Abstract
This study investigated the effect of temperature on taste and chemesthetic sensations produced by the prototypical salty and sour stimuli NaCl and citric acid. Experiment 1 measured the perceived intensity of irritation (burning, stinging) and taste (saltiness, sourness) produced on the tongue tip by brief (3 s) exposures to suprathreshold concentrations of NaCl and citric acid at 3 different temperatures (12, 34, and 42 °C). No significant effects of temperature were found on the taste or sensory irritation of either stimulus. Experiment 2 investigated the potential effects of temperature on sensory irritation at peri-threshold concentrations and its sensitization over time. Measurements were again made on the tongue tip at the same 3 temperatures. Heating was found to enhance the perception of irritation at peri-threshold concentrations for both stimuli, whereas cooling suppressed sensitization of irritation for NaCl but not for citric acid. These results (i) confirm prior evidence that perception of suprathreshold salty and sour tastes are independent of temperature; (ii) demonstrate that heat has only weak effects on sensory irritation produced by brief exposures to NaCl and citric acid; and (iii) suggest that sensitization of the irritation produced by NaCl and citric acid occur via different peripheral mechanisms that have different thermal sensitivities. Overall, the results are consistent with involvement of the heat-sensitive channel TRPV1 in the sensory irritation of both stimuli together with one or more additional channels (e.g., acid-sensing channel, epithelial sodium channel, TRPA1) that are insensitive to heat and may possibly be sensitive to cooling.
Keywords: chemesthesis, psychophysics, sensory irritation, taste, temperature
Introduction
It is well established that temperature modulates the perception of sweet, bitter, and umami tastes in humans (e.g., Bartoshuk et al. 1982; Green and Frankmann 1987; Green and Nachtigal 2015; Green et al. 2016; Green and Andrew 2017). These thermal effects are attributable at least in part to TRPM5 (Talavera et al. 2005, 2007), a heat-sensitive cation channel in the transduction cascade of the G-protein-coupled receptors that mediate these tastes (Liman 2014). The extent to which temperature affects salty or sour taste is less clear. Pangborn et al. (1970) reviewed the conflicting results of prior studies of salt taste and reported their own data showing that both threshold and suprathreshold sensitivity to NaCl was significantly reduced only at extreme solution temperatures (0 and 55 °C). A subsequent study of taste thresholds by McBurney et al. (1973) found that thresholds for detection of NaCl and HCl varied as a U-shaped function of temperature, but less so than did detection of the bitterness of QSO4 and the sweetness of dulcin. Two other studies conducted using suprathreshold concentrations reported that temperature had no effect on the sourness of citric acid but disagreed as to whether temperature affected the saltiness of NaCl (Moskowitz 1973; Green and Frankmann 1987). However, the study that found an effect on saltiness used an indirect measure of sensitivity (the intercept of the best-fitting power function of perceived taste intensity) which, contrary to other studies, predicted that saltiness is strongest at 50 °C and that bitterness is unaffected by temperature (Moskowitz 1973).
Because the receptors that serve salty and sour taste in humans are not yet fully understood (Breza and Contreras 2012; Liman et al. 2014; Roper 2015; Roper and Chaudhari 2017), the potential mechanisms of any temperature effects are also unclear. For example, a study of sour ageusia in 2 individuals indicated that both polycystic kidney disease channels (PKD1L3 and PKD2L1) and acid-sensing channels (ASICs) may be involved in human sour taste (Huque et al. 2009), and a protein (OTop1) was recently discovered in mice that is required to maintain zinc-sensitive proton conductances in acid-sensing taste cells (Tu et al. 2018). However, no data are available on the temperature sensitivity of OTop1, and in contrast to the human psychophysical data, an in vitro study of human variants of ASICs reported that channel activity was potentiated by cold (Askwith et al. 2001). Similarly, an in vitro study of the human epithelial sodium channel (ENaC), which is potentially involved in both sour and salty taste (Chandrashekar et al. 2010), showed that cooling from 30 to 15 °C increased the probability of channel opening by 2.3-fold (Chraïbi and Horisberger 2003). Finally, a TRPV1 variant (TRPV1t) has been proposed as a receptor for salt taste in rodents (Lyall et al. 2004; Lyall et al. 2005). However, there is as yet no evidence that TRPV1t is expressed in human taste cells, and subsequent behavioral and electrophysiological studies did not support its function as a salt taste receptor in rodents (Treesukosol et al. 2007; Breza and Contreras 2012; Smith et al. 2012).
