Abstract
Background:
Patients experience multiple sclerosis (MS) differently based on their disease type and other factors. This study aimed to explore the relative importance that patients with MS place on various attributes of MS drug therapies and to elucidate these patients' preferences regarding treatment characteristics such as administration, potential benefits, and side effects of the therapies.
Methods:
Focus groups were conducted in Vancouver, Canada, with 23 adult patients with MS. Participants were interviewed in three groups based on disease category and MS treatment experience: treatment-naive, non–treatment-naive relapsing-remitting and non–treatment-naive progressive MS.
Results:
Overall, the most important characteristics of MS drugs were effectiveness and side effects. As such, there is hesitancy about trying new-to-market drugs because the risks, benefits, and costs may not be well known. Participants valued stability in their treatment and generally did not want to take on the additional risk of trying a new drug if they felt that their current medication was providing benefit. Convenience and method of administration were secondary considerations that would generally be valued only if expected risks and benefits were considered equal or superior.
Conclusions:
This qualitative study shows that patients consider the impact and likelihood of benefits and side effects first and foremost when making drug treatment decisions and that other factors, such as convenience and method of administration, are of secondary concern.
Keywords: Drug treatment, Multiple sclerosis, Patient perspective, Patient preference, Qualitative study
Multiple sclerosis (MS) is a chronic immune-mediated demyelinating disease of the central nervous system that affects more than 2.3 million people worldwide and 93,500 people in Canada.1,2 Canada has one of the highest rates of MS globally, and it is estimated that the number of people living with MS in Canada will increase to approximately 133,600 in 2031.3 It is an unpredictable and heterogeneous disease with different phenotypes.4 Patients experience MS differently based on their disease type, which includes relapsing remitting (RRMS), primary progressive, and secondary progressive.5 Multiple sclerosis mainly affects young adults during the primary productive time of their life (typically between 15 and 40 years of age), placing a substantial burden on patients, health care systems, and society.
Although there is no definitive cure for MS, currently available disease-modifying therapies (DMTs) help to manage flare-ups, reduce the frequency of relapses, and control the symptoms.6,7 With beta-interferons and glatiramer acetate as the first and only DMTs initially for many years, treatment selection was limited. During the past 2 decades, an increasing number of new treatments have emerged, and many new drugs for managing MS are under development, providing patients and clinicians with more available treatment options with respect to the route and frequency of administration, and differences in potential benefits and side effects. Patient and physician preferences for different attributes of DMTs affect therapeutic choice.8,9
The risk-to-benefit trade-off is extremely relevant for patients with MS.10 While several of the available and forthcoming drug therapies demonstrate high levels of efficacy with respect to halting or slowing disease progression and reducing relapse rates, some DMTs also carry a risk of severe adverse effects.8,9,11 An additional consideration further complicating treatment decisions is that such treatments are most effective in young patients with RRMS who are not yet affected by high levels of disability but would be exposed to the risk of severe side effects without significant short-term clinical benefit, although with potential long-term slowing of disease progression.12
Despite the significance of these trade-offs in therapeutic decision making, there is limited research to date that has evaluated the relative importance of various treatment attributes from a patient perspective and how different treatment characteristics may affect treatment choice for patients, particularly in light of the rapidly evolving therapeutic armamentarium for MS. Webb et al13 reviewed attribute-based stated-preference studies in people living with MS and found 16 studies focusing on DMTs, of which only two used qualitative approaches that involved patients in the development of attributes, with the remaining studies relying on health care professionals and existing medical and social science literature. Given that previous research has highlighted the strengths/needs of using qualitative approaches in the development of attributes to be used in stated preference studies,14 this qualitative study drew on focus groups of patients with MS to understand their preferences regarding the different characteristics of available and emerging drug therapies for MS. In particular, we aimed to determine how patients value noninjectable drug administration relative to effectiveness, side effects, and potential adverse events. The results of this qualitative study were used to develop attributes that were subsequently used in the best-worst scaling study that was designed to quantify preferences for DMTs in patients with MS.15
Methods
Recruitment of Participants
We aimed to conduct two focus groups of approximately 11 patients with MS to determine patients' values and opinions regarding different drug therapy attributes. All patients registered with the MS Clinic at the University of British Columbia (UBC), which includes most patients with MS in British Columbia, were potential participants and were mailed an invitation letter to participate in the focus group study. Those who expressed an interest in participating were contacted directly by research personnel, and, at that point, the study was explained in more detail and informed consent was obtained if they were aged 19 years or older, able to speak English, and could travel to the study site to participate. The recruitment stopped when the target number was achieved (n = 23). Data regarding sociodemographics, MS duration and severity, current and previous medication use, and third-party payer coverage were collected to screen participants and allocate them to the appropriate cohort. Participants in the focus group received $75 for their time and parking cost. In addition, snacks and refreshments were served during the forum.
