Abstract
The mediodorsal thalamus is a higher order thalamic nucleus critical for many cognitive behaviors. Defined by its reciprocal connections with the prefrontal cortex, the mediodorsal thalamus receives strong projections from chemosensory cortical areas for taste and smell, gustatory cortex and piriform cortex. Recent studies indicate the mediodorsal thalamus is involved in experience-dependent chemosensory processes, including olfactory attention and discrimination and the hedonic perception of odor-taste mixtures. How novel and familiar chemosensory stimuli are represented within this structure remains unclear. Here, we compared the expression of c-Fos in the mediodorsal thalami of rats familiar with an odor, a taste, or an odor-taste mixture with those that sampled the stimuli for the first time. We found that familiar tastes or odor-taste mixtures induced significantly greater c-Fos expression in the mediodorsal thalamus than novel tastes or odor-taste mixtures, whereas novel odors induced greater c-Fos expression than familiar odors. These experience-dependent and modality-specific differences in c-Fos expression may relate to the behavioral relevance of the chemosensory stimulus, including odor neophobia. In a two-bottle brief-access preference task, rats preferred water to isoamyl acetate-odorized water over multiple days. However, after experience with isoamyl acetate mixed with sucrose (odor-taste mixture), the preference for water was eliminated. These findings demonstrate that experience with chemosensory stimuli modulates responses in the mediodorsal thalamus, suggesting this structure plays an integral role in communicating behaviorally relevant chemosensory information to higher order areas to guide food-related behaviors.
Keywords: familiar, mediodorsal thalamus, novel, odor, odor-taste, taste
Introduction
The mediodorsal thalamus is involved in a variety of cognitive functions, such as learning, memory, attention, and decision-making (Kawagoe et al. 2007; Plailly et al. 2008; Small et al. 2008; Veldhuizen and Small 2011; Han et al. 2013; Parnaudeau et al. 2013; Mitchell 2015), via its connectivity with the brainstem, basal forebrain, and primary sensory and higher order cortices (Groenewegen 1988; Kuroda and Price 1991a, 1991b; Ray and Price 1992; Krout and Loewy 2000; Collins et al. 2018). In rats, projections from chemosensory cortical areas, gustatory cortex and piriform cortex, overlap in the medial and central subnuclei of the mediodorsal thalamus (Price and Slotnick 1983; Kuroda et al. 1992; Shi and Cassell 1998). The mediodorsal thalamus does not project back to piriform cortex but does send projections to the gustatory cortex (Krettek and Price 1977b; Allen et al. 1991; Kuramoto et al. 2017) as well as the basolateral amygdala, orbitofrontal cortex, and other higher order regions important for both olfactory- and gustatory-dependent behaviors (Krettek and Price 1977a; Ray and Price 1992; Illig 2005; Kuramoto et al. 2017).
The extensive connectivity of the mediodorsal thalamus underlies its involvement in many chemosensory-based cognitive functions (see review by Courtiol and Wilson 2015). Neurons in the mediodorsal thalamus display odor-selective responses to orthonasal odor stimulation (Imamura et al. 1984; Courtiol and Wilson 2014), show anticipatory activity during odor sampling (Courtiol and Wilson 2016), and are modulated by changes in task structure (Kawagoe et al. 2007). Furthermore, attention to orthonasal odors increases the functional connectivity between the mediodorsal thalamus and the piriform cortex (Plailly et al. 2008; Courtiol and Wilson 2016). These findings support the hypothesis that the mediodorsal thalamus selectively routes and synchronizes behaviorally relevant sensory information to specific cortical networks depending on behavioral demand (Saalmann 2014; Courtiol and Wilson 2015; Nakajima and Halassa 2017).
Studies investigating the impact of mediodorsal thalamic damage have provided substantial insight into its role for chemosensory-based cognitive functions. Although humans and animals with extensive damage to the mediodorsal thalamus do not suffer from anosmia (Eichenbaum et al. 1980; Sela et al. 2009; Tham et al. 2009), lesions in this structure can disrupt odor discrimination (Eichenbaum et al. 1980), odor reversal learning (Slotnick and Risser 1990), cued-outcome associations (Mair et al. 2015), and most strikingly, the hedonic perception (pleasantness or disgustingness) of odor-taste mixtures (Tham et al. 2009, 2011). Sampling an odor and taste together leads to a robust association between the odor and the quality and value of the taste (Fanselow and Birk 1982; Holder 1991; Stevenson et al. 1995; Prescott et al. 2004; Gautam and Verhagen 2010; Green et al. 2012) that is resistant to extinction or interference (Sakai and Yamamoto 2001; Stevenson and Boakes 2004). In patients with damage to the mediodorsal thalamus, deficits in the hedonic perception of odors led to reduced food intake and weight loss (Rousseaux et al. 1996; Asai et al. 2008; Sela et al. 2009). People with lesions of the mediodorsal thalamus can distinguish odor qualities (such as “sweet” vs. “unsweet”) but report a reduced pleasantness of odor-taste mixtures (Tham et al. 2011). Thus, although not required for detecting odors, the mediodorsal thalamus is important for the perception of odor-taste mixtures.
Given the role of the mediodorsal thalamus in routing behaviorally relevant sensory information to higher order cortical networks, we hypothesized that novel and familiar chemosensory stimuli would drive different patterns of expression in the mediodorsal thalamus. To investigate how experience with tastes, odors, and odor-taste mixtures are represented in the mediodorsal thalamus, we examined the expression of the immediate early gene c-Fos as a correlate of neural activity between groups with novel or familiar chemosensory experience. We found that the response to a novel odor was greater than that to a familiar odor, while the response to a familiar taste or a familiar odor-taste mixture was greater than the response when novel. The results presented here demonstrate that the experience-dependent responses of neurons in the mediodorsal thalamus are modality specific and may relate to behavioral relevance. If true, the preference for an orally consumed odor should be influenced by pairing it with a palatable taste. To test this, we developed a two-bottle brief-access task to assess odor preferences over multiple sessions. We found that the preference for nonodorized water was maintained for days but was eliminated after the odor was paired with sucrose. These results provide further evidence that the mediodorsal thalamus is a key node in the network linking chemosensory signals and state information to guide behavior.