To fully understand the effect of temperature on perception of salts and acids also requires study of the chemesthetic sensations they evoke at moderate-to-high concentrations (Green and Gelhard 1989; Green 1991; Gilmore and Green 1993). Based on findings that capsaicin desensitization significantly reduces the burning and stinging sensations of NaCl and citric acid on the tongue (Green 1991; Gilmore and Green 1993; Dessirier et al. 2000, 2001), it is likely that the heat-sensitive channel TRPV1 (Caterina et al. 1997; Tominaga et al. 1998; Liu and Simon 2000) plays a role in generating these sensations, and thus that heat may increase their intensity similar to enhancement of the nociceptive sensations of capsaicin (Green 1986; Prescott et al. 1993). A more recent genetic study in humans also found evidence that TRPV1 contributes to the intensity of NaCl saltiness, which the authors proposed may contribute to the aversive qualities of salt taste (Dias et al. 2013). But it is also clear that TRPV1 is not the only nociceptive channel activated by concentrated solutions of salts and acids. For example, capsaicin desensitization can reduce the excitability of capsaicin-sensitive neurons and cause degeneration of intraepithelial nerve endings (Welk et al. 1983; Holzer 1991; Simone et al. 1998; Nolano et al. 1999), both of which would reduce input from any other receptors that are co-expressed with TRPV1. One such receptor is the nonselective cation channel TRPA1 (Story et al. 2003; Kobayashi et al. 2005), which has been reported in rodents to respond to chemical irritants and noxious cold (Story et al. 2003; Bandell et al. 2004). However, not all studies in rodents have found TRPA1 to be cold sensitive (McKemy 2005), and 2 studies of the human variant hTRPA1 came to opposite conclusions regarding its cold sensitivity (Moparthi et al. 2014; Chen 2015). In addition, a mouse variant of TRPA1 was reported to be insensitive to citric acid (Wang et al. 2011), and we could find no published evidence that NaCl is a ligand of TRPA1.
ENaC and ASIC channels are also potential contributors to the chemesthetic sensations of concentrated acids and salts. Both channels sense tissue acidosis and contribute to inflammatory pain (Deval et al. 2010; Delaunay et al. 2012), and Ugawa et al. (2005) reported co-localization in rat dorsal root ganglion of multiple ASIC variants and TRPV1. Consistent with the latter finding are data from Dessirier et al. (2000, 2001), which showed that treating the surface of the tongue with amiloride, a blocker of ENaC and ASIC channels, reduced the sensory irritation of both citric acid and NaCl. In agreement with those findings, Ugawa et al. (2002) and Jones et al. (2004) found that amiloride attenuates the pain from hydrochloric acid infused into the skin, and most relevant to the present investigation, Jones et al. (2004) also reported that the pain from hydrochloric acid in forearm skin did not differ significantly at temperatures of 4 and 40 °C. It has also been proposed based on experiments in mice that NaCl becomes aversive at high concentrations when the sour and bitter taste pathways are recruited via an amiloride-insensitive mechanism (Oka et al. 2013). However, the authors of the study propose that activating these pathways produces an aversive “ionic taste” rather than sensory irritation, and the concentrations of NaCl that were tested (≤1.0 M) would produce at most only weak sensory irritation in humans (Gilmore and Green 1993).
The objectives of this study were therefore to obtain the first data on the effect of solution temperature on the sensory irritation produced by NaCl and citric acid on the tongue while further investigating possible effects of temperature on salty and sour taste. In the first of 2 experiments, taste and irritation were measured as a function of temperature at suprathreshold concentrations that evoked both taste and chemesthetic sensations. The second experiment investigated the effects of temperature on the sensitivity to sensory irritation at peri-threshold concentrations and on the sensitization of irritation over time. Sensitization is a common property of chemosensory irritants in humans and has been demonstrated to occur for NaCl and citric acid (Green and Gelhard 1989; Gilmore and Green 1993; Dessirier et al. 2000; Green and McAuliffe 2000; Dessirier et al. 2001; Sudo et al. 2002; Merrill et al. 2008).
Materials and methods
Experiment 1
Subjects
Recruitment was conducted with flyers posted around the Yale University campus and through an online advertisement (Craigslist). A total of 34 adults (21 females [F], 13 males [M]; 18–45 years of age) were recruited, 30 (19 F, 11 M) of whom completed the study (3 did not return for the second testing session and 1 did not master the intensity rating task in the training and practice session, described later). Participation was limited to English speakers, self-reported healthy nonsmokers who had no known taste or smell disorders or deficiencies, and those were not pregnant and had no lip, cheek, or tongue piercings that might interfere with taste testing. Participants were asked to refrain from eating or drinking foods or beverages for at least 1 h prior to their scheduled session and to avoid hot/spicy food for 24 h before their session. The research protocol complied with the Declaration of Helsinki for medical research involving human subjects and was approved by the Human Investigations Committee of the Yale University IRB. Participants provided informed consent and were compensated for their participation.
Stimuli
The test stimuli were NaCl (Fisher Scientific) and citric acid (Sigma-Aldrich), and each delivered at 3 concentrations: 1.0, 2.0, and 2.8 M NaCl and 0.032, 0.056, and 0.10 M citric acid. All solutions were prepared weekly in 250 mL aliquots with deionized water (dH2O) and stored in airtight 250 mL flasks. Prior to each experimental session, the flasks were placed in 3 different circulated constant-temperature water baths set to temperatures of 12, 34, and 42 °C.