This study was approved by the research ethics board of the UBC.
Focus Group Interviews
Focus groups were conducted in Vancouver, Canada, with 23 patients with MS in 2013. Given that MS type and experience with DMTs could shape patients' values and opinions regarding different drug therapy attributes, participants were stratified into three groups based on their disease type and experience with treatments for MS (DMTs): treatment-naive MS, non–treatment-naive RRMS, and non–treatment-naive progressive MS. Each focus group session lasted approximately 90 minutes and was led by an experienced qualitative health researcher (N.J.H.) who guided the participants through a series of semistructured questions related to their MS drug use experiences and preferences (Appendix S1 (121.9KB, pdf) , which is published in the online version of this article at ijmsc.org). The focus group discussion guide was developed based on the present study objective and on discussion with MS physicians, nurses, and patients at the UBC MS Clinic, as well as some previous unpublished work in the area. The guide was meant merely to stimulate the discussion among the participants to determine their values and opinions regarding different drug therapy attributes. Each focus group began with the discussion leader verbally providing participants with information about oral and injectable treatments that were currently available or were under development, including mode and frequency of administration and what is known about possible side effects. Two patients with RRMS were unable to attend the scheduled focus group and thus were interviewed one-on-one by phone using the same questions as were posed in the focus group. Responses from these patients were then integrated with the corresponding focus group data for the analysis.
Data Analysis
All the discussions were audio-recorded and transcribed, and transcripts were imported into NVivo 9 software (QSR International, Doncaster, Australia) for thematic coding and analysis. Broad theme codes were developed based on the questions in the focus group guide, and subthemes were based on the content of the discussions. The coded transcripts were descriptively analyzed, and differences between participant types were noted, where appropriate.
Results
Characteristics of Study Participants
Table 1 shows the characteristics of the 23 patients with MS included in the study. Most of the participants were female and had non–treatment-naive RRMS.
Table 1.
Characteristics of study participants
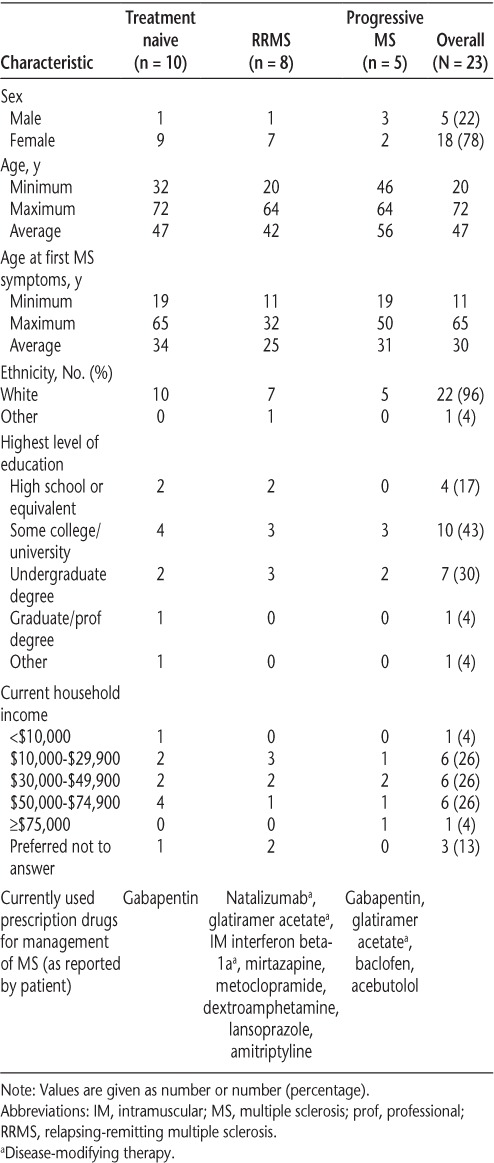
The following describes the opinions of the participants based on their comments in the specific focus group. Unless otherwise indicated, the responses were similar across focus groups; when responses did vary, the type of MS of the participants is noted. Quotations from participants are included verbatim except where clarifications are required, as indicated by [ ]. Given the similarity of the responses across focus groups, investigators concluded that saturation had been achieved. A summary of the participants' comments is provided in Table 2.
