Abstract
Study Design:
Systematic literature review.
Objectives:
The aims of this study were to (1) describe the clinical features, disabilities, and incidence of neurologic deficits of pyogenic spondylodiscitis prior to treatment and (2) compare the functional outcomes between patients who underwent medical treatment alone or in combination with surgery for pyogenic spondylodiscitis.
Methods:
A systematic literature review was performed using PubMed according to PRISMA guidelines. No year restriction was put in place. Statistical analysis of pooled data, when documented in the original report (ie, number of patients with desired variable and number of patients evaluated), was conducted to determine the most common presenting symptoms, incidence of pre- and postoperative neurologic deficits, associated comorbidities, infectious pathogens, approach for surgery when performed, and duration of hospitalization. Outcomes data, including return to work status, resolution of back pain, and functional recovery were also pooled among all studies and surgery-specific studies alone. Meta-analysis of studies with subgroup analysis of pain-free outcome in surgical and medical patients was performed.
Results:
Fifty of 1286 studies were included, comprising 4173 patients undergoing either medical treatment alone or in combination with surgery. Back pain was the most common presenting symptom, reported in 91% of patients. Neurologic deficit was noted in 31% of patients. Staphylococcus aureus was the most commonly reported pathogen, seen in 35% of reported cases. Decompression and fusion was the most commonly reported surgical procedure, performed in 80% of the surgically treated patients. Combined anterior-posterior procedures and staged surgeries were performed in 33% and 26% of surgeries, respectively. The meta-analysis comparing visual analog scale score at follow-up was superior among patients receiving surgery over medical treatment alone (mean difference −0.61, CI −0.90 to −0.25), while meta-analysis comparing freedom from pain in patients receiving medical treatment alone versus combined medical and surgical treatment demonstrated superior pain-free outcomes among surgical series (odds ratio 5.35, CI 2.27-12.60, P < .001), but was subject to heterogeneity among studies (I 2 = 56%, P = .13). Among all patients, freedom from pain was achieved in 79% of patients, and an excellent outcome was achieved in 73% of patients.
Conclusion:
Medical management remains first-line treatment of infectious pyogenic spondylodiscitis. Surgery may be indicated for progressive pain, persistent infection on imaging, deformity or neurologic deficits. If surgery is required, reported literature shows potential for significant pain reduction, improved neurologic function and a high number of patients returning to a normal functional/work status.
Keywords: discitis, spondylodiscitis, osteodiscitis, pyogenic, back pain, outcome
Introduction
Spondylodiscitis is an infectious disease of the intervertebral disc space and adjacent vertebral end plates.1 Most patients with discitis present initially with back pain and often have significant delays in time to diagnosis.2 Severity of back pain can be quite significant, resulting in poor quality of life and disability scores.3 Continued bone and disc destruction from prolonged, chronic infection may progress to significant spinal deformity in a subset of patients, with resultant worsening quality of life.4 The goals of this study were first to describe the clinical features, disabilities and neurologic deficits of pyogenic spondylodiscitis on presentation, and second to compare the functional outcomes between patients who underwent medical and surgical management for pyogenic spondylodiscitis.
Methods
Inclusion and Exclusion Criteria
In an effort to maximize inclusion of relevant literature regarding medical and surgical management of primary pyogenic spondylodiscitis, the following inclusion criteria were used: (1) study must contain at least 10 patients, (2) the primary focus of the study must be surgical or medical management with reported outcome of spontaneous discitis, (3) article must be written in English, (4) the study must involve human subjects, and (5) the articles must be found in MEDLINE journal categories. Case reports, reviews, technical notes, or studies involving primarily pediatric patients (age <18 years), vertebral abscess, iatrogenic discitis, or those focusing on one specific pathogen were excluded. If multiple studies were published by a single author, only the largest series was included.
Literature Search
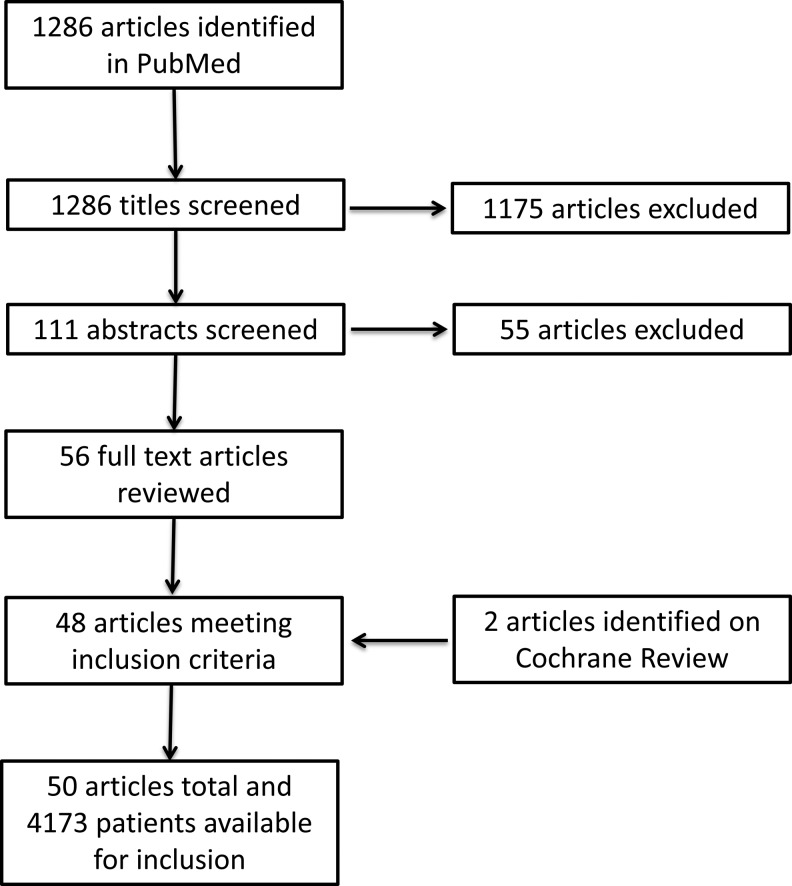
For the present systematic review, a focused search on PubMed was considered sufficient. A systematic review was performed on March 21, 2018 using PubMed with the following search term: (“discitis” OR “spondylodiscitis” OR “diskitis”) and (“management” OR “treatment” OR “surgery” OR “surgical”). Results were restricted to Medline journal categories regarding human subjects and published in English, of which 1286 articles resulted. Article titles were evaluated to identify those related to the medical or surgical management and outcomes of discitis or vertebral osteomyelitis. Studies not meeting the aforementioned inclusion criteria were excluded (Figure 1). A total of 111 abstracts were reviewed and application of inclusion criteria was repeated, which excluded an additional 55 studies. For the remaining 56 studies, we reviewed the full-text journal articles, and 48 achieved criteria for inclusion. Following, a search was performed on Cochrane Review for additional articles using the same inclusion and exclusion criteria, identifying 2 additional articles. Finally, 50 full-text journal articles were available for evaluation.
