Short abstract
Background
Obstructive sleep apnea is associated with pregnancy complications including gestational diabetes. Mechanisms underlying the association between obstructive sleep apnea and gestational diabetes remain to be elucidated.
Methods
Twenty-three participants with gestational diabetes underwent home sleep apnea testing. Obstructive sleep apnea was defined as an apnea hypopnea index > 5. Fasting morning blood samples were measured using multianalyte profiling (xMAP) multiplexed bead array immunoassay for Interleukin 6, tumor necrosis factor-alpha, and Interleukin 8.
Results
Age, body mass index, and gestational age at enrollment were 31 + 4.4 years, 35.7 + 7.4 kg/m2, and 28 ± 4 weeks, respectively. Participants were 52% Caucasian and 16% had obstructive sleep apnea. We observed positive correlations between apnea hypopnea index and Interleukin 6 (r = 0.62, p = 0.005), Interleukin 8 (r = 0.56, p = .56), and tumor necrosis factor-alpha (r = .58, p = .009). Women with obstructive sleep apnea had higher levels of Interleukin 6 (F = 5.01, p = .037) and Interleukin 8 (F = 6.33, p = .021) vs. women without obstructive sleep apnea.
Conclusion
These preliminary results indicate that in women with gestational diabetes, apnea hypopnea index is associated with an elevated inflammatory profile.
Keywords: Obstructive sleep apnea, pregnancy, gestational diabetes, inflammation, sleep-disordered breathing
Introduction
Obstructive sleep apnea (OSA), a condition on the spectrum of sleep-disordered breathing (SDB), has been linked to adverse metabolic outcomes in the general population as well as in pregnancy. Gestational diabetes is a known precursor of type II diabetes mellitus as women with a history of gestational diabetes are seven-fold more likely to develop type II diabetes later in life.1 Cross sectional,2 prospective,3 and population-based samples4,5 have shown that women with SDB were at a significantly increased risk of having gestational diabetes. These associations persisted after adjusting for body mass index, suggesting that the association is independent of obesity,6 a common condition in both disorders. On the other hand, there are scarce data on the prevalence of SDB in women with gestational diabetes. In a study consisting mainly of Black women, the prevalence of SDB has been described to be as high as 73%.7 Previously published data from this cohort show a significantly lower prevalence of SDB.8 The differences in prevalence estimates of SDB in women with gestational diabetes mellitus (GDM) may be related to differences in racial and ethnic distribution, the technology used to diagnose SDB (in-laboratory polysomnography vs. in-home sleep monitors), and the definition of the condition.
Mechanisms underlying the association between the two conditions have not been elucidated. Pro-inflammatory cytokines such as Interleukin 6 (IL-6), IL-8, and tumor necrosis factor-alpha (TNF-alpha) are potential culprits. These cytokines are secreted in various tissues, including adipose tissue, by activated macrophages, lymphocytes as well as epithelial and endothelial cells and phagocytes. They play a role in the adhesion of neutrophils and monocytes to the vascular endothelium as well as the regulation of T and natural killer cell proliferation and activation. In addition, these cytokines have been linked to obesity and OSA.9 Recent data from the Penn State Child Cohort10 suggest the release of pro-inflammatory cytokines partially explains the association between central obesity and OSA and that the association between OSA and pro-inflammatory markers are independent of obesity. It is also possible that changes in the inflammatory profiles of adolescents may be predictive of the development of OSA, suggesting potential causality.11 Furthermore, there may be some sex-specific differences in inflammatory profiles in OSA12 that may be related to the influence of female sex hormones.13
Pregnancy is associated with profound hormonal changes that can potentially accentuate the influence of female sex hormones on SDB. Thus, mechanisms underlying the association between OSA and metabolic outcomes such as GDM may be quite different in pregnancy than in the general population and are currently understudied. We have recently explored the potential role of the hypothalamic-pituitary-adrenal axis activation in this association and demonstrated that the cortisol awakening response was lower among pregnant women with OSA compared to those without,8 after adjusting for depression and stress. We hypothesized that women with OSA may have elevated levels of circulating pro-inflammatory cytokines. Hence, the aim of this study was to examine whether OSA status is associated with a higher inflammatory profile in a sample of pregnant women with GDM.
