Abstract
The appropriate development and regulation of neuronal morphology are important to establish functional neuronal circuits and enable higher brain function of the central nervous system. R-Ras, a member of the Ras family of small GTPases, plays crucial roles in the regulation of axonal morphology, including outgrowth, branching, and guidance. GTP-bound activated R-Ras reorganizes actin filaments and microtubules through interactions with its downstream effectors, leading to the precise control of axonal morphology. However, little is known about the upstream regulatory mechanisms for R-Ras activation in neurons. In this study, we found that brain-derived neurotrophic factor (BDNF) has a positive effect on endogenous R-Ras activation and promotes R-Ras-mediated axonal growth. RNA interference knockdown and overexpression experiments revealed that RasGRF1, a guanine nucleotide exchange factor (GEF) for R-Ras, is involved in BDNF-induced R-Ras activation and the promotion of axonal growth. Phosphorylation of RasGRF1 by protein kinase A at Ser916/898 is needed for the full activation of its GEF activity and to facilitate Ras signaling. We observed that BDNF treatment markedly increased this phosphorylation. Our results suggest that BDNF is one of the critical extrinsic regulators for R-Ras activation, and that RasGRF1 is an intrinsic key mediator for BDNF-induced R-Ras activation and R-Ras-mediated axonal morphological regulation.
Keywords: Brain-derived neurotrophic factor, R-Ras, RasGRF1, Axon
1. Introduction
Neurons project single axons that carry information to target cells, and they also have a set of multiple branched dendrites that receive signals from other neurons. The morphological structures of highly polarized neurons are essential to form complicated neuronal networks for proper function. Multiple steps of axonal development, including outgrowth, branching, and guidance, are strictly controlled by extrinsic cues and intrinsic signals [1]. Previous studies revealed that Ras and Rho family small GTPases function in the regulation of axonal morphology by controlling the dynamics of the cytoskeleton such as actin filaments and microtubules [2]. It is well known that members of the small GTPases serve as molecular switches of intracellular signaling pathways by cycling between a GDP-bound inactive state and a GTP-bound active state, and only activated forms interact with their downstream effectors, leading to different biological effects.
The Ras superfamily comprises a large group of structurally and functionally related small GTPases [3]. R-Ras-subfamily members, R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3, exhibit relatively high homology and form a distinct branch of the classical Ras subfamily [4]. Extensive studies have clarified the signaling pathways linking activated R-Ras to its downstream effectors. For example, R-Ras is involved in the activation of phosphatidylinositol 3-kinase (PI3K) but not of extracellular signal-regulated kinase (ERK), and in the regulation of integrin activity, which is important for cell-substrate adhesion [3], [5]. We previously reported that R-Ras regulates both actin and microtubule cytoskeletons in neurons during axonal development. We demonstrated that R-Ras is needed for the microtubule-dependent regulation of axonal morphology through PI3K signaling [6]. On the other hand, we recently found that afadin, an actin scaffold protein, is a binding partner for R-Ras, functioning as a regulator of axon branching through F-actin reorganization [7], [8]. Compared with the downstream mechanisms of R-Ras signaling, little is known about the upstream regulators for R-Ras activity in neurons. With regard to the molecular mechanisms of R-Ras inactivation, two major repulsive axon guidance factors, ephrins and semaphorins, inactivate R-Ras to induce axonal repulsion; ephrins inactivate R-Ras activity through tyrosine phosphorylation and p120RasGAP [9], whereas plexins, receptors for semaphorins, directly inactivate R-Ras [10], [11], [12]. However, it remains unclear which extracellular molecules or stimuli activate R-Ras in neurons.
Brain-derived neurotrophic factor (BDNF), one of the major neurotrophic factors, has long been known to play many vital roles in the brain, including in neuronal differentiation, survival, growth, synapse formation, and plasticity [13]. BDNF is abundantly and widely expressed in the mammalian brain, and its expression increases until reaching its maximum level after birth [14]. In neuropsychiatry, BDNF has been associated with several disorders such as depression and bipolar disorder [15], [16]. Therefore, many researchers have been investigating factors that can elevate BDNF levels in animals and humans based on the hypothesis that an increased BDNF level can improve brain health. Regarding neuronal morphological regulation, BDNF plays essential roles in the regulation of axonal differentiation, outgrowth, and branching [17], [18], [19]. However, to our knowledge, there is no report demonstrating the relationship between BDNF and R-Ras in the regulation of axonal morphology.
The RasGRF family, RasGRF1 and RasGRF2, harbor a complex array of structural motifs, including two pleckstrin homology (PH) domains, a coiled-coil and IQ motif, a Dbl homology (DH) domain acting as a catalyzer of GDP/GTP exchange on the Rac1 small GTPase, a Ras exchange motif, and CDC25 Ras exchange domain common to all Ras GEFs [20], [21]. Although RasGRF1 and RasGRF2 have the same domain architecture, RasGRF2 is unable to activate R-Ras due to sequence differences in their catalytic domains, which make RasGRF2 unable to recognize the lipid moieties bound to R-Ras [22]. Thus, we focused only on RasGRF1 as a candidate regulator for R-Ras activation and R-Ras-mediated axonal morphological changes in this study. Functional analyses of RasGRF1 KO mice have demonstrated specific involvement of this protein in memory formation [23], [24]. Moreover, at the cellular level, RasGRF1 was found to be phosphorylated at specific sites by a variety of intracellular kinases in response to several extracellular stimuli. Nerve growth factor (NGF), carbachol, forskolin, serotonin, and NMDA stimulation have been reported to increase phosphorylation of RasGRF1 at Ser916 in the mouse sequence or its equivalent (e.g., Ser898 in rats and Ser927 in humans) [25], [26], [27], [28]. This phosphorylation, which is mediated by protein kinase A (PKA), is necessary for a significant increase in its intrinsic GEF activity against Ras proteins. As for the mechanisms of R-Ras activation, elevation of the intracellular cyclic AMP (cAMP) level is known to induce R-Ras activation [8]. These results gave rise to the hypothesis that BDNF activates R-Ras through RasGRF1 phosphorylation, promoting axonal outgrowth. In the present study, we demonstrated that BDNF treatment triggers endogenous R-Ras activation through PKA-mediated RasGRF1 phosphorylation, which regulates R-Ras-mediated axonal growth in primary cultured rat cortical neurons.
