Abstract
Despite intense drug development in the last decade in metastatic urothelial carcinoma and the incorporation of novel compounds to the treatment armamentarium, chemotherapy remains a key treatment strategy for this disease. Platinum-based combinations are still the backbone of first-line therapy in most cases. The role of chemotherapy in the second line has been more ill-defined due to the complexity of this setting, where patient selection remains critical. Nevertheless, two regimens, one in monotherapy (i.e. vinflunine) and one in combination with antiangiogenics (i.e. docetaxel + ramucirumab) have shown efficacy. Immunotherapy through checkpoint inhibition has revealed remarkably durable benefit in a small proportion of patients in the first and second line and is currently the preferred partner for combinations with chemotherapy. Difficult populations such as patients with liver metastases or those progressing to checkpoint inhibition represent a medical challenge and selective ways of delivering cytotoxics, like the antibody–drug conjugates, might represent a valid alternative. This article reviews the current role of chemotherapy in the management of advanced urothelial carcinoma and the ongoing and coming studies involving this treatment strategy.
Keywords: advanced urothelial carcinoma, antiangiogenics, antibody–drug conjugates, chemotherapy, first line, second line
Introduction
Urothelial cancer (UC) represents a relevant medical challenge with around 430,000 new diagnoses and nearly 165,000 related deaths every year worldwide.1 Around 4% of the patients are diagnosed with de novo metastatic disease and nearly 50% of those with localized muscle-invasive UC, despite the best treatment, will recur with disease that is normally not curable with local therapies. Untreated metastatic UC (mUC) harbours a survival expectation of 4–6 months.2,3 Therefore, systemic treatments have a key role in the management of mUC. The introduction of chemotherapy regimens, such as the combination of methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) in the late 1980s and the doublet of gemcitabine and cisplatin (GC) in the late 1990s demonstrated response rates in the range of 40–60% and increased the median overall survival (OS) of these patients to nearly 15 months, leading to the incorporation of these regimens as standard therapy for mUC.4,5
Upon progression to first-line treatment, other chemotherapy regimens have been tested with variable degrees of success. Only vinflunine, a synthetic vinca alkaloid, has received approval in some world regions, not in the United States (US), based on the results of a phase III trial in this setting.6,7
More recently the concept of treatment maintenance, that succeeded in other tumour types, has been also examined in mUC. A phase II randomized placebo-controlled trial was able to demonstrate that vinflunine administered in a maintenance fashion to patients who had achieved disease control after first-line platinum-based chemotherapy, was able to delay relapse when compared with best supportive care (BSC).8
Additionally, chemotherapy has been tested in combination with a number of other strategies [e.g. epidermal growth factor receptor (EGFR) inhibitors and antiangiogenics] with different outcomes.
Recently, immunotherapy via modulation of the checkpoint pathways with monoclonal antibodies targeting the program cell death 1 protein (PD-1) or its ligand (PD-L1), has challenged chemotherapy. Pembrolizumab, an anti-PD-1, was superior to cytotoxic treatment in a randomized phase III trial in patients with mUC that had progressed to platinum-based chemotherapy while atezolizumab, an anti-PD-L1, failed when compared with chemotherapy in a similar setting.9,10 Moreover, contemporary studies are evaluating the role of combining chemotherapy with checkpoint inhibition and the results are highly awaited (IMvigor130: ClinicalTrials.gov identifier: NCT02807636, Keynote 361: ClinicalTrials.gov identifier: NCT02853305, Checkmate 901: ClinicalTrials.gov identifier: NCT03036098).
Lastly, recent studies are testing novel ways of delivering cytotoxic treatments. Accordingly, the antibody–drug conjugates (ADCs), that work with the premise of selectively introducing chemotherapy into UC cells, have shown promising results.11
So, chemotherapy remains a critical component of the treatment armamentarium of mUC, either as a single approach, in combination with immunotherapy or as part of novel ways of dispensing cytotoxics. This article will review the role of chemotherapy in the treatment of mUC in the different clinical settings.
First-line chemotherapy
The overall population of patients with mUC that are candidates to receive first-line chemotherapy is highly heterogenous. It includes patients with only nodal disease but also those with extensive visceral involvement. Moreover, the Eastern Cooperative Oncology Group (ECOG) performance status (PS) and laboratory abnormalities might be very variable among different patients and this will also compromise treatment outcomes. Long term survival with multi-agent chemotherapy has been reported almost exclusively in patients considered as having good prognosis. Different prognostic classifications that will inform the treating physician and help in the treatment selection process have been developed in this context. The majority of prognostic classifications have been developed from clinical trial cohorts or single-centre experiences. Bajorin and colleagues proposed a classification based on 229 patients treated with the M-VAC regimen on five consecutive trials, identifying two negative prognostic factors: Karnofsky PS < 80% and presence of visceral (lung, liver or bone) metastases. Patients with none, one and two risk factors, had a median OS of 33, 13 and 9 months; and a 5-year estimated survival of 33%, 11% and 0%, respectively.12 Subsequently, other nomograms were incorporated from cohorts including patients treated with cisplatin-based regimens;13,14 and other features such as hypoalbuminemia, anaemia, leucocytosis and number of metastatic sites, have been proven to impact in the survival of these patients. Finally, a nomogram from a real-world population was developed by the retrospective international study of invasive/advanced cancer of the urothelium (RISC) consortium.15 This study included over 1000 patients treated irrespectively with cisplatin or carboplatin-based regimens. Significant baseline prognostic factors included ECOG PS, body mass index, ethnicity, prior perioperative systemic treatment, visceral metastases and white blood counts.
Cisplatin-based treatment
In the early 1980s, cisplatin-based chemotherapy combinations were developed after the demonstration of anti-tumour activity with single agents such as fluorouracil, doxorubicin, methotrexate or cisplatin.16
In 1985, Sternberg and colleagues reported the activity of the M-VAC regimen showing in early trials an overall response rate (ORR) of 72%.4,17 Subsequent randomized phase III studies confirmed the superiority of M-VAC chemotherapy to single-agent cisplatin18 and CISCA (cisplatin, cyclophosphamide, and doxorubicin).19 In the US Intergroup trial,18 M-VAC demonstrated an OS benefit compared with cisplatin (median OS, 12.5 months versus 8.2 months, respectively), a progression-free survival (PFS) advantage (10 versus 4.3 months) and better ORR (39% versus 12%). Remarkably, 4% of the patients treated with M-VAC were continuously disease-free at 6 years.20 M-VAC was also superior when compared with CISCA both in terms of ORR (65% versus 46%) and OS (48.3 versus 36.1 weeks).19 Hence, M-VAC was adopted as the gold standard first-line chemotherapy.
Taking into account that M-VAC leads to considerable toxicities in a significant proportion of patients (usually leading to treatment delays and dose adjustments) including myelosuppression (25% rate of febrile neutropenia), infections and mucositis among others, as well as toxic deaths in up to 4% of patients,18–23 efforts were put into seeking an alternative regimen.
On one hand, intensification of this regimen was attempted, with the addition of granulocyte colony-stimulating factor (G-CSF) in order to improve outcomes and diminish haematological toxicity. On the other hand, less toxic new compounds were tested in this setting.
The initial results of a randomized phase III trial comparing high-dose [also known as dose-dense (DD)] M-VAC (DD-M-VAC; 2-weekly cycles) with G-CSF with standard M-VAC (4-week cycle) showed that the intensified regimen was not superior in terms of median OS (p = 0.122), despite obtaining a higher ORR (62% versus 50%) and longer PFS (9.1 versus 8.2 months).24,25 A borderline significant survival benefit was seen with DD-M-VAC at an updated analysis of this trial [5-year survival rate 21.8% versus 13.5%; hazard ratio (HR) 0.76, p = 0.042].25 Finally, patients receiving DD-M-VAC had fewer neutropenia and febrile neutropenia episodes, without increased nonhaematological toxicities.24 These results prompted a shift toward the preferential use of DD-M-VAC rather than conventional M-VAC when considering this four-drug regimen.
The development of the antimetabolite gemcitabine in the early 1990s26,27 and its promising activity in bladder cancer as a single agent28–31 and in synergy with cisplatin,32–34 led to the development of a phase III trial comparing GC with M-VAC, with a primary endpoint of OS improvement with the GC regimen. Response rates (GC, 49%; M-VAC, 46%), time to progression (TTP; 7.4 months in both arms) and OS [GC 13.8 months, M-VAC 14.8 months; HR 1.04; 95% confidence interval (CI), 0.82–1.32, p = 0.75] were similar in both arms.5 An updated analysis of this trial confirmed the survival results (GC 14.0 months versus M-VAC 15.2 months; HR of 1.09; 95% CI, 0.88–1.34, p = 0.66). Additionally, no significant differences were observed in survival rates at 24 months (GC, 25.0%; M-VAC, 31.0%), 48 months (GC, 16.4%; M-VAC, 17.3%), and 60 months (GC, 13.0%; M-VAC, 15.3%).35 Compared with M-VAC, GC was better tolerated. M-VAC had a higher incidence of grade 3/4 mucositis (22% versus 1%), grade 3/4 neutropenia (82% versus 71%), neutropenic fever (14% versus 2%), neutropenic sepsis (12% versus 1%), and toxic death rate (3% versus 1%). On the other hand, patients on GC had a higher incidence of grade 3/4 anaemia and thrombocytopaenia, but these did not translate into higher transfusion needs or bleeding rates.5 In summary, given the favourable toxicity profile of GC and the similar survival outcomes in comparison with M-VAC, GC got widely adopted and became the standard first-line therapy for UC in most institutions.
Furthermore, in an attempt to improve GC outcomes, and based on a phase I–II study conducted by the Spanish Oncology Genitourinary Group (SOGUG),36 a phase III randomized trial comparing GC with the triplet paclitaxel plus GC (PGC) was designed. This trial showed that the triplet strategy leads to an increased ORR (PGC 55.5% versus GC 43.6%) with no statistically significant differences in OS in the intention-to-treat (ITT) analysis (PGC 15.8 versus GC 12.7 months, p = 0.075) at a price of a higher toxicity in terms of febrile neutropaenia for the triplet (13.2% versus 4.3%, respectively; p < 0.001). Subgroup analysis suggested OS benefit for the eligible population and patients with primary bladder tumours only. Based on these results further research with triplets was abandoned and PGC is only scarcely used in very selected patients.37
Lastly, strategies such as dose intensification with other regimes have been also tested. One small phase III study conducted by the Hellenic group attempted to compare DD-M-VAC with a regimen of DD-GC suggesting the feasibility of the latter with a better toxicity profile and similar outcomes. Nevertheless, the study had to close prematurely due to low accrual rates and funding issues resulting in not reaching the predefined sample size and thus limiting the statistical validity and translation of these results to clinical practice.38
Regarding other cytotoxics with less toxicity, cisplatin-based chemotherapy has been compared with carboplatin combinations in different randomized trials. All of them revealed that carboplatin treatment achieves a lower response rate and shorter survival than its counterpart, restricting the use of this compound to patients with a compromised creatinine clearance or poor PS.39–42
More recently a number of studies (IMvigor130: ClinicalTrials.gov identifier: NCT02807636, Keynote 361: ClinicalTrials.gov identifier: NCT02853305, Checkmate 901: ClinicalTrials.gov identifier: NCT03036098). are challenging the role of chemotherapy in first-line patients comparing this strategy with immunotherapy via checkpoint inhibition or combinations of both strategies. While final results are highly awaited, an interim analysis has generated a warning from health regulatory authorities suggesting that a high level of PD-L1 expression could be a requisite for benefiting from checkpoint inhibition as monotherapy.