Methods
Setting
Pregnant women were recruited around the time of a diagnosis of gestational diabetes from a large hospital-based women’s practice. Women who met inclusion criteria were invited to participate and provided written informed consent (see Figure 1). Gestational diabetes was diagnosed based on the 3 hour glucose test between 24 and 28 weeks of gestation. Women with pre-gestational diabetes, i.e. type I or type II and those already diagnosed with OSA were not included in the study. Shift workers were also excluded. The study received ethical approval by the institutional review boards from Lifespan Health System IRB #363498 and Women and Infant’s Hospital of Rhode Island IRB #792474.
Figure 1.
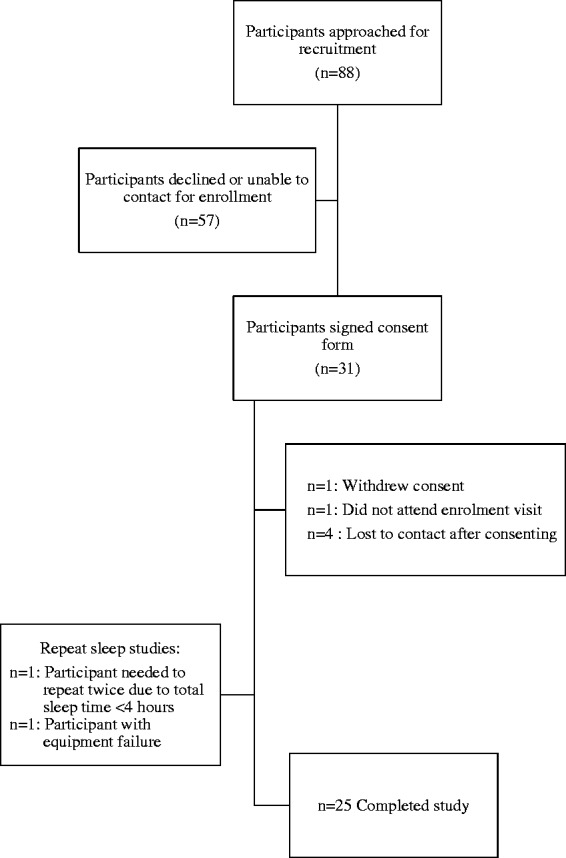
Consort diagram.
Respiratory assessment during sleep
In-home sleep studies were performed using the MediByte device (Medibyte, Braebon Inc., Kanata, ON, Canada). Medibyte® is a type III portable monitor (PM) for OSA and consists of two respiratory effort bands (chest and abdomen) equipped with respiratory inductive plethysmography technology, a nasal cannula pressure transducer for airflow detection, a finger pulse oximetry sensor (oxygen saturation and heart rate) as well as a body position sensor. The device is placed mid-sternum for comfort, contains a body position detection sensor, and is held in place via the abdominal and chest belts. The device operates on battery power (3.6 V) and has a sampling rate of 2000 Hz.
Upon completion of the study, data are downloaded from the MediByte PM into a chronologically readable format by using Pursuit software (Braebon Medical Corporation, Canada) for review and manual scoring. The device has been validated against in-laboratory polysomnography.14 All studies were scored by the same highly experienced polysomnography technician based on the 2012 AASM guidelines.15 Studies were included if participants had a minimum of 4 h of sleep. Apneas were defined as >90% flow limitation for at least 10 s. Hypopneas were defined as a 30% reduction in airflow for at least 10 s with 4% reduction in oxygen saturation. OSA was defined by an apnea hypopnea index (AHI) > 5 events per hour.
Laboratory testing
Women were given clear written instructions to refrain from eating overnight and exercise before blood sample was obtained. Fasting morning blood samples were obtained via peripheral venipuncture. Plasma was separated from blood and stored at −80°C. Samples were diluted 1:5 in a reaction buffer according to the manufacturer’s protocol. All samples were treated identically and all assays were performed simultaneously using the same platforms and reagents. Aliquots were used to measure cytokine levels of IL-6, IL-8, and TNF-alpha using an xMAP multiplexed bead array immunoassay. This approach allows for simultaneous quantification of multiple cytokines in solution by capturing them onto antibody coated spectrally distinct fluorescent microspheres and measuring fluorescence intensity using the Luminex-100 system (Luminex Corp., Austin, TX). All cytokine assay results fell within the linear dynamic range of the standards (fluorescent light units per milliliter = FLU/ml). However, the data were natural log transformed to reduce variance and outliers within the groups.