2. Materials and methods
2.1. Plasmids
The glutathione S-transferase (GST)-fused Ras-binding domain (RBD) of c-Raf-1, R-Ras shRNA expression vector, and control shRNA have been described previously [10], [11], [29]. The shRNA constructs for rat RasGRF1 were designed to target 21 nucleotides of the rat transcript. The target sequences were as follows: #A (nucleotides 723–743, 5´- GAACCAGGTGGTGTTCAGCAT -3´), #B (nucleotide 3541–3561, 5´- GACGGCCTGGTCAACTTCTCC -3´). These shRNAs were expressed using pSilencer-2.1 (Invitrogen). RasGRF1 cDNA was purchased from Addgene and subcloned into the pCXN2 vector with a Flag tag sequence.
2.2. Reagents and antibodies
The following reagents were purchased commercially: Poly-L-lysine, dbcAMP, forskolin, and the pharmacological Trk receptor inhibitor K252a (Sigma-Aldrich); recombinant human BDNF (PeproTech); and the pharmacological PKA inhibitor H-89 (Cell Signaling Technology). We used the following antibodies: a rabbit polyclonal antibody for R-Ras was obtained as described previously [8]. The other antibodies were purchased commercially: mouse monoclonal antibodies againstα-tubulin (B-5-1-2) and Flag (M2; Sigma-Aldrich); rabbit polyclonal antibodies against ERK and phospho-RasGRF1 (Ser916/898), and a rabbit monoclonal anti-T202/Y204 phospho-ERK antibody (Cell Signaling Technology); a rabbit polyclonal antibody against RasGRF1 (C-20; Santa Cruz Biotechnology); Alexa Fluor-conjugated secondary antibodies (Invitrogen); and horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako).
2.3. Cell culture and transfection
Cortical neurons were prepared from the embryonic day 19 rat brains as described previously [7], [8]. The dissociated neurons were seeded on poly-L-lysine-coated glass cover slips (circular, 13 mm in diameter) at a density of 2.5 × 104 cells or plastic dishes (60 mm in diameter) in DMEM containing 10% FBS, 4 mM glutamine, 100 units/mL of penicillin, and 0.1 mg/mL of streptomycin and cultured under humidified air containing 5% CO2 at 37 °C. After 4 h, the medium was replaced with Neurobasal medium (Invitrogen) containing 2% B27 supplement (Invitrogen), 0.5 mM GlutaMAX (Invitrogen), 50 units/mL penicillin, and 0.05 mg/mL streptomycin, and neurons were cultured under humidified air containing 5% CO2 at 37 °C. For knockdown and overexpression experiments, cortical neurons were transfected with the indicated plasmids at 1 d in vitro (DIV) with Lipofectamine 2000 according to the manufacturer's instructions. For analysis of R-Ras activity, 50 ng/mL BDNF was added to 3 DIV culture medium and incubated for 30 min. For neuronal morphological analysis, 50 ng/mL BDNF was added to 2 DIV culture medium and incubated for 24 h before fixation. For analysis of Ser916/898 phosphorylation level of RasGRF1, 50 ng/mL BDNF, 1 mM dbcAMP, 20 μM forskolin were added to 3 DIV culture medium and incubated for 30 min. Pretreatment with pharmacological inhibitors (100 nM K252a or 10 μM H-89) was performed for 45 min. All animal experiments were conducted according to the guidelines of the Kyoto University Research Committee.
2.4. Nucleofection
Cortical neurons (2.5 × 106 cells) were suspended in 100 μl of Nucleofector solution (Lonza), mixed with total 3 μg of plasmid for knockdown of endogenous RasGRF1 (YFP:shRNA,1:3), and nucleofected (program O-003) before plating using Nucleofector device (Lonza).
2.5. Pull-down assay
For the measurement of R-Ras activity, a pull-down assay was performed as described previously [29]. Cortical neurons (2.0 × 106 cells) at 3 DIV treated with 50 ng/mL BDNF for 30 min were lysed on dishes with ice-cold cell lysis buffer (25 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA, 25 mM NaF, 1 mM orthovanadate, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM dithiothreitol) containing 75 μg of GST-RBD of c-Raf-1.
2.6. Immunoblotting
Proteins were separated by SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with 3% low-fat milk in Tris-buffered saline (TBS) and then incubated with primary antibodies. The primary antibodies were detected with HRP-conjugated secondary antibodies and enhanced chemiluminescence (ECL) detection Kit (GE Healthcare). Images were captured using a LAS3000 analyzer (Fujifilm) equipped with Science Lab software (Fujifilm).
2.7. Intracellular cAMP assay
The cAMP concentration was measured using Cyclic AMP XP assay kit (Cell Signaling Technology). Briefly, 3 DIV cortical neurons (2.5 × 105 cells) were treated with 50 ng/mL of BDNF or 20 μM forskolin (positive control) for 15 min with or without pretreatment with 100 nM K252a for 45 min. Then, neurons were lysed with 100 μl of lysis buffer. Fifty microliters of cell lysate was transferred into microtubes, and the cAMP concentration was measured according to the protocol provided in the kit. In each experiment, a standard curve was generated in parallel and used to calculate the cAMP concentration.
2.8. Immunofluorescence microscopy
Immunofluorescence microscopy was performed as described previously [7], [8]. Primary cultured cortical neurons on cover slips were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. After residual paraformaldehyde had been quenched with 50 mM NH4Cl in PBS for 10 min, cells were permeabilized with 0.2% Triton X-100 in PBS for 10 min and incubated with 10% FBS in PBS for 30 min. Cells were incubated with the 1st antibodies for 1 h, followed by incubation with Alexa 594-conjugated 2nd antibody and Alexa 488-conjugated rabbit anti-GFP antibody for 1 h. The cells on cover slips were mounted in 90% glycerol containing 0.1% p-phenylenediamine dihydrochloride in PBS and photographed with a Leica DC350F digital camera system (Leica) equipped with a Nikon Eclipse E800 microscope (Nikon). The images were arranged and labeled using Photoshop CS5.1 (Adobe).
2.9. Data analysis
Densitometry analysis was performed with Multi Gauge, version 3.1 (Fujifilm). Morphometric analysis of axons was performed as described previously [7]. A cell was considered to have an axon if the length of one neurite was at least twice as long as any other process and was more than twice the diameter of the cell body, as described previously [30]. The total length of axon and the number of axon tips were measured. Only processes longer than 5 µm in length were included in the analysis [31]. Statistical significance was established at p < 0.05 by one-way analysis of variance (ANOVA) with post hoc test (Dunnett) or Student's t-test using KaleidaGraph (4.5.2; Synergy Software).
