Abstract
Study Design:
Review article.
Objectives:
A review of the literature on postoperative spinal infections, their diagnosis, and management.
Methods:
A systematic computerized Medline literature search was performed using PubMed, Cochrane Database of Systematic Reviews, and EMBASE. The electronic databases were searched for publication dates from the last 10 years. The searches were performed from Medical Subject Headings (MeSH) used by the National Library of Medicine. Specifically, MeSH terms “spine,” “infections,” “management,” and “diagnosis” were used.
Results:
Currently, the gold standard for diagnosis of postoperative spine infection is positive deep wound culture. Many of the current radiologic and laboratory tests can assist with the initial diagnosis and monitoring treatment response. Currently erythrocyte sedimentation rate, C-reactive protein, computed tomography scan, and magnetic resonance imaging with and without contrast are used in combination to establish diagnosis. Management of postoperative spine infection involves thorough surgical debridement and targeted antibiotic therapy.
Conclusions:
Postoperative spine infection is a not uncommon complication following surgery that may have devastating consequences for a patient’s short- and long-term health. A high index of suspicion is needed to make an early diagnosis.
Keywords: spine, infection, postoperative, diagnosis, management
Introduction
Postoperative spine infection can be a devastating complication after spine surgery in both the short term and long term. Infection places a patient at a high risk for pseudoarthrosis, chronic pain, return to operating room, adverse neurological sequelae, worsened long-term outcomes, and even death.1-6 Depending on the type of spine surgery being performed, the incidence of infection is highly variable, with ranges reported listed between 0% and 18%.4-6 There is a lower incidence of infection in a simple lumbar decompression or microdiscectomy (∼0.6% to 3%) compared with an instrumented fusion (∼6% to 18%).1-6 The surgical approach plays a role in infection as well. Posterior cervical surgery has a higher rate of infection than posterior lumbar surgery and anterior spinal surgery.
There are a multitude of risk factors that increase the rate of infection, only some of which are modifiable. In brief, modifiable risk factors that increase the chance of infection include obesity, smoking, malnutrition, administration of antibiotics (orally, intravenously (IV), or direct application at surgery site) and extended hospitalization.7-10 Nonmodifiable or minimally modifiable risk factors include advanced age, immunosuppression, urgent surgical need (ie, spinal trauma), spinal cord injury/myelopathy, neuromuscular scoliosis, and presence of diabetes mellitus.7,9,10 In addition to this, revision surgery is a nonmodifiable risk factor for postoperative spine infection, secondary to the excess of devitalized soft tissues. Patients should be counseled prior to undergoing elective spine surgery to optimize the modifiable risk factors present to reduce the rate of postoperative infection and other complications. Modifiable risk factors under surgeon control are operative time, retractor placement, and strict sterile technique.9,10 Preventive techniques will be further addressed in another review article in this focus issue. Article searches were performed from Medical Subject Headings (MeSH) used by the National Library of Medicine. Specifically, MeSH terms “spine,” “infections,” “management,” and “diagnosis” were used.
Classification
Postoperative spine infections can be classified by both the site of infection and the duration of the infection. Infections can be identified as superficial or deep. Superficial infections are limited to the skin and subcutaneous layers without violating the fascial layer. Deep infections extend below the lumbodorsal fascia, ligamentum nuchae, anterior abdominal fascia, or platysma (depending on surgical site).2-4 Infection can be further classified based on proximity to surgery. If the infection occurs within 3 weeks of the procedure it is classified as an acute infection, and if it occurs >3 weeks since surgery it is classified as delayed.2-4
Diagnosis
Presentation
The presentation of patients with a postoperative spine infection can vary significantly depending on the type of infection and the type of surgery performed. The most common symptom of infection is pain, which is usually insidious in onset about 1 month postoperatively.4 The pain may be localized axially near the area of the incision, but also may radiate to the extremities in a radicular pattern. The pain may also mimic the original preoperative symptoms, thus causing a confusing clinical picture.1-6
Most patients do not become systemically ill or septic from a postoperative spine infection, though acute infections with high virulence, such as methicillin-resistant Streptococcus aureus may present in this fashion. The most common physical sign of infection is erythema or swelling of the incision. However, it is quite frequent for infected wounds to appear benign. Obvious signs of infection would be wound dehiscence and purulent drainage from the wound. Wound drainage for greater than 1 week is a risk factor for deep infection. Fevers are present in less than half of patients (∼40%). Other signs and symptoms include fatigue and potentially even weight loss depending on the chronicity of the infection.