Glucose and C-peptide were also measured after an overnight fast. After the specimen was spun for 10 minutes at 3500 rpm and separated from cells within 3 hours of collection, it was then loaded on to the 5800 analyzer (Beckman Coulter) and tested. Serum was also collected for C-peptide measurement, transported at room temperature for testing and processed via immunoassay. The homeostatic model assessment (HOMA) for insulin resistance was then calculated using the formula: fasting insulin (microU/L) × fasting glucose (nmol/L)/22.5.
Statistical methods
Analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY) software. Descriptive analyses were performed to report demographics and comorbidities. Median values and interquartile range (IQR) were then used to describe non-parametric variables. Immune markers and AHI were significantly skewed and, therefore, were log transformed. Univariate analyses of variance were performed to examine differences in inflammatory markers by OSA status. Maternal body mass index (BMI) was examined as a covariate in the association with inflammatory markers. Inflammatory markers were transformed into Z-scores to better illustrate the data in the figure. Pearson correlation was performed to examine the correlation of AHI with inflammatory markers.
Results
Demographics and sleep characteristics
The median and IQR for age and BMI of participants was 30.5 (6.5) years and 36.1 (9.6) kg/m2, respectively, and 43% were nulliparous. Participants were 52% Caucasian, 18% Black, 8% Hispanic, and 30% Asian, American Indian, or multi-racial. Average gestational age at enrollment was 28 ± 4 weeks. Median gravidity was 2 ± 1.2 and median parity was 1 ± 0.97. Thirteen percent of all patients had chronic hypertension (see Table 1).
Table 1.
Maternal characteristics.
| Total sampleMedian (IQR) | OSA (n = 4)Median (IQR) | No OSA (n = 21)Median (IQR) | p | |
|---|---|---|---|---|
| Age (years) | 30.5 (6.5) | 34 (4.2) | 30 (6.2) | 0.51 |
| BMI at diagnosis (kg/m2) | 36.1 (9.6) | 39.9 (6.0) | 33.3 (8.5) | 0.11 |
| BMI antenatal (kg/m2) | 32.6 (8.4) | 36.4 (5.2) | 31.7 (10.6) | 0.11 |
| Gestational age at sampling | 30 (4) | 27.5 (2.25) | 31 (3.5) | 0.08 |
| Gravidity | 2 (2) | 2 (0.75) | 2 (2) | 0.98 |
| Parity | 1 (2) | 0.5 (1.5) | 1 (2) | 0.79 |
BMI: body mass index; IQR: interquartile range; OSA: obstructive sleep apnea.
Median AHI was 0.9 (IQR 1.95) events per hour. Median nadir oxygen saturation was 84% (IQR 9.5%) and time spent below 90% oxygen saturation was 0.35% (IQR 3.75%) of sleep time. There were no significant central or mixed apneic events.
Three patients had mild OSA (AHI 5–15 events per hour), and one patient had moderate OSA (AHI 15–30 events per hour). Average BMI in women with OSA was higher compared to women without OSA, but the difference did not reach statistical significance (42.7 + 19.3 vs. 38 + 12.9 kg/m2, p = 0.11). In addition, average age was also similar in the two groups (p = 0.5).
Pro-inflammatory cytokines and BMI
In this sample, maternal BMI was examined as a covariate for inflammatory markers and did not impact the markers tested: IL-6 (β = −.04, p = .86), IL-8 (β = −.14, p = .61) or TNF-alpha (β = .16, p = .60).
Pro-inflammatory cytokines and insulin resistance
There were no significant correlations between pro-inflammatory cytokines and insulin resistance measured by HOMA-IR (p-value > 0.4).
