Abstract
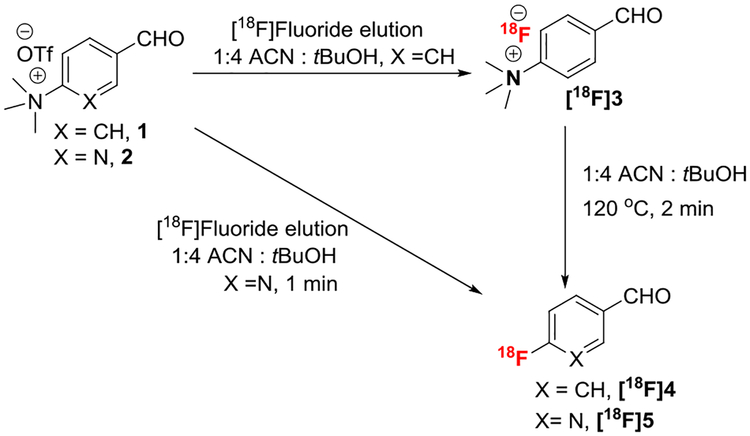
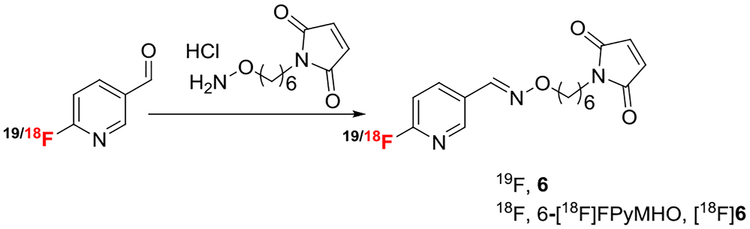
Following our recently published fluorine-18 labeling method, “Radio-fluorination on the Sep-Pak”, we have successfully synthesized 6-[18F] fluoronicotinaldehyde by passing a solution (1:4 acetonitrile: t-butanol) of its quaternary ammonium salt precursor, 6-(N,N,N-trimethylamino) nicotinaldehyde trifluoromethanesulfonate (2), through a fluorine-18 containing anion exchange cartridge (PS-HCO3). Over 80% radiochemical conversion was observed using 10 mg of precursor within 1 minute. The [18F] fluoronicotinaldehyde ([18F]5) was then conjugated with 1-(6-(aminooxy) hexyl)-1H-pyrrole-2,5-dione to prepare the fluorine-18 labeled maleimide functionalized prosthetic group, 6-[18F]fluoronicotinaldehyde O-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime, 6-[18F]FPyMHO ([18F]6). The current Sep-Pak method not only improves the overall radiochemical yield (50 ± 9%, decay-corrected, n = 9) but also significantly reduces the synthesis time (from 60–90 minutes to 30 minutes) when compared with literature methods for the synthesis of similar prosthetic groups.
