Mouse endothelial cells were cultured in Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum, transfected with 80 nm miRNAs, and treated with 10 ng/ml TNFα for 24 h.
miRNA and mRNA quantification
Total miRNAs from cells, tissues, and sera were isolated using an miRNeasy mini kit or an miRNeasy serum/plasma kit (Qiagen, Hilden, Germany), as reported previously (3). cDNAs for determining miRNAs were prepared from 1 μg of miRNAs using a miScript II RT kit. Quantitative real-time PCR (qRT-PCR) was performed with miScript SYBR Green PCR kit according to the manufacturer's instructions. The levels of primary, precursor, and mature miR-31 were analyzed using a miScript Primer Assay kit with miR-31–specific and universal primers. The relative levels of miR-31 were normalized to the housekeeping gene SNORD-95. In addition, total RNAs were isolated from cultured cells and vascular tissues using TRIzol reagent (Invitrogen) and were used to synthesize the first-strand cDNAs using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI), followed by quantitation of eNOS mRNA levels by qRT-PCR. The mRNA levels of eNOS were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used in this study are listed in Table S2.
Measurements of intracellular NO and cGMP
Intracellular NO levels were measured in situ using DAF-FM diacetate, as reported previously (3). HUVECs were transfected with 80 nm miR-31-5p mimic or inhibitor for 24 h and treated with TNFα for another 24 h. Cells were incubated with 5 μm (final concentration) DAF-FM diacetate for 30 min in a CO2 incubator. NO levels were determined using a confocal laser microscope. Cellular cGMP levels were determined using a cGMP assay kit (R&D Systems).
Assay of in vitro endothelial cell angiogenesis and trophoblast invasion
HUVECs were transfected with 80 nm miRNAs, followed by treatment with TNFα (10 ng/ml) or l-NAME (1 mm) for 24 h. HUVECs were incubated with or without vascular endothelial growth factor (VEGF, 10 ng/ml), and angiogenic properties were assessed as described previously (3). Endothelial cell proliferation was determined by [3H]thymidine incorporation assay. The cell migration assay was performed using Boyden chambers. Tube formation was determined after culturing HUVECs on a layer of growth factor-reduced Matrigel. Trophoblast invasion was assayed in a co-culture system of HUVECs and trophoblast-derived HTR-8/SVneo cells (3). For trophoblast invasion assay, HUVECs (2 × 104 cells) transfected with miRNAs and treated with TNFα were placed on the lower chambers of transwell plates with 6.5-mm diameter polycarbonate filters (Costar, New York), followed by treatment with or without l-NAME (1 mm), DETANO (100 μm), ODQ (50 μm), or 8-Br-cGMP (300 μm). Trophoblast-derived HTR-8/SVneo cells (5 × 103 cells) were placed into the upper wells pre-coated with 100 μl of growth factor-reduced Matrigel (Corning Inc., Corning, NY) and cultured for 5 h in a CO2 incubator as reported previously (3). Cells were fixed and stained with hematoxylin and eosin, and the upper surface of the filter was wiped with a cotton swab to remove nonmigrating cells. The cells that invaded to the lower side of the filter were observed using an inverted phase-contrast microscope (×100), and images were captured with a video graphic system. Cell invasion was quantified by counting the cells in all fields in each assay.
Measurement of vascular tension
Human umbilical arteries (∼40 mm from the insertion point in the placenta) from normal pregnancies were dissected, and the Warton's jelly and connective tissue were removed in ice-cold oxygenated Krebs-Ringer bicarbonate solution (in mm; NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.6, NaHCO3 25, glucose 11.1) as described previously (3). The normal and de-endothelialized vessels were cut into 3-mm rings and transfected with 80 nm of miRNAs in Opti-MEM reduced serum medium using Lipofectamine RNAiMAX. The vessel rings were pretreated with Bay 11-7082 (5 μm) in Dulbecco's modified Eagle's medium for 30 min and stimulated with TNFα (10 ng/ml) for 24 h. Some were used for analysis of miR-31-5p, eNOS mRNA and protein, and cGMP using qRT-PCR, Western blotting, and an ELISA kit. Others were mounted onto stainless steel wire stirrups (150 μm) in a multiwire myograph system (DMT-620, Aarhus, Denmark) and placed in 10-ml organ baths containing Krebs-Ringer solution. The rings were passively stretched at 30-min intervals in increments of 100 mg to reach optimal tone (200 mg) for human vessels. After the arterial rings had been stretched to their optimal resting tone, the contractile response to 100 mm KCl was determined. The response to a maximal dose of KCl was used to normalize responses to agonist across the vessel rings. The relaxant response of human vessels to CGRP-α was assessed as described previously (3).