Figure 1.
Flowchart outlining the systematic review process. The initial PubMed search resulted in 1286 articles. Subsequent screening and application of inclusion and exclusion criteria yielded 50 total articles to undergo detailed review and pooling of data. Two additional articles were identified after similar process through Cochrane Review.
Literature Review and Data Extraction
A protocol was registered with PROSPERO for this review (CRD42018092268), and the review follows the guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).5 When provided by the original article, demographic, clinical, pathological, surgical, and outcome data was extracted from selected studies. Demographic data included the number of patients, sex, age, and associated comorbidities. Clinical data included time to diagnosis, imaging modality, presenting symptoms, relevant laboratory values, culture source, and results. Outcome data included duration at follow-up, pain at follow-up, presence of neurologic deficits, and return to work. A composite score of excellent functional outcomes was created to capture the various reports of return to work with normal activity, freedom from pain at last follow-up, Oswestry Disability Index (ODI) score less than 20, or a Kirkaldy-Willis Score of 3 or greater. Surgery-specific series were further evaluated to determine type of surgery (decompression alone, decompression and fusion, combined anterior-posterior approach, or staged surgery).
Statistical Analysis
Pooled data were gathered from all available articles and continuous variables were summarized with means calculated based on the numbers of patients pooled from each article. When available, comparison of pain outcomes after medical and surgical treatment versus medical treatment alone was performed using Review Manager (RevMan), version 5.3 (Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2012). Odds ratios for individual studies and the sum of studies were computed using the Mantel-Haenszel test. Mean difference was determined using RevMan as well. Study heterogeneity was detected using the chi-square and I 2 test statistics. Given the limitations on the power of the chi-square test by the small number of studies in the analyses, significant heterogeneity was considered to be present when both the chi-square value was within 10% level of significance (P < .10) or the I 2 value exceeded 50%.
Results
Study Selection
The search term resulted in 1286 articles for review. Among these, 111 articles were selected for further review of abstracts based on title and relevance to inclusion and exclusion criteria. Application of inclusion and exclusion criteria to study abstracts yielded 56 articles. The full-text articles for these 56 studies were then reviewed and subjected to our inclusion criteria, after which 48 articles were eligible for systematic review.3,6-52 (Figure 1) Following an additional search on Cochrane Review, 2 additional studies53,54 were found, and a total of 50 articles were eligible for final systematic review (Tables 1 and 2) 16 studies reported both surgical and medical outcomes. All but 3 studies were retrospective in nature.
Table 1.
Summary of the 50 Studies Included in Pooled Data for Presenting Features of Pyogenic Spondylodiscitis.
| Demographics | Presentation | Lesion Location | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year | Paper Type | Patients (n) | Men (n) | Mean Age (Years) | Time to Diagnosis (Days) | Back Pain | Neurological Findings | Cervical | Thoracic | Lumbar |
| Aagaard, 2013 | Retrospective review | 100 | 37 | 60 | 32 | 25 | 26 | 65 | ||
| Asamoto, 2005 | Retrospective review | 21 | 13 | 66 | 19 | 12 | 5 | 7 | 11 | |