Keywords: aldehyde, fluorine-18, maleimide, Sep-Pak
1 |. INTRODUCTION
The goal of the present study is to expand the scope of our novel radiolabeling method,1,2 “Radiofluorination on the Sep-Pak”, to prepare useful fluorine-18 labeled synthons. Using this method, nucleophilic fluorine-18 labeling can be done rapidly without azeotropic drying of [18F]fluoride. Due to the versatility of the aldehyde functional group for radiolabeling of biomolecules, we decided to apply this method to prepare fluorine-18 labeled aldehyde. A variety of prosthetic groups containing aldehyde functionalities have been developed.3,4 Kugler et al and Lemaire et al reported labeling of 4-fluorobenzaldehydes and 2-fluorobenzaldehydes and compared the radiolabeling efficiencies of nitro- and trimethylammonium triflate-precursors.5,6 Although conversions were comparable, the reaction was much faster for the trimethylammonium triflate precursors. Moreover, purification of 2 or 4-[18F] fluorobenzaldehydes from the trimethylammonium triflate precursors was done simply by using a Sep-Pak. The same research group has demonstrated high radiochemical conversion using the pyridine derivative by halogen (Cl) exchange.5 More challenging fluorine-18 labelings at the meta-position to prepare 3-[18F]fluorobenzaldehydes have also been reported by our7 and other groups from a variety of precursors.8–10 Recently, Richarz et al reported fluorine-18 labeled benzaldehydes according to the “minimalist” radiolabeling protocol, without base and azeotropic drying of [18F]fluoride.11 In this approach, fluorine-18 was eluted with a quaternary ammonium triflate precursor in methanol and was evaporated at 70°C to 80°C. The residue was dissolved in the appropriate solvent, and the resulting solution was heated to obtain labeled aldehydes. The [18F] fluorobenzaldehyde, thus prepared using different methods, were used for a variety of coupling reactions to radiolabel a wide range of biomolecules.3,4
[18F]Fluorobenzaldehydes are also used in the synthesis of maleimide functionalized fluorine-18 labeling agents, another important class of radiolabeling synthons for site specific labeling of biomolecules via coupling with a thiol group. A variety of fluorine-18 labeled maleimide functionalized prosthetic groups have been developed over the last few years, eg, 2-[18F]fluoronicotinaldehyde O-(6-(2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime ([18F]FBAMPy),12 N-[2-(4-[18F]fluorobenzamido)ethyl]maleimide ([18F]FBEM),13,14 N-[6-(4-[18F]fluorobenzylidene)aminooxyhexyl]maleimide ([18F]FBAM),15 [18F]FDG-maleimidehexyloxime ([18F]FDG-MHO),16 1-[3-(2-[18F]fluoropyridin-3-oxy)propyl]maleimide ([18F]FPyME),17,18 and N-(2-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-ethyl)-6-fluoronicotinamide ([18F]FNEM).19
Preparation of any of these compounds requires a 2-step or 3-step synthesis with overall preparation times ranging from 60 to 90 minutes. The objective of this work is to improve the radiosynthesis of a maleimide functionalized radiolabeling agents by using our newly developed “Radiofluorination on the Sep-Pak” method. Herein, we report rapid synthesis of 6-[18F] fluoropyridinealdehyde ([18F]5) and its coupling with a maleimide functionalized amino-oxy ligand to produce a useful synthon ([18F]6) for site-specific radiolabeling of biomolecules in 30 minutes of total synthesis time.
2 |. MATERIALS AND METHODS
The precursor 4-formyl-N,N,N-trimethylanilinium trifluoromethanesulfonate (1) was purchased from ABX advanced biochemical compounds (Radeberg, Germany). The non-radioactive cold standard 6-fluoropyridinealdehyde was obtained from Sigma Aldrich (St. Louis, MO, USA). The precursor 6-(N,N,N-trimethylamino)nicotinaldehyde trifluoromethanesulfonate (2),19 1-(6-(aminooxy)hexyl)- 1H-pyrrole-2,5-dione13,20 and non-radioactive cold standard of fluorine-18 labeled maleimide derivative, fluoronicotinaldehyde O-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime, FPyMHO (6),13 were synthesized by literature methods. All other chemicals and solvents were received from Sigma Aldrich (St. Louis, MO, USA) and used without further purification. Anhydrous solvents were used for all radiolabeling reactions. Fluorine-18 was received from National Institutes of Health cyclotron facility (Bethesda, MD, USA). Chromafix 30-PS-HCO3 anion-exchange Sep-Pak cartridges were purchased from Macherey-Nagel (Düren, Germany) and the packing material was reduced to half (~20 mg). Luna (2) C18 column (10 × 250 mm, 5 μm) was obtained from Phenomenex (Torrance, CA, USA). Other columns and all other Sep-Pak cartridges used in this synthesis were obtained from Agilent Technologies (Santa Clara, CA, USA) and Waters (Milford, MA, USA), respectively. Oasis MCX Plus cartridge was conditioned by passing 5-mL acetonitrile. Sep-Pak plus C18 cartridges were conditioned with 5-mL ethanol, 10-mL air, and 10-mL water. Low resolution mass spectra (MS) were recorded on a 6130 Quadrupole LC/MS, Agilent Technologies instrument equipped with a diode array detector. 1H, 13C, and 19F NMR spectra were recorded on a 400-MHz Bruker spectrometer. Chemical shifts (ppm) are reported relative to the solvent residual peaks of acetonitrile (δ 1H, 2.50 ppm; 13C 118.26, 1.79), methanol (δ 1H, 1H, 3.34 ppm), and chloroform (δ 1H, 7.26 ppm). 19F NMR spectra are reported with reference to the trifluoroacetic acid (δ 19F, −76.72 ppm). High performance liquid chromatography (HPLC) purification and analytical HPLC analyses for radiochemical work were performed on an Agilent 1200 Series instrument equipped with multi-wavelength detectors along with a flow count radiodetector (Eckert & Ziegler, B-FC-3500 diode).
