Abstract
Two of the most popular positron emission tomography (PET) tracers, [11C]PE2I and [18F]FE-PE2I, used to quantify dopamine transporters (DAT), display dissimilar kinetic behavior in in vivo assays. This difference can be explained by comparing values of kinetic rate constants, which characterize interaction of these tracers with DAT sites in vitro. At the same time, this kinetic analysis showed that the overall binding mechanism is similar for these two tracers and includes a fast step of complex formation followed by a slow isomerization step of this complex. Comparison with previous PE2I data revealed that isomerization of the DAT complex with PE2I occurs three times faster than in the case of FE-PE2I, which leads to the slower onset of peak specific binding of the former tracer in the DAT-rich regions. Therefore, ligands with slower isomerization on-rate, including [18F]FE-PE2I, seem to be better tracers in vivo, and their properties can be predicted in vitro.
Keywords: Dopamine transporter, ligand binding kinetics, DAT, PET, PE2I, FE-PE2I, isomerization
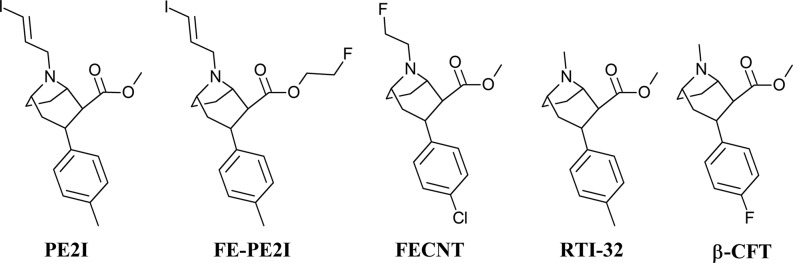
Among 11C-labeled radiotracers used for the visualization of dopamine transporter (DAT) in the brain in positron emission tomography (PET), [11C]PE2I (N-(3-iodoprop-2E-enyl)-2β-carbo[11C]methoxy-3β-(4-methylphenyl)nortropane, Scheme 1) has been widely used in diagnostic studies and neurodegenerative disease research.1−3 Other less frequent DAT imaging agents include [18F]FECNT,4 [11C]RTI-32,5 and [18F]CFT6 (Scheme 1), all of which share a bicyclic tropane core. Despite the good selectivity and high-binding affinity of [11C]PE2I,1 some limitations in its applicability were observed. These limitations are related to the slow kinetics and late peak formation in in vivo assays. These delays necessitate an imaging process of 70 min or more for accuracy,7 which is inconvenient for patients. Due to prolonged imaging time, more radioactive metabolites are formed.8 Therefore, a fluorine-labeled analogue of PE2I, [18F]FE-PE2I (N-(3-iodoprop-2E-enyl)-2β-carbo[18F]fluoroethoxy-3β-(4-methylphenyl)-nortropane, Scheme 1) was designed9,10 and tested in rodents,11 nonhuman primates,9,12 and humans.13 This ligand has a similar DAT affinity as PE2I, but it demonstrates faster in vivo kinetics, is therefore less metabolized, and appears slightly more selective.14 In addition, the possibility to label it with fluorine-18, an isotope with longer half-life than carbon-11, has made [18F]FE-PE2I an alternative tracer for DAT localization in the brain.14
Scheme 1. Structures of PE2I, FE-PE2I, FECNT, RTI-32, and β-CFT.
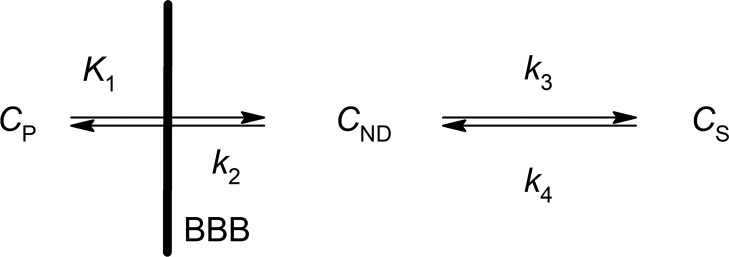
The different behaviors of these structurally similar radioligands, [11C]PE2I and [18F]FE-PE2I, were clearly demonstrated by the kinetic analysis of DAT quantification in the brain using the two-tissue compartment model (2-TCM) shown in Scheme 2.15 In this model, CP represents the tracer concentration in plasma, CND stands for the free and nonspecifically bound tracer in tissue (nondisplaceable compartment), and CS is the tracer specifically bound to the target site in the same tissue.