In summary, currently the standard first-line treatment for fit patients with mUC is chemotherapy based on cisplatin. This recommendation might be refined once we have the results of the ongoing studies with immunotherapy challenging chemotherapy in this setting. The choice of DD-M-VAC or GC is largely based on physician experience, institution guidelines and patient’s comorbidities. No definitive differences have been demonstrated between these two regimens in terms of efficacy although DD-M-VAC is associated with a worse toxicity profile.
Treatment of patients unfit for cisplatin
Despite the results previously summarized in the earlier paragraphs, it is estimated that 30–50%43–45 of the patients with mUC will not be able to receive cisplatin due to different reasons. The consensus definition of cisplatin ineligibility (or unfit patient) encompasses patients with mUC with any of the following criteria: ECOG PS of 2, creatinine clearance <60 ml/min, grade ⩾2 hearing loss, grade ⩾2 neuropathy, or New York Heart Association class III heart failure.46 Different chemotherapy regimens have been tested in cisplatin-unfit mUC patients, achieving an ORR of 30–40% and a median OS of 8–10 months.47–60 The majority of these trials had a small sample size and no control arm. The only phase III trial reported in this population compared two carboplatin-based strategies [methotrexate, carboplatin, and vinblastine (M-CAVI) versus gemcitabine/carboplatin (GCa)].56 The GCa regimen, while still associated with significant haematologic toxicity, was better tolerated than M-CAVI, and had a higher ORR (36.1% versus 21%, p = 0.01). Both regimens achieved similar outcomes in terms of OS (GCa 9.3 months, M-CAVI 8.1 months, p = 0.64) and PFS (GCa 5.8 months, M-CAVI 4.2 months, p = NS).56 Of note, patients with both impaired renal function and a PS of 2 were more likely to develop severe acute toxicities (including death) and thus, single-agent therapy or BSC has been widely adopted for this profile of UC patients.45
JASINT1 was a ‘proof of concept’ small phase II randomized study comparing vinflunine-carboplatin (VCa) with vinflunine-gemcitabine (VG) in patients unfit for cisplatin. Both doublets were similar in terms of disease control rate (77% for each), ORR (VCa 28.6% versus VG 44.1%, p = 0.21), PFS (VCa 6.1 versus VG 5.9 months), OS (VCa 12.8 versus VG 14 months, p = 0.86). Patients assigned to VG had lower rates of myelosuppression than those in the VCa arm, showing less G3/4 neutropenia (38% versus 68%), febrile neutropenia (3 versus 14%) and G3/4 thrombocytopenia (6 versus 21%).61 No further development of this combination in a phase III trial is currently planned. Table 1 summarizes the most relevant randomized studies conducted in the first line with chemotherapy in fit and unfit patients.
Table 1.
First-line randomized clinical trials testing chemotherapy in mUC.
| Author | Experimental arm | Control arm | Phase | n | ORR (%) | PFS (months) | OS (months) |
||
|---|---|---|---|---|---|---|---|---|---|
| Cisplatin-based treatment | Cisplatin-based combinations | Logothetis and colleagues19 | M-VAC | CISCA | 3 | 110 | 65 versus 46 | NR | 11.1 versus 8.3 |
| Loehrer and colleagues18; Saxman and colleagues20 | M-VAC | Cisplatin | 3 | 269 | 39 versus 12 | 10 versus 4.3 | 12.5 versus 8.2 | ||
| Von der Maase and colleagues5,35 | GC | M-VAC | 3 | 405 | 49 versus 46 (NS) |
7.4 versus 8.3 (NS) |
14 versus 15.2 (NS) |
||
| Sternberg and colleagues24,25 | DD-M-VAC | M-VAC | 3 | 263 | 64 versus 50 | 9.5 versus 8.1 | 15.1 versus 14.9 | ||
| Bamias and colleagues38 | DD-GC | DD-M-VAC | 3 | 130 | 65 versus 60 (NS) | 7.8 versus 8.5 (NS) |
18 versus 19 (NS) |
||
| Bellmunt and colleagues37 | PGC | GC | 3 | 626 | 55 versus 44 | 8.3 versus 7.6 (NS) | 15.8 versus 12.7 (NS) | ||
| Carboplatin versus cisplatin | Petrioli and colleagues41 | MVECa | MVEC | 2 | 57 | 41 versus 71 | 4.5 versus 8** | 9.5 versus 13 | |
| Bellmunt and colleagues39 | M-CAVI | M-VAC | 2 | 47 | 39 versus 52 (NS) | NR | 9 versus 16* | ||
| Dreicer and colleagues42 | Carbo-paclitaxel | M-VAC | 3 | 85 | 28 versus 36 (NS) | 5.2 versus 8.7 | 13.8 versus 15.4 (NS) | ||
| Dogliotti and colleagues40 | GCa | GC | 2 | 110 | 40 versus 49.1 | 7.7 versus 8.3 | 9.8 versus 12.8 | ||
| Unfit patients | De Santis and colleagues56 | GCa | M-CAVI | 2/3 | 238 | 41 versus 30 (NS)*** | 5.8 versus 4.2 p (NS) | 9.3 versus 8.1 (NS) | |
| De Santis (2015)61 | Vinflunine-gem | Vinflunine-carbo | 2 | 69 | 53 versus 43 (NS) | 5.9 versus 6.1 (NS) | 14 versus 12.8 (NS) | ||
carbo, carboplatin; CISCA, cisplatin, cyclophosphamide, and doxorubicin; DD, dose-dense; GC, gemcitabine and cisplatin; GCa, gemcitabine and carboplatin; gem, gemcitabine; mUC, metastatic urothelial cancer; M-CAVI, methotrexate, carboplatin, and vinblastine; M-VAC, methotrexate, vinblastine, doxorubicin, and cisplatin; MVECa, methotrexate, vinblastine, epirubicin, and carboplatin; MVEC, methotrexate, vinblastine, epirubicin, and cisplatin; NR, not reported; NS, difference not statistically significant; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PGC, paclitaxel plus GC.
based on median cancer-specific survival, OS not reported.
based on response duration, PFS not reported; ***ORR difference was statistically significant based on confirmed responses (36 versus 21%, p 0.01).
Both atezolizumab and pembrolizumab have challenged chemotherapy in this context in two single-arm phase II studies with encouraging results reporting survivals of 15.9 months and 11 months, respectively.62,63 Based on these data, and despite not yet having information from a randomized comparison with chemotherapy, these two immunotherapies received approval by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) in this context for all patients. Nevertheless, more recently this recommendation was restricted to patients with tumours expressing PD-L1 or unable to receive any platinum combination.64,65
Therefore, regimens such as GCa or monotherapy with single-agent cytotoxics should be considered in mUC patients unfit for cisplatin. Yet, atezolizumab and pembrolizumab represent valid alternatives in this setting proven PD-L1 expression or noneligibility for any platinum-containing regimen.
Maintenance therapy after platinum treatment
Despite the high ORR of first-line chemotherapy, the duration of response is usually limited and after progression, the prognosis is generally poor.66 Similarly to lung cancer, in which maintenance chemotherapy has a proven benefit in terms of OS,67 this strategy has been tested in mUC, but so far, although it remains attractive, has not led to a change of practice.68 Retrospective analyses have demonstrated interesting results with gemcitabine maintenance after standard first-line platinum-based chemotherapy.69–71 The MAJA trial represents the only prospective phase II randomized trial testing a chemotherapy agent as a maintenance strategy after first-line treatment. In this study, 88 patients were randomized to vinflunine versus BSC in patients not progressing to cisplatin-gemcitabine. Vinflunine maintenance showed an improvement in PFS (6.5 months versus 4.2 months, HR 0.59, p = 0.031), with an acceptable safety profile.8 However, no planned phase III trial is ongoing in the context of the quickly changing landscape of mUC after the introduction of immune checkpoint inhibitors.
Nonchemotherapy maintenance strategies with targeted therapies such as sunitinib,72 gefitinib,73 trastuzumab74 and lapatinib75 have been tested in this setting, but in contrast with vinflunine, they have not shown an improvement in PFS. Results from two phase III studies analysing bevacizumab (ClinicalTrials.gov identifier: NCT00942331) and avelumab (ClinicalTrials.gov identifier: NCT02603432) as maintenance therapies after front-line chemotherapy are awaited.
With the currently available evidence, maintenance cannot be recommended as a routine practice in patients with mUC after first-line treatment with platinum-based chemotherapy.
Second-line therapy
Introduction
Despite the high chemosensitivity of mUC in the first-line setting, the majority of the patients will progress and will require subsequent systemic treatments. Median PFS and OS are about 7–8 and 13–15 months, respectively, indicating an aggressive disease upon relapse. As well as in the first-line setting, a number of prognostic factors that stratify patients and help in predicting their outcomes have been identified in the second line. A retrospective analysis of patients treated with second-line vinflunine recognized ECOG PS > 0, haemoglobin < 100 g/l, and liver metastases as poor prognostic factors. The presence of none, one, two or three of these factors led to survivals of 14.2, 7.3, 3.8 and 1.7 months respectively.76 Moreover, a short TTP after first-line chemotherapy seems to have an adverse effect on sensitivity to second-line therapy as described more recently.77
In the last two decades, both single-agent regimens and combinations of cytotoxics have been tested with variable outcomes in the second-line setting. The first signal of activity was an American cooperative group multicentre phase II trial utilizing ifosfamide in a group of 56 patients with mUC and heavily pretreated. Ifosfamide demonstrated a promising ORR of 20%, including five complete responses. Despite this encouraging activity, the severe toxicity observed compromised further development of this regimen.78 Following that failed attempt, many compounds have been evaluated in patients with previously treated mUC both as a single agent or in combination. The majority of the single drug studies have been phase II trials testing taxanes, antimetabolites or vinca alkaloids. Most of these studies included fewer than 50 patients and the response rate (RR) ranged from 5% to 29% with a PFS of around 3–4 months and scarce impact in OS and quality of life. Trials conducted with a combination of cytotoxic agents revealed a higher activity (RR of 16–60%) although with more toxicity and without a clear translation in OS benefit. The size of these studies was very small with most of them including fewer than 30 patients and therefore limiting any formal conclusion.
The only randomized placebo-controlled phase III trial conducted in the second-line setting compared the vinca alkaloid vinflunine plus BSC with only BSC in a pure second-line context.6,79 In the eligible population the median OS was significantly longer for the experimental arm (6.9 versus 4.3 months, respectively) and this difference was statistically significant. Yet, in the intent-to-treat population, the 2-month survival advantage (6.9 months versus 4.6 months) was achieved but did not reach statistical significance.6,79 These results led to the approval of vinflunine by the EMA in 2009, although this drug has never been approved by the US FDA.
Thus, the development of compounds in the second line has been difficult. Commonly, patients’ poor ECOG PS, impaired renal function, comorbidities and advanced age, have compromised trial design, feasibility and patient accrual into trials. Also, some factors have jeopardized the progress in this context. First, the lack of a commonly accepted definition of second line. Many studies include patients who had received perioperative chemotherapy and then progressed in different time points, along with those who progressed after a first-line treatment for metastatic disease. It is well known that these are two very different populations in terms of prognosis. Second, the inclusion of patients with diverse UC locations such as bladder and upper genitourinary (GU) tract with distinctive biology and expected behaviour might bias the conclusion of some trials. Lastly, many of these studies lack a proper stratification by established prognostic parameters therefore introducing another bias that needs to be considered in the interpretation of the results limiting the possible comparison among trials.
In the following, we summarize the most relevant evidence of cytotoxic treatment in the second line presented by a family of drugs.
Taxanes
Despite a lack of solid data, the taxanes have been extensively utilized in the past in some world regions given the absence of other approved alternatives. Docetaxel, paclitaxel and nab-paclitaxel have been the drugs from this family tested either as single agents or in combination.