HPLC conditions:
Method A; Column: Phenomenex Luna (2) C18 column (10 × 250 mm, 5 μm). Mobile phase: A: water (0.1% TFA); B: acetonitrile (0.1% TFA). Isocratic: 45% B; flow rate of 4 mL/min.
Method B; Column: Agilent XDB C18 column (4.6 × 150 mm, 5 μm). Mobile phase: A: water (0.1% TFA); B: acetonitrile (0.1% TFA). Gradient: 20% to 40% B in 10 minutes; flow rate of 1 mL/min.
Method C; Column: Agilent XDB C18 column (4.6 × 150 mm, 5 μm). Mobile phase: A: water (0.1% TFA); B: acetonitrile (0.1% TFA). Isocratic: 60% B; flow rate of 1 mL/min.
2.1 |. Precursors and cold standard
2.1.1 |. 6-(N,N,N-trimethylamino) nicotinaldehyde trifluoromethanesulfonate (Scheme 1, 2)
SCHEME 1.
Synthesis of [18F]fluoroaryl aldehydes [18F]4 and [18F]5
SCHEME 2.
Synthesis of 6-[18F]fluoronicotinaldehyde O-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime, 6-[18F]PyMHO, ([18F]6)
The precursor was synthesized according to the literature method using 6-chloronicotinaldehyde instead of 2,3,5,6-tetrafluorophenyl 6-chloronicotinate.19 Briefly, to a tetrahydrofuran (THF, 2 mL) solution of 6-chloronicotinaldehyde (1.4 g, 9.9 mmol) under nitrogen was added 1 M THF solution of trimethylamine (11 mL, 11 mmol), and the reaction was stirred at room temperature for 24 hours. The off-white precipitate thus obtained was collected and washed with cold (4°C) THF. The solid was suspended in anhydrous dichloromethane (50 mL), and trimethylsilyl trifluoromethanesulfonate (2 mL) was added over 10 minutes, followed by stirring at room temperature for 20 minutes. The solvent was evaporated under reduced pressure and washed with diethyl ether to afford compound 2 as an off-white solid (1.7 g, 5.4 mmol, 55% yield) and was used without further purification.
1H NMR (400 MHz, Acetonitrile-d3) δ 10.21 (s, 1H), 9.10 (d, J = 2.1 Hz, 1H), 8.58 (dd, J = 8.6, 2.2 Hz, 1H), 8.08 (d, J = 8.6 Hz, 1H), 3.61 (s, 9H); 13C NMR (101 MHz, CD3CN) δ 190.08, 150.42, 141.31, 133.25, 117.35, 115.66, 55.11; 19F NMR (376 MHz, CD3CN) δ −79.32.
MS (ESI) calculated mass for the parent C11H14F3NO4S, 313.06, found 165 [M − OTf].
2.1.2 |. 1-(6-(Aminooxy)hexyl)-1H-pyrrole-2,5-dione
The compound was prepared according to the literature method.13,20
1H NMR (400 MHz, Methanol-d4) δ 6.82 (s, 2H), 4.04 (t, J = 6.4 Hz, 2H), 3.52 (t, J = 7.1 Hz, 2H), 1.76 to 1.65 (m, 2H), 1.65 to 1.55 (m, 2H), 1.53 to 1.41 (m, 2H), 1.41 to 1.30 (m, 2H).