Materials and methods
Animals
All experimental procedures were performed in accordance with university, state, and federal regulations regarding research animals and were approved by the University of Louisville Institutional Animal Care and Use Committee. Fifty-three 3-month-old female Long-Evans rats (250–300 g; Charles Rivers) were maintained on a 12/12-h light-dark cycle with ad libitum access to food and water unless otherwise specified.
Single-bottle experience task
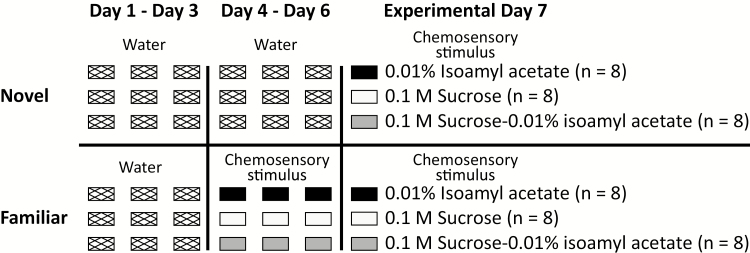
Single-housed rats (n = 48) were placed on a water regulation schedule, whereby access to distilled water was allowed for 1 h/day in the home cage. After 3 days of water regulation, the rats were placed in clean acrylic boxes with stainless-steel wire cage tops providing easy access to a single sipper tube (Figure 1). All liquid stimuli were mixed in distilled water. On training days (days 1–6), rats were given 30 min to drink 10 mL of liquid from the sipper tubes and then returned to their home cages with an additional 30 min of water access. Rats in the novel chemosensory groups received water for the first 6 days. On the seventh day, they were given one of the following: distilled water odorized with 0.01% isoamyl acetate (catalog no. W205508; Sigma–Aldrich) (novel odor), 0.1-M sucrose (catalog no. 470302–804; Wards Science) (novel taste), or a 0.01% isoamyl acetate-0.1-M sucrose mixture (novel odor-taste). Note that isoamyl acetate is tasteless at this concentration (Aimé et al. 2007; Samuelsen and Fontanini 2017). Rats in the familiar groups were given distilled water on days 1–3 and isoamyl acetate (familiar odor), sucrose (familiar taste), or the isoamyl acetate-sucrose mixture (familiar odor-taste) on days 4–7. To avoid the possibility of fluid intake-dependent differences in c-Fos expression, all rats were presented with 5 mL of liquid on the final experimental day (day 7). This volume was chosen because rats consistently drank more than 5 mL of liquid every day of training. Following the final experimental session, the rats were returned to their home cages and sacrificed 90 min later for c-Fos immunocytochemistry. The amount of liquid consumed during the single-bottle experience task is presented as the mean volume of fluid intake (mL) ± SEM.
Figure 1.
Schematic outline of the single-bottle experience task. Rats in the novel group were given 10 mL of water on days 1–6 and 0.01% isoamyl acetate (novel odor), 0.1-M sucrose (novel taste), or a mixture of 0.01% isoamyl acetate-0.1-M sucrose (novel odor-taste) on day 7. Rats in the familiar groups received 10-mL water on days 1–3 and 0.01% isoamyl acetate (familiar odor), 0.1-M sucrose (familiar taste), or a mixture of 0.01% isoamyl acetate-0.1-M sucrose (familiar odor-taste) on days 4–7. On the final experimental day (day 7), all rats received only 5 mL of liquid.
Immunocytochemistry
Rats were transcardially perfused with cold 0.1-M PBS and 4% paraformaldehyde. Brains were removed, postfixed for 24 h, cryoprotected in 30% sucrose, and sliced into 40-µm coronal sections. Free-floating sections were washed in 0.1-M PBS, blocked in a solution of 5% normal goat serum (catalog no. S-1000; Vector Laboratories) for 30 min, and incubated in a rabbit c-Fos primary antibody solution (sc-52, 1:2000; Santa Cruz Biotechnology) for 16–20 h at room temperature. The sections were then washed in 0.1-M PBS and incubated in a biotinylated goat anti-rabbit secondary antibody solution (catalog no. BA-1000, 1:400; Vector Laboratories) for 2 h. The sections were again washed with 0.1-M PBS and then incubated in ABC reagent (PK-6100; Vector Laboratories) for 1 h and stained with diaminobenzidine (catalog no. SK-4100; Vector Laboratories). The expression of c-Fos in the mediodorsal thalamus (sections from ~2.8–3.2 mm posterior to bregma) was quantified by an experimenter blind to experimental conditions using a custom macro in ImageJ (National Institutes of Health) to outline regions of interest (ROIs). There were no significant differences in the ROI areas across groups (Table 1). Expression values are averages from the left and right sides of three consecutive sections (Samuelsen and Meredith 2009, 2011). Immunocytochemistry data are presented as the mean numbers of c-Fos-positive nuclei ± SEMs.
Table 1.
