Abstract
Hallmarks of the difficult period of transition from hospital to home following stroke include stroke survivor and caregiver uncertainty about actionable steps toward recovery and prevention and unfamiliarity with related resources. Current research shows that interdisciplinary interventions focusing on patient experience and patient education enable health-care providers to activate and empower patients, potentially leading to better clinical outcomes. Tool kit approaches have been successfully used to aid patients through ongoing education after hospital discharge and to improve patient experience. In this article, we describe our efforts to iteratively develop and test personalized stroke management tool kits aimed at connecting stroke survivors and their caregivers to empowering resources, while soliciting feedback from patients and family members.
Keywords: patient education, patient activation, prevention tool kit, recovery tool kit, patient satisfaction, stroke education, stroke rehabilitation, design thinking
Introduction
Stroke survivors and their caregivers identify the transition from hospital to home as a significant challenge they face during the recovery phase after stroke. It is not uncommon for patients and their family members to express lack of familiarity with stroke resources and uncertainty about actionable steps needed for optimal stroke recovery, especially during the hospital-to-home transition. Inundated with information provided during the acute-care period, families report unmet information needs postdischarge (1 -3). Empowering patients with tailored information and resources gives them and their family members the practical knowledge, skills, and tools, which they can use to take greater charge of their own care—including care at critical periods of transition. Furthermore, patient empowerment has the potential to promote overall health by activating patients to use resources available to them (4).
Interventions focusing on patient education and activation can be used simultaneously to help improve the patient experience and clinical outcomes. Health education strategies increase patient and caregiver satisfaction, reduce anxiety, increase participation in health-care programs, and promote independence in activities of daily living (5). Effective patient education interventions are tailored to individual patient needs, use multiple components to improve self-management outcomes, and often employ multidisciplinary approaches (6). Programs teaching self-management skills have been shown to be more effective than information-only patient education in improving clinical outcomes (7). Prevention and recovery tool kits have been successfully utilized to educate and empower patients and families, resulting in improved outcomes. In a study evaluating the efficacy of home-delivered tool kits for injury prevention, the desired outcome of safety improvement was observed to be significantly higher in the experimental group (8). The use of prevention and recovery tool kits is not widespread, and further research is warranted for evaluating their impact on patient satisfaction and clinical outcomes.
Following months of iterative human-centered design (HCD) building upon conversations with stroke survivors, caregivers, and health-care providers, the Galva pilot project was initiated at MedStar National Rehabilitation Hospital (NRH) in Washington, DC It addressed the difficulties of transitioning from hospital to home using personalized stroke management tool kits tailored to stroke survivors and their caregivers. This article will describe the intervention and its outcomes.
Applying HCD
For the conception and development of this project, we used HCD methodologies. Human-centered design, as outlined by the International Organization for Standardization in ISO-9241-210, aims to solve problems by creating solutions that are informed by the end user’s needs (9). IDEO (IDEO.org) defines HCD as a research and design process that builds empathy by “generating tons of ideas, building a bunch of prototypes, sharing what you’ve made with the people you’re designing for, and eventually putting your innovative new solution out in the world” (10).
Over the course of 3 months, the health innovation fellows on our team identified major themes and needs in stroke care by shadowing physicians and interviewing rehabilitation specialists, including the remaining coauthors, as well as by surveying and speaking with stroke survivors and their primary caregivers. The difficulty of the hospital-to-home transition stood out starkly as one of the most prominent challenges identified through this needs assessment. Among the many ideas our team suggested and evaluated, the Galva project was selected as an optimal solution to this identified need based on clinical stakeholder feedback, feasibility, and financial resources. Human-centered design principles continued to shape Galva throughout the pilot phase. Feedback from users, including patients, caregivers, and clinicians, was used to iterate the project design and execution.
Conceptualization and Development of Personalized Stroke Management Tool Kits
We leveraged the principles of experiential learning and microlearning in the development of the stroke management tool kits. Experiential learning is the process of learning by doing. Microlearning is when the learner receives information in small chunks throughout a period of time (11,12).
Keeping these 2 learning theories in mind, we created prototypes of educational flashcards (see Figure 1), stroke “cheat sheets,” and tool kits (see Figure 2) containing “wellness items” such as salt-free spices, mindfulness coloring books, and grip strengtheners. The list of educational topics and wellness items that were ultimately included in the tool kits was informed by interviews and focus groups with rehabilitation specialists, neurologists, physiatrists, and stroke survivors and their primary caregivers.
