Abstract
Since its discovery, nerve growth factor (NGF) has long occupied a critical role in developmental and adult neuro-biology for its many important regulatory functions on the survival, growth and differentiation of nerve cells in the peripheral and central nervous system. NGF is the first discovered member of a family of neurotrophic factors, collectively indicated as neurotrophins, (which include brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin 4/5). NGF was discov-ered for its action on the survival and differentiation of selected populations of peripheral neurons. Since then, an enormous number of basic and human studies were undertaken to explore the role of purified NGF to prevent the death of NGF-receptive cells. These studies revealed that NGF possesses important therapeutic properties, after topical administration, on human cutaneous pressure ulcer, corneal ulcers, glaucoma, retinal maculopathy, Retinitis Pigmentosa and in pediatric optic gliomas and brain traumas. The aim of this review is to present our previous, recent and ongoing clinical studies on the ther-apeutic properties of NGF.
Keywords: Nerve growth factor (NGF), nerve cells, cutaneous cells, visual cells, cancer cells, brain traumas
1. Introduction
1.1. The NGF Discovery and Early Studies
The nerve growth factor (NGF) is the first discovered member of a family of neurotrophic factors, collectively indicated as neurotrophins, which include brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin 4/5 [1]. These factors share significant structural homologies and derive from a common ancestor gene [2]. Pioneering studies, started in the early 1950’s by R. Levi-Montalcini on laboratory animals and isolated cells, were focused on the biological action of NGF [3]. These studies have demonstrated the protective action of NGF not only in the survival of degenerating peripheral nerve cells, but also in the regulation of neurotransmitters and neuropeptides synthesis of sympathetic and sensory nerve cells [4, 5]. In vivo down-regulation of NGF, through the administration of NGF-antibodies or by peripheral nerve lesion causes a marked decrease in Substance P (SP) and Calcitonin-Gene Related Peptide (CGRP) synthesis [6, 7]. Indeed, both SP and CGRP gene expression are regulated by NGF [8], and their depletion, subsequent to NGF down-regulation, is parallel to the impairment in sensory perceptions, i.e. those described in diabetic neuropathy [9]. Exogenous NGF administration influences neuronal plasticity that allows the adult nervous system to modify its structure and functions in response to stimuli [10]. Moreover, it was also demonstrated that the constitutive synthesis of NGF in adult tissues correlates with peripheral nervous system (PNS) neurons phenotypic features, such as innervation density, cell body size, axonal terminal sprouting, dendritic growth, induction and/or inhibition of neuropeptides and neurotransmitters or transmitter-producing enzymes [11-14].
NGF exerts its action on the growth and survival of peripheral sensory and sympathetic neurons and on a number of brain neurons, particularly basal forebrain cholinergic neurons (BFCN) that are among the major NGF-target cells within the central nervous system (CNS) [4, 15, 16]. NGF, initially believed to act only on the growth and differentiation of peripheral sympathetic and sensory neurons, was found to interact with a number of other target cells within the nervous system as well as extra-neuronal targets (Table 1), such as mast cells, T and B lymphocytes, granulocytes, monocytes, keratinocytes, endothelial cells, hormones-secretory cells in the reproductive system [5, 17-20]. The NGF action on cells belonging to the immune and endocrine systems, suggests that the neurotrophin exert a modulatory role on neuro-immuno-endocrine mechanisms of vital importance in the regulation of homeostatic processes [21]. Accordingly, it has been shown that circulating and brain NGF levels undergo significant variations after exposure to stressful events, both in animal models and in humans [22].
Table 1.
NGF-responsive cells in the neuroendocrine and immune systems.
| Cell/Tissue Type | Functions Regulated by NGF | Refs. |
|---|---|---|
| (Neuro) Endocrine system | ||
| Pituitary | Possible regulation of corticotropes and lactotropes functions. Hormones secretion from the neural and intermediate lobes. | [4, 15] |
| Adrenal Medulla | Catecholamine production | [101, 132] |
| Testis (Leydig cells, Sertoli cells) | Steroid production, spermatogenesis | [113] |
| Ovary (Theca and Granulosa cells) | Steroid production, follicle maturation, ovulation | [39-41] |
| Immune system | ||
| Mast Cells | Survival, Differentiation, Cytokine production, maturation, degranulation, chemotaxis, viability, phagocytosis. | [21] |
| Monocytes | ||
| Granulocytes | ||
| Lymphocytes | ||
In the 1970’s, the existence of a NGF precursor (proNGF), was described as the product of the Ngf gene expression [23]. ProNGF is a polypeptide almost double the size of NGF, that includes a pro-region at the N-terminal and the so called “mature” NGF as a 118-120 aminoacid-long C-terminal portion [24]. ProNGF in the mouse is produced in two main protein variants (proNGF-A and proNGF-B), differing for the presence (proNGF-A) or the absence (proNGF-B) of a 66 aminoacid tail at the N-terminal portion of the pre-protein [25]. This murine proNGF is a product of the translation of different mRNA splicing variants [26] and/or the activation of at least two different pharmacologically active promoters [27]. A relevant novelty introduced in the NGF “saga” by studies carried out in the last twenty years, is that both proNGF and NGF can be retrogradely transported along neuronal processes toward the cell soma [28] and that proNGF has both neurotoxic and neurotrophic activities, depending on the different receptors challenged [29, 30]. The neurotrophic biological activity of NGF is mediated by two distinct receptors: the tropomyosin receptor kinase/tyrosine receptors kinase A (TrkA) and the p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor receptor superfamily [31-33], while proNGF can activate both the TrkA and a p75NTR-Sortilin receptor complex, respectively eliciting neurotrophic or pro-apoptotic signaling [29, 30].
