Short abstract
Background
Devic syndrome or neuromyelitis optica is an autoimmune neurological condition characterized by relapsing symptoms of optic neuritis and transverse myelitis. Women with neuromyelitis optica suffer from adverse pregnancy outcomes and high relapse rates during pregnancy and the postpartum period.
Methods
This case series describes 13 pregnancies in four women with neuromyelitis optica managed at a tertiary hospital in Toronto, Canada.
Results
In most cases, neurologic symptoms either worsened or developed for the first time during pregnancy or the postpartum period, and often responded to a combination of steroids, immunosuppressant medications, plasma exchange and intravenous immunoglobulin. The 13 pregnancies resulted in two miscarriages, three preterm and eight term births. One fetus whose mother was on gabapentin, prednisone and spironolactone, had congenital malformations (aplastic lung and fused fingers).
Conclusions
Despite high frequency of relapses in pregnancy and the postpartum period, with multidisciplinary team management, outcomes for women with neuromyelitis optica are encouraging.
Keywords: Complications, high-risk pregnancy, maternal-fetal medicine, drugs (medication), autoimmune demyelinating disease
Introduction
Devic syndrome or neuromyelitis optica (NMO) is a recurrent antibody-mediated inflammatory disorder characterized by clinical presentations of optic neuritis and transverse myelitis. It is differentiated from multiple sclerosis (MS) using the Wingerchuck criteria developed in 2006, and requires the presence of optic neuritis, transverse myelitis and at least two of: continuous spinal cord lesions extending more than 3 vertebral segments on magnetic resonance imaging (MRI), a brain MRI not meeting diagnostic criteria of MS, or presence of serum NMO-immunoglobulin G anti-aquaporin 4 antibodies (AQP4).1 Furthermore, the Wingerchuck criteria1 provide guidance on management of the disease.
Although there are less than 150 reported cases of NMO in pregnancy in the literature, there is evidence that pregnancy may be associated with disease exacerbation, relapse, and adverse maternal and fetal outcomes.2–5 In this paper, we describe outcomes of 13 pregnancies in four patients with NMO.
Methods
Patients with NMO were identified using a Special Pregnancy Program database that enlists medical, surgical, and obstetric diagnoses of all patients attending the Maternal-Fetal Medicine and High-Risk Obstetric clinics at Mount Sinai Hospital, Toronto, between the years 2000 and 2015. Preconception counseling, neurology, internal medicine, antepartum, intrapartum, and postpartum records were retrieved and maternal demographics including age, ethnicity, gravidity, parity, comorbidities, past obstetric history as well as details on neurological symptoms and disease progression were collected. Clinical outcomes such as NMO disease activity, maternal neurologic symptoms, development of preeclampsia, mode of delivery, fetal loss, preterm birth, infant’s status at birth and neonatal intensive care unit (NICU) admission status were retrieved from patient records and documented. A descriptive account of the four cases and 13 pregnancies is presented below.
Case series
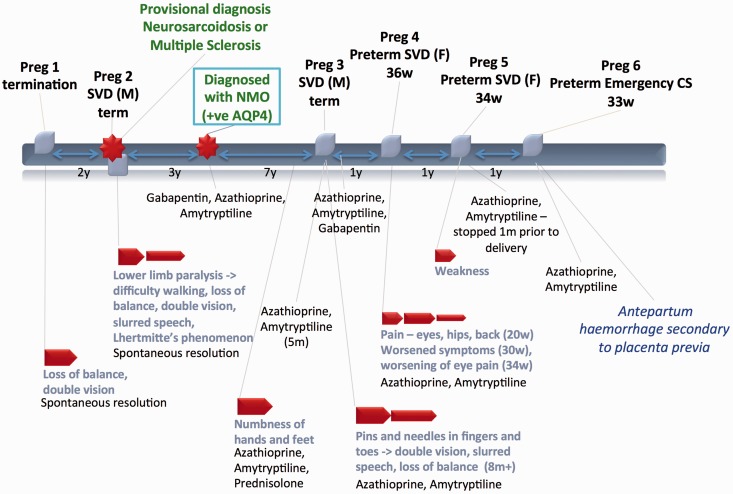
Case 1 (Figure 1)
Figure 1.
Case 1.
IV: intravenous; CS: cesarean section; SVD: spontaneous vaginal delivery.