Docetaxel
The two studies conducted in the late 1990s, one in Europe80 and in one in North America, examined for the first time the activity of docetaxel as a single agent in mUC in chemo-naïve and cisplatin pretreated patients respectively. McCaffrey and colleagues from Memorial Sloan Kettering Cancer Center in 1997 published data from a phase II study where 30 patients upon progression to a previous cisplatin-based chemotherapy, received 3-weekly docetaxel at 100 mg/m2. The ORR was 13.3% with a median duration of response between 3 and 8 months and a median OS of 9 months. Haematological toxicity was the most relevant adverse event with 70% of grade 4 neutropaenia and 50% of febrile neutropaenia (G-CSF was not utilized routinely in this trial).81 Docetaxel was also tested in a weekly fashion at doses of 30 mg/m2 on days 1 and 8 every 21 days in a single-arm phase II study in a similar population demonstrating better tolerability but modest activity with an ORR of 6% and median PFS and OS of 1.4 months, and 8.3 months respectively.82
Based on these preliminary, but insufficient signs of activity as a single agent, further trials attempted to demonstrate the efficacy of docetaxel in combination with other cytotoxics or alternative strategies. In 2001, Kregge and colleagues published the results of a small German phase II study where ifosfamide in combination with docetaxel revealed some promising activity and good tolerability. Out of 20 evaluable patients, the ORR was 25%, including 4 complete responses (CRs), all in patients with only isolated retroperitoneal lymph node disease, and with 50% of them having received previous chemotherapy only in the perioperative setting. Despite these promising activity results, the median OS was only 4 months.83
In 2012, Choueri and colleagues communicated the results of a phase III study where 142 patients with mUC with progression after platinum-based therapy were randomized to either docetaxel or the combination of docetaxel with vandetanib [an oral anti-EGFR, anti-vascular endothelial growth factor receptor (VEGFR)2 and anti-RET]. No statistically significant differences in PFS or OS were observed between both arms and worse toxicity was reported for the combination group.84 Subsequently, an open-label three-arm randomized controlled phase II study compared, in a cohort of 140 patients with mUC that had progressed during or within 12 months of platinum-based treatment, docetaxel versus docetaxel combined with either the monoclonal antibody (mAb) ramucirumab [a human immunoglobulin G1 (IgG1) against VEGFR-2] or icrucumab (a human IgG1 mAb that targets VEGFR-1). The study demonstrated the superiority for the docetaxel-ramucirumab arm but not for the icrucumab combination.85 These results led to a randomized, double-blind, phase III study that has been recently communicated (the RANGE study). This trial tested over 500 patients with mUC who progressed during or after platinum-based chemotherapy, the administration of 3-weekly docetaxel at 75 mg/m2 plus either intravenous ramucirumab 10 mg/kg or matching placebo. The study met its primary endpoint (investigator-assessed PFS), analyzed by ITT. The PFS was extended significantly in patients assigned to the ramucirumab plus docetaxel arm (median 4·07 months; 95% CI 2.96–4.47 versus 2·76 months, 2.60–2.96; HR 0.757, 95% CI 0.607–0.943; p = 0.0118). The OS data was immature at the time of this analysis and given the gatekeeping statistical design, a formal statistical analysis of response cannot be done, although a higher proportion of patients allocated to ramucirumab achieved a response compared with placebo (24.5% versus 14.0%), including nine CRs versus three in the placebo arm. The combination did not show additive toxic effects or impairment in the patient’s quality of life. These results seem to validate VEGFR-2 as an appropriate target in mUC and represent the first chemo-combination that achieves better results than chemotherapy alone in this patient population.86 Nevertheless, this trial has a short follow up (5 months) and the effect of not stratifying patients according to prognostic criteria and including patients previously treated with immunotherapy is unknown. More mature data are probably needed to validate the real impact of this combination.
Other strategies such as sequential treatment have been also explored with docetaxel with little success. Thus, a Greek phase II trial investigated GC followed by docetaxel and revealed a small effect for second-line docetaxel even when given straight away after four cycles of GC. The median TTP was 6.8 months and the median OS was 13 months.87
Paclitaxel
The antimicrotubule agent, paclitaxel, was first evaluated in UC in a phase II study published in 1994 by Roth and colleagues. This trial included a small cohort of 26 treatment-naïve mUC patients. The observed ORR of 42% (with 27% CR) was encouraging and led to further clinical development.88 This included the evaluation of single-agent paclitaxel in a number of studies in pretreated mUC patients. Nonetheless, the clinical activity of paclitaxel as monotherapy in the second-line setting was quite disappointing with a modest ORR in the range of 7–10% and a scarce impact in OS. In 1997, Papamichael and colleagues communicated the negative results of a small multicentre British study where 14 patients with previously treated mUC received paclitaxel at 200 mg/m2 every 3 weeks as a rescue therapy. The activity was very low with only one patient responding (7% ORR).89 Years later in 2002 in the US, Vaughn and colleagues published the results of a study where 31 mUC patients with progression to first-line treatment received weekly doses of paclitaxel at 80 mg/m2. Therapy was well tolerated with minimal haematological and nonhaematological toxicity but also with modest activity (10% ORR) and a median TTP of barely 2.2 months and a median OS of 7.2 months.77 More recently a small French Genitourinary Tumour Group (GETUG) study in the same setting, analyzed 55 patients in the role of weekly paclitaxel at 80 mg/m2 (on days 1, 8, and 15, of a 28-day cycle). Activity was similar to previous studies (9% ORR) with a stable disease (SD) rate of 38%, and so was the survival data (median OS close to 7 months) with a 1-year OS rate of 22%. More notably was the observation that most of the patients who achieved clinical benefit had a good baseline ECOG PS (0 or 1) and more than two-thirds of them (71%) had received previously adjuvant or neoadjuvant chemotherapy, emphasizing the relevance of patient selection in this clinical setting and the objectives to be set in such a challenging population.90
Following this, a number of combination studies with paclitaxel have been conducted. In 1999, Sweeney and colleagues communicated one of the first attempts of combining paclitaxel with other cytotoxics. A total of 13 patients with previously treated mUC, received paclitaxel plus ifosfamide; nevertheless, the combination failed to exceed the activity of each single agent separately.91 Subsequently a small Italian phase II study showed promising activity of the combination of paclitaxel and gemcitabine (GP). Out of 40 evaluable patients, the ORR was 60% with a 28% of CR. This activity was remarkably higher in those patients who had been previously treated in the perioperative setting compared with those who received prior chemotherapy for metastatic disease. The median OS for patients given GP after failing neoadjuvant or adjuvant M-VAC was 12 months (range, 2–43+), as compared with only 8 months (range, 2–28) for patients who had been treated after failure of prior therapy for metastatic disease. Around a third of the patients (32%) developed grade 3–4 neutropaenia, with febrile neutropaenia in three (7%) patients.92 Subsequently, two small randomized trials comparing GP up to 6 cycles versus continuous GP until progression were reported, showing no major differences between a fixed or a continuous strategy.93,94 Despite being randomized trials and the relatively high ORR (37–50%), considering their small sample, the mixed and imbalanced populations between the arms in terms of modality of first-line treatment and prior disease-free interval, the unclear benefit of retreatment with previously used agents bladder cancer (up to 50% of those patients were previously treated with gemcitabine), and that vinflunine had been approved in Europe based on more solid data; further research was not pursued under this combination.
Nab-paclitaxel
Nanoparticle albumin-bound (nab)-paclitaxel is a solvent-free formulation of paclitaxel with better tolerance than standard taxanes, and that does not require steroid premedications. This drug was first investigated in pretreated mUC in a Canadian phase II single-arm study. Out of 47 evaluable patients 27.7% responded including a 2.1% of CR. Tolerance was good overall, with fatigue, pain, alopecia and neuropathy as the most frequent adverse events.95 These encouraging results led to a phase III study recently communicated at the American Society of Clinical Oncology meeting in 2018 where 199 patients with mUC and either progression after treatment for systemic disease or within 12 months of concluding cisplatin-based perioperative therapy were randomized to nab-paclitaxel 260 mg/m2 or paclitaxel 175 mg/m2 both in a 3-weekly schedule. The primary endpoint of the study was PFS and secondary objectives were OS, ORR, toxicity and quality of life. This study was negative; both nab-paclitaxel and paclitaxel showed similar efficacy. The PFS was 3.35 and 3.02 months for the experimental and the control arm respectively and these differences were not statistically significant [HR 0.92 (0.68–1.23) p = 0.31]. Likewise, median OS did not differ significantly between arms [7.46 versus 8.77; HR 0.95 (0.7–1.3) p = 0.4] and the ORR was 21% and 23% for nab-paclitaxel and paclitaxel respectively. Moreover, toxicity was worse for the experimental arm, preventing this compound from further development.96
Cabazitaxel
Cabazitaxel is a novel semi synthetic taxane approved for the treatment of advanced prostate cancer. Despite some preliminary results suggesting activity of this compound in mUC, three studies (two single-arm phase II and one phase II–III) failed to demonstrate the efficacy of cabazitaxel in the second-line setting, and was stopped at intermediate analysis due to futility. These results preclude the further development of cabazitaxel in this setting.97–99
Vinca alkaloids
The microtubule-inhibiting vinca alkaloid, vinflunine, is the most extensively studied drug in the second-line setting. It was first evaluated in a two single-arm phase II studies in slightly different populations with ORRs of 15–18% and median duration of response (mDOR) of 6–9 months.100,101
Following this, a randomized phase III trial included 370 patients with mUC progressing after first-line platinum-based chemotherapy, excluding those who had received previous perioperative chemotherapy only. The majority of the patients had progressed within 6 months of receiving previous chemotherapy and more than two-thirds had visceral metastasis. The ITT analysis failed to show a statistically significant OS improvement for the experimental arm when compared with placebo (6.9 versus 4.6 months, p = 0.287) although a significant improvement in ORR (8.6 versus 0%, p = 0.006) and median PFS (3.0 versus 1.5 months, p = 0.001) was noted. Moreover, a multivariate Cox analysis adjusting for prognostic factors showed an increase in OS with vinflunine (p = 0.036) and a reduction in the risk of death by 23%. When the analysis was applied to the eligible population only (n = 357), the median OS improvement reached statistical significance (6.9 versus 4.3 months, p = 0.04). The most relevant grade 3 or 4 adverse events for vinflunine were haematological events [neutropenia (50%), febrile neutropenia (6%), anaemia (19%)] and out of the nonhaematological events, fatigue and constipation with 19% and 16% respectively were the most relevant. These initial data have been later reinforced for real-world data that have shown a median OS in the region of 10 months.7 Probably good patient selection and better management of this drug and its related adverse events explains these better outcomes over time.
Antimetabolites
Mostly, two drugs considered as antimetabolites have been evaluated in mUC: gemcitabine and the antifolate, pemetrexed.
Gemcitabine
Gemcitabine, a pyrimidine antimetabolite, has been tested in mUC upon progression to cisplatin-based chemotherapy in several studies. As a single agent, gemcitabine was evaluated in four small single-arm trials: three European and one Japanese.28,29,102,103 The two Italian studies evaluated the activity of this drug at weekly doses of 1200 mg/m2 or 1000 mg/m2 respectively for 3 weeks every 28 days in small single-centre experiences. Also, a multicentre German study analyzed a slightly different schema (gemcitabine 1250 mg/m2 days 1–8 every 21 days) in a similar population. Lastly, a Japanese phase II trial evaluated single-agent gemcitabine in patients with mUC who were previously treated with a platinum-based regimen. Gemcitabine was administered on a weekly basis at 1000 mg/m2 for 3 consecutive weeks followed by 1 week of rest. Overall, these studies had small sample sizes including 24–44 patients. They reported ORRs ranging from 11% to 25%, with CRs in the range of 4–13%. The median PFS stated varied from 3.1 to 4.9 and the median OS from 5 to 13 months. Despite limited activity, quality of life and control of tumour-related symptoms were improved significantly along with good tolerability. The most relevant grade 3–4 toxicities reported were leucopaenia (36%), thrombocytopaenia (11%) and anaemia (11%). Among nonhaematologic grade 3 adverse events, lung toxicity and skin toxicity (i.e. exanthema) were the most relevant at 11% and 4% respectively. The studies in combination with taxanes have already been discussed in previous sections.