Depending on the type of operation, specific signs and symptoms could be present as well. In anterior cervical surgery one of the presenting symptoms may be hypersalivation, dysphagia, and dysphonia due to a retropharyngeal abscess or esophageal perforation.4 Subcutaneous emphysema (the subcutaneous fat can feel like “bubble-wrap”) is pathopneumonic for an esophageal perforation. That said, infection and wound complications after an anterior cervical discectomy and fusion are very rare. After a lumbar microdiscectomy, significant pain with lumbar range of motion that is not improving over time, particularly forward flexion, is indicative of postprocedural diskitis. Epidural abscess would be considered if there are any neurological deficits present on exam such as loss of motor strength, sensation, or bowel/bladder changes.
Laboratory Tests
There are several laboratory tests that can be ordered to help guide the diagnosis of postoperative infection. The initial tests that should be ordered on clinical suspicion of postoperative infection should include a complete blood count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP).1-6 Blood cultures should be drawn from 2 sites prior to initiating antibiotics, even though they will be positive in less than half of postoperative spinal infections. While direct inoculation is the most common source of postoperative infection, hematogenous spread is second.
Used in isolation, the white blood cell (WBC) count is a poor marker for surgical site infection (SSI). The WBC value may be elevated, decreased, or normal depending on host immune system and type of pathogen. Less than 50% of cases of postoperative infection will have an elevated WBC. The use of perioperative steroids can contribute to an elevated WBC count, which further decreases the utility of this laboratory test. Assessing for a left shift is clinically useful in this scenario as steroid-induced leukocytosis is not associated with a left shift like infection is.
The ESR is a more sensitive test than WBC but is just as nonspecific for ruling in postoperative infection. ESR values must be interpreted with caution in the acute postoperative period. Depending on the extent of the surgery performed, it can take about 2 to 4 weeks for ESR values to peak and anywhere from 21 to 90 days to return to normal.11 Given that ESR is routinely elevated in the postoperative setting, it typically is not helpful for diagnosing an acute postoperative infection. One clinical utility of ESR is to follow the treatment course of a patient to help assess response. While nonspecific, ESR is useful to rule out an infection as there is unlikely to be an infection if the ESR is within normal limits. We find it useful to obtain preoperative ESR values on patients to determine their baseline, since trending information is far more predictive than absolute values. ESR has been shown to be both gender and age dependent so establishing baseline values preoperatively may increase the clinical utility of this test. Likewise, routinely obtaining inflammatory markers postoperatively (ie, postoperative day 3 following instrumented posterior spinal fusion) can establish a postoperative value for trending analysis.
CRP is the most sensitive indicator currently available to diagnose postoperative infection. CRP normalizes in a quicker and more reliable pattern postoperatively. CRP peaks on the second to third postoperative day and reliably returns to normal by 14 to 21 days postoperatively, at a rate of halving in value about every 3 days.11 If the CRP is elevated after this period, and even more specifically, if there is a second “bump” (ie, first elevation due to the normal inflammatory response to surgery, then a second elevation some number of days or weeks later), there is a high correlation with the presence of infection, with a sensitivity of 82%.12 Combined trending of ESR and CRP values can be the most predictive method for diagnosing and monitoring treatment response of postoperative spinal infections; however, no laboratory method has demonstrated excellent specificity/positive predictive value.
The most informative laboratory method for diagnosing postoperative spinal infection would be biopsy, typically percutaneously performed with computed tomography (CT) guidance. Cultures, in addition to blood and biopsy, should be obtained whenever there is suspicion for an extraspinal primary source, like a urinary tract infection. If the patient is experiencing urinary retention symptoms or dysuria especially if they had a perioperative in-dwelling catheter, urinalysis and urine culture should be obtained. If the patient has evidence of an upper respiratory infection or pneumonia, sputum cultures can be obtained. All cultures should be obtained prior to the initiation of antibiotics to increase the likelihood of finding a causative organism.