A 24-year-old African woman presented with balance and vision symptoms following termination of her first pregnancy. Her symptoms resolved spontaneously and she was not investigated further. Two years later she presented with complete lower extremity paralysis after a second pregnancy and spontaneous vaginal delivery (SVD) of a healthy male (M) infant. She remained hospitalized for six weeks over which time she had a relapsing-remitting course of symptoms. A provisional diagnosis of MS was made. Three years later her serum tested positive for AQP4 antibodies and her diagnosis was revised to NMO. She was treated with azathioprine, amitriptyline, and gabapentin with complete remission for seven years. She then had recurrence of paresthesias and was treated with prednisone.
During her third pregnancy, she was taken off gabapentin and experienced recurrence of peripheral paresthesias, visual complaints, and imbalance. She was treated successfully with azathioprine and amitriptyline. She had an uncomplicated term SVD of a healthy male infant and no further NMO-related symptoms. Gabapentin was added postpartum.
During her fourth pregnancy, she experienced visual and pain symptoms that were managed with azathioprine and amitriptyline. The symptoms progressively worsened until 36 weeks, when she delivered a preterm but healthy female (F) infant.
In her fifth pregnancy, she self-discontinued amitriptyline and her only symptom was generalized weakness. This pregnancy also resulted in preterm SVD at 34 weeks.
During her sixth pregnancy, the patient experienced third trimester antepartum hemorrhage believed to be secondary to placenta previa. She required emergency caesarean section (CS) at 33 weeks due to placental abruption, and delivered a preterm but healthy infant. There were no NMO-related symptoms in this pregnancy.
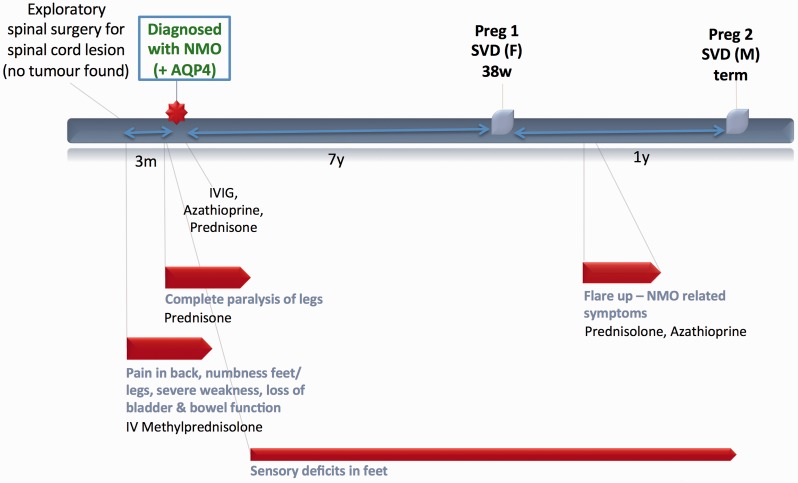
Case 2 (Figure 2)
A 26-year-old African-American woman initially presented with symptoms concerning for cauda equina. An MRI showed hyperintensities of unclear significance at the T4–T6 vertebral levels and exploratory spinal surgery found no apparent cause. Her symptoms responded partially to IV corticosteroids but there was progression to complete lower extremity paralysis within three months. She was eventually diagnosed with NMO when anti-AQP4 antibodies were detected in her serum. Over the next two years, her sensory deficits continued, however, her motor symptoms gradually recovered following courses of IV immunoglobulin (IVIG), azathioprine, prednisone, and extensive physical rehabilitation.
Many years later she underwent a healthy first pregnancy but experienced postpartum exacerbation of NMO requiring treatment with prednisone and azathioprine.
Figure 2.
Case 2.
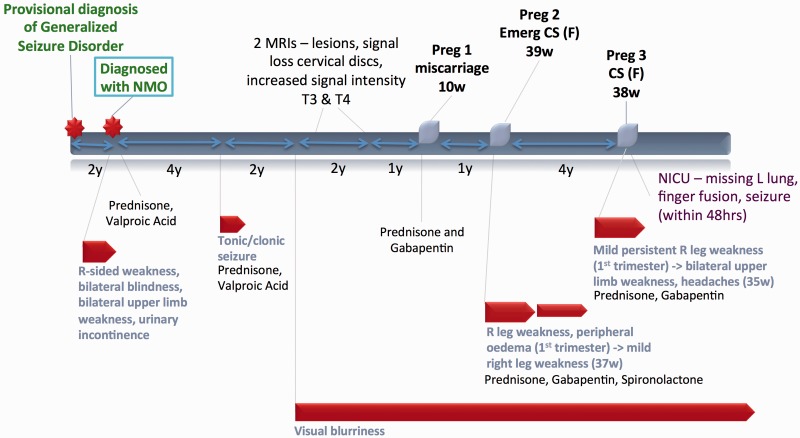
Figure 3.