Training and practice procedure
All recruited participants attended a practice session to become familiarized with the general version of the Labeled Magnitude Scale (gLMS; Green et al. 1993; Green et al. 1996; Bartoshuk et al. 2003) to practice using it to rate sensation intensity. After receiving instructions on how to use the gLMS, participants were asked to rate the intensities of 15 imagined sensations (e.g., the sweetness of milk, the pain of biting your tongue, the weight of a feather in your hand) that were also read to them by the experimenter. This procedure also enabled the experimenter to determine if participants grasped the concept of the gLMS and were using it as instructed (i.e., making their ratings in the context of “the strongest imaginable sensation of any kind”). Participants were then given practice rating actual sensations of various kinds (e.g., the coolness of a penny placed in the hand, the touch sensation from a cotton swab, the brightness of the ceiling light) before rating the sweetness, saltiness, sourness, bitterness, and umami taste of several gustatory stimuli (560 mM sucrose, 18 mM citric acid, 320 mM NaCl, 0.18 mM QHCl, 100 mM monopotassium glutamate (MPG) alone, and in 5 binary mixtures).
Data collection
There were 2 testing sessions: 1 stimulus (NaCl or citric acid) was sampled per session and the order of testing was counterbalanced across subjects. All 3 solution temperatures were presented in each session and also counterbalanced across subjects. For each temperature, the stimuli were presented in an ascending order of concentration to avoid possible adaptation effects.
Stimulus presentation was limited to the tongue tip, which offered better control of stimulus temperature and duration than sipping and expectorating solutions. Stimuli were sampled sequentially by dipping the tongue tip into 2 weigh boats containing ~7.5 mL of solution, which was pipetted into weigh boats at the beginning of each trial. Use of the weigh boats enabled the tongue tip to be submerged in approximately 1 cm of solution without making incidental contact with other surfaces (e.g., the sides of a medicine cup). The first weigh boat contained dH2O and the second contained the test stimulus. The experimenter signaled the subject to dip the tongue into the first solution for 3 s timed by a stopwatch, then verbally signaling the subject to “switch” to the second solution by lifting the tongue from the first solution and immediately dipping it into the second one. After 3 s, the experimenter instructed the participant to lift the tongue from the second solution and rate the intensity of sensory irritation followed by the intensity of taste (saltiness in the NaCl session and sourness in the citric acid session). The instructions were to make their ratings as quickly as possible with the tongue still extended, and to ignore as best they could sensations of temperature. There was a 1-min intertrial interval after each stimulus during which subjects rinsed at least 3 times with 37 °C dH2O to remove any lingering taste and to reduce the opportunity for sensitization of irritation over time.
Experiment 2
Subjects
Recruitment was conducted as in experiment 1. Individuals provided informed consent and were paid for their participation. Thirty-one adults (22 F, 9 M; 18–45 years of age) qualified for and completed the study out of a total of 34 who were recruited (2 did not return to complete all 6 sessions and 1 did not master the intensity rating task in the training and practice session). Participants were once again asked to refrain from eating or drinking food or beverages for at least 1 h prior to their scheduled session and to avoid hot/spicy food for 24 h before their session. The research protocol complied with the Declaration of Helsinki for medical research involving human subjects and was approved by the Human Investigations Committee of the Yale University IRB.
Stimuli
The test stimuli were 5 concentrations of NaCl (0.56, 1.0, 1.8, 2.0, and 2.4 M; Fisher Scientific) and citric acid (0.018, 0.032, 0.056, 0.10, and 0.18 M; Sigma-Aldrich). The concentrations chosen were based on pilot experiments conducted with laboratory personnel and were intended to evoke sensory irritation that ranged from imperceptible to clearly perceptible. All solutions were again prepared weekly in 250 mL aliquots with dH2O and stored in airtight 250 mL flasks until prior to each session. Solution temperatures were the same 3 used in experiment 1 (12, 34, and 42 °C).
Training and practice procedure
All participants were required to complete the same training and practice as in experiment 1.
Data collection
There were 6 testing sessions. One stimulus (NaCl or citric acid) was tested at one of the 3 temperatures (12, 34, or 42 °C) in each session. Participants completed all 3 sessions for one stimulus before sampling the other stimulus. The order of stimuli and temperatures tested were counterbalanced across subjects. The same sequential sampling procedure of experiment 1 was used again here.
Each session began with an ascending method of limits procedure to determine the lowest concentrations of NaCl or citric acid that produced clearly detectable sensory irritation at the temperature that was tested in that session. In addition to providing data on the effect of temperature on the perception of peri-threshold sensory irritation, the procedure was also intended to identify stimuli for measurements of sensitization over time. Using concentrations that initially produced very weak irritation maximized the opportunity for measuring sensitization while avoiding the induction of noxious sensations at the longest duration of exposure. Participants again submerged the tongue tip in the first weigh boat (containing dH2O) for 3 s before immediately dipping the tongue tip into the stimulus solution in the second weigh boat (beginning with the lowest concentration) for 3 s, timed by the experimenter. After sampling the test stimulus, the participants verbally reported whether or not they perceived a burning, stinging, or pricking sensation. When irritation was perceived, they were asked to describe its intensity verbally using the gLMS as a guide (e.g., “only barely detectable”; “more than barely detectable but less than weak”). Stimulus concentrations were presented in ascending order until the participants first reported irritation that they described as more than “barely detectable.”