| Ascione 2017 | Retrospective review | 30 | 13 | 64 | 28 | 30 | 5 | 0 | 2 | 20 |
| Bernard, 2015 | Randomized controlled trial | 351 | 242 | 61 | 337 | 57 | 52 | 96 | 246 | |
| Bettini, 2009 | Retrospective review | 56 | 21 | 48 | 34 | 56 | 18 | 3 | 8 | 48 |
| Butler, 2006 | Retrospective review | 48 | 24 | 59 | 47 | 14 | 4 | 11 | 30 | |
| Bydone, 2014 | Retrospective review | 118 | 57 | 67 | 61 | 35 | 40 | 43 | ||
| Cebrian, 2012 | Retrospective review | 108 | 49 | 68 | 97 | 27 | 4 | 38 | 66 | |
| Chung, 2011 | Retrospective review | 20 | 13 | 60 | 20 | 14 | 0 | 8 | 12 | |
| Citak, 2014 | Retrospective review | 183 | 99 | 63 | 80 | 14 | 66 | 97 | ||
| D’Agostino, 2010 | Prospective series | 81 | 51 | 58 | 55.4 | 79 | 12 | 10 | 59 | 12 |
| D’Aliberti, 2012 | Retrospective review | 40 | 29 | 56 | 39 | 16 | 13 | 18 | 9 | |
| Dennis, 2017 | Retrospective | 84 | 49 | 62 | 68 | 19 | 29 | 79 | 156 | |
| Eysel, 1997 | Retrospective review | 55 | 28 | 52 | ||||||
| Ha, 2007 | Retrospective review | 24 | 14 | 23 | 7 | 0 | 0 | 24 | ||
| Hadjipavlou, 2000 | Retrospective review | 101 | 76 | 46 | 40 | 10 | 33 | 55 | ||
| Heyde, 2006 | Retrospective review | 20 | 11 | 60 | 84 | 9 | ||||
| Homagk, 2016 | Retrospective review | 270 | 153 | 64 | 101 | 30 | 103 | 149 | ||
| Hopf, 1998 | Retrospective review | 72 | 39 | 52 | 0 | 40 | 49 | |||
| Karadimas, 2008 | Retrospective review | 163 | 101 | 56 | 13 | 72 | 88 | |||
| Kaya, 2014 | Retrospective review | 107 | 64 | 53 | 97 | 18 | 7 | 14 | 90 | |
| Kehrer, 2015 | Retrospective review | 298 | 182 | 66 | 33 | 38 | 95 | 203 | ||
| Klockner, 2003 | Retrospective review | 71 | 38 | 54 | 18 | 0 | 33 | 39 | ||
| Krodel, 1991 | Retrospective review | 24 | 50 | |||||||
| Legrand, 2001 | Retrospective review | 110 | 67 | 61 | 40 | 18 | 3 | 23 | 69 | |
| Lemaignen, 2017 | Retrospective review | 394 | 267 | 63 | 40 | 369 | 130 | 71 | 137 | 281 |
| Lin CP, 2012 | Retrospective review | 48 | 19 | 67 | 144 | 48 | 28 | 0 | 16 | 38 |
| Lin TY, 2014 | Retrospective review | 45 | 25 | 62 | 0 | 9 | 36 | |||
| Lin Y, 2015 | Retrospective review | 22 | 13 | 55 | 22 | 8 | 0 | 0 | 22 | |
| Lu, 2015 | Retrospective review | 28 | 13 | 60 | 6 | 0 | 2 | 28 | ||
| Mann, 2004 | Prospective series | 24 | 14 | 63 | 24 | 13 | 4 | 10 | 10 | |
| Nasto, 2014 | Retrospective review | 27 | 18 | 60 | 33 | 0 | 5 | 22 | ||
| Noh, 2017 | Retrospective review | 31 | 27 | 68 | 18 | 3 | 8 | 20 | ||
| Pee, 2008 | Retrospective review | 60 | 36 | 59 | 58 | 16 | 0 | 0 | 60 | |
| Rath, 1996 | Retrospective review | 43 | 24 | 60 | 46 | 35 | 0 | 19 | 24 | |
| Shinkel, 2003 | Retrospective review | 32 | 18 | 61 | 32 | 17 | 21 | 19 | 35 | |
| Schomacher, 2014 | Retrospective review | 37 | 24 | 62 | 37 | 6 | 6 | 25 | ||
| Shetty, 2016 | Retrospective review | 27 | 19 | 48 | 6 | |||||
| Shiban, 2014 | Retrospective review | 113 | 78 | 104 | 40 | |||||
| Shousha, 2012 | Retrospective review | 30 | 19 | 65 | 24 | 12 | 30 | 0 | 0 | |
| Sobottke, 2009 | Retrospective review | 20 | 14 | 43 | 23 | 3 | 2 | 6 | 12 | |
| Srinivasan, 2014 | Retrospective review | 48 | 29 | 56 | ||||||
| Suess, 2007 | Retrospective review | 24 | 15 | 65 | 9 | 24 | 7 | 3 | 14 | 7 |
| Tsai, 2017 | Retrospective review | 90 | 61 | 61 | ||||||
| Valancius, 2013 | Retrospective review | 196 | 106 | 52.5 | 177 | 47 | 9 | 42 | 125 | |
| Vcelak, 2014 | Retrospective review | 31 | 20 | 61 | 9 | 6 | 25 | |||
| Viale, 2009 | Prospective series | 48 | 36 | 60 | 9 | 6 | 9 | 29 | ||
| Wirtz, 2000 | Retrospective review | 59 | 29 | 61 | 90 | 46 | ||||
| Yaldz, 2015 | Retrospective review | 39 | 19 | 47 | 105 | 37 | 18 | 0 | 15 | 24 |
| Ziu, 2014 | Retrospective review | 102 | 87 | 45 | 102 | 26 | 9 | 24 | 59 | |
Table 2.
