Abstract
Background:
An interesting area of scientific research is the development of potential antiaging drugs. In order to pursue this goal, it is necessary to gather the specific knowledge about the adequate preclinical models that are available to evaluate the beneficial effects of new potential drugs. This review is focused on invertebrate and vertebrate preclinical models used to evaluate the efficacy of antiaging compounds, with the objective to extend life span and health span.
Methods:
Research and online content related to aging, antiaging drugs, experimental aging models is reviewed. Moreover, in this review, the main experimental preclinical models of organisms that have contributed to the research in the pharmacol-ogy of lifespan extension and the understanding of the aging process are discussed.
Results:
Dietary restriction (DR) constitutes a common experimental process to extend life span in all organisms. Besides, classical antiaging drugs such as resveratrol, rapamycin and metformin denominated as DR mimetics are also discussed. Likewise, the main therapeutic targets of these drugs include sirtuins, IGF-1, and mTOR, all of them being modulated by DR.
Conclusion:
Advances in molecular biology have uncovered the potential molecular pathways involved in the aging process. Due to their characteristics, invertebrate models are mainly used for drug screening. The National Institute on Aging (NIA) developed the Interventions Testing Program (ITP). At the pre-clinical level, the ITP uses Heterogeneous mouse model (HET) which is probably the most suitable rodent model to study potential drugs against aging prevention. The accelerated-senescence mouse P8 is also a mammalian rodent model for aging research. However, when evaluating the effect of drugs on a preclinical level, the evaluation must be done in non-human primates since it is the mammalian specie closest to humans. Research is needed to investigate the impact of new potential drugs for the increase of human quality of
Keywords: Aging, Resveratrol, sirtuins, IGF-1, mTOR, heterogeneous mice model, SAMP8
1. Introduction
Nowadays, life expectancy in the first-world countries has increased significantly due to the sanitary and alimentary strategies that governments have established [1]. Not long ago, the WHO reported that 125 million people were aged 80 or older in the world [1]. Consequently, a growing need to develop drugs for the prevention of age-related diseases has emerged, constituting one major sanitary, social and economic problem in the first-world countries. Among the many diseases associated with aging, we can emphasize the obesity-related type 2 diabetes mellitus (T2DM) and hypertension, which represent major risk factors for other serious conditions such as stroke and cardiovascular disease. Also, neuropathies, metabolic syndrome, arthritis, or hypercholesterolemia worsen during aging, as well as pathological factors such as chronic inflammation and carcinogenesis [2-4].
When trying to develop new antiaging drugs, it must be taken into account the inherent difficulties of assessing their effects in clinical trials, since these are very lengthy processes with a high number of variables [3]. It is noteworthy to mention that some of the drugs exhibiting antiaging effects are currently used in the cure of aging-associated diseases, e.g. metformin in diabetes’ treatment. However, we must differentiate between drugs aiming to reverse the aging process and geroprotective drugs, which prevent premature aging and allow lifespan extending. In both cases, the development of drugs for the treatment of chronic disorders, such as disturbances on cholesterol metabolism, rheumatoid arthritis, Parkinson’s disease (PD) and other neurodegenerative disorders would be a good therapeutic strategy. These drugs would exert very significant antiaging effects through improvement in the quality of life.
When a researcher is interested in the study of aging process, he/she must keep several considerations in mind:
It is important to understand the biochemical pathways related to the physiology of the aging process. Indeed, one of the main purposes of gerontology is the study of the biological process of aging. The physiology of aging involves complex mechanisms linked to a slow and progressive loss of cellular regulation, hormonal response, neurotransmitter activity, decrease of antioxidant enzymes and alterations on autophagy that lead to cellular and molecular damage [2, 3]. For instance, it is well known that lifespan increase depends on an efficient degradation of cytoplasmic damaged organelles and aberrant proteins, which are eliminated through the autophagy-lysosomal system [4]. Therefore, autophagy constitutes an important homeostatic mechanism that becomes altered in the aging process and needs to be studied properly. Fortunately, aging-related pathways are highly conserved in the evolution of the organisms whether they are worms, yeasts, flies or mammals, such as rodents and humans [5-10]. This provides a diverse array of experimental models to be used.
As it has been previously mentioned, aging research can also be conducted in many in vivo models, which have their own benefits and limitations. Hence, yeasts (Saccharomyces cerevisiae), nematodes (Caenorhabditis elegans), fruit flies (Drosophila melanogaster), short-living fishes (Nothobranchius furzeri), rodents (mice and rats), dogs and non-human primates are widely used depending on the objective of the study [5]. For instance, if we aim to study whether a drug specifically extends lifespan, experimental models such as worms, flies, yeast or rodents are useful due to their short lifespan. By contrast, to assess potential effects of drugs on diseases like T2DM or Alzheimer’s disease (AD), a closer approach to humans would be more appropriate, since this would allow longer and more elaborated treatments. Moreover, in many cases, it is also necessary to evaluate the potential side effects or toxicities of the drug of interest. For instance, if the aim is to search for tumorigenic effects or renal toxicity, a mammalian rodent model is required.
Finally, due to the lack of systemic complexity, in vitro studies have the disadvantage that they fail to show all mechanisms involved in the effect of the drug or its toxicity, as well as the influence of metabolism on its biodisponibility and elimination. As a consequence, they are not recommended for these studies.
2. PHYSIOLOGICAL TARGETS ASSOCIATED WITH THE AGING PROCESS
The first step in longevity research is to identify which molecular pathways are relevant to human aging. In a second step, it is important to develop safe drugs that will delay aging or aging-associated diseases by targeting the molecules involved in those pathways [4-9]. In a recent manuscript published by López-Otín and colleagues, they discuss the most important factors involved in the aging process in different organisms [11].
2.1. Genetic and Epigenetic Changes
The physiological process of aging is a complex mechanism. One of its main cellular hallmarks is the shortening of DNA’s ends (telomeres), which is initiated by the activity of specialized DNA polymerases known as telomerases [12-15]. In addition to telomere shortening, other factors are involved in the process of mammalian senescence, such as mitochondrial dysfunction, epigenetic alteration, genomic instability, altered intercellular communication and immune system activity alteration [11]. All these elements constitute potential targets for antiaging therapies [11]. Likewise, changes in DNA state, such as methylations and acetylations mediated by methyltransferases and histone acetylases, constitute epigenetic alterations that are involved in the aging process not only in mammalians but also in invertebrates [11-15]. As a result, we can consider that an increase in genetic alterations will be associated with the aging process. This statement can be of interest for those researchers aiming to detect genes involved in longevity. Also, heritable factors can affect positively or negatively the process of aging, thus influencing the resulting lifespan. For example, genetic background can favour age-associated diseases such as T2DM, hypertension associated with heart disease, neurodegenerative diseases and different types of cancer [16-21]. Some of the involved genes are the insulin-like growth factor 1 (igf1), the antioxidant superoxide dismutase (sod), several cytokine genes (tnf-α, il-1α, etc.) and the serum paraoxonase/arylterase 1 (pon1), an enzyme involved in lipid β-oxidation. Pon1 may have a prominent role in the development of metabolic diseases such as atherosclerosis [16]. Another important gene involved in the longevity process is the Forkhead box O3 (foxo3a). Several studies on humans from Japan, Germany, Italy and China demonstrated that in centenary subjects, foxo3a expression is linked to human longevity in both genders [17-21]. Overall, any drug that could modulate the expression or epigenetic modifications of these genes could be a promising strategy to improve age-related diseases and increase lifespan [21-24] (Table 1).
Table 1.
Relevant antiaging pathways modulated by the main drugs that mimics dietary restriction.
| Antiaging Strategy | Pathway (s) Modulated | Antiaging Effects |
|---|---|---|
| Dietary restriction | AMPK, SIRT-1, mTOR, PI3k/Akt pathway, IGF-1, FOXO, PGC-1α |
Decreases stress response, improves mitochondrial biogenesis, improves cellular maintenance, reduces inflammation. |
| Metformin | AMPK (Activation), SIRT1 activator via its effect on AMPK, PGC-1α |
Decreases stress response, improves mitochondrial biogenesis, improves cellular and genome maintenance, reduces inflammation. |
| Resveratrol | SIRT-1 (Activation), NF-κB inhibition, p53, FOXO, PGC-1α | Decreases stress response, improves mitochondrial biogenesis, improves cellular and genome maintenance, reduces inflammation. |
| RAPAMYCIN | mTOR (inhibition), inhibition of the eukaryotic translation initiation factor 4E (eIF4E)-binding proteins (4E-BPs). | Increases autophagy, improves mitochondrial biogenesis. |
| Selegiline/Deprenyl | SOD and CAT | Antioxidant effects. |
| Aspirin and Nordihydroguaiaretic acid | Antioxidant (SOD, CAT) and anti-inflammatory (COX-1 and NF-κB inhibition) | Decreases stress response, reduces inflammation. |
2.2. Caloric Intake
In addition to genetic and epigenetic changes, the aging process could be regulated by caloric intake, as it has been strongly supported by current available evidence. Thus, reducing caloric intake, also known as dietary restriction (DR) or caloric restriction (without causing malnutrition), results in the slowing of the aging process in an array of organisms, from yeast and flies to rodents and non-human primates [4, 8, 11, 24]. Notwithstanding, DR does not escape from controversy, since although it may be beneficial at the preclinical level, in some human studies its effectiveness is more questionable [25, 26].
Nonetheless, it might be possible that drugs that reproduce the effects of DR, also known as DR mimetics, could be a suitable strategy for anti-aging therapy [27]. The mechanism of how DR regulates longevity remains largely unknown, although several pathways that are highly conserved from prokaryotes to eukaryotes have been proposed [27-29]. Some of these pathways are IGF signalling (whose downregulation extends lifespan), sirtuin 1 (SIRT1) activation, the mammalian target of rapamycin (mTOR) kinase and AMP-activated protein kinase (AMPK). Likewise, DR could modulate lifespan trough reduction of oxidative damage, activation of transcription factors and the control of endoplasmic reticulum stress response [2, 8, 11, 24].
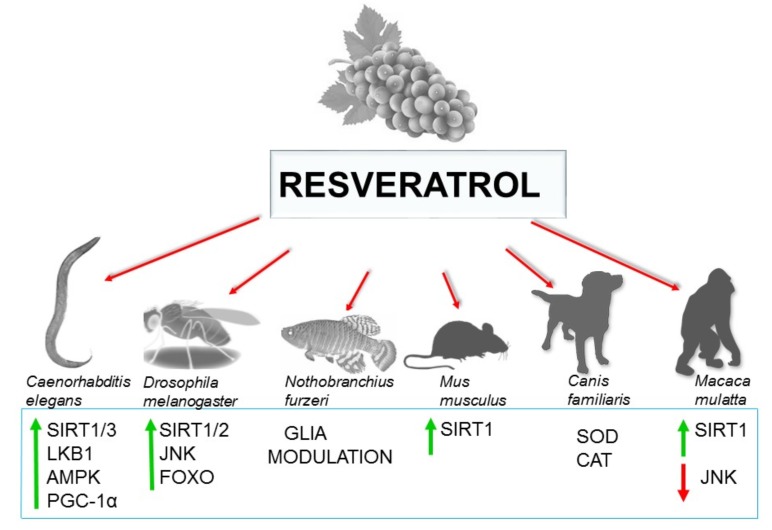
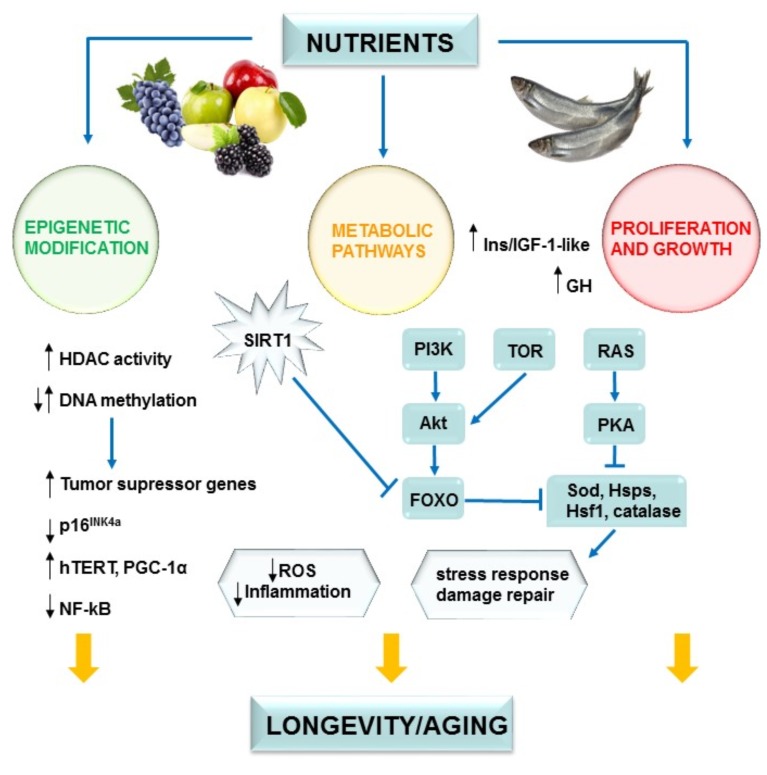
The silent information regulator 2 (Sir2) proteins, also known as SIRT1, are members of a highly conserved family of proteins that act as either nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases or mono-ADP-ribosyltransferases [2-4, 7,11, 30]. Sirtuins are found in a variety of species, ranging from bacteria to humans. SIRT1 is probably the best characterized sirtuin in the aging process [30-34]. It is well known that SIRT1 activation by DR or pharmacological intervention increases the acetylase activity of this enzyme, extending lifespan in yeasts, worms, flies and mammals [5]. Moreover, the SIRT1 activation and its regulation by resveratrol (RESV) is a well-known described mechanism of pharmacological intervention. It has been reported that this natural compound can deacetylate and modulate the activation of SIRT1 and activate the PPARγ coactivator 1α (PGC-1α) [31] (Fig. 1). In addition, PGC-1α exerts important physiological functions in the process of mitochondrial biogenesis, activation of antioxidant defenses and inhibition of amyloid-β (Aβ) peptides (the main component of amyloid plaques) production in Alzheimer’s disease (AD).