MS (ESI) calculated mass for the parent C10H16N2O3, 212, found 213 [M + H]+.
2.1.3 |. 6-Fluoronicotinaldehyde O-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime, FPyMHO, (Scheme 2, 6)
The compound was prepared according to the literature method for a similar compound.13
Briefly, to a solution of 1-(6-(aminooxy)hexyl)-1H-pyr-role-2,5-dione. HCl (0.025 g, 0.10 mmol) in dimethylformamide (10 mL) was added 6-fluoronicotinealdehyde (0.018 g, 0.14 mmol). The mixture was stirred for 30 minutes at room temperature. The solution was diluted with water and extracted with diethyl ether. The diethyl ether extract was washed with brine and dried (Na2SO4). The solvent was removed under vacuum, and the residue was purified by flash column chromatography (silica gel, hexane/EtOAc = 70/30) to afford 0.027 g (0.084 mmol, 84%) as a white powder.
1H NMR (400 MHz, Chloroform-d) δ 8.38 to 8.28 (m, 1H), 8.17 to 8.05 (m, 2H), 6.97 (dd, J = 8.6, 2.9 Hz, 1H), 6.70 (s, 2H), 4.18 (t, J = 6.6 Hz, 2H), 3.54 (t, J = 7.3 Hz, 2H), 1.80 to 1.56 (m, 4H), 1.52 to 1.30 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 170.84, 163.94 (d, J = 242.2 Hz), 146.77 (d, J = 15.3 Hz), 143.89, 138.46 (d, J = 8.3 Hz), 134.04, 126.78 (d, J = 4.7 Hz), 109.95 (d, J = 37.9 Hz), 74.50, 37.76, 28.86, 28.45, 26.49, 25.40. 19F NMR (376 MHz, Chloroform-d) δ −65.89. HRMS (ESI) calculated mass for the parent C16H18FN3O3Na [M + Na], 342.1224, found 342.1228.
2.2 |. Radiosynthesis
2.2.1 |. 4-[18F]fluorobenzaldehyde (Scheme 1, [18F]4)
Fluorine-18 labeled target water (370 – 1850 MBq) was diluted with 2 mL water and passed through an anion-exchange cartridge (Chromafix 30-PS-HCO3). The cartridge was washed with anhydrous acetonitrile (6 mL) and dried for 1 minute. The [18F]fluoride from the Sep-Pak was eluted (0.5 mL/min) with its quaternary ammonium triflate salt (1, 10 mg) in 0.5 mL 1:4 acetonitrile:t-butanol to produce compound [18F]3. The Sep-Pak was further eluted with 1-mL acetonitrile, and eluent was collected in the same vial. The reaction mixture was heated at 120°C for 2 minutes to produce compound [18F]4. No attempts were made to purify this product.
2.2.2 |. 6-[18F]fluoronicotinaldehyde (Scheme 1, [18F]5)
Fluorine-18 labeled target water (370–3700 MBq) was diluted with 2-mL water and passed through an anion-exchange cartridge (Chromafix 30-PS-HCO3). The cartridge was washed with anhydrous acetonitrile (6 mL) and dried under vacuum for 1 minute. The trapped [18F] fluoride from the Sep-Pak was eluted with the quaternary ammonium triflate (2, 7–10 mg) either in 0.5 mL 1:4 acetonitrile:t-butanol or in 0.5-mL acetonitrile (0.5 mL/min) through an activated Oasis MCX Plus cartridge to produce 6-[18F]fluoronicotinaldehyde ([18F]5). The cartridge was flushed with 1-mL acetonitrile, which was collected in the same vial for the next coupling reaction.