| Surgical Treatment | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| First Author, Year | Decompression or Decompression and Fusion | Patients (n) | Duration (Months) | Neurologic Deficits | Return to Work | Pain Free | Oswestry Disability Index <20% | Excellent Functional Outcome |
| Aagaard, 2013 | 94 | 3 | 26 | |||||
| Asamoto, 2005 | 21 | 11 | 11 | |||||
| Ascione, 2017 | 30 | 30 | 8 | 22 | 4 | 22 | ||
| Bernard, 2015 | 283 | 12 | 10 | |||||
| Bettini, 2009 | 3 | 56 | 12 | |||||
| Butler, 2006 | 0 | |||||||
| Bydone, 2014 | 118 | 15 | 23 | |||||
| Cebrian, 2012 | 108 | 6 | 35 | 35 | ||||
| Chung, 2014 | 20 | 20 | 36 | 4 | 20 | 20 | 20 | |
| Citak, 2011 | 102 | 183 | ||||||
| D’Agostino, 2010 | 10 | 72 | 65 | |||||
| D’Aliberti, 2012 | 40 | 40 | 43 | 13 | 17 | 17 | ||
| Eysel, 1997 | 55 | 42 | ||||||
| Ha, 2007 | 1 | |||||||
| Hadjipavlou, 2000 | 62 | 47 | 14 | |||||
| Heyde, 2006 | 37 | 3 | ||||||
| Homagk, 2016 | ||||||||
| Hopf, 1998 | 72 | 38 | 1 | |||||
| Karadimas, 2008 | 93 | 163 | 12 | 22 | ||||
| Kaya, 2014 | 94 | 94 | ||||||
| Klockner, 2003 | 71 | 71 | 64 | |||||
| Krodel, 1991 | 36 | |||||||
| Legrand, 2001 | 99 | 3 | 42 | 42 | ||||
| Lemaignen, 2017 | 58 | 378 | 5 | 26 | ||||
| Lin CP, 2012 | 48 | 48 | 64 | 32 | 32 | 32 | ||
| Lin TY, 2014 | 45 | 45 | 24 | |||||
| Lin Y, 2015 | 22 | 22 | 31 | 0 | 19 | 19 | ||
| Lu, 2015 | 28 | 28 | 20 | 0 | 10 | 15 | 25 | |
| Mann, 2004 | 24 | 15 | 15 | |||||
| Nasto, 2014 | 12 | 27 | 9 | |||||
| Pee, 2008 | 60 | 60 | 36 | 5 | ||||
| Rath, 1996 | 45 | 41 | 15 | |||||
| Shinkel, 2003 | 32 | 20 | 49 | 10 | ||||
| Schomacher, 2014 | 37 | 37 | 20 | |||||
| Shiban, 2014 | 106 | 3 | 16 | |||||
| Shousha, 2012 | 30 | 27 | 28 | |||||
| Sobottke, 2009 | 11 | 13 | ||||||
| Srinivasan, 2014 | 48 | |||||||
| Suess, 2007 | 24 | 24 | 18 | |||||
| Tsai, 2017 | 43 | |||||||
| Valancius, 2013 | 117 | 80 | 12 | 64 | 64 | |||
| Vcelak, 2014 | 31 | 30 | 12 | 27 | 27 | |||
| Wirtz, 2000 | 59 | 26 | ||||||
| Yaldz, 2015 | 12 | 39 | 96 | 1 | ||||
| Ziu, 2014 | 24 | 66 | 7 | |||||
Demographic and Presenting Clinical Data
Demographic and clinical data for the 4173 patients from the 50 studies included in this systematic review are summarized in Table 3. The mean patient age at presentation was 58.3 years, and 60% of the patients were men. The mean time to diagnosis from initial symptom onset was 54.93 days. Back pain was the most commonly reported presenting symptom, occurring in 91% (2101/2299) of patients. Other commonly observed findings included fever (35%, 748/2125) and focal neurologic deficits (29%, 1009/3422. Among the patients treated surgically, back pain (88%, 674/770) and focal neurologic deficits (39%, 475/1224) were the most commonly reported findings. Concurrent infection (35%, 207/834) and cardiovascular disease (24%, 260/1083) were the most common comorbidities associated with infectious discitis. Other common comorbidities included intravenous drug abuse (IVDA) (15%, 226/1379), diabetes mellitus (20%, 651/3272), immunosuppression (11%, 274/2429), and end stage renal disease (9%, 111/1263) (Table 3).
Table 3.
| Demographic and Clinical Data (n = Number of Studies) | |||
|---|---|---|---|
| No. of patients (n = 50) | 4173 | ||
| Percent men (n = 48) | 60 | ||
| Age (years) (n = 46) | 58.28 | ||
| Time to diagnosis (days) (n = 16) | 54.93 | ||
| Length of follow-up (months) (n = 33) | 26.14 | ||
| Comorbidities | Number | Total | Percent |
| Concurrent infection (n = 14) | 207 | 834 | 35 |
| Cardiovascular disease (n = 13) | 260 | 1083 | 24 |
| Intravenous drug abuse (n = 14) | 226 | 1379 | 15 |
| Diabetes (n = 33) | 651 | 3727 | 20 |
| Immunosuppression (n = 20) | 274 | 2429 | 11 |
| End-stage renal disease (n = 17) | 111 | 1263 | 9 |
| Back pain on presentation (n = 28) | 2101 | 2299 | 91 |
| Fever on presentation (n = 21) | 748 | 2125 | 35 |
| Neurologic deficits on presentation (n = 38) | 1009 | 3422 | 29 |
Laboratory and diagnostic data are summarized in Table 4. C-reactive protein (CRP) was elevated (>10 mg/L) in 85% (1083/1272) of patients, and erythrocyte sedimentation rate (ESR) was elevated in 69% (464/672) of patients. Leukocytosis was found in 43% (457/1052) of patients. Magnetic resonance imaging (MRI) and nuclear bone scan were diagnostic in 94% (980/1045) and 93% (14/15) of patients, respectively. Computed tomography (CT) was positive in in 87% (127/146) of patients and plain radiographs in 70% (129/185) of patients. Infection occurred in the cervical region in 12% of reported sites (459/3824), 31% in the thoracic spine (1219/3919), and 62% in the lumbar spine (2463/3943). Blood cultures were positive in only 51% of 930 reported cases, compared to 57% (149/261) of percutaneous biopsies and 58% (296/511) of surgical biopsies. Commonly isolated pathogens were Staphylococcus aureus (38%, 1268/3360), Mycobacterium tuberculosis (15%, 126/866), and Streptococcus species (12%, 181/1472). Blood cultures were negative in 28% of cases (Table 4).
Table 4.
| Laboratory and Diagnostic Data (n = Number of Studies) | Number | Total | Percent |
|---|---|---|---|
| Imaging | |||
| MRI positive (n = 14) | 980 | 1045 | 94 |
| Bone scan positive (n = 1) | 14 | 15 | 93 |
| CT positive (n = 4) | 127 | 146 | 87 |
| X-ray positive (n = 4) | 129 | 185 | 70 |
| Levels involved | |||
| Cervical (n = 40) | 459 | 3824 | 12 |
| Thoracic (n = 42) | 1219 | 3919 | 31 |
| Lumbar (n = 42) | 2863 | 3943 | 62 |
| Associated abscess (n = 18) | 567 | 1924 | 29 |
| Labs | |||
| Elevated CRP (>10 mg/L) (n = 16) | 1083 | 1272 | 85 |
| Elevated ESR (>20 mm/h) (n = 12) | 464 | 672 | 69 |
| Leukocytosis (n = 16) | 457 | 1052 | 43 |
| Culture yield | |||
| Surgical biopsy positive (n = 13) | 296 | 511 | 58 |
| Percutaneous biopsy positive (n = 8) | 149 | 261 | 57 |
| Blood culture positive (n = 13) | 475 | 930 | 51 |
| Negative cultures (n = 27) | 447 | 1583 | 28 |
| Culture results | |||
| Staphylococcus aureus (n = 42) | 1268 | 3360 | 38 |
| Mycobacterium tuberculosis (n = 23) | 126 | 866 | 15 |
| Gram-negative rods (n = 28) | 214 | 1810 | 12 |
| Streptococcus (n = 26) | 181 | 1472 | 12 |
| Enterococcus (n = 22) | 55 | 913 | 6 |
Abbreviations: CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging.