2. NGF AND CENTRAL NERVOUS SYSTEM
Within the CNS, the greatest amount of NGF is produced in the cortex, hippocampus and pituitary gland, although significant quantities of this neurotrophin are also produced in other areas, including the basal ganglia, thalamus, spinal cord and retina [34]. The first study suggesting the presence of NGF and/or its receptors in the CNS was published in 1984 [35]. Subsequent investigations demonstrated that NGF administration directly into the brain can be transported to the BFCN neurons, where it can improve experimentally-induced cholinergic dysfunctions [16, 35]. NGF regulates the development and the phenotypic maintenance of cholinergic neurons in the basal forebrain [16, 36] and the striatum [37, 38], as well as of noradrenergic neurons in the hypothalamus [39, 40]. Because the degeneration of BFCN and the decline of cognitive abilities are hallmarks of the Alzheimer’s disease (AD) [41, 42], it was hypothesized that NGF might be of therapeutic value for AD patients. Indeed, based on these evidences, possible clinical application for NGF in neurodegenerative diseases as AD and Parkinson disease (PD) has been proposed and verified earlier [43, 44].
2.1. NGF and Neurological Diseases
The history of clinical trials on NGF started in the early 1990’s, when, supported by laboratory findings on the role of NGF on CNS neurons (see below), patients affected by PD and AD were treated by intracerebroventricular (ICV) injection of murine NGF [43, 45, 46]. Soon after these early clinical studies, recombinant human NGF (rhNGF) was developed and used in a series of large clinical trials on patients affected by peripheral neuropathies [47-49], where moderate side effects, such as myalgias and injection site hyperalgesia, were evidenced in both healthy subjects [50, 51] and in patients affected by diabetic polyneuropathy [47, 48]. The overall relative failure of these trials was mainly attributed to the low dosage of rhNGF, which was insufficient to give appreciable therapeutic outcomes due to the limitations of side effects related to the altered pain perception [47]. A comprehensive review, resuming the early development of NGF-based clinical trials, has been recently published, with extensive discussion of the successes and failures in the early pharmacological history of NGF [44]. In the present review, we will focus on the latest advancements in the pharmacology of NGF and especially in the experience of our group in ophthalmological, dermatological and neurological clinical use of the neurotrophin.
2.2. Alzheimer’s Disease
The synthesis/release of NGF from cortical neurons and glia as well as NGF-regulated functions in selected neuronal populations, i.e. the synthesis of acetylcholine in BFCNs, could be markedly affected in brain neurodegenerative disorders [41, 52], while exogenous administration of NGF was found to be able to protect degenerating neurons (for a recent comprehensive review see:[44]). NGF is able to promote the survival of BFCN, known to degenerate in age-related disorders (such as AD), leading to the hypothesis that intracerebral administration of NGF might reduce or prevent brain neuronal degeneration of these neurons [44]. Unfortunately, there is a major difficulty in delivering NGF directly to brain neurons, due to the poor permeability of NGF to the blood–brain barrier when injected systemically [53]. Clinical trials were performed in patients with AD and Parkinson disease (PD) and published in the 1990s by Swedish scientists [43, 46, 54]. The results showed partial beneficial effects after ICV administration of NGF by implantable infusion systems, such as a marked transient increase in uptake and binding of [11C] -nicotine in the frontal and temporal cortex, a persistent increase in cortical blood flow, a progressive decrease of slow wave EEG activity and the improvement of verbal episodic memory tests. ICV administration of NGF also resulted in some negative side effects, such as reversible weight loss during the NGF infusion period and the development of back pain symptoms after the beginning of ICV infusion. Such symptoms may reflect the NGF-mediated hyperactivation of nociceptive transmission system in the spinal cord [55]. Studies published in the recent years and carried out on laboratory animals reported that NGF can be safely delivered into the brain by the olfactory pathway [56-59] or by ocular administration [60, 61] and the NGF that reached its target neuron was biologically active. These observations suggested that both the olfactory and the ocular pathways might represent promising noninvasive route for NGF delivery to NGF-responsive brain neurons.