3. Results
3.1. BDNF treatment increases endogenous R-Ras activity and promotes R-Ras-mediated axonal growth
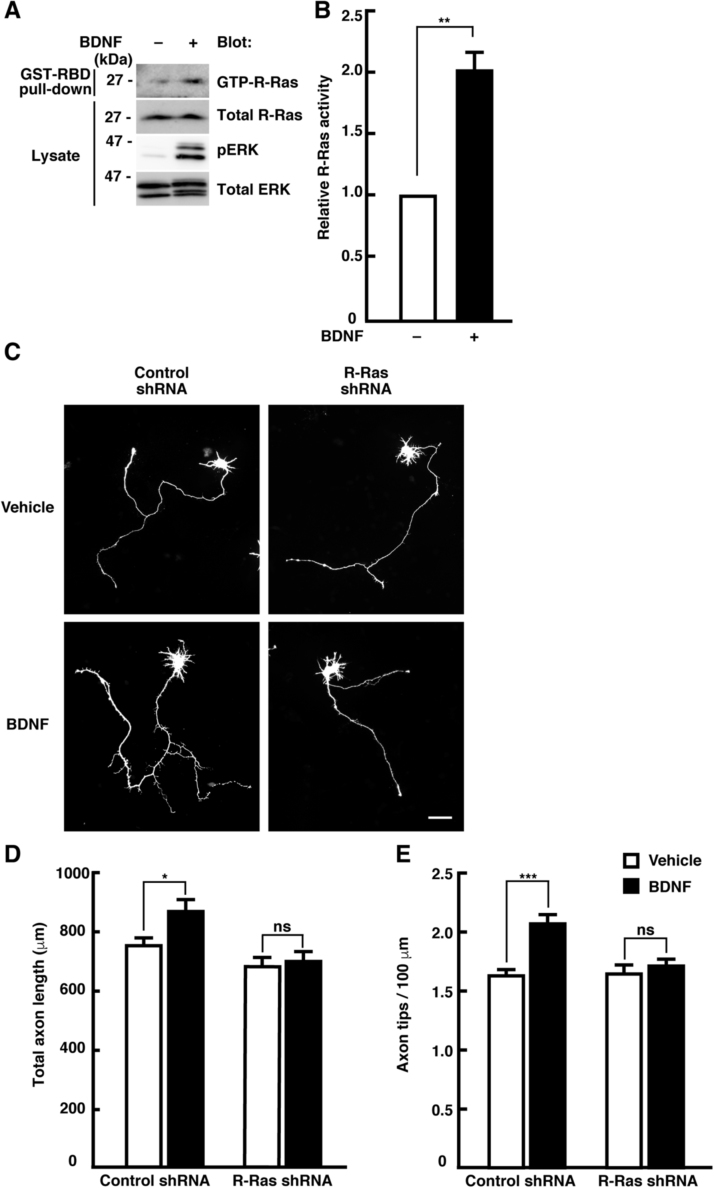
In primary cultured cortical neurons, R-Ras localizes at the axon and its activity increases after plating, and then peaks when neuronal polarization and axonal formation occurs [29]. To identify the upstream regulators of R-Ras activation, we focused on molecules that have been reported to have positive effects on axonal growth. BDNF, a neurotrophin, has been reported to positively regulate axonal outgrowth and branching [18], [19]. Thus, we examined the effects of BDNF on R-Ras activation by a pull-down assay with the GST-fused Ras-binding domain (RBD) of c-Raf-1, which can selectively isolate active R-Ras [32]. After a 30-min treatment with 50 ng/mL of BDNF, the level of GTP-bound R-Ras increased (Fig. 1A-B). We next examined whether R-Ras is involved in the BDNF-induced axonal morphological changes using a short hairpin RNA (shRNA) expression vector designed to target the endogenous R-Ras transcript [29]. Cortical neurons were transfected with yellow fluorescent protein (YFP) and shRNA at 1 DIV, and 50 ng/mL of BDNF was added to the culture medium at 2 DIV. After a 24-h incubation, the axonal morphology was analyzed at 3 DIV (Fig. 1C-E). In neurons transfected with control shRNA, BDNF treatment significantly increased not only axonal length, but also branching, which is consistent with previous reports [18], [19]. In contrast, expression of R-Ras specific shRNA blocked the BDNF-induced promotion of axonal growth. These results suggest that BDNF promotes R-Ras activation and axonal growth through R-Ras activation.
Fig. 1.
BDNF activates R-Ras and promotes R-Ras-mediated axonal growth. (A) Cortical neurons at 3 DIV were treated with 50 ng/mL of BDNF or vehicle control for 30 min. Activated R-Ras was isolated with GST-Ras-binding domain (RBD) of c-Raf-1 and detected by the R-Ras specific antibody. (B) Densitometry analysis was performed, and GTP-R-Ras/total R-Ras ratio was determined. Relative R-Ras activity compared to vehicle-treated cells is shown. Data are presented as the means ± SEM of four independent preparations, **p < 0.01. (C) Neurons were transfected with YFP and shRNAs at 1 DIV, then 50 ng/mL of BDNF was added to 2 DIV culture medium, and incubated for 24 h before fixation. Scale bar, 50 µm. (D, E) The effects on axon length (D) and branching (E) were quantitatively analyzed and expressed as means ± SEM of 54 cells in three independent preparations, *p < 0.05; ***P < 0.001; ns, not significantly different.