Fig. (1).
Signal transduction, nutrient sensing as well as epigenetic profile are nutrient dependent and converge on longevity regulation.
mTOR kinase is a multiprotein complex involved in the regulation of cellular metabolism [34]. Since mTOR activation accelerates the aging process in all organisms, its pharmacological inhibition using rapamycin might constitute an interesting approach to increase lifespan [34, 35]. On another front, AMPK activation favours healthy aging and it is involved in cell metabolism regulation. Indeed, metformin, an AMPK-activator drug which is used in T2DM treatment, could be another pharmacological approach to increase lifespan [13]. Moreover, AMPK could also activate SIRT1 [28].
2.3. Mitochondrial Dysfunction
Mitochondria constitute master regulators of cellular energetics and are involved in free radical production [11, 35-39]. According to the free radical theory of aging proposed by Denham Harman in 1956, aging favours continuous mitochondrial dysfunction, the consequence of which is an increase in reactive oxygen species (ROS) production [37]. This process is cumulative and damages other biological structures, favouring further continuous mitochondrial deterioration through positive feedback [11, 35].
However, recent studies question the role of ROS in the aging process and the efficacy of antioxidants as anti-aging compounds [35-37]. To explain this discrepancy, the concept of “mitohormesis” has been introduced. This term tries to explain why the relationship between ROS generation and aging is not linear, and emphasizes that physiological concentrations of cellular ROS are necessary for life. Thus, eliminating ROS may be detrimental and could explain the fact that some antioxidants showed no improvements in lifespan.
Moreover, the participation of mitochondria in cellular physiological functions is complex [39]. Mitochondria are involved in the regulation of metabolic pathways, including cytosolic protein synthesis that may be altered in the aging process. They are also involved in the process of adequate proteostasis, maintaining chaperones that repair misfolded proteins and proteases that remove proteins damaged [40-42]. Likewise, mitochondrial-associated membranes (MAMs) are important signalling sites, allowing continuous interconnection with the endoplasmic reticulum (ER), having key roles in physiological processes such as apoptosis, autophagy, Ca2+ transport, inflammation and lipid synthesis [41, 42].
2.4. Protein Alteration and Endoplasmic Reticulum Stress
As it has been previously mentioned, some protein alterations, such as protein misfolding, are also involved with the aging process. For example, accumulations of Aβ protein in AD and increased brain levels of α-synuclein in Parkinson’s disease have been reported [7]. This is usually regulated by the ubiquitin-proteasomal enzymes, autophagy and molecular chaperones, which prevent the accumulation of toxic levels of unfolded or misfolded proteins [4]. Consequently, the accumulation of altered proteins is often caused by dysfunctions in those processes that are supposed to prevent it, but have modified their activity due to aging.
Finally, the association between ER stress response and the longevity process remains poorly understood [40]. ER is involved in the synthesis and processing of secretory and membrane proteins. Its stress is characterized by the accumulation of misfolded proteins in the ER lumen, and the activation of a stress signal pathway known as the unfolded protein response (UPR) [40-42]. The accumulation of unfolded or misfolded proteins occurs in aging-associated diseases such as AD and PD [42]. Interestingly, lifespan in C. elegans is shortened by inhibition of stress sensors in the UPR signalling pathways, such as inositol-requiring enzyme 1[36]. Accordingly, inhibition of ER stress by chemical drugs could improve the process of aging [36].
3. INVERTEBRATES AS PRECLINICAL EXPERI-MENTAL MODELS IN AGING RESEARCH
Although mammals are the ones most commonly used as experimental models for testing life-extending agents, the preclinical development of antiaging drugs usually starts in invertebrate organisms like nematodes, yeasts and flies [28]. One of the main advantages of these organisms is their short life expectancy, which allows a fast evaluation of the potential efficacy of the tested drugs.
3.1. Caenorhabditis elegans as a Model of Aging
C. elegans is a suitable preclinical experimental model for the study of aging, mainly due to its short lifespan (around 20 days) under normal growth conditions [43] (Table 2). This allows the performance of genetic dissection of the mechanisms affecting aging and lifespan modification [44-47]. Moreover, several genes and signalling pathways associated with neurological disorders in humans have orthologues in C. elegans. For instance, genetic modifications increasing the levels of proteins like Aβ 42 (transgenic strain CL2006) or poly-glutamine (polyQ) in this worm, make C. elegans a useful model to study the molecular mechanisms involved in neurodegenerative processes such as AD, PD and Huntington’s disease (HD) [44-46]. Regarding to these brain diseases, interestingly, there is a high degree of evolutionary conservation of biochemical pathways between C. elegans and mammals [45-51].
Table 2.
Summary of preclinical model’s strengths and limitations in antiaging research.
| Models of Aging | Strengths | Limitations |
|---|---|---|
| Caenorhabditis elegans | Short life expectancy, fast evaluation of the potential efficacy of the tested drugs. Lower research costs. Simple neuronal system | Invertebrate model. Toxicity studies. Studies on metabolism of drugs. |
| Drosophila melanogaster | Short life expectancy, fast evaluation of the potential efficacy of the tested drugs. Lower research costs. | Invertebrate model. Toxicity studies. Studies on metabolism of drugs. |
| Saccharomyces cerevisiae | Short life expectancy, fast evaluation of the potential efficacy of the tested drugs. Lower research costs. | Invertebrate model. Toxicity studies. Studies on metabolism of drugs. |
| Nothobranchius furzeri | Appropriate for anti-aging drug research testing | Organs are quite different to those in humans. Studies on aging-associated diseases, such as T2DM and AD. |
| Senescence prone inbred strains | Appropriate for anti-aging drug research testing and neurodegenerative diseases, such as AD. | Significant differences at a pharmacokinetical drug level. Lifespan extension could also vary between rodent’s genders. |
| HET mice | Developed by the National Institute on Aging interventions testing program as the most adequate mammal mice model in drug research. | Differences at a pharmacokinetical drug level. Lifespan extension could also vary between rodent’s genders. |
| klotho mutant mice | Common features with human aging process, such as short lifespan, hypokinesia, infertility, arteriosclerosis, osteoporosis and brain alterations related to neurological disorders. | Differences at a pharmacokinetical drug level. |
| Rodent models of progeria | Effects of premature aging. Reduction in time, labor and costs for lifespan studies, as well as the ability to target accelerated aging to specific organs. | Differences at a pharmacokinetical drug level. |
| Dogs | Useful in studies with drugs related with AD. | Cognitive evaluation and behavioral studies. |
| Nonhuman primate models of aging | Evaluate behavioral or cognitive decline tests. Allow the investigation of molecular mechanisms of neurodegenerative diseases. Best extrapolation of the results to our specie. | Expensive. Isolation in cages is difficult. |
The insulin/IGF-1 signalling pathway is probably the most studied and frequently conserved mechanism that controls aging and lifespan in this worm. Daf-2 gene is the homolog of mammalian IGF-1 receptor genes for invertebrates. Thus, decreased Daf-2 signalling induces an increase in C. elegans lifespan and constitutes a suitable target for aging research. Another lifespan-involved gene is clk-1, which was characterized and found to be conserved among eukaryotes, including humans, rodents, and yeast (homologue of the Saccharomyces cerevisiae COQ7 gene) [51]. The clk-1 gene plays a prominent role in the biosynthesis of the mitochondrial electron transport component Coenzyme Q (CoQ, ubiquinone) [52]. Coenzyme Q is also involved in other processes such as fatty acid β-oxidation, uridine synthesis and antioxidant activities. In mammals, CoQ levels tend to decrease with aging, and this could explain, at least in part, the differences found in aging processes among different organisms, as well as the susceptibility to aging or aging-related diseases of some tissues such as the brain, which is especially sensible to oxidative stress. Accordingly, lower levels of CoQ levels have been found in cerebrospinal fluid of AD [53]. In this regard, Varela-López and colleagues reported in an excellent revision that CoQ10 supplementation could be a suitable compound to prevent lifespan shortening due to its effect on mitochondrial function and antioxidant defences in mammals [54].
Giving support to the free radical theory of aging, increases in antioxidant enzymes such as SOD and catalase (CAT) have been found to increase worm lifespan [55, 56]. Notwithstanding, some reports have concluded that damage from ROS is unlikely to be a major primary determinant of aging in C. elegans [57, 59]. For instance, Van Raamsdonk and Hekimi showed that the knockout of sod-2, the main mitochondrial SOD in C. elegans, increases lifespan [60].
This invertebrate model has been used to study numerous nutritional natural compounds (Fig. 1). Among them, RESV, a natural polyphenol compound found in red wine [28] stands out (Fig. 2). It has been found that a dietary supplementation with RESV extends lifespan in C. elegans, hypothetically through SIRT1 activation [28]. Interestingly, a screening with two natural compounds structurally similar to resveratrol such as quercetin and piceatannol, also demonstrated SIRT1 activation. In addition, the antiaging effects of RESV observed in C. elegans (Fig. 1) might be attributed to its capacity for reducing oxidative stress through direct ROS-scavenging activity and mitochondrial dysfunction minimization [28]. Furthermore, in a recent study, Shen and colleagues evidenced that piceatannol significantly extended C. elegans’ lifespan via the insulin/ IGF-1 signalling and sir-2.1-dependent pathways.
Fig. (2).
Resveratrol increase lifespan in vertebrates and invertebrates preclinical models mainly through SIRT1 activation.
Likewise, recent studies reported that the beneficial effects of RESV could be the result from the activation of SIRT3, liver kinase B1 (LKB1) and AMPK [30-32]. Indeed, RESV improves mitochondrial function and prevents against metabolic alterations by activating PGC-1α through SIRT1 [30-32] Table 1. In addition, it was reported that C. elegans produces specific small molecules to control adult lifespan through the activation of SIRT1, giving support to the hypothesis that sirtuins play an important role in regulating the cellular functions associated to lifespan [45, 47]. Taking all this data into account, it is clear that RESV exerts multiple beneficial cellular effects through a complex mechanism of action.
Metformin is another drug with potential antiaging properties [13]. Preclinical data in C. elegans suggests that metformin mimics DR and increases lifespan and health span through the activation of AMPK [28, 35] (Table 1). Recently, Cabreiro and colleagues suggested that the effects of metformin on C. elegans lifespan are indirect, through metabolic modulation of intestinal microbiome (e.g. E. coli) present in in vitro cultures of C. elegans [52]. The authors stated that Metformin alters the bacterial folate cycle, decreasing the S-adenosylmethionine levels in C. elegans, and thus slowing down its aging process by both AMPK activation and DR [52].
In turn, Robida-Stubbs and colleagues reported that rapamycin also increased lifespan in C. elegans, targeting mTOR and regulating SKN-1 (Protein skinhead-1) and DAF-16 (Abnormal DAuer Formation-16) [33]. Moreover, autophagy proved to be a key mechanism, since the effects of rapamycin were lost when the gene encoding the autophagy modulator BEC-1 (the worm orthologue of mammalian beclin 1) was suppressed [33].
In addition to the three above-mentioned drugs, C. elegans is a suitable preclinical model widely used to assess other potential molecules that mimic DR [28]. For example, molecules such as Celecoxib, aspirin, allantoin, trichostatin A, LY-294002 or geldanamycin, as well as natural products like flavonoids, have been evaluated in this model [44-50]. Therefore, the invertebrate C. elegans model has proved to be an effective approach for the evaluation of new drugs in the research field of aging.
3.2. Drosophila Melanogaster as a Model of Aging
Drosophila melanogaster (DM) is a useful invertebrate model for the evaluation of antiaging drugs such as RESV, metformin, rapamycin, deprenyl, and lamotrigine, both in basic and in applied research [61-75] Table 2. Indeed, most of the research on lifespan and aging is focused in the DM model since the established vertebrate models are too long-lived [54]. In addition, the high degree of functional conservation in proteins between insects and humans makes DM more interesting for preclinical research (Table 2).
Proshkina and colleagues reviewed the main genes that have been identified as positive regulators of lifespan and longevity when they are overexpressed in DM [14]. According to their discussed data, the majority of longevity-associated genes are involved in the control of metabolism, such as IGF-1R, phosphatidylinositide 3-kinase (PI3K), protein kinase B (PKB), AMPK and mTOR [4]. Therefore, the main pathways involved in the regulation of the process of lifespan and health span are also present in DM [64-66]. Note that the insulin receptor in DM is homologous to mammalian insulin and IGF-1 receptors. Furthermore, DM is useful for the study of many pathological mechanisms involved in neurological disorders, especially AD, and since genetic manipulation in DM is feasible, transgenic flies can be generated in order to study some diseases such as AD and PD [66]. Indeed, the overexpression of mutant human Tau in DM nervous system allowed the detection of a pathologic increase in Tau phosphorylation [70]. On another front, Spindler and colleagues showed that the beta-adrenergic receptor has an important role in DM aging process [71]. The authors used two β-blockers, metoprolol and nebivolol, which allowed them to conclude that the β-receptor is fundamental in lifespan regulation [71]. Likewise, Jordens and colleagues reported that the effects of deprenyl on lifespan in DM are mediated through an increase in antioxidant enzyme activity [75].