2.3. Brain Traumas
NGF has been preclinically tested in models of traumatic brain injury (TBI), with positive effects on the neurological deficits following brain injury [62]. Moreover, NGF levels in the cerebro-spinal fluid (CSF) of TBI patients have a positive correlation with neurological outcomes [63]. Two clinical studies used ICV infusion of murine NGF in children with TBI. In the first study [64] two infants were treated with NGF infused into the right cerebral ventricle for 10 days starting 30 days after a hypoxic-ischemic brain injury. It was detected an improvement in the comatose status, increased alpha/theta ratio in the EEG, reduction of malacic areas and improvement, in one child only, of the regional cerebral perfusion in right temporal and occipital cortices, as measured by SPECT. In a further study [65], 2 infants affected by hypoxic-ischemic brain damage, were treated with NGF ICV infusion, starting 4 months after TBI. Again an improvement in EEG and SPECT parameters was detected with a concomitant increase of doublecortin, a protein expressed by newly formed neurons, in the CSF. Thus, it was demonstrated that NGF displayed a potential in the treatment of TBI secondary to hypoxic-ischemic brain insult. A recent case-report, showed the outcome of intranasal NGF delivery in a patient with a severe traumatic TBI was reported [66]. Intranasal NGF improved PET/CT, SPECT/CT and MRI assessments, EEG and Visual Evoked Potential and clinical conditions. The authors did not report the development of significant side-effects, despite the treatment with intranasal NGF (0.1 mg/kg, twice a day for 10 consecutive days) was repeated 4 times in a period of 6 months. Thus, intranasal NGF administration appears to be a promising and safe rescuing strategy treatment in children with neurological impairment after TBI.
3. NGF AND EPITHELIAL DISEASES
3.1. Cutaneous Wound Healing
The potential therapeutic role for NGF on cutaneous ulcers and lesion was demonstrated in preclinical studies [67], that hypothesized a potential role for the neurotrophin secreted in the saliva on the natural healing process induced by licking. NGF is produced by keratinocytes [68] and actively participates in the maintenance of epidermal trophism [69]. NGF treatment in rodents significantly accelerated the rate of wound healing by promoting wound re-epithelialization, the formation of granulation tissue, collagen production and accelerating fibroblast migration [70].
Topical NGF exerted a healing effect on human pressure ulcers, as demonstrated around the beginning of the new century [71, 72]. Nineteen patients were treated with daily topical application of purified murine NGF. It was reported that NGF accelerated the healing process, in comparison with patients treated with conventional therapies. The cutaneous lesions in the NGF-treated patients displayed a marked peripheral scar indicating that the healing process was taking place and that the rate of recovering was not related to the severity of the ulcer, the age of the patient or the site of the ulcer [71, 72]. The action of NGF on cutaneous ulcer, was also assessed in patients affected by chronic vasculitis secondary to rheumatoid arthritis (RA) [73]. The RA-associated skin ulcers were treated with topical NGF in four patients that showed a rapid improvement after the first 2–3 weeks of NGF application. This healing action was followed by a significant reduction of local pain and inflammation. Thereafter, the ulcer improves progressively, reaching complete healing after eight weeks of NGF treatment [73]. It is worth noticing that these ulcers did not respond to previously-applied conventional treatments and were stable or worsening for a minimum of 1 month before the application of NGF [73]. Likewise, diabetes-associated leg cutaneous ulcer responded positively to topical NGF [74]. Even chronic ulcers with damage extending below the hypodermis and muscle layers responded to the NGF by healing in a few weeks. The first clear reduction of the size of the ulcer was noted after 8 weeks of NGF administration, and the follow-up study indicated that the NGF stimulated the proliferation and/or differentiation of local immune cells, blood vessel and even neurite outgrowth.
It is worth noticing that, differently from what reported for subcutaneous injection of NGF in both healthy [75] and diseased [76] patients, the application of NGF on the skin surface did not exert any particular pain or sensitization, as reported in the studies reviewed in this chapter [71-74]. This could indicate that topically applied NGF primarily exerts its activity on cells belonging to the epidermal and dermal tissues (i.e. keratinocytes, fibroblasts) [77, 78] and/or on endothelial cells [20], instead of acting on NGF receptors located on sensory nerve terminals or on immune cells (such as mast cells) able to secrete sensitizing peptides with potential nociceptive activity [79]. However, it cannot be excluded that the topical NGF also contributed to the re-establishment of a normal sensory and sympathetic innervation of the damaged skin, inducing an indirect beneficial effect over the skin trophism, mediated by the action of neuropeptides released from peripheral nerve terminals [33].