Mean ROI
| Mean ROI (± SEM) | Mediodorsal thalamus |
|---|---|
| Novel odor | 3 422 556 ± 173 728 pixels2 |
| Novel taste | 3 714 441 ± 239 070 pixels2 |
| Novel odor-taste | 3 494 673 ± 190 184 pixels2 |
| Experience odor | 3 491 726 ± 154 118 pixels2 |
| Experience taste | 3 480 133 ± 127 177 pixels2 |
| Experience odor-taste | 3 621 013 ± 160 919 pixels2 |
Two-bottle brief-access odor preference task
Odor preference was assessed using a computer-controlled custom two-bottle brief-access apparatus. The rats (n = 5) were placed on a water regulation regimen (as above) and trained to drink water in the test chamber. After 3 days of habituation, the rats were acclimatized for 5 min to the test chamber before the two-bottle brief-access task was initiated under the control of custom-written LabVIEW scripts (National Instruments). A session began with the opening of the two port doors. One port allowed access to a sipper tube containing water, and the other allowed access to a sipper tube containing water (days 1–3), 0.01% isoamyl acetate (days 4–6 and day 8), or a mixture of 0.1-M sucrose-0.01% isoamyl acetate (day 7). The bottles were counterbalanced such that chemosensory stimuli were presented five times at each port. Once opened, the rat had 15 s to initiate a trial by licking either bottle. If no contact was made with either bottle, the shutters closed and a new trial began. Once either bottle was licked during the initial 15 s, the shutters remained open for an additional 15 s. Each individual lick was recorded by a grounded circuit. At the completion of a trial, the shutters closed, a 15-s intertrial interval began, and two different bottles were moved into position. The data are presented as the mean numbers of licks per 15-s trial and as preference ratios, calculated as (B1 − B2)/(B1 + B2), where B1 is the total number of licks for the counterbalanced water and B2 is the total number of licks for the counterbalanced stimulus. A positive preference ratio indicates a preference for water, whereas a negative preference ratio indicates a preference for the stimulus.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software). ROI areas are presented as mean pixels2 ± SEM. A one-way ANOVA was used to determine whether ROI areas differed across groups. Significant differences in c-Fos expression between groups were determined with independent t-tests. Significant differences in mean fluid intake between the three novel groups and the familiar odor group were determined with independent t-tests and Dunn–Sidak correction for familywise errors. Comparisons of mean fluid intake as well as mean numbers of licks between groups and days were determined using two-way repeated-measures ANOVAs, and comparisons among preference ratios were made using a one-way ANOVA. Post hoc analyses included Holm–Sidak tests for multiple comparisons to correct for familywise errors.
Results
Single-bottle experience task
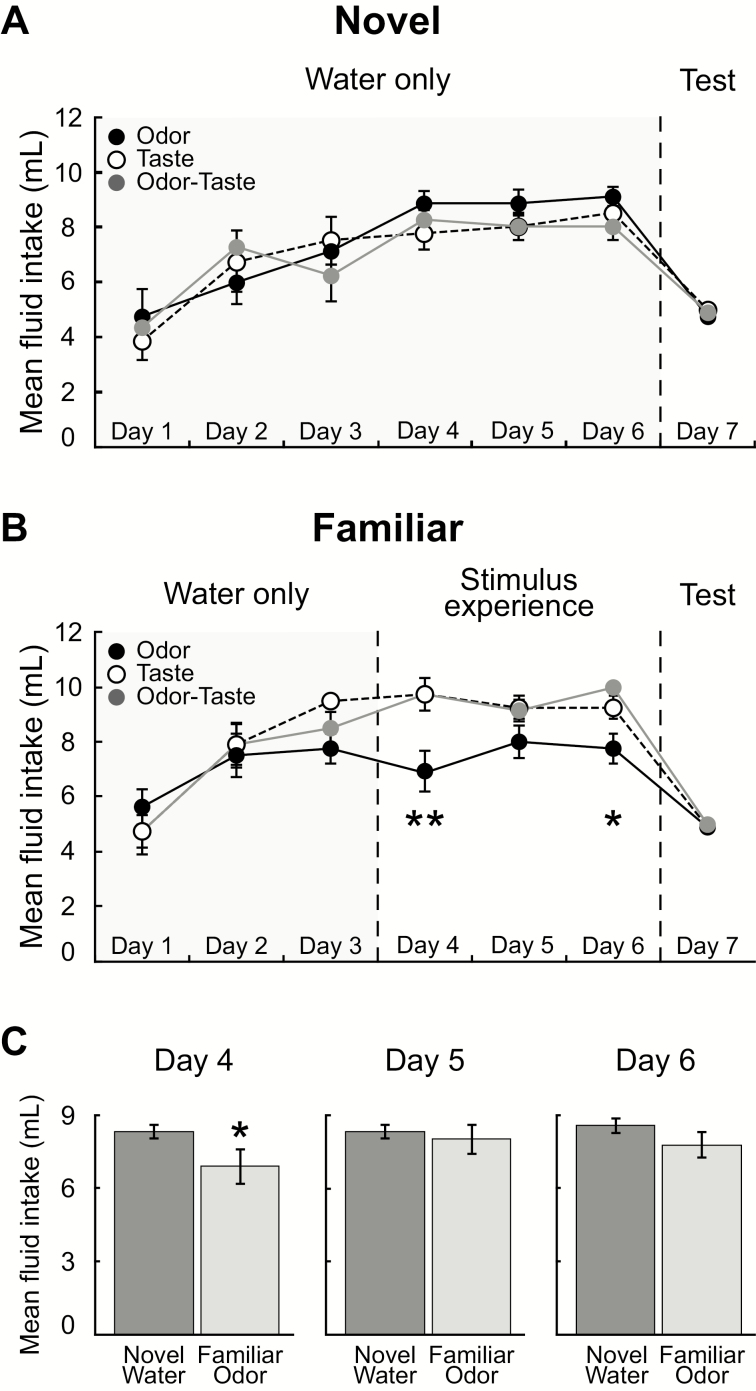
To determine how experience impacts mediodorsal thalamic responses to an odor, a taste, and an odor-taste mixture, we compared rats that had a single chemosensory experience (novel group) to those with many days of experience (familiar group). The rats in the novel group were given water for six consecutive days. The results of a two-way repeated-measure ANOVA comparing novel group’s mean fluid intake over multiple days (Figure 2A) revealed a significant main effect across days [F(5,105) = 29.16, P < 0.0001], but no difference between stimuli [F(2,21) = 0.2566, P = 0.78] or interaction between stimulus and day [F(10,105) = 1.175, P = 0.32]. The rats in the familiar group were given experience with chemosensory stimuli for four consecutive days. The results of a two-way repeated-measure ANOVA comparing the mean fluid intake over multiple days revealed a significant main effect across days [F(5,105) = 23.78, P < 0.0001] but no difference between stimuli [F(2,21) = 2.449, P = 0.1107] (Figure 2B). We found a significant interaction between stimulus and day [F(10,105) = 1.927, P = 0.0494]. On the first day of chemosensory stimulus access (day 4) in the familiar group, rats drank significantly less isoamyl acetate-odorized water than rats given sucrose (t(126) = 3.146, P < 0.01) or the isoamyl acetate-sucrose mixture (t(126) = 2.288, P < 0.05); the only other significant difference was on day 6 between rats that sampled the odorized water and those that sampled the mixture (t(126) = 2.574, P < 0.05). Next, we compared the mean fluid intake of the familiar odor group to the mean fluid intake of the novel groups. Because all three novel groups received water during experimental days 4–6, their mean fluid intake was combined. The results of an independent t-test found that rats in the novel group drank significantly more than rats in the familiar odor group on experimental day 4 (t(30) = 2.274, P = 0.0151), but not on day 5 (t(30) = 0.4932, P = 0.31) or day 6 (t(30) = 1.377, P = 0.0893) (Figure 2C). As most of the rats in the taste and odor-taste mixture groups drank all 10 mL of the solution during training, we were unable to determine sampling differences between these. Regardless, these results show that rats drank significantly more of a novel taste and a novel odor-taste mixture than of a novel odorized solution, in agreement with previous findings that rats display neophobia to novel orally consumed odors (Miller et al. 1986; Lin et al. 2009).