3.2. RasGRF1 is required for the BDNF-induced promotion of axonal growth
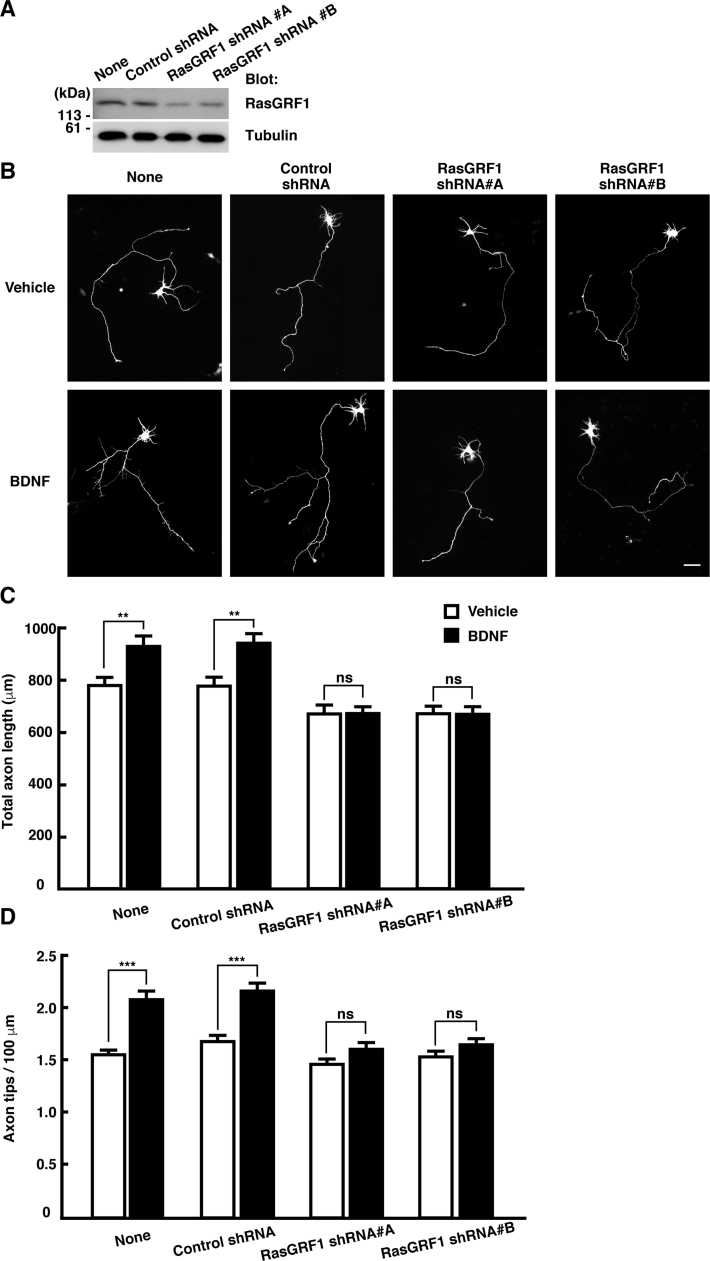
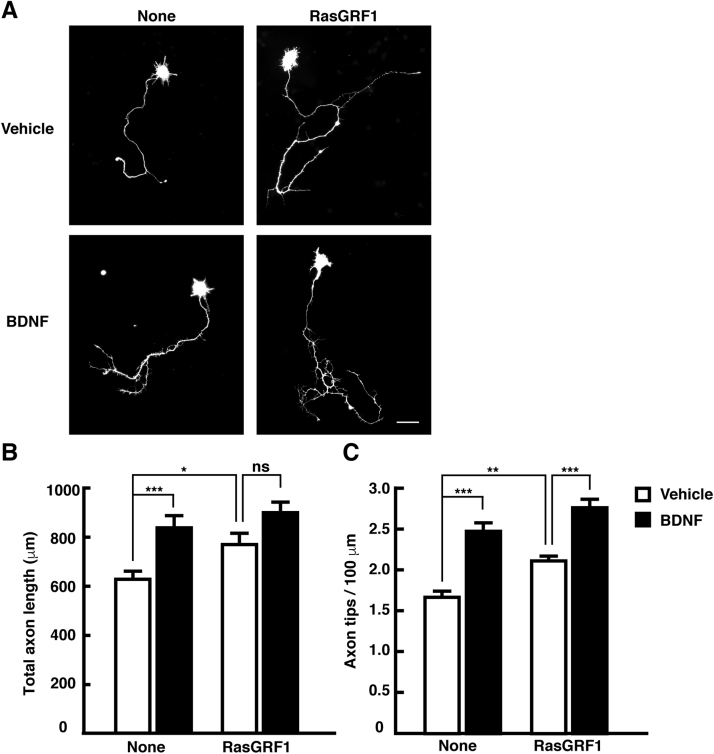
We next investigated the molecular events that connect extracellular BDNF stimulation to intracellular R-Ras activation. In general, activation of Ras family small GTPases requires GDP-GTP exchange catalyzed by guanine nucleotide exchange factors (GEFs). Thus, we sought to identify a GEF linking BDNF signaling to the regulation of R-Ras activity. RasGRF1, one of the GEFs for Ras family proteins, is highly expressed in the brain and acts as an important component of signaling pathways for CNS functions such as the regulation of synaptic plasticity, learning, and memory [20], [21]. It has been reported that RasGRF1 can activate R-Ras [22]. Additionally, several reports have demonstrated that RasGRF1 directly interacts with three Trk receptors, TrkA, TrkB, and TrkC, which are receptors for neurotrophins [33], [34]. These findings raised the possibility that RasGRF1 is involved in BDNF-TrkB signaling, leading to R-Ras activation and the promotion of axonal growth. To explore this possibility, we generated two kinds of shRNA designed to target two different regions of the RasGRF1 transcript. These shRNAs effectively reduced the amount of endogenous RasGRF1 in cortical neurons (Fig. 2A). Expression of RasGRF1-specific shRNAs blocked the promotion of axonal growth induced by BDNF treatment (Fig. 2B-D). We next performed the overexpression experiment to examine the involvement of RasGRF1 in the regulation of axonal morphology. Cortical neurons were transfected with YFP and Flag-tagged RasGRF1 at 1 DIV, and then treated with 50 ng/mL of BDNF at 2 DIV. After a 24-h incubation, the axonal morphology was analyzed. As shown in Fig. 3, ectopic expression of RasGRF1 promoted axonal growth, i.e., increased axon length and branching. In addition, BDNF treatment slightly promoted axonal growth in neurons transfected with RasGRF1, but a significant difference was observed only in axonal branching. Collectively, these results suggest that RasGRF1 positively regulates the BDNF-induced promotion of axonal growth.
Fig. 2.
Knockdown of endogenous RasGRF1 suppresses the BDNF-induced promotion of axonal growth. (A) Neurons were nucleofected with the indicated shRNAs before plating and lysed at 3 DIV. The effects of RasGRF1 shRNAs were verified by SDS-PAGE and immunoblotting. (B) Neurons were transfected with YFP and shRNAs at 1 DIV, then 50 ng/mL of BDNF was added to 2 DIV culture medium, and incubated for 24 h before fixation. Scale bar, 50 µm. (C, D) The effects on axon length (C) and branching (D) were quantitatively analyzed and expressed as means ± SEM of 54–55 cells in three independent preparations, **P < 0.01; ***P < 0.001; ns, not significantly different.
Fig. 3.
Overexpression of RasGRF1 promotes axonal growth. (A) Neurons were transfected with YFP and RasGRF1 at 1 DIV, then 50 ng/mL of BDNF was added to 2 DIV culture medium, and incubated for 24 h before fixation. Scale bar, 50 µm. (B, C) The effects on axon length (B) and branching (C) were quantitatively analyzed and expressed as means ± SEM of 57 cells in three independent preparations, *p < 0.05; **P < 0.01; ***P < 0.001; ns, not significantly different.