2.2.3 |. 6-[18F]fluoronicotinaldehyde O-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl) oxime, 6-[18F]PyMHO, (Scheme 2, [18F]6)
To the above solution of [18F]5 was added a solution of 1-(6-(aminooxy)hexyl)-1H-pyrrole-2,5-dione in (15 mg, 117 mM) in 1:1 methanol:1 N aqueous HCl (400 μL), and the resulting mixture was heated at 80°C for 5 minutes. The product was purified by either Sep-Pak or HPLC using semi-prep column. For Sep-Pak purification, the mixture was diluted with 8 mL of water and passed through an activated Sep-Pak plus C18 cartridge. The cartridge was washed with water (10 mL) followed by 15% acetonitrile in water (10 mL). The product was eluted with 1.5-mL acetonitrile to obtain the final compound 6-[18F]PyMHO ([18F]6). For HPLC purification, the crude reaction mixture was diluted with 3-mL water and injected on the HPLC (conditions: method A). To the collected HPLC fraction was added 10-mL water and passed through an activated Sep-Pak plus C18 cartridge, and the cartridge was washed with 5 mL of water. The trapped product [18F]PyMHO, ([18F]6) was eluted from the Sep-Pak cartridge with 1.5-mL acetonitrile. The identity and purity of the product were confirmed by analytical HPLC.
3 |. RESULTS AND DISCUSSION
The precursor 2 (Scheme 1),19 1-(6-(aminooxy)hexyl)-1H-pyrrole-2,5-dione,13,20 and the non-radioactive cold standard13 (Scheme 2, 6) of fluorine-18 maleimide derivative were synthesized by literature methods. In this study, packing material of the anion-exchange cartridge (Chromafix 30-PS-HCO3) was reduced to half (~20 mg), which is capable of effectively trapping all the fluorine-18 from the target water. The elution efficiency was slightly better (10%−15%) compared with the unmodified anion-exchange cartridge. We wanted to test the [18F] fluoride elution efficiency using a quaternary ammonium triflate precursor (Scheme 1, 1) of benzaldehyde in different solvents with the intention to directly incorporate the fluoride in the product. Attempts to elute [18F]fluoride from the Sep-Pak with 1 in acetonitrile were unsuccessful (Table 1); however, a solution of 1 in a mixture of acetonitrile and t-butanol (1:4) eluted fluorine-18 as [18F]fluoride salt [18F]3 (Scheme 1, Table 1). Quantitative conversion of [18F]3 to the desired product, 6-[18F] fluorobenzaldehyde ([18F]4), was observed when the eluted solution of [18F]3 (1:4 acetonitrile: t-butanol) was heated directly at 120°C for 2 minutes (Scheme 1). However, over 80% of activity was incorporated into the product within 1 minute by passing 10 mg of quaternary ammonium triflate precursor (2) in acetonitrile through the Sep-Pak containing [18F]fluoride. Fluoride elution efficiency was tested using different conditions (Table 1). As previously observed for another product,1 Sep-Pak elution of the labeled-product was slightly better with mixed solvents (1:4 acetonitrile: t-butanol) and an increased amount of precursor. The current Sep-Pak method of fluorination is at room temperature in absence of any base; therefore, as expected no other major by-product (Figure 1A) was observed by analytical HPLC. Most of the unreacted precursor was removed by passing the crude reaction mixture through an activated Oasis MCX plus cartridge. In a patent application, Olberg and Svadberg reported 33% elution of radioactivity as a product ([18F]5) using 20.3 mg of quaternary ammonium triflate precursor (2) in 1:1 acetonitrile and t-butanol mixture.21
TABLE 1.
Elution conditions from the Sep-Pak (Chromafix 30-PS-HCO3)
| Precursor | Amount, mg | Solvent, 1 mL | Product | Eluted from the Sep-Pak, %a |
|---|---|---|---|---|
| 1 | 5 | Acetonitrile | [18F]3 | 0b |
| 1:4, acetonitrile: t-butanol | [18F]3 | 88 | ||
| Methanol | [18F]3 | 99c11 | ||
| 1 | 1 | 1:4, acetonitrile: t-butanol | [18F]3 | 65 |
| 1 | 10c | DMSO | [18F]4 | 66–775,6 |
| 2 | 15 | Acetonitrile | [18F]5 | 89 |
| 1:4, acetonitrile: t-butanol | [18F]5 | 91 | ||
| 2 | 10 | Acetonitrile | [18F]5 | 80 ± 5b,d |
| 1:4, acetonitrile: t-butanol | [18F]5 | 85 ± 3b,d | ||
| 2 | 5 | 1:4, acetonitrile: t-butanol | [18F]5 | 63 |
| 2 | 10c | DMSO | [18F]5 | 80 ± 65 |
Radiolabeling was performed with 370 to 740 MBq of fluorine-18, precursor in 0.5-mL solvent, then flush the cartridge with 1-mL acetonitrile.