Figure 2.
Mean fluid intake (±SEM) during the single-bottle experience task. (A) There was no difference in the mean fluid intake between rats in the novel groups for any day of training. (B) On their first day of exposure to chemosensory stimuli (day 4), rats in the familiar group drank significantly less odorized water (0.01% isoamyl acetate; black) than those given a novel taste (0.1-M sucrose; white) or an odor-taste mixture (0.01% isoamyl acetate-0.1-M sucrose; gray). On day 6, rats drank significantly less odorized water than the odor-taste mixture. (C) On the fourth experimental day, rats in the familiar odor group drank significantly less odorized water compared with the amount of water consumed by rats in novel groups (left). There was no difference on experimental day 5 (middle) or day 6 (right). All groups received only 5 mL of liquid on the final day. *P < 0.05; **P < 0.01.
Expression of c-Fos in mediodorsal thalamus
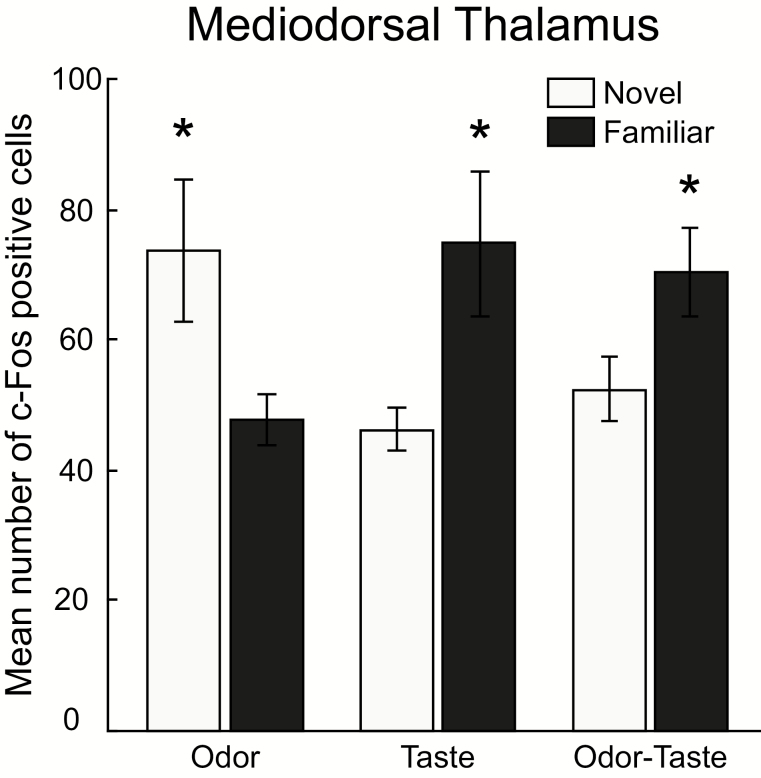
Chemosensory-induced c-Fos expression in the mediodorsal thalamus differed with experience (Figures 3 and 4). After consuming isoamyl acetate-odorized water, rats in the novel odor group had significantly greater c-Fos expression than familiar odor rats (73.7 ± 10.9 vs. 47.9 ± 3.9 cells, respectively; t(14) = 2.24, P = 0.0422). After drinking the sucrose solution, rats in the familiar taste group had significantly greater expression than those in the novel taste group (74.9 ± 11.3 vs. 46.42 ± 3.46 cells, respectively; t(14) = 2.42, P = 0.0296). Furthermore, rats familiar with the isoamyl acetate-sucrose mixture (familiar odor-taste) had significantly greater c-Fos expression than those in the novel odor-taste group (70.7 ± 6.8 vs. 52.6 ± 5.0 cells, respectively; t(14) = 2.15, P = 0.0491).
Figure 3.
Mean number of c-Fos-positive cells (±SEM) in the mediodorsal thalamus in response to novel and familiar chemosensory stimuli. Rats that sampled a novel odor (0.01% isoamyl acetate, light gray bars) had greater expression of c-Fos than those with chemosensory experience (dark gray bars). However, rats with experience with a taste (0.1-M sucrose) or an odor-taste mixture had significantly greater c-Fos expression than rats in the novel taste or the novel odor-taste mixture groups, respectively. *P < 0.05.
