Abstract
Neurodegeneration (NDG) is linked with the progressive loss of neural function with intellectual and/or motor impairment. Several diseases affecting older individuals, including
Alzheimer's disease, Amyotrophic Lateral Sclerosis, Huntington’s disease, Parkinson's disease, stroke, Multiple Sclerosis and many others, are the most relevant disorders associated with NDG. Since other pathologies such as refractory epilepsy, brain infections, or hereditary diseases such as “neurodegeneration with brain iron accumulation”, also lead to chronic brain inflammation with loss of neural cells, NDG can be said to affect all ages. Owing to an energy and/or oxygen supply imbal-ance, different signaling mechanisms including MAPK/PI3K-Akt signaling pathways, glutamatergic synapse formation, and/or translocation of phosphatidylserine, might activate some central executing mechanism common to all these pathologies and also related to oxidative stress. Hypoxia inducible factor 1-α (HIF-1α) plays a twofold role through gene activation, in the sense that this factor has to “choose” whether to protect or to kill the affected cells. Most of the afore-mentioned process-es follow a protracted course and are accompanied by progressive iron accumulation in the brain. We hypothesize that the neuroprotective effects of iron chelators are acting against the generation of free radicals derived from iron, and also induce sufficient -but not excessive- activation of HIF-1α, so that only the hypoxia-rescue genes will be activated. In this regard, the expression of the erythropoietin receptor in hypoxic/inflammatory neurons could be the cellular “sign” to act upon by the na-sal administration of pharmacological doses of Neuro-EPO, inducing not only neuroprotection, but eventually, neurorepair as well
Keywords: Neurodegeneration, HIF-1, MDR-1, refractory epilepsy, Neuro-EPO, neuroprotection
1. Introduction
The Earth´s population nowadays is more than 7 billion, and life expectancy is around 69 years asymmetrically distributed into two main groups: a) 80 years for developed countries and b) 57-67 years for developing undeveloped countries respectively.
Never before in the history of mankind have people expected to live to be seventy or even longer. At least five factors can account for this phenomenon [1].
The drastic decline in the mortality rate in all age groups around the world.
Improvements in health-care standards and sanitary conditions.
The increase in the general standard of living in many regions of the world.
Enhanced food production and more efficient distribution.
The implementation of more effective development policies.
Today, for the first time in history, most people can reasonably expect to live until the age of 60 or even longer. This will require fundamental changes, not only in what we do, but in how we conceive of the very process of ageing [2]. The world's population is aging at a rapid pace, and between 2000 and 2050, people over 60y will double from 11% to 22%. Furthermore, public institutions need to take a more comprehensive approach to tackle the issue of the aging process of the world`s population, more specifically the morbi-mortality associated with neurodegenerative processes.
So the question arises, “What must we do that we have not done yet?”
Firstly, we should try to understand what does our brain require in order to overcome the loss of its normal homeostasis, or rather in order to prevent this loss, which innumerable processes are triggered off that can evolve to stages actually incompatible with the wonderful miracle of life.
The central nervous system (CNS) degenerative processes - collectively known as “neurodegeneration”- are currently linked with the progressive loss of neural function associated with intellectual and/or motor impairment. However, the underlying mechanisms of this process are not yet fully understood. In several diseases affecting older individuals, including Alzheimer's disease (AD), Amyotrophic Lateral Sclerosis (ALS), Huntington’s disease (HD), Parkinson's disease (PD), stroke, Multiple Sclerosis (MS) - and many others- hypoxia could play a central role in triggering “neurodegeneration”. Perhaps we could include some other pathologies in this neurodegenerative category, such as refractory epilepsy, CNS infections or some hereditary diseases, all leading to chronic brain inflammation and neuron death. Thus, neurodegenerative processes might actually be said to affect all ages.
HIF-1α could be the main actor responsible for both protective and deleterious effects during the development of the most relevant disorders associated with neurodegeneration. Hypoxia-inducible factor-1α (HIF-1α) was clearly shown to have a twofold role as “protective transcription factor”, or “killer factor” (when associated with p53), depending on the severity of hypoxia [3].
In the first scenario, HIF-1α induces the expression of EPO-R in the hypoxic tissue. In this regard and “as previously mentioned” HIF-1α -induced high overexpression of EPO-R is necessary for a successful treatment with exogenously administered human recombinant erythropoietin (rHu-Epo) [4].
Perhaps, our challenge will be to develop novel strategies, ranging from therapeutic and nutritional interventions to the adoption of more wholesome life-styles, thus allowing for both neuroprotection and neurorepair [5]. Furthermore, it has to be conceded that the normal mechanistic processes during aging are not easy to differentiate from those underlying some NDG diseases as recently discussed [6].
However, the main central mechanism responsible for this process of neurodegeneration still remains to be identified. To date, the etiology of most neurodegenerative disorders is assumed to be multifactorial in nature, whereby complex interactions between environmental and endogenous factors, together with genetic susceptibility conditions, finally come into play [7]. This “multifactorial” concept compels us to consider that different signaling pathways might activate some central executing mechanism common to all these pathologic entities. One of these central pathways is undoubtedly related to hypoxia, which in turn produces energy imbalance, oxidative stress and inflammation.
Taking into account that moderate free radical generation is a common outcome of normal aerobic cellular metabolism, any imbalance in the antioxidant mechanisms, as well as free radical overproduction that involves iron accumulation, poses a high risk of brain cell death, thus leading to neurodegeneration. Furthermore, as previously described, the brain is the organ that shows the lowest antioxidant activity accounting for only about 10% of the antioxidant activity of the liver. In contrast, the human brain concentrates high levels of iron in certain specific regions, and consequently neurons are more susceptible to oxidative damage than many other cells of the body [8].
2. Brain Hypoxia and HIF-1
Since the brain is a great energy consumer and so it is particularly susceptible to hypoxia. Consequently, severe and prolonged oxygen deprivation can contribute to brain damage by inducing cell death and NDG. Mammalian cells, in order to adapt to a hypoxic microenvironment, activate or initiate physiological responses to hypoxia that are mediated by hypoxia-inducible factors (HIFs). Two hypoxia-inducible factor HIF-1 protein subunits have been described: the inducible HIF-1〈 and the constitutive HIF-1β. Under normoxic conditions, HIF-1〈 is synthesized and degraded by the ubiquitin-proteasome system [9].
Furthermore, the maintenance of normal brain function depends on the continuous supply of oxygen. Therefore, it is crucial for the body to be capable of detecting and rapidly responding to hypoxia. Therefore, many of the long-term hypoxic responses are orchestrated by the transcriptional complex HIF-1〈/β, which plays a key role in cellular and systemic oxygen homeostasis. Under hypoxic conditions, HIF-1〈 protein becomes stabilized and rapidly accumulates in the cytosol. HIF-1〈 then binds with the β subunit and the complex is finally translocated into the nucleus where it serves as a transcriptional activator of over 100 genes [10]. Interestingly, HIF-1 induces the transcription of vascular endothelial growth factor (VEGF), erythropoietin (EPO) and their corresponding receptors (VEGR-R and EPO-R), which increases oxygen availability by promoting erythropoiesis and angiogenesis. Furthermore, HIF-1〈 may also activate the genes involved in glucose transport and metabolism. The role of HIF-1α is also important for normal homeostasis in the face of oxygen deprivation [11, 12]. In addition, it was observed that mild hypoxia can induce tolerance to a more severe hypoxic lesion afterwards by activating the “hypoxia-inducible factor 1-α” (HIF-1α), thus allowing for adaptive modifications required for a better and earlier recovery of the affected tissue. In fact, under ischemic conditions, HIF-1α activation in cells represents an endogenous biological protective mechanism which induces protection against global cerebral ischemia or future lethal insults [13, 14]. Moreover, it is reported that in a wide spectrum of diseases, including some NDG-diseases, HIF-1α is up-regulated as a brain neuroprotective response element against stressors such as ROS [15] and inflammation [16, 17].
However, the role of HIF-1α goes beyond this adaptive response, since it is also involved in the regulation of other pathologic processes including the modulation of the apoptotic process, angiogenesis and progression and pharmacoresistance of cancer. Furthermore, HIF-1〈 inhibition reverses multidrug resistance in colon cancer cells via downregulation P-glycoprotein (P-gp), the product of the multidrug-resistance (MDR-1) gene [18].
As previously mentioned, and on an absolutely speculative level, if neurodegenerative processes are “naturally” observed during the course of normal ageing, similar mechanisms might also be active during the development of neurodegenerative “pathologies”, regardless of their age of presentation. Interestingly, neurodegenerative diseases in the elderly are quite common and their actual impact on the normal process of brain ageing remains to be elucidated, because many aspects of the normal aging process are not clearly different from pathologic processes occurring at this age [6].
According to this thorough review, perhaps, we should consider NDG in the elderly population as a “normal” process. Thus, the concept of “Neurodegeneration” could only be invoked if NDG were observed in younger individuals, as an anticipated and/or accelerated process, which would be otherwise “normal” in the elderly.
Perhaps, we should also ask ourselves whether, as part of a circular process, hypoxic intrauterine conditions during pregnancy, might gradually recur with the normal process of ageing, or eventually precipitate earlier during the development of pathologic processes. If this were the case, the use of biological tools that restore and/or induce normal tissue O2 balance, such as erythropoietin, would be fully justified in the light of its well-documented proliferative, anti-inflammatory and anti-apoptotic properties.
3. HIF-1, Hypoxia and neurodegenerative diseases
Under hypoxic conditions, HIF-1〈 is known to be involved in neuron protection through an increase in glucose uptake (through an insulin-independent mechanism), because the enhanced glycolytic flux during hypoxia requires the transcriptional activation of genes encoding glucose transporters and the corresponding glycolytic enzymes [19]. During glucose deprivation, the energy-related metabolic stress can produce the intracellular activation of the oxidative catabolism of glucose, which includes glycolysis, pentose phosphate pathway and tricarboxylic acid cycle. All these metabolic alternative pathways can induce the altered metabolism of β-amyloid precursor protein (APP) that also can contribute to amyloidosis in both insulin-resistant diabetes type 2 and AD [20, 21].
We know that the brain consumes at least about 20-30% of total oxygen, and needs 120 g of glucose to be supplied daily to fulfill its normal function. A decreased rate of aerobic glycolysis in the brain producing the loss of cell survival mechanisms can be further combined with other pathogenic processes leading to neurodegeneration and including overexpression of APP and the imbalance in the production and clearance of the associated of β-amyloid peptide (Aβ) [22].
The activation of the afore mentioned genes is mediated by HIF-1α and includes a shunt from the normal pyruvate metabolism of the tricarboxylic acid cycle (TCA) or Krebs cycle to glycolysis, and all these mechanisms, thereby are boosting the antioxidant defense system [15].
With reference to Alzheimer´s disease (AD), some intriguing published data point to both neuroprotective and detrimental effects associated with the hypoxia-inducible factor (HIF-1〈). In this sense, decreased brain levels of HIF-1α and glucose transporters, associated with the increased phosphorylation of tau protein and the formation of neurofilaments have been described, than the age-matched controls [21].
A preclinical study demonstrated that HIF-1〈 induces an increase in the expression of BACE1, the major protease catalyzing the β-cleavage of APP, thus suggesting a direct link between HIF-1〈 and AD. Moreover, HIF-1〈-deficient mice show a reduced BACE1 expression in some brain areas, such as the hippocampus and brain cortex. These results point to an important role of hypoxia/HIF-1〈 in the modulation of the amyloidogenic processing of APP [23].