Case 3.
Prior to her next pregnancy, she came off all immunosuppressants and had an uncomplicated second pregnancy without NMO symptoms.
Case 3 (Figure 3)
A 21-year-old African woman who had previously been diagnosed with generalized seizures, presented with hemiparesis, loss of vision, and bladder symptoms over a three-week period. After a complete workup, she was diagnosed with NMO and started prednisone therapy with good response. Her only subsequent neurologic symptoms included a seizure four years later, and later recurrence of visual symptoms. MRIs performed in subsequent years revealed signal loss within the cervical discs on T2-weighted imaging and signal hyperintensity in the thoracic T3 and T4 vertebrae.
The patient later conceived but had first trimester spontaneous abortion. During her second pregnancy, she experienced recurrence of NMO symptoms with progressively worsening visual complaints and hemiparesis requiring use of a walker. She was treated with gabapentin and prednisone and eventually delivered a healthy infant at term by CS for failure to progress. There was no progression of symptoms postpartum.
During her third pregnancy she was maintained on a regimen of gabapentin and prednisone. Although her symptoms persisted, in late third trimester, she had exacerbation of weakness now involving both upper limbs and experienced new headaches. No changes were made to her medications, and she eventually delivered a female infant by CS at term. The infant had congenital fusion of her digits and left lung aplasia. She went on to require NICU admission for respiratory failure and seizures.
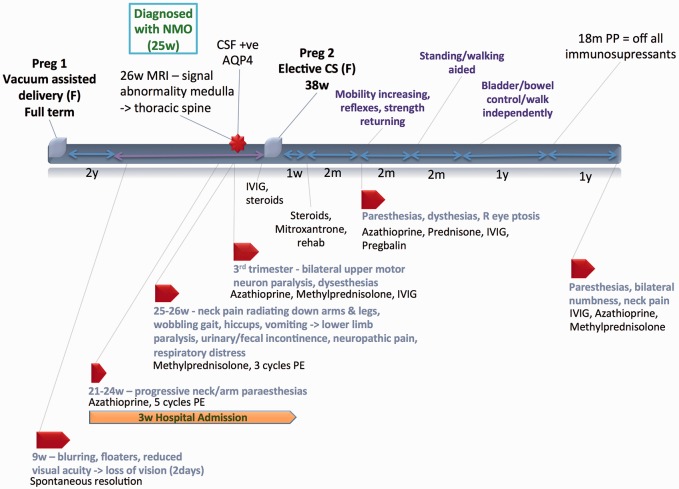
Case 4 (Figure 4)
Figure 4.
Case 4.
A 28-year-old woman with a prior uncomplicated pregnancy presented in her first trimester with transient progressive visual symptoms that spontaneously resolved. Three months later she was admitted to hospital with progressive neck and arm paresthesias. Despite treatment with azathioprine and plasma exchange, she progressed to dysesthesias involving upper and lower extremities, gait changes, hiccups and vomiting, and eventually had complete lower limb paralysis with bladder and bowel incontinence. MRI studies revealed extensive signal abnormalities in her medulla and thoracic spine. Cerebrospinal fluid (CSF) tested positive for AQP4. During the course of the pregnancy, her symptoms fluctuated with motor and sensory involvement, even after high doses of IV methylprednisolone, numerous cycles of plasma exchange, IVIG, and azathioprine. Notably, fetal surveillance was always reassuring throughout. She went on to deliver a healthy infant by CS at term. Postpartum, azathioprine was stopped, steroids tapered and Mitoxantrone started, along with physical rehabilitation.
Over the next six months the patient had fluctuating but gradually improving symptoms and was eventually walking with assistance. Azathioprine and monthly IVIG were used for sustained improvement. Bladder and bowel continence was eventually regained. By 18 months, she was walking independently and off immunosuppressive therapy in complete remission.
She relapsed again two years later with similar symptoms to her initial presentation. MRIs revealed hyperintensities at the T11 vertebral level. She was restarted on IV methylprednisolone, IVIG, azathioprine, and prednisone with some recovery.
Discussion
Devic syndrome in pregnancy was first reported in 19856 in the case of a woman who successfully delivered without complications after multiple treatments with plasmapheresis. Her symptoms included optic neuritis and limb weakness progressing to paralysis. In our case series, we describe four patients with 13 pregnancies that were managed at a tertiary care hospital in Toronto, Canada. Three of the four patients showed either a relapsing and remitting pattern or progressive worsening during their respective pregnancies. In two of the four patients, NMO-related symptoms appeared de-novo, during or soon after a pregnancy.