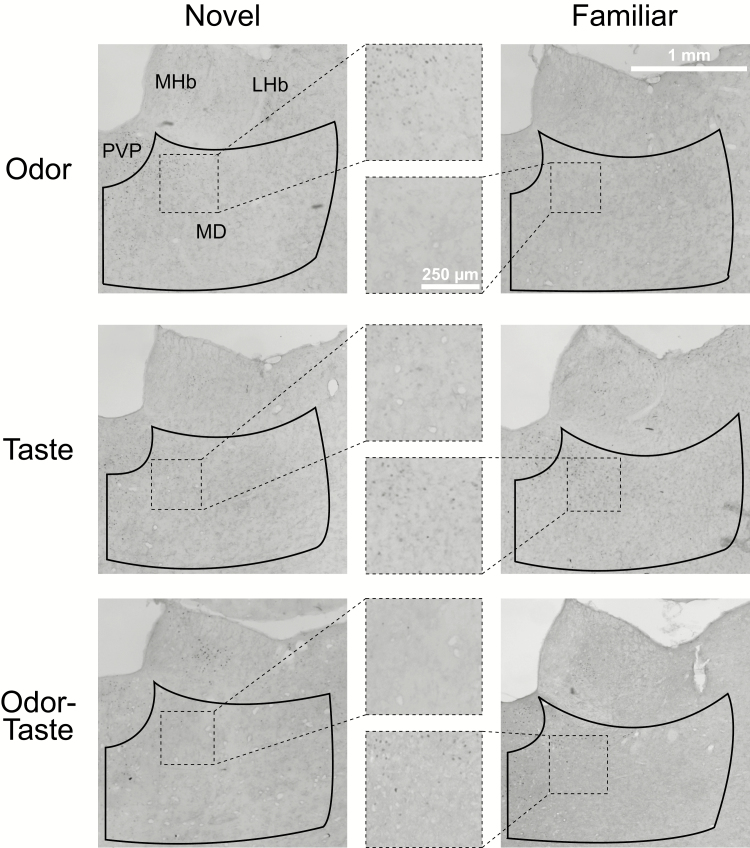
Figure 4.
Representative images of mediodorsal thalamus and ROIs. Expression of c-Fos in mediodorsal thalami of the novel groups (left column) and familiar groups (right column) in response to an odor (top), a taste (middle), or an odor-taste mixture (bottom). Middle column displays enlarged images of the boxed areas. Greater expression of c-Fos is observed after exposure to odorized water that is novel than when familiar, whereas c-Fos expression is greater after exposure to familiar taste alone or an odor-taste mixture than with a novel taste or a novel odor-taste mixture, respectively. There were no significant differences in ROI areas across sections or groups. Scale bar: 1-mm low magnification; 250-µm high magnification. Abbreviations: LHb, lateral habenular nucleus; MD, mediodorsal thalamus; MHb, medial habenular nucleus; PVP, paraventricular thalamic nucleus.
Two-bottle brief-access odor preference task
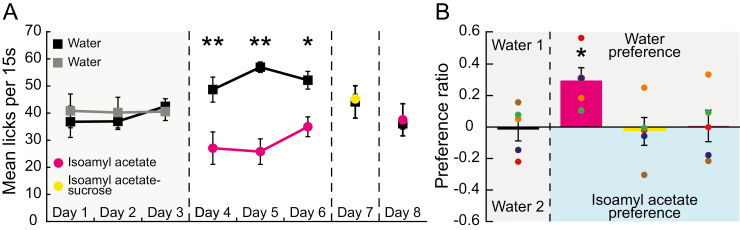
Although rodents display robust chemosensory-related neophobia (Lin et al. 2009, 2012), the presentation of an odor mixed with a pleasant taste can change odor preferences (Fanselow and Birk 1982; Gautam and Verhagen 2010). The results of the single-bottle experience task (Figure 2) show that rats initially avoided a novel odorized solution but consumed it on subsequent days. To examine how the preference for an orally consumed odor changes over time and with experience, we employed a two-bottle brief-access preference task. This task allows the rats to choose between two simultaneously presented liquid stimuli within a set amount of time. The results of a two-way repeated-measures ANOVA comparing the mean number of licks for water and chemosensory stimuli across days revealed a significant main effect of stimulus [F(1,8) = 15.15, P = 0.0046], no difference across days [F(7,56) = 0.7821, P = 0.6050], and a significant interaction between stimulus and day [F(7,56) = 4.34, P = 0.0005] (Figure 5A). A post hoc analysis comparing the mean number of licks between the two bottles for each experimental day showed a significant preference for water over that odorized with isoamyl acetate on day 4 (t(64) = 3.44, P < 0.01), day 5 (t(64) = 4.96, P < 0.01), and day 6 (t(64) = 2.731, P < 0.05). We found that rats did not prefer water to an isoamyl acetate-sucrose mixture (day 7: t(64) = 0.1798, P > 0.05). Importantly, after experience with the odor-taste mixture (day 7), there was no significant difference in the number of licks for water over the odorized water (observed on days 4–7) (day 8: t(64) = 0.2291, P > 0.05).
Figure 5.
Experience with an odor-taste mixture changes odor preference. (A) Mean licks per 15 s (±SEM) during the two-bottle brief-access task. Rats given the choice between 0.01% isoamyl acetate-odorized water and water, significantly prefer to drink water (days 4–6). There was no preference when given the choice between 0.01% isoamyl acetate mixed with 0.1-M sucrose (odor-taste) and water (day 7). The day after an experience with the odor-taste mixture (day 8), the preference for water over odorized water was eliminated. (B) Preference ratios (± SEMs) for each two-bottle choice. Preference ratios were averaged for days 1–3 (water vs. water) and days 4–6 (water vs. isoamyl acetate). Prior to experience with the odor-taste mixture, the preference for water over isoamyl acetate (left magenta bar) was significantly greater than the preference ratios for water/water (black bar), water/isoamyl acetate-sucrose (yellow bar), and water/isoamyl acetate after odor-taste experience (right magenta bar). Colored circles represent an individual rats’ average preference ratio for each two-bottle choice. *P < 0.05; **P < 0.01.
To determine whether preferences changed with experience, preference ratios were calculated. Briefly, the preference ratio represents which of the two bottles (water or stimulus) was sampled more during each two-bottle choice; a positive preference ratio indicates a preference for water, and a negative ratio indicates a preference for the stimulus. The preference ratios for water versus water (days 1–3) and water versus odorized water (days 4–6) were averaged, as there were no significant differences across days. The results of a one-way ANOVA indicated a significant difference between preference ratios [F(3,16) = 3.323, P = 0.0465] (Figure 5B). A post hoc analysis revealed that the preference ratio for water/isoamyl acetate-odorized water (prior to sampling an odor-taste mixture) was significantly different from all other two-bottle choices (water/water: t(16) = 2.597, P < 0.05; water/sucrose-isoamyl acetate: t(16) = 2.692, P < 0.05; water/isoamyl acetate: t(16) = 2.413, P < 0.05).