Astrocyte activation by the amyloid beta peptide can be reduced by increased levels of HIF-1〈 [24]. Furthermore, by means of a gene therapy procedure that induced the expression of the human HIF-1〈 gene, it was demonstrated that HIF-1α can significantly reduce the Aβ-protein induced-apoptosis of primary cultured hippocampal neurons [25].
In contrast, it was also demonstrated that cerebral ischemia and stroke induce the overexpression of HIF-1〈, which (in its proapoptotic role) increases the expression of beta-secretase, resulting in the overexpression of BACE1, associated with the amyloidogenic process [23]. Also, under the same conditions, the production of reactive oxygen species can induce inflammation with the reduced expression of genes required to maintain synaptic structure and function [26]. All these findings are in accordance with the previously described two-fold role attributed to HIF-1α, in which moderate hypoxia induces protection, but under severe hypoxia, high levels of HIF-1α can activate the p53 tumor-suppressor gene, and thus induce cell apoptosis [2].
Since cerebral hypo-perfusion (inducing hypoxia) has been recently associated with both accelerated cognitive decline and an increased risk of dementia in the general population [27], the afore-mentioned effects of HIF-1α on glycolysis and glucose uptake by themselves, are not enough to prevent the NDG observed after stroke or brain ischemia.
Since Aβ activates astrocytes and decreases HIF-1〈 expression, these authors concluded that HIF-1〈 is not necessary for glial activation. However, cells lacking HIF-1〈 gene expression are not rescued from oxidative stress induced by desferoxamine (DFO). This iron chelator stabilizes HIF-1〈 by inhibiting both pyrolyl-hydroxylases (PHD) and the proteasome, and thus enhance several HIF-dependent genes such as those involved in the pentose shunt, and finally producing more NADPH to limit ROS accumulation [28] and DFO-induced HIF-1〈 expression involves a cascade of signaling events including ROS generation [29].
Contrary to these observations, in another system DFO administration to erythroid progenitors, reduced intracellular ROS levels, suppressed apoptosis, and restored the differentiation of these precursors to mature erythroblasts, in iron overload cultured cells [30]. Again, all these controversial results could be accounted for by the different intracellular concentrations of DFO, HIF-1〈 or ROS generated in each experimental model.
Mitochondria are perhaps the main producers of ROS and they are also the main targets of oxidative damage, as described in several neurodegenerative diseases including AD [31]. Under physiologic conditions, mitochondrial generation of moderate ROS levels regulates several metabolic pathways, and mitochondrial ROS production is not only essential to the stabilization and activation of the HIF-1α protein [32], but ROS can also be the putative signaling molecule mediating between a cellular O2-sensor (PHD) and HIF-1α [33]. During hypoxia the increased production of H2O2 from mitochondria requires both electron transport at complex III and HIF-1α stabilization, indicating that moderate mitochondrial ROS production is directly related withy HIF-1〈 stabilization. Interestingly, in response to DFO, HIF-1α stabilization could also be produced in a mitochondrion-independent manner.
In this respect, it is important to remark that the microenvironmental and intracellular levels of ROS are vital to cell homeostasis. Excessive ROS concentrations can not only kill pathogens but our own ROS-producing cells as well. If not controlled by physiologic antioxidant mechanisms, ROS production can produce chronic inflammatory tissue injury [34].
During hypoxia the increased production of H2O2 from mitochondria requires both electron transport at complex III and HIF-1α stabilization, indicating that moderate mitochondrial ROS production is directly related withy HIF-1〈 stabilization. Interestingly, in response to DFO, HIF-1α stabilization could also be produced in a mitochondrion-independent manner [35].
Additionally, it was recently suggested that in the neuroinflammatory process observed during mild cognitive impairment (MCI) in AD, the production of pro-inflammatory cytokines, such as TNF-α and interleukin 1 beta (IL-1β) can up-regulate HIF-1〈 expression, through the inhibition of the PHD enzymes, thus generating a double mechanism of neurotoxicity, by the increased Aβ production and by the neuroinflammatory process itself, with the consequent up-regulation of HIF-1〈 [36].
Recently it was reported that the intranasal administration of DFO to APP/PS1 mice improves cognition and inhibits both Aβ plaque formation and tau hyperphosphorylation. The authors suggest that HIF-1〈 activation, associated with the specific HIF-1α gene expression is involved in the neuroprotective properties of DFO [37]. Since the HIF-1α signaling pathway has a neuroprotective effect, it could be postulated that the drugs that induce an increase in the intracellular concentrations of HIF-1〈 might represent a promising therapeutic target for the treatment of neurodegenerative disorders. Therefore, HIF-1〈 activators may be potential drugs for the management of neurologic diseases.
M30 is the name of a new drug which might act as an activator of the HIF-1α pathway. It was demonstrated that this drug increases the transcriptional rates of the HIF-1-responsive genes, achieving neuroprotection [38]. This compound is an 8-hydroxyquinoline derivative that exerts its physiological effects through the chelation of metal ions. In addition, the neuroprotective effects of iron chelators could result, at least in part, from the inhibition of PHDs and from the sequestering of redox-active iron, thus preventing the formation of hydroxyl radicals. Therefore, several iron chelators derived from 8-hydroxyquinolines have been developed and the compound M30 has proven to be the most potent, nontoxic, lipophilic, and BBB-permeable iron-selective chelator. In addition to the antioxidant properties of 8-hydroxyquinolines derivatives, these compounds have also shown anti-inflammatory properties through the inhibition of NF-κB activation. Inflammatory processes can generate an increase in NO through activation of the iNOS enzyme, whose gene expression is regulated by NF-κB.
The anti-inflammatory properties of 8-hydroxyquinoline derivatives lend support to the postulation of these drugs as potential neuroprotective agents. Thus, in a preclinical model of familial AD using APPswe/PSEN1 mice, it was reported that M30 treatment reduced Aβ plaque formation associated with an increase in HIF-1α expression [39]. Moreover, in other preclinical model of ALS, M30 has also proven to be effective by increasing the survival of G93A-SOD-1-ALS mice, and delaying disease onset, suggesting that this drug might act through the activation of the HIF-1α signaling pathway, with the subsequent transcription of the HIF-1-dependent genes [40].
On the other hand, M30 orally administered to mice for 14 days at a dose of 2.5 mg/kg/day exerts its neuroprotective effects against the neurotoxin MPTP (1-metil-4-fenil-1,2,3,6-tetrahidropiridina), suggesting a beneficial effect of this compound in experimental preclinical PD models [41].
Furthermore, DFO confers neuroprotection on dopaminergic neurons and improves motor deficits in the preclinical model of PD 6-hydroxydopamine (6-OHDA)-induced rat model of parkinsonism by inducing HIF-1α expression. In vitro studies also confirm that DFO has neuroprotective effects against 1-methyl-4-phenylpyridiniumion (MPP+), and rotenone through the inhibition of oxidative stress and the enhanced expression of HIF-1〈 and its signaling pathway [42].
In this context, prolonged hypoxia may activate the mitophagy, a selectively protective mechanism remove damaged or unwanted mitochondria for both mitochondrial quantity and quality control, in concert with inhibition of mitochondrial biogenesis [43]. Moreover, mitochondrial autophagy can be induced by hypoxia in a HIF-1α-dependent pathway that requires expression of BNIP3, a protein involved in programmed cell death pathway, which is required for autophagy induced by serum and O2 deprivation, or by treatment with iron chelators or other inhibitors of prolyl-hydroxylases. In this regards, mitochondrial autophagy has been proposed as an adaptive metabolic response that promotes the survival of cells under conditions of prolonged hypoxia which could be necessary to prevent increased levels of reactive oxygen species and cell death [17, 44]. Besides, the systemic chronic administration of the novel multifunctional brain permeable iron-chelator M30, results in the up-regulation of hypoxia-inducible factor HIF-1〈 protein levels, and its consequent stimulation of a wide spectrum of described HIF-1 dependent genes, as well as a new set of genes directly related with the fine control of electron transport, glucose utilization, and glucose sensing at mitochondrial level. So, in a HIF-1-dependent manner, several genes can be upregulated as PGC-1α (proliferator-activated receptor γ coactivator-1α), Tfam (mitochondrial transcription factor), NFT (neurotrophic factor) and SIRT1 (NAD-dependent protein deacetylase, which were suggested to be related with mitochondrial biogenesis [45].
Thus DFO is probably the most widely used chelating drug at preclinical stages for the management of iron overload [43]. However, since DFO exhibits poor blood-brain barrier (BBB) permeability, which can limit its use for the treatment of NDG disorders, the intranasal administration of this drug constitutes a suitable strategy for the direct delivery of the drug to the CNS, thus diminishing potential systemic side-effects [44]. Moreover, DFO could exert beneficial protective effects in both AD and PD [36, 45].
Additionally, 3-nitropropionic acid (3-NP), a mitochondrial toxin that irreversibly inhibits the succinate dehydrogenase (complex II) activity [46] produces selective striatal lesions in rodents that closely resemble many of the histological and neurochemical features observed in Huntington’s disease (HD). In this regard, pre-treatment of C6 astroglial cells with compounds that activate the HIF-1α signaling pathway, such as CoCl2, mimosine and DFO, attenuates the cytotoxic effects induced by the afore-mentioned mitochondrial complex-II inhibitor 3-NP [47].
In addition to M30, other 8-hydroxyquinolineses derivatives such as clioquinol and VK-28 also show antineurodegenerative properties. Kaur and col. were the first researchers to report that clioquinol protects against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced dopaminergic nigral cell loss. Likewise, this compound improved pre-clinical behavioral and pathologic symptoms in a transgenic murine model of Huntington`s disease. Moreover clioquinol decreased the accumulation of the huntingtin aggregate and enhanced motor functions and survival. Finally, this drug also improved cognitive function and decreased the Aβ plaque burden in the TgCRND8 mouse model of AD [48].
VK-28 and clioquinol can act as iron chelators with properties similar to those of DFO but with better access to the CNS [49]. More recently, it was demonstrated that VK-28 (5-[4-(2-hydroxyethyl) piperazine-1-ylmethyl]-quinoline-8-ol), has a protective effect against iron toxicity after intracerebral hemorrhage and it is more effective and less toxic than DFO. In this study, VK-28 decreased cell death and ROS production both in vitro and in vivo. VK-28 decreased iron-deposition and microglial activation surrounding the hemorraegic are in vivo, and improved neurologic function [50].
Even though the mechanism responsible for the clioquinol neuroprotective effect is still unclear, it was demonstrated that clioquinol (10-50 μM) increased the function of HIF-1α protein, leading to the also increased expression of its target genes: vascular endothelial growth factor (VEGF) and erythropoietin (EPO) [51] and this property can be the bases of the mentioned neuroprotective affect.
Intraventricular or intraperitoneal administration of the iron chelator VK-28 induces neuroprotection against 6-OHDA neurotoxicity, and prevents oxidative stress, probably due to its iron-chelating properties [52].
However, these are not the only drugs used for this purpose. In this regard, free radical production in mitochondria is dependent on the activity of Qi or Qo sites in the complex III, and it is related with the consecutive production of H2O2. It was demonstrated that ROS is released into the matrix and/or the intermembrane space/cytosol [53], while H2O2 can be released to the cytosol. Under this condition, cytosolic catalase activity can prevent HIF-1α stabilization, suggesting that the antioxidant activity in the cytosol could be a better regulatory factor for HIF-1α stabilization. Furthermore, as demonstrated by Guzy et al. [35], drugs such as myxothiazol, stigmatellin that inhibit ROS formation also inhibit HIF-1〈 stabilization during hypoxia, suggesting that not all antioxidant drugs will always lead to HIF-1α stabilization.