Pemetrexed
Pemetrexed acts by inhibiting the enzyme, thymidylate synthetase, resulting in decreased availability of the thymidine necessary for pyrimidine synthesis. Pemetrexed also inhibits dihydrofolate reductase and glycinamide ribonucleotide formyl transferase (a folate-dependent enzyme involved in purine synthesis). Folate and vitamin B12 nutritional status affects the toxicity of pemetrexed, including rates of neutropenic fever. This compound has been extensively used in the US and other world regions as an option for the treatment of mUC until the recent availability of other compounds.
Pemetrexed was first tested in mUC in 2003 as part of its initial clinical development and responses of around 30% were reported in an untreated population.104 This led to a single-cohort phase II study assessing the efficacy and safety of pemetrexed as a second-line chemotherapy for metastatic disease or first-line chemotherapy of metastatic disease that had recurred within 12 months of adjuvant therapy. The final reported ORR was 27.7% and the toxicity profile with a previous load of vitamin B12 and folic acid was manageable. No substantial differences in terms of efficacy were reported for patients who received treatment for metastatic disease or those relapsing after adjuvant treatment. In terms of disease control (DC), pemetrexed was associated with a DC rate of 48.9% after two cycles and a median OS of 9.6 months and a 1-year survival of 41.8%.105
However, a subsequent single institution study conducted at Memorial Sloan Kettering Cancer Center (MSKCC) in a similar population could not be completed due to futility after the analysis of the first 13 patients enrolled and only 1 response (ORR 8%). The toxicity profile was slightly worse than in the previous study. Multiple reasons could potentially explain these differences in activity, including patient selection, previous treatments, trial design, etc. To address this discrepancy a retrospective analysis of over 120 patients with mUC who received pemetrexed at MSKCC outside of a clinical trial between 2008 and 2013 was performed by Bambury and colleagues. They also looked for potential prognostic factors for OS in this population. The ORR in this group was 5% (95% CI 1–9%) with a median duration of response of 8 months. Median PFS and OS were 2.4 and 6.7 months respectively. The only two factors that had independent prognostic significance after multivariable analysis were the ECOG PS (p < 0.01), the liver metastases (p = 0.02), and the neutrophil–lymphocyte ratio (p < 0.01). Pemetrexed was overall well tolerated and only 15% of the patients required dose reductions mostly due to either fatigue or haematological toxicity. Febrile neutropaenia and grade 3–4 thrombocytopaenia was reported in 5% and 8% respectively, both lower than stated in previous studies.106
Other agents
Oxaliplatin
Other cytotoxics have been explored in second-line mUC with no robust outcomes. Thus, oxaliplatin was evaluated in a study where patients were assigned to one of two different arms based on their sensitivity to cisplatin. No patient in the so-called ‘cisplatin-resistant’ arm responded to oxaliplatin and only 1 out of 10 did in the ‘cisplatin sensitive’ arm at a modest activity of 10% ORR.107
Ixabepilone
The microtubule stabilizer, ixabepilone, showed slight activity after previous chemotherapy treatment with an ORR of 11.9% (90% CI, 5.3%, 26.5%) and a median OS of 8 months in a cohort of 45 mUC patients. Haematological toxicity, fatigue, and sensory neuropathy were the most common side effects.108
So, in summary, multiple attempts with several cytotoxic drugs have been performed in the last three decades in second-line mUC with limited success. Response rates in the range of 0–60% in unselected populations have been documented in phase II, single agent and combination studies. When taken to the phase III setting, only two compounds have shown reasonable signs of activity (Tables 2 and 3). Vinflunine, that succeeded in the per protocol analysis when compared with BSC and the combination of docetaxel-ramucirumab that revealed greater PFS and ORR than docetaxel alone. The former was associated with a PFS of 3 months and a median OS around 7 months while the latter achieved a PFS of 4.07 months and an ORR of 26%.
Table 2.
Second-line single-arm studies.
| Author | Chemotherapy | Phase | n | ORR (%) | PFS/TTP* (months) | OS/CSS** (months) |
|
|---|---|---|---|---|---|---|---|
| Single-agent chemotherapy | McCaffrey and colleagues81 | Docetaxel (3 weekly) | 2 | 30 | 13.3 | 4 | 9 |
| Kim and colleagues82 | Docetaxel (weekly) | 2 | 31 | 6 | 1.4 | 8.3 | |
| Papamichael and colleagues89 | Paclitaxel (3 weekly) | 2 | 14 | 7 | NR | NR | |
| Vaughn (2002)77 |
Paclitaxel (weekly) | 2 | 31 | 10 | 2.2 | 7.2 | |
| Joly and colleagues90 | Paclitaxel (weekly) | 2 | 55 | 9 | 3 | 7 | |
| Ko and colleagues95 | Nab-Paclitaxel | 2 | 48 | 28 | 6 | 9.8 | |
| Hoffman-Censits and colleagues97 | Cabazitaxel | 2 | 14 | 0 | NR | NR | |
| Arranz Arija and colleagues98 | Cabazitaxel | 2 | 71 | 5 | 2.1 | 4.3 | |
| Culine and colleagues100 | Vinflunine | 2 | 51 | 18 | 3 | 6.6 | |
| Vaughn and colleagues101 | Vinflunine | 2 | 175 | 15 | 2.8 | 8.2 | |
| Lorusso and colleagues29 | Gemcitabine | 2 | 35 | 23 | 3.8* | 5 | |
| Gebbia and colleagues28 | Gemcitabine | 2 | 24 | 29 | NR | 13 | |
| Albers and colleagues102 | Gemcitabine | 30 | 11 | 4.9* | 8.7** | ||
| Akaza and colleagues103 | Gemcitabine | 2 | 46 | 25 | 3.1 | 12.6 | |
| Sweeney and colleagues105 | Pemetrexed | 2 | 47 | 28 | 2.9* | 9.6 | |
| Galsky (2007)106 |
Pemetrexed | 2 | 13 | 8 | NR | NR | |
| Winquist and colleagues107 | Oxaliplatin | 2 | 20 | 5 | 1.4* | 6.9 | |
| Dreicer and colleagues108 | Ixabepilone | 2 | 45 | 12 | 2.7 | 8 | |
| Chemotherapy combinations | Krege and colleagues83 | Docetaxel-ifosfamide | 2 | 22 | 25 | NR | 4 |
| Sweeney and colleagues91 | Paclitaxel-ifosfamide | 2 | 13 | 15 | NR | 8 | |
| Sternberg and colleagues92 | Paclitaxel-gemcitabine | 2 | 41 | 60 | 6.4 | 14.4 | |
| Bellmunt and colleagues109 | Docetaxel + B-701 | 1b | 19 | 16 | 3.2 | 6.9 |
CSS, cancer-specific survival; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
TTP reported when PFS data is not available. **CSS reported when OS not available.
Table 3.
Second-line randomized clinical trials with chemotherapy in mUC.
| Author [Year] | Experimental arm | Control arm | Phase | n | ORR (%) | PFS/TTP* (months) | OS/CSS**
(months) |
|
|---|---|---|---|---|---|---|---|---|
| Chemotherapy (CT) alone | Fechner and colleagues94 | Gem-paclitaxel q2w cont | Gem-paclitaxel q3w x6 | 2 | 30 | 39 versus 50 | 6 versus 11 (NS) | 9 versus 13 (NS) |
| Albers (2010)93 | Gem-paclitaxel q3w cont | Gem-paclitaxel q3w x6 | 3 | 102 | 41.5versus 37.5 (NS) | 3.1 versus 4 (NS) | 8 versus 7.8 (NS) | |
| Bellmunt and colleagues6,79 | Vinflunine | BSC | 3 | 370 | 8.6 versus 0 | 3 versus 1.5 | 6.9 versus 4.6† (NS) | |
| Bellmunt and colleagues99 | Cabazitaxel | Vinflunine | 2 | 70 | 13 versus 30 (NS) | 1.9 versus 2.9 | 5.5 versus 7.6 (NS) | |
| Sridhar and colleagues96 | Nab-paclitaxel | Paclitaxel | 2 | 160 | 22 versus 25 | 3.35 versus 3.02 (NS) | 7.46 versus 8.77 (NS) | |
| CT + targeted therapy | Choueiri and colleagues84 | Docetaxel-vandetanib | Docetaxel | 2 | 142 | 7 versus 11 | 2.56 versus 1.58 (NS) | 5.85 versus 7.03 (NS) |
| Petrylak and colleagues86 | Docetaxel-ramucirumab | Docetaxel | 3 | 530 | 24.5 versus 14 | 4.07 versus 2.76 | NR | |
| Rosenberg and colleagues151
|
Docetaxel-apatorsen | Docetaxel | 2 | 99 | 16 versus 11 (NS) | 1.8 versus 1.6 (NS) | 6.4 versus 5.9 |
BSC: best supportive care; CSS: cancer-specific survival; CT, chemotherapy; gem, gemcitabine; mUC, metastatic urothelial cancer; NR, not reported; NS, difference not statistically significant; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
TTP reported when PFS data is not available.
CSS reported when OS not available.
Results from the intention-to-treat population.
These data have been recently challenged by the arrival of immunotherapy in this setting. As further reviewed, a number of phase II trials conducted with immune checkpoint inhibitors in the second line, revealed ORRs close to 20–25% with a median OS of around 10 months. Remarkably, they all had a small group of long survivors beyond the 24-month mark and a more favourable toxicity profile when compared with chemotherapy. The two phase III studies subsequently conducted comparing chemotherapy with pembrolizumab and atezolizumab respectively had opposite results. Pembrolizumab was able to overcome chemotherapy (taxanes or vinflunine by investigator choice) and achieved a higher ORR and median OS. Nonetheless, atezolizumab failed to show superiority versus chemotherapy in a group of patients with high expression of PD-L1 being the study considered formally negative despite clinical benefit in the ITT analysis and the observation of a group of long survivors beyond the 24 month mark. These drugs have been approved by the US FDA and EMA and should also be considered standard options in the second line or beyond.
Role of chemotherapy in the immunotherapy era
Since 2014, multiple clinical trials analysing the role of immune-oncology (IO) agents in mUC have been published.9,10,62,63,110–119 Overall, five drugs targeting either PD-1 or PDL-1 (atezolizumab, avelumab, durvalumab, nivolumab and pembrolizumab) have been approved in the US after progression to platinum-based chemotherapy following single-arm phase I–II trials.111,114,116,118,119 In Europe atezolizumab, pembrolizumab and nivolumab have received regulatory approval. Subsequently, two phase III randomized trials comparing respectively pembrolizumab9 and atezolizumab10 with chemotherapy were performed. In the KEYNOTE-045 trial, pembrolizumab demonstrated an increased OS and ORR, but no PFS advantage, over chemotherapy.9 On the other hand, the IMVIGOR 211 study comparing atezolizumab with chemotherapy with a hierarchical fixed-sequence procedure in a prespecified biomarker-selected population, was negative. Atezolizumab did not improve OS, PFS nor ORR when compared with investigator’s choice chemotherapy.10 Despite these conflicting results, taking into consideration the tolerability profile of immunotherapy, and its duration of response, IO agents have superseded and replaced chemotherapy in the second-line setting. However, there is still space for debating whether all patients progressing to front-line platinum-based chemotherapy should be treated with IO.