While the role of metformin on aging in DM is unclear, a recent study showed beneficial antiaging effects when this compound was administered in obese flies [65, 72]. Other drugs like pioglitazone showed antiaging properties targeting the insulin-signalling pathway, whereas RESV effects in DM could be explained through Sir2 activation of a NAD+-dependent class III histone deacetylase [66]. Bearing in mind that Sir2 expression in DM modulates apoptotic and survival pathways through activation of Jun N-terminal kinase (JNK) and FOXO [73], the activation of SIRT1 may have a double effect in DM: antiaging and pro-apoptotic. Nevertheless, the role of RESV and sirtuins in fruit fly aging are still highly controversial in the field. Regarding rapamycin, it has been reported that this compound impairs motor performance and exerts pathophysiological effects in a DM model of Friedreich’s ataxia [74]. However, rapamycin effects in DM lifespan are superior to those of DR restriction. Therefore, the antiaging pathways or genes activated by rapamycin seem to be different to those activated with DR in DM model [66].
3.3. Saccharomyces cerevisiae as a Model of Aging
The yeast Saccharomyces cerevisiae constitutes a common preclinical aging model used for biomedicine and aging research [76-79]. One of the advantages of using yeast cells as experimental models is that the basic metabolic pathways involved in neurodegenerative processes are well conserved Table 2. For instance, S. cerevisiae has been used as a preclinical model to study human aging associated to neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), PD, and HD [77]. Furthermore, S. cerevisiae allows easier quantification and analysis of longevity than other preclinical models [78] (Table 2).
Perhaps, one inconvenient of the S. cerevisiae model is the lack of the insulin-signalling pathway. However, precursor pathways such as serine/threonine kinase AKT/ PKB and the cAMP-dependent protein kinase A (PKA) can be found in this model. Moreover, although DR has been reported to extend lifespan in a broad range of organisms such as yeasts, worms, fruit flies, mice, rats, and monkeys, Huberts and colleagues demonstrated that DR did not induce lifespan extension in S. cerevisiae [79]. For this reason, the authors suggest that studies on dietary restriction in yeast S. cerevisiae should be interpreted with caution. Nonetheless, the mTOR signalling pathway and SIR2 (mammalian SIRT1) are still the most studied longevity targets in the antiaging field in yeasts.
4. VERTEBRATE PRECLINICAL EXPERIMENTAL MODELS ON AGING RESEARCH
4.1. The Fish Nothobranchius furzeri as Model for Aging Research
The annual fish Nothobranchius furzeri, known as “turquoise-killifish” and native of middle-east Africa, is a potential useful model for aging studies, mainly due to their short lifespan (2 to 6-8 months) [80-84] (Table 2). Some of the most interesting studies performed in this model were conducted by Terzibasi and colleagues, reporting that DR causes an increase in fish longevity and health span, as well as an improved learning and delayed neurodegeneration [83]. Moreover, Kirschner and colleagues performed the first analysis of genes involved in lifespan and health span in this fish [85]. On another front, D'Angelo and colleagues studied in depth the brain regulation of brain derived neurotrophic factor (BDNF) and published an important work of neuroanatomical structures between this model and mammals [86].
Although few preclinical studies with drugs have been performed in this fish, Valenzano and colleagues were the first scientists who described the antiaging properties of RESV in N. furzeri [80-85]. They demonstrated that RESV supplementation in food extends vertebrate lifespan and improves fish cognition [80]. Likewise, when Fluoro-Jade B staining (widely used to detect degenerating neurons) was performed in RESV-treated old fishes, labelling was absent. It was suggested that RESV exerted glial protective effects rather than a direct action over neurons [83]. Yet, few drugs apart from RESV have been evaluated in N. furzeri [87].
4.2. Rodents as Models for Aging Research
The National Institute on Aging (NIA) developed the Interventions Testing Program (ITP) for the scientific community working in aging research [88]. This program offers adequate protocols to reproduce studies, as well as interventions to identify or evaluate potential compounds, diets and supplements that may have an impact on lifespan and promote healthy aging in rodent models [88-95].
Three main laboratories are evaluating potential compounds capable of preventing or delaying multiple aging diseases in mice: The Jackson Laboratory in Bar Harbor, Maine (TJL), the University of Michigan at Ann Arbor (UM), and the University of Texas Health Science Center at San Antonio (UT) [88-90]. In these preclinical studies, genetically heterogeneous mice (HET) were obtained by crossing progeny CB6F1 females with C3D2F1 males [88-92]. According to these laboratories, HET is probably the most suitable rodent model to study potential drugs against aging prevention [94] (Table 2).
Several compounds with previously reported antiaging properties have been evaluated in HET, including rapamycin, RESV, Green Tea Extract, curcumin and other additional drugs. Results with rapamycin were probably the most satisfactory, as the drug proved effective even when the treatment started late in mice life [93]. Moreover, the antiaging effects were observed both in males and females. Although the exact biochemical mechanisms involved in these effects remain to be elucidated, the inhibition of mTOR by rapamycin is involved in the regulation of antiaging effects in flies, worms, and yeast, leading to lifespan extension [2, 4, 8, 11, 93]. Moreover, the deletion of ribosomal S6 protein kinase (S6K) 1 gene, a downstream target of mTOR, increases lifespan and improves aging effects in female mice [29]. Bearing in mind that the downregulation of mTOR by DR could also be involved in mice lifespan extension, the effects of rapamycin may be similar to those exerted by a DR diet on body metabolism. In fact, it was reported that rapamycin decreased weight gain and adiposity in rats [96]. Finally, the analgesic drugs aspirin and nordihydroguaiaretic acid also increased lifespan of HET males [90]. However, the molecular mechanism involved in this process was not mediated by DR [90]. Probably, the antioxidant and antiinflammatory properties of both drugs could account for the reported antiaging effects.
On the other hand, although RESV has shown promising effects in invertebrate models, no antiaging effects in HET has been achieved with this compound [92]. Thus, it remains to be ascertained whether SIRT1 activation by RESV could really explain the widely described antiaging properties of this drug on invertebrates and mammals. Moreover, although previous reported data suggests that statins may have an antiaging effect, simvastatin was also evaluated in HET with negative results [92]. Curcumin is another compound proven to be ineffective in HET despite previous studies demonstrating beneficial effects in murine models of AD and diabetes, as well as oxaloacetate, which increased lifespan in C. elegans by a mechanism similar to DR [93].
Another well-established murine model for aging-associated disorders are the senescence prone inbred strains (SAMP), originally generated from AKR/J mice [96-103]. These mice, developed by Dr. Takeda, show features of accelerated aging, including shortened life expectancy, memory loss and presence of Aβ depositions compared to the SAMR (senescence-resistant inbred strains) [31,32]. In addition to accelerated senescence, there are some SAMP strains which also manifest pathobiological phenotypes that are often used as characteristic features to differentiate the strains [96-99].
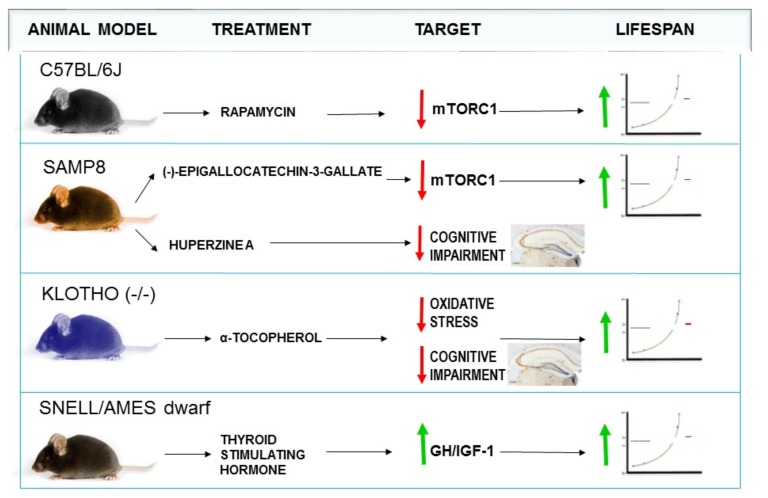
Fujitsuka and colleagues reported that DR activates ghrelin signalling and improves health and lifespan in different murine models (SAMP8 and klotho-deficient mice) [97]. In turn, Porquet and colleagues also demonstrated the efficacy of RESV as lifespan and health span increaser in this mice strain, probably through the SIRT1 pathway activation [31]. Regarding the downregulation of insulin and/or IGF-1, the administration of tetrahydroxystilbene glucoside to SAMP8 improved memory and prolonged lifespan by decreasing the activity of this pathway [3, 6, 98]. Moreover, the administration of (−)-epigallocatechin-3-gallate to SAMP8 mice improved insulin resistance through the down-regulation of the mTORC1 pathway [99] (Fig. 3). mTOR is a kinase with a catalytic subunit of two functionally and structurally distinct complexes. Current evidence indicates that mTORC1 acts as a nutrient sensor that can be activated by insulin, and its inhibition by rapamycin increases longevity and postpones age-related diseases [3]. However, the inhibition of mTORC2 is likely deleterious to health, because it causes an impairment of glucose homeostasis and of the immune system. Huperzine A is another compound evaluated in SAMP8, and it proved to be an antioxidant with beneficial effects in AD treatment [104]. All these studies support the usefulness of SAMP8 as a model for drug development against aging-associated metabolic diseases [99-104].
Fig. (3).
Effect of some drug treatments in mice models of aging.
Another interesting murine model of aging is the klotho mutant mice, characterized by a deletion in the klotho gene. Klotho mutant mice show common features with human aging process, such as short lifespan, hypokinesia, infertility, arteriosclerosis, osteoporosis and brain alterations related to neurological disorders [105-107]. Indeed, the klotho (KLOTHO) protein appears to have antiaging properties, and several researchers supported the hypothesis that klotho functions as a human aging-suppression molecule [107]. This notion is supported by the fact that the overexpression of the klotho gene extends lifespan in mice [105-107]. Although few pharmacological studies have been performed in this model, Kuro and colleagues reported an increase in brain oxidative stress and memory loss in klotho mutant mice that was improved by the administration of the antioxidant α-tocopherol [107]. Furthermore, it has been suggested that drugs which increase the brain levels of KLOTHO protein could be a promising strategy for treating AD [105].
The Snell and Ames dwarf mice are also interesting models of delayed aging and increased longevity since they show an average increase in life expectancy of the 40% [108]. Snell dwarf mice live longer than their normal siblings, showing significant increases in both average and maximal lifespan [108-111]. Both mice are characterized by the lack of growth hormone (GH) producing cells in the pituitary gland [109]. Thus, mutations in the Snell dwarf gene Pit1 (pituitary transcription factor 1) reduced GH, prolactin, and thyroid stimulating hormone (TSH) production. The Ames dwarf gene Prop1 (Prophet of Pit1) encodes for transcription factors that control pituitary development, thus causing alterations in the hormonal system, decreasing the levels of GH, prolactin and TSH [108-111]. In turn, GH is involved in the regulation of IGF-1 secretion in the majority of tissues. Therefore, increasing life expectancy could be related with a decrease in GH, which, in turn, would decrease the IGF-1 signalling pathway [109-111]. These mice models of aging confirm the importance of the IGF-1 decrease in lifespan extension, and point to the deficient regulation of GH through Prop-1 and Pit-1 mutations as one of the most important effects on longevity [109, 110]. Moreover, it has been recently shown that TSH treatment partially restores life extension of the Snell mice, suggesting that thyroid hormone and GH/IGF-1 play a role in lifespan extension [108]. The IGF-1 knockout mice are not viable (they die in the first week after birth), it may be possible that a partial or a moderate loss of the Igf1r gene decreases insulin and IGF-1 signalling, thus extending lifespan in mice [112].
Mice with fat-specific disruption of the insulin receptor gene (FIRKO), constitute another suitable preclinical model. These mice show reduced insulin receptor expression in adipose tissue and a lower expression of genes involved in oxidative stress, which could explain their higher lifespan [112, 113]. An additional rodent model to study the aging process and age-related diseases are the OXYS rats [114]. This rat strain is characterized by an overproduction of oxygen radicals. Furthermore, OXYS rats show an age-related mitochondrial dysfunction, being the most probable cause of their enhanced oxidative stress and accelerated senescence [114]. It has been reported that OXYS rats treated with rapamycin 0.1 or 0.5 mg/kg of body weight per day from the age of 1.5 to 3.5 months show an improvement in some aging-related parameters, such as locomotion and exploratory activity, in addition to inhibit tau phosphorylation, which is a sign of brain damage [114]. Notwithstanding, rapamycin and related analogous compounds (i.e., everolimus) have a potential risk of presenting adverse side effects, including metabolic alterations (hypertriglyceridemia), pain, haematological cell alterations, diarrhoea and others. Moreover, it is well known their ability to induce immunosuppression, thus increasing the risk of infections [115].
Some studies aiming to evaluate antiaging drugs have been performed in wild-type mice. For instance, Arriola-Apelo and colleagues reported that an intermittent rapamycin treatment in female C57BL/6 mice extends lifespan. In addition, it decreases the risk and the occurrence of undesirable side effects caused by the drug [116]. Moreover, this study demonstrated that a close monitoring of the dose of administered rapamycin at preclinical level could be a potential treatment of diseases related with the aging process.
Finally, selegiline (deprenyl) is another drug that has been reported to have an antiaging effect. It was originally developed as an antidepressant, and later proved to be a MAO B inhibitor for PD treatment [117-121] Table 1. The first preclinical evidences of its antiaging effects were reported by Knoll and colleagues, who showed that repeated administration of deprenyl prolonged lifespan of male rats [118] (Table 2). Further studies carried out by Kitani and colleagues in Fischer 344/Du (F344/Du) rats confirmed the lifespan-increasing properties of this drug [117]. The upregulation of antioxidant SOD and CAT activities in the majority of animal tissues may be the mechanism involved in this antiaging effect [119-121]. In addition, it has been hypothesized that the beneficial effects of deprenyl are more complex, and could be mediated by homeostatic mechanisms which regulate the aging process through a neuro-immuno-endocrine axis [122].