In the main task, the first weigh boat contained either (i) H2O only (the baseline condition: no pre-exposure to the stimulus), which was sampled for 3 s, or (ii) the test stimulus (sensitization condition), which was sampled for 3, 7, or 12 s, timed by the experimenter. At the experimenter’s verbal signal, the participant lifted the tongue from the first weigh boat and dipped it into a second weight boat (postexposure stimulus) for 3 s that contained the same test stimulus at the same temperature. The postexposure enabled assessment of any sensitization that resulted from the immediately preceding exposure. To avoid possible lingering effects of sensitization on the baseline condition, the H2O pre-exposure condition was presented first followed by the 3, 7, and 12 s pre-exposures. As with experiment 1, there was a 1-min intertrial interval during which subjects rinsed at least 3 times with 37 °C dH2O.
Data analysis
In both experiments, the gLMS data were normalized by converting to log10 and analyzed with repeated-measures ANOVAs using the General Linear Models module of Statistica v.13. Significant interactions were followed-up with Tukey Honestly Significant Difference (HSD) post hoc tests.
Results
Experiment 1
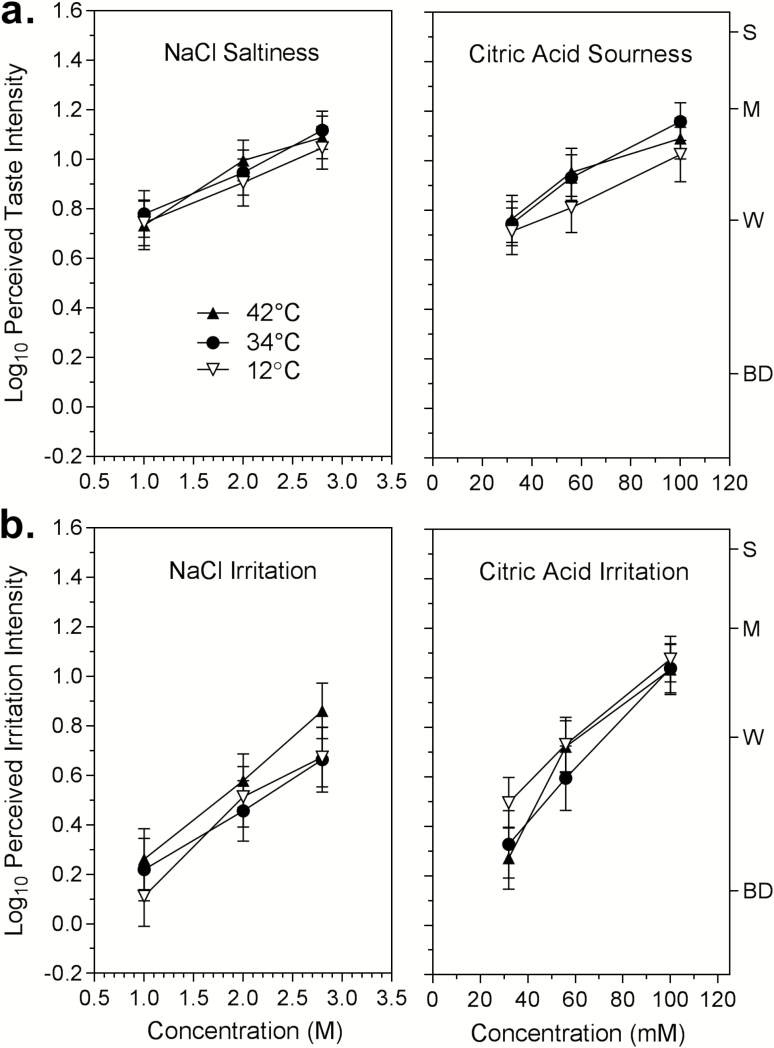
Figure 1 contains the log10-mean ratings of the taste and sensory irritation of NaCl and citric acid as a function of concentration. Figure 1a shows that the sourness of citric acid and the saltiness of NaCl were perceived to be similarly intense at the concentrations tested. An ANOVA on the taste data showed there was no main effect of temperature (F2,58 = 2.11; P = 0.13) nor any significant interactions with temperature. Analysis of the sensory irritation data (Figure 1b) also found no main effect of temperature (F2,58 = 2.58; P = 0.41) or significant interactions with temperature. But unlike taste intensity, there was a main effect of stimulus (F1,29 = 7.47; P < 0.05) and a significant stimulus × concentration interaction (F2,58 = 3.61; P < 0.05). The latter effects were independent of temperature and resulted from stronger irritation produced by citric acid compared with NaCl, and the tendency for the difference in irritation between stimuli to increase with concentration. However, the slopes of the functions for sensory irritation for both NaCl and citric acid were steeper than for saltiness and sourness, indicating that as concentration increased, irritation contributed more to the sensory impact of both stimuli. This tendency is consistent with the recruitment of lingual nociceptive afferent fibers of the trigeminal nerve (Sudo et al. 2002; Sudo et al. 2003).
Figure 1.