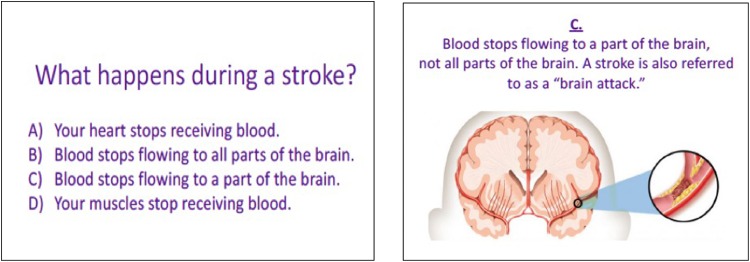
Figure 1.
Prototype of flashcard in quiz format.
Figure 2.

Stroke management tool kits with wellness items, flashcards, and stroke “cheat sheets.”
Project Pilot
The Galva pilot was conducted at NRH with the help of an interdisciplinary team of social workers, rehabilitation specialists, stroke coordinators, and health-care innovation consultants. It lasted for 6 months and involved 3 stages.
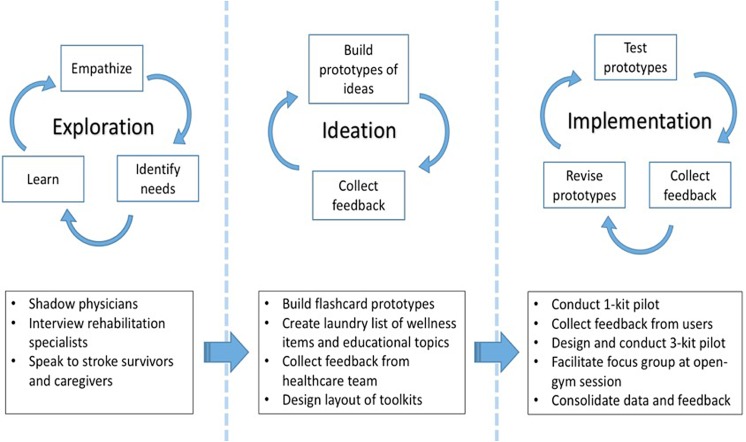
The first stage involved a nonpersonalized 1-kit model which delivered tool kits to stroke survivors who had already completed outpatient rehabilitation and their primary caregivers. The second stage involved a personalized 3-kit model, with each kit containing different combinations of wellness/comfort items and educational flashcards tailored to each patient’s unique stroke profile. The first kit, which was delivered directly to the patient and/or caregiver in the hospital, contained comfort items (eg, blanket, earplugs, motivational calendar, etc) and flashcards with general information about stroke. The second and third kits primarily contained wellness items (eg, brain puzzles and recipes) and educational flashcards aimed at maintaining physical and mental well-being. The third kit also contained personalized “stroke cheat sheets” which outlined local resources and a list of digital health apps based on the patient’s zip code and comorbidities. The final stage of the project pilot was a focus group that was facilitated during an adaptive “open gym” session for traumatic brain injury (TBI) and stroke survivors. We asked participants to answer a 3-item survey, exploring: (a) which tool kit they would prefer receiving, (b) what type of educational materials they’d like to have in the kit, and (c) at what point during their recovery would they prefer to receive the kits. A summary of our pilot and methodology can be seen in Figure 3.
Figure 3.
Summary of human-centered design (HCD)-inspired methodology.
Collecting Data and Learned Insights
Ten stroke survivors who had completed outpatient rehabilitation were recruited and enrolled in the 1-kit pilot. The stroke survivors who were included in the project had a full-blown stroke and not a transient ischemic attack (TIA). These stroke survivors were able to give consent during enrollment or had at least 1 family member who was able to give consent. Those who were ultimately enrolled were communicative, oriented, and alert. Feedback was collected via phone calls approximately 1.5 weeks after the kit was delivered. Seven (70%) of the 10 enrollees gave feedback. More than half (5 of 7) of the respondents used and read the educational flashcards at least once. Clinical stakeholders had a 100% response rate when the fellows asked for advice and logistical support for the pilot, suggesting a high level of clinical buy-in for the tool kits. When asked how the kits improved their confidence about stroke recovery, patients/caregivers on average gave a 4.5 of 5 rating (5 being very confident). Some of the most liked wellness items included the mindfulness coloring book, brain games book, recipe cards, jar opener, healthy snack, and salt-free seasoning. Our respondents reported the most helpful educational topics to be caregiver self-care, general stroke information, stroke exercises, healthy eating, support groups, local activities, medical equipment, and clinical trials. During a follow-up phone call, 1 respondent said, “The box [tool kit] is a very good idea. I think different stroke victims will use it differently. We are always looking for new activities and ideas that one can do alone or in a group.” Another respondent recommended personalizing the support groups listed in the educational flashcards by zip code.