4.3. Rodent Models of Progeria
Premature aging-like syndromes in humans constitute an unusual group of rare genetic diseases [123-126], in which the effects of premature aging could affect several organs and tissues as well as exhibit some symptoms seen in the physiological aging [127]. These syndromes include clinically and genetically heterogeneous diseases such as ataxia-telangiectasia, Bloom syndrome, Cockayne syndrome, Fanconi anaemia, Hutchinson-Gilford syndrome, Rothmund-Thomson syndrome, trichothiodystrophy, xeroderma pigmentosum, and Werner syndrome (also known as adult progeria) [124-135]. The scientific community has been devoted on developing animal models capable of reproducing all symptomology of these diseases in order to understand their pathogenesis and, design and develop therapeutic drugs capable of halting them [132]. In general, the advantages of working with progeria rodents include the reduction in time, labor and costs for lifespan studies, as well as the ability to target accelerated aging on specific organs (Table 2). Moreover, premature aging mice mutants may display more pronounced aging features than normal mice (e.g. neurodegeneration in AD, which is not a very pronounced aging feature in wild type mice), and they are useful to investigate underlying mechanisms. One of these models are the Ercc1 mutant mice, which show a deficiency in the DNA excision-repair gene Ercc due to a deletion mutation in one allele [124]. This causes a slight retardation in embryonic and early post-natal development, as well as a reduced lifespan of only 4–6 months [124]. These Ercc1 mutant mice suffer skin, liver and kidney malfunctions, neurodegeneration, arrested development, osteoporosis, bone marrow pathologies, progressive ataxia, loss of vision and hearing and early death [134]. Recently, Vermeij and colleagues reported that dietary restriction in Ercc1 mutant mice increased resistance to DNA damage-induced stress and improved antioxidant defences, altered insulin and other hormonal signalling pathways and extended lifespan by 180% [126]. Likewise, DR in Xpg−/− mouse mutant, another nucleotide excision repair-deficient progeroid mutant mice, induced an increase of median lifespan of approximately 80% [126, 135]. Therefore, DR could be a suitable antiaging intervention to treat progeroid syndromes associated with defective DNA-repair [126].
Another model of progeria is the Lmna−/− mice, which carry an autosomal recessive mutation in the Lmna gene [127]. Lmna gene encodes the protein LMNA, also known as Lamin A/C, which belongs to the lamin family of proteins. These cytoskeletal proteins form a complex meshwork in the internal nuclear envelope [128]. Mutations in laminin genes LMNA, LMNB1 and LMNB2 are responsible for several diseases that are known as laminopathies, which include premature aging syndromes. Thus, Lmna−/− mice develop a phenotype resembling Hutchinson-Gilford Progeria, with marked growth retardation, skin and bone dysfunctions and premature death approximately by 4-6 weeks of age [127-129].
Ramos and colleagues showed that Lmna–/– mice fed with a diet supplemented with rapamycin lived significantly longer than animals which were fed with an identical diet without rapamycin (56% increase in mean lifespan) [128]. Moreover, rapamycin improved cardiac and muscle function, probably through autophagy-enhancing mechanisms, since rapamycin inhibits mTORC1 hyperactivation in Lmna−/− mice [128]. This remarks the importance of understanding the links between mTORC1 signalling and autophagy in progeria-like diseases, as well as in physiological aging. In turn, Liu and colleagues reported that RESV treatment in Zmpste24-/- mice (a model with prelamin A accumulation and a phenotype of premature aging) resulted in rescued adult stem cell decline and improved body weight loss, trabecular bone structure and bone mineral density [127]. Mice treated with RESV also showed increased lifespan as compared to untreated mice. Thus, this study suggests a therapeutic strategy based on SIRT1 pathway activation by RESV for Hutchinson-Gilford Progeria. In contrast, Strandgren and colleagues demonstrated that RESV treatment did not improve skeletal abnormalities in a mouse model with the osteoblast and osteocyte-specific inducible transgenic expression of the most common Hutchinson-Gilford Progeria mutation [133]. It is noteworthy that the phenotypes in LmnaHG/+ mice closely resemble those in Zmpste24–/– mice [133]. Finally, Fong and colleagues demonstrated that the farnesyltransferase inhibitor ABT-100 delays the onset of many progeroid symptoms and significantly improves lifespan in Zmpste24−/− mice [129]. Likewise, Yang and colleagues reported that farnesyltransferase inhibitors controlled body weight, prevented loss of adipose tissue, improved bone mineralization, and ameliorated disease phenotypes in LmnaHG/+mice [130].
4.4. Dogs as a Model of Aging
Although dogs are not often used in geroscience, they are suitable models due to their brain similarities with humans, showing similar age-related cognitive decline, presence of oxidative damage and Aβ deposits [136-138]. For this reason, dogs are often used to study drugs with potential efficacy on cognition and in AD research (Table 2). In addition, the use of dogs has some advantages: they are easy to handle and they allow predictive validity in extrapolating the results to clinical trials in humans, due to high similarities in pharmacokinetics and metabolism of drugs [136,137]. Beagles are probably the most widely used dogs in preclinical research, having an average life expectancy of 14 years.
Although there are some studies assessing the effects of DR in dogs [136], studies evaluating the antioxidant effects of some drugs are probably the most detailed. Thus, some research groups have evaluated the effect of antioxidant compounds such as lipoic acid and performed treatments with antioxidant diets, including a broad spectrum of antioxidants and mitochondrial co-factors [136]. In general, the results were contradictory: whereas some studies reported cognitive improvements, others described no cognitive benefits in aged beagles [138, 139].
Finally, it is worth to mention that the Dog Aging Project, directed by Promislow and Kaeberlein from the University of Washington [140]. This project aims to enhance the longevity and health span in people’s pets, since this could improve people’s quality of life. To reach this goal, the Dog Aging Project has two major aims: they are performing a longitudinal study of aging in dogs and an intervention trial with rapamycin to prevent disease and extend healthy longevity in middle-aged dogs.
4.5. Nonhuman Primate Models of Aging
Choosing primates as an experimental model has several disadvantages. For instance, it is an expensive animal model which carries health risks and whose isolation in cages is difficult (Table 2). Besides, choosing a nonhuman primate (NHP) model with short life expectancy is mandatory in order to not extend excessively the studies with drugs [141-149]. Obviously, the main advantage of NHP is that they are the closest model to humans, and therefore the results will be better reproduced in our species [142].
The Rhesus macaque (Macaca mulatta), is an example of NHP used in aging research, but its relatively long lifespan (around 40 years) is an important inconvenience in studies with drugs due to the high costs associated [142]. Nonetheless, Jimenez-Gomez and colleagues reported that chronic RESV administration (2-year) in M. mulatta under an obesity-inducing diet associated with insulin resistance improved the metabolic body status [150]. Authors concluded that the administration of RESV was safe in this NHP and the effects of the drug would probably be mediated through the inhibition of JNK activity, which inhibits serine phosphorylation of IRS-1. SIRT1 activation could also contribute to beneficial effects of RESV. In turn, Colman and colleagues reported positive effects of CR in this model of NHP [145].
The common marmoset (Callithrix jacchus) is a widely used NHP model in biomedical aging research, due to its short lifespan and its small size [142]. Marmosets also exhibit some age-related characteristic diseases similar to those observed in humans, like neurological diseases, cancer and diabetes. Furthermore, when compared with other NHP, C. jacchus is much more docile and does not transmit diseases to humans, thus offering practical advantages. Ross and colleagues reported that long-term rapamycin-treated marmosets had a reduced mTOR signalling, finding no negative side effects at previously used doses [146]. Likewise, Lelegren and colleagues reported that a long-term rapamycin treatment (14 months) in the common marmoset induced the activation of some of the molecular components responsible for the regulation of proteostasis associated with a significant reduction of mTOR [141]. Marmosets are also useful as preclinical models in the development of drugs for the treatment of PD [144]. Thus, Bourdenx and colleagues developed and administered a recombinant viral vector overexpressing mutated α-synuclein, a major constituent of Lewy bodies, in order to evaluate the effects of aging on the development of PD [144]. Likewise, numerous studies have been conducted in this NHP model to evaluate the effect of the neurotoxin MPTP, as a preclinical model for PD [145]. Furthermore, Palazzi and colleagues reported the appearance of Aβ deposits in the brains of marmosets, so this NHP could be a useful model to evaluate potential dugs for the treatment of sporadic AD related to aging [149]. One of the advantages of this experimental NHP model is that it allows evaluating the efficacy of drugs affecting cognitive processes which may decrease with aging, with an easier extrapolation to humans than invertebrate models.
DR studies have also been performed in another NHP model, specifically the grey mouse lemur (Microcebus murinus), which originates from Madagascar and has a life expectancy of about 10 years. This NHP is used in the “RESTRIKAL” study, which investigates the long-term effects of DR and/or supplementation of a mimetic compound of RESV on the aging process and lifespan [151]. Marchal and colleagues demonstrated in this NHP that 1 year of treatment with both strategies was safe; however, RESV produced an activation of energy metabolism, while DR decreased energy expenditure [151-154].
Conclusion
This review discusses the most common and appropriate preclinical models used in aging research, and the main biochemical mechanisms involved in the aging process.
The major biochemical pathways associated with aging are the activation of sirtuins (specifically SIRT1), and the inhibition of IGF-1 and mTOR pathways. Therefore, the development of drugs that increase lifespan and health span depends on the elucidation of these pathways which, are highly conserved in all species from prokaryotes to eukaryotes.
Most compounds assessed in aging research are based in drugs that mimic and induce the health effects of DR. Consequently, most preclinical research in experimental models of aging is based on RESV studies, rapamycin and, to a lesser extent, metformin. The development of DR mimetic drugs is difficult, since it is also necessary to evaluate the toxic effects of a specific chronic treatment on different organs and tissues. Thus, studies in invertebrates such as C. elegans, Drosophila and S. cerevisae are useful for the performance of initial screening of new compounds and confirmation on their potential applications in aging. The main advantage on the use of invertebrate models is the rapid generation of sufficient amount of data in a short period of time and the lower research costs when compared to studies in mammals. Furthermore, several studies suggest that DR induced lifespan extension in invertebrates is mediated by the down-regulation of nutrient signalling pathways. This extends longevity by activating stress-resistance transcription factors that regulate the expression of genes involved in protection against oxidative stress, DNA repair, and metabolism. Both yeast and C. elegans are powerful invertebrate models for aging research studies, as it is demonstrated by the fact that most processes that modulate aging (e.g., insulin signalling and mitochondrial alteration, sirtuins, TOR, caloric restriction) have been originally and extensively investigated in these models. In addition, C. elegans has some important advantages as compared to other invertebrate models, such as their simple neuronal system and the fact that some genes are homologous to those of human. Moreover, mutant lines with aging phenotypes have been developed in order to investigate the role of specific proteins in specific particular aging processes. In spite of this, the extrapolation of results obtained in invertebrates to humans is not possible without assessing further animal models. Consequently, mammalian models are essential in the development of molecules with antiaging effects, as well as to improve aging-associated conditions such as T2DM, coronary and neurodegenerative diseases.
Although significant progresses in N. furzeri have been achieved in geroscience, this preclinical model still presents many limitations. Firstly, the model is not a good choice when studying aging-associated diseases such as T2DM and AD. Moreover, there are limitations to the studies of organs, which logically are quite different to those in humans. However, the short lifespan of N. furzeri is one of the main advantages that this model can offer in the discovery of genes, proteins or pathways associated to vertebrate aging. Likewise, N. furzeri could also be appropriate for antiaging drug research testing.
Although rodents are genetically much closer to humans than invertebrates, they still present significant differences at a pharmacokinetic drug level. Moreover, we have to be cautious about age and DR in this model. Theoretically, DR can increase lifespan, although this effect is not uniform depending of mouse strain. For instance, male C57BL/6J mice respond to DR with an increase in median and maximum lifespan, while other mice strains do not. In addition, some age related diseases appear at 24 months of age in C57BL/6J mice, which is equivalent to 70 years old in humans. Therefore, lifespan and health span studies in rodents should be performed on this time period. Moreover, an important focus for future research is the fact that the degree of lifespan extension could also vary between the gender of rodents. Most authors point to HET mice developed by the National Institute on Aging interventions testing program as the most adequate mammal mice model in drug research [88-95]. The studies performed in HET mice allowed the evaluation of compounds hypothesized to delay aging and also prevent diseases associated with the aging process in other preclinical models. Hence, the ITP program has assessed the efficacy of various antiaging compounds such as anti-inflammatory drugs, Green Tea Extract, Curcumin, RESV, rapamicyn, etc. Notwithstanding, the SAMP8 mice is also useful as model of aging, since it allows the reproduction of chronic diseases closely related to humans. This model also allows the retrieval of different tissues such as heart, liver and brain for further analysis. Moreover, it shows cognitive loss and develops a symptomatology similar to AD. Likewise, it can be used to study the emergence of T2DM in mice under a high fat diet or to reproduce some neurological diseases after the administration of neurotoxins, such as PD or epilepsy.
Regarding dogs as experimental models in aging, they are especially useful in studies with drugs related with AD; however, some important limitations exist, mostly associated with cognitive evaluation and behavioural studies.
Prior to any clinical trial, the validation of an antiaging drug in NHP will probably be essential. In addition, NHP are important models to evaluate behavioural alterations or cognitive decline that cannot be investigated in other animal models. Therefore, they represent an extremely valuable tool to improve our knowledge in aging diseases and to establish new potential therapeutic strategies for aging and aging-related diseases like AD. Indeed, although they have their intrinsic limitations, NHP models could allow the investigation of molecular mechanisms underlying AD, as well as its histopathological modifications using the closest models to human physiology [149, 153]. Currently, shorter-lived primate models as the common marmoset are available for preclinical studies. Likewise, recent studies of aging research with NHP have demonstrated the safety and efficacy of DR and some antiaging drugs such as RSV and rapamycin [141, 146, 150, 151]. Notwithstanding, it is important to keep in mind that all animal models reproduce only some aspects of the aging process and it is not possible to recapitulate the entire human clinical picture [142].