The results of the two-bottle brief-access odor preference task agree with previous studies investigating taste modulation of orally consumed odor preferences (Fanselow and Birk 1982; Gautam and Verhagen 2010). Furthermore, this task shows that the initial avoidance of a novel orally consumed odor stimulus continues when rats are allowed to choose between odorized water and water, but the preference for water over odorized water was eliminated after sampling the odor-taste mixture.
Discussion
The results of this study provide evidence that responses to chemosensory stimuli in the mediodorsal thalamus differ based on experience. Specifically, a novel odor produced greater c-Fos expression than a familiar odor, whereas a familiar taste or odor-taste mixture resulted in greater expression than when novel. These differences may be related to the behavioral relevance of the chemosensory stimuli, where both odor neophobia and experience with odor-taste mixtures shape subsequent odor preference. This hypothesis was supported by the single-bottle experience task, which found that the rats avoided odorized water only on the first day of experience. Furthermore, the results of the two-bottle brief-access odor preference task showed that, when given a choice between odorized water and water alone, rats significantly preferred water for many days. However, pairing the odor with a palatable taste eliminated the preference for water over odorized water. This is in line with research documenting that previous experience shapes our preferences for foods (Sclafani 2001; Verhagen and Engelen 2006). Taken together, our findings suggest that the mediodorsal thalamus communicates experience-dependent chemosensory information to guide behavior.
The hedonics of novel chemosensory stimuli vary within and across modalities (Miller and Holzman 1981; Knaden and Hansson 2014; Li and Liberles 2015). Whereas rats exhibit pronounced neophobia to orally consumed odors (Miller et al. 1986; Lin et al. 2009), this does not occur for sucrose, which is inherently palatable (Miller and Holzman 1981). The results of the single-bottle experience task fit the classic definition of neophobia (Barnett 1958; Best et al. 1978; Miller et al. 1986; Lin et al. 2009); rats avoided novel isoamyl acetate-odorized water on the first day of experience, but on subsequent days, they consumed the odorized water similarly to rats given other chemosensory stimuli or nonodorized water. In the two-bottle brief-access odor preference task, rats were given a set time to choose between isoamyl acetate-odorized water and water. They sampled water significantly more than odorized water until they were given experience with an isoamyl acetate-sucrose mixture. On subsequent trials, rats sampled water and odorized water similarly. Taken together, these behavioral results suggest that a novel orally consumed odor is avoided due to neophobia, and this initial avoidance continues when rats are given a choice. Only after the odor had been paired with a palatable taste does the preference for water and odorized water equilibrate.
A recent behavioral experiment found that rats generalize distilled water as more similar to quinine than to sucrose, suggesting that distilled water may have a “bitterness” quality and be unpleasant (Loney et al. 2012). Because we presented all of the stimuli in distilled water, the possibility arises that the odor potentiated the “bitterness” of distilled water and that pairing the odor with sucrose subsequently increased the comparative “bitterness” of the distilled water. If the “bitterness” of distilled water were the primary factor for our behavioral results, we would expect that, after experience with the odor-taste mixture, rats would continue to sample the distilled water more than odorized water, as the odor would still potentiate the “bitterness” of distilled water. However, we found that the preference for water is eliminated after experience with the odor-taste mixture. Our interpretation of the behavioral tasks supports previous findings showing that the affective value of an orally consumed odor is altered after being paired with a palatable taste (Fanselow and Birk 1982; Stevenson et al. 1995; Prescott et al. 2004; Gautam and Verhagen 2010; Green et al. 2012).
Rats are aroused by novel situations, and arousal-related behaviors are reduced with experience (Capdevila et al. 2007; Sobolewski et al. 2010, 2015). The circumplex model of affect proposes that behavioral states arise from a combination of valence (attractiveness/aversiveness) and arousal (alertness/indifference) (reviewed by Posner et al. 2005), where a pleasant stimulus in a novel situation would evoke a different response than the same pleasant stimulus in a familiar situation. At the risk of over simplifying this model, the summation of stimulus valence and situational arousal equals behavioral relevance. In our experiments, the sampling of a neutral/aversive chemosensory stimulus (isoamyl acetate) for the first time (heightened arousal) was likely more behaviorally relevant than the same stimulus presented after experience. Conversely, sampling a pleasant chemosensory stimulus (sucrose or isoamyl acetate-sucrose) in a familiar situation (moderate arousal) was likely more behaviorally relevant than sampling that same stimulus in a novel situation. Both are behaviorally relevant but for different reasons: novel odorized water should be avoided, whereas a sucrose or isoamyl acetate-sucrose solution should be consumed.
Recent electrophysiology experiments indicate that, during heightened states of arousal, higher order thalamic nuclei communicate sensory information to the cortex to guide behavior (Kawagoe et al. 2007; Sobolewski et al. 2010, 2015; Miller et al. 2017; Schmitt et al. 2017). In work by Schmitt et al. (2017), mice were presented with a cue signaling which of two simultaneously presented sensory stimuli, a light or tone, should be attended to in order to receive a reward. When the cue was low-frequency white noise, mice attended to the tone, and when it was high-frequency white noise, they attended to the light. The researchers report that the connections from the mediodorsal thalamus to the prefrontal cortex sustain the representation of the salient stimulus, regardless of sensory modality, and contend that the mediodorsal thalamus controls the functional connectivity of cortical circuits depending on the stimulus important for the task. These works support the hypothesis that the mediodorsal thalamus routes and synchronizes behaviorally relevant sensory information to specific cortical networks on the basis of behavioral need (Saalmann 2014; Courtiol and Wilson 2015; Nakajima and Halassa 2017).
In the present experiment, we used sucrose as the taste component of the odor-taste mixture. This raises an interesting question: How would the mediodorsal thalamus respond if an aversive taste stimulus, such as citric acid or quinine, was used instead? Given the results of the present study, we can venture two hypotheses. The first is that the responses by mediodorsal thalamus would resemble those of the novel and familiar odor groups. Although the odor-taste mixture would be “negative” for both groups, the novelty of the odor-taste stimulus would elicit a heightened state of arousal and drive significantly higher c-Fos expression compared with the familiar group. The second possibility is that the “negative” nature of the odor-taste mixture would drive similarly high levels of c-Fos expression in both the novel and familiar odor-taste groups. This result would suggest that, regardless of chemosensory experience, the negative affect of an aversive odor-taste mixture is potent enough to elicit similar levels of c-Fos expression in both groups. Future experiments will aim to elucidate the relationship between mediodorsal thalamic responses to pleasant/aversive odor-taste mixtures and choice.