Scheme 2. 2-TCM Used To Describe the Binding of Radiotracers in Brain.
Analysis found that the parameter k3 differed significantly for these two tracers ([11C]PE2I and [18F]FE-PE2I) in DAT-rich brain regions such as the caudate and putamen, characterized by values 1.04 ± 0.77 and 0.70 ± 0.45 min–1 for [11C]PE2I, and 0.28 ± 0.12 and 0.24 ± 0.18 min–1 for [18F]FE-PE2I.14 At the same time, the k4 values were very low and rather similar for both ligands, ranging from 0.01 to 0.04 min–1.14
These differences observed in in vivo experiments cannot be explained by binding of PE2I and FE-PE2I to other monoamine transporters and dopamine receptors in brain, as both ligands display superior affinity and selectivity for DAT sites.9,16 The dissimilarities between the two tracers also cannot be explained by the formation of radioactive metabolites, as the main degradation products of [11C]PE2I and [18F]FE-PE2I in vivo are rather similar polar compounds,17,18 i.e., the metabolites differ only by the fluoromethylene moiety, which have lower affinity than their parent compounds since DAT binding site is sensitive to polar groups. Also, the kinetics of the formation of main metabolites of the two tracers is almost identical, e.g., the oxidation of benzylic carbon, which produces the most interfering metabolites is very little if at all affected by the difference in ester substituent.14 Consequently, the off-target activity and metabolism cannot explain the different in vivo time curves when comparing [11C]PE2I and [18F]FE-PE2I.
Following these considerations, we suggest that the different pharmacokinetic behavior of [11C]PE2I and [18F]FE-PE2I in brain could be related to kinetic differences of their interaction with DAT sites, which can also be seen in in vitro experiments. To demonstrate this, we studied kinetics of FE-PE2I interaction with DAT sites in vitro and compared these results with kinetic data for PE2I interaction with DAT sites that were thoroughly studied previously.19,20 A well-established method, developed to study the binding kinetics of ligands to various membrane proteins, were used in these experiments.21,22
Previous studies with PE2I revealed that two kinetically distinct steps can be differentiated in the overall ligand binding process according to Scheme 3.
Scheme 3. Two-Step Binding of PE2I to DAT.
FE-PE2I displays a similar mechanism.
In Scheme 3, PE2I-DAT stands for the rapidly forming complex in true equilibrium. This fast step is followed by the slow step of the PE2I-DAT* complex isomerization, and the proposed methods of kinetic analysis allow for the determination of the parameters KL, ki, and k–i.20 The presence of the slow, complex isomerization process significantly shifts the equilibrium between the free and ligand-bound DAT sites. Therefore, it is possible that this process also affects tracer trafficking between compartments CND and CS in Scheme 2.
In this Letter, we investigated the kinetic mechanism of the FE-PE2I interaction with DAT sites in vitro, and these results revealed that the fluoroethyl derivative of PE2I can also induce the isomerization step; however, the minor change of the ligand structure affects, indeed, the ligand binding kinetics and may be responsible for different peak formation time in the case of these tracers.