3.3. Ser916/898 phosphorylation of RasGRF1 is enhanced by BDNF treatment
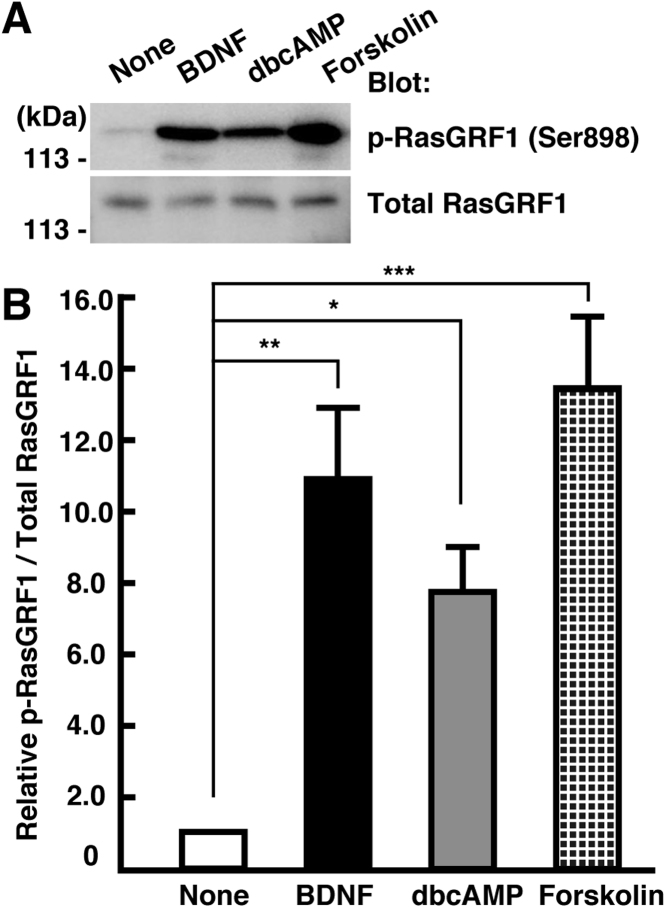
The GEF activity of RasGRF1 is fully activated by its phosphorylation at Ser916 in the mouse sequence, equivalent to Ser898 in rats. This phosphorylation is triggered by many extracellular stimuli such as carbachol, forskolin, and serotonin [25], [27]. In addition, nerve growth factor (NGF) stimulation of endogenous Trk receptors induces RasGRF1 Ser916/898 phosphorylation and promotes Ras-dependent neurite outgrowth [26]. These previous findings raised the hypothesis that BDNF-TrkB signaling also promotes the Ser916/898 phosphorylation of RasGRF1. To address this hypothesis, 3 DIV cortical neurons were treated with 50 ng/mL of BDNF for 30 min, and cell lysates were immunoblotted with the anti-pSer916/898 antibody. We also treated neurons with 1 mM dibutyryl cAMP (dbcAMP), a membrane-permeable cAMP analogue, or 20 μM forskolin, an activator of adenylyl cyclase, as positive controls for Ser916/898 phosphorylation. We found that treatment with BDNF, as well as dbcAMP and forskolin, markedly increased Ser916/898 phosphorylation (Fig. 4A-B). This suggests that Ser916/898 is phosphorylated by BDNF treatment in cultured cortical neurons.
Fig. 4.
Effects of BDNF on Ser916/898 phosphorylation of RasGRF1. (A) Cortical neurons at 3 DIV were treated with 50 ng/mL of BDNF, 1 mM dbcAMP, and 20 μM forskolin for 30 min. Phosphorylated RasGRF1 (Ser916/898) was analyzed by immunoblotting with the anti-pSer916/898 antibody. (B) Densitometry analysis was performed, and p-RasGRF1/total RasGRF1 ratio was determined. Relative levels of p-RasGRF1/total RasGRF1 compared to vehicle-treated cells are shown. Data are presented as the means ± SEM of four independent preparations, *p < 0.05; **P < 0.01; ***P < 0.001.
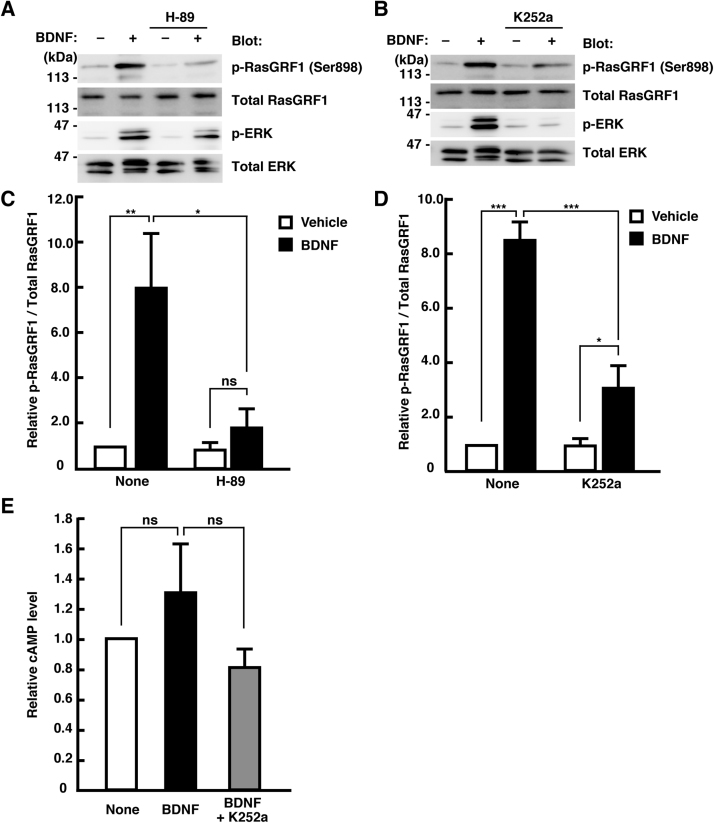
3.4. BDNF-induced Ser916/898 phosphorylation of RasGRF1 is mediated by PKA
Next, we sought to identify the intracellular molecular mechanisms underlying the phosphorylation of RasGRF1 after BDNF treatment. As the sequence around Ser916/898 is a consensus PKA substrate sequence and this phosphorylation is reported to be induced by PKA [25], [35], we examined whether BDNF-induced Ser916/898 phosphorylation of RasGRF1 is regulated by PKA using a pharmacological inhibitor. In the presence of a PKA inhibitor, H-89, there was no significant increase in Ser916/898 phosphorylation of RasGRF1 induced by BDNF treatment (Fig. 5A, C). Moreover, pretreatment with a Trk receptor inhibitor, K252a, partially blocked the BDNF-induced phosphorylation (Fig. 5B, D). These data suggest that BDNF-Trk signaling regulates PKA activity, thereby promoting Ser916/898 phosphorylation of RasGRF1. A previous study using fluorescence resonance energy transfer (FRET) imaging sensors expressed individually in cultured neurons revealed that bath application of BDNF increased the cAMP level and PKA activity [36]. To quantify the changes in the intracellular cAMP concentration after BDNF treatment under our culture conditions, we utilized a competition enzyme-linked immunoassay kit. As neurons treated with forskolin exhibit a significant increase in the intracellular cAMP level [37], we used forskolin as a positive control for cAMP increase. Following the addition of forskolin to the culture medium, a significant increase in the intracellular cAMP level was noted (data not shown), indicating that this assay system can be used to measure the changes in cAMP level under our experimental conditions. The intracellular cAMP level in BDNF-treated neurons was higher than that in control or K252a pretreated neurons, although the difference was not significant (Fig. 5E). This may be because this assay method utilized whole cell lysate, and local and transient elevation of cAMP after BDNF treatment may not have been clearly detected. Collectively, these results indicate that BDNF-TrkB signaling triggers cAMP/PKA activation, resulting in the promotion of Ser916/898 phosphorylation of RasGRF1.