Western blot analysis
HUVECs were suspended in RIPA buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS) and incubated on ice for 30 min for complete cell lysis. Cell debris was removed by centrifugation at 12,000 × g for 15 min. Cell lysates (30 μg of protein) were separated by SDS-PAGE, and target protein levels were determined by Western blot analysis (7).
Measurement of eNOS mRNA stability
HUVECs were transfected with 80 nm control siRNA, miR-31-5p mimic, or miR-31-5p inhibitor and stimulated with TNFα (10 ng/ml) for 10 h. Cells were further incubated with 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB, 20 μg/ml, Sigma) for the indicated time periods. Total mRNAs were isolated using TRIzol reagent, and eNOS mRNA levels were determined by qRT-PCR.
Reporter gene assay
The plasmid psiCHECK-2–eNOS-3′-UTR was constructed by ligation of the PCR product (430 nucleotides) of the human eNOS 3′-UTR into a psiCHECK-2 vector at XhoI and NotI sites as described previously (4). The mutant psiCHECK-2–eNOS-3′-UTR was prepared by site-directed mutagenesis (Fig. 1B). HUVECs were transfected with 2 μg/ml of either psiCHECK-2–eNOS-3′-UTR or its mutant vector or with pGL3-human eNOS promoter (1.6 kb)-Luc construct (7) alone or in combination with miR-31-5p mimic or inhibitor using Lipofectamine 3000 (Invitrogen). HUVECs were transfected with 2 μg/ml psiCHECK-2 vector containing either WT eNOS 3′-UTR or its mutant or pGL3-human eNOS promoter (1.6 kb)-Luc construct (7) alone or in combination with or without miRNAs using Lipofectamine 3000 (Invitrogen). After 4 h of incubation, cells were recovered in fresh medium for 24 h, followed by incubation with TNFα for 24 h. Reporter gene activity was assayed by a luciferase assay system or a dual-luciferase report assay kit (Promega, Madison, WI).
Isolation of exosomes
Serum samples (1 ml) were centrifuged at 1500 × g for 10 min to remove cells and cell debris. The collected supernatant was centrifuged again at 17,000 × g for 15 min to remove large vesicles, and the supernatant was spun again in an ultracentrifuge at 100,000 × g for 1.5 h at 4 °C. The pellets containing exosomes were carefully separated from the exosome-depleted supernatant and resuspended in PBS. Both the exosome suspension and the exosome-depleted serum were processed to extract miRNAs.
Statistical analysis
Quantitative data are expressed as mean ± S.D. of at least three independent experiments performed in triplicate, except for presenting as mean ± S.E. of the mean (S.E.) for vascular tone. Statistical analyses were performed with GraphPad Prism 6.0 for Windows (GraphPad Software). Statistical significance was determined using either the unpaired Student's t test or one-way analysis of variance followed by Tukey's post-hoc test, depending on the number of experimental groups analyzed. Significance was established at a p value < 0.05.
Author contributions
S. K. conceptualization; S. K. and K.-S. L. data curation; S. K. software; S. K. formal analysis; S. K., S. C., J. K., D.-K. L., M. P., W. P., T.-H. K., M.-H. W., H. L., S. R., K.-S. H., Y.-G. K., and Y.-M. K. validation; S. K., K.-S. L., and J. K. investigation; S. K. visualization; S. K. methodology; S. K. writing-original draft; S. K. and Y.-M. K. project administration; S. K. and Y.-M. K. writing-review and editing; J. Y. H. resources; Y.-M. K. supervision.
Supplementary Material
Acknowledgment
We thank the Central Research Laboratory of Kangwon National University for use of a liquid scintillation counter (Packard, Tri-Carb 2810TR).
This work was supported by National Research Foundation of Korea (NRF) Grant 2016M3A9B6903103 funded by the Korea Government (MSIP). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S7 and Tables S1–S2.
- miRNA
- microRNA
- miR
- microRNA
- 3′-UTR
- 3′-untranslated regions
- CGRP-α
- calcitonin gene-related peptide-α
- DAF-FM
- 4-amino-5-methylamino-2′,7′-difluorofluorescein
- DETANO
- DETA NONOate
- DRB
- 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- eNOS
- endothelial nitric-oxide synthase
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HUVEC
- human umbilical vein endothelial cell
- IFN
- interferon
- IL
- interleukin
- LPS
- lipopolysaccharide
- l-NAME
- NG-nitro-l-arginine methyl ester
- NO
- nitric oxide
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- qRT-PCR
- quantitative real-time PCR
- sGC
- soluble guanylyl cyclase
- TNFα
- tumor necrosis factor-α
- VEGF
- vascular endothelial growth factor
- HAEC
- human aortic endothelial cell
- MAPK
- mitogen-activated protein kinase
- pri-miRNA
- primary miRNA
- pre-miRNA
- precursor miRNA.