Interestingly, the topical application of a liposomal gel with lactoferrin, the most potent natural iron chelator, to ulcerative lesions on the lower limbs of patients presenting with chronic venous insufficiency, proved to be effective for the complete scarring of these refractory ulcerative haemosiderinic dyschromic lesions, with complete restoration of muscle, vessel, nerve and skin tissues in 7 of 9 cases [54].
The exact mechanisms responsible for the neuroprotective effects of iron chelators observed in several preclinical models of neurological disorders are not yet totally clear. We might hypothesize that several but not all these compounds could inhibit the production of free radicals derived from iron, and, simultaneously, induce sufficient -but not excessive- activation of HIF-1α, for the transcriptional activation of the hypoxia-rescue genes.
Amyotrophic lateral sclerosis (ALS), first described by Charcot in 1869, is a neurodegenerative disease of unknown etiology which affects, either simultaneously or sequentially, the upper and lower motor neurons, and it is the most common form of motor neuron disease affecting adults (incidence 1-5 per 100,000). Although most ALS cases are sporadic (sALS), 5-10% are familial (fALS), typically associated with genetic mutations mainly involving the gene coding for the superoxide dismutase 1 (SOD-1), an antioxidant enzyme whose activity preserves against oxidative stress, one of the main mechanisms by which motor neuron death occurs.
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the biological capacity of the detoxifying systems to remove ROS or repair the ROS-induced damage. ROS accumulation may be an important factor that reduces the ability of the cell to cope with an underlying pathologic situation. In fact, mutations of the anti-oxidant enzyme SOD-1 account for this disease only in a minority of familial cases [55].
Gagliardi et al. (2010) [56] found abnormally high levels of SOD-1 transcripts in the spinal cord, brain stem and lymphocytes of sALS patients as well. This observation needs to be accounted for.
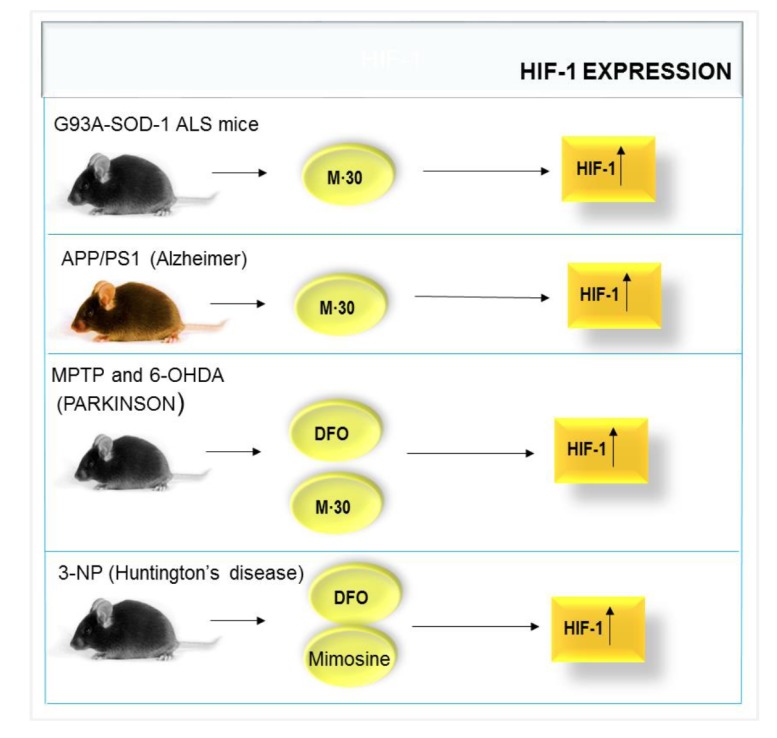
Could this high level of SOD-1 transcripts constitute an unsuccessful compensatory response mechanism, secondary to a potential SOD-1 loss of function? In this regard, it was demonstrated in transgenic mice expressing either wild-type (wt) or mutant G93A variant of human SOD-1 that wtSOD-1 was present in the cytoplasm and in the nucleus of motor neurons, whereas mutant SOD-1 was mainly confined to the cytoplasm. Similar results were obtained in immortalized motor neurons (NSC34 cells) expressing either wtSOD-1 or G93A-SOD-1. An impairment of proteasome activity was detected only in the cytoplasm of both in vivo and in vitro experiments from mutant G93A-SOD-1 variant, associated with more extensive DNA damage [57] (Fig. 1).
Fig. (1).
Neurodegenerative diseases treated with M-30, DFO or Mimosine that increase HIF-1 activation with neuroprotective effects.
The analysis of CSF and serum from both familial and sporadic ALS patients showed increased concentrations of oxidative stress-induced damage products [58, 59]. In this connection, evidence of oxidative damage to proteins [60], lipids [61] and DNA [62] has been reported in tissues from ALS patients. Furthermore, because ferritin can be overexpressed as a consequence of an iron overload and also oxidative stress, the measurement of both isoforms of ferritin (L & H) in CSF may be useful for the clinical evaluation of the disease and its progression [63].
Different enzymatic pathways may contribute to the generation of reactive oxygen species (ROS) in sALS. Most probably, in sALS, the increase in the intracytoplasmatic Ca2+ concentration, due to glutamate-mediated excitotoxicity, enhances the activity of some enzymes such as phospholipase A2, neuronal nitric oxide synthase (nNOS) and xanthine oxidase. In this setting, mitochondria will be affected as well.
Under oxidative stress, the overall morphology of mitochondria undergoes typical changes. These organelles become smaller, their crests are disrupted and their membranes break down, and edema and vacuolization ensue during the development of these morphological changes. All these ultrastructural modifications were observed in an experimental model of minimal motor lesion induced by CoCl2 cortical injection [64], and reverted by the intranasal administration of human recombinant erythropoietin (rHu-Epo). Interestingly, all these lesions were also associated with high HIF-1α nuclear translocation and EPO-receptor overexpression [65].
One of the most important consequences of oxidative stress is the damage to RNA species. Chang et al. (2008) [66] reported that mRNA oxidation primarily occurs in motor neurons and spinal cord oligodendrocytes of mutG93A-SOD-1 mice at early stages of the disease. Moreover, the translation of these oxidized mRNA species also decreases. Some mRNA species seem to be highly susceptible to oxidation, including those involved in the mitochondrial electron transport chain, protein biosynthesis, protein folding and degradation pathways, myelination, etc. [56]. Interestingly, aberrant oxidation of wt-SOD-1 present in sALS patients confers pathologic properties similar to those observed in mut-SOD-1, such as the inhibition of axonal transport [67]. All these data lend support to the hypothesis that ALS is also a disease where there is an imbalance between oxidative stress, oxygen deprivation, iron overload and HIF-1α signaling.
4. Epilepsy, inflammation and neuro-degeneration: hypoxia as the leading actor of the play
The vital and essential need for adequate O2 supplementation in tissues has evolved significantly in the higher mammals, which have actually developed highly sophisticated regulatory mechanisms capable of tolerating and/or adapting their metabolisms to transient energy deficiencies due to both hypoxia and nutrient starvation. Under all these conditions of energy imbalance, hypoxia-inducible factor-1 (HIF-1α) activation plays a central role in the sensing of hypoxia.
The inducible property of HIF-1α is translated into the overexpression of a large list of gene targets, related to vasomotor control, angiogenesis, erythropoiesis, iron metabolism, cell proliferation/cell cycle control, energy metabolism, cell death [9], or pharmacoresistance by induction of the multidrug resistant P-glycoprotein (P-gp) in different tumors [68] was well as in several areas from hypoxic brain [69].
The concept that seizures generate a condition that produces acute brain hypoxia and ischemia has been well recognized [70]. Interestingly, during the time course following both brain hypoxia and seizures, some common sequential events including neurotoxicity, depolarization, inflammation and apoptosis have been observed [71].
Seizure activity in both experimental models of epilepsy and in patients with pharmacoresistant epilepsy is known to induce the overexpression of specific factors associated with hypoxia, such as the vascular endothelial growth factor (VEGF) [72-74], P-glycoprotein [75]. HIF-1α was detected in postmortem studies of patients presenting with refractory epilepsy and hippocampal sclerosis [76]. Even though ARNT has been traditionally defined as a constitutive and non-inducible factor, it has been documented that treatment of kainic acid (KA), induces a marked increase in ARNT protein levels in both the cytosolic and organellar fractions, predominantly in microglia and partly in astrocytes, and showing a similar pattern to that observed in the immunoreactivity of heme-oxygenase 1 [77]. So, both hypoxia and glutamatergic neurotoxicity could simultaneously increase the expression of both components of the HIF-1 complex (HIF-1α and HIF-1β).
In all patients presenting with refractory epilepsy (RE), a common clinical feature is the persistent repetitive seizures, which are not controlled by antiepileptic drugs (AEDs). In these cases, irrespectively of excitotoxicity and inflammation, it could be argued that a repetitive seizure acts as a trigger of new subsequent hypoxic-ischemic events. Consequently, a large list of HIF-1-responsive genes could be overexpressed in this setting [9] including the MDR-1 gene, which produces the pharmacoresistant phenotype found not only in tumor cells [78] but also in brain cells affected by epilepsy [79, 80].
In this connection, our group has demonstrated the overexpression of P-glycoprotein (P-gp) an ABC-transporter product of the MDR-1 gene, in the brain cortex and hippocampus in rodent models of epilepsy and brain hypoxia [75, 81-83].
Furthermore, after inducing intermittent hypoxia, we observed significant astroglial hyperplasia and hypertrophy in the parietal brain cortex and hippocampus associated with HIF-1α and P-gp overexpression [84]. As recently reported, experimental intermittent hypoxias in mice increase the amount of phosphorylated tau protein in the hippocampus. In these mice, hyperactivity in Y-maze tests was also observed [85].
Interestingly, ABC-transporters, particularly P-glycoprotein (P-gp), in addition to their classical drug- efflux mechanism, can also act as a “hydrophobic vacuum cleaner”, causing lipid substrates to diffuse into the membrane bilayer, to be subsequently extruded through a central channel (present in this transporter) into the extracellular space in an ATP-dependent process [86]. Additionally, the “floppase model” accounts for the translocation of lipids, mainly phospholipids, from the inner to the outer leaflet of biological membranes [87]. Phosphatidylserine (PS) is the most abundant anionic phospholipid class on the inner leaflet of the plasma membrane of neural tissues, accounting for 13-15% of the total phospholipid content in the human brain cortex [88].
5. Phosphatidylserine exposure: Is it a new epileptogenic mechanism?
PS is a constitutive and negatively- charged component of the inner leaflet of the plasma membrane. Its high concentration on this side of the membrane allows the binding and subsequent activity of several calcium-dependent proteins involved in neural signaling pathways. The activation of Akt/PI3K, Raf-1 and protein kinase Cα requires their own translocation from the cytosol to the plasma membrane, for which their interaction with PS is critically important [89].
Akt, also known as protein kinase B (PKB), is a serine/threonine kinase which plays a role in mediating neuronal survival and it can be activated by NMDA receptors [90]. Normally, the activation of Akt by trophic factors depends on PI3K signaling, resulting in an anti-apoptotic effect, thorough the transcriptional control of molecules that promote cell survival, and the regulation of cell metabolism
[91]. Taking all this evidence into account, the loss of PS on the inner leaflet of the plasma membrane might contribute to the activation of proapoptoric signaling pathways and, consequently, an increase in the apoptotic cell rate.
Furthermore, the translocation of PS to the external side of the membrane represents an important signal that stimulates the immune mechanism recently described as “find me and eat me” [92].