Shown are the log10-mean ratings of the perceived intensity of (a) the saltiness of NaCl and the sourness of citric acid, and (b) the sensory irritation produced by each stimulus at 3 concentrations and 3 different temperatures. Temperature had no significant effect on either sensation for either stimulus. Letters on the right y axis denote semantic labels of perceived intensity on the gLMS: BD = barely detectable; W= weak; M = moderate; S = strong. Vertical lines represent the standard errors of the means.
Experiment 2
The data obtained in the sensitivity portion of this experiment show that at peri-threshold concentrations the irritation produced by both stimuli was enhanced by heat (Figure 2). Separate repeated-measures ANOVAs found significant effects of temperature for both stimuli (NaCl: F2,60 = 6.73, P < 0.005; citric acid: F2,60 = 3.42, P < 0.05). Post hoc analyses showed that compared with 12 °C, sensitivity was significantly higher at 42 °C for NaCl, and at both 34 and 42 °C for citric acid.
Figure 2.
The averages of the lowest concentrations of NaCl and citric acid that produced sensations of irritation that were more than just “barely detectable” are plotted as a function of solution temperature. Asterisks denote warmer temperatures at which the average concentration was significantly lower than the mean concentration at 12 °C (Tukey HSD, P < 0.05). Vertical lines represent the standard errors of the means.
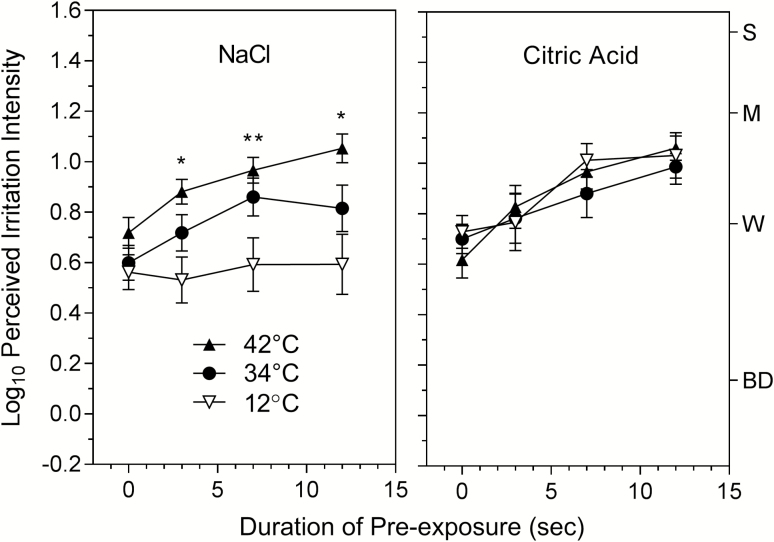
In contrast, temperature had a selective effect on sensitization of irritation over time. Figure 3 shows that for NaCl, sensitization of irritation was completely blocked at 12 °C but grew in intensity at 34 and 42 °C, whereas sensitization was independent of temperature for citric acid. An ANOVA on the data for both stimuli found there was a main effect of temperature (F1,30 = 7.14, P < 0.002) and significant interactions between stimulus and temperature (F2,60 = 10.62; P < 0.0002) and between temperature and time (F6,180 = 3.28, P < 0.005). To further investigate the effect of temperature on sensitization for NaCl, a separate ANOVA on those data showed the expected main effects of temperature (F2,60 = 17.33; P < 0.00001) and time (F3,90 = 4.53; P < 0.005) and a significant interaction between these factors (F6,180 = 2.35; P < 0.05). Analysis of the citric acid data yielded only a significant effect of time (F3,90 = 12.09; P < 0.00001), which confirmed the temperature independence of sensitization over time. Although the rank order of log-mean irritation ratings for NaCl in the baseline condition (pre-exposure = 0) was consistent with the effect of temperature on sensitization, post hoc tests found no significant effect of temperature for either stimulus (P > 0.05) in this condition. This was the expected result because in the detection task the test concentrations were determined separately for each temperature, which effectively “corrected” for any effects of temperature.
Figure 3.
Shown are the log10-mean ratings of the perceived intensity of sensory irritation produced by NaCl and citric acid at 3 different solution temperatures as a function of the duration of pre-exposure to the same stimulus. Sensitization was significant for both stimuli but was significantly affected by temperature only for NaCl. Asterisks indicate significant differences (Tukey HSD, P < 0.05) in sensitization between 12 and 42°C only (*) and between 12 and both 34 and 42 °C (**). Letters on the right y axis denote semantic labels of perceived intensity on the gLMS: BD = barely detectable; W = weak; M = moderate; S = strong. Vertical lines represent the standard errors of the means.
Discussion
Saltiness and sourness
The taste data support prior evidence that at concentrations of NaCl and citric acid that produce weak-to-moderate tastes, temperatures within the innocuous range do not significantly affect saltiness (Pangborn et al. 1970; Green and Frankmann 1987) or sourness (Green and Frankmann 1987). The data do not speak to the results of Pangborn et al. (1970), which indicated that more extreme temperatures (50 and near 0 °C) that strongly stimulate thermal nociceptors (e.g., Van Hees and Gybels 1981; Treede et al. 1998; Caterina and Julius 2001; Stucky et al. 2009) can interfere with salt taste perception. Because 50 °C exceeds the threshold for heat pain on the tongue tip (Green 1985), and because cutaneous pain can mask innocuous touch (Apkarian et al. 1994; Bolanowski et al. 2000), it is reasonable to expect that noxious heat and cold can also mask taste.