As a higher order thalamic rely, the mediodorsal thalamus is reciprocally connected with many regions of the brain (see reviews by Courtiol and Wilson 2015; Mitchell 2015). Based on the distribution of these connections and its cytoarchitecture, the mediodorsal thalamus is divided into three anatomically distinct portions: medial, central, and lateral (Krettek and Price 1977b; Groenewegen 1988). Projections from the chemosensory cortical areas for taste and smell, gustatory cortex and piriform cortex, overlap in the medial and central subdivision of the mediodorsal thalamus (Price and Slotnick 1983; Kuroda et al. 1992; Shi and Cassell 1998). The amygdala, a limbic region important for processing affectively salient stimuli (Baxter and Murray 2002), also projects to the medial subdivision of mediodorsal thalamus (Krettek and Price 1977a; McDonald 1987). Electron microscopy (EM) studies show that the axonal projections from these key chemosensory-related regions form different synaptic connections with neurons in mediodorsal thalamus; where amygdalar projections primarily form large synapses, gustatory cortical projections primarily form small synapses, and axons from piriform cortex make both large and small synapses (Kuroda and Price 1991a; Kuroda et al. 1992; Pelzer et al. 2017). Interestingly, a recent study by Pelzer et al. (2017) determined that the large synapses formed by piriform axons are functionally similar to the large “driver” synapses between sensory neocortical regions and thalamus (see review (Sherman and Guillery 2011; Bickford 2016). These different cortical and amygdalar synaptic connections are sure to mediate many aspects of mediodorsal thalamic function, including experience-dependent responses to chemosensory stimuli. Future experiments specifically labeling the projections from piriform cortex, gustatory cortex, and amygdala would help to elucidate the role of these regions in driving experience-dependent chemosensory responses across the different subdivisions of the mediodorsal thalamus.
In conclusion, the results of this study show that a novel odor stimulus, but a familiar taste or odor-taste mixture, produced greater c-Fos expression in the mediodorsal thalamus. Our results align with previous findings indicating that this structure exhibits experience-dependent responses to sensory information and support the premise that the mediodorsal thalamus is integral in communicating state and sensory information to higher order areas to guide chemosensory behaviors. Future research will investigate how the chemosensory cortical areas, gustatory cortex and piriform cortex, modulate the connectivity between the mediodorsal thalamus and other higher order cortical regions in guiding food choices.
Funding
This work was supported by the National Institute of Deafness and Other Communication Disorders at the National Institutes of Health [R03 DC014319].
Conflict of interests
The authors declare no conflict of interest.
References
- Aimé P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, Julliard AK.. 2007. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav Brain Res. 179:258–264. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF.. 1991. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 311:1–16. [DOI] [PubMed] [Google Scholar]
- Asai H, Udaka F, Hirano M, Ueno S.. 2008. Odor abnormalities caused by bilateral thalamic infarction. Clin Neurol Neurosurg. 110:500–501. [DOI] [PubMed] [Google Scholar]
- Barnett SA. 1958. Experiments on neophobia in wild and laboratory rats. Br J Psychol. 49:195–201. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA.. 2002. The amygdala and reward. Nat Rev Neurosci. 3:563–573. [DOI] [PubMed] [Google Scholar]
- Best MR, Domjan M, Haskins WL.. 1978. Long-term retention of flavor familiarization: effects of number and amount of prior exposures. Behav Biol. 23:95–99. [DOI] [PubMed] [Google Scholar]
- Bickford ME. 2016. Thalamic circuit diversity: modulation of the driver/modulator framework. Front Neural Circuits. 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila S, Giral M, Ruiz de la Torre JL, Russell RJ, Kramer K.. 2007. Acclimatization of rats after ground transportation to a new animal facility. Lab Anim. 41:255–261. [DOI] [PubMed] [Google Scholar]
- Collins DP, Anastasiades PG, Marlin JJ, Carter AG.. 2018. Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron. 98:366–379.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol E, Wilson DA.. 2014. Thalamic olfaction: characterizing odor processing in the mediodorsal thalamus of the rat. J Neurophysiol. 111:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol E, Wilson DA.. 2015. The olfactory thalamus: unanswered questions about the role of the mediodorsal thalamic nucleus in olfaction. Front Neural Circuits. 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol E, Wilson DA.. 2016. Neural representation of odor-guided behavior in the rat olfactory thalamus. J Neurosci. 36:5946–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Shedlack KJ, Eckmann KW.. 1980. Thalamocortical mechanisms in odor-guided behavior. I. Effects of lesions of the mediodorsal thalamic nucleus and frontal cortex on olfactory discrimination in the rat. Brain Behav Evol. 17:255–275. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Birk J.. 1982. Flavor-flavor associations induce hedonic shifts in taste preference. Anim Learn Behav. 10:223–228. [Google Scholar]
- Gautam SH, Verhagen JV.. 2010. Evidence that the sweetness of odors depends on experience in rats. Chem Senses. 35:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J.. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ. 1988. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 24:379–431. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JH, Kim MJ, Jung MW.. 2013. Neural activity in mediodorsal nucleus of thalamus in rats performing a working memory task. Front Neural Circuits. 7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder MD. 1991. Conditioned preferences for the taste and odor components of flavors: blocking but not overshadowing. Appetite. 17:29–45. [DOI] [PubMed] [Google Scholar]
- Illig KR. 2005. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 488:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Onoda N, Takagi SF.. 1984. Odor response characteristics of thalamic mediodorsal nucleus neurons in the rabbit. Jpn J Physiol. 34:55–73. [DOI] [PubMed] [Google Scholar]
- Kawagoe T, Tamura R, Uwano T, Asahi T, Nishijo H, Eifuku S, Ono T.. 2007. Neural correlates of stimulus-reward association in the rat mediodorsal thalamus. Neuroreport. 18:683–688. [DOI] [PubMed] [Google Scholar]