NMO is known to have a higher prevalence in women of Asian, African American, and Hispanic descent.1 We observed this trend in our case series, with three of the four women being African or African American. Most published observational studies show a median age of onset in the late 30s–40s, however, in our case series, the median age of disease onset was lower at 27 years (24.75–28.25). With a higher index of suspicion for the disease, likely leading to earlier diagnosis, there is an opportunity to better examine the relationship between NMO and pregnancy prospectively over time.
Studies suggest that NMO exacerbates in pregnancy2,7,8 but unlike MS, where a specific immune target has yet to be identified, NMO is a distinct autoimmune disease with a specific antibody target. Unlike most other autoimmune diseases that improve in pregnancy, NMO can develop de novo or worsen in pregnancy and the postpartum period, as was seen in three out of four of our cases. In fact, of the 13 total reported pregnancies, only four pregnancies did not develop NMO-related symptoms during pregnancy or postpartum.
There are some possible explanations for increased NMO activity in pregnancy, including rising AQP4 titers during the third trimester and postpartum,9 accelerated demyelination of astrocytes due to their susceptibility in pregnancy and a potential role of the hyperestrogenemic or hyperprolactenemic states of pregnancy. Other suggested hypotheses include changes in T-helper cell function in pregnancy, akin to diseases such as systemic lupus10 or Sjogren’s, both of which are actually more common in patients with NMO.11
With regard to the effect that NMO has on pregnancy, we showed that 15% of the pregnancies studied resulted in miscarriage, which is comparable to the 13% spontaneous miscarriage rate seen in a retrospective cohort of AQP4 positive women from the National NMO Service (Oxford, UK)5 and higher than expected given that the median age of our patients was 27 years old. Three of the 8 pregnancies were preterm live births occurring at 36, 34, and 33 weeks (mean gestational age 34.3 weeks, SD 1.25).
Regarding fetal and neonatal outcomes, we observed one case of multiple congenital anomalies (aplastic left lung and fusion of fingers) in a female infant delivered at 38 weeks, who then experienced a seizure two days after birth. Although the mother received gabapentin, spironolactone and prednisone during the pregnancy, none of these medications have been previously associated with the observed fetal anomalies or neonatal seizures.
In our case series, six treatment strategies were used including oral and intravenous corticosteroids, azathioprine, IVIG, plasma exchange, gabapentin/pregabalin, and mitoxantrone. Other drugs such as amitriptyline were also used as adjuvant analgesics for neuropathic pain. A summary of the evidence for use of these modalities in pregnancy and lactation is shown in Table 1. Corticosteroids such as prednisone are commonly used during pregnancy, are generally considered safe outside of the first trimester,12–15 and formed the mainstay of treatment for NMO. IVIG was also used successfully in two pregnancies in our series and has also shown promise in several other reports of NMO in pregnancy.16 Plasma exchange is deemed safe in pregnancy17 and was used successfully in one of our reported pregnancies. Azathioprine is an immunosuppressant with benefits generally believed to outweigh the risks in pregnancy when used for disease modulation.18 It was successfully used in 7 of the 13 pregnancies in our series and has also been shown in other reports to reduce disease progression and disability in NMO in pregnancy.19 Gabapentin has routinely been used for the treatment of neuropathic pain associated with NMO,20 and is generally considered safe in pregnancy.21 In our series gabapentin was used in four pregnancies. Of these, two relapsed with recurrence of symptoms, one patient delivered an infant with congenital anomalies and neonatal seizures and one resulted in a miscarriage. Although the adverse fetal outcomes cannot directly be attributed to gabapentin, its efficacy in managing symptoms of NMO remains uncertain. Pregabalin is believed to be more effective than gabapentin in the treatment of neuropathic pain in NMO patients22 and could be considered as an alternative. We report two pregnancies in which no medications were taken due to stable ongoing disease activity that resulted in two healthy term deliveries. A study by Shi et al.19 showed similar outcomes with eight pregnant women with NMO, with stable disease activity that resulted in eight live births without complications. After careful discussion of the risks and benefits and the knowledge that symptoms of NMO often worsen in pregnancy, an approach involving conservative (non-medicated) management may be an option for those with stable disease activity.23,24
Table 1.