The absence of a significant effect of heat on weak-to-moderate tastes does not support a significant role for the proposed salt taste receptor TRPV1t (Lyall et al. 2004; Lyall et al. 2005). Similarly, the lack of a significant effect of cold suggests either that any contribution of cold-sensitive ASIC (Askwith et al. 2001) or ENaC channels (Chraïbi and Horisberger 2003) to sourness is relatively minor, or that the effect of cold on these channels is too small to be perceived at the concentrations we tested. The concentrations chosen in this study ensured that both taste and sensory irritation would be perceived, which resulted in relatively strong salty and sour tastes. Thus the results do not conflict with those of McBurney et al. (1973), which showed that the thresholds for detection of salty and sour tastes vary as U-shaped functions of temperature.
Because the data were collected exclusively on the tongue tip, the possibility cannot be ruled out that temperature may be a more significant factor in the posterior oral cavity, where taste is mediated by the glossopharyngeal and greater superficial petrosal nerves. But evidence against regional differences comes from prior studies in which little or no effects of innocuous temperatures were found during whole mouth exposures to suprathreshold salty and sour stimuli (Pangborn et al. 1970; Green and Frankmann 1987).
It is important to consider that this results do not agree with electrophysiological studies in animal models that have found significant effects of temperature on the responses of peripheral gustatory neurons to moderate concentrations of salts and acids (e.g., Nakamura and Kurihara 1988, 1991; Lu et al. 2016); for a review, see Lemon (2017). The disagreement may result from species differences in the receptor mechanisms for these tastes. Alternatively, interactions between gustatory and thermal (trigeminal) inputs may modify the effect of temperature on the response of central gustatory neurons. This possibility is supported by data showing that subsets of neurons in the mouse NTS that respond to NaCl and HCl do not exhibit the characteristic inverted U-shaped response to temperature that has generally been found in chorda tympani neurons (Wilson and Lemon 2013).
Sensory irritation
The evidence of increased sensitivity at peri-threshold concentrations of NaCl and citric acid for warmer temperatures in experiment 2, and the absence of a significant effect of temperature at suprathreshold levels in experiment 1, indicates that the effect of heat on sensory irritation produced by both stimuli is relatively weak. This outcome is consistent with the study by Jones et al. (2004) cited earlier, which found that the pain evoked by infusion of hydrochloric acid into forearm skin was not significantly different at temperatures of 4 and 40 °C. Both results suggest that TRPV1 plays a greater role in the irritancy of NaCl and citric acid at low, peri-threshold concentrations. The results are also consistent with the fact that cross-desensitization of the sensory irritation produced by NaCl and citric acid is only partial (Green 1991; Gilmore and Green 1993; Dessirier et al. 2000, 2001), which suggests that TRPV1 is not the only chemo-nociceptor involved in the irritancy of these stimuli. Because the cold sensitivity of TRPA1 remains unclear (Moparthi et al. 2014; Chen 2015), the absence of an effect of cold at either peri-threshold or suprathreshold concentrations does not rule out a possible role of hTRPA1 in the irritancy of either stimulus. Nor do the results rule out contributions by ASIC and/or ENaC. As noted earlier, Dessirier et al. (2000, 2001) reported that amiloride weakly but significantly reduced the irritancy of NaCl and citric acid, and ASIC and ENaC have been reported in some studies to be activated by cold (Askwith et al. 2001; Chraïbi and Horisberger 2003). However, any enhancement of the activity of these channels at colder temperatures appears to be less than the effects of heat on TRPV1 at peri-threshold levels. On the other hand, the absence in experiment 1 of a significant effect of heat on the irritation produced during 3-s stimulus exposures also implies that the enhancement of TRPV1 by heat becomes a smaller fraction of the total nociceptive signal as stimulation intensifies, perhaps as a result of the recruitment of channels that are insensitive to heat and which may be sensitive to cooling.
Sensitization of sensory irritation
The marked effect of temperature on sensitization of NaCl irritation was unexpected. Its occurrence only for NaCl implies that sensitization arises from peripheral mechanisms that differ for NaCl and citric acid. The possibility that the effect of temperature is attributable to sensitization of TRPV1 is supported by data from Ahern et al. (2005), indicating that cations can increase the response of TRPV1 to its ligands and also to temperature. Sensitization to NaCl irritation may therefore result from an interaction between the excitatory effects of Na+ and heat on TRPV1. Such an interaction would be expected to begin at the onset of stimulation and intensify over time. Rapid development of sensitization is consistent with significant sensitization after just a single, 3-s pre-exposure to NaCl (Figure 3), and with nonsignificant trends toward increased irritation from NaCl at 42 °C during the 3-s exposures in experiment 1 (Figure 1), and in the baseline condition of experiment 2 (Figure 3).