4. NGF AND OCULAR DISORDERS
NGF, TrkA and p75NTR are expressed in the anterior ocular segment in physiological states [80-82]. NGFmRNA is expressed in the cornea, iris, ciliary body, and lens [83] and NGF is normally detectable in the aqueous humor [83]. NGF has also been quantified in human tears, indicating that the lacrimal gland basally releases NGF, suggesting a role in the maintenance of the tear film homeostasis [84]. NGF and its receptors, TrkA and p75NTR, are also expressed by the rat lacrimal gland tissue [85, 86]. The roles played by NGF in the homeostasis of the eye and in vision are, therefore, crucial and have been widely investigated both in vitro and in vivo [80, 87]. Pharmacodynamic studies showed that NGF administered as eye drops, affected not only the ocular surface, but was also able to reach the retina, the optic nerve and the brain [60, 61, 80, 88]. Moreover, in vitro studies have shown that NGF is produced, released and utilized by conjunctival cells (both epithelial cells, goblet cells, immune cells and fibroblasts) [89]. NGF and NGF receptors are also widely expressed in the central visual pathway (lateral geniculate nucleus and visual cortex) as well as in the optic nerve and retina [90, 91]. It has also been shown that NGF and NGF receptors are expressed by cells of the posterior segment, including vitreous and choroid [83, 92], and topical NGF administration has been shown to modulate the development and differentiation of the retina and the optic nerve, and to promote the survival and recovery of retinal ganglion cells (RGCs) [10, 93].
4.1. Corneal Ulcers
NGF is produced and released by the corneal cells of rodents and humans [89, 94], while exogenous NGF administration has been shown to play a critical role in migration and proliferation of these cells [80, 94-96]. NGF has gained attention for the treatment of human patients with chronic epithelial defects, as topical eye NGF administration restores corneal integrity in human patients with immune or neurotrophic corneal ulcers [81]. Corneal epithelial cells are among the most densely innervated cells of the mammalian organism and alteration of their innervation can cause corneal damages, including the deficit of the ocular surface with consequent visual impairments [97, 98]. However, the first clear evidence that NGF exerts such a protective role in human cornea was published by Lambiase and co-workers who demonstrated that topical eye NGF administration in humans affected by corneal neurotrophic keratitis stimulate corneal healing [81, 95]. The studies demonstrated that the healing action of NGF was not impaired by the severity of corneal ulcer, the depth of stromal lesion or by the clinical history of the patients. The healing action was equally effective and the eye topical NGF administration induced a complete healing after three-to-six weeks of treatment, in patients suffering for corneal ulceration for a mean of 45±24 days and unresponsive to the conventional therapies so far administered [81]. Moreover, the NGF topical administration enhanced both corneal transparency and tear film production, improving the visual function [80, 95, 96]. Taken together, these results indicate that eye topical NGF treatment acts as a pleiotropic factor for damaged corneal surface through different mechanisms, such as stimulation of corneal innervation and healing, modulation of corneal stem cells, refining of both stromal and endothelial cells [18, 80, 96]. Moreover, eye NGF topical administration enhanced tear release in humans and bulldogs suffering of dry eye, a chronic ocular surface disease characterized by the impairment of tear film quantity and/or quality [99, 100]. None of the reviewed studies reported the occurrence of significant side effects, after topical ocular treatment with NGF. The possible occurrence of unpleasant side effects was however specifically addressed in a study published in 2007 [101], evaluating the effect of topical treatment with NGF on 11 patients with neurotrophic keratopathy. All patients had healing of corneal ulcers between 9 and 43 days after initiation of treatment. The ocular discomfort, reported as a moderate and tolerable painful sensation, lasted less than an hour after the instillation of eye drops and then disappeared, even when NGF treatment was continued after the healing of corneal ulcers. None of the patients developed systemic symptoms during treatment or during follow-up. Of note, after an almost 30 years-long story of NGF clinical trials [8], the first orphan designation (EU/3/15/1586), a status assigned to a medicine intended for use against a rare condition (affecting not more than five in 10,000 people in the European Union) or where the medicine is unlikely to generate sufficient profit to justify research and development costs, was recently granted by the European Commission to Dompé Farmaceutici S.p.A., Italy, for recombinant human NGF for the treatment of human neurotrophic keratitis. Recombinant human nerve growth factor has then been authorized in the EU as Oxervate since 6 July 2017. The release of EU authorization and the beginning of commercialization of recombinant human NGF, further confirm its safety and efficacy as eye drops in human clinics.