Table 2.
Summary of findings
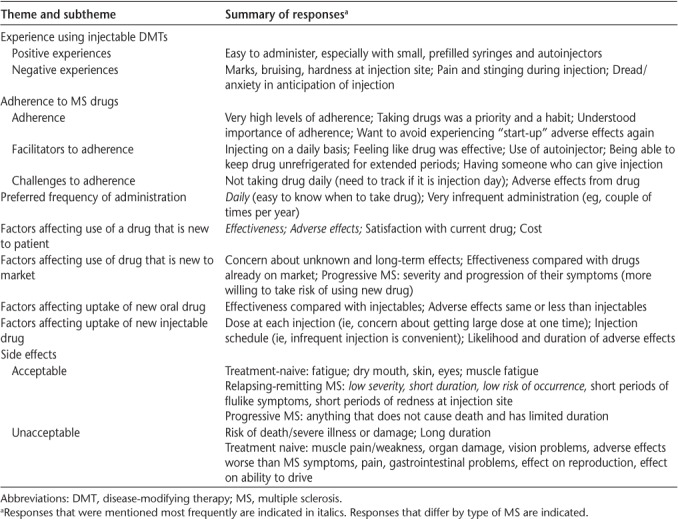
Experience Using Injectable DMTs
Positive Experiences
Participants who had positive experiences with injectable drugs had little difficulty in injecting themselves. They described the injections as “remarkably easy” and “quite straightforward.” Some mentioned that they were nervous before they started but that after one or two injections they felt comfortable with the process, and one participant said that injecting herself gave her a sense of control over the needle that alleviated her fear of needles. Participants found injections much easier when the needle was smaller, when syringes came prefilled, and especially when the drug had an injector: “[T]he injector itself made all the difference in the world for me.”
Negative Experiences
Treatment-naive participants anticipated that they would get marks on their skin and flulike symptoms if they used injectable drugs. There was also concern that the effectiveness of currently available treatments is too low given the potential side effects. Participants who had negative experiences directly related to taking medication by injection described marks, bruising, and hardness/scar tissue at the injection site (“I used to get those big red marks, like loonie-sized [diameter, 26.5 mm] marks all over myself”), pain and stinging during the injection, and feeling dread or anxiety in anticipation of the injection. One participant found it difficult to prepare the medication because she had to mix the contents of two vials.
Although not related to the route of administration, participants disliked using their injectable drugs because of side effects, including flulike symptoms, insomnia, fatigue, depression, and “feeling crappy.” Several participants stopped using their drugs because their symptoms were not being managed, and two participants who had been taking interferon beta-1b said that over time they developed antibodies to the medication that made it ineffective.
Adherence to MS Drugs
Adherence
Participants reported very high levels of adherence. Taking their medications was described as a priority and a commitment, and something that had become part of their routine. Some illustrative comments: “I was super committed, oh, yeah. I took it seriously.” “It's very much a routine.”
Participants also recognized that adherence was to their advantage: “I mean, it's my life. I'm taking it for a reason. So, I mean, it's my only hope to get anywhere better. So I better do it properly. There's no way I'm missing it, any part of it. Especially since it's only, like, supposed to reduce relapses by, like, 33 percent.”
