Abstract
Aiming to unravel interspecific differences in olfactory preferences, we performed comparative studies of odor valence in flies, mice, and humans. Our analysis suggests a model where flies and mice share similar olfactory preferences, but neither species share odor preferences with humans. This model contrasts with a previous study by Mandairon et al., which suggested that the olfactory preferences of mice and humans are similar. A probabilistic examination revealed that underpowered studies can result in spurious significant correlations, which can account for the differences between both studies. Future analyses aimed at dissecting the olfactory preferences across species need to test large numbers of odorants to stress-test the model proposed here and identify robust associations.
Keywords: fly, human, mouse, odor preferences, olfaction
Animals perceive myriad odors as attractive or neutral and yet others as aversive. Olfactory cues can also elicit changes in behavior and physiology, thus playing an instrumental role in survival, reproduction, and species-specific adaptations to different ecological niches (Niimura 2012; Li and Liberles 2015). Of the many roles of olfaction, it has recently been proposed that assessing the valence of odors is its key function in humans (Yeshurun and Sobel 2010). However, such perceptions and rating of odorants across a hedonic scale can be innate, learned, and modulated by the internal state of the individual, or even be context dependent (Yeshurun and Sobel 2010; Li and Liberles 2015; Saraiva et al. 2016). Moreover, although many molecular mechanisms underlying olfaction are conserved between species, rapid evolutionary dynamics ensure the creation of highly species-specific repertoires and relative abundances of olfactory receptors (ORs), which ultimately shape the olfactory preferences and abilities in different animals (Niimura 2012; Ibarra-Soria et al. 2017).
Previous studies comparing olfactory preferences between flies–humans and mouse–humans revealed that the perception of odor intensity and perceptual valence are conserved in these species pairs, respectively, and that judgments of odor quality are different in fruit flies–humans (Keller and Vosshall 2007; Mandairon et al. 2009). Taken together, these observations suggest that distinct aspects of olfactory perception can be either species specific or conserved across these evolutionarily distant species pairs. However, these prior studies used small sample sizes (10 or less odorants) and different criteria to select the odorants, which could affect the interpretations of these findings. This is mainly due to highly combinatorial nature of the olfactory systems, allied to the vast arrays of odorants present in nature and the large receptor repertoires equipping individual species (Malnic et al. 1999; Nara et al. 2011; Knaden and Hansson 2014; Li and Liberles 2015). Fortuitously, the odor valences (i.e., olfactory preferences) of larger panels of odorants (73–480) have recently been characterized in flies (Drosophila melanogaster), mice (Mus musculus), and humans (Homo sapiens) (Knaden et al. 2012; Keller and Vosshall 2016; Saraiva et al. 2016). Using these data as a starting point, we performed comparative studies aimed at dissecting the differences in olfactory preferences among these 3 species.
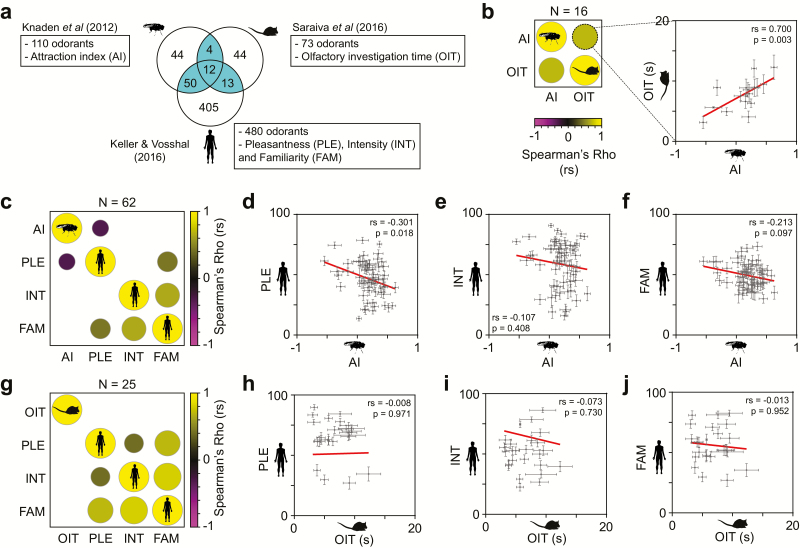
We started by compiling the odor valence scores for all possible combinations of overlapping odorants between flies, mice, and humans (Figure 1a). Our first comparison was between the attraction indexes (AI) of flies and the olfactory investigation times (OIT) of mice for the 16 overlapping odorants and found a strong positive correlation (rs = 0.700, P = 0.003, Figure 1b and Supplementary File 2) between the olfactory preferences of these species. Next, we focused on the set of 62 intersecting odorants between flies and humans, and compared the AI of flies to 3 measures of human odor perception: pleasantness (PLE), intensity (INT), and familiarity (FAM) (Figure 1c–f). We found that AI of flies correlates negatively with the human-rated PLE (rs = −0.301, P = 0.018, Figure 1d and Supplementary File 2), but not with INT or FAM (Figure 1e,f). We observed that the human odor valence parameters tend to correlate positively with each other (Figure 1c,g, Supplementary Figure 1b, and Supplementary File 2), in line with previous studies (Distel et al. 1999; Keller and Vosshall 2016). Subsequently, we tested the odor valence scores for the intersecting 25 odorants between the mouse and human studies, and found no significant correlation between the mouse OIT and the human-rated PLE, INT, or FAM (Figure 1g–j and Supplementary File 2).