n ≥ 3.
Literature method (radiochemical yield).
71 ± 3% isolated yield.
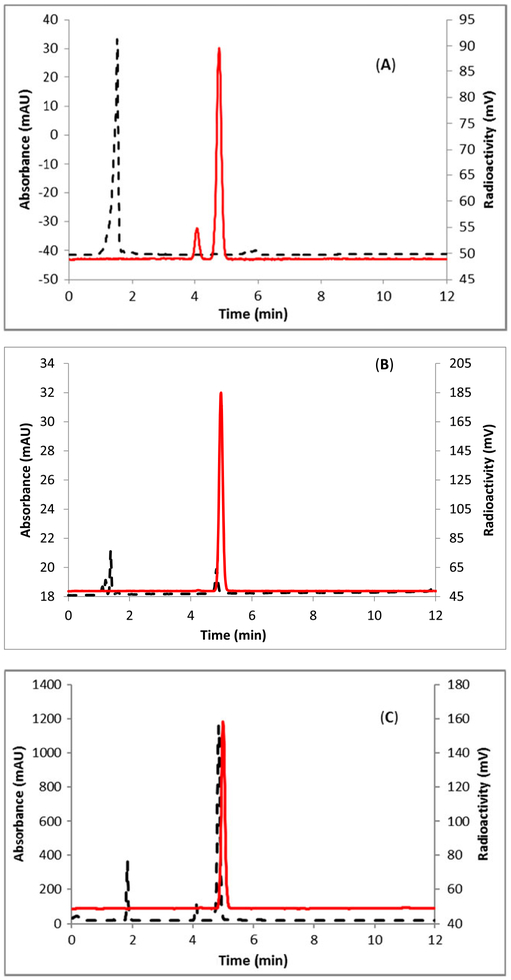
FIGURE 1.
HPLC analysis of ([18F]5). A, Crude reaction mixture; B, Sep-Pak purified product co-injected with the non-radioactive standard. Method A, solid line, in-line radiodetector; dotted line, UV detector at 254 nm
The elution efficiency using 2 to produce [18F]5 was 85 ± 3% (uncorrected, n = 6) in a <5-minute synthesis time with a radiochemical purity of >98% by analytical HPLC. The identity of the product ([18F]5) was confirmed by comparing its HPLC retention time with co-injected, authentic nonradioactive 6-fluoronicotinaldehyde (Figure 1B). The fluorine-18 incorporation of current method is comparable with literature method5 but in shorter times.
The synthesis of the maleimide functionalized labeled synthon ([18F]6) as well as the nonradioactive cold standard (6) is outlined in Scheme 2. The progress of the radiolabeling reaction was monitored by analytical HPLC, and >90% radiochemical conversion was observed at 80°C in 5 minutes. The product ([18F]6) was purified by using either Sep-Pak or HPLC. The compound purified by Sep-Pak was 90% radiochemically pure (Figure 2A). The overall isolated radiochemical yield was 50 ± 9% (decay-corrected, n = 9) in a 30-minute synthesis time with a molar activity of 259 to 370 GBq/μmol. In a typical radiosynthesis starting from 3626 MBq of [18F]fluoride, 1443 MBq of product was obtained. The product was stable in solution up to 2 hours of post-synthesis. The crude product was also purified by HPLC to remove the impurity peaks (Figure 2B). The overall isolated radiochemical yield was 43 ± 3% (decay-corrected, n = 3) in a 45-minute synthesis time with a comparable molar activity. The identity of the product [18F]6 (Scheme 2) was confirmed by comparing its HPLC retention time with co-injected, authentic nonradioactive standard (Figure 2C). T. M. Moore et al reported another derivative of this thiol-reactive prosthetic group, 2-[18F]FBAMPy, with a decay corrected yield of 30 ± 4% in 80 minutes of synthesis time.12
FIGURE 2.