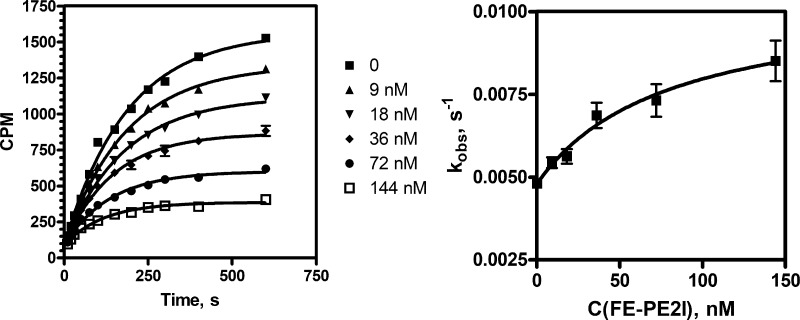
Kinetic experiments were made to investigate the mechanism of FE-PE2I interaction with DAT sites in vitro, and the results were compared to the interaction of this tracer with DAT sites in brain. The kinetic curves that characterize FE-PE2I binding are shown in Figure 1 (left panel), and the pseudo first order observed rate constants kobs were calculated at different ligand concentrations. As the values of these rate constants increase hyperbolically with increasing ligand concentration (Figure 1, right panel), it can be concluded that FE-PE2I interaction with DAT follows the reaction mechanism shown in Scheme 3, as this ligand induces the slow isomerization step, characterized by rate constants ki and k–i.21 Principles of this experimental approach were described in detail in our previous works,20 and the results obtained were used for calculation of kinetic parameters KL, ki, and k–i for FE-PE2I interaction with DAT by using eqs 2 and 3 (see Supporting Information).
Figure 1.
Influence of various concentrations of FE-PE2I on the kinetics of 3 nM [3H]PE2I binding to DAT (left); observed rate constant versus FE-PE2I concentration plot (right).
The results of this kinetic analysis are listed in Table 1, together with similar data from our previous kinetic study made with PE2I. Consequently, the overall kinetic mechanism of interaction of FE-PE2I and PE2I with DAT is analogous; however, the process is described by different kinetic parameters.
Table 1. Equilibrium and Kinetic Constants of PE2I and FE-PE2I Binding to DAT.
| compd | Ki, nM | KL, nM | ki, min–1 | k–i, min–1 | Kisom |
|---|---|---|---|---|---|
| FE-PE2I | 23 ± 3 | 79 ± 33 | 0.34 ± 0.06 | 0.15 ± 0.09 | 0.44 |
| PE2Ia | 5 ± 1 | 37 ± 24 | 1.2 ± 0.5 | 0.14 ± 0.05 | 0.12 |
Data from ref (20).
The most important feature of this kinetic mechanism is the presence of the slow isomerization step, quantified by rate constants ki and k–i.21 This additional step also functions as an equilibrium process in which the monomolecular rate constants ki and k–i allow us to calculate the equilibrium constant of isomerization Kisom = k–i/ki (Table 1). However, differing from the first equilibrium binding step that can be shifted by changing the ligand concentration, the isomerization step is a monomolecular equilibrium, and the half-life of the process leading to this equilibrium state remains independent of ligand concentration. This half-life is determined by the slowest step of a reversible process that is characterized by the off-rate constant k–i, which is similar in both PE2I and FE-PE2I and therefore remains below 5 min for both ligands in vitro.
The presence of the isomerization step also changes the physical meaning of the overall ligand affinity, which is observed by conventional binding or displacement studies and characterized by the Ki value. In the case of ligands, which do not initiate the slow isomerization step, the parameters Ki and KL should have the same value and meaning because the isomerized complex L-DAT* is absent (Scheme 3). However, when the isomerization step is present, the apparent affinity of parameter Ki has a more complex meaning
| 1 |
and Ki is a function of the isomerization equilibrium constant Kisom.20 As the Ki values for FE-PE2I and PE2I in Table 1 were determined experimentally by the conventional ligand displacement method under true equilibrium conditions, these values agree with the observed differences of Kisom values. The large relative errors of ki and KL in Table 1 can be attributed to this indirect method of quantification, and the kinetic parameters from direct measurement of [3H]PE2I binding kinetics20 support the PE2I data.