Finally, taking into account all the preclinical results published so far, we emphasize that rapamycin is the most promising drug in the field of geroscience. In addition to its antiaging effect, it has shown neuroprotective effects in experimental models of AD and PD. We also believe that mTOR could be a promising target for drug development in antiaging research, neurodegenerative diseases and Hutchinson–Gilford progeria syndrome. In spite of this, we must not forget the potential side effects of rapamycin in a chronic treatment. The use of safer drugs, or a combinatory drug treatment such as rapamicyn with metformin and resveratrol, may be advantageous in chronic treatments aiming to extend human health and lifespan or preventing aging-related diseases.
Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation project, SAF-2016-33307 and PI2016/01, CB06/05/0024 (CIBERNED) and the European Regional Development Founds. Research team from UB and URV belongs to 2014SGR-525 from Generalitat de Catalunya. ESL belong to 2014SGR-1023. NIA 1R15AG050292 (GC).
The author, ESL, acknowledges the support of the Spanish Ministry for the PhD scholarship FPI-MICINN (BES-2012-056083). The authors are grateful to Roxanne Rowles for the English revision of the manuscript.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- ALS
Amyotrophic Lateral Sclerosis
- AMPK
AMP-activated protein kinase
- Aβ
Amyloid-β protein
- CAT
Catalase
- CoQ
Coenzime Q
- DM
Drosophila melanogaster
- DR
Dietary Restriction
- ER
Endoplasmic Reticulum
- GH
Growth Hormone
- HD
Huntington disease
- HET
Heterogeneous Mice Model
- IGF-1
Insulin Growth Factor 1
- IL-1β
Interleukin-1beta
- IL-6
Interleukin-6
- ITP
Interventions Testing Program level
- JNK
Jun N-terminal Kinase
- LKB1
Liver Kinase B1
- mTOR
Mammalian Target of Rapamycin
- NAD
Nicotinamide adenine nucleotide
- NHP
Non Human Primate
- NIA
National Institute on Aging
- PD
Parkinson’s disease
- PKB
Protein Kinase B
- Pon1
paraoxonase1
- RESV
Resveratrol
- ROS
Reactive oxygen species
- SIRT1
Sirtuin 1
- SOD
Superoxide dismutase
- T2DM
Type 2 Diabetes Mellitus
- TNFα
Tumor necrosis factors alpha
- TSH
Thyroid Stimulating Hormone
- UPR
Unfolded Protein Response
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1. World Health Organization. World report on ageing and health. 1- 260 . 2015.
- 2.Bitto A., Wang A.M., Bennett C.F., Kaeberlein M. Biochemical Genetic Pathways that Modulate Aging in Multiple Species. Cold Spring Harb. Perspect. Med. 2015;5(11):a025114. doi: 10.1101/cshperspect.a025114. [http://dx. doi.org/10.1101/cshperspect.a025114]. [PMID: 26525455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazucanti C.H., Cabral-Costa J.V., Vasconcelos A.R., Andreotti D.Z., Scavone C., Kawamoto E.M. Longevity Pathways (mTOR, SIRT, Insulin/IGF-1) as Key Modulatory Targets on Aging and Neurodegeneration. Curr. Top. Med. Chem. 2015;15(21):2116–2138. doi: 10.2174/1568026615666150610125715. [http://dx.doi.org/10.2174/1568026615666150610125715]. [PMID: 26059361]. [DOI] [PubMed] [Google Scholar]
- 4.Knuppertz L., Osiewacz H.D. Orchestrating the network of molecular pathways affecting aging: Role of nonselective autophagy and mitophagy. Mech. Ageing Dev. 2016;153:30–40. doi: 10.1016/j.mad.2016.01.003. [http://dx. doi.org/10.1016/j.mad.2016.01.003]. [PMID: 26814678]. [DOI] [PubMed] [Google Scholar]
- 5.Fusco S., Maulucci G., Pani G. Sirt1: def-eating senescence? Cell Cycle. 2012;11(22):4135–4146. doi: 10.4161/cc.22074. [http://dx.doi.org/10.4161/cc. 22074]. [PMID: 22983125]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarente L., Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408(6809):255–262. doi: 10.1038/35041700. [http://dx. doi.org/10.1038/35041700]. [PMID: 11089983]. [DOI] [PubMed] [Google Scholar]
- 7.Hartl F.U. Protein Misfolding Diseases. Annu. Rev. Biochem. 2017;86:21–26. doi: 10.1146/annurev-biochem-061516-044518. [http://dx.doi.org/10.1146/annurev-biochem-061516-044518]. [PMID: 28441058]. [DOI] [PubMed] [Google Scholar]
- 8.de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157(7):1515–1526. doi: 10.1016/j.cell.2014.05.031. [http://dx.doi. org/10.1016/j.cell.2014.05.031]. [PMID: 24949965]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang B.L. Sirt1's systemic protective roles and its promise as a target in antiaging medicine. Transl. Res. 2011;157(5):276–284. doi: 10.1016/j.trsl.2010.11.006. [http://dx.doi.org/10.1016/j.trsl.2010.11.006]. [PMID: 21497775]. [DOI] [PubMed] [Google Scholar]
- 10.Hill J.O., Wyatt H., Phelan S., Wing R. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J. Nutr. Educ. Behav. 2005;37(4):206–210. doi: 10.1016/s1499-4046(06)60248-0. [http://dx. doi.org/10.1016/S1499-4046(06)60248-0]. [PMID: 16029692]. [DOI] [PubMed] [Google Scholar]
- 11.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [http://dx.doi.org/10.1016/j.cell.2013.05.039]. [PMID: 23746838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenart P., Krejci L. Reprint of “DNA, the central molecule of aging”. Mutat. Res. 2016;788:25–31. doi: 10.1016/j.mrfmmm.2016.04.002. [http://dx.doi.org/10.1016/ j.mrfmmm.2016.04.002]. [PMID: 27133220]. [DOI] [PubMed] [Google Scholar]
- 13.Novelle M.G., Ali A., Diéguez C., Bernier M., de Cabo R. Metformin: A Hopeful Promise in Aging Research. Cold Spring Harb. Perspect. Med. 2016;6(3):a025932. doi: 10.1101/cshperspect.a025932. [http://dx.doi.org/10. 1101/cshperspect.a025932]. [PMID: 26931809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proshkina EN, Shaposhnikov MV, Sadritdinova AF, Kudryavtseva AV, Moskalev AA. Basic mechanisms of longevity: A case study of Drosophila pro-longevity genes. 2015. [DOI] [PubMed]
- 15.Honjoh S., Nishida E. Two sides of lifespan regulating genes: pro-longevity or anti-longevity? J. Biochem. 2011;149:381–388. doi: 10.1093/jb/mvr026. [DOI] [PubMed] [Google Scholar]
- 16.Anselmi C.V., Malovini A., Roncarati R., Novelli V., Villa F., Condorelli G., Bellazzi R., Puca A.A. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. doi: 10.1089/rej.2008.0827. [http://dx. doi.org/10.1089/rej.2008.0827]. [PMID: 19415983]. [DOI] [PubMed] [Google Scholar]
- 17.Willcox B.J., Donlon T.A., He Q., Chen R., Grove J.S., Yano K., Masaki K.H., Willcox D.C., Rodriguez B., Curb J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [http:// dx.doi.org/10.1073/pnas.0801030105]. [PMID: 18765803]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flachsbart F., Caliebe A., Kleindorp R., Blanché H., von Eller-Eberstein H., Nikolaus S., Schreiber S., Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA. 2009;106(8):2700–2705. doi: 10.1073/pnas.0809594106. [http://dx.doi.org/10.1073/pnas.0809594106]. [PMID: 19196970]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Wang W.J., Cao H., Lu J., Wu C., Hu F.Y., Guo J., Zhao L., Yang F., Zhang Y.X., Li W., Zheng G.Y., Cui H., Chen X., Zhu Z., He H., Dong B., Mo X., Zeng Y., Tian X.L. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum. Mol. Genet. 2009;18(24):4897–4904. doi: 10.1093/hmg/ddp459. [http://dx.doi.org/10.1093/hmg/ddp459]. [PMID: 19793722]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleindorp R., Flachsbart F., Puca A.A., Malovini A., Schreiber S., Nebel A. Candidate gene study of FOXO1, FOXO4, and FOXO6 reveals no association with human longevity in Germans. Aging Cell. 2011;10(4):622–628. doi: 10.1111/j.1474-9726.2011.00698.x. [http://dx.doi.org/10.1111/ j.1474-9726.2011.00698.x]. [PMID: 21388494]. [DOI] [PubMed] [Google Scholar]
- 21.Sierra M.I., Fernández A.F., Fraga M.F. Epigenetics of Aging. Curr. Genomics. 2015;16(6):435–440. doi: 10.2174/1389202916666150817203459. [http://dx.doi.org/10.2174/ 1389202916666150817203459]. [PMID: 27019618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taormina G., Mirisola M.G. Longevity: epigenetic and biomolecular aspects. Biomol. Concepts. 2015;6(2):105–117. doi: 10.1515/bmc-2014-0038. [http://dx.doi.org/10.1515/bmc-2014-0038]. [PMID: 25883209]. [DOI] [PubMed] [Google Scholar]
- 23.Salminen A., Kauppinen A., Kaarniranta K. AMPK/Snf1 signaling regulates histone acetylation: Impact on gene expression and epigenetic functions. Cell. Signal. 2016;28(8):887–895. doi: 10.1016/j.cellsig.2016.03.009. [http:// dx.doi.org/10.1016/j.cellsig.2016.03.009]. [PMID: 27010499]. [DOI] [PubMed] [Google Scholar]
- 24.Govindaraju D., Atzmon G., Barzilai N. Genetics, lifestyle and longevity: Lessons from centenarians. Appl. Transl. Genomics. 2015;4:23–32. doi: 10.1016/j.atg.2015.01.001. [http://dx.doi.org/10.1016/j.atg.2015.01.001]. [PMID: 26937346]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxmen A. Calorie restriction falters in the long run. Nature. 2012;488(7413):569. doi: 10.1038/488569a. [http://dx.doi.org/10.1038/488569a]. [PMID: 22932356]. [DOI] [PubMed] [Google Scholar]
- 26.Phelan J.P., Rose M.R. Why dietary restriction substantially increases longevity in animal models but won’t in humans. Ageing Res. Rev. 2005;4(3):339–350. doi: 10.1016/j.arr.2005.06.001. [http://dx.doi.org/10.1016/j.arr. 2005.06.001]. [PMID: 16046282]. [DOI] [PubMed] [Google Scholar]
- 27.Azzu V., Valencak T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology. 2017;63(4):327–336. doi: 10.1159/000454924. [http://dx.doi.org/10.1159/000454924]. [PMID: 28118636]. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell S.J., Scheibye-Knudsen M., Longo D.L., de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829. [http://dx.doi.org/10.1146/annurev-animal-022114-110829]. [PMID: 25689319]. [DOI] [PubMed] [Google Scholar]
- 29.Holmes D.J. Naturally long-lived animal models for the study of slow aging and longevity. Ann. N. Y. Acad. Sci. 2004;1019:483–485. doi: 10.1196/annals.1297.088. [http://dx.doi.org/10.1196/annals.1297.088]. [PMID: 15247070]. [DOI] [PubMed] [Google Scholar]
- 30.Pallàs M., Casadesús G., Smith M.A., Coto-Montes A., Pelegri C., Vilaplana J., Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr. Neurovasc. Res. 2009;6(1):70–81. doi: 10.2174/156720209787466019. [http:// dx.doi.org/10.2174/156720209787466019]. [PMID: 19355928]. [DOI] [PubMed] [Google Scholar]
- 31.Porquet D., Casadesús G., Bayod S., Vicente A., Canudas A.M., Vilaplana J., Pelegrí C., Sanfeliu C., Camins A., Pallàs M., del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordr.) 2013;35(5):1851–1865. doi: 10.1007/s11357-012-9489-4. [http://dx.doi.org/10.1007/s11357-012-9489-4]. [PMID: 23129026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cristòfol R., Porquet D., Corpas R., Coto-Montes A., Serret J., Camins A., Pallàs M., Sanfeliu C. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J. Pineal Res. 2012;52(3):271–281. doi: 10.1111/j.1600-079X.2011.00939.x. [http://dx.doi.org/10.1111/j.1600-079X. 2011.00939.x]. [PMID: 22085194]. [DOI] [PubMed] [Google Scholar]
- 33.Robida-Stubbs S., Glover-Cutter K., Lamming D.W., Mizunuma M., Narasimhan S.D., Neumann-Haefelin E., Sabatini D.M., Blackwell T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15(5):713–724. doi: 10.1016/j.cmet.2012.04.007. [http://dx.doi.org/10.1016/j.cmet.2012.04.007]. [PMID: 22560223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emran S., Yang M., He X., Zandveld J., Piper M.D. Target of rapamycin signalling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging (Albany N.Y.) 2014;6(5):390–398. doi: 10.18632/aging.100665. [http://dx.doi.org/10.18632/aging. 100665]. [PMID: 24861087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onken B., Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [http://dx.doi.org/10.1371/journal.pone.0008758]. [PMID: 20090912]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guha S., Natarajan O., Murbach C.G., Dinh J., Wilson E.C., Cao M., Zou S., Dong Y. Supplement timing of cranberry extract plays a key role in promoting Caenorhabditis elegans healthspan. Nutrients. 2014;6(2):911–921. doi: 10.3390/nu6020911. [http://dx.doi.org/10.3390/nu6020911]. [PMID: 24566444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann. N. Y. Acad. Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [http://dx.doi.org/10.1196/annals.1354.003]. [PMID: 16803965]. [DOI] [PubMed] [Google Scholar]
- 38.Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766. doi: 10.1016/j.cmet.2014.01.011. [http://dx.doi.org/10.1016/j.cmet.2014.01.011]. [PMID: 24561260]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magwere T., West M., Riyahi K., Murphy M.P., Smith R.A., Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006;127(4):356–370. doi: 10.1016/j.mad.2005.12.009. [http://dx.doi.org/10.1016/j.mad. 2005.12.009]. [PMID: 16442589]. [DOI] [PubMed] [Google Scholar]