The start-up side effects that accompanied initiating a new drug therapy motivated some of the participants with RRMS to adhere because they did not want to endure these multiple times. Others adhered because they did not want to experience MS symptoms that were under control as long as they remained adherent to their medication. One participant said, “I think that for me, what makes me stick to my routine and do my injection every day was a bad experience I had going off of it.... [My neurologist] said, ‘you know what, go off of it, give it a try.’ So I did and 6 months later I relapsed hard and ended up in the hospital for several months. So now because of that I'm doing it. I'm not going to go back to the place I was at when I had to be in the hospital.”
Facilitators to Adherence
Participants with progressive MS and RRMS identified two of the same factors that facilitated adherence. Patients in both groups reported that injecting on a daily basis allowed the injections to become very routine and decreased the chance of missing a dose. “[Adhering is as] easy as can be. Because I take it every night ... taking it every day is very, very simple.” In addition, one participant in each of these groups said it was easier to adhere if they knew the medicine was making a difference in controlling their disease (ie, the drug was effective).
Participants in the RRMS group identified three additional factors that facilitated improved adherence: the administration of an injectable drug with an auto-injector, making injecting easier and less nerve-racking; the ability to keep the medication unrefrigerated for up to 30 days, increasing portability and availability; and having someone willing and able to give the injection, providing support and making it easier for the injection to be administered. As noted by one participant, “'Cause it's just—makes your life easier if someone can help you, and everyone in my life will help me.”
Challenges to Adherence
Treatment-naive participants speculated that the occurrence of side effects would potentially hinder adherence, as would running out of sites on the body to inject, pain around the injection site, infections caused by the injection, and travel (due to inconvenience).
Participants in the RRMS and progressive MS groups reported that adherence is more difficult when the injection is not required daily, particularly when the drug is used on different days each week (eg, every other day). In the RRMS group, participants discussed the challenges of being adherent when the drug caused side effects, especially flulike symptoms, although only one participant reported actually missing doses because of side effects. Traveling with the drug was also reported to cause some difficulty.
Preferences on Frequency of Administration
In consideration of drugs under development by Sanofi Genzyme (Cambridge, MA), participants were asked their opinions about using an injectable drug that would be administered as a cluster of three injections at the start of a year and then a single injection at the end of the year. Mixed sentiments were expressed about the proposed administration schedule. Several participants said that daily injections were preferable because of the ease of keeping track of when doses need to be taken. However, others in the groups liked the idea of infrequent administration: one due to not liking needles and others because it eliminated the need to both carry the drug and remember to take it. Three participants thought that the infrequent administration schedule would be psychologically beneficial because it would allow them to focus less on their disease and feel less like a sick person. One participant said, “If it was the one where you just had to go for twice a year, then that makes a difference....You don't have to think about it for the rest of the year. I can just go on and pretend everything's fine.” Another stated, “Well, I think for me part of the thing about taking the injectables was that it was, like, it made me believe that I was sick, like, I've got to take the injection.”
They were also asked about having the option of using an orally administered drug that required daily administration. Participants said that they would find the daily administration very convenient and easy to keep track of. However, there was no discussion differentiating between once or twice administration. Importantly, however, participants felt that they could adapt to any administration schedule if there was real benefit derived from the treatment, as this was the primary consideration.
Factors Affecting Use of Drug New to Patient
Participants were asked what they would take into consideration when deciding whether to switch to a new drug. In this scenario, the drug was not new in the marketplace, just new to the patient.
Effectiveness and Side Effects
Across all focus groups, by far the two most important factors that would affect their decision to switch to a new drug were the drug's potential effectiveness and potential side effects. Participants were more willing to accept the potential for side effects if there was a greater chance of potential benefit associated with the drug therapy. (”There's no sense having something that's 50% effective if it's going to make you feel lousy.”) Importantly, there was some differentiation between the chance for improvement and the amount of improvement (eg, a 50% chance of improvement vs. a 50% improvement in symptoms), suggesting that it is important to differentiate between these.
One treatment-naive participant explained that her current condition would affect her willingness to accept greater uncertainty associated with starting a new drug. When experiencing more severe symptoms, she said she would accept potential side effects more readily; otherwise, additional side effects on top of MS symptoms seemed too burdensome.