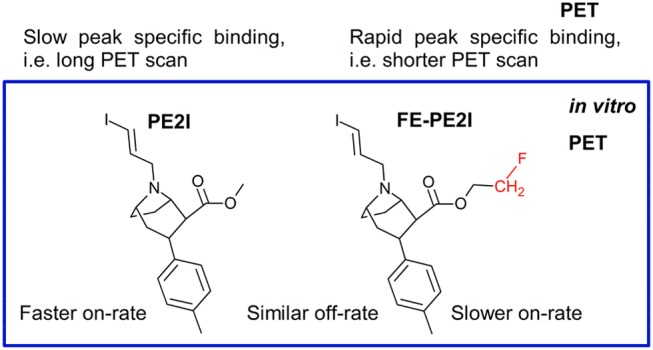
It is important to emphasize that the in vitro kinetic on-rate constants (ki) of FE-PE2I and PE2I coincide with the respectable in vivo rate constants (k3) from PET experiments (Schemes 2 and 3).14 In addition, the off-rate of both ligands in vivo is the same, as in our in vitro data. It was proposed that the faster in vivo kinetics of FE-PE2I, if compared with PE2I, could be caused by different affinities of these ligands in vivo,12 calculated as the ratio of k4 and k3 in Scheme 2, but instead seems to be related to their differences in on-rate. Since the off-rates of both ligands are the same, the pharmacokinetic difference between the ligands should be caused by their different on-rates. Due to the slower isomerization constant (ki or k3), for FE-PE2I the rate of association to the transporter, relative to its dissociation, is lower than for PE2I. This relative difference corresponds in vivo to more rapid kinetics of dissociation from the DAT for [18F]FE-PE2I as compared with [11C]PE2I. Differences in on-rate, rather than off-rate, determines the contribution of kinetic effect as in the case of other cocaine derivatives,23 which is often neglected in drug discovery.24
In PET, this phenomenon is more pronounced in DAT-rich regions, where the dissociating PE2I from one DAT complex binds rapidly to another DAT, resulting in a cascade of association and dissociation steps before being washed out of the receptor-rich regions. The association of FE-PE2I with DAT is much weaker, this tracer diffuses more rapidly back to the blood. The main reason for this difference is that the concentration of the tracer is exceptionally low compared to the concentration of the receptor in PET experiments, which contrasts with standard in vitro conditions. Therefore, the receptors compete with one another to bind the tightly bound ligand. This difference explains why the peak specific binding of [11C]PE2I is 70 min and that [18F]FE-PE2I is between 20 and 40 min in PET runs.14 Comparison of time–activity curves of both tracers in DAT rich regions show how different the behaviors of these tracers in vivo are, while binding to a region with low DAT concentration is almost identical. This suggest that the difference must be caused by the specific binding to DAT and that their kinetic constants need to be different. Target occupancy is directly dependent on the kinetic rate constants of association and dissociation, while ligand potency alone cannot describe the pharmacokinetics.25
Carbon-11 tracers typically have higher specific activity than their fluorine-18 analogues, as is the case with tropane derivatives, where the produced [11C]PE2I has approximately 40% higher specific activity than [18F]FE-PE2I.14 Due to the relatively low density of transporters in brain, the radiotracer should have as high specific activity as possible since increased amount of injected material can perturb the dopaminergic system. Therefore, in some instances [11C]PE2I is advantageous over [18F]FE-PE2I in spite of its less favorable DAT binding kinetics.
In summary, it is possible that the kinetic parameters, which characterize the ligand–DAT interaction in vitro could indeed model the pharmacodynamic behavior of PET tracers in vivo. In other words, ligands with fast on-rate and slow off-rate occupy the target-rich region for a longer time before being washed out. It is also important to recapitulate that the isomerization equilibrium is independent of ligand concentration; therefore, the ligand trafficking into the slowly dissociating state (or isomerized state) cannot be controlled by tracer concentration in CNS and CP compartments. However, the Kisom value also affects the value of the conventional parameter Ki. Therefore, it is not surprising that several ligands, characterized by high affinity and very low Ki values, were discarded as potential PET tracers.26
This study demonstrates that in vitro ligand binding kinetic experiments can be used to assess the pharmacokinetic properties of imaging agents in PET analysis. The formation of stable complexes between the tracer and its target, characterized by rapid on-rate and slow off-rate, translates to a long onset of peak specific binding in target-rich regions that results in long PET scans for robust outputs. Conversely, if a tracer complex dissociates too rapidly, the potency of the tracer is lost. Therefore, a compromise between kinetic parameters of the off-rate and on-rate is needed for optimal PET performance. Therefore, based on the results of this Letter, we can suggest that rapid on-rate (or slow off-rate) could be a common reason, why several promising ligands have been dismissed from clinical use due to the late peak formation in in vivo assays. Ligand binding kinetics could be the main culprit of tracer failure, where the initial explanation has been high affinity,27 affinity difference between in vitro and in vivo,26,28 or slow brain kinetics.28,29 Application of in vitro kinetic experiments may be an important tool for indicative estimation of the molecular imaging applicability of tracers before costly in vivo experiments.