Using patient and clinical stakeholder feedback from the 1-kit pilot, we developed a model that included a series of 3 tool kits delivered in the hospital during inpatient rehabilitation and after hospital discharge. In contrast to the 1-kit model, these tool kits were personalized based on the patient’s stroke profile. Ten stroke survivors undergoing inpatient rehabilitation were enrolled in the second pilot. These were patients who had a full-blown stroke. Those who did not have a point of contact were not included in the study nor were those who had severe cognitive and communication deficits. All 10 received the first tool kit. Eight received the second tool kit. Six received the third and final tool kit. Delivery of kits was spaced out by an average of 1.5 weeks, and feedback was collected via phone calls approximately 1.5 weeks after the kit was delivered. Attrition occurred due to a lack of responsiveness to follow-up phone calls. Six of the 10 enrollees gave feedback at least once. More than half (4 of 6) of our respondents used and read the educational flashcards at least once. Our users reported the most helpful wellness items to be books featuring motivational stroke survivor stories, earplugs, eye masks, blankets, mindfulness coloring books, and hand exercise balls. Educational topics that resonated the most with our users included symptoms of stroke and how stroke happens. One user preferred the educational flashcards that were written in quiz format, also noting that “they helped clear up some misconceptions, which is important.”
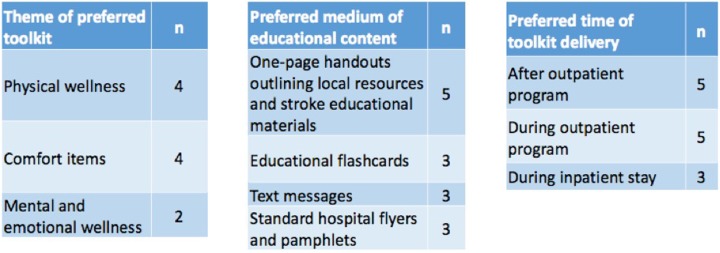
Due to our low sample size of tool kit users resulting from time and logistical constraints, we conducted a 3-item survey to collect supplemental data at an adaptive open-gym session offered for TBI and stroke survivors at the MedStar NRH. Three sample tool kits were presented to a total of 10-survey participants (stroke survivors and caregivers), along with a brief survey. Figure 4 details the survey results. The preferred tool kit themes were physical wellness (containing items promoting healthy eating and exercise) and comfort items. A 1-page handout outlining local resources and relevant stroke educational materials was the most popular educational material, and the majority of respondents preferred to receive the tool kits during or after outpatient therapy.
Figure 4.
Summary of survey from open-gym session. Respondents were free to select more than 1 answer.
Discussion
Stroke survivors and caregivers tended to prefer flashcards and succinct 1-page stroke fact sheets over conventional flyers and brochures as the optimal method for delivering stroke education. The concept of a tangible resource that users could “work with” was important to them, as was the personalization that aligned with their comorbidities and local stroke resources. One caregiver’s testimony praised the project’s pairing of microlearning techniques with experiential practice via tangible wellness items. When asked about the tool kit, the caregiver indicated she planned to prepare one of the stroke- and diabetes-friendly recipes included within that weekend. Motivating behavior change lies at the core of prevention, and user feedback like this indicates that there is potential for the tool kits to empower patients and/or their caregivers.
When collecting feedback, it was observed that frequent follow-up calls to patients (once per week) resulted in user fatigue. Three of the 10 three-kit pilot participants became less responsive over time when contacted for feedback about each tool kit, ostensibly due to a “telemarketer effect.”