On the one hand, it is well known that only a minority of patients with mUC (around 20–25%) will respond to immunotherapy.9,10 Moreover, the so-called hyperprogression phenomena, consisting of rapid growing of the tumour after administration of immunotherapy, has been described by three different groups in 9–29% of patients treated.120–122 Whether hyperprogression in mUC reflects a detrimental effect of immunotherapy or it is just enlightening the natural history of some patients with bad prognosis, remains to be established. Yet, it is well known that a subset of patients will quickly develop progressive disease while on immunotherapy and subsequently their general status will decline, making it unlikely for them to receive further chemotherapy. In IMvigor210, patients treated with second-line atezolizumab were more likely to progress on first radiological assessment (done at 9 weeks) when having liver metastases, low haemoglobin (<10 g/dl), high tumour burden (per sum of target lesion diameter) and PS ECOG > 0.123 Patients with these bad prognostic indicators, or those who could not tolerate pseudoprogression or hyperprogressive disease should be selected carefully for second-line treatment, more likely chemotherapy if able to receive it.
Furthermore, although only useful as hypothesis-generating, the subgroup analysis of IMVIGOR211 revealed that patients with renal pelvis tumours seemed to derive greater benefit of chemotherapy, but this finding is inconsistent with the KEYNOTE-045 trial, in which pembrolizumab seemed to derive a better survival in patients with upper tract tumours. On the other hand, both phase III trials, supported that patients with limited lymph node spread achieved better outcomes in terms of survival and response rates, when treated with either pembrolizumab or atezolizumab, a finding consistent with earlier PD-1/PD-L1 inhibitors studies.9,10
The previously mentioned clinical characteristics have not been established as predictive factors of response but rather as prognostic factors and may only be reflecting a complex molecular biological basis of UC. Although solid predictive molecular biomarkers which could potentially help clinicians to select patients for the approved treatments are lacking, there is hope for precision medicine in the coming years.124
The PD-L1 biomarker in patients with mUC progressing to platinum-based treatment has been proven to be consistently inconsistent,125 and currently, it is insufficient on its own to identify patients who would benefit most from one strategy or the other.
Mutations in DNA damage and repair (DDR) genes may predict responses both to chemotherapy126,127 and immunotherapy128–131 in patients with UC. Perhaps, patients with defects in this pathway could benefit from a combination strategy.
In recent years, whole genome and RNA sequencing has allowed researchers to identify different molecular subtypes of muscle-invasive bladder cancer.132 These subtypes have a prognostic significance, but more notably, they may display different sensitivities to therapeutic strategies. For example, luminal tumours appear to be particularly sensitive to immune checkpoint blockade.133 On the other hand, basal-squamous subgroups harbour the worst prognosis, but benefit the most from neoadjuvant chemotherapy,134,135 although whether these results will replicate in the metastatic setting and allow clinicians to a better optimization of therapies, remains an open question. Contrary to the basal subtype, luminal-papillary tumours have a better prognosis but seem to respond worse to cisplatin-based neoadjuvant chemotherapy,135 and these patients are likely to achieve better outcomes with fibroblast growth factor receptor 3 (FGFR3) inhibitors, since this target is overexpressed in this subset.136–138 Finally, small cell/neuronal tumours are also initially highly chemosensitive, but responses are usually short, and thus, novel approaches for this subtype are needed.139
The impact of chemotherapy in patients progressing both to platinum-based chemotherapy and checkpoint inhibitors remains uncertain, however preliminary data suggest that checkpoint inhibitor exposure does not appear to confer resistance to chemotherapy and that salvage chemotherapy as a third-line agent after checkpoint inhibitor failure may derive a clinical benefit.140,141 A small retrospective study showed that in this population, 92% of the patients achieved disease control with chemotherapy, mainly driven by stabilizations.141 Another European multicentric retrospective analysis showed, despite the relatively high proportion of responses, that only a third of these patients eventually received further systemic treatment after checkpoint inhibitor progression.140
The only prospective study that tested chemotherapy in patients previously treated with checkpoint inhibitors was the RANGE trial comparing docetaxel in combination with ramucirumab with docetaxel plus placebo, although only 7% of the patients included in the trial had been previously exposed to checkpoint inhibitors86 and thus analysis of this subgroup remains limited by the small sample size.
As mentioned previously, patients who are unfit to receive cisplatin as a first-line therapy, represent an unmet medical need. Currently, two checkpoint inhibitor agents, pembrolizumab and atezolizumab, have prospective single-arm data with encouraging results in this population,62,63 and subsequently, both the US FDA and EMA approved these agents for this profile of patients, implying that both checkpoint inhibitor agents and carboplatin-based regimens coexisted as a therapeutic option for the same population, in the absence of a predictive biomarker that could guide choosing the preferred treatment.
More recently, both the US FDA and EMA have restricted both agents to PD-L1-positive patients (defined as combined positive score (CPS) ⩾ 10 per DAKO 22C3 in the case of pembrolizumab, and as immunohistochemistry (IC) ⩾ 5% per VENTANA SP142 for atezolizumab). This decision was taken on the basis of an assessment conducted by the data monitoring committee for the phase III IMvigor130 (ClinicalTrials.gov identifier: NCT02807636) and KEYNOTE-361 (ClinicalTrials.gov identifier: NCT02853305), which are examining the efficacy of atezolizumab and pembrolizumab respectively, alone or in combination with chemotherapy as a front-line strategy. The analysis of these data showed, indeed, a decreased OS in patients with PD-L1 low status in the single-agent immunotherapy arms compared with chemotherapy.64
Thus, in the absence of further data, patients unfit for cisplatin should be considered for carboplatin-based chemotherapy when PD-L1 status is low and their ECOG PS is acceptable for this treatment. Instead, immunotherapy should be contemplated in patients with PD-L1 high tumours or those unable to receive any platinum-based combination.
Moreover, in both first-line phase II trials, atezolizumab and pembrolizumab ORR were low in patients with liver (ORR 8–17%) or visceral (ORR 14–23%) metastases,62,63 and perhaps, these patients should be considered for front-line chemotherapy which leads to higher ORR (around 40%).56
Strikingly, the US FDA and EMA communications are somehow conflicting with the previous phase II studies, in which atezolizumab led to better survival rates in PDL-1 negative patients63 and CPS ⩾ 10 per DAKO 22C3 enriched for response to first-line pembrolizumab, but did not preclude a response in biomarker-negative patients,62 underlining the importance of randomized controlled trials and the inconsistency of PD-L1 status as a biomarker in UC. Thus, the complete results of the ongoing phase III trials in the first line are eagerly awaited in order to help clarify the role of PD-L1 expression in mUC.
Role of chemotherapy combinations and non-IO novel approaches
Chemotherapy + IO combinations
Immunotherapy derives a prolonged benefit in those patients who respond to this treatment strategy (ranging 13–31% in an unselected population).9,10,62,63,110–114,116,118,119 Conversely, platinum-based chemotherapy leads to a high proportion of response, but the duration of response is generally short and the proportion of patients alive after 5 years is poor.4,5,17–19,37,56 Moreover, cytotoxics such as gemcitabine have been shown to lead to immunogenic cell death. In an MC38 model, gemcitabine showed additive/synergistic effects with muDX400 (murine anti-PD-1).142 Also, as mentioned previously, combining chemotherapy with IO agents may be useful in the presence of somatic mutations in DDR genes, since these alterations have been correlated with high responses to chemotherapy and immunotherapy.126–131
A phase II trial testing ipilimumab, a CTLA4 inhibitor, in combination with GC showed that combing immunotherapy and chemotherapy is feasible and seems particularly effective in patients harbouring DDR genes alterations.130
Different phase III trials testing the combination of PD-1 or PD-L1 inhibitors with platinum-based chemotherapy are ongoing. Both IMVIGOR130 (ClinicalTrials.gov identifier: NCT02807636) and KEYNOTE 361 (ClinicalTrials.gov identifier: NCT02853305) are testing atezolizumab and pembrolizumab, under a similar design. Both studies allow cisplatin-fit and unfit patients, and patients can be allocated to three different arms: a checkpoint inhibitor alone; a checkpoint inhibitor + platinum-based chemotherapy followed by checkpoint inhibitor maintenance after 4–6 cycles if there is no progressive disease; and standard of care platinum-based chemotherapy. In addition, the Checkmate 901 trial (ClinicalTrials.gov identifier: NCT03036098) is similar to the previous studies but introduces an IO combination arm with nivolumab + ipilimumab (without chemotherapy) instead of a checkpoint inhibitor monotherapy arm.
Chemotherapy + non-IO combinations
Non-IO targeted therapies have so far, vastly failed to demonstrate improvement of outcomes in mUC patients. Antiangiogenic agents alone are not active in unselected patients72,84,143 and some of them are highly toxic in combination with chemotherapy.144 However mAbs targeting the VEGFR family can be safely combined with chemotherapy and can improve chemotherapy efficacy. A phase II study combining gemcitabine-carboplatin chemotherapy with bevacizumab demonstrated an ORR of 49%. Median OS was 13.9 months (above the expected) but the median PFS of 6.5 months did not reach the predesignated value.145 Also, a phase II trial investigating bevacizumab in combination with cisplatin-gemcitabine demonstrated a promising median OS of 19.1 months.146 These results provided enough argument for investigating bevacizumab in UC, and a phase III study of GC with or without bevacizumab was conducted (ClinicalTrials.gov identifier: NCT00942331). The results of this trial have not yet been communicated.
More recently, as discussed in previous sections, the results of a phase II and a phase III trial, testing the combination of second-line docetaxel with antiangiogenics against VEGFR-2 such as ramucirumab, demonstrated the efficacy and feasibility of this approach.85,86
As the biological and molecular knowledge of UC expands, novel targeted therapies are likely to arise, particularly in biomarker-selected patients. Alterations in fibroblast growth factor receptor (FGFR)3 are found in approximately one-fifth of patients with locally advanced or metastatic bladder cancer.147 These alterations appear to be enriched mostly in luminal-papillary tumours,137 which at the same time, seem to be less responsive to immunotherapy.118,119 Early trials have demonstrated that inhibition of FGFR3 is effective in biomarker-selected patients,148–150 and a combination with docetaxel has already been tested in a phase I trial109 and a phase II expansion is currently enrolling patients with FGFR3 mutations or fusions.