HPLC analysis of 6-[18F]PyMHO ([18F]6), A, purified by Sep-Pak method; B, purified by HPLC method; C, co-injected with the non-radioactive standard. Method C, solid line, in-line radiodetector; dotted line, UV detector at 254 nm
4 |. CONCLUSION
We have successfully prepared 6-[18F] fluoronicotinaldehyde ([18F]5) on the Sep-Pak within a very short synthesis time (<5 minutes). Using [18F]5, another useful thiol reactive prosthetic group, [18F] FPyMHO ([18F]6), was synthesized with an decay-corrected radiochemical yield of 50 ± 9% in 30-minute synthesis time. This simple and fast radiolabeling procedure at room temperature without azeotropic drying of fluorine-18 has potential applications in the preparation of other useful fluorine-18 labeled synthons.
ACKNOWLEDGEMENTS
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding information
National Cancer Institute; National Institutes of Health, Grant/Award Number: HHSN261200800001E
REFERENCES
- 1.Basuli F, Zhang X, Jagoda EM, Choyke PL, Swenson RE. Facile room temperature synthesis of fluorine-18 labeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester without azeotropic drying of fluorine-18. Nucl Med Biol. 2016;43(12):770–772. https://doi.org/10.1016/j.nucmedbio.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basuli F, Zhang X, Woodroofe CC, Jagoda EM, Choyke PL, Swenson RE. Fast indirect fluorine-18 labeling of protein/peptide using the useful 6-fluoronicotinic acid-2,3,5,6-tetrafluorophenyl prosthetic group: a method comparable to direct fluorination. J Label Compd Radiopharm. 2017;60(3):168–175. https://doi.org/10.1002/jlcr.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preshlock S, Tredwell M, Gouverneur V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev. 2016;116(2):719–766. https://doi.org/10.1021/acs.chemrev.5b00493 [DOI] [PubMed] [Google Scholar]
- 4.van der Born D, Pees A, Poot AJ, Orru RVA, Windhorst AD, Vugts DJ. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem Soc Rev. 2017;46(15):4709–4773. https://doi.org/10.1039/C6CS00492J [DOI] [PubMed] [Google Scholar]
- 5.Kügler F, Ermert J, Coenen HH. Labeling of benzodioxin piper-azines with fluorine-18 as prospective radioligands for selective imaging of dopamine D4 receptors. J Label Compd Radiopharm. 2013;56(12):609–618. https://doi.org/10.1002/jlcr.3074 [DOI] [PubMed] [Google Scholar]
- 6.Lemaire C, Libert L, Plenevaux A, Aerts J, Franci X, Luxen A. Fast and reliable method for the preparation of ortho- and para-[18F]fluorobenzyl halide derivatives: key intermediates for the preparation of no-carrier-added PET aromatic radiopharmaceuticals. Journal of Fluorine Chemistry. 2012;138:48–55. https://doi.org/10.1016/j.jfluchem.2012.03.015 [Google Scholar]
- 7.Basuli F, Wu H, Griffiths GL. Syntheses of meta-[18F]fluorobenzaldehyde and meta-[18F]fluorobenzylbromide from phenyl(3-Formylphenyl) iodonium salt precursors. J Label Compd Radiopharm. 2011;54(4):224–228. https://doi.org/10.1002/jlcr.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH. Copper-catalyzed [18F]fluorination of (mesityl)(aryl) iodonium salts. Org Lett. 2014;16(12):3224–3227. https://doi.org/10.1021/ol501243g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tredwell M, Preshlock SM, Taylor NJ, et al. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew Chem Int Ed. 2014;53(30):7751–7755. https://doi.org/10.1002/anie.201404436 [DOI] [PubMed] [Google Scholar]