Evidence that PS externalization can be reversible was shown in in-vivo studies of experimental heart ischemia using 99mTc-Annexin V, in which phosphatidylserine exposure occurred continuously for at least 6 h after a single ischemia-reperfusion insult. In these experiments, PS returned to its normal location 24h after the initial injury [93]. All these data suggest that if a first mild insult, without the progression to inflammation, the PS-dependent apoptotic signal can be avoided if O2, nutrients, energy or antiapoptortic signaling pathways are promptly supplied, restored or activated.
One very intriguing aspect of macrophage recognition of PS represents the activity of the TIM receptor family, the natural receptors of PS. Within this family, TIM-1, TIM-3 and TIM-4 are the most active receptors in the recognition of exposed PS. Recently, in the context of a murine brain hypoxia–ischemia (H/I) model, it was described that the TIM-3 receptor is highly expressed in hypoxic brain [94], suggesting that the hypoxic condition might be responsible for PS translocation. Interestingly, TIM-2 receptor fails to recognize PS and no evidence is available as to whether TIM-2 could be induced by HIF-1. However, TIM-2 is a specific ferritin receptor in different tissues, including CNS cells [95], which allow the massive iron influx to the cells expressing TIM-2. If brain hypoxia is simultaneously produced under different inflammatory conditions, all TIM-receptors and transferrin-1 receptor (Trf1-R) will be upregulated.
Trf1-R is induced by HIF-1, but it is also a ferritin receptor. If a “mixed” situation (hypoxia plus inflammation) occurred simultaneously, all TIM-receptors and Trf1-R could be overexpressed and therefore become activated. In this particular situation, external PS would be recognized by macrophages - and/or some other complementary system - eventually leading to phagocytosis of viable cells. Moreover, in this process referred to as “Primary phagocytosis”, high iron overloads could be produced [96].
According to these concepts, all the neurodegenerative processes previously mentioned, including epilepsy [97], could show high iron concentrations in the basal ganglia or high ferritin concentrations in CSF, plasma and brain tissue [98]. Furthermore, in cultured glial cells subjected to acute stimulation with three pro-inflammatory cytokines, an increased mRNA expression of HIF-1 and mdr-1 genes was observed [99].
Differential cell-specific effects of HIF-1α induction were also reported in cell survival studies of cortical neuron as compared with astrocyte. While mild hypoxic insults induce neuronal expression of HIF-1α, promoting neuronal survival, without any astrocytic expression of HIF-1α. Oddly enough, more potent hypoxic stimuli promoted neuron death through hypoxia, but simultaneously protected astrocytes [100].
On the other hand, a more recent study on samples of brain tissue showed that the mRNA and protein levels of HIF-1〈 were highly up-regulated in brain tissues from refractory epileptic patients, as compared with those of control subjects [101], as well as in an experimental status epilepticus (SE) model induced by pilocarpine [102].
In conclusion, hypoxia plus inflammation could turn on the overexpression of the HIF-1α and MDR-1 genes, and also induce iron overload, with differential impacts on each type of brain cell. Perhaps, NBIA (Neurodegeneration with Brian Iron Accumulation) disorders are the best examples of neurodegenerative syndromes related to high iron concentrations in the brain [103]. The affected white matter observed in some of these entities might also constitute an independent source of iron leading to abnormal iron accumulation, as recently suggested by different reports on the role of iron overload in multiple sclerosis and other disorders affecting white matter [104].
In this context of NBIA pathogenesis, mitochondrial dysfunction could lead to functional hypoxia, enhancing compensatory cellular iron uptake mechanisms through the activation of HIF-1 [105]. Furthermore, since mitochondrial biogenesis in neurons can also occur in response to hypoxia (in order to maintain energy production), an increase in iron uptake might also be required for the production of new heme-enzymes (involved in energy production, such as cytochromes) to be supplied to these newly-formed mitochondria [106].
Thus a wide range of both intrinsic and extrinsic proapoptotic factors could come into play after PS translocation secondary to hypoxia or some other injury. Some of these mechanisms can also be observed in the inflammatory process developing during the natural progression not only of neurodegenerative diseases, but also of pharmacoresistant epilepsies, in which P-gp is reported to be overexpressed. Particularly in the case of the so-called “mesial temporal sclerosis” (MTS), in which significant pathologic changes involve not only the hippocampus but also the amygdala and entorhinal cortex [107], the progressive neurodegenerative process involving cell death by phagocytosis, might also result from exposure of phosphatidylserine (PS) or other “eat-me” signals [96].
Normally, PS modulates the properties and function of several membrane-bound receptors that play a key role in neuronal function and neurotransmission. PS fulfils this function by increasing the binding affinity of both AMPA-glutamate [108], as well as of the benzodiazepine receptors, where for example, interaction with the anionic domain of the membrane, increases the affinity of this receptor for flunitrazepam [109]. In this regard, it was demonstrated that lipid imbalance is indeed a problem not only in the setting of a typical neurodegenerative disorder such as Parkinson´s disease (PD), but also in epilepsy [110].
The Drosophila called “easily shocked”, present mutations in the ethanolamine kinase gene and cannot synthesize
PE, leading to PS accumulation and membrane leakiness with a decrease in ion channel activity. In these “eas-flies” a transient paralysis following a brief mechanical shock is observed and adopted as an epileptic model. These “eas-flies”, present mutations in the ethanolamine kinase gene, and thus cannot use ethanolamine to synthesize PE, thus inducing PS accumulation. This phospholipid imbalance can cause membrane leakiness or a decrease in ion channel activity, which leads, in either case, to the transient paralytic phenotype observed in this model [111].
Similar results were reported in worms that over-express α-synuclein, and present a deletion in the phosphatidylserine decarboxylase enzyme (psd1Δ) gene, also generating low amounts of phosphatidyl-ethanolamine and increased amounts of PS [112]. Furthermore, because lipid disequilibrium could be a problem for both PD and/or epilepsy, lipid-related genes were identified from sequencing studies, and in this regard, mutations in FASN (fatty acid synthase) and PLCB1 (phospholipase C isoform β1) were recently detected from a small number of individuals with a severe form of childhood epilepsy [113].
More recently, it was hypothesized that de novo formation of ion channels by naturally unfolded proteins (NUPs), namely α-synuclein and stefin B, increases neuronal excitability [114]. Furthermore, some reports indicate that α-synuclein gene multiplication or increased concentrations of α-synuclein in CSF, were detected in patients with epilepsy, and in experimental epileptic models [115, 116]. How can we account for the increased cytosolic concentrations of these naturally unfolded proteins? Is this phenomenon related to an abnormal PS/PE ratio, to PS external exposure with concomitant loss of the natural binding sites for these proteins on the inner side of the membrane?
We believe that the progressive expression and activity of P-gp in hypoxic and/or epileptic neurons could increase the amount of phosphatydylserine (PS) translocated to the external side of the plasma membrane. At this stage, we hypothesize a loss of BZD affinity for GABA receptors, increased α-synuclein accumulation and decreased ion channel activity. In addition, the concomitant and direct external exposure of the anionic domain of PS on membrane could produce its depolarization, which will induce a lower convulsive threshold, facilitating the occurrence of new seizures. So, P-gp overexpression induced by HIF-1〈 and PS translocation, mediated by P-gp activation, might constitute a new epileptogenic mechanism which is not controlled by the AEDs designed to date.
6. Intranasal administration of Erytro-poietin (EPO) as a new therapeutic strategy
In 1893 Friedrich Miescher hypothesized that erythropoiesis occurs as a result of a decrease in the oxygen concentration in the bone marrow. Paul Carnot and Catherine Deflandre (1906) raised the hypothesis that a humoral factor could be responsible for the regulation of hemopoiesis. It was necessary to wait 42 years for this humoral factor to be finally identified as Erythropoietin (EPO) [117]. Despite these historical facts, the credit for the discovery of EPO often goes to Allan Jacob Erslev, who published the first scientific article on the existence of EPO in 1953 [118]. At this time, researchers from the University of Chicago demonstrated that EPO is formed in the kidney, and 20 years later, and for the first time, the same group isolated EPO from human urine [119].
The interaction of EPO with its receptor (EPO-R) not only acts as the main regulator of red blood cell (RBC) production (erythropoiesis) in response to hypoxia, but also plays a central role in the protection of different nonhematopoietic cells such as brain, vascular endothelial cells, and even solid tumors from hypoxia. The activation of EPO-R can also protect different cells against several injuries, inducing a broad range of cellular responses aimed at preserving cells from death, and/or repairing damaged tissues [120]. Some in-vitro experiments have demonstrated that EPO plays an antiapoptotic and/or protective role after different types of insults [121-123].
During the biotechnological production of rHu-EPO (human recombinant EPO), several isoforms are obtained with different contents of sialic acid. When the content of sialic acid molecules of protein is less than 10 per mole of EPO, this EPO cannot be used as an erythropoietic agent, since it is rapidly degraded in the liver. However, this protein is able to cross the BBB (Blood Brain Barrier), and it is referred to as Neuro-EPO, due to its similarity to that which is synthesized in the mammalian brain [124].
Neuro-EPO has been effective in preclinical studies in different biomodels of neurodegenerative diseases. Moreover, neuro-EPO has been developed as a new product for the treatment of the acute phase of cerebral infarction [125-127]. Besides, this compound is currently used in both non-transgenic and transgenic models of Alzheimer's disease [128]. Neuro-EPO is indeed a promising molecule for several reasons. First, it lacks erythropoietic activity and its biological effect is thus limited to cytoprotection. Second, it can exert its protective effects not only on neurons but on glial cells as well.
Of interest, glial cells play a key role in the clearance of Aβ. The intranasal (IN) route of application will also prove to be beneficial in AD, allowing for an easy and reliable drug delivery route, even in seriously compromised AD patients. Today the group led by Garcia-Rodríguez is currently designing controlled clinical trials with Neuro-EPO for patients presenting with AD [128]. The aim is to offer a safe and effective therapeutic alternative for the management and/or prevention of this disease.
In other in vivo study, it was demonstrated that, irrespectively of the sialic acid content of rHu-EPO, the nasal administration of this drug was able to recover the spontaneous motor activity (SMA) in rats subjected to focal brain ischemia [65]. The potentially protective role of the EPO/EPO-R axis activation in hypoxic brain cells has been recently revised, and the anti-apoptotic, anti-inflammatory, anti-oxidant, and/or cell-proliferative effects of EPO are well documented [129].
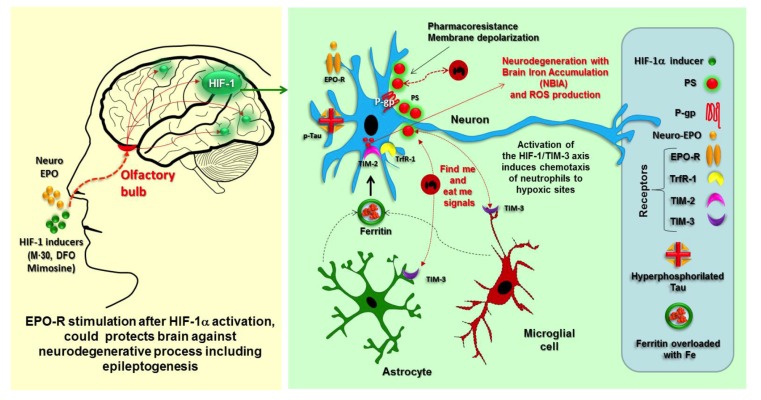
The use of EPO in all types of cells expressing EPO-R after a hypoxic-ischemic event might constitute a potential strategy for neuroprotection, neurorepair, or neurogenesis of damaged brain areas. The activation of EPO-R has also been postulated as inducing neuroprotection by reducing the release of glutamate through the modulation of voltage-insensitive calcium channels via phospholipase C [130]. Furthermore, the activation of EPO-R by EPO could also reverse PS translocation [131] (Fig. 2).
Fig. (2).