- 40.Martínez G., Duran-Aniotz C., Cabral-Miranda F., Vivar J.P., Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16(4):615–623. doi: 10.1111/acel.12599. [http://dx.doi.org/10.1111/ acel.12599]. [PMID: 28436203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carreras-Sureda A., Pihán P., Hetz C. The unfolded protein response: at the intersection between endoplasmic Reticulum Function and mitochondrial bioenergetics. Front. Oncol. 2017;7:55. doi: 10.3389/fonc.2017.00055. [http://dx.doi.org/10.3389/fonc.2017.00055]. [PMID: 28421160]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hetz C., Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014;15(4):233–249. doi: 10.1038/nrn3689. [http://dx.doi.org/10.1038/nrn3689]. [PMID: 24619348]. [DOI] [PubMed] [Google Scholar]
- 43.Tissenbaum H.A. Using C. elegans for aging research. . Invertebr. Reprod. Dev. . 2015; 59 ((sup1)):59 –63 . doi: 10.1080/07924259.2014.940470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye X., Linton J.M., Schork N.J., Buck L.B., Petrascheck M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell. 2014;13(2):206–215. doi: 10.1111/acel.12163. [http://dx.doi.org/10. 1111/acel.12163]. [PMID: 24134630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., Barclay J.W., Burgoyne R.D., Morgan A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chem. Cent. J. 2015;9:65. doi: 10.1186/s13065-015-0143-y. [http:// dx.doi.org/10.1186/s13065-015-0143-y]. [PMID: 26617668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu R.H., Harn H.J., Liu S.P., Chen C.S., Chang W.L., Chen Y.M., Huang J.E., Li R.J., Tsai S.Y., Hung H.S., Shyu W.C., Lin S.Z., Wang Y.C. n-butylidenephthalide protects against dopaminergic neuron degeneration and α-synuclein accumulation in Caenorhabditis elegans models of Parkinson’s disease. PLoS One. 2014;9(1):e85305. doi: 10.1371/journal.pone.0085305. [http://dx.doi.org/10.1371/journal.pone.0085305]. [PMID: 24416384]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Kwon G, Park J, Kim JK, Lim YH. SIR-2.1-dependent lifespan extension of Caenorhabditis elegans by oxyresveratrol and resveratrol. Exp. Biol. Med. (Maywood) 2016. [DOI] [PMC free article] [PubMed]
- 48.Van Raamsdonk J.M., Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5(2):e1000361. doi: 10.1371/journal.pgen.1000361. [http://dx.doi.org/10. 1371/journal.pgen.1000361]. [PMID: 19197346]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreno-Arriola E., El Hafidi M., Ortega-Cuéllar D., Carvajal K. AMP-Activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 Transcriptional regulators. PLoS One. 2016;11(1):e0148089. doi: 10.1371/journal.pone.0148089. [http:// dx.doi.org/10.1371/journal.pone.0148089]. [PMID: 26824904]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ching T.T., Chiang W.C., Chen C.S., Hsu A.L. Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell. 2011;10(3):506–519. doi: 10.1111/j.1474-9726.2011.00688.x. [http://dx.doi.org/10.1111/j.1474-9726.2011.00688.x]. [PMID: 21348927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaghan R.M., Barnes R.G., Fisher K., Andreou T., Rooney N., Poulin G.B., Whitmarsh A.J. A nuclear role for the respiratory enzyme CLK-1 in regulating mitochondrial stress responses and longevity. Nat. Cell Biol. 2015;17(6):782–792. doi: 10.1038/ncb3170. [http://dx.doi.org/ 10.1038/ncb3170]. [PMID: 25961505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cochemé H.M., Noori T., Weinkove D., Schuster E., Greene N.D., Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [http://dx.doi.org/10.1016/j.cell.2013.02.035]. [PMID: 23540700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isobe C., Abe T., Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J. Neurol. 2010;257(3):399–404. doi: 10.1007/s00415-009-5333-x. [http://dx.doi.org/10.1007/s00415-009-5333-x]. [PMID: 19784856]. [DOI] [PubMed] [Google Scholar]
- 54.Varela-López A., Giampieri F., Battino M., Quiles J.L. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21(3):373. doi: 10.3390/molecules21030373. [http://dx.doi.org/10.3390/molecules21030373]. [PMID: 26999099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Büchter C., Zhao L., Havermann S., Honnen S., Fritz G., Proksch P., Wätjen W. TSG (2,3,5,4′-Tetrahydroxystilbene-2-O- β -D-glucoside) from the Chinese Herb polygonum multiflorumi life span and stress resistance of Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2015;2015:124357. doi: 10.1155/2015/124357. [http://dx.doi.org/10.1155/ 2015/124357]. [PMID: 26075030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Chávez J.L., Coballase-Urrutia E., Nieto-Camacho A., Delgado-Lamas G. Antioxidant capacity of “Mexican arnica” Heterotheca inuloides Cass natural products and some derivatives: their anti-inflammatory evaluation and effect on C. elegans life span. Oxid. Med. Cell. Longev. 2015;2015:843237. doi: 10.1155/2015/843237. [http://dx.doi. org/10.1155/2015/843237]. [PMID: 25821555]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinh J., Angeloni J.T., Pederson D.B., Wang X., Cao M., Dong Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS One. 2014;9(7):e103290. doi: 10.1371/journal.pone.0103290. [http://dx.doi.org/10.1371/journal.pone.0103290]. [PMID: 25062095]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pant A., Mishra V., Saikia S.K., Shukla V., Asthana J., Akhoon B.A., Pandey R. Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp. Gerontol. 2014;57:81–95. doi: 10.1016/j.exger.2014.05.007. [http://dx.doi.org/10. 1016/j.exger.2014.05.007]. [PMID: 24835194]. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Onken B., Chen H., Xiao S., Liu X., Driscoll M., Cao Y., Huang Q. Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J. Agric. Food Chem. 2014;62(15):3422–3431. doi: 10.1021/jf500210p. [http://dx.doi.org/10.1021/jf500210p]. [PMID: 24701969]. [DOI] [PubMed] [Google Scholar]
- 60.Van Raamsdonk J.M., Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5(2):e1000361. doi: 10.1371/journal.pgen.1000361. [http://dx.doi.org/10. 1371/journal.pgen.1000361]. [PMID: 19197346]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen P., Yue Y., Sun Q., Kasireddy N., Kim K.H., Park Y. Piceatannol extends the lifespan of Caenorhabditis elegans via DAF-16. Biofactors. 2017;43(3):379–387. doi: 10.1002/biof.1346. [http://dx.doi.org/ 10.1002/biof.1346]. [PMID: 28128482]. [DOI] [PubMed] [Google Scholar]
- 62.Wan Q.L., Zheng S.Q., Wu G.S., Luo H.R. Aspirin extends the lifespan of Caenorhabditis elegans via AMPK and DAF-16/FOXO in dietary restriction pathway. Exp. Gerontol. 2013;48(5):499–506. doi: 10.1016/j.exger.2013.02.020. [http://dx.doi.org/10.1016/j.exger.2013.02.020]. [PMID: 23485446]. [DOI] [PubMed] [Google Scholar]
- 63.Bass T.M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [http://dx.doi.org/10.1016/j.mad.2007.07.007]. [PMID: 17875315]. [DOI] [PubMed] [Google Scholar]
- 64.Frankel S., Ziafazeli T., Rogina B. dSir2 and longevity in Drosophila. Exp. Gerontol. 2011;46(5):391–396. doi: 10.1016/j.exger.2010.08.007. [http://dx.doi.org/ 10.1016/j.exger.2010.08.007]. [PMID: 20728527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirazi F., Farmakiotis D., Yan Y., Albert N., Do K.A., Kontoyiannis D.P. Diet modification and metformin have a beneficial effect in a fly model of obesity and mucormycosis. PLoS One. 2014;9(9):e108635. doi: 10.1371/journal.pone.0108635. [http://dx.doi.org/10.1371/journal.pone. 0108635]. [PMID: 25268492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jafari M. Drosophila melanogaster as a model system for the evaluation of anti-aging compounds. Fly (Austin) 2010;4(3):253–257. doi: 10.4161/fly.4.3.11997. [http://dx.doi.org/10.4161/fly.4.3.11997]. [PMID: 20473034]. [DOI] [PubMed] [Google Scholar]
- 67.Chandrashekara K.T., Shakarad M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(9):965–971. doi: 10.1093/gerona/glr103. [http://dx.doi.org/10.1093/gerona/glr103]. [PMID: 21719611]. [DOI] [PubMed] [Google Scholar]
- 68.Wood J.G., Rogina B., Lavu S., Howitz K., Helfand S.L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [http://dx.doi.org/10.1038/nature02789]. [PMID: 15254550]. [DOI] [PubMed] [Google Scholar]
- 69.Avanesian A., Khodayari B., Felgner J.S., Jafari M. Lamotrigine extends lifespan but compromises health span in Drosophila melanogaster. Biogerontology. 2010;11(1):45–52. doi: 10.1007/s10522-009-9227-1. [http://dx.doi. org/10.1007/s10522-009-9227-1]. [PMID: 19430925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouleau S., Tricoire H. Drosophila models of Alzheimer’s disease: advances, limits, and perspectives. J. Alzheimers Dis. 2015;45(4):1015–1038. doi: 10.3233/JAD-142802. [http://dx.doi.org/10.3233/JAD-142802]. [PMID: 25697708]. [DOI] [PubMed] [Google Scholar]
- 71.Spindler S.R., Mote P.L., Li R., Dhahbi J.M., Yamakawa A., Flegal J.M., Jeske D.R., Li R., Lublin A.L. β1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. Age (Dordr.) 2013;35(6):2099–2109. doi: 10.1007/s11357-012-9498-3. [http://dx.doi.org/10. 1007/s11357-012-9498-3]. [PMID: 23314750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jafari M., Khodayari B., Felgner J., Bussel I.I., Rose M.R., Mueller L.D. Pioglitazone: an anti-diabetic compound with anti-aging properties. Biogerontology. 2007;8(6):639–651. doi: 10.1007/s10522-007-9105-7. [http://dx.doi.org/10.1007/s10522-007-9105-7]. [PMID: 17628757]. [DOI] [PubMed] [Google Scholar]
- 73.Griswold A.J., Chang K.T., Runko A.P., Knight M.A., Min K.T. Sir2 mediates apoptosis through JNK-dependent pathways in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105(25):8673–8678. doi: 10.1073/pnas.0803837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calap-Quintana P., Soriano S., Llorens J.V., Al-Ramahi I., Botas J., Moltó M.D., Martínez-Sebastián M.J. TORC1 inhibition by rapamycin promotes antioxidant defences in a Drosophila Model of Friedreich’s Ataxia. PLoS One. 2015;10(7):e0132376. doi: 10.1371/journal.pone.0132376. [http://dx.doi.org/10.1371/journal.pone.0132376]. [PMID: 26158631]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jordens R.G., Berry M.D., Gillott C., Boulton A.A. Prolongation of life in an experimental model of aging in Drosophila melanogaster. Neurochem. Res. 1999;24(2):227–233. doi: 10.1023/a:1022510004220. [http://dx.doi. org/10.1023/A:1022510004220]. [PMID: 9972869]. [DOI] [PubMed] [Google Scholar]
- 76.Yang H., Baur J.A., Chen A., Miller C., Adams J.K., Kisielewski A., Howitz K.T., Zipkin R.E., Sinclair D.A. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6(1):35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [http://dx.doi.org/10.1111/j.1474-9726.2006.00259.x]. [PMID: 17156081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller-Fleming L., Giorgini F., Outeiro T.F. Yeast as a model for studying human neurodegenerative disorders. Biotechnol. J. 2008;3(3):325–338. doi: 10.1002/biot.200700217. [http://dx.doi.org/10.1002/biot.200700217]. [PMID: 18228539]. [DOI] [PubMed] [Google Scholar]
- 78.Kaeberlein M. Lessons on longevity from budding yeast. A review on the molecular basis involved in aging process. Nature. 2010;464(7288):513–519. doi: 10.1038/nature08981. [http://dx.doi.org/10.1038/nature08981]. [PMID: 20336133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huberts D.H., González J., Lee S.S., Litsios A., Hubmann G., Wit E.C., Heinemann M. Calorie restriction does not elicit a robust extension of replicative lifespan in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2014;111(32):11727–11731. doi: 10.1073/pnas.1410024111. [http:// dx.doi.org/10.1073/pnas.1410024111]. [PMID: 25071164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valenzano D.R., Cellerino A. Resveratrol and the pharmacology of aging: a new vertebrate model to validate an old molecule. Cell Cycle. 2006;5(10):1027–1032. doi: 10.4161/cc.5.10.2739. [http://dx.doi.org/10.4161/cc.5.10. 2739]. [PMID: 16687936]. [DOI] [PubMed] [Google Scholar]
- 81.Valenzano D.R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [http://dx.doi.org/10.1016/j.cub.2005. 12.038]. [PMID: 16461283]. [DOI] [PubMed] [Google Scholar]