Novel Laboratory Tests
In addition to the standard tests, serum amyloid A (SAA), which has traditionally been considered to have a role in amyloidosis, has been used to track postoperative spine infection. SAA has a very short half-life of around 50 minutes, which enables a very rapid decrease postoperatively after a peak is reached on day 3.13 This enables SAA to be of great clinical utility when evaluating for early postoperative infection when the CRP and ESR will still be elevated. Another benefit to SAA as a marker for infection after spine surgery is that it is unaffected by corticosteroid administration, which is commonly given after spine surgery.13 At this point, the use of SAA in the diagnosis of postoperative infection has great promise, but limited clinical validation.
Presepsin is another biomarker with possible utility in diagnosing postoperative spine infection. Presepsin is a biomarker that has been used in the diagnosis of bacterial sepsis. A recent prospective study identified that presepsin levels return to baseline 1 week after surgery (∼126 pg/mL) if there is no infectious complications and that patients with infections had presepsin levels greater than 300 pg/mL 1 week postoperatively.14 Presepsin will need to be studied further prior to broad implementation, but it has great potential as a diagnostic tool for postoperative spine infection.
Other novel inflammatory markers that have been evaluated for SSI have not been proven effective. Procalcitonin (PCT) has an established clinical utility for diagnosis of bacterial infection and wound complications in some orthopedic procedures. However, when PCT is used for evaluation of spinal infection, the sensitivity is lower than that of CRP.15 The addition of a PCT laboratory test did not significantly alter the likelihood of making a diagnosis of postoperative spine infection when compared with the standard inflammatory markers.
Imaging
Plain Radiographs
While basic radiographs may lack early sensitivity in postoperative spine infection, advanced imaging can be quite useful; however, separating normal postoperative change from infection can be a real challenge. Regardless, plain radiographs should be obtained to assess for any hardware failure. In the setting of discitis, endplate erosion and loss of disc height may be evident (Figure 1A and B).16,17 Unfortunately, the earliest these changes would be expected would be around 6 weeks postoperatively. In long-standing infections there may be the presence of instability on flexion and extension radiographs and/or segmental kyphosis. In latent infections there may also be lucencies around orthopedic hardware.16,17 Radiographs are also beneficial because they avoid metal artifact that occur with imaging modalities.
Figure 1.
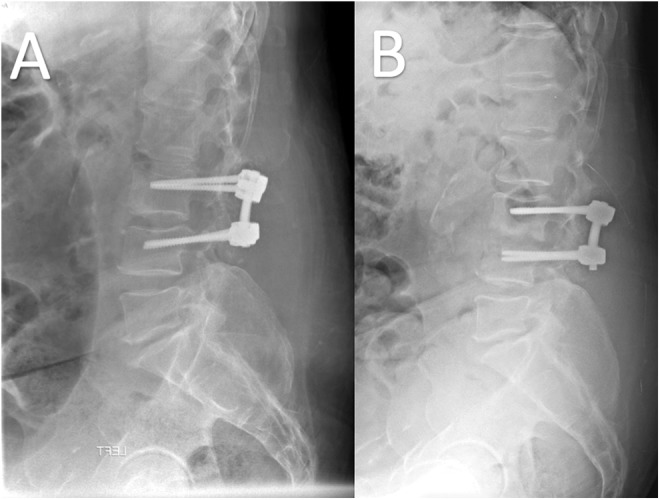
Lumbar spine radiographs taken 1 month postoperatively (A) and 2 months postoperatively (B). The 2-month postoperative radiograph shows significant L3-4 endplate erosions indicative of infection.
Computed Tomography
Computed tomography scan is the modality of choice for evaluation of bone. It can also give information on soft tissue collections. Early changes include erosion and destructive changes to the bony endplates as well as disk space narrowing. Lucencies can also be seen around orthopedic implants. This can be seen earlier on CT scan than plain radiographs. Soft tissue fluid collections could represent a postoperative abscess, hematoma, or a sterile seroma. Hemorrhage can be identified by seeing blood/serum level on cross-sectional imaging or by measuring Hounsfield Units (HU) on CT scan. The HU of blood is 13 to 50, the HU of clotted blood is 50 to 75, and the HU of cerebrospinal is approximately 15. The other clinical utility for CT scan is to obtain a CT-guided aspiration/biopsy in cases of postoperative diskitis/osteomyelitis for culture (Figure 2).16,17 This would allow for targeted antibiotic therapy if the blood cultures are negative. Cultures should be taken prior to the initiation of antimicrobial therapy.
Figure 2.