HIF-1 dependent progressive neurodegenerative process could be avoided if EPO is nasally administered.
All these data clearly indicate that HIF-1α is one of the leading actors in the mechanisms related not only to hypoxia and neurodegenerative processes, but also, to excitotoxicity and inflammation, all conditions in which the MDR-1 gene can be induced to be overexpressed, generating the refractory epilepsy phenotype, and- perhaps also- epileptogenesis itself [83].
Finally, and with reference to the potential intranasal administration of EPO, it is important to remark that the up / down-regulation of HIF-1alpha (functioning either as a “protective” or a “deleterious” factor) should be evaluated within in the context of the micro-environment conditions, most specially in the presence of the systemic input of peripheral factors inducing either “brain” ageing or rejuvenation. According to two interesting studies, the same fresh plasma from young mice had detrimental effects when administered to other young mice [132], as opposed to the neuroprotective effects observed when this plasma was administered to aged mice [133]. Furthermore, these experiments suggest that the reactivation of the latent plasticity which lies “dormant” in the aged CNS might help to rejuvenate and regenerate synapses and impaired cognitive functions in the elderly. This might also have promising implications for the extension of lifespan [134].
Conclusion
“Hypoxia-inducible factor-1α (HIF-1α) was clearly shown to have a twofold role as “protective transcription factor”, or “proapoptotic factor”, depending on the severity of hypoxia, and associated environmental conditions. Progressive neurodegenerative processes show mechanism that includes iron accumulation, free radical production, excitotoxicity, inflammation, macrophage activation, all leading to the loss of neuronal functions. Due to the complexity of the balance between the protective and harmful effects of HIF1, in the context of hypoxia with inflammation, it could be necessary to articulate complex therapeutic actions, aimed at decreasing the inflammatory burden, and simultaneously stabilization of a moderate amount of HIF-1α, and stimulate the HIF-dependent rescue genes functional expression. Therefore, agents with a wide range of therapeutic goals and effects are urgently needed. Since Neuro-EPO lacks its primary erythropoietic activity, it is limited to cytoprotection not only of neurons, but also of glial cells which play a key role in eliminating Aβ. The intranasal (IN) route of Neuro-EPO administration will also be a great advantage in several neurodegenerative diseases, allowing an easy and reliable route of brain access of these drugs, even in severely compromised patients. In base of all described here, we suggest that the design of controlled clinical trials for management of NGD with the IN combined administration of both iron-chelators and Neuro-EPO, could provide a safe and effective strategy for the treatment and / or prevention of some of the diseases mentioned above”.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Toledano I. AL. Hacia el equilibrio de la población mundial (Cátedra de Bioética) Book. 2004 Publisher: Desclée De Brouwer, Edition: Softcover, Language: Spanish 440 pages. ISBN13. 2004. [Google Scholar]
- 2.WHO World report on ageing and health. www.who.int/ageing/ publications/world-report-2015
- 3.Piret J.P., Mottet D., Raes M., Michiels C. Is HIF-1〈 a pro- or an anti-apoptotic protein? Biochem. Pharmacol. 2002;64(5-6):889–892. doi: 10.1016/s0006-2952(02)01155-3. [http://dx.doi.org/10.1016/S0006-2952(02)01155-3]. [PMID: 12213583]. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez P.E., Fares R.P., Risso J.J., Bonnet C., Bouvard S., Le-Cavorsin M., Georges B., Moulin C., Belmeguenai A., Bodennec J., Morales A., Pequignot J.M., Baulieu E.E., Levine R.A., Bezin L. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc. Natl. Acad. Sci. USA. 2009;106(24):9848–9853. doi: 10.1073/pnas.0901840106. [http://dx.doi.org/10.1073/pnas. 0901840106]. [PMID: 19497871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez C., García F.A., Amaro G.D., García R.J.C. Neuroprotección en enfermedades Neuro y Heredo degenerativas; García Rodríguez, J.C., Ed.; OmniaScience: Barcelona, España, 2014. De la Neuroprotección a la Neurorestauración. Evidencias de las Potencialidades de la Neuro-eritropoyetina (Neuro-EPO). . [Google Scholar]
- 6.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–186. doi: 10.1038/nature20411. [http://dx.doi.org/10.1038/ nature20411]. [PMID: 27830812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliore L., Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009;674(1-2):73–84. doi: 10.1016/j.mrgentox.2008.09.013. [http://dx.doi.org/10.1016/j.mrgentox.2008.09.013]. [PMID: 18952194]. [DOI] [PubMed] [Google Scholar]
- 8.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365(6):537–547. doi: 10.1056/NEJMra1011165. [http://dx.doi.org/10.1056/NEJMra 1011165]. [PMID: 21830968]. [DOI] [PubMed] [Google Scholar]
- 10.Sharp F.R., Bernaudin M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004;5(6):437–448. doi: 10.1038/nrn1408. [http://dx.doi.org/10. 1038/nrn1408]. [PMID: 15152194]. [DOI] [PubMed] [Google Scholar]
- 11.Freeman R.S., Barone M.C. Targeting hypoxia-inducible factor (HIF) as a therapeutic strategy for CNS disorders. Curr. Drug Targets CNS Neurol. Disord. 2005;4(1):85–92. doi: 10.2174/1568007053005154. [http://dx.doi.org/10. 2174/1568007053005154]. [PMID: 15723616]. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y., Feng L., Zhou Y., Sheng J., Long D., Li S., Li Y. Systematic review with meta-analysis: HIF-1〈 attenuates liver ischemia-reperfusion injury. Transplant. Rev. (Orlando) 2015;29(3):127–134. doi: 10.1016/j.trre.2015.05.001. [http://dx.doi.org/10.1016/j.trre.2015.05.001]. [PMID: 26007634]. [DOI] [PubMed] [Google Scholar]
- 13.Chen S., Sang N. Hypoxia-Inducible Factor-1: A critical player in the survival strategy of cells. J. Cell. Biochem. 2016;117(2):267–278. doi: 10.1002/jcb.25283. [http://dx.doi.org/10.1002/jcb.25283]. [PMID: 26206147]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu T., Zhan L., Liang D., Hu J., Lu Z., Zhu X., Sun W., Liu L., Xu E. Hypoxia-inducible factor 1α mediates neuroprotection of hypoxic postconditioning against global cerebral ischemia. J. Neuropathol. Exp. Neurol. 2014;73(10):975–986. doi: 10.1097/NEN.0000000000000118. [http://dx.doi. org/10.1097/NEN.0000000000000118]. [PMID: 25192050]. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [http://dx.doi.org/10.1016/j.cmet. 2006.02.002]. [PMID: 16517405]. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia D., Ardekani M.S., Shi Q., Movafagh S. Chapter 21 Hypoxia and its Emerging Therapeutics in Neurodegenerative, Inflammatory and Renal Diseases http://dx.doi.org/10.5772/66089. In Hypoxia and Human Diseases. INTECH, 2017. 2017. Hypoxia and its Emerging Therapeutics in Neurodegenerative, Inflammatory and Renal Diseases. . [Google Scholar]
- 17.Zhang H., Bosch-Marce M., Shimoda L.A., Tan Y.S., Baek J.H., Wesley J.B., Gonzalez F.J., Semenza G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [http://dx. doi.org/10.1074/jbc.M800102200]. [PMID: 18281291]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Chen J., Ding Z., Peng Y., Pan F., Li J., Zou L., Zhang Y., Liang H. HIF-1〈 inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One. 2014;9(6):e98882. doi: 10.1371/journal.pone.0098882. [http://dx.doi.org/10.1371/journal.pone. 0098882]. [PMID: 24901645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587–597. doi: 10.1016/j.tins.2013.07.001. [http://dx. doi.org/10.1016/j.tins.2013.07.001]. [PMID: 23968694]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Liu F., Grundke-Iqbal I., Iqbal K., Gong C.X. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J. Neurochem. 2009;111(1):242–249. doi: 10.1111/j.1471-4159.2009.06320.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06320.x]. [PMID: 19659459]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Liu F., Iqbal K., Grundke-Iqbal I., Gong C.X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582(2):359–364. doi: 10.1016/j.febslet.2007.12.035. [http://dx.doi.org/10.1016/j.febslet.2007.12.035]. [PMID: 18174027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashok B.S., Ajith T.A., Sivanesan S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2017;44(3):327–334. doi: 10.1111/1440-1681.12717. [http://dx.doi.org/10.1111/ 1440-1681.12717]. [PMID: 28004401]. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Zhou K., Wang R, Cui J., Lipton S.A., Liao F.F., Xu H., Zhang Y.W. Hypoxia-inducible factor 1alpha (HIF- 1alpha)-mediated hypoxia increases BACE1 expression and betaamyloid generation. J. Biol. Chem. . 2007; 13,282(15): 10873–80 . doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 24.Schubert D., Soucek T., Blouw B. The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur. J. Neurosci. 2009;29(7):1323–1334. doi: 10.1111/j.1460-9568.2009.06712.x. [http://dx.doi.org/10.1111/j.1460-9568.2009.06712.x]. [PMID: 19519624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai X., Kong W., Liu L., Yu W., Zhang Z., Sun Y. A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis. Neural Regen. Res. 2014;9(11):1145–1153. doi: 10.4103/1673-5374.135317. [http://dx.doi.org/10.4103/1673-5374.135317]. [PMID: 25206774]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazan N.G., Palacios-Pelaez R., Lukiw W.J. Hypoxia signaling to genes: significance in Alzheimer’s disease. Mol. Neurobiol. 2002;26(2-3):283–298. doi: 10.1385/MN:26:2-3:283. [http://dx.doi.org/10.1385/MN:26:2-3:283]. [PMID: 12428761]. [DOI] [PubMed] [Google Scholar]
- 27.Wolters F.J., Zonneveld H.I., Hofman A., van der Lugt A., Koudstaal P.J., Vernooij M.W., Ikram M.A., Heart-Brain Connection Collaborative Research Group Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017;136(8):719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Reyes I., Chandel N.S. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov. 2014;4(12):1371–1373. doi: 10.1158/2159-8290.CD-14-1228. [http://dx.doi.org/10.1158/2159-8290.CD-14-1228]. [PMID: 25477105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Cui Y., Shi M., Zhang Q., Wang Q., Chen X. Deferoxamine promotes MDA-MB-231 cell migration and invasion through increased ROS-dependent HIF-1〈 accumulation. Cell. Physiol. Biochem. 2014;33(4):1036–1046. doi: 10.1159/000358674. [http://dx.doi.org/10. 1159/000358674]. [PMID: 24732598]. [DOI] [PubMed] [Google Scholar]