An important factor recognized by the 2 fellows during the course of this pilot was that the post-acute period during which a stroke survivor is still an inpatient is a highly sensitive time for both stroke survivors and caregivers. The inpatient and early outpatient periods proved to be less optimal than late outpatient or beyond in terms of engagement with the tool kits. This assessment was informed by user responsiveness (answering phone calls, speaking to study coordinators, etc), reported level of engagement with each kit, and directly stated preference. One caregiver explained that she hadn’t used most of the items in the 3 tool kits since her husband, “hasn’t been home for three months” and was “very weak and having a hard time getting interested.” The caregiver further explained: “I don’t have the energy right now but when things settle down when he’s home…I’m looking forward to sharing the information with him.” Another caregiver, when asked why he didn’t use the first tool kit, responded, “I have two jobs, and I have to feed her and give her medicine. I don’t have time.” Stroke survivors and caregivers enrolled in the 1-kit pilot reported greater use of the wellness items and educational content as compared to the 3-kit pilot enrollees. Moreover, at the open-gym session, more than half of the votes cast by 10 stroke survivors and caregivers indicated a preference for stroke management tool kits to be delivered during later stages of outpatient therapy or beyond. This suggests that those in the stages of late outpatient rehabilitation and beyond may be more receptive to the tool kits and similar interventions.
It is worth noting that patients with TIA may be the optimal candidates to receive similar tool kits, as their recent ischemic event is an incentive for them to take immediate action to prevent a future stroke, yet they experience less cognitive impairment than those recovering from a major stroke (13). As patients with TIA retain their independence following hospitalization, caregivers do not become as substantially involved as with stroke survivors, and thus the removal of caregiver burden from the equation may further increase the likelihood and ease with which patients with TIA can embrace lifestyle changes. Transient ischemic attack survivors who adopt healthy changes in their diet and physical activity can significantly reduce their risk of stroke. The potential impact of the Galva project to patients with TIA became apparent after the team received a phone call from a patient who had a TIA and had heard of the tool kits but never received them. When asked about how the tool kits could have helped, the patient with TIA said: “I know I have to do something drastic. The hospital gave me 1- or 2-page handouts. They didn’t really tell me how to do things; they just told me what to do.”
To the best of our knowledge, this article is the first to study the impact of personalized prevention and recovery tool kits on stroke survivors and their caregivers. Among the limitations of this pilot study were limited number of participants and a limited time period in which to collect the data. Despite these limitations, the response from patients and caregivers was overwhelmingly positive. Future considerations for similar interventions should include increasing the number of patients and expanding the length of the study to collect information at additional data points (eg, 3 or 6 months poststroke). Spacing out the telephone surveys may decrease user fatigue when collecting feedback. Creating a 2-kit model serving patients with TIA or stroke survivors who have completed outpatient rehabilitation and collecting data on these groups would be a recommended modification for a future study design based on this pilot. Human-centered design should be considered as a methodological tool when designing similar health interventions. More research assessing interdisciplinary interventions are needed, and innovative health strategies that leverage patient education and activation should be adopted by health-care providers to achieve this.
Conclusion
We have found that personalized stroke management tool kits have the potential to improve patient experience and satisfaction among stroke survivors and their families. Experiential learning, via tangible wellness items and information provided using microlearning techniques, shows promise in empowering patients and their families and creating an impetus to more actively engage in their own care and recovery. In 1 patient’s words, “I think the kits are a wonderful idea, and that virtually all [acquired] brain injury patients would benefit from receiving such kits upon their release from the hospital.” Providing ongoing support through tangible resources can show patients how to enact positive change, rather than simply telling them they need to do something—a modified approach that may make a tremendous difference in improving patients’ overall health.
Acknowledgments
The authors would like to thank the stroke clinical teams, rehabilitation specialists, and subject matter experts at the MedStar Washington Hospital Center (MWHC), MedStar National Rehabilitation Hospital, and MedStar Institute for Innovation, respectively, for making this project possible. Special appreciation goes to Dr Amie Hsia of MWHC and Dr Mark Smith, executive sponsor of the HFA fellowship. Both served as clinical mentors throughout the project pilot. The authors would also like to thank Harsh Thakkar for assisting the Galva team during the adaptive open-gym session at MedStar National Rehabilitation Hospital and Mandy Dorn, director of HFA, for editing and proofreading this manuscript.