Satisfaction with Current Drug
For participants with RRMS, decision making about switching drugs was also strongly affected by their satisfaction with their current drugs. If the current drug was working well to control their symptoms and they were experiencing few or no side effects, then they would be less inclined to try something new and risk an outcome that was inferior to their current therapy. One participant stated, “Well I guess it depends how you're doing right now with your drug. Like, if there's issues or you've got stuff that you're unhappy with your drug, you'd probably be more interested in trying something new that could potentially be better. So I think if I was in that position I'd be okay with it. But if you've got no troubles with where you're at, then it's like, why would you change?”
Cost
For those with no or poor extended medical insurance (ie, supplemental plan to fill gaps in existing provincial medical plans), cost could be a barrier to using a drug. Participants also speculated that if they did not have drug insurance then cost would be a significant consideration in deciding whether to switch drugs. However, most participants did seem to have adequate drug coverage, and a few said that they were willing to pay any cost if a drug was highly effective and stopped the progression and symptoms of their disease. However, overall there was widespread consensus within all groups that the frequency and mode of administration were extremely minor considerations in selecting a drug, if they were factors at all.
Factors Affecting Use of a Drug New to Market
Participants were asked about their willingness to switch to a drug that was released onto the market only recently. In the progressive MS group, two participants said that although they would be concerned about unknown or long-term effects, they do not have the luxury of waiting to see how the drug does over the long term. One remarked, “If it was a perfect world, I'd wait awhile till it, actually, like 5 years, 10 years after the drug was developed, to see if it actually worked. Unfortunately, MS doesn't get better by itself. So I'm pretty much stuck in a boat where if it comes out on the market and it seems to work, I pretty much have to try it.”
Similarly, one participant with RRMS said that the disease is so “insidious” that you have to try whatever may help and would thus be willing to use a novel drug. In addition, one participant in the progressive MS group and two in the treatment-naive group said that they were willing to try anything and had previously participated in clinical trials (unrelated to MS). Some participants revealed that they would try a novel drug but only if trials showed the drug to be both very effective and actually more effective than other drugs already on the market with known histories.
In contrast to these supporters of novel drugs, most participants with RRMS stated that they would not be interested in trying new therapies because of their concerns about side effects and long-term effects that may not have been detected during the trial period. One person said, “I'm not all over that because sometimes there are serious side effects that people don't know about that come up like a couple years down the road.”
Factors Affecting Uptake of a New Treatment
Participants would be receptive to taking a daily oral drug if it is at least as effective as currently available injectable drugs and the side effect profile is comparable or better. Although there was recognition that daily oral administration would be easier and more convenient, participants were not willing to sacrifice effectiveness or take on the risk of additional side effects only for convenience or avoidance of injections.
Considering the use of a new injectable drug that would have a cluster of injections at the start of the year and a single injection at the end of the year, participants were generally wary of the effect of receiving such a large dose of the medication during the injection cluster (note: the receipt of the large dose at the beginning of a year was the perception of the patients, which is not necessarily correct). Participants said that they assumed it was better to have more steady levels of the drug administered over time rather than one “massive” dose. The primary concern about receiving a large dose at one time was the intensity or duration of adverse effects: “If all things were equal, I would 100% get a couple of shots in the beginning of the year and then one at the end of the year ... but I would be really concerned about having side effects “
There were also concerns that after receiving the upfront dose, the person would be committed to that drug for a prolonged period even if the person's medical situation changed: “What happens if in those 9 or 10 months you have to take some other medication.... You don't know how they're going to interact together.”
Participants thought that the administration schedule sounded convenient but found it difficult to speculate on their willingness to try a new injectable drug without having more information on the likelihood and expected duration of side effects. However, there was agreement that the convenience of administration would not outweigh the importance of effectiveness and risk of side effects. One participant said, “I like the fact that it's twice a year, but like everybody else says, I hate the fact that it has side effects that are adverse to your health and all that. So I would probably consider it, but I'd probably be hesitant before I would start it.”
Side Effects
Potential side effects of currently available drugs vary widely in their severity and duration. Participants listed the side effects they consider tolerable and not tolerable, and discussed how tolerance is affected by the risk of occurrence and effectiveness of the drug.