- 30.Taoka K., Kumano K., Nakamura F., Hosoi M., Goyama S., Imai Y., Hangaishi A., Kurokawa M. The effect of iron overload and chelation on erythroid differentiation. Int. J. Hematol. 2012;95(2):149–159. doi: 10.1007/s12185-011-0988-3. [http://dx.doi.org/10.1007/s12185-011-0988-3]. [PMID: 22193844]. [DOI] [PubMed] [Google Scholar]
- 31.Moreira P.I., Duarte A.I., Santos M.S., Rego A.C., Oliveira C.R. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J. Alzheimers Dis. 2009;16(4):741–761. doi: 10.3233/JAD-2009-0972. [http://dx.doi.org/10.3233/JAD-2009-0972]. [PMID: 19387110]. [DOI] [PubMed] [Google Scholar]
- 32.Klimova T., Chandel N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15(4):660–666. doi: 10.1038/sj.cdd.4402307. [http://dx.doi.org/10.1038/sj.cdd.4402307]. [PMID: 18219320]. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D.X., Gutterman D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [http://dx.doi. org/10.1152/ajpheart.01283.2006]. [PMID: 17237240]. [DOI] [PubMed] [Google Scholar]
- 34.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue Injury. Antioxid. Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [http://dx.doi.org/10.1016/j.cmet.2005.05.001]. [PMID: 16054089]. [DOI] [PubMed] [Google Scholar]
- 36.Iyalomhe O., Swierczek S., Enwerem N., Chen Y., Adedeji M.O., Allard J., Ntekim O., Johnson S., Hughes K., Kurian P., Obisesan T.O. The Role of Hypoxia-Inducible Factor 1 in Mild Cognitive Impairment. Cell. Mol. Neurobiol. 2017;37(9):3110–3123. doi: 10.1007/s10571-016-0440-6. Epub ahead of print [PMID: 27858285]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo C., Zhang Y.X., Wang T., Zhong M.L., Yang Z.H., Hao L.J., Chai R., Zhang S. Intranasal deferoxamine attenuates synapse loss via up-regulating the P38/HIF-1〈 pathway on the brain of APP/PS1 transgenic mice. Front. Aging Neurosci. 2015;7:104. doi: 10.3389/fnagi.2015.00104. [http://dx.doi.org/10.3389/fnagi.2015.00104]. [PMID: 26082716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youdim M.B. Multi target neuroprotective and neurorestorative anti-Parkinson and anti-Alzheimer drugs ladostigil and m30 derived from rasagiline. Exp. Neurobiol. 2013;22(1):1–10. doi: 10.5607/en.2013.22.1.1. [http:// dx.doi.org/10.5607/en.2013.22.1.1]. [PMID: 23585716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mechlovich D., Amit T., Bar-Am O., Mandel S., Youdim M.B., Weinreb O. The novel multi-target iron chelator, M30 modulates HIF-1〈-related glycolytic genes and insulin signaling pathway in the frontal cortex of APP/PS1 Alzheimer’s disease mice. Curr. Alzheimer Res. 2014;11(2):119–127. doi: 10.2174/1567205010666131212112529. [http://dx.doi.org/10.2174/ 1567205010666131212112529]. [PMID: 24359498]. [DOI] [PubMed] [Google Scholar]
- 40.Kupershmidt L., Weinreb O., Amit T., Mandel S., Carri M.T., Youdim M.B. Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2009;23(11):3766–3779. doi: 10.1096/fj.09-130047. [http://dx.doi.org/10.1096/fj. 09-130047]. [PMID: 19638399]. [DOI] [PubMed] [Google Scholar]
- 41.Gal S., Zheng H., Fridkin M., Youdim M.B. Restoration of nigrostriatal dopamine neurons in post-MPTP treatment by the novel multifunctional brain-permeable iron chelator-monoamine oxidase inhibitor drug, M30. Neurotox. Res. 2010;17(1):15–27. doi: 10.1007/s12640-009-9070-9. [http://dx.doi.org/10.1007/s12640-009-9070-9]. [PMID: 19609632]. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Li X., Xie W., Jankovic J., Le W., Pan T. Neuroprotection of deferoxamine on rotenone-induced injury via accumulation of HIF-1 alpha and induction of autophagy in SH-SY5Y cells. Neurochem. Int. 2010;57(3):198–205. doi: 10.1016/j.neuint.2010.05.008. [http://dx.doi.org/10.1016/ j.neuint.2010.05.008]. [PMID: 20546814]. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K.I., Dang C.V., Semenza G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–420. doi: 10.1016/j.ccr.2007.04.001. [http://dx.doi.org/10.1016/j.ccr.2007.04.001]. [PMID: 17482131]. [DOI] [PubMed] [Google Scholar]
- 44.Wu H., Chen Q. Hypoxia activation of mitophagy and its role in disease pathogenesis. Antioxid. Redox Signal. 2015;22(12):1032–1046. doi: 10.1089/ars.2014.6204. [http://dx.doi.org/10.1089/ars.2014.6204]. [PMID: 25526784]. [DOI] [PubMed] [Google Scholar]
- 45.Youdim M.B., Oh Y.J. Promise of neurorestoration and mitochondrial biogenesis in Parkinson’s disease with multi target drugs: an alternative to stem cell therapy. 2013. [DOI] [PMC free article] [PubMed]
- 45.Guo C., Wang T., Zheng W., Shan Z.Y., Teng W.P., Wang Z.Y. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34(2):562–575. doi: 10.1016/j.neurobiolaging.2012.05.009. [http://dx.doi.org/10.1016/j.neurobiolaging.2012. 05.009]. [PMID: 22717236]. [DOI] [PubMed] [Google Scholar]
- 46.Alston T.A., Mela L., Bright H.J. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc. Natl. Acad. Sci. USA. 1977;74(9):3767–3771. doi: 10.1073/pnas.74.9.3767. [http://dx.doi.org/10.1073/pnas.74.9.3767]. [PMID: 269430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y.T., Ju T.C., Yang D.I. Induction of hypoxia inducible factor-1 attenuates metabolic insults induced by 3-nitropropionic acid in rat C6 glioma cells. J. Neurochem. 2005;93(3):513–525. doi: 10.1111/j.1471-4159.2005.03032.x. [http://dx.doi.org/10.1111/j.1471-4159.2005.03032.x]. [PMID: 15836611]. [DOI] [PubMed] [Google Scholar]
- 48.Grossi C., Francese S., Casini A., Rosi M.C., Luccarini I., Fiorentini A., Gabbiani C., Messori L., Moneti G., Casamenti F. Clioquinol decreases amyloid-beta burden and reduces working memory impairment in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2009;17(2):423–440. doi: 10.3233/JAD-2009-1063. [http://dx.doi. org/10.3233/JAD-2009-1063]. [PMID: 19363260]. [DOI] [PubMed] [Google Scholar]
- 49.Shachar D.B., Kahana N., Kampel V., Warshawsky A., Youdim M.B.H. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46(2):254–263. doi: 10.1016/j.neuropharm.2003.09.005. [http://dx.doi.org/10.1016/j. neuropharm.2003.09.005]. [PMID: 14680763]. [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Wan J, Lan X, Han X, Wang Z, Wang J. Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice. 2017. [DOI] [PMC free article] [PubMed]
- 51.Choi S.M., Choi K.O., Park Y.K., Cho H., Yang E.G., Park H. Clioquinol, a Cu(II)/Zn(II) chelator, inhibits both ubiquitination and asparagine hydroxylation of hypoxia-inducible factor-1alpha, leading to expression of vascular endothelial growth factor and erythropoietin in normoxic cells. J. Biol. Chem. 2006;281(45):34056–34063. doi: 10.1074/jbc.M603913200. [http://dx.doi.org/10.1074/jbc.M603913200]. [PMID: 16973622]. [DOI] [PubMed] [Google Scholar]
- 52.Shachar D.B., Kahana N., Kampel V., Warshawsky A., Youdim M.B. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46(2):254–263. doi: 10.1016/j.neuropharm.2003.09.005. [http://dx.doi.org/10.1016/j. neuropharm.2003.09.005]. [PMID: 14680763]. [DOI] [PubMed] [Google Scholar]
- 53.Muller F.L., Liu Y., Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [http://dx.doi.org/10. 1074/jbc.M407715200]. [PMID: 15317809]. [DOI] [PubMed] [Google Scholar]
- 54.Brizzio E., Castro M., Narbaitz M., Borda N., Carbia C., Correa L., Mengarelli R., Merelli A., Brizzio V., Sosa M., Biancardi B., Lazarowski A. Ulcerated hemosiderinic dyschromia and iron deposits within lower limbs treated with a topical application of biological chelator. Veins and Lymphatics. 2012;1(e6):18–26. [Google Scholar]
- 55.Saccon R.A., Bunton-Stasyshyn R.K.A., Fisher E.M.C., Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–2358. doi: 10.1093/brain/awt097. [http://dx.doi.org/10.1093/ brain/awt097]. [PMID: 23687121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagliardi S., Cova E., Davin A., Guareschi S., Abel K., Alvisi E., Laforenza U., Ghidoni R., Cashman J.R., Ceroni M., Cereda C. SOD1 mRNA expression in sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 2010;39(2):198–203. doi: 10.1016/j.nbd.2010.04.008. [http://dx.doi.org/10. 1016/j.nbd.2010.04.008]. [PMID: 20399857]. [DOI] [PubMed] [Google Scholar]
- 57.Sau D., De Biasi S., Vitellaro-Zuccarello L., Riso P., Guarnieri S., Porrini M., Simeoni S., Crippa V., Onesto E., Palazzolo I., Rusmini P., Bolzoni E., Bendotti C., Poletti A. Mutation of SOD1 in ALS: a gain of a loss of function. Hum. Mol. Genet. 2007;16(13):1604–1618. doi: 10.1093/hmg/ddm110. [http://dx.doi.org/10.1093/hmg/ddm110]. [PMID: 17504823]. [DOI] [PubMed] [Google Scholar]
- 58.Lyras L., Evans P.J., Shaw P.J., Ince P.G., Halliwell B. Oxidative damage and motor neurone disease difficulties in the measurement of protein carbonyls in human brain tissue. Free Radic. Res. 1996;24(5):397–406. doi: 10.3109/10715769609088038. [http://dx.doi.org/10.3109/ 10715769609088038]. [PMID: 8733944]. [DOI] [PubMed] [Google Scholar]
- 59.Mitsumoto H., Santella R.M., Liu X., Bogdanov M., Zipprich J., Wu H.C., Mahata J., Kilty M., Bednarz K., Bell D., Gordon P.H., Hornig M., Mehrazin M., Naini A., Flint Beal M., Factor-Litvak P. Oxidative stress biomarkers in sporadic ALS. Amyotroph. Lateral Scler. 2008;9(3):177–183. doi: 10.1080/17482960801933942. [http://dx.doi.org/ 10.1080/17482960801933942]. [PMID: 18574762]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw P.J., Ince P.G., Falkous G., Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann. Neurol. 1995;38(4):691–695. doi: 10.1002/ana.410380424. [http://dx.doi.org/10.1002/ana.410380424]. [PMID: 7574472]. [DOI] [PubMed] [Google Scholar]
- 61.Simpson E.P., Henry Y.K., Henkel J.S., Smith R.G., Appel S.H. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62(10):1758–1765. doi: 10.1212/wnl.62.10.1758. [http://dx.doi.org/10.1212/WNL.62.10.1758]. [PMID: 15159474]. [DOI] [PubMed] [Google Scholar]
- 62.Bogdanov M., Brown R.H., Matson W., Smart R., Hayden D., O’Donnell H., Flint B.M., Cudkowicz M. Increased oxidative damage to DNA in ALS patients. Free Radic. Biol. Med. 2000;29(7):652–658. doi: 10.1016/s0891-5849(00)00349-x. [http://dx.doi.org/10.1016/S0891-5849(00)00349-X]. [PMID: 11033417]. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y., Gao L., Wang D., Zang D. Elevated levels of ferritin in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 2016;136(2):145–150. doi: 10.1111/ane.12708. [http://dx.doi.org/10. 1111/ane.12708]. [PMID: 27804118]. [DOI] [PubMed] [Google Scholar]