Glossary
ABBREVIATIONS
- 2-TCM
two-tissue compartment model
- CND
nondisplaceable compartment
- CP
concentration in plasma
- CPM
counts per minute
- CS
concentration of target-bound radioligand
- DAT
dopamine transporter
- FE-PE2I
(N-(3-iodoprop-2E-enyl)-2β-carbofluoroethoxy-3β-(4-methylphenyl)nortropane
- Ki
inhibition constant
- ki
on-rate constant of isomerized ligand–receptor complex
- k–i
off-rate rate constant of isomerized ligand–receptor complex
- Kisom
dissociation constant of isomerization
- KL
equilibrium binding constant
- kobs
observed pseudo-first-order rate constant
- PE2I
N-(3-iodoprop-(2E)-enyl)-2β-carbomethoxy-3β-(4′-iodophenyl)nortropane
- RTI
Research Triangle Institute
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00504.
Experimental procedures of ligand binding kinetics assay (PDF)
Author Contributions
S.K. performed the equilibrium and kinetic assays. S.K. and J.J. designed the kinetic experiments and wrote the manuscript. S.K., O.L., and J.H. were involved with the synthesis of PE2I and FE-PE2I.
This work was funded by Grant IUT20–15 from the Estonian Ministry of Education and Research.
The authors declare no competing financial interest.
Supplementary Material
References
- Emond P.; Garreau L.; Chalon S.; Boazi M.; Caillet M.; Bricard J.; Frangin Y.; Mauclaire L.; Besnard J.; Guilloteau D. Synthesis and Ligand Binding of Nortropane Derivatives: N-Substituted 2β-Carbomethoxy-3β-(4‘-Iodophenyl)Nortropane and N-(3-Iodoprop-(2E)-Enyl)-2β-Carbomethoxy-3β-(3‘,4‘-Disubstituted Phenyl)Nortropane. New High-Affinity and Selective Compounds for the Do. J. Med. Chem. 1997, 2623 (96), 1366–1372. 10.1021/jm960795d. [DOI] [PubMed] [Google Scholar]
- Garreau L.; Emond P.; Belzung C.; Guilloteau D.; Frangin Y.; Besnard J.-C.; Chalon S. N-(3-Iodoprop-2E-Enyl)-2β-Carbomethoxy-3β-(3′,4′-Dichlorophenyl)Nortropane (β-CDIT), a Tropane Derivative: Pharmacological Characterization as a Specific Ligand for the Dopamine Transporter in the Rodent Brain. J. Pharmacol. Exp. Ther. 1997, 282 (1), 467–474. [PubMed] [Google Scholar]
- Halldin C.; Erixon-Lindroth N.; Pauli S.; Chou Y.-H.; Okubo Y.; Karlsson P.; Lundkvist C.; Olsson H.; Guilloteau D.; Emond P.; et al. [11C]PE2I: A Highly Selective Radioligand for PET Examination of the Dopamine Transporter in Monkey and Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2003, 30 (9), 1220–1230. 10.1007/s00259-003-1212-3. [DOI] [PubMed] [Google Scholar]
- Goodman M. M.; Keil R.; Shoup T. M.; Eshima D.; Eshima L.; Kilts C.; Votaw J.; Camp V. M.; Votaw D.; Smith E.; et al. Fluorine-18-FPCT: A PET Radiotracer for Imaging Dopamine Transporters. J. Nucl. Med. 1997, 38, 119–126. [PubMed] [Google Scholar]
- Meyer J. H.; Krüger S.; Wilson A. A.; Christensen B. K.; Goulding V. S.; Schaffer A.; Minifie C.; Houle S.; Hussey D.; Kennedy S. H. Lower Dopamine Transporter Binding Potential in Striatum during Depression. NeuroReport 2001, 12 (18), 4121–4125. 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Laakso A.; Bergman J.; Haaparanta M.; Vilkman H.; Solin O.; Hietala J. [18F]CFT ([18F]WIN 35,428), a Radioligand to Study the Dopamine Transporter with PET: Characterization in Human Subjects. Synapse 1998, 28 (3), 244–250. . [DOI] [PubMed] [Google Scholar]