4.2. Glaucoma
Glaucoma, one of the leading causes of blindness worldwide, is a chronic and progressive optic neuropathy characterized by degeneration of the retinal ganglion cells (RGC) and loss of axons of the optic nerve with a progressive and consequent deficit of the peripheral and central visual field [102]. The main risk factor for the development of this disease is the elevated intraocular pressure (IOP). However, despite successful management of ocular hypertension, up to 20% glaucoma patients show progression of visual field defects with RGC and optic nerve degeneration. Thus, an approach that would involve neuroprotection with exogenous neurotrophic factors could improve the treatment of this challenging disease.
Pharmacodynamic studies have shown that NGF eye drops results in the ability to target the optic nerve and brain areas such as the basal forebrain [61, 88]. Recently, it has been demonstrated that murine NGF administered topically to the eye rescued RGCs from apoptosis in a rat model of glaucoma [103]. It has also been shown, in humans with advanced glaucoma, that NGF eye drop treatment induced long lasting improvements in the visual field, optic nerve function as measured by electro-functional parameters, contrast sensitivity, and visual acuity [100]. It was shown, in 1979, that the retinal cells are receptive to the action of NGF [104]. NGF also induces modification of pre-synaptic elements in adult visual system [10, 105], which prevents the shift in ocular dominance distribution of visual cortical neurons, promotes functional recovery of RGC after ischemia [93], delays retinal degeneration in rodents with inherited retinopathy [106, 107], reduces retinal damages in rabbits with ocular hypertension, while injection of antibody against NGF exacerbate the damaging effect on retinal cells [108]. Together these observations lead to the hypothesis that ocular NGF administration would protect damaged retinal cells in glaucomatous patients. Using a rodent model of glaucoma, induced through injection of hypertonic saline into the episcleral vein, we observed that the levels of NGF and NGF-receptors in the retina were significantly down regulated, while eye NGF administration reduced this loss and promoted recovery of damaged RGC [100, 109].
These experimental results supported the testing of NGF eye-drop administration in three patients suffering of glaucoma with progressive visual field defects [100]. The patients underwent baseline psychosensorial (static perimetry and foveal contrast sensitivity) and electro functional (pattern lectroretinigram and visual-evoked potentials) tests and then they were followed weekly during a three-months NGF treatment. Significantly less RGC loss was observed with NGF treatment associated with inhibition of cell death by apoptosis. Patients treated with NGF demonstrated long lasting improvements in visual field, optic nerve function, contrast sensitivity, and visual acuity. Concomitant investigations using the same NGF doses and batch in glaucomatous rabbits (experimental ocular hypertension) indicated that the NGF treatment exerted neuroprotective effects by reducing RGC death, as observed through a reduced apoptotic mechanism, in line with the improvement of all visual function parameters monitored in advanced glaucoma patients [100].
4.3. Retinitis Pigmentosa
In 1990, Faktorovich and coworkers reported that growth factors might be involved in the protection of retinal cell degeneration in rodents with retinitis pigmentosa (RP), a genetic disorder characterized by the progressive photoreceptor degeneration leading to the loss of vision [110]. The potential therapeutic role of NGF in animal models of RP was later demonstrated [32, 90, 107]. One of the first indications of NGF’s ability to protect photoreceptors was published in 1996 using the mouse strain C3H, characterized by the progressive photoreceptor degeneration during early postnatal life [106]. The authors demonstrated that intravitreal NGF administration was able to delay photoreceptor degeneration. These observations and the NGF action on retinal cells were confirmed and extended in the Royal College of Surgeon (RCS) rat model, a strain characterized by autosomal recessive mutation of the retinal dystrophy gene [111, 112]. Studies carried out in mice and rats affected by RP showed that topical NGF administration was able to delay photoreceptor degeneration [107]. The protective action of NGF on RCS photoreceptors was also indicated by findings showing that eye NGF administration enhances both gene and receptor expression in visual cortex and geniculate nucleus [90]. Recently, results of clinical trials on RP patients treated with 10 daily administration of murine NGF as eye-drops have been published [113]. The study supported the safety of the use of NGF in RP patients, reporting only a transient tolerable local corneal irritation as an adverse effect. As for the efficacy, 3 out of 8 patients reported a subjective feeling of improved visual performance, associated to a temporary enlargement of the visual field and to improved macular focal electroretinogram [113].
4.4. Macular Degeneration
Retinal cells are known to express both TrkA and p75NTR receptors and to be receptive to the action of NGF [114]. Age-related macular degeneration (ARMD) is a human ocular pathology characterized by an oval-shaped highly pigmented yellow spot in the macula (the center of retina), progressively worsening with age [115]. The effect of NGF topical application was tested in a 94 years old female patient with ARMD by several years and unable to respond to all current available therapies and close to complete vision lost [116]. The visual acuity in this patient was progressively worsening in spite of previous surgical and pharmacological treatments. The patient was treated with NGF eye drops for 6 consecutive months. Weekly clinical examination revealed the absence of NGF related undesired effects. Moreover, a clear improvement of visual acuity and of electro functional parameters was monitored 3 months after the beginning of the treatment [116].