References
- 1. Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., and Kim V. N. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 10.1038/nature01957 [DOI] [PubMed] [Google Scholar]
- 2. Pankratz F., Hohnloser C., Bemtgen X., Jaenich C., Kreuzaler S., Hoefer I., Pasterkamp G., Mastroianni J., Zeiser R., Smolka C., Schneider L., Martin J., Juschkat M., Helbing T., Moser M., et al. (2018) MicroRNA-100 suppresses chronic vascular inflammation by stimulation of endothelial autophagy. Circ. Res. 122, 417–432 10.1161/CIRCRESAHA.117.311428 [DOI] [PubMed] [Google Scholar]
- 3. Kim J., Lee K. S., Kim J. H., Lee D. K., Park M., Choi S., Park W., Kim S., Choi Y. K., Hwang J. Y., Choe J., Won M. H., Jeoung D., Lee H., Ryoo S., et al. (2017) Aspirin prevents TNFα-induced endothelial cell dysfunction by regulating the NF-κB–dependent miR-155/eNOS pathway: role of an miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 104, 185–198 10.1016/j.freeradbiomed.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 4. de Jager S. C. A., Meeuwsen J. A. L., van Pijpen F. M., Zoet G. A., Barendrecht A. D., Franx A., Pasterkamp G., van Rijn B. B., Goumans M. J., and den Ruijter H. M. (2017) Preeclampsia and coronary plaque erosion: manifestations of endothelial dysfunction resulting in cardiovascular events in women. Eur. J. Pharmacol. 816, 129–137 10.1016/j.ejphar.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 5. Choi S., Kim J., Kim J. H., Lee D. K., Park W., Park M., Kim S., Hwang J. Y., Won M. H., Choi Y. K., Ryoo S., Ha K. S., Kwon Y. G., and Kim Y. M. (2017) Carbon monoxide prevents TNFα-induced eNOS downregulation by inhibiting NF-κB–responsive miR-155-5p biogenesis. Exp. Mol. Med. 49, e4036 10.1038/emm.2017.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conrad K. P., Miles T. M., Benyo D. F. (1998) Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 40, 102–111 10.1111/j.1600-0897.1998.tb00398.x [DOI] [PubMed] [Google Scholar]
- 7. Lee K. S., Kim J., Kwak S. N., Lee K. S., Lee D. K., Ha K. S., Won M. H., Jeoung D., Lee H., Kwon Y. G., and Kim Y. M. (2014) Functional role of NF-κB in expression of human endothelial nitric-oxide synthase. Biochem. Biophys. Res. Commun. 448, 101–107 10.1016/j.bbrc.2014.04.079 [DOI] [PubMed] [Google Scholar]
- 8. Li Y., Wang Y., Ding X., Duan B., Li L., and Wang X. (2016) Serum levels of TNFα and IL-6 are associated with pregnancy-induced hypertension. Reprod. Sci. 23, 1402–1408 10.1177/1933719116641760 [DOI] [PubMed] [Google Scholar]
- 9. Alexander B. T., Cockrell K. L., Massey M. B., Bennett W. A., and Granger J. P. (2002) Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric-oxide synthase expression. Am. J. Hypertens. 15, 170–175 10.1016/S0895-7061(01)02255-5 [DOI] [PubMed] [Google Scholar]
- 10. Jiang F., Li J., Wu G., Miao Z., Lu L., Ren G., and Wang X. (2015) Upregulation of microRNA-335 and microRNA-584 contributes to the pathogenesis of severe preeclampsia through downregulation of endothelial nitric-oxide synthase. Mol. Med. Rep. 12, 5383–5390 10.3892/mmr.2015.4018 [DOI] [PubMed] [Google Scholar]
- 11. Huang P. L., Huang Z., Mashimo H., Bloch K. D., Moskowitz M. A., Bevan J. A., and Fishman M. C. (1995) Hypertension in mice lacking the gene for endothelial nitric-oxide synthase. Nature 377, 239–242 10.1038/377239a0 [DOI] [PubMed] [Google Scholar]
- 12. Rudic R. D., Shesely E. G., Maeda N., Smithies O., Segal S. S., and Sessa W. C. (1998) Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 101, 731–736 10.1172/JCI1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li F., Hagaman J. R., Kim H. S., Maeda N., Jennette J. C., Faber J. E., Karumanchi S. A., Smithies O., and Takahashi N. (2012) eNOS deficiency acts through endothelin to aggravate sFlt-1–induced pre-eclampsia–like phenotype. J. Am. Soc. Nephrol. 23, 652–660 10.1681/ASN.2011040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taganov K. D., Boldin M. P., Chang K. J., and Baltimore D. (2006) NF-κB–dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yentrapalli R., Merl-Pham J., Azimzadeh O., Mutschelknaus L., Peters C., Hauck S. M., Atkinson M. J., Tapio S., and Moertl S. (2017) Quantitative changes in the protein and miRNA cargo of plasma exosome-like vesicles after exposure to ionizing radiation. Int. J. Radiat. Biol. 93, 569–580 10.1080/09553002.2017.1294772 [DOI] [PubMed] [Google Scholar]