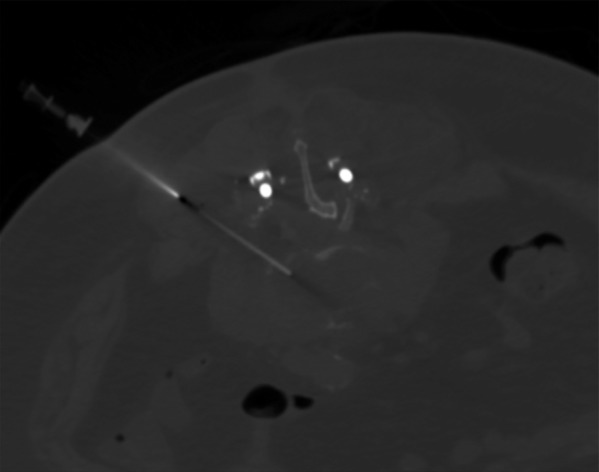
Computed tomography–guided biopsy of the infected disc for gram stain, culture, and speciation to determine antibiotic sensitivity.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) with gadolinium contrast is traditionally thought to be the most sensitive modality for evaluation of postoperative infection. MRI with gadolinium contrast has been shown to have a sensitivity of 93% and specificity of 97% for postprocedural diskitis, even in cases when hardware has been placed.16,17 There is a high false positive rate of MRI as an increase in signal intensity/edema may be a normal postoperative finding rather than an indication of an infection. Findings must be interpreted based on the postoperative timing because of the potentially confounding noninfectious causes. MRI findings that correlate with infectious causes are rim-enhancing fluid collections, ascending epidural collections, evidence of bony destruction, and progressive marrow signal changes. Gadolinium-enhanced T1-weighted images also provide additional evidence of infection (Figure 3).16,17
Figure 3.
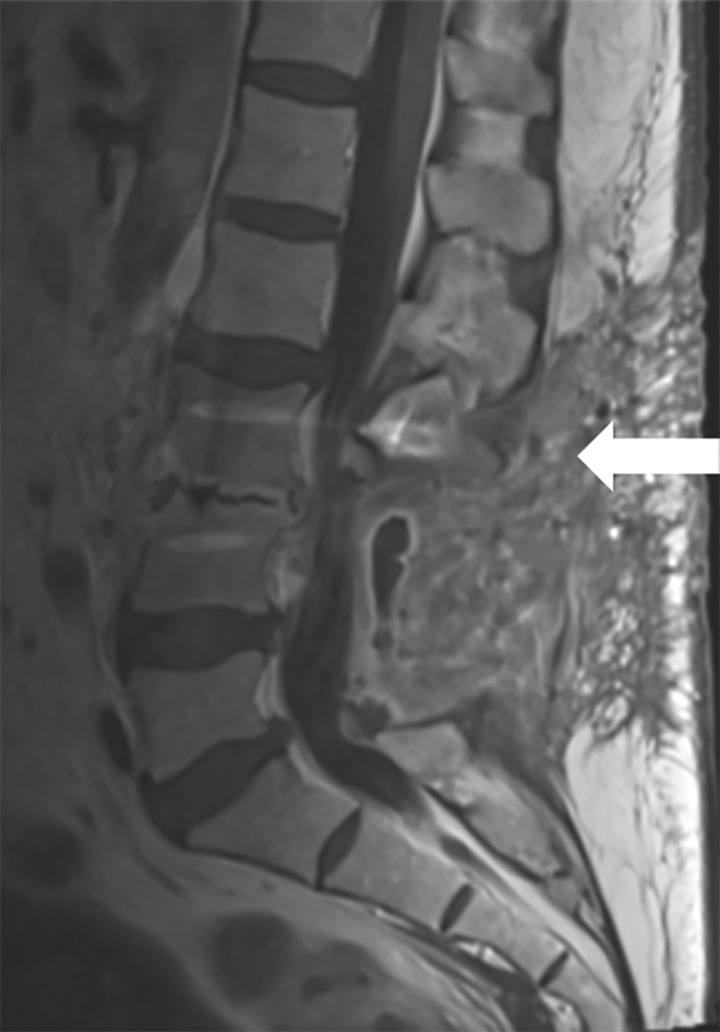
Sagittal T1 postcontrast magnetic resonance imaging scan of the lumbar spine showing rim-enhancing fluid collection dorsal to laminectomy (arrow).