- Hirvonen J.; Johansson J.; Teräs M.; Oikonen V.; Lumme V.; Virsu P.; Roivainen A.; Någren K.; Halldin C.; Farde L.; et al. Measurement of Striatal and Extrastriatal Dopamine Transporter Binding with High-Resolution PET and [11C]PE2I: Quantitative Modeling and Test–retest Reproducibility. J. Cereb. Blood Flow Metab. 2008, 28, 1059–1069. 10.1038/sj.jcbfm.9600607. [DOI] [PubMed] [Google Scholar]
- Shetty H. U.; Zoghbi S. S.; Liow J.-S.; Ichise M.; Hong J.; Musachio J. L.; Halldin C.; Seidel J.; Innis R. B.; Pike V. W. Identification and Regional Distribution in Rat Brain of Radiometabolites of the Dopamine Transporter PET Radioligand [11C]PE2I. Eur. J. Nucl. Med. Mol. Imaging 2007, 34 (5), 667–678. 10.1007/s00259-006-0277-1. [DOI] [PubMed] [Google Scholar]
- Schou M.; Steiger C.; Varrone A.; Guilloteau D.; Halldin C. Synthesis, Radiolabeling and Preliminary in Vivo Evaluation of [18F]FE-PE2I, a New Probe for the Dopamine Transporter. Bioorg. Med. Chem. Lett. 2009, 19 (16), 4843–4845. 10.1016/j.bmcl.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Stepanov V.; Krasikova R.; Raus L.; Loog O.; Hiltunen J.; Halldin C. An Efficient One-Step Radiosynthesis of [18F]FE-PE2I, a PET Radioligand for Imaging of Dopamine Transporters. J. Labelled Compd. Radiopharm. 2012, 55 (6), 206–210. 10.1002/jlcr.2927. [DOI] [Google Scholar]
- Bang J.-I.; Jung I. S.; Song Y. S.; Park H. S.; Moon B. S.; Lee B. C.; Kim S. E. PET Imaging of Dopamine Transporters with [18F]FE-PE2I: Effects of Anti-Parkinsonian Drugs. Nucl. Med. Biol. 2016, 43 (2), 158–164. 10.1016/j.nucmedbio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Varrone A.; Steiger C.; Schou M.; Takano A.; Finnema S. J.; Guilloteau D.; Gulyás B.; Halldin C. In Vitro Autoradiography and in Vivo Evaluation in Cynomolgus Monkey of [18F]FE-PE2I, a New Dopamine Transporter PET Radioligand. Synapse 2009, 63 (10), 871–880. 10.1002/syn.20670. [DOI] [PubMed] [Google Scholar]
- Lizana H.; Johansson L.; Axelsson J. E.; Larsson Strömvall A.; Ögren M.; Linder J.; Halldin C.; Varrone A.; Mo S. J. Whole-Body Biodistribution and Dosimetry of the Dopamine Transporter Radio Ligand 18F-FE-PE2I in Human Subjects. J. Nucl. Med. 2018, 117, 1275–1280. 10.2967/jnumed.117.197186. [DOI] [PubMed] [Google Scholar]
- Varrone A.; Toth M.; Steiger C.; Takano A.; Guilloteau D.; Ichise M.; Gulyas B.; Halldin C. Kinetic Analysis and Quantification of the Dopamine Transporter in the Nonhuman Primate Brain with 11C-PE2I and 18F-FE-PE2I. J. Nucl. Med. 2011, 52 (1), 132–139. 10.2967/jnumed.110.077651. [DOI] [PubMed] [Google Scholar]
- Gunn R. N.; Gunn S. R.; Cunningham V. J. Positron Emission Tomography Compartmental Models. J. Cereb. Blood Flow Metab. 2001, 21 (6), 635–652. 10.1097/00004647-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Emond P.; Guilloteau D.; Chalon S. PE2I: A Radiopharmaceutical for in Vivo Exploration of the Dopamine Transporter. CNS Neurosci. Ther. 2008, 14 (1), 47–64. 10.1111/j.1755-5949.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty H. U.; Zoghbi S. S.; Liow J. S.; Ichise M.; Hong J.; Musachio J. L.; Halldin C.; Seidel J.; Innis R. B.; Pike V. W. Identification and Regional Distribution in Rat Brain of Radiometabolites of the Dopamine Transporter PET Radioligand [11C]PE2I. Eur. J. Nucl. Med. Mol. Imaging 2007, 34 (5), 667–678. 10.1007/s00259-006-0277-1. [DOI] [PubMed] [Google Scholar]