4.5. Optic Gliomas
At the interface between ophthalmology and neuro-oncology, optic gliomas (OG) are tumors involving the optic nerve and optic chiasm, often associated with neurofibromatosis type 1 [117]. The NGF eye-drop delivery has been described in patients affected by OGs, [118-120], inducing improvements in electrophysiological parameters (electroretinographic photopic negative response amplitude and visual-evoked potentials), effect that were not observed in placebo-treated patients. Furthermore, visual field worsening was only observed in placebo-treated patients, while the majority of NGF-treated subjects showed significant visual field enlargement [120].
5. THERAPEUTIC APPROACH TO REGULATE ENDOGENOUS NGF ACTIVITY
The possibility to use non-pharmacologic approaches, such as physical therapies, to modulate endogenous NGF synthesis and activity, has been studied [121, 122]. The main aim for this unconventional approach was to circumvent the possible undesired side effects elicited by NGF administration, obtaining therapeutic outcomes based on the re-establishment of a correct functionality of the NGF physiology. Among different physical therapies, electroacupuncture (EA) was found to be effective in regulating NGF activity in a wide spectrum of disease models [123]. EA is a Western derivative of the traditional Chinese acupuncture (TCA), using electrical currents delivered through the needles to generate a selective activation of C-, A-delta- and A-beta sensory fibers, while in TCA manual stimulation of the needles activates preferentially an A-delta fibers-mediated response [123, 124]. This approach presented several advantages, from a clinical standpoint, mainly because it is easy and safe to translate into the clinical practice. Both traditional Chinese acupuncture and EA have been proven effective for pain management [31, 124] and in a wide spectrum of diseases, including infections and inflammations, dysfunction of the autonomic nervous system, peripheral and central nervous system diseases, metabolic disorders [123, 124], actually listed in the 1998 NIH Consensus Statement [125]. The neurophysiological correlates of these techniques are actually subjects of extensive investigations [31, 124, 126, 127]. From a Western perspective, EA is a potent form of sensory stimulation. It has been suggested that EA, activating sensory receptors through repetitive muscle contractions, results in the activation of physiological processes similar to those resulting from aerobic physical exercise [124]. Both EA and physical exercise stimulate the release of endogenous opioids, which seems to be essential in the induction of acupuncture-mediated functional changes of different organ systems [123, 124]. As for the effects of EA on the modulation of endogenous production and activity of NGF, we address the readers to a recent comprehensive review [126]. However, reports relevant to the topic of the present review, can be briefly summarized as follows. We demonstrated that EA increased NGF synthesis in the retina of a rat strain affected by an inherited retinopathy, strongly resembling human Retinitis Pigmentosa [128]. This effect was parallel to a partial recovery of the retinal normal morphological features and strongly resemble the effects obtained through a direct administration of NGF in the same experimental model [107]. We also applied EA in an experimental model of diabetic neuropathy [129], demonstrating that the early increase of spinal and peripheral NGF, associated with the development of thermal hyperalgesia, could be corrected by EA. Thus, EA was proposed as a possible supporting therapy, to overcome the hyperalgesic effects of peripheral-injected NGF [129], such as those reported in clinical trials that studied the effects of NGF in patients affected by peripheral neuropathies [47, 49]. By modulating NGF and BDNF in the hippocampus, EA was found to be effective in counteracting the stress-induced alterations in anxiety-related behavior in mice and in decreasing hypothalamic NGF [130]. More recently, we found that EA was effective in restoring the diabetes-induced impairment in spatial learning and memory, in the hippocampal long-term potentiation, in the release of proNGF from hippocampal cells of diabetic rats [127] and in the decrease of NGF/proNGF ratio in the brain [131]. We also demonstrated that the action of EA in the brain of diabetic rats was due to its regulatory action on the muscarinic response to acetylcholine [127], known to regulate endogenous NGF synthesis and release in the brain [132], pointing at the use of physical therapies, such as acupuncture, as supportive to the studied muscarinic agonists or cholinesterase inhibitors for the treatment of Alzheimer’s disease [133, 134].