Medication use in pregnancy.
| Medication | Evidence for use in pregnancy | Evidence for use in breastfeeding |
|---|---|---|
| Corticosteroids(e.g. prednisone, methylprednisolone) |
|
|
| IVIG |
|
|
| Amitriptyline |
|
|
| Azathioprine |
|
|
| Mitoxantrone |
|
|
| Gabapentin |
|
IUGR: intrauterine growth restriction; IQ: intelligence quotient.
Our case series is a limited examination of the relationship between NMO and pregnancy since it involves 13 pregnancies in four women, some of which were not managed at our hospital. The data, which were obtained retrospectively from a chart review, have limitations characteristic of a retrospective analysis. Some of these limitations include missing data and insufficient information with regards to doses, duration of therapies, and details on some clinical outcomes. Furthermore, the relapsing and remitting nature of the disease in some pregnancies poses a challenge when trying to accurately assess trends in progression, recovery, response to therapy, and when trying to establish associations.
To our knowledge, our case series is the first Canadian series describing pregnancy outcomes in women with NMO. It adds important information to the limited existing literature on the topic pertaining to the effect pregnancy has on NMO and the course of the disease during pregnancy. With multidisciplinary management, the prognosis for pregnancies in women with NMO is reassuring. This study also highlights a need for prospective data gathering on pregnancy outcomes in women with NMO, possibly through establishment of an international registry. Information gleaned from such a registry would be vital in assisting healthcare providers in the management of pregnancies in these women.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
REB approval was obtained from Mount Sinai Hospital, Toronto – REB#16-0218-C.
Guarantor
AW
Contributorship
All authors have contributed to the production of this paper.
References
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–815. [DOI] [PubMed] [Google Scholar]
- 2.Fragoso YD, Adoni T, Bichuetti DB, et al. Neuromyelitis optica and pregnancy. J Neurol 2013; 260: 2614–2619. [DOI] [PubMed] [Google Scholar]
- 3.Rubio T J, Amaya G PF. Plasma exchange therapy for a severe relapse of Devic's disease in a pregnant woman: a case report and concise review. Clin Neurol Neurosurg 2016; 148: 88–90. [DOI] [PubMed] [Google Scholar]
- 4.Igel C, Garretto D, Robbins MS, et al. Neuromyelitis optica in pregnancy complicated by posterior reversible encephalopathy syndrome, eclampsia and fetal death. J Clin Med Res 2015; 7: 193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nour MM, Nakashima I, Coutinho E, et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology 2016; 86: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilera AJ, Carlow TJ, Smith KJ, et al. Lymphocytaplasmapheresis in Devic's syndrome. Transfusion 1985; 25: 54–56. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Wang Y, Zhou Y, et al. Pregnancy in neuromyelitis optica spectrum disorder: a multicenter study from South China. J Neurol Sci 2017; 372: 152–156. [DOI] [PubMed] [Google Scholar]
- 8.Bourre B, Marignier R, Zephir H, et al. Neuromyelitis optica and pregnancy. Neurology 2012; 78: 875–879. [DOI] [PubMed] [Google Scholar]
- 9.Toji H, Naito K, Yamawaki T, et al. Relapse of neuromyelitis optica during pregnancy: transition of the anti-aquaporin 4 antibodies titer. Clin Exp Neuroimmunol 2015; 6: 67–69. [Google Scholar]
- 10.Pittock SJ, Lennon VA, de Seze J, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol 2008; 65: 78–83. [DOI] [PubMed] [Google Scholar]
- 11.Zhong YH, Zhong ZG, Zhou Z, et al. Comparisons of presentations and outcomes of neuromyelitis optica patients with and without Sjogren's syndrome. Neurol Sci 2017; 38: 271–277. [DOI] [PubMed] [Google Scholar]
- 12.Kallen B. Maternal drug use and infant cleft lip/palate with special reference to corticoids. Cleft Palate. Craniofac J 2003; 40: 624–628. [DOI] [PubMed] [Google Scholar]
- 13.Pirson Y, Van Lierde M, Ghysen J, et al. Retardation of fetal growth in patients receiving immunosuppressive therapy. N Engl J Med 1985; 313: 328. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael SL, Shaw GM, Ma C, et al. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol 2007; 197: 585. ISSN 0002-9378. [DOI] [PubMed] [Google Scholar]