Pro-inflammatory cytokines and SDB status
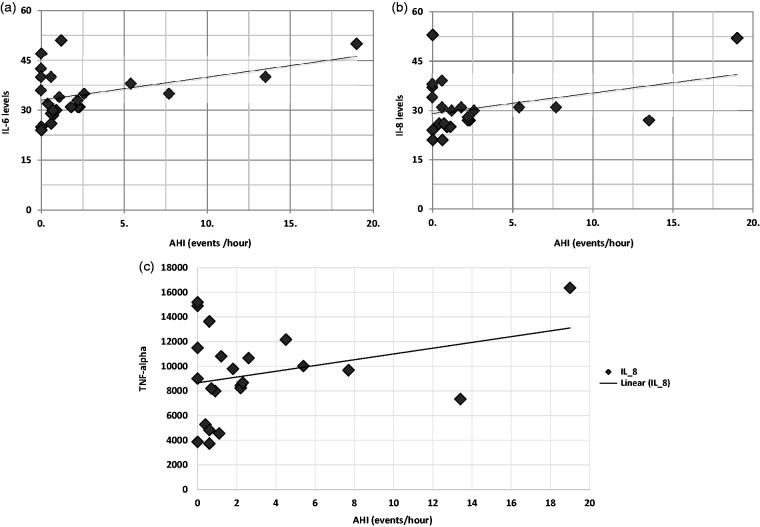
There was a significant positive association between the AHI and IL-6 (r = 0.62, p = .005), IL-8 (r = 0.56, p = .013), and TNF-alpha (r = .58, p = .009) (Figure 2(a) to (c)).
Figure 2.
(a) Correlation of pro-inflammatory markers with apnea hypopnea index: Interleukin-6. (b) Correlation of pro-inflammatory markers with apnea hypopnea index: Interleukin-8. (c) Correlation of pro-inflammatory markers with apnea hypopnea index: tumor necrosis factor-alpha.
AHI: apnea hypopnea index; IL-6, IL-8: Interleukin 6, 8; TNF-alpha: tumor necrosis factor-alpha.
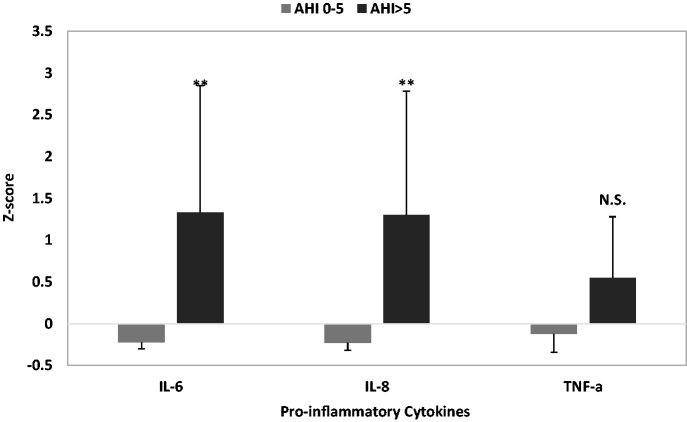
Pro-inflammatory cytokines were then examined based on OSA status. Compared to women without a diagnosis of OSA, women with OSA had significantly higher levels of IL-6 (F = 5.01, p = .037) and IL-8 (F = 6.33, p = .021). There was no significant difference in levels of TNF-alpha between the two groups (F = 1.23, p = .28). See Figure 3.
Figure 3.
Inflammatory marker levels in participants with and without OSA presented as Z scores.
**p < .01. AHI: apnea hypopnea index.
Discussion
Our study shows that women with gestational diabetes who have OSA have a more enhanced pro-inflammatory profile compared to women without a diagnosis of OSA. There appears to be a dose–response association between Il-6, IL-8, TNF-alpha, and OSA severity, as these cytokines were all positively correlated with AHI. In this sample of pregnant women with overweight or obesity, neither BMI nor the degree of insulin resistance correlated with the inflammatory profile, and hence, it appears that the association between inflammatory cytokines and OSA is independent from obesity and insulin resistance. Our sample is racially diverse; however, the impact of race on the prevalence of SDB or the degree of inflammation in this population cannot be examined. As previously noted, an in-laboratory study of primarily African American women with gestational diabetes showed a significantly higher prevalence of SDB.7 Although the difference in prevalence may also be due to the differences in methodology in defining SDB, racial differences cannot be excluded. It is also possible that race may impact an individual’s inflammatory profile;16 however, this determination cannot be made in this study due to sample size.