CONCLUSION
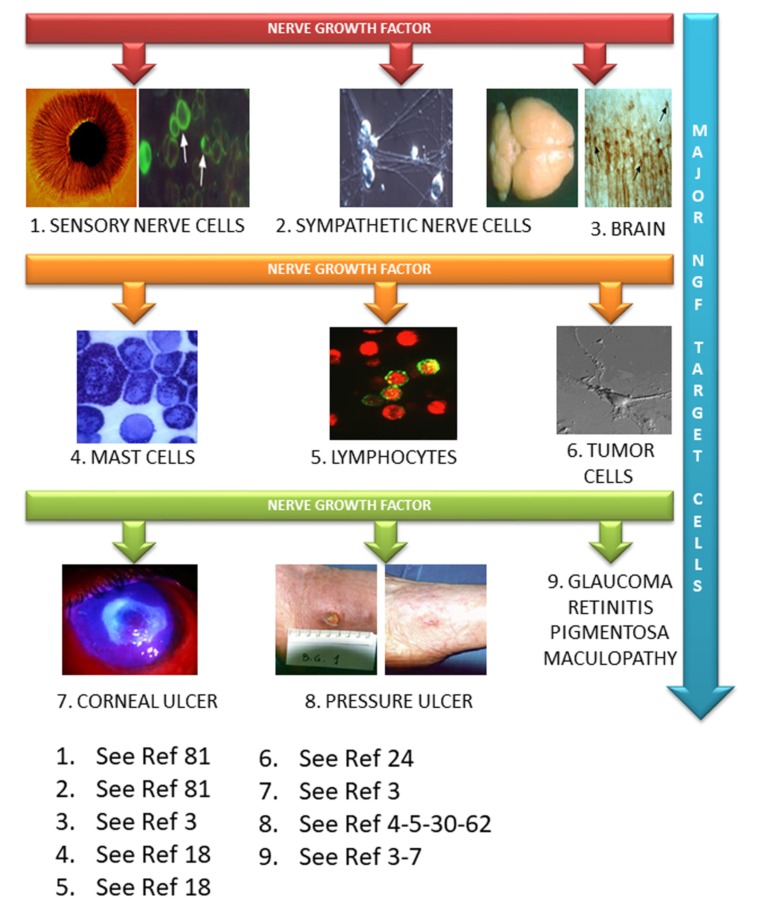
NGF might have potential therapeutic properties on peripheral and central nervous system’s diseases but also on the visual system and on cutaneous wound healing (Fig. 1) [44]. As we have reported in this brief review, NGF is now known to have pharmacological potential, for the treatment of diseases affecting the anterior and the posterior segment of the eye, epithelial derangements, central neurodegenerative and traumatic diseases. The results of early trials, despite encouraging, evidenced that the clinical use of NGF delivered ICV or subcutaneously, could be hampered by the development of side effects, such as injection site hyperalgesia, myalgias and arthralgias [48, 49], linked to the action of the neurotrophin on the sensory and sympathetic systems [44]. Nevertheless, the basic and clinical research on NGF identified novel delivery routes, safest dosages, and widened the spectrum of diseases that could benefit from NGF-based therapy. A promising and yet unexplored new field for possible clinical use of NGF is suggested by the basic studies on the role of NGF in the regulation of immune system functions [19, 135].
Fig. (1).
Cells in the central and peripheral nervous systems, immune system as well as epithelial and cancer cells, all responsive to NGF, have been studied as potential targets for a NGF-based pharmacology.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONs
- ARMD
Age-related macular degeneration
- AD
Alzheimer’s disease
- BFCN
Basal forebrain cholinergic neurons
- BDNF
Brain-derived neurotrophic factor
- CGRP
Calcitonin-Gene Related Peptide
- CNS
Central nervous system
- CSF
Cerebro-spinal fluid
- EA
Electroacupuncture
- ICV
Intra- cerebro-ventricular
- NGF
Nerve growth factor
- NT-3
Neurotrophin-3
- proNGF
NGF precursor
- OG
Optic gliomas
- PD
Parkinson disease
- PNS
Peripheral Nervous System
- RGC
Retinal ganglion cells
- RP
Retinitis Pigmentosa
- RA
Rheumatoid arthritis
- RCS
Royal College of Surgeon
- SP
Substance P
- TBI
Traumatic brain injury
- TrkA
Tyrosine kinase A
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lewin G.R., Barde Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [http://dx.doi.org/10.1146/ annurev.ne.19.030196.001445]. [PMID: 8833445]. [DOI] [PubMed] [Google Scholar]
- 2.Barde Y.A. The nerve growth factor family. Prog. Growth Factor Res. 1990;2(4):237–248. doi: 10.1016/0955-2235(90)90021-b. [http://dx.doi.org/10.1016/0955-2235 (90)90021-B]. [PMID: 2133291]. [DOI] [PubMed] [Google Scholar]
- 3.Shooter E.M. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 2001;24:601–629. doi: 10.1146/annurev.neuro.24.1.601. [http://dx.doi.org/10.1146/ annurev.neuro.24.1.601]. [PMID: 11283322]. [DOI] [PubMed] [Google Scholar]
- 4.Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987;6(5):1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [http://dx.doi.org/10.1002/j. 1460-2075.1987.tb02347.x]. [PMID: 3301324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi-Montalcini R., Dal Toso R., della Valle F., Skaper S.D., Leon A. Update of the NGF saga. J. Neurol. Sci. 1995;130(2):119–127. doi: 10.1016/0022-510x(95)00007-o. [http://dx.doi.org/10.1016/0022-510X(95)00007-O]. [PMID: 8586974]. [DOI] [PubMed] [Google Scholar]