- 64.Caltana L., Merelli A., Lazarowski A., Brusco A. Neuronal and glial alterations due to focal cortical hypoxia induced by direct cobalt chloride (CoCl2) brain injection. Neurotox. Res. 2009;15(4):348–358. doi: 10.1007/s12640-009-9038-9. [http://dx.doi.org/10.1007/s12640-009-9038-9]. [PMID: 19384568]. [DOI] [PubMed] [Google Scholar]
- 65.Merelli A., Caltana L., Girimonti P., Ramos A.J., Lazarowski A., Brusco A. Recovery of motor spontaneous activity after intranasal delivery of human recombinant erythropoietin in a focal brain hypoxia model induced by CoCl2 in rats. Neurotox. Res. 2011;20(2):182–192. doi: 10.1007/s12640-010-9233-8. [http://dx.doi.org/10.1007/s12640-010-9233-8]. [PMID: 21116766]. [DOI] [PubMed] [Google Scholar]
- 66.Chang Y., Kong Q., Shan X., Tian G., Ilieva H., Cleveland D.W., Rothstein J.D., Borchelt D.R., Wong P.C., Lin C.L. Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PLoS One. 2008;3(8):e2849. doi: 10.1371/journal.pone.0002849. [http://dx.doi.org/10.1371/journal.pone.0002849]. [PMID: 18682740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosco D.A., Morfini G., Karabacak N.M., Song Y., Gros-Louis F., Pasinelli P., Goolsby H., Fontaine B.A., Lemay N., McKenna-Yasek D., Frosch M.P., Agar J.N., Julien J.P., Brady S.T., Brown R.H., Jr Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010;13(11):1396–1403. doi: 10.1038/nn.2660. [http://dx.doi.org/10.1038/nn. 2660]. [PMID: 20953194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Comerford K.M., Wallace T.J., Karhausen J., Louis N.A., Montalto M.C., Colgan S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–3394. [PMID: 12067980]. [PubMed] [Google Scholar]
- 69.DeMars K.M., Yang C., Hawkins K.E., McCrea A.O., Siwarski D.M., Candelario-Jalil E. Spatiotemporal changes in P-glycoprotein levels in brain and peripheral tissues following ischemic stroke in Rats. J. Exp. Neurosci. 2017;11:1179069517701741. doi: 10.1177/1179069517701741. [http://dx.doi.org/10.1177/1179069517701741]. [PMID: 28469478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bateman L.M., Li C.S., Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(Pt 12):3239–3245. doi: 10.1093/brain/awn277. [http://dx.doi.org/10.1093/ brain/awn277]. [PMID: 18952672]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moseley B.D., Nickels K., Britton J., Wirrell E. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia. 2010;51(7):1219–1224. doi: 10.1111/j.1528-1167.2009.02490.x. [http://dx.doi.org/10.1111/j.1528-1167.2009.02490.x]. [PMID: 20067502]. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez P.E., Fares R.P., Risso J.J., Bonnet C., Bouvard S., Le-Cavorsin M., Georges B., Moulin C., Belmeguenai A., Bodennec J., Morales A., Pequignot J.M., Baulieu E.E., Levine R.A., Bezin L. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc. Natl. Acad. Sci. USA. 2009;106(24):9848–9853. doi: 10.1073/pnas.0901840106. [http://dx.doi.org/10.1073/pnas. 0901840106]. [PMID: 19497871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicoletti J.N., Shah S.K., McCloskey D.P., Goodman J.H., Elkady A., Atassi H., Hylton D., Rudge J.S., Scharfman H.E., Croll S.D. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151(1):232–241. doi: 10.1061/j.neuroscience.2007.09.083. [http:// dx.doi.org/10.1016/j.neuroscience.2007.09.083]. [PMID: 18065154]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikitidou L., Kanter-Schlifke I., Dhondt J., Carmeliet P., Lambrechts D., Kokaia M. VEGF receptor-2 (Flk-1) overexpression in mice counteracts focal epileptic seizures. PLoS One. 2012;7(7):e40535. doi: 10.1371/journal.pone.0040535. [http://dx.doi.org/10.1371/journal.pone.0040535]. [PMID: 22808185]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazarowski A., Ramos A.J., García-Rivello H., Brusco A., Girardi E. Neuronal and glial expression of the multidrug resistance gene product in an experimental epilepsy model. Cell. Mol. Neurobiol. 2004;24(1):77–85. doi: 10.1023/B:CEMN.0000012726.43842.d2. [http://dx.doi.org/10.1023/B: CEMN.0000012726.43842.d2]. [PMID: 15049512]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feast A., Martinian L., Liu J., Catarino C.B., Thom M., Sisodiya S.M. Investigation of hypoxia-inducible factor-1α in hippocampal sclerosis: a postmortem study. Epilepsia. 2012;53(8):1349–1359. doi: 10.1111/j.1528-1167.2012.03591.x. [http://dx.doi.org/10.1111/j.1528-1167.2012.03591.x]. [PMID: 22812626]. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura Y., Takata K., Kakimura J., Umeki M., Azukawa S., Suzuki S., Taniguchi T. Aryl hydrocarbon receptor nuclear translocator is induced by kainic acid in rat hippocampal glial cells. Neurosci. Lett. 2000;291(2):117–120. doi: 10.1016/s0304-3940(00)01412-9. [http://dx.doi.org/10.1016/ S0304-3940(00)01412-9]. [PMID: 10978588]. [DOI] [PubMed] [Google Scholar]
- 78.Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [http://dx.doi.org/10.1016/0005-2736(76)90160-7]. [PMID: 990323]. [DOI] [PubMed] [Google Scholar]
- 79.Lazarowski A., Sevlever G., Taratuto A., Massaro M., Rabinowicz A. Tuberous sclerosis associated with MDR1 gene expression and drug-resistant epilepsy. Pediatr. Neurol. 1999;21(4):731–734. doi: 10.1016/s0887-8994(99)00074-0. [http://dx.doi.org/10.1016/S0887-8994(99)00074-0]. [PMID: 10580886]. [DOI] [PubMed] [Google Scholar]
- 80.Lazarowski A., Massaro M., Schteinschnaider A., Intruvini S., Sevlever G., Rabinowicz A. Neuronal MDR-1 gene expression and persistent low levels of anticonvulsants in a child with refractory epilepsy. Ther. Drug Monit. 2004;26(1):44–46. doi: 10.1097/00007691-200402000-00010. [http://dx. doi.org/10.1097/00007691-200402000-00010]. [PMID: 14749549]. [DOI] [PubMed] [Google Scholar]
- 81.Ramos A.J., Lazarowski A., Villar M.J., Brusco A. Transient expression of MDR-1/P-glycoprotein in a model of partial cortical devascularization. Cell. Mol. Neurobiol. 2004;24(1):101–107. doi: 10.1023/B:CEMN.0000012728.19117.73. [http://dx.doi.org/10.1023/B:CEMN.0000012728.19117.73]. [PMID: 15049514]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazarowski A., Caltana L., Merelli A., Rubio M.D., Ramos A.J., Brusco A. Neuronal mdr-1 gene expression after experimental focal hypoxia: a new obstacle for neuroprotection? J. Neurol. Sci. 2007;258(1-2):84–92. doi: 10.1016/j.jns.2007.03.004. [http://dx.doi.org/10.1016/j.jns.2007. 03.004]. [PMID: 17459414]. [DOI] [PubMed] [Google Scholar]
- 83.Lazarowski A., Czornyj L., Lubienieki F., Girardi E., Vazquez S., D’Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48(Suppl. 5):140–149. doi: 10.1111/j.1528-1167.2007.01302.x. [http://dx.doi.org/10.1111/j.1528-1167. 2007.01302.x]. [PMID: 17910594]. [DOI] [PubMed] [Google Scholar]
- 84.Aviles-Reyes R.X., Angelo M.F., Villarreal A., Rios H., Lazarowski A., Ramos A.J. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J. Neurochem. 2010;112(4):854–869. doi: 10.1111/j.1471-4159.2009.06535.x. [http://dx.doi. org/10.1111/j.1471-4159.2009.06535.x]. [PMID: 20002528]. [DOI] [PubMed] [Google Scholar]
- 85.Yagishita S., Suzuki S., Yoshikawa K., Iida K., Hirata A., Suzuki M., Takashima A., Maruyama K., Hirasawa A., Awaji T. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol. Brain. 2017;10(1):2. doi: 10.1186/s13041-016-0282-7. [http://dx.doi.org/10.1186/s13041-016-0282-7]. [PMID: 28057021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higgins C.F., Gottesman M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [http://dx.doi. org/10.1016/0968-0004(92)90419-A]. [PMID: 1374941]. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Garcia M.A., Feria-Romero I.A., Fernando-Serrano H., Escalante-Santiago D., Grijalva I., Orozco-Suarez S. Genetic polymorphisms associated with antiepileptic metabolism. Front. Biosci. (Elite Ed.) 2014;6:377–386. doi: 10.2741/E713. [http://dx.doi.org/10.2741/713]. [PMID: 24896213]. [DOI] [PubMed] [Google Scholar]
- 88.Svennerholm L. Distribution and fatty acid composition of normal human brain. J. Lipid Res. 1968;9:570–579. [PMID: 4302302]. [PubMed] [Google Scholar]
- 89.Huang B.X., Akbar M., Kevala K., Kim H.Y. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011;192(6):979–992. doi: 10.1083/jcb.201005100. [http://dx.doi.org/10.1083/jcb.201005100]. [PMID: 21402788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [http://dx.doi.org/10.1016/S0092-8674(00)80405-5]. [PMID: 9346240]. [DOI] [PubMed] [Google Scholar]
- 91.Rivera-Cervantes M., Feria-Velasco A.I., Junyent F., Camins Espuny A., Beas-Zárate C. Intracellular Pathways Associated with Neuronal Survival and Death in Epilepsy. [Google Scholar]
- 92.Ravichandran K.S. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [http://dx.doi.org/10.1084/jem.20101157]. [PMID: 20805564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kenis H., Zandbergen H.R., Hofstra L., Petrov A.D., Dumont E.A., Blankenberg F.D., Haider N., Bitsch N., Gijbels M., Verjans J.W., Narula N., Narula J., Reutelingsperger C.P. Annexin A5 uptake in ischemic myocardium: demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J. Nucl. Med. 2010;51(2):259–267. doi: 10.2967/jnumed.109.068429. [http://dx.doi.org/10. 2967/jnumed.109.068429]. [PMID: 20124049]. [DOI] [PubMed] [Google Scholar]
- 94.Koh H.S., Chang C.Y., Jeon S.B., Yoon H.J., Ahn Y.H., Kim H.S., Kim I.H., Jeon S.H., Johnson R.S., Park E.J. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J. Neurochem. 2008;107(6):1495–1505. doi: 10.1111/j.1471-4159.2008.05678.x. [DOI] [PubMed] [Google Scholar]
- 95.Todorich B., Zhang X., Slagle-Webb B., Seaman W.E., Connor J.R. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J. Neurochem. 2008;107(6):1495–1505. doi: 10.1111/j.1471-4159.2008.05678.x. [http://dx.doi.org/10.1111/ j.1471-4159.2008.05678.x]. [PMID: 19014383]. [DOI] [PubMed] [Google Scholar]
- 96.Neher J.J., Neniskyte U., Brown G.C. Primary phagocytos is of neurons by inflamed microglia: potential roles in neurodegeneration. Front. Pharmacol. 2012:3. doi: 10.3389/fphar.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorter J.A., Mesquita A.R., van Vliet E.A., da Silva F.H., Aronica E. Increased expression of ferritin, an iron-storage protein, in specific regions of the parahippocampal cortex of epileptic rats. Epilepsia. 2005;46(9):1371–1379. doi: 10.1111/j.1528-1167.2005.11505.x. [http://dx.doi.org/10.1111/ j.1528-1167.2005.11505.x]. [PMID: 16146431]. [DOI] [PubMed] [Google Scholar]