It is unclear why sensitization was not also temperature dependent for citric acid given that H+ potentiates the sensitivity of TRPV1 to heat and to endogenous agonists (Caterina et al. 1997; Tominaga et al. 1998). One possibility is that sensitization of NaCl irritation depends on diffusion of the stimulus to receptors in the lingual epithelium, which might be more rapid at warmer temperatures. However, modulation of diffusion by temperature would also be expected to affect the threshold for NaCl irritation, but the effects of temperature were similar for NaCl and citric acid (Figure 2). Alternatively, under the present experimental conditions sensitization to citric acid may have resulted primarily from a breakdown in the buffering effect of saliva during exposure to aqueous solutions. As the tongue tip remained in the solution, saliva would become more dilute and be progressively rinsed away, which would increase the availability of protons to receptors over time. In this view, longer stimulus exposures would be equivalent to raising the concentration of citric acid, which in experiment 1 led to increases in sensory irritation that were independent of temperature. Underlying this temperature independence may be an increasing involvement by ASIC channels. Stronger activation of these channels over time might also help sustain sensitization at cooler temperatures, where activation of TRPV1 would be expected to decline.
Summary and conclusions
Consistent with prior evidence, at suprathreshold concentrations salty and sour tastes were not significantly affected by solution temperature, nor was the sensory irritation produced by brief exposures to NaCl or citric acid. However, in a detection task the sensitivity to irritation increased with temperature for both stimuli, and at peri-threshold concentrations sensitization of irritation over time was highly temperature dependent for NaCl but not for citric acid. The relatively weak effect of heat on initial, suprathreshold irritation and the stimulus specificity of the effect of heat on sensitization were unexpected, given prior evidence that the irritancies of both stimuli are mediated in part by the heat-sensitive nociceptive channel TRPV1. The results suggest that in humans, one or more chemo-nociceptive channels that are insensitive to heat but which may be sensitive to cooling (e.g., ASIC, ENaC, TRPA1) play an increasing role in the sensory irritation of NaCl and citric acid at higher concentrations.
Funding
This work was supported in part by a grant from The National Institutes of Health [RO1-DC05002].
References
- Ahern GP, Brooks IM, Miyares RL, Wang XB.. 2005. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 25:5109–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Stea RA, Bolanowski SJ.. 1994. Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res. 11:259–267. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM.. 2001. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci U S A. 98:6459–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A.. 2004. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 41:849–857. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Rennert K, Rodin J, Stevens JC.. 1982. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav. 28:905–910. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ.. 2003. Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer. 14:125–138. [Google Scholar]
- Bolanowski SJ, Maxfield LM, Gescheider GA, Apkarian AV.. 2000. The effects of stimulus location on the gating of touch by heat- and cold-induced pain. Somatosens Mot Res. 17:195–204. [DOI] [PubMed] [Google Scholar]
- Breza JM, Contreras RJ.. 2012. Anion size modulates salt taste in rats. J Neurophysiol. 107:1632–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D.. 2001. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 24:487–517. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D.. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389:816–824. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS.. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 464:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. 2015. The evolutionary divergence of TRPA1 channel: heat-sensitive, cold-sensitive and temperature-insensitive. Temperature (Austin). 2:158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraïbi A, Horisberger JD.. 2003. Dual effect of temperature on the human epithelial Na+ channel. Pflugers Arch. 447:316–320. [DOI] [PubMed] [Google Scholar]
- Delaunay A, Gasull X, Salinas M, Noël J, Friend V, Lingueglia E, Deval E.. 2012. Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc Natl Acad Sci U S A. 109:13124–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Iodi-Carstens M, Carstens E.. 2000. Sensory properties of citric acid: psychophysical evidence for sensitization, self-desensitization, cross-desensitization and cross-stimulus-induced recovery following capsaicin. Chem Senses. 25:769–780. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Iodi-Carstens M, Yao E, Carstens E.. 2001. Oral irritation by sodium chloride: sensitization, self-desensitization, and cross-sensitization to capsaicin. Physiol Behav. 72:317–324. [DOI] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E.. 2010. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 128:549–558. [DOI] [PubMed] [Google Scholar]
- Dias AG, Rousseau D, Duizer L, Cockburn M, Chiu W, Nielsen D, El-Sohemy A.. 2013. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem Senses. 38:137–145. [DOI] [PubMed] [Google Scholar]
- Gilmore MM, Green BG.. 1993. Sensory irritation and taste produced by NaCl and citric-acid: effects of capsaicin desensitization. Chem Senses. 18:257–272. [Google Scholar]
- Green BG. 1985. Heat pain thresholds in the oral-facial region. Percept Psychophys. 38:110–114. [DOI] [PubMed] [Google Scholar]
- Green BG. 1986. Sensory interactions between capsaicin and temperature in the oral cavity. Chem Senses. 11:371–382. [Google Scholar]
- Green BG. 1991. Capsaicin cross-desensitization on the tongue - psychophysical evidence that oral chemical irritation is mediated by more than one sensory pathway. Chem Senses. 16:675–689. [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM.. 1993. Derivation and Evaluation of a Semantic Scale of Oral Sensation Magnitude with Apparent Ratio Properties. Chem Senses. 18:683–702. [Google Scholar]
- Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J.. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21:323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Alvarado C, Andrew K, Nachtigal D.. 2016. The effect of temperature on umami taste. Chem Senses. 41:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Andrew K.. 2017. Stimulus-dependent effects of temperature on bitter taste in humans. Chem Senses. 42:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Frankmann SP.. 1987. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses. 12:609–619. [Google Scholar]