Pro-inflammatory cytokines have been shown to be up-regulated in non-pregnant patients with OSA. Our study is consistent with findings from previous studies of non-pregnant patients that have shown a significant elevation in IL-6,17,18 IL-8, and TNF-alpha17 in patients with OSA compared to those without. Data have not been entirely consistent with these findings; IL-6 and TNF-alpha were similar in morbidly obese subjects with and without metabolic syndrome and OSA in all three groups,19 despite matching for body mass index and sex. However, hormonal status was not addressed in that sample. A large study that examined sex differences in inflammatory profile measured by chemiluminescence in relation to OSA showed that TNF-alpha was the predominant cytokine in women with OSA, whereas leptin predominated in men with OSA.12 It is possible, as Hirotsu et al. have concluded, that discrepancies in the available data may be driven by unmeasured factors such as hormonal status, as it is likely that inflammation is modulated by female sex hormones such as estrogens. In an experimental study, intermittent hypoxia in an OSA mouse model led to considerable increases in gene expression of both IL-6 and IL-8 in the heart and brain,13 an effect that was mitigated by oophorectomy, suggesting that female sex hormones may mediate or enhance inflammation in response to intermittent hypoxia and OSA. As estrogen levels increase significantly in pregnancy and are many fold higher by the late second trimester, it is likely that high estrogen levels promote the inflammatory state observed in patients with OSA. More research is needed to further understand the role of estrogens in the association between OSA and inflammation in pregnancy.
It is biologically plausible that OSA leads to an enhanced inflammatory state; however, data in this study are cross-sectional and do not prove causality. A reverse directionality is also possible. It can be argued that a pronounced inflammatory state can be associated with changes in the upper airway that result in edema and anatomical and physiological changes that could predispose to OSA as temporal relationships are demonstrated in longitudinal cohorts.11 Hence, our findings may imply that women with gestational diabetes who have OSA have a higher inflammatory profile than those without OSA. However, they could also be consistent with the fact that women with gestational diabetes with a higher inflammatory state are more likely to have OSA.
Enhanced inflammation may be associated with adverse outcomes and cardiovascular complications outside of pregnancy. Associations of OSA with adverse metabolic and cardiovascular outcomes in pregnancy, such as gestational diabetes and preeclampsia, and negative neonatal outcomes such as preterm birth, have been demonstrated in numerous studies.4,5,20,21 However, mechanisms linking the associations have not been elucidated. Although alterations in placental function22,23 and evidence of placental hypoxia23 have been demonstrated, mechanisms proximal to the placenta have not been explicated. Data show that oxidative stress does not appear to be a significant marker of OSA in pregnancy24 and this may be related to higher levels of estradiol in pregnancy, which have been shown to suppress oxidative stress.25 Based on current data, knowledge of the impact of female sex hormones, and previous literature linking OSA,18 preeclampsia26 and preterm labor27 to inflammation, it is possible that inflammatory pathways may mediate these adverse outcomes. However, data from this study only pertain to women with gestational diabetes and cannot be generalized to uncomplicated pregnancies, though it could be argued that the lack of an association between inflammatory markers and the degree of insulin resistance suggests that the enhanced inflammatory profile is related to disordered breathing and is independent of glucose metabolism.
Strengths of this study include the objective determination of a diagnosis of SDB. Studies defining SDB based on questionnaire data usually show a much higher prevalence of SDB compared to studies that objectively measure SDB. Results from this study are preliminary and should be interpreted in light of a number of limitations. First, our main limitation is the sample size (due to lack of funding), which may have impacted our findings and our ability to detect statistically significant differences. In addition, the study is restricted to patients with gestational diabetes. Findings will need to be replicated in larger samples as well as in patients with a diagnosis of OSA independent from gestational diabetes. However, as this study is the first to our knowledge to examine the effect of SDB on inflammatory cytokines in pregnancy, it will help to inform the design of future studies that are appropriately powered to detect differences. Next, OSA status was determined using in-home rather than an in-laboratory sleep study and may have underestimated the prevalence of OSA. Finally, we did not include measures of estrogens in pregnancy. Future larger studies that examine inflammation as a key candidate mechanism linking OSA and adverse pregnancy outcomes are needed and should take into consideration hormonal status.
Results from this pilot study indicate that pregnant women with gestational diabetes and OSA display elevated inflammatory cytokines compared to patients with gestational diabetes who do not meet criteria for OSA. Future research is needed to replicate these findings in a larger sample and among pregnant patients without gestational diabetes. Results from this area of research may identify mechanisms that explain associations among OSA and adverse pregnancy outcomes.