Treatment and Outcomes
Decompression with instrumented fusion was the most commonly performed intervention reported (79%, 1049/1321), compared to decompression alone (22%, 223/1024) based on the respective series reporting each variable. Combined anterior and posterior approach was performed in 33% (448/1364) of surgical patients, and staged surgery was performed in 26% (128/485) of surgical patients. Repeat surgery was necessary in 13% (119/891) of patients among the surgery-specific series (Table 5).
Table 5.
Surgical Procedures Performed Among Surgical Reports.
| Surgery Demographics (n = Number of Studies) | Number | Total | Percent |
|---|---|---|---|
| Decompression and fusion among surgical patients (n = 27) | 1049 | 1321 | 79 |
| Treated surgically (n = 46) | 2133 | 3384 | 63 |
| Decompression alone among surgical patients (n = 22) | 223 | 1024 | 22 |
| Anterior and posterior combined approach (n = 22) | 448 | 1364 | 33 |
| Staged surgery (n = 13) | 128 | 485 | 26 |
| Reoperation rate (n = 24) | 119 | 891 | 13 |
Follow-up was reported in 33 articles, with an average follow-up of 26.14 months. Outcome was reported in various ways (no pain, treatment success, etc), but functional outcome was reported sparingly and using various metrics (return to work, ODI, visual analog scale [VAS] score, Kirkaldy-Willis [KW]). Four studies (all surgical) reported return-to-work outcomes, with 88% (83/94) of patients reported as returning to normal work activities at last follow-up. Neurologic deficits decreased among all patients from 29% at presentation to 12% at follow-up, and from 39% among all surgical patients to 15% at follow-up. A composite score was made to identify all patients with a KW score of 3 or greater, ODI <20%, pain freedom, or return to normal work status. Excellent functional outcomes were reported in 73% (895/1234) of all patients, and in 68% (376/555) of surgical patients. Mortality rate was 8% among all patients, and 6% among surgical patients.
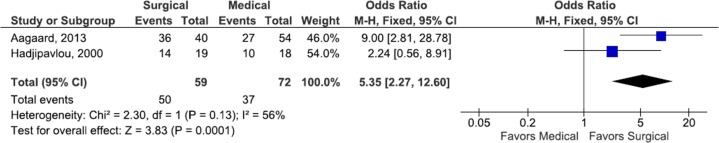
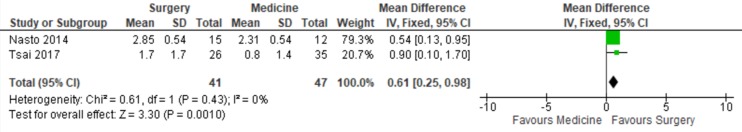
Two studies reported pain outcome of surgical and medical management cohorts separately at last follow-up, with 85% (50/59) of surgical patients reporting freedom from pain compared with 52% (37/72) of medical patients (odds ratio [OR] 5.35, CI 2.27-12.60, P < .001)6,20 (Figure 2). Two articles also reported VAS at follow-up in medical and surgical patients, with surgical patients experiencing a significantly lower VAS score compared with medical patients (mean difference −0.61, CI −0.98 to −0.25).35,47 Significant study heterogeneity was observed in both meta-analyses (Figures 2 and 3).
Figure 2.
Forest plot of the odds ratios (ORs) of pain free outcomes among surgical and medical cohorts with infectious spondylodiscitis. The estimated OR and 95% confidence interval (CI) of each included study are represented by the center of the squares and the horizontal line, respectively. The summary OR and 95% CI are represented by the diamond. Heterogeneity and overall effect are given below the summary statistics.
Figure 3.
Forest plot of the mean difference in visual analog score among surgical and medical cohorts with infectious spondylodiscitis. The estimated mean difference and 95% confidence interval (CI) of each included study are represented by the center of the squares and the horizontal line, respectively. The summary mean difference and 95% CI are represented by the diamond. Heterogeneity and overall effect are given below the summary statistics.
Discussion
Spondylodiscitis is an infectious disease of the intervertebral disc space and adjacent bone that occurs secondary to hematogenous seeding or direct spread from an adjacent infection site.1 Though epidural abscess may be seen in patients with pyogenic discitis, neurologic symptoms in the setting of abscesses are often dealt with through prompt surgical evacuation and not necessarily as a means of source control, spinal stabilization, or resolution of infection, and thus must be thought of separately from management of vertebral osteomyelitis and discitis.55 Thus, we did not include articles primarily discussing management of epidural abscess.