- 82.Kim Y., Nam H.G., Valenzano D.R. The short-lived African turquoise killifish: an emerging experimental model for ageing. Dis. Model. Mech. 2016;9(2):115–129. doi: 10.1242/dmm.023226. [http://dx.doi.org/10.1242/ dmm.023226]. [PMID: 26839399]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terzibasi E., Valenzano D.R., Cellerino A. The short-lived fish Nothobranchius furzeri as a new model system for aging studies. Exp. Gerontol. 2007;42(1-2):81–89. doi: 10.1016/j.exger.2006.06.039. [http://dx.doi.org/10.1016/ j.exger.2006.06.039]. [PMID: 17049789]. [DOI] [PubMed] [Google Scholar]
- 84.Genade T., Lang D.M. Resveratrol extends lifespan and preserves glia but not neurons of the Nothobranchius guentheri optic tectum. Exp. Gerontol. 2013;48(2):202–212. doi: 10.1016/j.exger.2012.11.013. [http://dx.doi.org/10.1016/ j.exger.2012.11.013]. [PMID: 23220248]. [DOI] [PubMed] [Google Scholar]
- 85.Kirschner J., Weber D., Neuschl C., Franke A., Böttger M., Zielke L., Powalsky E., Groth M., Shagin D., Petzold A., Hartmann N., Englert C., Brockmann G.A., Platzer M., Cellerino A., Reichwald K. Mapping of quantitative trait loci controlling lifespan in the short-lived fish Nothobranchius furzeri--a new vertebrate model for age research. Aging Cell. 2012;11(2):252–261. doi: 10.1111/j.1474-9726.2011.00780.x. [http://dx.doi.org/10.1111/j.1474-9726.2011.00780.x]. [PMID: 22221414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Angelo L., De Girolamo P., Lucini C., Terzibasi E.T., Baumgart M., Castaldo L., Cellerino A. Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J. Comp. Neurol. 2014;522(5):1004–1030. doi: 10.1002/cne.23457. [http://dx.doi.org/10.1002/cne.23457]. [PMID: 23983038]. [DOI] [PubMed] [Google Scholar]
- 87.Reichwald K., Lauber C., Nanda I., Kirschner J., Hartmann N., Schories S., Gausmann U., Taudien S., Schilhabel M.B., Szafranski K., Glöckner G., Schmid M., Cellerino A., Schartl M., Englert C., Platzer M. High tandem repeat content in the genome of the short-lived annual fish Nothobranchius furzeri: a new vertebrate model for aging research. Genome Biol. 2009;10(2):R16. doi: 10.1186/gb-2009-10-2-r16. [http://dx.doi.org/10.1186/gb-2009-10-2-r16]. [PMID: 19210790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller R.A., Harrison D.E., Astle C.M., Floyd R.A., Flurkey K., Hensley K.L., Javors M.A., Leeuwenburgh C., Nelson J.F., Ongini E., Nadon N.L., Warner H.R., Strong R. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [http://dx.doi.org/10.1111/j.1474-9726.2007.00311.x]. [PMID: 17578509]. [DOI] [PubMed] [Google Scholar]
- 89.Nadon N.L. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell. 2006;5(1):9–15. doi: 10.1111/j.1474-9726.2006.00185.x. [http://dx.doi.org/10.1111/j.1474-9726.2006.00185.x]. [PMID: 16441838]. [DOI] [PubMed] [Google Scholar]
- 90.Strong R., Miller R.A., Astle C.M., Floyd R.A., Flurkey K., Hensley K.L., Javors M.A., Leeuwenburgh C., Nelson J.F., Ongini E., Nadon N.L., Warner H.R., Harrison D.E. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [http:// dx.doi.org/10.1111/j.1474-9726.2008.00414.x]. [PMID: 18631321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., Pahor M., Javors M.A., Fernandez E., Miller R.A. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [http://dx.doi.org/10. 1038/nature08221]. [PMID: 19587680]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller R.A., Harrison D.E., Astle C.M., Baur J.A., Boyd A.R., de Cabo R., Fernandez E., Flurkey K., Javors M.A., Nelson J.F., Orihuela C.J., Pletcher S., Sharp Z.D., Sinclair D., Starnes J.W., Wilkinson J.E., Nadon N.L., Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [http://dx.doi.org/10.1093/gerona/glq178]. [PMID: 20974732]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strong R., Miller R.A., Astle C.M., Baur J.A., de Cabo R., Fernandez E., Guo W., Javors M., Kirkland J.L., Nelson J.F., Sinclair D.A., Teter B., Williams D., Zaveri N., Nadon N.L., Harrison D.E. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(1):6–16. doi: 10.1093/gerona/gls070. [http://dx.doi.org/10.1093/gerona/ gls070]. [PMID: 22451473]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nadon N.L., Strong R., Miller R.A., Nelson J., Javors M., Sharp Z.D., Peralba J.M., Harrison D.E. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr.) 2008;30(4):187–199. doi: 10.1007/s11357-008-9048-1. [http://dx.doi.org/10.1007/s11357-008-9048-1]. [PMID: 19424842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scarpace P.J., Matheny M., Strehler K.Y., Toklu H.Z., Kirichenko N., Carter C.S., Morgan D., Tümer N. Rapamycin normalizes serum leptin by alleviating obesity and reducing leptin synthesis in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(7):891–899. doi: 10.1093/gerona/glu230. [http://dx.doi.org/10.1093/gerona/glu230]. [PMID: 25617379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009;34(4):639–659. doi: 10.1007/s11064-009-9922-y. [http://dx.doi. org/10.1007/s11064-009-9922-y]. [PMID: 19199030]. [DOI] [PubMed] [Google Scholar]
- 97.Fujitsuka N., Asakawa A., Morinaga A., Amitani M.S., Amitani H., Katsuura G., Sawada Y., Sudo Y., Uezono Y., Mochiki E., Sakata I., Sakai T., Hanazaki K., Yada T., Yakabi K., Sakuma E., Ueki T., Niijima A., Nakagawa K., Okubo N., Takeda H., Asaka M., Inui A. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry. 2016;21(11):1613–1623. doi: 10.1038/mp.2015.220. [http://dx.doi.org/10.1038/ mp.2015.220]. [PMID: 26830139]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ling S., Xu J.W. Biological Activities of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside in antiaging and antiaging-related disease treatments. Oxid. Med. Cell. Longev. 2016;2016:4973239. doi: 10.1155/2016/4973239. [http://dx.doi.org/10.1155/2016/4973239]. [PMID: 27413420]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu H.W., Chan Y.C., Wang M.F., Wei C.C., Chang S.J. Dietary (-)-Epigallocatechin-3-gallate Supplementation counteracts aging-associated skeletal muscle insulin resistance and fatty liver in senescence-accelerated mouse. J. Agric. Food Chem. 2015;63(38):8407–8417. doi: 10.1021/acs.jafc.5b02501. [http://dx.doi.org/10.1021/acs.jafc.5b02501]. [PMID: 26152236]. [DOI] [PubMed] [Google Scholar]
- 100.Zhang G.R., Cheng X.R., Zhou W.X., Zhang Y.X. Age-related expression of calcium/calmodulin-dependent protein kinase II A in the hippocampus and cerebral cortex of senescence accelerated mouse prone/8 mice is modulated by anti-Alzheimer’s disease drugs. Neuroscience. 2009;159(1):308–315. doi: 10.1016/j.neuroscience.2008.06.068. [http://dx.doi.org/ 10.1016/j.neuroscience.2008.06.068]. [PMID: 18721865]. [DOI] [PubMed] [Google Scholar]
- 101.Porquet D., Andrés-Benito P., Griñán-Ferré C., Camins A., Ferrer I., Canudas A.M., Del Valle J., Pallàs M. Amyloid and tau pathology of familial Alzheimer’s disease APP/PS1 mouse model in a senescence phenotype background (SAMP8). Age (Dordr.) 2015;37(1):9747. doi: 10.1007/s11357-015-9747-3. [http://dx.doi.org/10.1007/s11357-015-9747-3]. [PMID: 25663420]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morley J.E., Farr S.A., Kumar V.B., Armbrecht H.J. The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer’s disease. Curr. Pharm. Des. 2012;18(8):1123–1130. doi: 10.2174/138161212799315795. [http://dx.doi.org/10.2174/138161212799315795]. [PMID: 22288401]. [DOI] [PubMed] [Google Scholar]
- 103.Butterfield D.A., Poon H.F. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005;40(10):774–783. doi: 10.1016/j.exger.2005.05.007. [http://dx.doi.org/10.1016/j.exger.2005.05.007]. [PMID: 16026957]. [DOI] [PubMed] [Google Scholar]
- 104.Huang L., Su T., Li X. Natural products as sources of new lead compounds for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2013;13(15):1864–1878. doi: 10.2174/15680266113139990142. [http://dx.doi.org/10.2174/ 15680266113139990142]. [PMID: 23931437]. [DOI] [PubMed] [Google Scholar]
- 105.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., McGuinness O.P., Chikuda H., Yamaguchi M., Kawaguchi H., Shimomura I., Takayama Y., Herz J., Kahn C.R., Rosenblatt K.P., Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [http:// dx.doi.org/10.1126/science.1112766]. [PMID: 16123266]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murakami S. Stress resistance in long-lived mouse models. Exp. Gerontol. 2006;41(10):1014–1019. doi: 10.1016/j.exger.2006.06.061. [http://dx.doi.org/10.1016/ j.exger.2006.06.061]. [PMID: 16962277]. [DOI] [PubMed] [Google Scholar]
- 107.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y.I. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [http://dx.doi.org/10.1038/36285]. [PMID: 9363890]. [DOI] [PubMed] [Google Scholar]
- 108.Bartke A., Brown-Borg H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [http://dx.doi.org/10. 1016/S0070-2153(04)63006-7]. [PMID: 15536017]. [DOI] [PubMed] [Google Scholar]
- 109.Sadagurski M., Landeryou T., Cady G., Kopchick J.J., List E.O., Berryman D.E., Bartke A., Miller R.A. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell. 2015;14(6):1045–1054. doi: 10.1111/acel.12382. [http://dx.doi.org/10. 1111/acel.12382]. [PMID: 26268661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dominick G., Berryman D.E., List E.O., Kopchick J.J., Li X., Miller R.A., Garcia G.G. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology. 2015;156(2):565–575. doi: 10.1210/en.2014-1690. [http://dx.doi.org/10.1210/en.2014-1690]. [PMID: 25456069]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khodr C.E., Clark S.M., Hurley D.L., Phelps C.J. Long-term, homologous prolactin, administered through ectopic pituitary grafts, induces hypothalamic dopamine neuron differentiation in adult Snell dwarf mice. Endocrinology. 2008;149(4):2010–2018. doi: 10.1210/en.2007-1426. [http://dx.doi.org/10.1210/en.2007-1426]. [PMID: 18096658]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katic M., Kennedy A.R., Leykin I., Norris A., McGettrick A., Gesta S., Russell S.J., Bluher M., Maratos-Flier E., Kahn C.R. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6(6):827–839. doi: 10.1111/j.1474-9726.2007.00346.x. [http://dx. doi.org/10.1111/j.1474-9726.2007.00346.x]. [PMID: 18001293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bluher M. Fat tissue and long life. Obes. Facts. 2008;1(4):176–182. doi: 10.1159/000145930. [http://dx.doi.org/10.1159/000145930]. [PMID: 20054178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolosova N.G., Vitovtov A.O., Muraleva N.A., Akulov A.E., Stefanova N.A., Blagosklonny M.V. Rapamycin suppresses brain aging in senescence-accelerated OXYS rats. Aging (Albany N.Y.) 2013;5(6):474–484. doi: 10.18632/aging.100573. [http://dx.doi.org/10.18632/aging.100573]. [PMID: 23817674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang G.R., Wu Y.Y., Chiu Y.S., Chen W.Y., Liao J.W., Hsu H.M., Chao T.H., Hung S.W., Mao F.C. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin. Pharmacol. Toxicol. 2009;105(3):188–198. doi: 10.1111/j.1742-7843.2009.00427.x. [http://dx.doi.org/10.1111/j.1742-7843. 2009.00427.x]. [PMID: 19496779]. [DOI] [PubMed] [Google Scholar]
- 116.Arriola A.S.I., Pumper C.P., Baar E.L., Cummings N.E., Lamming D.W. Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(7):876–881. doi: 10.1093/gerona/glw064. [http://dx.doi.org/10.1093/gerona/ glw064]. [PMID: 27091134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitani K., Kanai S., Carrillo M.C., Ivy G.O. (-)Deprenyl increases the life span as well as activities of superoxide dismutase and catalase but not of glutathione peroxidase in selective brain regions in Fischer rats. Ann. N. Y. Acad. Sci. 1994;717:60–71. doi: 10.1111/j.1749-6632.1994.tb12073.x. [http://dx.doi.org/10.1111/j.1749-6632.1994.tb12073.x]. [PMID: 8030852]. [DOI] [PubMed] [Google Scholar]
- 118.Knoll J. The striatal dopamine dependency of life span in male rats. Longevity study with (-)deprenyl. Mech. Ageing Dev. 1988;46(1-3):237–262. doi: 10.1016/0047-6374(88)90128-5. [http://dx.doi.org/10.1016/0047-6374(88)90128-5]. [PMID: 3147347]. [DOI] [PubMed] [Google Scholar]
- 119.Jordens R.G., Berry M.D., Gillott C., Boulton A.A. Prolongation of life in an experimental model of aging in Drosophila melanogaster. Neurochem. Res. 1999;24(2):227–233. doi: 10.1023/a:1022510004220. [http://dx.doi. org/10.1023/A:1022510004220]. [PMID: 9972869]. [DOI] [PubMed] [Google Scholar]
- 120.Stoll S., Hafner U., Kränzlin B., Müller W.E. Chronic treatment of Syrian hamsters with low-dose selegiline increases life span in females but not males. Neurobiol. Aging. 1997;18(2):205–211. doi: 10.1016/s0197-4580(97)00009-2. [http://dx.doi.org/10.1016/S0197-4580(97)00009-2]. [PMID: 9258898]. [DOI] [PubMed] [Google Scholar]