Nuclear Medicine
Nuclear medicine can be used as an adjunct for diagnosis of postoperative spine infection. These imaging modalities do not add much clinical utility as they have very poor sensitivity and are often not used clinically. Gallium-67 can identify the presence of postoperative disk space infection earlier than technetium-99. Sequentially obtaining both technetium-99 and gallium-67 studies increases the sensitivity and can help establish a diagnosis when MRI is unable to be obtained. Indium-111–labeled WBC are used infrequently due to poor specificity.16,17
Novel Imaging Studies
Recently there has been use of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for evaluation of postoperative spinal infection. 18F-FDG PET/CT has been showed to both be more sensitive and specific for the diagnosis of postoperative spinal infection with a sensitivity of 100% and specificity of 79%, compared with MRI.18,19 In addition, FDG-PET/CT was also able to better localize the foci of infection than MRI.18 FDG-PET/CT demonstrated limited areas of abnormal metabolic activities immediately adjacent to the center of spinal infection compared with MRI, which showed abnormality over a broader area.19 FDG-PET/CT is also very clinically useful when there is hardware present as there is less artifact from metallic implants than MRI. In addition, FDG-PET/CT can more aptly differentiate between spondylodiscitis and endplate degeneration. The drawbacks to using this imaging modality is the great expense and the limited clinical studies in relation to postoperative spine infection. In the future, FDG-PET/CT may be used to assess treatment efficacy and termination of antibiotic therapy.
Management
Initial Presentation
SSI in spine surgery can be difficult to manage and often multiple debridements and long-term antibiotics are required for treatment. The initial treatment should focus on the clinical stability of the patient. Once clinical suspicion is present for SSI, the patient should have blood cultures drawn and antibiotics held. The laboratory tests and imaging modalities discussed before can help establish a diagnosis of SSI. If a patient remains stable, antibiotics should be held until after operating room cultures are obtained unless a positive culture was obtained from another intervention (blood culture or CT-guided aspiration). After cultures are obtained, broad spectrum antibiotics covering both gram positive, gram negative, and anaerobic bacteria should be initiated. An infectious disease doctor should be consulted for comanagement of the patient and to guide antibiotic therapy. If the patient presents in sepsis or septic shock the patient should have emergent surgery and broad spectrum antibiotics should be initiated directly after blood cultures are taken while the patient is being resuscitated. In addition, if the patient has a rapidly changing neurological exam the patient should be taken to the operating room emergently for an irrigation and debridement to decompress any neurologic structures.
Surgical Debridement
The principles of open surgical debridement are exploration of the wound to establish if the infection is deep versus superficial. This is followed by a thorough debridement of necrotic and infected tissue. Early involvement of a plastic surgeon is helpful in management of postoperative spine infection, as sometimes multiple debridements are necessary.20 Poor local soft tissue vascularity can occur as a result of many debridements. It is recommended that antibiotic-impregnated beads be used in these cases to ensure adequate antibiotic concentration in the surgical wound that is compromised by decreased perfusion and decreased delivery of IV antibiotics to the infection site.20 For early postoperative infection (<3 months), in cases where spinal instrumentation is present the current recommendation is not the remove the hardware to avoid destabilizing the spine.21,22 Bone graft that is loose at time of debridement should be removed, but any graft material that is adherent to bony structures should be left in place. For late postoperative infection hardware removal is more necessary for a variety of reasons. One reason for removal of hardware is that spinal anchorage points and the region directly beneath the rods are relatively inaccessible without removal. Assuming a solid fusion has occurred, removal of hardware can allow a more adequate debridement of the wound. In addition, late-onset infections are often indolent and caused by organisms, like coagulase-negative Staphylococci or Propionibacterium acnes, which are likely to cause biofilm formation. Di Silvestre et al showed that if the implants are not removed in delayed infection there is a 50% chance that the infection can remain.23 The benefit of eradicating the biofilm must be weighed against the risk of removing fixation prior to osseous fusion. In cases where a fusion has occurred the hardware can be removed; however, with long fusions it is possible to fracture the fusion mass, lose alignment, or to settle into a position of kyphosis. In cases where a fusion has not occurred, autograft and/or allograft can be used to achieve bony fusion. There is not an increase in postoperative infection rate with the use of allograft.