Fig. 5.
Involvement of PKA and Trk receptor in BDNF-induced Ser916/898 phosphorylation of RasGRF1. (A, B) Cortical neurons at 3 DIV were pretreated with 10 μM H-89 (A) or 100 nM K252a (B) for 45 min, and then treated with 50 ng/mL of BDNF for 30 min. Phosphorylated RasGRF1 (Ser916/898) was analyzed by immunoblotting with the anti-pSer916/898 antibody. (C, D) Densitometry analysis was performed, and p-RasGRF1/total RasGRF1 ratio was determined. Relative levels of p-RasGRF1/total RasGRF1 compared to vehicle-treated cells are shown. Data are presented as the means ± SEM of four independent preparations, *p < 0.05; **P < 0.01; ***P < 0.001; ns, not significantly different. (E) Cortical neurons at 3 DIV were treated with 50 ng/mL of BDNF for 15 min with or without pretreatment with 100 nM K252a for 45 min. Then, the intracellular cAMP concentration was measured using the Cyclic AMP XP assay kit. Relative cAMP levels compared to vehicle-treated cells are shown. Data are presented as the means ± SEM of four independent preparations, ns, not significantly different.
4. Discussion
The findings presented here support a positive role of BDNF in endogenous R-Ras activation. Moreover, we demonstrated that RasGRF1, one of the guanine nucleotide exchange factors (GEFs) for Ras family small GTPases, is involved in the BDNF-induced R-Ras activation and R-Ras-mediated axonal growth. Thus, our results provide new insight into the detailed molecular mechanisms of how endogenous R-Ras activity is regulated in response to extracellular stimuli in neurons.
We previously reported that R-Ras, a Ras family small GTPase, plays essential roles in axonal formation, elongation, and guidance [10], [11], [29]. Activated R-Ras specifically interacts with its downstream effectors, leading to the regulation of actin or microtubule cytoskeletons [6], [7], [8]. Regarding the regulatory mechanisms of endogenous R-Ras activity, ephrins, repulsive axon guidance molecules, inactivate R-Ras activity through R-Ras tyrosine phosphorylation and p120RasGAP to induce axonal repulsion [9]. Plexins, receptors for semaphorins, have also been reported to directly inactivate R-Ras in cultured neurons, inducing axonal repulsion [10], [11], [12]. It is well known that repulsive guidance factors, including ephrins and semaphorins, have negative effects on axonal growth such as retraction of axonal structure [38], [39]. Recent studies raised the possibility that extracellular factors, which play positive roles in axonal growth, may have the ability to activate R-Ras activity. In light of the previous observation that BDNF promotes axonal differentiation, outgrowth, and branching [17], [18], [19], we focused on BDNF as a candidate upstream regulator for R-Ras activation and R-Ras-mediated axonal growth in this study.
The importance of precise control of neuronal morphology in brain function has long been recognized, as axonal morphological disruption is likely responsible for disease progression. In the animal model of epilepsy, aberrant axonal branches were directed to inappropriate regions in the granule cells in the dentate gyrus [40]. In addition, axonal loss is known to be an early pathological feature in many neurodegenerative disorders [41]. BDNF has been widely studied and recognized as an important neurotrophic factor in the regulation of neuronal growth, survival, and morphology [13]. It is also known that a low circulating BDNF level is associated with a wide range of neuropsychiatric disorders, including depression and bipolar disorder [15], [16]. However, the detailed downstream molecular mechanisms of BDNF-induced neuronal growth are not well understood. In the current study, a pull-down assay and morphological observations revealed that BDNF regulates axonal morphology through R-Ras activation, and this activity may be important for the precise neuronal network formation. Given the relationships between axonal morphology and brain function, BDNF-induced positive effects on R-Ras activation and R-Ras-mediated axonal growth at the cellular level may partially account for the beneficial effects of BDNF on brain health. Additional studies are required to further elucidate the importance of BDNF-R-Ras signaling in vivo, which will enhance our understanding of the physiological role of BDNF and its therapeutic potential for improving brain health.
RasGRF1, one of the GEFs for Ras family small GTPases, is highly expressed in the brain, and has important functions for learning and memory [20], [21]. At the cellular level, RasGRF1-mediated signaling pathways are linked to diverse cellular functions such as the control of cell growth and neurite outgrowth. In neurons, RasGRF1 was reported to be expressed in postsynaptic densities [42]. In addition, RasGRF1 is a binding partner of plasticity related gene 3 (PRG3), and functions in PRG3-induced filopodia formation and axonal growth [43]. These observations suggest that RasGRF1 plays a role in the regulation of neuronal morphology. Our knockdown and overexpression experiments indicated that RasGRF1 is involved in the BDNF-induced promotion of axonal growth. The ectopic expression of RasGRF1 alone significantly promoted axonal growth, suggesting that RasGRF1 has positive effects on axonal growth via interactions with endogenous R-Ras or some other small GTPases, such as Rac1, under basal conditions.