- 98.Mills E., Dong X.P., Wang F., Xu H. Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med. Chem. 2010;2(1):51–64. doi: 10.4155/fmc.09.140. [http://dx.doi.org/10.4155/ fmc.09.140]. [PMID: 20161623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Lemos M.L., de la Torre A.V., Petrov D., Brox S., Folch J., Pallàs M., Lazarowski A., Beas-Zarate C., Auladell C., Camins A. Evaluation of hypoxia inducible factor expression in inflammatory and neurodegenerative brain models. Int. J. Biochem. Cell Biol. 2013;45(7):1377–1388. doi: 10.1016/j.biocel.2013.04.011. [http://dx.doi.org/10.1016/j.biocel. 2013.04.011]. [PMID: 23603149]. [DOI] [PubMed] [Google Scholar]
- 100.Vangeison G., Carr D., Federoff H.J., Rempe D.A. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J. Neurosci. 2008;28(8):1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.5323-07.2008]. [PMID: 18287515]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang G., Zhou R., He X., Shi Z., Huang M., Yu J., Wang X. Expression levels of microRNA-199 and hypoxia-inducible factor-1 alpha in brain tissue of patients with intractable epilepsy. Int. J. Neurosci. 2016;126(4):326–334. doi: 10.3109/00207454.2014.994209. [http://dx.doi.org/10.3109/ 00207454.2014.994209]. [PMID: 25539181]. [DOI] [PubMed] [Google Scholar]
- 102.Li J., Jiang G., Chen Y., Chen L., Li Z., Wang Z., Wang X. Altered expression of hypoxia-Inducible factor-1α participates in the epileptogenesis in animal models. Synapse. 2014;68(9):402–409. doi: 10.1002/syn.21752. [http://dx.doi.org/10.1002/syn.21752]. [PMID: 24889205]. [DOI] [PubMed] [Google Scholar]
- 103.Hogarth P. Neurodegeneration with brain iron accumulation: diagnosis and management. J. Mov. Disord. 2015;8(1):1–13. doi: 10.14802/jmd.14034. [http:// dx.doi.org/10.14802/jmd.14034]. [PMID: 25614780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams R., Buchheit C.L., Berman N.E., LeVine S.M. Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 2012;120(1):7–25. doi: 10.1111/j.1471-4159.2011.07536.x. [http://dx.doi.org/10.1111/j.1471-4159.2011.07536.x]. [PMID: 22004421]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee D.W., Andersen J.K. Role of HIF-1 in iron regulation: potential therapeutic strategy for neurodegenerative disorders. Curr. Mol. Med. 2006;6(8):883–893. doi: 10.2174/156652406779010849. [http://dx.doi.org/10.2174/ 156652406779010849]. [PMID: 17168739]. [DOI] [PubMed] [Google Scholar]
- 106.Schneider S.A., Dusek P., Hardy J., Westenberger A., Jankovic J., Bhatia K.P. Genetics and pathophysiology of neurodegeneration with brain iron accumulation (NBIA). Curr. Neuropharmacol. 2013;11(1):59–79. doi: 10.2174/157015913804999469. [PMID: 23814539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al Sufiani F., Ang L.C. Neuropathology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012;2012:624519. doi: 10.1155/2012/624519. [http://dx.doi. org/10.1155/2012/624519]. [PMID: 22957233]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baudry M., Massicotte G., Hauge S. Phosphatidylserine increases the affinity of the AMPA/quisqualate receptor in rat brain membranes. Behav. Neural Biol. 1991;55(2):137–140. doi: 10.1016/0163-1047(91)80134-z. [http://dx.doi. org/10.1016/0163-1047(91)80134-Z]. [PMID: 1647760]. [DOI] [PubMed] [Google Scholar]
- 109.Levi de Stein M., Medina J.H., De Robertis E. In vivo and in vitro modulation of central type benzodiazepine receptors by phosphatidylserine. Brain Res. Mol. Brain Res. 1989;5(1):9–15. doi: 10.1016/0169-328x(89)90012-0. [http:// dx.doi.org/10.1016/0169-328X(89)90012-0]. [PMID: 2538706]. [DOI] [PubMed] [Google Scholar]
- 110.Waugh M.G. PIPs in neurological diseases. Biochim. Biophys. Acta. 2015;1851(8):1066–1082. doi: 10.1016/j.bbalip.2015.02.002. [http://dx.doi.org/10.1016/ j.bbalip.2015.02.002]. [PMID: 25680866]. [DOI] [PubMed] [Google Scholar]
- 111.Kroll J.R., Tanouye M.A. Rescue of easily shocked mutant seizure sensitivity in Drosophila adults. J. Comp. Neurol. 2013;521(15):3500–3507. doi: 10.1002/cne.23364. [http://dx.doi.org/10.1002/cne.23364]. [PMID: 23682034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pavlidis P., Ramaswami M., Tanouye M.A. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79(1):23–33. doi: 10.1016/0092-8674(94)90397-2. [http://dx.doi.org/10.1016/0092-8674(94)90397-2]. [PMID: 7923374]. [DOI] [PubMed] [Google Scholar]
- 113.Witt S.N. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 114.Surguchov A., Surgucheva I., Sharma M., Sharma R., Singh V. Pore-Forming Proteins as Mediators of Novel Epigenetic Mechanism of Epilepsy. 2017. [DOI] [PMC free article] [PubMed]
- 115.Puschmann A., Wszolek Z.K., Farrer M., Gustafson L., Widner H., Nilsson C. Alpha-synuclein multiplications with parkinsonism, dementia or progressive myoclonus? Parkinsonism Relat. Disord. 2009;15(5):390–392. doi: 10.1016/j.parkreldis.2008.08.002. [http://dx.doi.org/10.1016/j.parkreldis.2008. 08.002]. [PMID: 18824390]. [DOI] [PubMed] [Google Scholar]
- 116.Rong H., Jin L., Wei W., Wang X., Xi Z. Alpha-synuclein is a potential biomarker in the serum and CSF of patients with intractable epilepsy. Seizure. 2015;27:6–9. doi: 10.1016/j.seizure.2015.02.007. [http://dx.doi.org/10.1016/j. seizure.2015.02.007]. [PMID: 25891920]. [DOI] [PubMed] [Google Scholar]
- 117.Bonsdorff B.V., Jalavisto E. A humoral mechanism in anoxic erythrocytosis. Nord. Med. 1948;40:1830. [PMID: 18225175]. [Google Scholar]
- 118.Erslev A. Humoral regulation of red cell production. Blood. 1953;8(4):349–357. [PMID: 13032205]. [PubMed] [Google Scholar]
- 119.Miyake T., Kung C.K., Goldwasser E. Purification of human erythropoietin. J. Biol. Chem. 1977;252(15):5558–5564. [PMID: 18467]. [PubMed] [Google Scholar]
- 120.Lappin T. The cellular biology of erythropoietin receptors. Oncologist. 2003;8(Suppl. 1):15–18. doi: 10.1634/theoncologist.8-suppl_1-15. [http://dx.doi.org/10.1634/ theoncologist.8-suppl_1-15]. [PMID: 12626783]. [DOI] [PubMed] [Google Scholar]
- 121.Pregi N., Vittori D., Pérez G., Leirós C.P., Nesse A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim. Biophys. Acta. 2006;1763(2):238–246. doi: 10.1016/j.bbamcr.2005.12.011. [http://dx.doi.org/10.1016/j.bbamcr.2005. 12.011]. [PMID: 16500719]. [DOI] [PubMed] [Google Scholar]
- 122.Pregi N., Wenker S., Vittori D., Leirós C.P., Nesse A. TNF-alpha-induced apoptosis is prevented by erythropoietin treatment on SH-SY5Y cells. Exp. Cell Res. 2009;315(3):419–431. doi: 10.1016/j.yexcr.2008.11.005. [http://dx.doi.org/10.1016/j.yexcr.2008.11.005]. [PMID: 19056379]. [DOI] [PubMed] [Google Scholar]
- 123.Callero M.A., Pérez G.M., Vittori D.C., Pregi N., Nesse A.B. Modulation of protein tyrosine phosphatase 1B by erythropoietin in UT-7 cell line. Cell. Physiol. Biochem. 2007;20(5):319–328. doi: 10.1159/000107518. [http://dx.doi.org/10.1159/000107518]. [PMID: 17762161]. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Rodriguez J.C., Sosa-Teste I. The nasal route as a potential pathway for delivery of erythropoietin in the treatment of acute ischemic stroke in humans. Sci. World J. 2009;9:970–981. doi: 10.1100/tsw.2009.103. [http://dx.doi.org/10.1100/tsw.2009.103]. [PMID: 19768354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sosa T.I., García Rodríguez J.C., García S.J.D., Santana J., Subirós M.N. González. T. C.; Rodríguez, C.Y.; Cruz, R.J. Intranasal administration of recombinant human erythropoietin exerts neuroprotective effects on post-ischemic brain injury in Mongolian gerbils. Pharmacologyonline. 2006;1:100–112. [Google Scholar]
- 126.Rodríguez Cruz Y., Mengana T. Y.; Muñoz, C. A.; Subirós, M. N.; González-Quevedo, A.; Sosa, T. I.; García, R.J.C. Treatment with nasal neuro-EPO improves the neurological, cognitive, and histological state in a gerbil model of focal ischemia. Sci. World J. 2010;10:2288–2300. doi: 10.1100/tsw.2010.215. [http://dx.doi.org/10.1100/tsw.2010.215]. [PMID: 21103798]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia-Rodríguez J.C., Rodríguez-Cruz Y. The therapeutic potential of Neuro-EPO administered nasally on acute cerebrovascular disease. Curr. Psychopharmacol. 2012;1:228–232. [http://dx.doi. org/10.2174/2211556011201030228]. [Google Scholar]
- 128.Rodríguez Cruz Y., Strehaiano M., Rodríguez O.T., García R.J.C., Maurice T. An Intranasal formulation of erythropoietin (Neuro-EPO) Prevents memory deficits and amyloid toxicity in the APPSwe transgenic mouse model of Alzheimer’s Disease. J. Alzheimers Dis. 2017;55(1):231–248. doi: 10.3233/JAD-160500. [http://dx.doi.org/10.3233/ JAD-160500]. [PMID: 27662300]. [DOI] [PubMed] [Google Scholar]
- 129.Merelli A., Czornyj L., Lazarowski A. Erythropoietin as a new therapeutic opportunity in brain inflammation and neurodegenerative diseases. Int. J. Neurosci. 2015;125(11):793–797. doi: 10.3109/00207454.2014.989321. [http://dx. doi.org/10.3109/00207454.2014.989321]. [PMID: 25405533]. [DOI] [PubMed] [Google Scholar]
- 130.Sekiguchi M., Sato K., Kozaki S., Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. JBC. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [http://dx.doi.org/10.1074/jbc.M105832200]. [DOI] [PubMed] [Google Scholar]
- 131.Merelli A., Czornyj L., Rocha L., Lazarowski A. 2016. Erythropoietin as Potential Neuroprotective and Antiepileptogenic Agent in Epilepsy and Refractory Epilepsy. [Google Scholar]
- 132.Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A., Lucin K.M., Czirr E., Park J.S., Couillard-Després S., Aigner L., Li G., Peskind E.R., Kaye J.A., Quinn J.F., Galasko D.R., Xie X.S., Rando T.A., Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [http://dx.doi.org/10.1038/nature10357]. [PMID: 21886162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D., Wabl R., Udeochu J., Wheatley E.G., Zou B., Simmons D.A., Xie X.S., Longo F.M., Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [http://dx.doi.org/10. 1038/nm.3569]. [PMID: 24793238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bouchard J., Villeda S.A. Aging and brain rejuvenation as systemic events. J. Neurochem. 2015;132(1):5–19. doi: 10.1111/jnc.12969. [http://dx.doi. org/10.1111/jnc.12969]. [PMID: 25327899]. [DOI] [PMC free article] [PubMed] [Google Scholar]