Primary closure can be performed if the underlying tissue appears healthy. This should be done over suction drains. If the surgical site does not appear healthy the wound should be packed and assessed again 2 to 5 days later during a scheduled debridement. The use of negative pressure wound therapy via a vacuum-assisted closure (VAC) has been shown to lessen morbidity to the patient. The number of surgical debridements for deep spine infection decreases from an average of 2.2 debridements when using VAC therapy compared to a range of 2.7 to 4.7 debridements for standard packing dressing.24 VAC therapy can be used to facilitate secondary intention closures as well over time if surgical debridement is too morbid for the patient.
Antibiotic Therapy
Equally important to multiple debridements is the continuation of antibiotic therapy. As discussed before, antibiotics should not be administered prior to culture results if the patient is stable. If the patient is septic and unstable, antibiotics should be administered empirically to help prevent further clinical decline. Broad spectrum antibiotics should be initiated prior to obtaining final culture results. The duration of antibiotic therapy is controversial and dependent on the type of infection being treated. For patients with postoperative infection in the absence of hardware, a shorter course of antibiotics is typically used. Postoperative discitis/osteomyelitis is generally treated with >3 months of antibiotics depending on the inflammatory markers. In cases of deep infection with hardware in place, most commonly patients are placed on at least a 4- to 6-week IV antibiotic course. These antibiotics are tailored to culture results and inflammatory markers. If ESR/CRP are rising after the discontinuation of antibiotics and fusion has occurred, it is recommended to remove the hardware at this point. There is controversy if long-term oral suppressive antibiotic therapy is also needed. Kowalski et al showed that treatment failure was lower in the group that received suppressive therapy in addition to IV antibiotics (22%) in comparison to the group that only received IV antibiotics (83%).25 This study was in a mixed group of patients including early and late infection, in addition to retention of instrumentation and explanation of hardware. Contrary to this study, Clark et al showed that 100% of patients presenting with delayed postoperative infection treated with debridement and hardware removal followed by 72 hours of IV antibiotics and 7 days of oral antibiotics had complete resolution of their infection.26 There is no clear consensus on the duration of antibiotic regimen; however, our recommendation is that if hardware is retained, long-term IV antibiotics should be followed by a course of oral suppressive antibiotics and a shorter treatment course may be appropriate if instrumentation is removed.
Adjunctive Treatments
Hyperbaric oxygen therapy has also been used as an adjunct in the treatment of spinal infection. The theory behind this treatment is that hyperbaric oxygen has beneficial effects treating infection due to restoring intramedullary bone oxygen tension, restoring phagocyte killing to normal levels, stimulation of neovascularization of healing wound edges, induction of vasodilation in healing tissues, and subjugation of biofilm formation.27 There are no known side effects to this adjunct treatment. More research is needed to assess if hyperbaric oxygen decreases the number of revision surgeries/debridements or leads to better long-term outcomes when compared with standard treatment alone.
Conclusions
Postoperative spine infection can have devastating consequences for a patient’s short-term and long-term health. A high index of suspicion is needed to make an early diagnosis. Currently, the gold standard for diagnosis of postoperative spine infection is positive deep culture. Many of the radiologic and laboratory tests currently available are confusing and misleading. Promising results for novel imaging and laboratory tests have the possibility to make diagnosis of postoperative spine infection easier. Management of postoperative spine infection revolves around multiple debridement and targeted antibiotic therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This Supplement was supported by funding from AOSpine North America.