RasGRF1 becomes phosphorylated at several sites by a variety of intracellular kinases in response to several extracellular stimuli. NGF, forskolin, serotonin, and NMDA stimulation are known to trigger the phosphorylation of RasGRF1 at Ser916/898 [25], [26], [27], [28]. This phosphorylation is dependent on PKA activity and is associated with a significant increase in its intrinsic GEF activity against Ras proteins. Replacement of Ser916/898 with alanine inhibits the full activation of GEF activity induced by carbachol in NIH-3T3/hm1 fibroblasts, suggesting that this phosphorylation is necessary for the maximum GEF activity [25]. The Ser916/898 phosphorylation site in RasGRF1 is conserved in rodents and humans, but is absent in RasGRF2. Therefore, this phosphorylation-mediated regulatory mechanism is one of the unique signal transduction pathways of RasGRF1 that cannot be replaced by RasGRF2. In the present study, we newly found that BDNF-TrkB signaling also induced phosphorylation of RasGRF1 at Ser916/898 by PKA. Moreover, we observed a slight increase in the intracellular cAMP level after BDNF treatment, which is consistent with previous reports [36]. Therefore, it is reasonable to consider BDNF-TrkB stimulation as the trigger of the local increase in cAMP level, leading to PKA activation and PKA-induced RasGRF1 phosphorylation, which in turn, fully activate R-Ras. Several other putative phosphorylation sites have been identified or suggested in RasGRF1. RasGRF1 is known to be phosphorylated at tyrosine residues by Trk receptors, Src, and Ack1, leading to regulation of its GEF activity [34], [44], [45], [46]. For example, Src-induced tyrosine phosphorylation increases Rac-GEF activity but not Ras-GEF activity. On the other hand, Ack1-induced tyrosine phosphorylation increases Ras-GEF activity but not Rac-GEF activity. These reports suggest that RasGRF1 possesses multiple tyrosine phosphorylation sites with distinct effects. Furthermore, the morphology of PC12 cells was reported to be regulated by coordinated activation of H-Ras and Rac1 induced by RasGRF1 [47], suggesting that harmonized regulation of Ras and Rac activity by RasGRF1 is required for effective control of cell morphology. To date, however, the functional relationships between phosphorylation events on RasGRF1 at serines and tyrosines are unknown. As RasGRF1 has many phosphorylated sites induced by different extracellular stimuli, RasGRF1 may orchestrate several signaling cascades required for small GTPases-mediated biological effects. Future studies should investigate the molecular mechanisms underlying the cross-talk between serine and tyrosine phosphorylation of RasGRF1 that regulate diverse extracellular stimulation-initiated morphological changes in neurons.
Acknowledgements
We would like to thank Dr. J. Miyazaki and Dr. T. Saito for providing the EYFP expression plasmid. This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [18K06215 (to H.K), 17J02057 (to K.U)].
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.11.011.
Appendix A. Transparency document
Supplementary material
References
- 1.O’Donnell M., Chance R.K., Bashaw G.J. Axon growth and guidance: receptor regulation and signal transduction. Annu. Rev. Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall A., Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinbara K., Goldfinger L.E., Hansen M., Chou F.-L., Ginsberg M.H. Ras GTPases: integrins' friends or foes? Nat. Rev. Mol. Cell Biol. 2003;4:767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K., Asano T., Endo T. Novel small GTPase M-Ras participates in reorganization of actin cytoskeleton. Oncogene. 1997;15:2409–2417. doi: 10.1038/sj.onc.1201416. [DOI] [PubMed] [Google Scholar]
- 5.Marte B.M., Rodriguez-Viciana P., Wennström S., Warne P.H., Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr. Biol. 1997;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y., Oinuma I., Katoh H., Kaibuchi K., Negishi M. Sema4D/plexin-B1 activates GSK-3β through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7:704–709. doi: 10.1038/sj.embor.7400737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasawa N., Negishi M., Oinuma I. R-Ras controls axon branching through afadin in cortical neurons. Mol. Biol. Cell. 2012;23:2793–2804. doi: 10.1091/mbc.E12-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umeda K., Iwasawa N., Negishi M., Oinuma I. A short splicing isoform of afadin suppresses the cortical axon branching in a dominant-negative manner. Mol. Biol. Cell. 2015;26:1957–1970. doi: 10.1091/mbc.E15-01-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dail M., Richter M., Godement P., Pasquale E.B. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J. Cell Sci. 2006;119:1244–1254. doi: 10.1242/jcs.02842. [DOI] [PubMed] [Google Scholar]
- 10.Oinuma I., Ishikawa Y., Katoh H., Negishi M. The semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 11.Oinuma I., Katoh H., Negishi M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J. Neurosci. 2004;24:11473–11480. doi: 10.1523/JNEUROSCI.3257-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyofuku T., Yoshida J., Sugimoto T., Zhang H., Kumanogoh A., Hori M., Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat. Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- 13.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 14.Murer M.G., Yan Q., Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 15.Lang U.E., Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell. Physiol. Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 16.Cunha A.B., Frey B.N., Andreazza A.C., Goi J.D., Rosa A.R., Gonçalves C.A., Santin A., Kapczinski F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci. Lett. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 17.Shelly M., Cancedda L., Heilshorn S., Sumbre G., Poo M.M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Granseth B., Fukushima Y., Sugo N., Lagnado L., Yamamoto N. Regulation of thalamocortical axon branching by BDNF and synaptic vesicle cycling. Front. Neural Circuits. 2013;7:202. doi: 10.3389/fncir.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata K., Shintani N., Hayata-Takano A., Kamo T., Higashi S., Seiriki K., Momosaki H., Vaudry D., Vaudry H., Galas L., Kasai A., Nagayasu K., Nakazawa T., Hashimoto R., Ago Y., Matsuda T., Baba A., Hashimoto H. PACAP enhances axon outgrowth in cultured hippocampal neurons to a comparable extent as BDNF. PLoS One. 2015;10:e0120526. doi: 10.1371/journal.pone.0120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feig L.A. Regulation of neuronal function by Ras-GRF exchange factors. Genes Cancer. 2011;2:306–319. doi: 10.1177/1947601911408077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Medarde A., Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim. Biophys. Acta. 2011;1815:170–188. doi: 10.1016/j.bbcan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Gotoh T., Tian X., Feig L.A. Prenylation of target GTPases contributes to signaling specificity of ras-guanine nucleotide exchange factors. J. Biol. Chem. 2001;276:38029–38035. doi: 10.1074/jbc.M104658200. [DOI] [PubMed] [Google Scholar]