The treatment-naive participants provided the following list of unacceptable side effects, each of which was cited by at least one participant: any organ damage; gastrointestinal problems; vision problems; dizziness/headaches (because it impairs driving); side effects that are worse than the actual MS symptoms; pain; dry mouth because they would drink more and use the washroom more, which may complicate MS; and effect on ability to reproduce. They considered the following side effects to be acceptable and, thus, would not affect their decision to discontinue treatment: fatigue; dry mouth, skin, or eyes; itchy skin; and muscle fatigue but not muscle pain or weakness.
The acceptable risk of side effects varied with the severity of the side effects for this group. For serious side effects, such as bleeding in the brain, participants said that they would accept a 1% or 2% risk (although their understanding of 1% or 2% was not explored). For less serious side effects, such as flulike symptoms or hair loss, acceptable risk was as high as 60% for some. Overall, treatment-naive participants felt that for any side effects, a risk of less than 10% would be tolerable.
For participants with RRMS, there seemed to be a tolerance for short periods of flulike symptoms (eg, 1 week is acceptable, 3 months is not), short periods of redness at the injection site, and, in general, side effects with low severity, short duration, and low risk of occurrence. These participants also said that they would accept a less than 2% risk of side effects occurring.
Three participants with progressive MS said that if the drug worked to control their disease they would accept any side effect as long as it does not cause death and is of a limited duration. One remarked, “If you can guarantee me that I'm going to feel better, I don't mind. I'll be on my back for a month or so if I know that 10 months of the year I'm fine.” Others in the group said that they would accept anything less than a 2% risk of serious side effects occurring and a higher risk for mild side effects.
Discussion
In this focus group study, potential effectiveness and side effects (eg, severity, duration, and likelihood of occurrence) of MS drugs emerged as the most important attributes to the patients with MS. In general, of those who were using an injectable therapy, participants did not find it difficult to use injectable drugs and were not willing to sacrifice the potential benefits or the stability of their current injectable therapies or to take on an increased risk of side effects for the sake of greater convenience in route or frequency of administration. Although some participants found the possibility of receiving injections twice yearly appealing, there were concerns about potential side effects and the impact of future therapy. Many participants stated that they prefer daily administration because it allows use of the medication to become routine and eliminates concern about losing track of when the drug needs to be taken. Adherence to injectable drugs, as well as overall satisfaction with the drugs, was enhanced with the ability to keep the medication unrefrigerated for extended periods and the availability of prefilled autoinjectors. Although participants did identify side effects from their current drugs, those using MS drugs generally felt that they were getting a benefit from their medication that made it worth enduring the side effects they were experiencing. However, there was dissatisfaction with the generally low level of effectiveness of MS drugs.
In deciding whether to switch to a new drug, participants would consider their satisfaction with their current medication and the potential effectiveness and side effects of the new drug. If the current drug was working well to control their symptoms and they were experiencing few or no side effects, participants would be less inclined to try something new and risk an outcome that was inferior to their current therapy. Some participants would also factor costs into the decision-making process. Because of a desire to avoid uncertainty and maintain stability in their lives, there was hesitancy about trying new drugs unless the new drug is superior to currently available drugs (in terms of effectiveness and side effects) or the current drugs are not working. Otherwise, participants preferred to avoid the uncertainty of switching to a new therapy (ie, what kind of side effects will occur, will the drug work) that is either new to them or new to the market. Although all the participants had concerns about unknown effects that may take years to identify for newly marketed drugs, participants with progressive MS expressed a greater willingness to try novel drugs than participants with RRMS. However, due to the relatively small sample size, this apparent difference may be a product of random variation rather than a real difference between the two MS populations, which should be explored in future studies. Participants felt that they could adapt to any drug characteristics and were unwilling to choose a drug based on convenience, conditional on receiving a benefit from the treatment. However, if the more convenient drugs were equally as effective as current drugs and had comparable side effects, then participants expressed interest in considering switching medications.