Docetaxel has also been combined in a phase II trial with the Hsp27 inhibitor, apatorsen, demonstrating increased activity in comparison with docetaxel, although median OS (6.4 months for the combination arm) remains to be short. The levels of Hsp27 seem to be prognostic, but not predictive of apatorsen efficacy.151 Contrary to this finding, survival was not improved by combining this drug with first-line GC.152
Finally, novel epigenetic modulating agents are being tested in bladder cancer among other tumours. Epigenetic changes such as DNA methylation and histone modification are capable of altering gene expression without modifying the DNA sequence. In mUC, promoter hypermethylation affects many genes153 and reversal of this hypermethylation can lead to tumour suppressor gene re-expression. At the same time, preclinical data suggest cisplatin resistance might be avoided by concurrent administration of a DNA hypomethylating agent.154,155 SPIRE is a phase Ib/IIa trial evaluating the safety and activity of the DNA methyltransferase inhibitor guadecitabine (SGI-110) in combination with GC chemotherapy (ISRCTN16332228).156
Antibody–drug conjugates
The antibody–drug conjugates (ADCs) represent an innovative way of delivering chemotherapy. The structure of any ADC comprises a mAb that binds to a specific antigen highly expressed in the cancer cell surface, a cytotoxic compound and a linker molecule. Once the mAb binds, the antigen is internalized by endocytosis and release the cytotoxic drug (also known as the payload) after a process of lysosomal degradation. The payloads are broadly divided into two families, those disrupting tubulin homeostasis (i.e. aurastatins and maytansines) and those acting on DNA (i.e. SN-38 calicheamicins, pyrrolobenzodiazepines and duocarmycins). The formers have had more development and represent a high percentage of the payloads in the ADCs.157 The maytansines include emtasine (DM1) and ravtansine (DM4) and the auristatins comprise monomethyl auristatin E (MMAE) and F (MMAF). Toxicity of ADCs will be determined by each payload utilized but overall include haematological, hepatic, ocular and peripheral neuropathy. Currently, there are a number of ADCs in the advanced phases of clinical development in mUC including enfortumab vedotin (ASG-22CE/ASG-22ME), sacituzumab govitecan (IMMU-132) and ASG-15ME. The development of the ADC, enfortumab vedotin, is based on the knowledge that UC cells almost universally express a type I transmembrane protein named nectin-4, whose expression in normal tissue is limited.158 This drug consists of a human anti-nectin-4 mAb link to the tubulin disrupting agent, MMAE. Strong preclinical activity of this compound in UC animal models supported further clinical development. A recent update of a phase I trial with this agent (ClinicalTrials.gov identifier: NCT02091999), focused exclusively in the cohort that received enfortumab vedotin at the recommended phase II dose (n = 112) showed a confirmed ORR of 33% (95% CI 24.7–42.9) with eight additional unconfirmed Partial responses (PRs) pending confirmation.11 More notably, significant activity was observed in two difficult populations, those patients who had progressed to previous checkpoint inhibitor therapy (32% ORR; n = 84) and those with liver metastases and progression to checkpoint inhibitors (26% ORR; n = 23). The drug was overall well tolerated with hyponatraemia and vomiting as the two most common grade 3 adverse events, although hyperglycaemia was identified as a class adverse event. There are two trials with enfortumab vedotin, a single-arm phase II study and a randomized phase III study versus chemotherapy that are presently enrolling patients (ClinicalTrials.gov identifiers: NCT0329333 and NCT03474107). Combination studies have been initiated utilizing enfortumab vedotin along with either atezolizumab or pembrolizumab (ClinicalTrials.gov identifier: NCT03288545). This solid clinical development has granted enfortumab vedotin the breakthrough designation by the US FDA. The two other ADCs (IMMU-132 and ASG-15ME) bind respectively to the glycoprotein trop-2 and the SLIT and NTRK-like (SLITRK) 6 type I transmembrane protein.159 The former is a human trophoblast cell-surface antigen, with a high expression in muscle-invasive bladder tumours compared with nonmuscle invasive and healthy urothelium. The latter is a transmembrane protein primarily found in the brain, associated with hearing and vision development and expressed almost universally in tumours of both the lower and upper urinary tract. Notably, SLITRK6 is expressed in over 50% of nontransitional cell histologies.160 IMMU-132 consists of an anti-trop-mAb conjugated with the active metabolite of irinotecan (SN-38). In a phase I/II study for multiple tumours (ClinicalTrials.gov identifier: NCT01631552) activity of this drug was observed in mUC patients with an ORR of 38%.161 In a larger cohort, remarkable responses in patients with liver metastases were reported (39% ORR).160 The ADC ASG-15ME comprises an SLITRK6-antibody conjugated to the tubule disrupting agent MMAE.162 Preliminary efficacy of this compound showed remarkable activity including responses in patients previously treated with checkpoint inhibitors and those with liver metastases along with a good safety profile.163 Thus, ADCs appear to be a promising treatment strategy to deliver chemotherapy in a selective and less toxic mode. This group of compounds has revealed encouraging activity and an acceptable safety profile. Moreover, their efficacy does not seem confined to good prognosis patients as they have shown notable responses in patients with liver metastases. Future studies will determine the real value of ADCs in mUC.
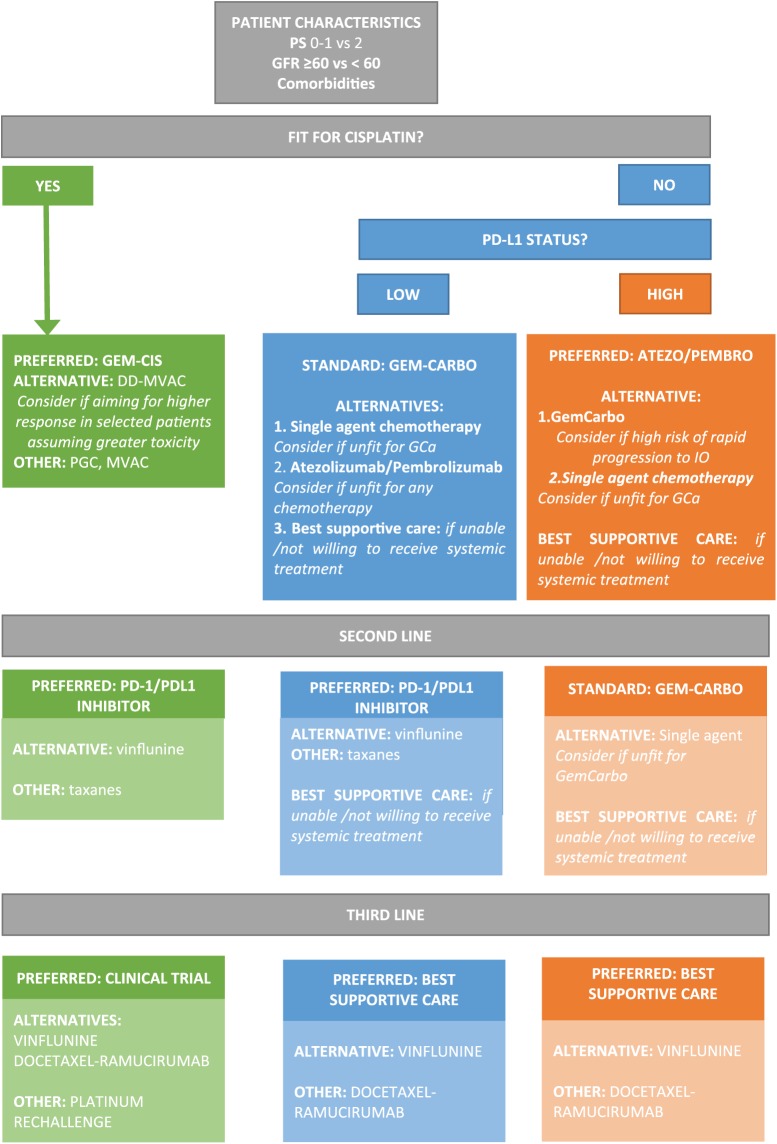
In summary, chemotherapy has a relevant role in the treatment of mUC in different clinical settings. Cisplatin-based combinations remain the preferred treatment option in first-line fit mUC patients. In patients deemed unfit for platinum, both carboplatin-based combinations, single-agent chemotherapy regimens or immunotherapy are valid options. However, the use of the latter in the first line is currently restricted to those tumours with PD-L1 expression or unable to tolerate any platinum combination. In the second-line setting, chemotherapy with vinflunine or with the combination of docetaxel and ramucirumab, would represent valid options. Yet, the recently incorporated checkpoint inhibitors are a good alternative in this setting, achieving long term benefit in selected patients with better safety profiles. Pembrolizumab has demonstrated superiority when compared with chemotherapy in this setting. No validated predictive biomarkers of response exist yet to help treatment choice. Based on these data, a potential treatment algorithm has been built (Figure 1) to guide patient treatment. More likely, the results of the ongoing studies with combinations of chemotherapy and immunotherapy will demonstrate the additive value of both strategies and also the novel ways of delivering cytotoxics will open a new opportunity for patients with mUC as well as drugs targeting particular molecular abnormalities such as fibroblast growth factor receptor gene alterations or defects on DNA damage repair genes.
Figure 1.
Proposed treatment algorithm for patients with metastatic urothelial carcinoma.
PS, performance status; GFR, glomerular filtration rate; GEM-CIS, gemcitabine and cisplatin; GEM-CARBO / GCa, gemcitabine and carboplatin; MVAC, metrotexate, vinblastine, doxorubicin and cisplatin; DD-MVAC, dose dense MVAC; PGC, paclitaxel, gemcitabine and cisplatin; PD-1, programmed death-1; PD-L1, programmed death-ligand 1.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr. Ignacio Duran has participated in compensated advisory boards for BMS, Roche-Genentech, IPSEN, Astra-Zeneca, MSD Oncology, Seattle Genetics, Pharmacyclics, Jansen Oncology, Bayer and Novartis. He has also received honoraria for participating in educational activities with BMS, IPSEN, Roche-Genentech, Janssen Oncology, MSD Oncology, the NCCN and Astellas Pharma. Part of his travel/registration expenses to medical meetings have been covered by Astellas Pharma, Astra-Zeneca and Roche-Genentech. Dr. Duran’s institution has received research funding from Roche-Genentech, Astra-Zeneca, Janssen Oncology and Astellas Pharma.
ORCID iD: Ignacio Duran  https://orcid.org/0000-0001-8571-7163
https://orcid.org/0000-0001-8571-7163
Contributor Information
Alfonso Gómez De Liaño, Medical Oncology Department, Complejo Hospitalario Universitario Insular-Materno Infantil, Las Palmas de Gran Canaria, Spain.
Ignacio Duran, Servicio de Oncologia Medica, Medical Oncology Department, Hospital Universitario Marques de Valdecilla, Edificio Sur, 2a Planta, Despacho 277, 39008 Santander, Spain.
References
- 1. International Agency for Research on Cancer, World Health Organisation. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012, http://globocan.iarc.fr (2012, accessed 1 September 2018).