In the pathogenesis of discitis, bacteria seed the arterioles of the vertebral end plates, resulting in pathogen deposition into the relatively avascular intervertebral disc or adjacent vertebral endplate.2,8 The poor vascularity of this region results in a relatively safe haven for seeding bacteria and consequently often requires prolonged antibiotic treatment.8,56 This can be complicated by the natural history of osteomyelitis (a frequent accompanying feature of discitis), in which bacterial growth and purulence within vascular channels results in increased intraosseous pressure and further worsening blood flow.57
Diagnosis of discitis begins with the clinical presentation, whereby the vast majority of patients (91% in this series) present with back pain. Serum studies have low specificity for disease and may be unreliable in diagnosis.57 Still, ESR and CRP are frequently elevated, and C-reactive protein levels may be useful in determining clinical response after diagnosis is made.57 Ultimately diagnosis requires advanced imaging, with MRI demonstrating superiority in specificity compared with CT.1,57,58 Antibiotics are typically withheld until after percutaneous or surgical biopsy has been performed, unless a patient demonstrates evidence of septicemia or neurologic deficit.56 Withholding antibiotics is of particular importance as biopsy yield can decrease considerably following initiation of antibiotics.56 Staphylococcus aureus was the most common organisms isolated among pyogenic causes of discitis. This organism was found in 38% of the cases in this systematic review. Of particular concern with pyogenic discitis is the rising prevalence of methicillin-resistant species of Staph aureus and the implications associated with prolonged disease due to poor bone penetrance of vancomycin.2
With treatment resistant pathogens arises the possibility of prolonged infection and continued destruction of the vertebral end plates and disc, which can result in progressive spinal deformity and poor functional outcomes.1,4,56,57,59 Among historical series, in which tuberculosis accounted for a substantial portion of spondylodiscitis cases, as many as 61% of patients developed spinal deformity.2,8 With further osseous and discoligamentous destruction and deformity, kyphosis and worsened focal spinal alignment may result in increased back pain and overall global sagittal malalignment, which has been repeatedly shown to increase disability scores and reduce quality of life.60 Among patients with deformity, and in particular segmental kyphosis, surgery offers an opportunity for debridement, source control, and correction of deformity.
Unfortunately, reporting of functional outcome or return-to-work data among both medical and surgical literature is lacking. In the current review functional outcomes were reported in only 20 studies (13 surgical) and return to work status was reported in only 4 studies.3,13,34,41 Among the included surgical series, 88% of patients were able to return to normal work activity. Surgical patients experienced excellent outcomes in 68% of those reported at follow-up, which is comparable to all patients where an excellent outcome was achieved in 73% of patients. Pain outcomes and VAS scores for both surgical and medical management were investigated by 2 separate meta-analyses. While the results from the available data suggest improved pain ratings with surgery compared with the medical treatment alone, significant heterogeneity (I 2 = 56%, P = .13; I 2 = 0%, P = .43) was detected in both studies preventing definitive determination of the influence of surgery on pain outcomes. For both analyses, these results can be attributed to the small sample sizes and it must be noted that lack of control of treatment strategy (operative vs nonoperative) and surgical procedure performed further obscures interpretation of outcome data.
The reports by Nasto et al35 and Tsai et al47 suggest a more rapid recovery and improvement in functional scores following surgery when compared with medical treatment alone, but with diminishing significance over time. These results suggest that surgery may have a role in enhancing patient’s short-term recovery but may be unlikely to change long-term results as most patients will have substantial improvement in back pain regardless of treatment modality. Lemaignen et al30 also demonstrated that surgery was protective of functional outcome on univariate analysis but not on multivariate analysis. These findings support the need for additional research to delineate what role surgery may have in improving functional outcomes.
This study is also limited by the availability of pooled data from mostly retrospective studies, each with their own inherent biases and a general lack of uniformity in reporting of outcomes. The lack of consistent reporting of outcomes for surgical and medical series, as well as for particular surgical procedures (instrumentation vs decompression alone), limits our ability to perform further meta-analysis or subgroup analysis. Furthermore, the retrospective case series may suffer from selection bias as patients who were treated surgically tended to be those who suffered intractable back pain, neurologic deficit or progressive spinal deformity, and thus outcome comparison between medical and surgical treatments may be inherently biased. Furthermore, the limitation of the search to English may lead to reporting bias. No randomized studies were identified in this literature review comparing surgical and medical therapies. Future prospective studies limiting comparison of surgical and medical patients to those with similar clinical features may reduce errors due to selection bias and provide further insight into clinical outcomes.
Conclusions
Spondylodiscitis is a disease with increasing prevalence that can result in poor functional outcome and progressive spinal deformity. According to this study, most patients with spondylodiscitis present with back pain. MRI and bone scan are the most sensitive imaging modalities for detecting infectious spondylodiscitis, and Staph aureus is the most common organism isolated from cultures. Medical treatment is often first-line management for infectious discitis, but surgery may be indicated in cases of significant or worsening neurologic deficit, recalcitrant infections, or progressive spinal deformity. Functional outcomes suggest surgery may result in a greater reduction in pain compared to medical treatment alone, but substantial patient heterogeneity prevents definitive conclusions. Further, this meta-analysis included retrospective studies, and so its conclusions should be interpreted with caution. Direct comparison in prospective trials is needed to reduce selection bias and definitively determine the appropriate treatment algorithm.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: James Harrop, MD, receives speaking honorarium from Globus and is a consultant for Ethicon. Christopher I. Shaffrey, MD, is a consultant for Medtronic, Nuvasive, Zimmer Biomet, K2M, Stryker, and In Vivo; receives royalties from Medtronic, Nuvasive, and Zimmer Biomet; has patents with Medtronic, Nuvasive, and Zimmer Biomet; is a stockholder in Nuvasive; and receives grants from the NIH, Department of Defense, ISSG, DePuy Synthes, and AO. Justin S. Smith, MD, PhD, is a consultant for Zimmer Biomet, Nuvasive, K2M, Allsource and a past consultant for Cerapedics; receives honorarium for teaching from Zimmer Biomet, Nuvasive, and K2M; receives royalties from Zimmer Biomet; receives research study group support from DePuy Synthes/ISSG; and receives fellowship funding from NREF and AOSpine.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This Supplement was supported by funding from AOSpine North America.