Previous research concurs with this study in suggesting that both efficacy and side effects influence patients' decisions on whether to switch treatments (81% and 65%, respectively).10 Route and frequency of administration (oral vs. injected) is also likely to be of increasing importance as oral agents for MS are developed (eg, dimethyl fumarate, fingolimod, teriflunomide). Similarly, recent studies suggest that route and frequency of administration (oral vs. injectable) are important attributes to patients with MS, and they would switch to oral treatments if they were equally effective as injectable treatments and had comparable side effects.16,17 However, there is a paucity of studies that have assessed the relationship between current therapy and preferences of patients with MS, particularly in treatment-naive, recently diagnosed patients. The inclusion of the treatment-naive subgroup in this study offered the opportunity to begin investigating these potential differences.
Previous research has generally indicated a ceiling effect when assessing which efficacy attributes are important to patients. Notably, most patients (≥90%) consider reducing disability progression, relapse rates, and brain lesions as important characteristics of drug treatment, as well as improving cognition.10 Similarly, many patients seem to be willing to trade greater efficacy for severe (even deadly) adverse effects. Not surprisingly, as the risk of death increases, the preference for enhanced efficacy concomitantly increases. In their exploratory mixed methods study of preferences of patients with MS for DMTs that included patients with RRMS and clinically isolated syndrome, Kremer et al18 identified 34 attributes that were then quantified using a best-worst scaling study. Consistent with the present study findings, Kremer et al18 found that the most important attributes of DMTs for patients with MS are the benefit (halting disease progression and improving quality of life) and avoidance of adverse effects as opposed to usability issues (eg, route and frequency of administration of DMTs).18 Similarly, a discrete-choice experiment conducted by Johnson et al19 to evaluate the benefit-risk trade-offs of treatment attributes associated with natalizumab found that the most important attribute of DMTs for patients with MS was slowing disability progression (in years) and, in fact, they seemed willing to tolerate a nontrivial risk of death for delaying disability progression.
Contrary to most previous studies in MS that developed attributes used in stated preference studies by relying on perspectives of health care professionals or existing literature without involving people with MS,13 this qualitative study drew on the patients with MS focus group discussions to identify preferences regarding the different characteristics of available and emerging drug therapies for MS. In particular, this study provides insights on how patients value noninjectable drug administration relative to effectiveness, side effects, and potential adverse events. As discussed earlier, the results of these focus group discussions were used to inform a quantitative study using stated-choice methods to determine the relative preferences of patients with MS for each of the identified important treatment characteristics.15 Then, both the qualitative and quantitative results can be used to inform regulation, development, and marketing of drugs so as to improve alignment with the preferences of patients and to inform the development of further research on this subject.
As with all focus group studies, there are inherent limitations that need to be acknowledged.20 Focus groups may be influenced by “dominant voices overriding other voices” that may or may not represent the thinking of all members. Although we attempted to minimize the dominant voice issue by making the focus groups homogenous (eg, grouping participants by MS type and MS treatment experience), it is worth noting that MS is an unpredictable and heterogeneous disease and that participants in these groups could still have different MS experiences. Moreover, despite the focus group discussion being moderated by a trained biocultural anthropologist (N.J.H.), it is possible (and difficult to know the extent to which) her presence may have affected the participants' responses. Furthermore, owing to the small, nonrepresentative sample, the analyses, interpretation, and conclusions may not be generalizable to all patients with MS, although theoretical and data saturation was reached.
PRACTICE POINTS
People with MS have different treatment preferences, which can partly be explained by their experiences with the disease or with their treatment.
The most important characteristics of MS drugs for people with MS are effectiveness and side effects.
People with MS desire to avoid uncertainty and maintain stability in their lives; there is hesitancy about trying new drugs unless the new drug is superior to currently available drugs (in terms of effectiveness and side effects) or the current drugs are not working.
Supplementary Material
Financial Disclosures
Dr. Lynd has received consultant fees from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Pfizer Canada, the British Columbia Ministry of Health, and the British Columbia Pharmacy. Dr. Marra has received advisory board fees from Boehringer Ingelheim, Glaxo, Pfizer Canada, and the Canadian Agency for Drugs Working Group. Dr. Traboulsee has received speaker fees and/or advisory board fees from Biogen, F. Hoffman-La Roche, Genzyme, and EMD Serono. The other authors declare no conflicts of interest.
Funding/Support
This research received funding from Genzyme Canada. Genzyme Canada had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit for publication.