Figure 1.
Interspecific differences of odor valence in fruit flies, mice, and humans. (a) Study design: the Venn diagram indicates the number of odorants (highlighted in ocean blue) for which odor valence scores overlap in flies (Knaden et al. 2012), mice (Saraiva et al. 2016), and humans (Keller and Vosshall 2016). For each species, the name and abbreviation of the parameters measured are specified as follows: fly attraction index (AI), mouse olfactory investigation time (OIT) and human-rated pleasantness (PLE), intensity (INT), and familiarity (FAM). (b) Correlogram matrix (left) and correlation plot (right) comparing the fly AI with mouse OIT for the 16 overlapping odorants. (c) Correlogram for the 62 overlapping odorants between fly AI and the human PLE, INT, and FAM. To the right are the corresponding correlation plots between fly AI and human-rated PLE (d), INT (e), and FAM (f). (g) Correlogram for the 25 overlapping odorants between mice OIT and humans PLE, INT, and FAM. To the right are the corresponding correlation plots between mouse OIT and human-rated PLE (h), INT (i), and FAM (j). In all correlograms, only significant (P < 0.05) correlations are plotted, and the circle size and color indicate the magnitude and direction of the correlation (Spearman rho, rs). Blank cells correspond to nonsignificant correlations.
A limitation of the present study is the difference in the combinations of intersecting odorants used for the 3-way interspecific comparisons. To address this, we performed an analysis using the odor valence ratings for all twelve overlapping odorants between the 3 studies (Supplementary Figure 1a,b, Supplementary File 2). Consistent with the results above, we observe a strong positive correlation (rs = 0.657, P = 0.024) between flies and mice, but no correlation between mice and humans (Supplementary Figure 1b). Moreover, the negative correlation observed above between flies AI and rated PLE in humans (Figure 1c) is no longer maintained, probably due to the ~5.5-fold reduction in odorant sample size.
Although our data are consistent with a previous study showing that the judgments of odor quality between flies and humans are species specific (Keller and Vosshall 2007), it contradicts the previously reported finding by Mandairon et al. (2009) that a positive correlation exists between the olfactory preferences of mice and humans. Because both studies include odorants covering many different chemical structures and perceived odors in humans, what could explain these differences?