- 2. Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute, https://seer.cancer.gov/csr/1975_2015/ (2017, SEER data submission, posted to the SEER website, 8 April, accessed 1 September 2018). [Google Scholar]
- 3. de Vos FY, de Wit R. Choosing chemotherapy in patients with advanced urothelial cell cancer who are unfit to receive cisplatin-based chemotherapy. Ther Adv Med Oncol 2010; 2: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 1989; 64: 2448–2458. [DOI] [PubMed] [Google Scholar]
- 5. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- 6. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27: 4454–4461. [DOI] [PubMed] [Google Scholar]
- 7. Castellano D, Puente J, de Velasco G, et al. Safety and effectiveness of vinflunine in patients with metastatic transitional cell carcinoma of the urothelial tract after failure of one platinum-based systemic therapy in clinical practice. BMC Cancer 2014; 14: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Donas J, Font A, Perez-Valderrama B, et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): a multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol 2017; 18: 672–681. [DOI] [PubMed] [Google Scholar]
- 9. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg JE, Sridhar SS, Zhang J, et al. Updated results from the enfortumab vedotin phase 1 (EV-101) study in patients with metastatic urothelial cancer (mUC). J Clin Oncol 2018; 36(Suppl. 15): abstract 4504. [Google Scholar]
- 12. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999; 17: 3173–3181. [DOI] [PubMed] [Google Scholar]
- 13. Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013; 105: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galsky MD, Moshier E, Krege S, et al. Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin-based chemotherapy. Cancer 2013; 119: 3012–3019. [DOI] [PubMed] [Google Scholar]
- 15. Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Eur Urol 2017; 71: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yagoda A. Chemotherapy of urothelial tract tumors. Cancer 1987; 60(Suppl. 3): 574–585. [DOI] [PubMed] [Google Scholar]
- 17. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985; 133: 403–407. [DOI] [PubMed] [Google Scholar]
- 18. Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1992; 10: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 19. Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 1990; 8: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 20. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997; 15: 2564–2569. [DOI] [PubMed] [Google Scholar]
- 21. Boutan-Laroze A, Mahjoubi M, Droz JP, et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced carcinoma of the bladder. The French Federation of Cancer Centers experience. Eur J Cancer 1991; 27: 1690–1694. [DOI] [PubMed] [Google Scholar]
- 22. Connor JP, Olsson CA, Benson MC, et al. Long-term follow-up in patients treated with methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) for transitional cell carcinoma of urinary bladder: cause for concern. Urology 1989; 34: 353–356. [DOI] [PubMed] [Google Scholar]
- 23. Tannock I, Gospodarowicz M, Connolly J, et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J Urol 1989; 142(2 Pt 1): 289–292. [DOI] [PubMed] [Google Scholar]
- 24. Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 2001; 19: 2638–2646. [DOI] [PubMed] [Google Scholar]
- 25. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006; 42: 50–54. [DOI] [PubMed] [Google Scholar]
- 26. Abbruzzese JL, Grunewald R, Weeks EA, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 1991; 9: 491–498. [DOI] [PubMed] [Google Scholar]
- 27. Hertel LW, Boder GB, Kroin JS, et al. Evaluation of the antitumor activity of gemcitabine (2’,2’-difluoro-2’-deoxycytidine). Cancer Res 1990; 50: 4417–4422. [PubMed] [Google Scholar]
- 28. Gebbia V, Testa A, Borsellino N, et al. Single agent 2’,2’-difluorodeoxycytidine in the treatment of metastatic urothelial carcinoma: a phase II study. Clin Ter 1999; 150: 11–15. [PubMed] [Google Scholar]
- 29. Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 1998; 34: 1208–1212. [DOI] [PubMed] [Google Scholar]
- 30. Pollera CF, Ceribelli A, Crecco M, et al. Weekly gemcitabine in advanced bladder cancer: a preliminary report from a phase I study. Ann Oncol 1994; 5: 182–184. [DOI] [PubMed] [Google Scholar]
- 31. Stadler WM, Kuzel T, Roth B, et al. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 1997; 15: 3394–3398. [DOI] [PubMed] [Google Scholar]
- 32. Mead GM, Russell M, Clark P, et al. A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer 1998; 78: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore MJ, Winquist EW, Murray N, et al. Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1999; 17: 2876–2881. [DOI] [PubMed] [Google Scholar]
- 34. von der Maase H, Andersen L, Crino L, et al. Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trial. Ann Oncol 1999; 10: 1461–1465. [DOI] [PubMed] [Google Scholar]
- 35. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–4608. [DOI] [PubMed] [Google Scholar]
- 36. Bellmunt J, Guillem V, Paz-Ares L, et al. Phase I-II study of paclitaxel, cisplatin, and gemcitabine in advanced transitional-cell carcinoma of the urothelium. Spanish Oncology Genitourinary Group. J Clin Oncol 2000; 18: 3247–3255. [DOI] [PubMed] [Google Scholar]
- 37. Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol 2012; 30: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013; 24: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 39. Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 1997; 80: 1966–1972. [DOI] [PubMed] [Google Scholar]
- 40. Dogliotti L, Carteni G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol 2007; 52: 134–141. [DOI] [PubMed] [Google Scholar]
- 41. Petrioli R, Frediani B, Manganelli A, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer 1996; 77: 344–351. [DOI] [PubMed] [Google Scholar]
- 42. Dreicer R, Manola J, Roth BJ, et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer 2004; 100: 1639–1645. [DOI] [PubMed] [Google Scholar]
- 43. Sonpavde G, Watson D, Tourtellott M, et al. Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer 2012; 10: 1–5. [DOI] [PubMed] [Google Scholar]
- 44. Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006; 107: 506–513. [DOI] [PubMed] [Google Scholar]
- 45. Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl 2016; 14: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol 2011; 29: 2432–2438. [DOI] [PubMed] [Google Scholar]
- 47. Carles J, Nogue M, Domenech M, et al. Carboplatin-gemcitabine treatment of patients with transitional cell carcinoma of the bladder and impaired renal function. Oncology 2000; 59: 24–27. [DOI] [PubMed] [Google Scholar]
- 48. Bellmunt J, de Wit R, Albanell J, et al. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur J Cancer 2001; 37: 2212–2215. [DOI] [PubMed] [Google Scholar]
- 49. Ricci S, Galli L, Chioni A, et al. Gemcitabine plus epirubicin in patients with advanced urothelial carcinoma who are not eligible for platinum-based regimens. Cancer 2002; 95: 1444–1450. [DOI] [PubMed] [Google Scholar]
- 50. Vaughn DJ, Manola J, Dreicer R, et al. Phase II study of paclitaxel plus carboplatin in patients with advanced carcinoma of the urothelium and renal dysfunction (E2896): a trial of the Eastern Cooperative Oncology Group. Cancer 2002; 95: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 51. Linardou H, Aravantinos G, Efstathiou E, et al. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: phase II study of the Hellenic Co-operative Oncology Group. Urology 2004; 64: 479–484. [DOI] [PubMed] [Google Scholar]
- 52. Bamias A, Lainakis G, Kastritis E, et al. Biweekly carboplatin/gemcitabine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: report of efficacy, quality of life and geriatric assessment. Oncology 2007; 73: 290–297. [DOI] [PubMed] [Google Scholar]
- 53. Carles J, Esteban E, Climent M, et al. Gemcitabine and oxaliplatin combination: a multicenter phase II trial in unfit patients with locally advanced or metastatic urothelial cancer. Ann Oncol 2007; 18: 1359–1362. [DOI] [PubMed] [Google Scholar]
- 54. Calabro F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 2009; 115: 2652–2659. [DOI] [PubMed] [Google Scholar]
- 55. Culine S, Flechon A, Guillot A, et al. Gemcitabine or gemcitabine plus oxaliplatin in the first-line treatment of patients with advanced transitional cell carcinoma of the urothelium unfit for cisplatin-based chemotherapy: a randomized phase 2 study of the French Genitourinary Tumor Group (GETUG V01). Eur Urol 2011; 60: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 56. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bellmunt J, Albanell J, Gallego OS, et al. Carboplatin, methotrexate, and vinblastine in patients with bladder cancer who were ineligible for cisplatin-based chemotherapy. Cancer 1992; 70: 1974–1979. [DOI] [PubMed] [Google Scholar]
- 58. Small EJ, Fippin LJ, Ernest ML, et al. A carboplatin-based regimen for the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer 1996; 78: 1775–1780. [DOI] [PubMed] [Google Scholar]
- 59. Turkolmez K, Beduk Y, Baltaci S, et al. Gemcitabine plus vinorelbine chemotherapy in patients with advanced bladder carcinoma who are medically unsuitable for or who have failed cisplatin-based chemotherapy. Eur Urol 2003; 44: 682–686. [DOI] [PubMed] [Google Scholar]
- 60. Galsky MD, Iasonos A, Mironov S, et al. Phase II trial of dose-dense doxorubicin plus gemcitabine followed by paclitaxel plus carboplatin in patients with advanced urothelial carcinoma and impaired renal function. Cancer 2007; 109: 549–555. [DOI] [PubMed] [Google Scholar]
- 61. De Santis M, Wiechno PJ, Bellmunt J, et al. Vinflunine-gemcitabine versus vinflunine-carboplatin as first-line chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma: results of an international randomized phase II trial (JASINT1). Ann Oncol 2015; 27(3): 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 63. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. United States Food and Drug Administration. FDA Alerts Health Care Professionals and Oncology Clinical Investigators about an Efficacy Issue Identified in Clinical Trials for Some Patients Taking Keytruda (pembrolizumab) or Tecentriq (atezolizumab) as Monotherapy to Treat Urothelial Cancer with Low Expression of PD-L1, 2018. https://www.fda.gov/Drugs/DrugSafety/ucm608075.htm (accessed 16 August 2018).
- 65. Gourd E. EMA restricts use of anti-PD-1 drugs for bladder cancer. Lancet Oncol 2018; 19: e341. [DOI] [PubMed] [Google Scholar]
- 66. Nadal R, Bellmunt J. New treatments for bladder cancer: when will we make progress? Curr Treat Options Oncol 2014; 15: 99–114. [DOI] [PubMed] [Google Scholar]
- 67. Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31: 2895–2902. [DOI] [PubMed] [Google Scholar]
- 68. Hoffman-Censits J, Wong YN. Perioperative and maintenance therapy after first-line therapy as paradigms for drug discovery in urothelial carcinoma. Clin Genitourin Cancer 2015; 13: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Muto S, Abe H, Noguchi T, et al. Maintenance monotherapy with gemcitabine after standard platinum-based chemotherapy in patients with advanced urothelial cancer. Int J Urol 2015; 22: 490–494. [DOI] [PubMed] [Google Scholar]
- 70. Kalogirou C, Svistunov A, Krebs M, et al. Maintenance monotherapy with Gemcitabine following cisplatin-based primary combination chemotherapy in surgically treated advanced urothelial carcinoma: a matched-pair single institution analysis. Mol Clin Oncol 2016; 4: 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mitsuzuka K, Yamashita S, Namiki S, et al. Low-dose maintenance gemcitabine-carboplatin chemotherapy could be an alternative to continuous standard chemotherapy for patients with metastatic urothelial carcinoma. Int J Urol 2014; 21: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 72. Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer 2014; 120: 692–701. [DOI] [PubMed] [Google Scholar]
- 73. Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol 2009; 20: 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 2015; 51: 45–54. [DOI] [PubMed] [Google Scholar]
- 75. Powles T, Huddart RA, Elliott T, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol 2017; 35: 48–55. [DOI] [PubMed] [Google Scholar]
- 76. Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28: 1850–1855. [DOI] [PubMed] [Google Scholar]
- 77. Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20: 937–940. [DOI] [PubMed] [Google Scholar]
- 78. Witte RS, Elson P, Bono B, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol 1997; 15: 589–593. [DOI] [PubMed] [Google Scholar]
- 79. Bellmunt J, Fougeray R, Rosenberg JE, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol 2013; 24: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 80. de Wit R, Kruit WH, Stoter G, et al. Docetaxel (Taxotere): an active agent in metastatic urothelial cancer; results of a phase II study in non-chemotherapy-pretreated patients. Br J Cancer 1998; 78: 1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–1857. [DOI] [PubMed] [Google Scholar]
- 82. Kim YS, Lee SI, Park SH, et al. A phase II study of weekly docetaxel as second-line chemotherapy in patients with metastatic urothelial carcinoma. Clin Genitourin Cancer 2016; 14: 76–81. [DOI] [PubMed] [Google Scholar]
- 83. Krege S, Rembrink V, Borgermann C, et al. Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol 2001; 165: 67–71. [DOI] [PubMed] [Google Scholar]