- Amini N.; Nakao R.; Schou M.; Halldin C. Identification of PET Radiometabolites by Cytochrome P450, UHPLC/Q-ToF-MS and Fast Radio-LC: Applied to the PET Radioligands [11C]Flumazenil, [18F]FE-PE2I, and [11C]PBR28. Anal. Bioanal. Chem. 2013, 405 (4), 1303–1310. 10.1007/s00216-012-6541-2. [DOI] [PubMed] [Google Scholar]
- Stepanov V.; Järv J. Slow Isomerization Step in the Interaction between Mouse Dopamine Transporter and Dopamine Re-Uptake Inhibitor N-(3-Iodoprop-2E-Enyl)-2β-Carbo-[3H]Methoxy-3β-(4′-Methylphenyl)Nortropane. Neurosci. Lett. 2006, 410 (3), 218–221. 10.1016/j.neulet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kukk S.; Järv J. Differentiating between Drugs with Short and Long Residence Times. MedChemComm 2016, 7 (8), 1654–1656. 10.1039/C6MD00269B. [DOI] [Google Scholar]
- Strickland S.; Palmer G.; Massey V. Determination of Dissociation Constants and Specific Rate Constants of Enzyme-Substrate (or Protein-Ligand) Interactions from Rapid Reaction Kinetic Data. J. Biol. Chem. 1975, 250 (11), 4048–4052. [PubMed] [Google Scholar]
- Tummino P. J.; Copeland R. A. Residence Time of Receptor– Ligand Complexes and Its Effect on Biological Function. Biochemistry 2008, 47 (20), 5481–5492. 10.1021/bi8002023. [DOI] [PubMed] [Google Scholar]
- Kukk S.; Järv J. Small Structural Changes at the N -Position of the Tropane Core Control the Mechanism of Nortropane Derivatives Binding to Dopamine Transporter. ChemistrySelect 2018, 3 (23), 6581–6584. 10.1002/slct.201801532. [DOI] [Google Scholar]
- Folmer R. H. A. Drug Target Residence Time: A Misleading Concept. Drug Discovery Today 2018, 23 (1), 12–16. 10.1016/j.drudis.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Tonge P. J. Drug-Target Kinetics in Drug Discovery. ACS Chem. Neurosci. 2018, 9 (1), 29–39. 10.1021/acschemneuro.7b00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike V. W. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 23 (18), 1818–1869. 10.2174/0929867323666160418114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson H.; Halldin C.; Swahn C. G.; Farde L. Quantification of [11C]FLB 457 Binding to Extrastriatal Dopamine Receptors in the Human Brain. J. Cereb. Blood Flow Metab. 1999, 19 (10), 1164–1173. 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Ashworth S.; Rabiner E. A.; Gunn R. N.; Plisson C.; Wilson A. A.; Comley R. A.; Lai R. Y. K.; Gee A. D.; Laruelle M.; Cunningham V. J. Evaluation of 11 C-GSK189254 as a Novel Radioligand for the H 3 Receptor in Humans Using PET. J. Nucl. Med. 2010, 51, 1021–1029. 10.2967/jnumed.109.071753. [DOI] [PubMed] [Google Scholar]
- Horti A. G.; Villemagne V. L. The Quest for Eldorado: Development of Radioligands for in Vivo Imaging of Nicotinic Acetylcholine Receptors in Human Brain. Curr. Pharm. Des. 2006, 12 (30), 3877–3900. 10.2174/138161206778559605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.