- 121.Ruehl W.W., Entriken T.L., Muggenburg B.A., Bruyette D.S., Griffith W.C., Hahn F.F. Treatment with L-deprenyl prolongs life in elderly dogs. Life Sci. 1997;61(11):1037–1044. doi: 10.1016/s0024-3205(97)00611-5. [http://dx.doi. org/10.1016/S0024-3205(97)00611-5]. [PMID: 9307048]. [DOI] [PubMed] [Google Scholar]
- 122.Kitani K., Minami C., Isobe K., Maehara K., Kanai S., Ivy G.O., Carrillo M.C. Why (--)deprenyl prolongs survivals of experimental animals: increase of anti-oxidant enzymes in brain and other body tissues as well as mobilization of various humoral factors may lead to systemic anti-aging effects. Mech. Ageing Dev. 2002;123(8):1087–1100. doi: 10.1016/s0047-6374(01)00392-x. [http://dx.doi.org/10.1016/S0047-6374(01)00392-X]. [PMID: 12044958]. [DOI] [PubMed] [Google Scholar]
- 123.Mariño G., Ugalde A.P., Fernández A.F., Osorio F.G., Fueyo A., Freije J.M., López-Otín C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. USA. 2010;107(37):16268–16273. doi: 10.1073/pnas.1002696107. [http://dx.doi.org/10.1073/pnas. 1002696107]. [PMID: 20805469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Waard M.C., van der Pluijm I., Zuiderveen Borgesius N., Comley L.H., Haasdijk E.D., Rijksen Y., Ridwan Y., Zondag G., Hoeijmakers J.H., Elgersma Y., Gillingwater T.H., Jaarsma D. Age-related motor neuron degeneration in DNA repair-deficient Ercc1 mice. Acta Neuropathol. 2010;120(4):461–475. doi: 10.1007/s00401-010-0715-9. [http://dx. doi.org/10.1007/s00401-010-0715-9]. [PMID: 20602234]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Boer J., Andressoo J.O., de Wit J., Huijmans J., Beems R.B., van Steeg H., Weeda G., van der Horst G.T., van Leeuwen W., Themmen A.P., Meradji M., Hoeijmakers J.H. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296(5571):1276–1279. doi: 10.1126/science.1070174. [http://dx.doi.org/10.1126/science.1070174]. [PMID: 11950998]. [DOI] [PubMed] [Google Scholar]
- 126.Vermeij W.P., Dollé M.E., Reiling E., Jaarsma D., Payan-Gomez C., Bombardieri C.R., Wu H., Roks A.J., Botter S.M., van der Eerden B.C., Youssef S.A., Kuiper R.V., Nagarajah B., van Oostrom C.T., Brandt R.M., Barnhoorn S., Imholz S., Pennings J.L., de Bruin A., Gyenis Á., Pothof J., Vijg J., van Steeg H., Hoeijmakers J.H. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [http://dx.doi.org/10.1038/nature19329]. [PMID: 27556946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu B., Ghosh S., Yang X., Zheng H., Liu X., Wang Z., Jin G., Zheng B., Kennedy B.K., Suh Y., Kaeberlein M., Tryggvason K., Zhou Z. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16(6):738–750. doi: 10.1016/j.cmet.2012.11.007. [http://dx.doi.org/ 10.1016/j.cmet.2012.11.007]. [PMID: 23217256]. [DOI] [PubMed] [Google Scholar]
- 128.Ramos F.J., Chen S.C., Garelick M.G., Dai D.F., Liao C.Y., Schreiber K.H., MacKay V.L., An E.H., Strong R., Ladiges W.C., Rabinovitch P.S., Kaeberlein M., Kennedy B.K. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012;4(144):144ra103. doi: 10.1126/scitranslmed.3003802. [http://dx.doi. org/10.1126/scitranslmed.3003802]. [PMID: 22837538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fong L.G., Frost D., Meta M., Qiao X., Yang S.H., Coffinier C., Young S.G. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science. 2006;311(5767):1621–1623. doi: 10.1126/science.1124875. [http://dx.doi.org/10.1126/science.1124875]. [PMID: 16484451]. [DOI] [PubMed] [Google Scholar]
- 130.Yang S.H., Meta M., Qiao X., Frost D., Bauch J., Coffinier C., Majumdar S., Bergo M.O., Young S.G., Fong L.G. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Invest. 2006;116(8):2115–2121. doi: 10.1172/JCI28968. [http://dx.doi.org/10.1172/JCI28968]. [PMID: 16862216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mendelsohn A.R., Larrick J.W. Rapamycin as an antiaging therapeutic? targeting mammalian target of rapamycin to treat Hutchinson-Gilford progeria and neurodegenerative diseases. Rejuvenation Res. 2011;14(4):437–441. doi: 10.1089/rej.2011.1238. [http://dx.doi.org/10.1089/ rej.2011.1238]. [PMID: 21851176]. [DOI] [PubMed] [Google Scholar]
- 132.Jaarsma D., van der Pluijm I., van der Horst G.T., Hoeijmakers J.H. Cockayne syndrome pathogenesis: lessons from mouse models. Mech. Ageing Dev. 2013;134(5-6):180–195. doi: 10.1016/j.mad.2013.04.003. [http://dx.doi.org/10. 1016/j.mad.2013.04.003]. [PMID: 23591128]. [DOI] [PubMed] [Google Scholar]
- 133.Strandgren C., Nasser H.A., McKenna T., Koskela A., Tuukkanen J., Ohlsson C., Rozell B., Eriksson M. Transgene silencing of the Hutchinson-Gilford progeria syndrome mutation results in a reversible bone phenotype, whereas resveratrol treatment does not show overall beneficial effects. FASEB J. 2015;29(8):3193–3205. doi: 10.1096/fj.14-269217. [http://dx.doi.org/10.1096/fj.14-269217]. [PMID: 25877214]. [DOI] [PubMed] [Google Scholar]
- 134.Niedernhofer L.J., Garinis G.A., Raams A., Lalai A.S., Robinson A.R., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W., Theil A.F., Vermeulen W., van der Horst G.T., Meinecke P., Kleijer W.J., Vijg J., Jaspers N.G., Hoeijmakers J.H. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444(7122):1038–1043. doi: 10.1038/nature05456. [http://dx.doi.org/10.1038/nature05456]. [PMID: 17183314]. [DOI] [PubMed] [Google Scholar]
- 135.Barnhoorn S., Uittenboogaard L.M., Jaarsma D., Vermeij W.P., Tresini M., Weymaere M., Menoni H., Brandt R.M., de Waard M.C., Botter S.M., Sarker A.H., Jaspers N.G., van der Horst G.T., Cooper P.K., Hoeijmakers J.H., van der Pluijm I. Cell-autonomous progeroid changes in conditional mouse models for repair endonuclease XPG deficiency. PLoS Genet. 2014;10(10):e1004686. doi: 10.1371/journal.pgen.1004686. [http://dx.doi.org/10.1371/journal.pgen.1004686]. [PMID: 25299392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kealy R.D., Lawler D.F., Ballam J.M., Mantz S.L., Biery D.N., Greeley E.H., Lust G., Segre M., Smith G.K., Stowe H.D. Effects of diet restriction on life span and age-related changes in dogs. J. Am. Vet. Med. Assoc. 2002;220(9):1315–1320. doi: 10.2460/javma.2002.220.1315. [http://dx. doi.org/10.2460/javma.2002.220.1315]. [PMID: 11991408]. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt F., Boltze J., Jäger C., Hofmann S., Willems N., Seeger J., Härtig W., Stolzing A. Detection and Quantification of β-Amyloid, pyroglutamyl Aβ, and tau in aged canines. J. Neuropathol. Exp. Neurol. 2015;74(9):912–923. doi: 10.1097/NEN.0000000000000230. [http://dx.doi.org/10. 1097/NEN.0000000000000230]. [PMID: 26247394]. [DOI] [PubMed] [Google Scholar]
- 138.Christie L.A., Opii W.O., Head E., Araujo J.A., de Rivera C., Milgram N.W., Cotman C.W. Short-term supplementation with acetyl-L-carnitine and lipoic acid alters plasma protein carbonyl levels but does not improve cognition in aged beagles. Exp. Gerontol. 2009;44(12):752–759. doi: 10.1016/j.exger.2009.08.012. [http://dx.doi.org/10.1016/j.exger.2009. 08.012]. [PMID: 19735717]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Araujo J.A., Landsberg G.M., Milgram N.W., Miolo A. Improvement of short-term memory performance in aged beagles by a nutraceutical supplement containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine. Can. Vet. J. 2008;49(4):379–385. [PMID: 18481547]. [PMC free article] [PubMed] [Google Scholar]
- 140.Araujo J.A., Landsberg G.M., Milgram N.W., Miolo A. Improvement of short-term memory performance in aged beagles by a nutraceutical supplement containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine. Can. Vet. J. 2008;49(4):379–385. [PMID: 18481547]. [PMC free article] [PubMed] [Google Scholar]
- 141.Lelegren M., Liu Y., Ross C., Tardif S., Salmon A.B. Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol. Aging Age Relat. Dis. 2016;6:31793. doi: 10.3402/pba.v6.31793. [http://dx.doi. org/10.3402/pba.v6.31793]. [PMID: 27341957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fischer K.E., Austad S.N. The development of small primate models for aging research. ILAR J. 2011;52(1):78–88. doi: 10.1093/ilar.52.1.78. [http:// dx.doi.org/10.1093/ilar.52.1.78]. [PMID: 21411860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tardif S.D., Mansfield K.G., Ratnam R., Ross C.N., Ziegler T.E. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [http://dx.doi.org/10.1093/ilar.52.1.54]. [PMID: 21411858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bourdenx M., Nilsson A., Wadensten H., Fälth M., Li Q., Crossman A.R., Andrén P.E., Bezard E. Abnormal structure-specific peptide transmission and processing in a primate model of Parkinson’s disease and l-DOPA-induced dyskinesia. Neurobiol. Dis. 2014;62:307–312. doi: 10.1016/j.nbd.2013.10.016. [http://dx.doi.org/10.1016/j.nbd.2013.10. 016]. [PMID: 24148855]. [DOI] [PubMed] [Google Scholar]
- 145.Colman R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W., Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [http://dx.doi.org/10.1126/science.1173635]. [PMID: 19590001]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ross C., Salmon A., Strong R., Fernandez E., Javors M., Richardson A., Tardif S. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus). Aging (Albany N.Y.) 2015;7(11):964–973. doi: 10.18632/aging.100843. [http://dx. doi.org/10.18632/aging.100843]. [PMID: 26568298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Philippens I.H., ’t Hart B.A., Torres G. The MPTP marmoset model of parkinsonism: a multi-purpose non-human primate model for neurodegenerative diseases. Drug Discov. Today. 2010;15(23-24):985–990. doi: 10.1016/j.drudis.2010.08.009. [http://dx.doi.org/10.1016/j.drudis.2010.08.009]. [PMID: 20732446]. [DOI] [PubMed] [Google Scholar]
- 148.Yun J.W., Ahn J.B., Kang B.C. Modeling Parkinson’s disease in the common marmoset (Callithrix jacchus): overview of models, methods, and animal care. Lab. Anim. Res. 2015;31(4):155–165. doi: 10.5625/lar.2015.31.4.155. [http://dx.doi.org/10.5625/lar.2015.31.4.155]. [PMID: 26755918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palazzi X., Switzer R., George C. Natural occurrence of amyloid-Abeta deposits in the brain of young common marmosets (Callithrix jacchus): a morphological and immunohistochemical evaluation. Vet. Pathol. 2006;43(5):777–779. doi: 10.1354/vp.43-5-777. [http://dx.doi.org/10.1354/ vp.43-5-777]. [PMID: 16966460]. [DOI] [PubMed] [Google Scholar]
- 150.Jimenez-Gomez Y., Mattison J.A., Pearson K.J., Martin-Montalvo A., Palacios H.H., Sossong A.M., Ward T.M., Younts C.M., Lewis K., Allard J.S., Longo D.L., Belman J.P., Malagon M.M., Navas P., Sanghvi M., Moaddel R., Tilmont E.M., Herbert R.L., Morrell C.H., Egan J.M., Baur J.A., Ferrucci L., Bogan J.S., Bernier M., de Cabo R. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18(4):533–545. doi: 10.1016/j.cmet.2013.09.004. [http://dx.doi.org/10.1016/j.cmet.2013.09. 004]. [PMID: 24093677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marchal J., Dal-Pan A., Epelbaum J., Blanc S., Mueller S., Wittig Kieffer M., Metzger F., Aujard F. Calorie restriction and resveratrol supplementation prevent age-related DNA and RNA oxidative damage in a non-human primate. Exp. Gerontol. 2013;48(9):992–1000. doi: 10.1016/j.exger.2013.07.002. [http://dx.doi.org/10.1016/j.exger.2013.07.002]. [PMID: 23860387]. [DOI] [PubMed] [Google Scholar]
- 152.Geula C., Nagykery N., Wu C.K. Amyloid-beta deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol. 2002;103(1):48–58. doi: 10.1007/s004010100429. [http://dx.doi.org/10.1007/s004010100429]. [PMID: 11837747]. [DOI] [PubMed] [Google Scholar]
- 153.Bernier M., Wahl D., Ali A., Allard J., Faulkner S., Wnorowski A., Sanghvi M., Moaddel R., Alfaras I., Mattison J.A., Tarantini S., Tucsek Z., Ungvari Z., Csiszar A., Pearson K.J., de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany N.Y.) 2016;8(5):899–916. doi: 10.18632/aging.100942. [http://dx.doi.org/10.18632/ aging.100942]. [PMID: 27070252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mattison J.A., Roth G.S., Beasley T.M., Tilmont E.M., Handy A.M., Herbert R.L., Longo D.L., Allison D.B., Young J.E., Bryant M., Barnard D., Ward W.F., Qi W., Ingram D.K., de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [http://dx.doi.org/10.1038/nature11432]. [PMID: 22932268]. [DOI] [PMC free article] [PubMed] [Google Scholar]