- 15.Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ Can Med Assoc J 2011; 183: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada K, Tsuji S, Tanaka K. Intermittent intravenous immunoglobulin successfully prevents relapses of neuromyelitis optica. Intern Med 2007; 46: 1671–1672. [DOI] [PubMed] [Google Scholar]
- 17.Kronbichler A, Brezina B, Quintana LF, et al. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review. Autoimmun Rev 2016; 15: 38–49. [DOI] [PubMed] [Google Scholar]
- 18.Zare-Shahabadi A, Langroodi HG, Azimi AR, et al. Neuromyelitis optica and pregnancy. Acta Neurol Belg 2016; 116: 431–438. [DOI] [PubMed] [Google Scholar]
- 19.Shi B, Zhao M, Geng T, et al. Effectiveness and safety of immunosuppressive therapy in neuromyelitis optica spectrum disorder during pregnancy. J Neurol Sci 2017; 377: 72–76. [DOI] [PubMed] [Google Scholar]
- 20.Qian P, Lancia S, Alvarez E, et al. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol 2012; 69: 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H, Goel A, Bernard N, et al. Pregnancy outcomes following gabapentin use: results of a prospective comparative cohort study. Neurology 2013; 80: 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz R. Management of non-obstetric pain during pregnancy. Review article. Rev Colomb Anestesiol 2012; 40: 213–223. [Google Scholar]
- 23.Ringelstein M, Harmel J, Distelmaier F, et al. Neuromyelitis optica and pregnancy during therapeutic B cell depletion: infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartum. Mult Scler 2013; 19: 1544–1547. [DOI] [PubMed] [Google Scholar]
- 24.Akiba R, Oshitari T, Yokouchi H, et al. Spontaneous recovery of neuromyelitis optica spectrum disorder during pregnancy. Neuroophthalmology 2015; 39: 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ost L, Wettrell G, Bjorkhem I, et al. Prednisolone excretion in human milk. J Pediatr 1985; 106: 1008–1011. [DOI] [PubMed] [Google Scholar]
- 26.McElhatton PR, Garbis HM, Elefant E, et al. The outcome of pregnancy in 689 women exposed to therapeutic doses of antidepressants. A collaborative study of the European Network of Teratology Information Services (ENTIS). Reprod Toxicol 1996; 10: 285–294. [DOI] [PubMed] [Google Scholar]
- 27.Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 1997; 336: 258–262. [DOI] [PubMed] [Google Scholar]
- 28.Bennett PN and World Health Organization. Regional Office for Europe. Drugs and human lactation : a guide to the content and consequences of drugs, micronutrients, radiopharmaceuticals, and environmental and occupational chemicals in human milk. New York, NY: Elsevier, 1988.
- 29.Cleary BJ, Kallen B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defect Res A 2009; 85: 647–654. [DOI] [PubMed] [Google Scholar]
- 30.Motta M, Ciardelli L, Marconi M, et al. Immune system development in infants born to mothers with autoimmune disease, exposed in utero to immunosuppressive agents. Am J Perinatol 2007; 24: 441–447. [DOI] [PubMed] [Google Scholar]
- 31.Biggioggero M, Borghi MO, Gerosa M, et al. Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus 2007; 16: 651–656. [DOI] [PubMed] [Google Scholar]
- 32.Coelho J, Beaugerie L, Colombel JF, et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study. Gut 2011; 60: 198–203. [DOI] [PubMed] [Google Scholar]
- 33.Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108: 433–440. [DOI] [PubMed] [Google Scholar]
- 34.de Meij TG, Jharap B, Kneepkens CM, et al. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther 2013; 38: 38–43. [DOI] [PubMed] [Google Scholar]
- 35.Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy. Gastroenterology 2012; 142: S149–S14S. [Google Scholar]
- 36.Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol 2014; 72: 132–141. [DOI] [PubMed] [Google Scholar]
- 37.Azuno Y, Kaku K, Fujita N, et al. Mitoxantrone and etoposide in breast milk. Am J Hematol 1995; 48: 131–132. [DOI] [PubMed] [Google Scholar]
- 38.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK epilepsy and pregnancy register. J Neurol Neurosurg Psychiatr 2006; 77: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiby G, Daltveit AK, Engelsen BA, et al. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014; 261: 579–588. [DOI] [PubMed] [Google Scholar]
- 40.Guttuso T, Jr., Shaman M, Thornburg LL. Potential maternal symptomatic benefit of gabapentin and review of its safety in pregnancy. Eur J Obstet Gynecol Reprod Biol 2014; 181: 280–283. [DOI] [PubMed] [Google Scholar]
- 41.Ohman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia 2005; 46: 1621–1624. [DOI] [PubMed] [Google Scholar]