- 84. Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 2012; 30: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Petrylak DP, Tagawa ST, Kohli M, et al. Docetaxel as monotherapy or combined with ramucirumab or icrucumab in second-line treatment for locally advanced or metastatic urothelial carcinoma: an open-label, three-arm, randomized controlled phase II trial. J Clin Oncol 2016; 34: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 86. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017; 390: 2266–2277. [DOI] [PubMed] [Google Scholar]
- 87. Boukovinas I, Androulakis N, Vamvakas L, et al. Sequential gemcitabine and cisplatin followed by docetaxel as first-line treatment of advanced urothelial carcinoma: a multicenter phase II study of the Hellenic Oncology Research Group. Ann Oncol 2006; 17: 1687–1692. [DOI] [PubMed] [Google Scholar]
- 88. Roth BJ, Dreicer R, Einhorn LH, et al. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 1994; 12: 2264–2270. [DOI] [PubMed] [Google Scholar]
- 89. Papamichael D, Gallagher CJ, Oliver RT, et al. Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. Br J Cancer 1997; 75: 606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Joly F, Houede N, Noal S, et al. Do patients with advanced urothelial carcinoma benefit from weekly paclitaxel chemotherapy? A GETUG phase II study. Clin Genitourin Cancer 2009; 7: E28–E33. [DOI] [PubMed] [Google Scholar]
- 91. Sweeney CJ, Williams SD, Finch DE, et al. A Phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer 1999; 86: 514–518. [DOI] [PubMed] [Google Scholar]
- 92. Sternberg CN, Calabro F, Pizzocaro G, et al. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 2001; 92: 2993–2998. [DOI] [PubMed] [Google Scholar]
- 93. Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol 2011; 22: 288–294. [DOI] [PubMed] [Google Scholar]
- 94. Fechner G, Siener R, Reimann M, et al. Randomised phase II trial of gemcitabine and paclitaxel second-line chemotherapy in patients with transitional cell carcinoma (AUO Trial AB 20/99). Int J Clin Pract 2006; 60: 27–31. [DOI] [PubMed] [Google Scholar]
- 95. Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol 2013; 14: 769–776. [DOI] [PubMed] [Google Scholar]
- 96. Sridhar SS, Blais N, Tran B, et al. Randomized phase II trial comparing nab-paclitaxel (Nab-P) to paclitaxel (P) in patients (pts) with advanced urothelial cancer progressing on or after a platinum containing regimen (NCT02033993). J Clin Oncol 2018; 36(Suppl. 15): abstract 4505. [Google Scholar]
- 97. Hoffman-Censits JH, Vaughn DJ, Lin J, et al. A phase II study of cabazitaxel in patients with urothelial carcinoma who have disease progression following platinum-based chemotherapy. J Clin Oncol 2014; 32(Suppl. 15): abstract e15519. [Google Scholar]
- 98. Arranz Arija J, Garcia del Muro X, Perez-Valderrama B, et al. JEVTCC: phase II trial of cabazitaxel (Cbz) in patients (pt) with advanced or metastatic transitional-cell carcinoma (mTCC), who progressed before 12 months after cisplatin-based chemotherapy—A Spanish Oncologic Genitourinary Group (SOGUG) study. J Clin Oncol 2015; 33(Suppl. 15): abstract 4525. [Google Scholar]
- 99. Bellmunt J, Kerst JM, Vazquez F, et al. A randomized phase II/III study of cabazitaxel versus vinflunine in metastatic or locally advanced transitional cell carcinoma of the urothelium (SECAVIN). Ann Oncol 2017; 28: 1517–1522. [DOI] [PubMed] [Google Scholar]
- 100. Culine S, Theodore C, De Santis M, et al. A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer 2006; 94: 1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer 2009; 115: 4110–4117. [DOI] [PubMed] [Google Scholar]
- 102. Albers P, Siener R, Hartlein M, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma - prognostic factors for response and improvement of quality of life. Onkologie 2002; 25: 47–52. [DOI] [PubMed] [Google Scholar]
- 103. Akaza H, Naito S, Usami M, et al. Efficacy and safety of gemcitabine monotherapy in patients with transitional cell carcinoma after Cisplatin-containing therapy: a Japanese experience. Jpn J Clin Oncol 2007; 37: 201–206. [DOI] [PubMed] [Google Scholar]
- 104. Paz-Ares L, Bezares S, Tabernero JM, et al. Review of a promising new agent–pemetrexed disodium. Cancer 2003; 97(Suppl. 8): 2056–2063. [DOI] [PubMed] [Google Scholar]
- 105. Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 2006; 24: 3451–3457. [DOI] [PubMed] [Google Scholar]
- 106. Galsky MD, Mironov S, Iasonos A, et al. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs 2007; 25: 265–270. [DOI] [PubMed] [Google Scholar]
- 107. Winquist E, Vokes E, Moore MJ, et al. A Phase II study of oxaliplatin in urothelial cancer. Urol Oncol 2005; 23: 150–154. [DOI] [PubMed] [Google Scholar]
- 108. Dreicer R, Li S, Manola J, et al. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology Group. Cancer 2007; 110: 759–763. [DOI] [PubMed] [Google Scholar]
- 109. Bellmunt J, Picus J, Kohli M, et al. FIERCE-21: Phase 1b/2 study of docetaxel + b-701, a selective inhibitor of FGFR3, in relapsed or refractory (R/R) metastatic urothelial carcinoma (mUCC). J Clin Oncol 2018; 36(Suppl. 15): abstract 4534. [Google Scholar]
- 110. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–562. [DOI] [PubMed] [Google Scholar]
- 111. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016; 34: 3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016; 17: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol 2017; 35: 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017; 18: 212–220. [DOI] [PubMed] [Google Scholar]
- 115. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 2017; 3: e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018; 19: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Petrylak DP, Powles T, Bellmunt J, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol 2018; 4: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017; 18: 312–322. [DOI] [PubMed] [Google Scholar]
- 120. Saada-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017; 28: 1605–1611. [DOI] [PubMed] [Google Scholar]
- 121. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 123. Joseph RW, Loriot Y, Perez-Gracia JL, et al. Clinical characteristics associated with early progression or long-term response from the phase II IMVIGOR210 study: atezolizumab in locally advanced or metastatic urothelial carcinoma. J Urol 2018; 199: e1039. [Google Scholar]
- 124. Maleki Vareki S, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol 2017; 116: 116–124. [DOI] [PubMed] [Google Scholar]
- 125. Di Nunno V, De Luca E, Buttigliero C, et al. Immune-checkpoint inhibitors in previously treated patients with advanced or metastatic urothelial carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018; 129: 124–132. [DOI] [PubMed] [Google Scholar]
- 126. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015; 68: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017; 23: 3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol 2018; 36: 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mouw KW, Goldberg MS, Konstantinopoulos PA, et al. DNA damage and repair biomarkers of immunotherapy response. Cancer discov 2017; 7: 675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Galsky MD, Wang H, Hahn NM, et al. Phase 2 trial of gemcitabine, cisplatin, plus ipilimumab in patients with metastatic urothelial cancer and impact of dna damage response gene mutations on outcomes. Eur Urol 2018; 73: 751–759. [DOI] [PubMed] [Google Scholar]
- 131. Necchi A, Briganti A, Bianchi M, et al. Preoperative pembrolizumab (pembro) before radical cystectomy (RC) for muscle-invasive urothelial bladder carcinoma (MIUC): interim clinical and biomarker findings from the phase 2 PURE-01 study. J Clin Oncol 2018; 36(Suppl. 5) abstract 4507. [DOI] [PubMed] [Google Scholar]
- 132. McConkey DJ, Choi W. Molecular subtypes of bladder cancer. Curr Oncol Rep 2018; 20: 77. [DOI] [PubMed] [Google Scholar]
- 133. Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018; 554: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014; 25: 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017; 72: 544–554. [DOI] [PubMed] [Google Scholar]
- 136. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017; 171: 540–556.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McConkey DJ, Choi W, Dinney CP. New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur Urol 2014; 66: 609–610. [DOI] [PubMed] [Google Scholar]
- 139. Alanee S, Alvarado-Cabrero I, Murugan P, et al. Update of the International Consultation on Urological Diseases on bladder cancer 2018: non-urothelial cancers of the urinary bladder. World J Urol. Epub ahead of print 1 August 2018. DOI: 10.1007/s00345-018-2421-5. [DOI] [PubMed] [Google Scholar]
- 140. Gomez de Liano Lista A, Necchi A, Lavaud P, et al. 862P - Treatment and outcome after Immune checkpoint inhibitors (ICI) in metastatic Urothelial Carcinoma (mUC): a European perspective. Ann Oncol 2017; 28(Suppl. 5): v295–v329. [Google Scholar]
- 141. Szabados B, van Dijk N, Tang YZ, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol 2018; 73: 149–152. [DOI] [PubMed] [Google Scholar]
- 142. Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015; 26: 812–817. [DOI] [PubMed] [Google Scholar]
- 143. Jones RJ, Hussain SA, Protheroe AS, et al. Randomized phase II study investigating pazopanib versus weekly paclitaxel in relapsed or progressive urothelial cancer. J Clin Oncol 2017; 35: 1770–1777. [DOI] [PubMed] [Google Scholar]
- 144. Geldart T, Chester J, Casbard A, et al. SUCCINCT: an open-label, single-arm, non-randomised, phase 2 trial of gemcitabine and cisplatin chemotherapy in combination with sunitinib as first-line treatment for patients with advanced urothelial carcinoma. Eur Urol 2015; 67: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Balar AV, Apolo AB, Ostrovnaya I, et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 2013; 31: 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04–75. J Clin Oncol 2011; 29: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 147. Ross JS, Wang K, Khaira D, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016; 122: 702–711. [DOI] [PubMed] [Google Scholar]
- 148. Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov 2018; 8: 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Joerger M, Cassier PA, Penel N, et al. Rogaratinib in patients with advanced urothelial carcinomas prescreened for tumor FGFR mRNA expression and effects of mutations in the FGFR signaling pathway. J Clin Oncol 2018; 36(Suppl. 15): abstract 4513. [Google Scholar]
- 150. Siefker-Radtke AO, Necchi A, Park SH, et al. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). J Clin Oncol 2018; 36(Suppl. 15): abstract 4503. [Google Scholar]
- 151. Rosenberg JE, Hahn NM, Regan MM, et al. Apatorsen plus docetaxel versus docetaxel alone in platinum-resistant metastatic urothelial carcinoma (Borealis-2). Br J Cancer 2018; 118: 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bellmunt J, Eigl BJ, Senkus E, et al. Borealis-1: a randomized, first-line, placebo-controlled, phase II study evaluating apatorsen and chemotherapy for patients with advanced urothelial cancer. Ann Oncol 2017; 28: 2481–2488. [DOI] [PubMed] [Google Scholar]
- 153. Dudziec E, Gogol-Doring A, Cookson V, et al. Integrated epigenome profiling of repressive histone modifications, DNA methylation and gene expression in normal and malignant urothelial cells. PLoS One 2012; 7: e32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shang D, Liu Y, Matsui Y, et al. Demethylating agent 5-aza-2’-deoxycytidine enhances susceptibility of bladder transitional cell carcinoma to Cisplatin. Urology 2008; 71: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 155. Christoph F, Kempkensteffen C, Weikert S, et al. Methylation of tumour suppressor genes APAF-1 and DAPK-1 and in vitro effects of demethylating agents in bladder and kidney cancer. Br J Cancer 2006; 95: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Crabb S, Danson SJ, Catto JWF, et al. SPIRE - combining SGI-110 with cisplatin and gemcitabine chemotherapy for solid malignancies including bladder cancer: study protocol for a phase Ib/randomised IIa open label clinical trial. Trials 2018; 19: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lambert JM, Morris CQ. Antibody-Drug Conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Ther 2017; 34: 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016; 76: 3003–3013. [DOI] [PubMed] [Google Scholar]
- 159. Avellini C, Licini C, Lazzarini R, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017; 8: 58642–58653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tagawa S, Faltas B, Lam E, et al. 858P - Sacituzumab govitecan (IMMU-132) for patients with pretreated metastatic urothelial uancer (UC): interim results. Ann Oncol 2017; 28(Suppl. 5): v295–v329. [Google Scholar]
- 161. Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab Govitecan, a novel antibody–drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 2016; 14: e75–e79. [DOI] [PubMed] [Google Scholar]
- 162. Morrison K, Challita-Eid PM, Raitano A, et al. Development of ASG-15ME, a novel antibody-drug conjugate targeting SLITRK6, a new urothelial cancer biomarker. Mol Cancer Ther 2016; 15: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 163. Petrylak D, Heath E, Sonpavde G, et al. 780PD - Interim analysis of phase 1 dose escalation trial of the antibody-drug conjugate ASG15E in patients with metastatic urothelial cancer. Ann Oncol 2016; 27: 266–295. [Google Scholar]