- 23.Brambilla R., Gnesutta N., Minichiello L., White G., Roylance A.J., Herron C.E., Ramsey M., Wolfer D.P., Cestari V., Rossi-Arnaud C., Grant S.G.N., Chapman P.F., Lipp H.P., Sturani E., Klein R. A role for the Ras signalling pathway in synaptic transmission and long- term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 24.Giese K.P., Friedman E., Telliez J.B., Fedorov N.B., Wines M., Feig L.A., Silva A.J. Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1) Neuropharmacology. 2001;41:791–800. doi: 10.1016/s0028-3908(01)00096-x. [DOI] [PubMed] [Google Scholar]
- 25.Mattingly R.R. Phosphorylation of serine 916 of Ras-GRF1 contributes to the activation of exchange factor activity by muscarinic receptors. J. Biol. Chem. 1999;274:37379–37384. doi: 10.1074/jbc.274.52.37379. [DOI] [PubMed] [Google Scholar]
- 26.Yang H., Cooley D., Legakis J.E., Ge Q., Andrade R., Mattingly R.R. Phosphorylation of the Ras-GRF1 exchange factor at Ser916 / 898 reveals activation of ras signaling in the cerebral cortex. J. Biol. Chem. 2003;278:13278–13285. doi: 10.1074/jbc.M209805200. [DOI] [PubMed] [Google Scholar]
- 27.Norum J.H., Méthi T., Mattingly R.R., Levy F.O. Endogenous expression and protein kinase A-dependent phosphorylation of the guanine nucleotide exchange factor Ras-GRF1 in human embryonic kidney 293 cells. FEBS J. 2005;272:2304–2316. doi: 10.1111/j.1742-4658.2005.04658.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt J.M., Guire E.S., Saneyoshi T., Soderling T.R. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 2005;25:1281–1290. doi: 10.1523/JNEUROSCI.4086-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oinuma I., Katoh H., Negishi M. R-Ras controls axon specification upstream of glycogen synthase kinase-3β through integrin-linked kinase. J. Biol. Chem. 2007;282:303–318. doi: 10.1074/jbc.M607979200. [DOI] [PubMed] [Google Scholar]
- 30.Yin D.-M., Huang Y.-H., Zhu Y.-B., Wang Y. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J. Neurosci. 2008;28:8832–8843. doi: 10.1523/JNEUROSCI.1291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marler K.J.M., Becker-Barroso E., Martinez A., Llovera M., Wentzel C., Poopalasundaram S., Hindges R., Soriano E., Comella J., Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J. Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Triest M., De Rooij J., Bos J.L. Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol. 2001;333:343–348. doi: 10.1016/s0076-6879(01)33068-9. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald J.I.S., Verdi J.M., Meakin S.O. Activity-dependent interaction of the intracellular domain of rat TrkA with intermediate filament proteins, the β-6 proteasomal subunit, Ras-GRF1, and the p162 subunit of eIF3. J. Mol. Neurosci. 1999;13:141–158. doi: 10.1385/JMN:13:1-2:141. [DOI] [PubMed] [Google Scholar]
- 34.Robinson K.N., Manto K., Buchsbaum R.J., MacDonald J.I.S., Meakin S.O. Neurotrophin-dependent tyrosine phosphorylation of ras guanine-releasing factor 1 and associated neurite outgrowth is dependent on the HIKE domain of TrkA. J. Biol. Chem. 2005;280:225–235. doi: 10.1074/jbc.M410454200. [DOI] [PubMed] [Google Scholar]
- 35.Pearson R.B., Kemp B.E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 36.Shelly M., Cancedda L., Lim B.K., Popescu A.T., Cheng P. lin, Gao H., Poo M.M. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shelly M., Lim B.K., Cancedda L., Heilshorn S.C., Gao H., Poo M.M. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 38.Meima L., Moran P., Matthews W., Caras I.W. Lerk2 (ephrin-B1) is a collapsing factor for a subset of cortical growth cones and acts by a mechanism different from AL-1 (ephrin-A5) Mol. Cell. Neurosci. 1997;9:314–328. doi: 10.1006/mcne.1997.0621. [DOI] [PubMed] [Google Scholar]
- 39.Dent E.W., Barnes A.M., Tang F., Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J. Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parent J.M., Yu T.W., Leibowitz R.T., Geschwind D.H., Sloviter R.S., Lowenstein D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neukomm L.J., Freeman M.R. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 2014;24:515–523. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturani E., Abbondio A., Branduardi P., Ferrari C., Zippel R., Martegani E., Vanoni M., Denis-Donini S. The Ras guanine nucleotide exchange factor CDC25Mm is present at the synaptic junction. Exp. Cell Res. 1997;235:117–123. doi: 10.1006/excr.1997.3660. [DOI] [PubMed] [Google Scholar]
- 43.Broggini T., Schnell L., Ghoochani A., Mateos J.M., Buchfelder M., Wiendieck K., Schäfer M.K., Eyupoglu I.Y., Savaskan N.E. Plasticity Related Gene 3 (PRG3) overcomes myelin-associated growth inhibition and promotes functional recovery after spinal cord injury. Aging (Albany NY) 2016;8:2463–2487. doi: 10.18632/aging.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiyono M., Kaziro Y., Satoh T. Induction of Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25Mm following phosphorylation by the nonreceptor tyrosine kinase Src. J. Biol. Chem. 2000;275:5441–5446. doi: 10.1074/jbc.275.8.5441. [DOI] [PubMed] [Google Scholar]
- 45.Kiyono M., Kato J., Kataoka T., Kaziro Y., Satoh T. Stimulation of Ras guanine nucleotide exchange activity of Ras-GRF1/CDC25Mm upon tyrosine phosphorylation by the Cdc42-regulated kinase ACK1. J. Biol. Chem. 2000;275:29788–29793. doi: 10.1074/jbc.M001378200. [DOI] [PubMed] [Google Scholar]
- 46.Talebian A., Robinson-Brookes K., MacDonald J.I.S., Meakin S.O. Ras guanine nucleotide releasing factor 1 (RasGrf1) enhancement of Trk receptor-mediated neurite outgrowth requires activation of both H-Ras and Rac. J. Mol. Neurosci. 2013;49:38–51. doi: 10.1007/s12031-012-9847-9. [DOI] [PubMed] [Google Scholar]
- 47.Yang H., Mattingly R.R. The Ras-GRF1 exchange factor coordinates activation of H-Ras and Rac1 to control neuronal morphology. Mol. Biol. Cell. 2006;17:2177–2189. doi: 10.1091/mbc.E05-10-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material