ORCID iD: Samuel Cho  http://orcid.org/0000-0003-3826-1786
http://orcid.org/0000-0003-3826-1786
References
- 1. Beiner JM, Grauer J, Kwon BK, Vaccaro AR. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15:E14. [DOI] [PubMed] [Google Scholar]
- 2. Bible JE, Biswas D, Devin CJ. Postoperative infections of the spine. Am J Orthop (Belle Mead NJ). 2011;40:E264–E271. [PubMed] [Google Scholar]
- 3. Pawar AY, Biswas SK. Postoperative spine infections. Asian Spine J. 2016;10:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaudhary SB, Vives MJ, Basra SK, Reiter MF. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med. 2007;30:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parchi PD, Evangelisti G, Andreani L, et al. Postoperative spine infections. Orthop Rev (Pavia). 2015;7:5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharif S, Gulzar F. Postoperative infections of the spine. World Spinal Column J. 2015;1:19–26. [Google Scholar]
- 7. Brown EM, Pople IK, de louvois J, et al. ; British Society for Antimicrobial Chemotherapy Working Party on Neurosurgical Infections. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine (Phila Pa 1976). 2004;29:938–945. [DOI] [PubMed] [Google Scholar]
- 8. Liu G, Chen S, Fang J, et al. Vancomycin microspheres reduce postoperative spine infection in an in vivo rabbit model. BMC Pharmacol Toxicol. 2016;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazennec JY, Fourniols E, Lenoir T, et al. ; French Spine Surgery Society. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res. 2011;97(6 suppl):S107–S116. [DOI] [PubMed] [Google Scholar]
- 10. Anderson PA, Savage JW, Vaccaro AR, et al. Prevention of surgical site infection in spine surgery. Neurosurgery. 2017;80(3S):S114–S123. [DOI] [PubMed] [Google Scholar]
- 11. Mok JM, Pekmezci M, Piper SL, et al. Use of C-reactive protein after spinal surgery: comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976). 2008;33:415–421. [DOI] [PubMed] [Google Scholar]
- 12. Kunakornsawat S, Tungsiripat R, Putthiwara D, et al. Postoperative kinetics of C-reactive protein and erythrocyte sediment rate in one-, two-, and multilevel posterior spinal decompressions and instrumentations. Global Spine J. 2017;7:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Front Med (Lausanne). 2014;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koakutsu T, Sato T, Aizawa T, Itoi E, Kushimoto S. Postoperative changes in presepsin level and values predictive of surgical site infection after spinal surgery: a single center, prospective observational study [published online August 14, 2017]. Spine (Phila Pa 1976). doi:10.1097/BRS.0000000000002376. [DOI] [PubMed] [Google Scholar]
- 15. Forsberg JA, Elster EA, Andersen RC, et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi D, Roemer FW, Mian A, Gharaibeh M, Müller B, Guermazi A. Imaging features of postoperative complications after spinal surgery and instrumentation. AJR Am J Roentgenol. 2012;199:W123–W129. [DOI] [PubMed] [Google Scholar]
- 17. Herrera IH, de la Presa RM, Gutiérrez RG, Ruiz EB, Benassi JMG. Evaluation of the postoperative lumbar spine. Radiologia. 2013;55:12–23. [DOI] [PubMed] [Google Scholar]
- 18. Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013;2013:623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakahara M, Ito M, Hattori N, et al. 18F-FDG-PET/CT better localizes active spinal infection than MRI for successful minimally invasive surgery. Acta Radiol. 2015;56:829–836. [DOI] [PubMed] [Google Scholar]
- 20. Lall RR, Wong AP, Lall RR, Lawton CD, Smith ZA, Dahdaleh NS. Evidence-based management of deep wound infection after spinal instrumentation. J Clin Neurosci. 2015;22:238–242. [DOI] [PubMed] [Google Scholar]
- 21. Hegde V, Meredith DS, Kepler CK, Huang RC. Management of postoperative spinal infections. World J Orthop. 2012;3:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Núñez-Pereira S, Pellisé F, Rodríguez-Pardo D, et al. Implant survival after deep infection of an instrumented spinal fusion. Bone Joint J. 2013;95-B:1121-1126. [DOI] [PubMed] [Google Scholar]
- 23. Di Silvestre MD, Bakaloudis G, Lolli F, Giacomini S. Late-developing infection following posterior fusion for adolescent idiopathic scoliosis. Eur Spine J. 2011;20(suppl 1):S121–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Canavese F, Gupta S, Krajbich JI, Emara KM. Vacuum-assisted closure for deep infection after spinal instrumentation for scoliosis. J Bone Joint Surg Br. 2008;90:377–381. [DOI] [PubMed] [Google Scholar]
- 25. Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Mandrekar JN, Osmon DR. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis. 2007;44:913–920. [DOI] [PubMed] [Google Scholar]
- 26. Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976). 1999;24:1909–1912. [DOI] [PubMed] [Google Scholar]
- 27. Larsson A, Uusijärvi J, Lind F, Gustavsson B, Saraste H. Hyperbaric oxygen in the treatment of postoperative infections in paediatric patients with neuromuscular spine deformity. Eur Spine J. 2011;20:2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]