- 10.Chun J-H, Pike VW. Single-step syntheses of no-carrier-added functionalized [18F]fluoroarenes as labeling synthons from diaryliodonium salts. Org Biomol Chem. 2013;11(37):6300–6306. https://doi.org/10.1039/c3ob41353e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richarz R, Krapf P, Zarrad F, Urusova EA, Neumaier B, Zlatopolskiy BD. Neither azeotropic drying, nor base nor other additives: a minimalist approach to 18F-labeling. Org Biomol Chem. 2014;12(40):8094–8099. https://doi.org/10.1039/C4OB01336K [DOI] [PubMed] [Google Scholar]
- 12.Moore TM, Akula MR, Kabalka GW. Fluorine-18 radiochemistry: a novel thiol-reactive prosthetic group, [18F]FBAMPy. Natural Science. 2016;8(01):1–7. [Google Scholar]
- 13.Berndt M, Pietzsch J, Wuest F. Labeling of low-density lipoproteins using the 18F-labeled thiol-reactive reagent N-[6-(4-[18F] fluorobenzylidene)aminooxyhexyl]maleimide. Nucl Med Biol. 2007;34(1):5–15. https://doi.org/10.1016/j.nucmedbio.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Kiesewetter DO, Jacobson O, Lang L, Chen X. Automated radio-chemical synthesis of [18F]FBEM: a thiol reactive synthon for radiofluorination of peptides and proteins. Appl Radiat Isot. 2011;69(2):410–414. https://doi.org/10.1016/j.apradiso.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kniess T, Kuchar M, Pietzsch J. Automated radiosynthesis of the thiol-reactive labeling agent N-[6-(4-[18F]fluorobenzylidene) aminooxyhexyl]maleimide ([18F]FBAM). Appl Radiat Isot. 2011;69(9):1226–1230. https://doi.org/10.1016/j.apradiso.2011.03.043 [DOI] [PubMed] [Google Scholar]
- 16.Wuest F, Berndt M, Bergmann R, van den Hoff J, Pietzsch J. Synthesis and application of [18F]FDG-maleimidehexyloxime ([18F]FDG-MHO): a [18F]FDG-based prosthetic group for the chemoselective 18F-labeling of peptides and proteins. Bioconjug Chem. 2008;19(6):1202–1210. https://doi.org/10.1021/bc8000112 [DOI] [PubMed] [Google Scholar]
- 17.de Bruin B, Kuhnast B, Hinnen F, et al. 1-[3-(2-[18F] Fluoropyridin-3-yloxy)propyl]pyrrole-2,5-dione: design, synthesis, and radiosynthesis of a new [18F]fluoropyridine-based maleimide reagent for the labeling of peptides and proteins. Bioconjug Chem. 2005;16(2):406–420. https://doi.org/10.1021/bc0497463 [DOI] [PubMed] [Google Scholar]
- 18.Cavani M, Bier D, Holschbach M, Coenen HH. Efficient synthesis of [18F]FPyME: a new approach for the preparation of maleimide-containing prosthetic groups for the conjugation with thiols. J Label Compd Radiopharm. 2017;60(1):87–92. https://doi.org/10.1002/jlcr.3469 [DOI] [PubMed] [Google Scholar]
- 19.Yue X, Yan X, Wu C, et al. One-pot two-step radiosynthesis of a new 18F-labeled thiol reactive prosthetic group and its conjugate for insulinoma imaging. Mol Pharm. 2014;11(11):3875–3884. https://doi.org/10.1021/mp5001857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DS, Hammaker JR, Tedder ME. A convenient synthesis of N-(tert-butyloxycarbonyl)aminooxy ethers. Tetrahedron Lett. 2000;41(10):1531–1533. https://doi.org/10.1016/S0040-4039(99)02331-X [Google Scholar]
- 21.Olberg DE, Svadberg A. International patent WO/2017/072200 May. 2017;4.