Author Biographies
King John Pascual was a 2016-2017 Healthcare Innovation Fellow at Health for America at MedStar Health. Before joining the HFA fellowship, he taught high school science in the South Bronx and completed his master's degree in science education in New York City. He is a co-leader for the Galva project.
Ekaterina Vlasova was a 2016-2017 Healthcare Innovation Fellow at Health for America at MedStar Health. Before joining HFA, she conducted research in integrative medicine approaches across Europe, Southeast Asia, and South America. She is a co-leader for the Galva project.
Kimberly J Lockett is a licensed social worker with 12 years of experience at MedStar National Rehabilitation Hospital. There she is an inpatient case manager on the stroke and brain injury units. She was a clinical rehabilitation mentor and liaison for the Galva project.
Judson Richardson is a clinical social worker with 10 years of experience at MedStar National Rehabilitation Hospital. There he is the co-director of outpatient day rehabilitation programs for neurological conditions such as strokes, TBI, and spinal cord injuries. He was a clinical rehabilitation mentor and liaison for the Galva project.
Michael Yochelson is the chief medical officer at Shepherd Center in Atlanta, Georgia. Prior to this role, he was chief medical officer at MedStar National Rehabilitation Network where he also served as the founding program director of the Brain Injury Medicine Fellowship and was a physician advisor for the 2016-2017 Health for America fellowship. His clinical area of practice is neurorehabilitation with a particular interest in cognitive and neurobehavioral issues and spasticity management after brain injury and stroke.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the 2016-2017 Health for America (HFA) at MedStar Health fellowship.
ORCID iD: King John Pascual, BA, MAT  http://orcid.org/0000-0003-2339-3831
http://orcid.org/0000-0003-2339-3831
References
- 1. Eames S, Hoffmann T, Worrall L, Read S. Stroke patients’ and carers’ perception of barriers to accessing stroke information. Top Stroke Rehabil. 2010;17:69–78. [DOI] [PubMed] [Google Scholar]
- 2. Perry L, Middleton S. An investigation of family caregivers’ needs following stroke survivors’ discharge from acute hospital care in Australia. Disabil Rehabil. 2011;33:1890–1900. [DOI] [PubMed] [Google Scholar]
- 3. Cameron V. Best practices for stroke patient and family education in the acute care setting: a literature review. Medsurg Nurs. 2013;22:51–5. [PubMed] [Google Scholar]
- 4. Funnel MM, Anderson RM, Arnold MS, Barr PA, Donnelly M, Johnson PD. Empowerment: an idea whose time has come in diabetes education. Diabetes Educ. 1991;17:37–41. [DOI] [PubMed] [Google Scholar]
- 5. Yuen EY, Knight T, Ricciardelli LA, Burney S. Health literacy of caregivers of adult care recipients: a systematic scoping review. Health Soc Care Community. 2016. [DOI] [PubMed] [Google Scholar]
- 6. Barnason S, White-Williams C, Rossi LP, Centeno M, Crabbe DL, Lee KS; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Evidence for therapeutic patient education interventions to promote cardiovascular patient self-management: a scientific statement for healthcare professionals from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2017;10:e000025. [DOI] [PubMed] [Google Scholar]
- 7. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–75. [DOI] [PubMed] [Google Scholar]
- 8. Sznajder M, Leduc S, Janvrin MP, Bonnin MH, Aegerter P, Baudier F. Home delivery of an injury prevention kit for children in four French cities: a controlled randomized trial. Inj Prev. 2003;9:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ISO-9241-210. Ergonomics of human-system interaction: human-centered design for interactive systems (formerly known as 13407). Switzerland: International Standards Organization; 2010. [Google Scholar]
- 10. IDEO.org. What is human-centered design? http://www.designkit.org/human-centered-design. Accessed June 2017.
- 11. Hug T. Didactics of Microlearning. Berlin, Germany: Waxmann; 2016:p10. [Google Scholar]
- 12. Dudley D, Cotton WG, Peralta LR. Teaching approaches and strategies that promote healthy eating in primary school children: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2015;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Rooij FG, Kessels RP, Richard E, De Leeuw FE, van Dijk EJ. Cognitive impairment in transient ischemic attack patients: a systematic review. Cerebrovasc Dis. 2016;24:1–9. [DOI] [PubMed] [Google Scholar]