Acknowledgments
We would like to acknowledge all women who agreed to participate in the study and Beth Hott for her assistance with the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially funded by The CHEST Foundation Women’s Lung Health Research Award. GB is funded by the National Institutes of Health R01HD078515 and R01HL130702, and has received research equipment from Respironics.
Ethical approval
Written consent was obtained from participants for publication.
Guarantor
GB.
Contributorship
MHB: design, analysis, interpretation, writing. MC: design, interpretation, critical review of the manuscript. SA: design, analysis, interpretation, critical review of the manuscript. MLK: critical review of the manuscript. RM: data acquisition and interpretation, critical review of manuscript. SMDLM: data acquisition and interpretation, critical review of manuscript. GB: design, analysis, interpretation, data acquisition and interpretation, writing, critical review of the manuscript. All authors contributed to, reviewed, and edited the manuscript and approved the final version.
References
- 1.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 2.Bourjeily G, Raker CA, Chalhoub M, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 2010; 36: 849–855. [DOI] [PubMed] [Google Scholar]
- 3.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol 2012; 120: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourjeily G, Danilack VA, Bublitz MH, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med 2017; 38: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med 2016; 12: 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luque-Fernandez MA, Bain PA, Gelaye B, et al. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care 2013; 36: 3353–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reutrakul S, Zaidi N, Wroblewski K, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab 2013; 98: 4195–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bublitz M, De La Monte S, Martin S, et al. Childhood maltreatment and inflammation among pregnant women with gestational diabetes mellitus: a pilot study. Obstet Med 2017; 10: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lima FF, Mazzotti DR, Tufik S, et al. The role inflammatory response genes in obstructive sleep apnea syndrome: a review. Sleep Breath 2016; 20: 331–338. [DOI] [PubMed] [Google Scholar]
- 10.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab 2016; 311: E851–E858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Increased inflammation from childhood to adolescence predicts sleep apnea in boys: a preliminary study. Brain Behav Immun 2017; 64: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirotsu C, Albuquerque RG, Nogueira H, et al. The relationship between sleep apnea, metabolic dysfunction and inflammation: the gender influence. Brain Behav Immun 2017; 59: 211–218. [DOI] [PubMed] [Google Scholar]
- 13.Torres M, Palomer X, Montserrat JM, et al. Effect of ovariectomy on inflammation induced by intermittent hypoxia in a mouse model of sleep apnea. Respir Physiol Neurobiol 2014; 202: 71–74. [DOI] [PubMed] [Google Scholar]
- 14.Driver HS, Pereira EJ, Bjerring K, et al. Validation of the MediByte(R) type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can Respir J 2011; 18: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sam S. Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. Horm Mol Biol Clin Investig 2018; 33: PMID: 29522417. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Liu J, Xiong S, et al. The change of interleukin-6 and tumor necrosis factor in patients with obstructive sleep apnea syndrome. J Tongji Med Univ 2000; 20: 200–202. [DOI] [PubMed] [Google Scholar]
- 18.Cizza G, Piaggi P, Lucassen EA, et al. Obstructive sleep apnea is a predictor of abnormal glucose metabolism in chronically sleep deprived obese adults. PLoS One 2013; 8: e65400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salord N, Gasa M, Mayos M, et al. Impact of OSA on biological markers in morbid obesity and metabolic syndrome. J Clin Sleep Med 2014; 10: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol 2017; 129: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis JM, Mogos MF, Salemi JL, et al. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014; 37: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourjeily G, Curran P, Butterfield K, et al. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med 2015; 43: 81–87. [DOI] [PubMed] [Google Scholar]
- 23.Ravishankar S, Bourjeily G, Lambert-Messerlian G, et al. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol 2015; 18: 380–386. [DOI] [PubMed] [Google Scholar]
- 24.Khan N, Lambert-Messerlian G, Monteiro JF, et al. Oxidative and carbonyl stress in pregnant women with obstructive sleep apnea. Sleep Breath 2018; 22: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laouafa S, Ribon-Demars A, Marcouiller F, et al. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep 2017; 40: PMID: 28633495. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson KK, Meeker JD, McElrath TF, et al. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol 2017; 216: 527.e1–527.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Shazly S, Makhseed M, Azizieh F, et al. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol 2004; 52: 45–52. [DOI] [PubMed] [Google Scholar]