References
- 1. Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. [DOI] [PubMed] [Google Scholar]
- 2. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–412. [DOI] [PubMed] [Google Scholar]
- 3. Lu ML, Niu CC, Tsai TT, Fu TS, Chen LH, Chen WJ. Transforaminal lumbar interbody debridement and fusion for the treatment of infective spondylodiscitis in the lumbar spine. Eur Spine J. 2015;24:555–560. [DOI] [PubMed] [Google Scholar]
- 4. Batirel A, Erdem H, Sengoz G, et al. The course of spinal tuberculosis (Pott disease): results of the multinational, multicentre Backbone-2 study. Clin Microbiol Infect. 2015;21:1008e9–1008.e18. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 6. Aagaard T, Roed C, Dragsted C, Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006-2011. Scand J Infect Dis. 2013;45:417–424. [DOI] [PubMed] [Google Scholar]
- 7. Asamoto S, Doi H, Kobayashi N, et al. Spondylodiscitis: diagnosis and treatment. Surg Neurol. 2005;64:103–108. [DOI] [PubMed] [Google Scholar]
- 8. Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–882. [DOI] [PubMed] [Google Scholar]
- 9. Bettini N, Girardo M, Dema E, Cervellati S. Evaluation of conservative treatment of non-specific spondylodiscitis. Eur Spine J. 2009;18(suppl 1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976). 2006;31:2695–2700. [DOI] [PubMed] [Google Scholar]
- 11. Bydon M, De la Garza-Ramos R, Macki M, et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 2014;82:e807–e814. [DOI] [PubMed] [Google Scholar]
- 12. Cebrián Parra JL, Saez-Arenillas Martin A, Urda Martinez-Aedo AL, Soler Ivañez I, Agreda E, Lopez-Duran Stern L. Management of infectious discitis. Outcome in one hundred and eight patients in a university hospital. Int Orthop. 2012;36:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung TC, Yang SC, Chen HS, Kao YH, Tu YK, Chen WJ. Single-stage anterior debridement and fibular allograft implantation followed by posterior instrumentation for complicated infectious spondylitis: report of 20 cases and review of the literature. Medicine (Baltimore). 2014;93:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Citak M, Backhaus M, Kälicke T, Hilal Z, Muhr G, Frangen TM. Myths and facts of spondylodiscitis: an analysis of 183 cases. Acta Orthop Belg. 2011;77:535–538. [PubMed] [Google Scholar]
- 15. D’Agostino C, Scorzolini L, Massetti AP, et al. A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection. 2010;38:102–107. [DOI] [PubMed] [Google Scholar]
- 16. D’Aliberti G, Talamonti G, Villa F, Debernardi A. The anterior stand-alone approach (ASAA) during the acute phase of spondylodiscitis: results in 40 consecutively treated patients. Eur Spine J. 2012;21(suppl 1):S75–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hey HWD, Ng LWN, Tan CS, et al. Spinal implants can be inserted in patients with deep spine infection: results from a large cohort study. Spine (Phila Pa 1976). 2017;42:E490–E495. [DOI] [PubMed] [Google Scholar]
- 18. Eysel P, Hopf C, Vogel I, Rompe JD. Primary stable anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis? Results of a comparative study. Eur Spine J. 1997;6:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ha KY, Shin JH, Kim KW, Na KH. The fate of anterior autogenous bone graft after anterior radical surgery with or without posterior instrumentation in the treatment of pyogenic lumbar spondylodiscitis. Spine (Phila Pa 1976). 2007;32:1856–1864. [DOI] [PubMed] [Google Scholar]
- 20. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000;25:1668–1679. [DOI] [PubMed] [Google Scholar]
- 21. Heyde CE, Boehm H, El Saghir H, Tschöke SK, Kayser R. Surgical treatment of spondylodiscitis in the cervical spine: a minimum 2-year follow-up. Eur Spine J. 2006;15:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Homagk L, Homagk N, Klauss JR, Roehl K, Hofmann GO, Marmelstein D. Spondylodiscitis severity code: scoring system for the classification and treatment of non-specific spondylodiscitis. Eur Spine J. 2016;25:1012–1020. [DOI] [PubMed] [Google Scholar]
- 23. Hopf C, Meurer A, Eysel P, Rompe JD. Operative treatment of spondylodiscitis—what is the most effective approach? Neurosurg Rev. 1998;21:217–225. [DOI] [PubMed] [Google Scholar]
- 24. Karadimas EJ, Bunger C, Lindblad BE, et al. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008;79:650–659. [DOI] [PubMed] [Google Scholar]
- 25. Kaya S, Ercan S, Kaya S, et al. Spondylodiscitis: evaluation of patients in a tertiary hospital. J Infect Dev Ctries. 2014;8:1272–1276. [DOI] [PubMed] [Google Scholar]
- 26. Kehrer M, Pedersen C, Jensen TG, Hallas J, Lassen AT. Increased short- and long-term mortality among patients with infectious spondylodiscitis compared with a reference population. Spine J. 2015;15:1233–1240. [DOI] [PubMed] [Google Scholar]
- 27. Klockner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976). 2003;28:1036–1042. [DOI] [PubMed] [Google Scholar]
- 28. Krödel A, Stürz H, Siebert CH. Indications for and results of operative treatment of spondylitis and spondylodiscitis. Arch Orthop Trauma Surg. 1991;110:78–82. [DOI] [PubMed] [Google Scholar]
- 29. Legrand E, Flipo RM, Guggenbuhl P, et al. Management of nontuberculous infectious discitis. Treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68:504–509. [DOI] [PubMed] [Google Scholar]
- 30. Lemaignen A, Ghout I, Dinh A, et al. Characteristics of and risk factors for severe neurological deficit in patients with pyogenic vertebral osteomyelitis: a case-control study. Medicine (Baltimore). 2017;96:e6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin CP, Ma HL, Wang ST, Liu CL, Yu WK, Chang MC. Surgical results of long posterior fixation with short fusion in the treatment of pyogenic spondylodiscitis of the thoracic and lumbar spine: a retrospective study. Spine (Phila Pa 1976). 2012;37:E1572–E1579. [DOI] [PubMed] [Google Scholar]