- 16. Baylis C., Mitruka B., and Deng A. (1992) Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J. Clin. Invest. 90, 278–281 10.1172/JCI115849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cartwright J. E., and Balarajah G. (2005) Trophoblast interactions with endothelial cells are increased by interleukin-1β and tumour necrosis factor α and involve vascular cell adhesion molecule-1 and α4β1. Exp. Cell Res. 304, 328–336 10.1016/j.yexcr.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 18. Walford G., and Loscalzo J. (2003) Nitric oxide in vascular biology. J. Thromb. Haemost. 1, 2112–2118 10.1046/j.1538-7836.2003.00345.x [DOI] [PubMed] [Google Scholar]
- 19. Cuhlmann S., Van der Heiden K., Saliba D., Tremoleda J. L., Khalil M., Zakkar M., Chaudhury H., Luong le A., Mason J. C., Udalova I., Gsell W., Jones H., Haskard D. O., Krams R., and Evans P. C. (2011) Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-κB regulation that promotes arterial inflammation. Circ. Res. 108, 950–959 10.1161/CIRCRESAHA.110.233841 [DOI] [PubMed] [Google Scholar]
- 20. Laresgoiti-Servitje E. (2013) A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 94, 247–257 10.1189/jlb.1112603 [DOI] [PubMed] [Google Scholar]
- 21. Gangwar R. S., Rajagopalan S., Natarajan R., and Deiuliis J. A. (2018) Noncoding RNAs in cardiovascular disease: pathological relevance and emerging role as biomarkers and therapeutics. Am. J. Hypertens. 31, 150–165 10.1093/ajh/hpx197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R. R., Heyll K., Gremse F., Kiessling F., Grommes J., Weber C., and Schober A. (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Invest. 122, 4190–4202 10.1172/JCI61716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian F. J., An L. N., Wang G. K., Zhu J. Q., Li Q., Zhang Y. Y., Zeng A., Zou J., Zhu R. F., Han X. S., Shen N., Yang H. T., Zhao X. X., Huang S., Qin Y. W., and Jing Q. (2014) Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc. Res. 103, 100–110 10.1093/cvr/cvu070 [DOI] [PubMed] [Google Scholar]
- 24. Martinez E. C., Lilyanna S., Wang P., Vardy L. A., Jiang X., Armugam A., Jeyaseelan K., and Richards A. M. (2017) MicroRNA-31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J. Mol. Cell. Cardiol. 112, 27–39 10.1016/j.yjmcc.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 25. Liu X., Cheng Y., Chen X., Yang J., Xu L., and Zhang C. (2011) MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J. Biol. Chem. 286, 42371–42380 10.1074/jbc.M111.261065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sunderland N. S., Thomson S. E., Heffernan S. J., Lim S., Thompson J., Ogle R., McKenzie P., Kirwan P. J., Makris A., and Hennessy A. (2011) Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56, 192–199 10.1016/j.cyto.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 27. Vaughan J. E., and Walsh S. W. (2012) Activation of NF-κB in placentas of women with preeclampsia. Hypertens. Pregnancy 31, 243–251 10.3109/10641955.2011.642436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luppi P., Tse H., Lain K. Y., Markovic N., Piganelli J. D., and DeLoia J. A. (2006) Preeclampsia activates circulating immune cells with engagement of the NF-κB pathway. Am. J. Reprod. Immunol. 56, 135–144 10.1111/j.1600-0897.2006.00386.x [DOI] [PubMed] [Google Scholar]