Although these discrepancies could arise from the different experimental protocols used to assess the odor preferences in the 2 mouse studies (Mandairon et al. 2009; Saraiva et al. 2016), it is more likely that these are due to the dissimilar and overall low number of odorants tested in both studies. Mandairon et al. (2009) tested 2 combinations of 9 and 10 odorants each, which yielded significant positive correlations (R = 0.56 and R = 0.58, respectively), calculated using linear regression (Mandairon et al. 2009). In the present study, we assessed a single combination of 25 odorants, and despite the increased (~2.6-fold) sample size, we find no evidence to support that mice and humans have similar olfactory preferences (Figure 1g–j). To test whether these differences could be due to the different statistical methods used, we performed statistical analyses (Spearman’s rho, Pearson’s rho, linear regression) using the raw data from the study by Mandairon et al. (2009). Surprisingly, we did not find any significant statistical association between and mouse OIT and human PLE for either combination of 9 and 10 odorants (Supplementary File 2). However, when combining all 19 odorants from the study by Mandairon et al. (2009), one statistical method does support a positive association between both (rs = 0.536, P = 0.0206, Supplementary File 2). We thus hypothesized that these differences in results could be due to subsampling. To assess this, we computed the spearman correlation coefficient (rs) and corresponding P value for all the possible combinations of 2–25 odorants selected from our pool of 25. Of the ~33.5 million possible combinations, only 0.89% (299 255) have a significant (P < 0.05) rs, and as expected, an even smaller proportion (0.52% or 175 491 combinations) showed significant rs ≥ 0.56 (Supplementary File 3, Supplementary Figure 1c). Interestingly, a similar number of combinations (0.31% or 154 054) also displayed significant rs ≤ −0.56. We then calculated the probability of obtaining a significant rs ≥ 0.56 or rs ≤ −0.56, for all possible combinations of 2–25 odorants. We found that in our dataset, there is a low probability (≤0.01) of finding subsets of 9–10 odorants with a significant rs ≥ 0.56 or rs ≤ −0.56, and that this probability decreases to zero as we reach subsets of 21 and 18 odorants, respectively (Supplementary Figure 1d, Supplementary File 3). These results suggest that the association between mouse OIT and human-rated PLE reported by Mandairon et al. (2009) could be affected by subsampling, which often can lead to spurious correlations. Finally, due to the complexity of the olfactory combinatorial code and the virtually infinite number of odorous chemicals present in nature, we cannot presently exclude the possibility that larger or different subsets of odors might yield results that differ from the model proposed in this study.
In conclusion, our analysis supports a model where odor preferences of flies correlate positively with the ones of mice and negatively with the ones of humans, but does not support the hypothesis that humans and mice prefer the same odors. Future studies investigating similarities and differences in olfactory preferences across species will need to test larger numbers of odorants to robustly establish differences of olfactory preferences among species and stress-test the model proposed here.
Author contributions
D.M. analyzed data and wrote the paper; M.M. analyzed data; A.S. contributed analysis tools, and L.R.S. conceived and supervised the project, analyzed data, and wrote the paper.
Conflict of interest
The authors declare that no competing interests exist.
Supplementary Material
Acknowledgments
We thank Eman Abou-Moussa, Bernice Lo, and Shoshana Helfer for helpful comments and discussions, and Markus Knaden and Nathalie Mandairon for providing us with the data reported in the studies by Knaden et al. (2012) and Mandairon et al. (2009).
References
- Distel H, Ayabe-Kanamura S, Martínez-Gómez M, Schicker I, Kobayakawa T, Saito S, Hudson R. 1999. Perception of everyday odors – correlation between intensity, familiarity and strength of hedonic judgement. Chem Senses. 24:191–199. [DOI] [PubMed] [Google Scholar]
- Ibarra-Soria X, Nakahara TS, Lilue J, Jiang Y, Trimmer C, Souza MA, Netto PH, Ikegami K, Murphy NR, Kusma M, et al. 2017. Variation in olfactory neuron repertoires is genetically controlled and environmentally modulated. eLife. 6:e21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Vosshall LB. 2016. Olfactory perception of chemically diverse molecules. BMC Neurosci. 17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Vosshall LB. 2007. Influence of odorant receptor repertoire on odor perception in humans and fruit flies. Proc Natl Acad Sci USA. 104:5614–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaden M, Hansson BS. 2014. Mapping odor valence in the brain of flies and mice. Curr Opin Neurobiol. 24:34–38. [DOI] [PubMed] [Google Scholar]
- Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. 2012. Spatial representation of odorant valence in an insect brain. Cell Rep. 1:392–399. [DOI] [PubMed] [Google Scholar]
- Li Q, Liberles SD. 2015. Aversion and attraction through olfaction. Curr Biol. 25:R120–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. 1999. Combinatorial receptor codes for odors. Cell. 96:713–723. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Poncelet J, Bensafi M, Didier A. 2009. Humans and mice express similar olfactory preferences. PLoS One. 4:e4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara K, Saraiva LR, Ye X, Buck LB. 2011. A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci. 31:9179–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y. 2012. Olfactory receptor multigene family in vertebrates: from the viewpoint of evolutionary genomics. Curr Genomics. 13:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva LR, Kondoh K, Ye X, Yoon KH, Hernandez M, Buck LB. 2016. Combinatorial effects of odorants on mouse behavior. Proc Natl Acad Sci USA. 113:E3300–E3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N. 2010. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 61:219–241, C1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.