- 32. Lin TY, Tsai TT, Lu ML, et al. Comparison of two-stage open versus percutaneous pedicle screw fixation in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord. 2014;15:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin Y, Li F, Chen W, Zeng H, Chen A, Xiong W. Single-level lumbar pyogenic spondylodiscitis treated with mini-open anterior debridement and fusion in combination with posterior percutaneous fixation via a modified anterior lumbar interbody fusion approach. J Neurosurg Spine. 2015;23:747–753. [DOI] [PubMed] [Google Scholar]
- 34. Mann S, Schütze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus. 2004;17:E3. [DOI] [PubMed] [Google Scholar]
- 35. Nasto LA, Colangelo D, Mazzotta V, et al. Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. Spine J. 2014;14:1139–1146. [DOI] [PubMed] [Google Scholar]
- 36. Noh SH, Zhang HY, Lim HS, Song HJ, Yang KH. Decompression alone versus fusion for pyogenic spondylodiscitis. Spine J. 2017;17:1120–1126. [DOI] [PubMed] [Google Scholar]
- 37. Pee YH, Park JD, Choi YG, Lee SH. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine. 2008;8:405–412. [DOI] [PubMed] [Google Scholar]
- 38. Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. [DOI] [PubMed] [Google Scholar]
- 39. Schinkel C, Gottwald M, Andress HJ. Surgical treatment of spondylodiscitis. Surg Infect (Larchmt). 2003;4:387–391. [DOI] [PubMed] [Google Scholar]
- 40. Schomacher M, Finger T, Koeppen D, et al. Application of titanium and polyetheretherketone cages in the treatment of pyogenic spondylodiscitis. Clin Neurol Neurosurg. 2014;127:65–70. [DOI] [PubMed] [Google Scholar]
- 41. Shetty AP, Aiyer SN, Kanna RM, Maheswaran A, Rajasekaran S. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. Int Orthop. 2016;40:1163–1170. [DOI] [PubMed] [Google Scholar]
- 42. Shiban E, Janssen I, Wostrack M, et al. A retrospective study of 113 consecutive cases of surgically treated spondylodiscitis patients. A single-center experience. Acta Neurochir (Wien). 2014;156:1189–1196. [DOI] [PubMed] [Google Scholar]
- 43. Shousha M, Boehm H. Surgical treatment of cervical spondylodiscitis: a review of 30 consecutive patients. Spine (Phila Pa 1976). 2012;37:E30–E36. [DOI] [PubMed] [Google Scholar]
- 44. Sobottke R, Zarghooni K, Krengel M, et al. Treatment of spondylodiscitis in human immunodeficiency virus-infected patients: a comparison of conservative and operative therapy. Spine (Phila Pa 1976). 2009;34:E452–E458. [DOI] [PubMed] [Google Scholar]
- 45. Srinivasan D, Terman SW, Himedan M, Dugo D, La Marca F, Park P. Risk factors for the development of deformity in patients with spinal infection. Neurosurg Focus. 2014;37:E2. [DOI] [PubMed] [Google Scholar]
- 46. Suess O, Weise L, Brock M, Kombos T. Debridement and spinal instrumentation as a single-stage procedure in bacterial spondylitis/spondylodiscitis. Zentralbl Neurochir. 2007;68:123–132. [DOI] [PubMed] [Google Scholar]
- 47. Tsai TT, Yang SC, Niu CC, et al. Early surgery with antibiotics treatment had better clinical outcomes than antibiotics treatment alone in patients with pyogenic spondylodiscitis: a retrospective cohort study. BMC Musculoskelet Disord. 2017;18:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valancius K, Hansen ES, Høy K, Helmig P, Niedermann B, Bünger C. Failure modes in conservative and surgical management of infectious spondylodiscitis. Eur Spine J. 2013;22:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Včelák J, Chomiak J, Toth L. Surgical treatment of lumbar spondylodiscitis: a comparison of two methods. Int Orthop. 2014;38:1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wirtz DC, Genius I, Wildberger JE, Adam G, Zilkens KW, Niethard FU. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis—an evaluation of 59 cases. Arch Orthop Trauma Surg. 2000;120:245–251. [DOI] [PubMed] [Google Scholar]
- 51. Yaldz C, Özdemir N, Yaman O, Feran HG, Tansug T, Minoglu M. A retrospective study of 39 patients treated with anterior approach of thoracic and lumbar spondylodiscitis: clinical manifestations, anterior surgical treatment, and outcome. Medicine (Baltimore). 2015;94:e2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ziu M, Dengler B, Cordell D, Bartanusz V. Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users. Neurosurg Focus. 2014;37:E3. [DOI] [PubMed] [Google Scholar]
- 53. Ascione T, Balato G, Di Donato SL, et al. Clinical and microbiological outcomes in haematogenous spondylodiscitis treated conservatively. Eur Spine J. 2017;26(suppl 4):489–495. [DOI] [PubMed] [Google Scholar]
- 54. Viale P, Furlanut M, Scudeller L, et al. Treatment of pyogenic (non-tuberculous) spondylodiscitis with tailored high-dose levofloxacin plus rifampicin. Int J Antimicrob Agents. 2009;33:379–382. [DOI] [PubMed] [Google Scholar]
- 55. Baker AS, Ojemann RG, Swartz MN, Richardson EP., Jr Spinal epidural abscess. N Engl J Med. 1975;293:463–468. [DOI] [PubMed] [Google Scholar]
- 56. Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133–139. [DOI] [PubMed] [Google Scholar]
- 57. Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. [DOI] [PubMed] [Google Scholar]
- 58. Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50:777–798. [DOI] [PubMed] [Google Scholar]
- 59. Colmenero JD, Jiménez-Mejias ME, Sánchez-Lora FJ, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blondel B, Schwab F, Ungar B, et al. Impact of magnitude and percentage of global sagittal plane correction on health-related quality of life at 2-years follow-up. Neurosurgery. 2012;71:341–348. [DOI] [PubMed] [Google Scholar]