- 29. Fichtlscherer S., Rössig L., Breuer S., Vasa M., Dimmeler S., and Zeiher A. M. (2001) Tumor necrosis factor antagonism with etanercept improves systemic endothelial vasoreactivity in patients with advanced heart failure. Circulation 104, 3023–3025 10.1161/hc5001.101749 [DOI] [PubMed] [Google Scholar]
- 30. Li H., Wallerath T., and Förstermann U. (2002) Physiological mechanisms regulating the expression of endothelial-type NO synthase. Nitric Oxide 7, 132–147 10.1016/S1089-8603(02)00127-1 [DOI] [PubMed] [Google Scholar]
- 31. Salomon C., Guanzon D., Scholz-Romero K., Longo S., Correa P., Illanes S. E., and Rice G. E. (2017) Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 102, 3182–3194 10.1210/jc.2017-00672 [DOI] [PubMed] [Google Scholar]
- 32. Sarker S., Scholz-Romero K., Perez A., Illanes S. E., Mitchell M. D., Rice G. E., and Salomon C. (2014) Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 12, 204 10.1186/1479-5876-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vargas A., Zhou S., Éthier Chiasson M., Flipo D., Lafond J., Gilbert C., and Barbeau B. (2014) Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 28, 3703–3719 10.1096/fj.13-239053 [DOI] [PubMed] [Google Scholar]
- 34. Shah T. J., and Walsh S. W. (2007) Activation of NF-κB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am. J. Obstet. Gynecol. 196, 48.e1–8 [DOI] [PubMed] [Google Scholar]
- 35. Peraçoli J. C., Rudge M. V., and Peraçoli M. T. (2007) Tumor necrosis factor-α in gestation and puerperium of women with gestational hypertension and pre-eclampsia. Am. J. Reprod. Immunol. 57, 177–185 10.1111/j.1600-0897.2006.00455.x [DOI] [PubMed] [Google Scholar]
- 36. Yan S., Xu Z., Lou F., Zhang L., Ke F., Bai J., Liu Z., Liu J., Wang H., Zhu H., Sun Y., Cai W., Gao Y., Su B., Li Q., et al. (2015) NF-κB–induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat. Commun. 6, 7652 10.1038/ncomms8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diejomaoh M. F., Omu A. E., Taher S., Al-Busiri N., Fatinikun T., Fernandes S., and Al-Othman S. (2003) Nitric oxide metabolites in preterm and induced labor. Gynecol. Obstet. Invest. 56, 197–202 10.1159/000074452 [DOI] [PubMed] [Google Scholar]
- 38. Sladek S. M., Magness R. R., and Conrad K. P. (1997) Nitric oxide and pregnancy. Am. J. Physiol. 272, R441–R463 [DOI] [PubMed] [Google Scholar]
- 39. Shaamash A. H., Elsnosy E. D., Makhlouf A. M., Zakhari M. M., Ibrahim O. A., and EL-dien H. M. (2000) Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int. J. Gynaecol. Obstet. 68, 207–214 10.1016/S0020-7292(99)00213-1 [DOI] [PubMed] [Google Scholar]
- 40. Fu Q., Liu X., Liu Y., Yang J., Lv G., and Dong S. (2015) MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric-oxide synthase. Int. J. Mol. Med. 36, 1417–1425 10.3892/ijmm.2015.2355 [DOI] [PubMed] [Google Scholar]
- 41. Henderson J. T., O'Connor E., and Whitlock E. P. (2014) Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann. Intern. Med. 161, 613–614 10.7326/L14-5020-5,10.7326/L14-5020-4 [DOI] [PubMed] [Google Scholar]
- 42. Zhai D., Guo Y., Smith G., Krewski D., Walker M., and Wen S. W. (2012) Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. Am. J. Obstet. Gynecol. 207, 57.e1–9 [DOI] [PubMed] [Google Scholar]
- 43. Moore M. J., Scheel T. K., Luna J. M., Park C. Y., Fak J. J., Nishiuchi E., Rice C. M., and Darnell R. B. (2015) miRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat. Commun. 6, 8864 10.1038/ncomms9864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marin V., Kaplanski G., Grès S., Farnarier C., and Bongrand P. (2001) Endothelial cell culture: protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods 254, 183–190 10.1016/S0022-1759(01)00408-2 [DOI] [PubMed] [Google Scholar]
- 45. Dong Q. G., Bernasconi S., Lostaglio S., De Calmanovici R. W., Martin-Padura I., Breviario F., Garlanda C., Ramponi S., Mantovani A., and Vecchi A. (1997) A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler. Thromb. Vasc. Biol. 17, 1599–1604 10.1161/01.ATV.17.8.1599 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.