References
- 1.Multiple Sclerosis International Federation Atlas of MS 2013: Mapping multiple sclerosis around the world. https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf Published 2013. Accessed September 20, 2018.
- 2.Statistics Canada Neurological conditions, by age group and sex, household population aged 0 and over, 2010/2011. Ottawa, Canada: Canadian Community Health Survey (CCHS); https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310046701 Published 2011. Accessed September 20, 2018. [Google Scholar]
- 3.Amankwah N, Marrie RA, Bancej C et al. Multiple sclerosis in Canada 2011 to 2031: results of a microsimulation modelling study of epidemiological and economic impacts. Health Promot Chronic Dis Prev Can. 2017;37:37–48. doi: 10.24095/hpcdp.37.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermersch P, Berger T, Gold R et al. The clinical perspective: how to personalise treatment in MS and how may biomarkers including imaging contribute to this? Mult Scler. 2016;22:18–33. doi: 10.1177/1352458516650739. [DOI] [PubMed] [Google Scholar]
- 5.What is MS? MS Society of Canada website. https://mssociety.ca/about-ms/what-is-ms 2016. Accessed November 30, 2017.
- 6.Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354 doi: 10.1136/bmj.i3518. i3518. [DOI] [PubMed] [Google Scholar]
- 7.Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. doi: 10.1016/S0140-6736(16)31320-4. [DOI] [PubMed] [Google Scholar]
- 8.Kieseier BC, Stüve O. A critical appraisal of treatment decisions in multiple sclerosis—old versus new. Nat Rev Neurol. 2011;7:255–262. doi: 10.1038/nrneurol.2011.41. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis T, Lublin F. Neurotherapeutics in multiple sclerosis: novel agents and emerging treatment strategies. Mt Sinai J Med. 2008;75:157–167. doi: 10.1002/msj.20030. [DOI] [PubMed] [Google Scholar]
- 10.Calfee JE. A Representative Survey of MS Patients on Attitudes Toward the Benefits and Risks of Drug Therapy. Washington, DC: AEI-Brookings Joint Center for Regulatory Studies; 2006. [Google Scholar]
- 11.Toussirot É, Bereau M. The risk of progressive multifocal leukoencephalopathy under biological agents used in the treatment of chronic inflammatory diseases. Inflamm Allergy Drug Targets. 2014;13:121–127. doi: 10.2174/1871528113666140224103712. [DOI] [PubMed] [Google Scholar]
- 12.Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol. 2011;10:1026–1034. doi: 10.1016/S1474-4422(11)70228-9. [DOI] [PubMed] [Google Scholar]
- 13.Webb EJ, Meads D, Eskyte I et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient. 2018;11:391–402. doi: 10.1007/s40271-017-0296-y. [DOI] [PubMed] [Google Scholar]
- 14.Coast J, Al-Janabi H, Sutton EJ et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21:730–741. doi: 10.1002/hec.1739. [DOI] [PubMed] [Google Scholar]
- 15.Lynd LD, Traboulsee A, Marra CA et al. Quantitative analysis of multiple sclerosis patients' preferences for drug treatment: a best-worst scaling study. Ther Adv Neurol Disord. 2016;9:287–296. doi: 10.1177/1756285616648060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utz KS, Hoog J, Wentrup A et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014;7:263–275. doi: 10.1177/1756285614555335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson LS, Loucks A, Gipson G et al. Patient preferences for attributes of multiple sclerosis disease-modifying therapies: development and results of a ratings-based conjoint analysis. Int J MS Care. 2015;17:74–82. doi: 10.7224/1537-2073.2013-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer IE, Evers SM, Jongen PJ, van der Weijden T, van de Kolk I, Hiligsmann M. Identification and prioritization of important attributes of disease-modifying drugs in decision making among patients with multiple sclerosis: a nominal group technique and best-worst scaling. PloS One. 2016;11 doi: 10.1371/journal.pone.0164862. e0164862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson FR, Van Houtven G, Özdemir S et al. Multiple sclerosis patients—benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256:554–562. doi: 10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 20.Smithson J. Using and analysing focus groups: limitations and possibilities. Int J Social Res Method. 2000;3:103–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