- Knaden M, Hansson BS.. 2014. Mapping odor valence in the brain of flies and mice. Curr Opin Neurobiol. 24:34–38. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL.. 1977a. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 172:687–722. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL.. 1977b. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 171:157–191. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD.. 2000. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 424:111–141. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Pan S, Furuta T, Tanaka YR, Iwai H, Yamanaka A, Ohno S, Kaneko T, Goto T, Hioki H.. 2017. Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: a single neuron-tracing study using virus vectors. J Comp Neurol. 525:166–185. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Murakami K, Kishi K, Price JL.. 1992. Distribution of the piriform cortical terminals to cells in the central segment of the mediodorsal thalamic nucleus of the rat. Brain Res. 595:159–163. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Price JL.. 1991a. Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 303:513–533. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Price JL.. 1991b. Ultrastructure and synaptic organization of axon terminals from brainstem structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 313:539–552. [DOI] [PubMed] [Google Scholar]
- Li Q, Liberles SD.. 2015. Aversion and attraction through olfaction. Curr Biol. 25:R120–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, Arthurs J, Reilly S.. 2012. Taste neophobia and c-Fos expression in the rat brain. Brain Res. 1448:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S.. 2009. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 1251:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, Blonde GD, Eckel LA, Spector AC.. 2012. Determinants of taste preference and acceptability: quality versus hedonics. J Neurosci. 32:10086–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Miller RL, Wormwood BA, Francoeur MJ, Onos KD, Gibson BM.. 2015. The neurobiology of thalamic amnesia: contributions of medial thalamus and prefrontal cortex to delayed conditional discrimination. Neurosci Biobehav Rev. 54:161–174. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. 1987. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J Comp Neurol. 262:46–58. [DOI] [PubMed] [Google Scholar]
- Miller JS, Nonneman AJ, Kelly KS, Neisewander JL, Isaac WL.. 1986. Disruption of neophobia, conditioned odor aversion, and conditioned taste aversion in rats with hippocampal lesions. Behav Neural Biol. 45:240–253. [DOI] [PubMed] [Google Scholar]
- Miller RLA, Francoeur MJ, Gibson BM, Mair RG.. 2017. Mediodorsal thalamic neurons mirror the activity of medial prefrontal neurons responding to movement and reinforcement during a dynamic DNMTP task. eNeuro. 4:ENEURO.0196-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Holzman AD.. 1981. Neophobia: generality and function. Behav Neural Biol. 33:17–44. [DOI] [PubMed] [Google Scholar]
- Mitchell AS. 2015. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev. 54:76–88. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Halassa MM.. 2017. Thalamic control of functional cortical connectivity. Curr Opin Neurobiol. 44:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C.. 2013. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 77:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer P, Horstmann H, Kuner T.. 2017. Ultrastructural and functional properties of a giant synapse driving the piriform cortex to mediodorsal thalamus projection. Front Synaptic Neurosci. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J, Howard JD, Gitelman DR, Gottfried JA.. 2008. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 28:5257–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Russell JA, Peterson BS.. 2005. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 17:715–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J, Johnstone V, Francis J.. 2004. Odor-taste interactions: effects of attentional strategies during exposure. Chem Senses. 29:331–340. [DOI] [PubMed] [Google Scholar]
- Price JL, Slotnick BM.. 1983. Dual olfactory representation in the rat thalamus: an anatomical and electrophysiological study. J Comp Neurol. 215:63–77. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL.. 1992. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol. 323:167–197. [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Muller P, Gahide I, Mottin Y, Romon M.. 1996. Disorders of smell, taste, and food intake in a patient with a dorsomedial thalamic infarct. Stroke. 27:2328–2330. [DOI] [PubMed] [Google Scholar]
- Saalmann YB. 2014. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci. 8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T.. 2001. Effects of excitotoxic brain lesions on taste-mediated odor learning in the rat. Neurobiol Learn Mem. 75:128–139. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Fontanini A.. 2017. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci. 37:244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M.. 2009. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 1263:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M.. 2011. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 180:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM.. 2017. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 545:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. 2001. Psychobiology of food preferences. Int J Obes Relat Metab Disord. 25(Suppl 5):S13–S16. [DOI] [PubMed] [Google Scholar]
- Sela L, Sacher Y, Serfaty C, Yeshurun Y, Soroker N, Sobel N.. 2009. Spared and impaired olfactory abilities after thalamic lesions. J Neurosci. 29:12059–12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW.. 2011. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 106:1068–1077. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD.. 1998. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 399:440–468. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Risser JM.. 1990. Odor memory and odor learning in rats with lesions of the lateral olfactory tract and mediodorsal thalamic nucleus. Brain Res. 529:23–29. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F.. 2008. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 57:786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski A, Kublik E, Swiejkowski DA, Kamiński J, Wróbel A.. 2015. Alertness opens the effective flow of sensory information through rat thalamic posterior nucleus. Eur J Neurosci. 41:1321–1331. [DOI] [PubMed] [Google Scholar]
- Sobolewski A, Kublik E, Swiejkowski DA, Lęski S, Kamiński JK, Wróbel A.. 2010. Cross-trial correlation analysis of evoked potentials reveals arousal-related attenuation of thalamo-cortical coupling. J Comput Neurosci. 29:485–493. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Boakes RA.. 2004. Sweet and sour smells: learned synaesthesia between the senses of taste and smell BT—the handbook of multisensory processing. Cambridge (MA): MIT Press. [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA.. 1995. The acquisition of taste properties by odors. Learn Motiv. 26:433–455. [Google Scholar]
- Tham WW, Stevenson RJ, Miller LA.. 2009. The functional role of the medio dorsal thalamic nucleus in olfaction. Brain Res Rev. 62:109–126. [DOI] [PubMed] [Google Scholar]
- Tham WW, Stevenson RJ, Miller LA.. 2011. The impact of mediodorsal thalamic lesions on olfactory attention and flavor perception. Brain Cogn. 77:71–79. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Small DM.. 2011. Modality-specific neural effects of selective attention to taste and odor. Chem Senses. 36:747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Engelen L.. 2006. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev. 30:613–650. [DOI] [PubMed] [Google Scholar]