- Green BG, Gelhard B.. 1989. Salt as an oral irritant. Chem Senses. 14:259–271. [Google Scholar]
- Green BG, McAuliffe BL.. 2000. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol Behav. 68:631–639. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D.. 2015. Temperature affects human sweet taste via at least two mechanisms. Chem Senses. 40:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. 1991. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 43:143–201. [PubMed] [Google Scholar]
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG.. 2009. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 4:e7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB.. 2004. Acid-induced pain and its modulation in humans. J Neurosci. 24:10974–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K.. 2005. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 493:596–606. [DOI] [PubMed] [Google Scholar]
- Lemon CH. 2017. Modulation of taste processing by temperature. Am J Physiol Regul Integr Comp Physiol. 313:R305–R321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER. 2014. TRPM5. Handb Exp Pharmacol. 222:489–502. [DOI] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C.. 2014. Peripheral coding of taste. Neuron. 81:984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA.. 2000. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol Behav. 69:363–378. [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Contreras RJ.. 2016. Temperature influences chorda tympani nerve responses to sweet, salty, sour, umami, and bitter stimuli in mice. Chem Senses. 41:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA.. 2004. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 558:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA.. 2005. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses. 30(Suppl 1):i42–i43. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Collings VB, Glanz LM.. 1973. Temperature dependence of human taste responses. Physiol Behav. 11:89–94. [DOI] [PubMed] [Google Scholar]
- McKemy DD. 2005. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AW, Cuellar JM, Judd JH, Carstens MI, Carstens E.. 2008. Effects of TRPA1 agonists mustard oil and cinnamaldehyde on lumbar spinal wide-dynamic range neuronal responses to innocuous and noxious cutaneous stimuli in rats. J Neurophysiol. 99:415–425. [DOI] [PubMed] [Google Scholar]
- Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, Johanson U, Zygmunt PM.. 2014. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A. 111:16901–16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz HR. 1973. Effect of solution temperature on taste intensity in humans. Physiol Behav. 10:289–292. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K.. 1988. Temperature dependence of amiloride-sensitive and -insensitive components of rat taste nerve response to NaCl. Brain Res. 444:159–164. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K.. 1991. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol. 261:R1402–R1408. [DOI] [PubMed] [Google Scholar]
- Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR.. 1999. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 81:135–145. [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS.. 2013. High salt recruits aversive taste pathways. Nature. 494:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn RM, Chrisp RB, Bertoler LL.. 1970. Gustatory, salivary, and oral thermal responses to solutions of sodium chloride at 4 temperatures. Atten Percept Psychophys. 8:69–75. [Google Scholar]
- Prescott J, Allen S, Stephens L.. 1993. Interactions between oral chemical irritation, taste and temperature. Chem Senses. 18:389–404. [Google Scholar]
- Roper SD. 2015. The taste of table salt. Pflugers Arch. 467:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N.. 2017. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 18:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR.. 1998. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 18:8947–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Treesukosol Y, Paedae AB, Contreras RJ, Spector AC.. 2012. Contribution of the TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. Am J Physiol Regul Integr Comp Physiol. 303:R1195–R1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. . 2003. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 112:819–829. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM.. 2009. Roles of transient receptor potential channels in pain. Brain Res Rev. 60:2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S, Sudo M, Simons CT, Dessirier JM, Carstens E.. 2002. Sensitization of trigeminal caudalis neuronal responses to intraoral acid and salt stimuli and desensitization by nicotine. Pain. 98:277–286. [DOI] [PubMed] [Google Scholar]
- Sudo S, Sudo M, Simons CT, Dessirier JM, Iodi Carstens M, Carstens E.. 2003. Activation of neurons in trigeminal caudalis by noxious oral acidic or salt stimuli is not reduced by amiloride. Brain Res. 969:237–243. [DOI] [PubMed] [Google Scholar]
- Talavera K, Ninomiya Y, Winkel C, Voets T, Nilius B.. 2007. Influence of temperature on taste perception. Cell Mol Life Sci. 64:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B.. 2005. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 438:1022–1025. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D.. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21:531–543. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN.. 1998. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 80:1082–1093. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC.. 2007. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol. 292:R1799–R1809. [DOI] [PubMed] [Google Scholar]
- Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER.. 2018. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 359:1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S.. 2002. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 110:1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Yamamura H, Shimada S.. 2005. In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Res Mol Brain Res. 136:125–133. [DOI] [PubMed] [Google Scholar]
- Van Hees J, Gybels J.. 1981. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry. 44:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER.. 2011. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 137:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welk E, Petsche U, Fleischer E, Handwerker HO.. 1983. Altered excitability of afferent C-fibres of the rat distal to a nerve site exposed to capsaicin. Neurosci Lett. 38:245–250. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH.. 2013. Modulation of central gustatory coding by temperature. J Neurophysiol. 110:1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]