- 6.Kessler J.A., Black I.B. Nerve growth factor stimulates the development of substance P in sensory ganglia. Proc. Natl. Acad. Sci. USA. 1980;77(1):649–652. doi: 10.1073/pnas.77.1.649. [http://dx.doi.org/10.1073/pnas.77.1. 649]. [PMID: 6153799]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLean D.B., Bennett B., Morris M., Wheeler F.B. Differential regulation of calcitonin gene-related peptide and substance P in cultured neonatal rat vagal sensory neurons. Brain Res. 1989;478(2):349–355. doi: 10.1016/0006-8993(89)91515-1. [http://dx.doi.org/10.1016/0006-8993(89)91515-1]. [PMID: 2466532]. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R.M., Harmar A.J. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337(6205):362–364. doi: 10.1038/337362a0. [http://dx.doi.org/10.1038/337362a0]. [PMID: 2911387]. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson D. R., Fernyhough P., Diemel L. T. Neurotrophins and peripheral neuropathy. 1996. [DOI] [PubMed]
- 10.Carmignoto G., Canella R., Candeo P., Comelli M.C., Maffei L. Effects of nerve growth factor on neuronal plasticity of the kitten visual cortex. J. Physiol. 1993;464:343–360. doi: 10.1113/jphysiol.1993.sp019638. [http://dx.doi.org/10. 1113/jphysiol.1993.sp019638]. [PMID: 8229806]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falckh P.H., Harkin L.A., Head R.J. Nerve growth factor mRNA content parallels altered sympathetic innervation in the spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol. 1992;19(8):541–545. doi: 10.1111/j.1440-1681.1992.tb00502.x. [http://dx.doi.org/10.1111/j.1440-1681.1992.tb00502.x]. [PMID: 1526060]. [DOI] [PubMed] [Google Scholar]
- 12.Lindholm D., Castrén E., Berzaghi M., Blöchl A., Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain--implications for neuronal plasticity. J. Neurobiol. 1994;25(11):1362–1372. doi: 10.1002/neu.480251105. [http://dx.doi.org/10.1002/ neu.480251105]. [PMID: 7852991]. [DOI] [PubMed] [Google Scholar]
- 13.White D. M., Ehrhard P., Hardung M., Meyer D. K., Zimmermann M., Otten U. Substance P modulates the release of locally synthesized nerve growth factor from rat saphenous nerve neuroma. 1987. [DOI] [PubMed]
- 14.Whittemore S.R., Ebendal T., Lärkfors L., Olson L., Seiger A., Strömberg I., Persson H. Development and regional expression of beta nerve growth factor messenger RNA and protein in the rat central nervous system. Proc. Natl. Acad. Sci. USA. 1986;83(3):817–821. doi: 10.1073/pnas.83.3.817. [http://dx.doi.org/10.1073/pnas.83.3.817]. [PMID: 3456170]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 1986;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [http://dx.doi.org/10.1523/JNEUROSCI.06-08-02155. 1986]. [PMID: 3746405]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hefti F., Dravid A., Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984;293(2):305–311. doi: 10.1016/0006-8993(84)91237-x. [http://dx.doi.org/10. 1016/0006-8993(84)91237-X]. [PMID: 6697222]. [DOI] [PubMed] [Google Scholar]
- 17.Dissen G.A., Hirshfield A.N., Malamed S., Ojeda S.R. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology. 1995;136(10):4681–4692. doi: 10.1210/endo.136.10.7664689. [http://dx.doi.org/10. 1210/endo.136.10.7664689]. [PMID: 7664689]. [DOI] [PubMed] [Google Scholar]
- 18.Manni L., Rocco M.L., Bianchi P., Soligo M., Guaragna M., Barbaro S.P., Aloe L. Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors. 2013;31(4):115–122. doi: 10.3109/08977194.2013.804073. [http://dx.doi.org/10.3109/08977194.2013.804073]. [PMID: 23777359]. [DOI] [PubMed] [Google Scholar]
- 19.Minnone G., De Benedetti F., Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 2017;18(5):E1028. doi: 10.3390/ijms18051028. [http://dx.doi.org/10.3390/ijms18051028]. [PMID: 28492466]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raychaudhuri S.K., Raychaudhuri S.P., Weltman H., Farber E.M. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch. Dermatol. Res. 2001;293(6):291–295. doi: 10.1007/s004030100224. [http://dx.doi.org/10.1007/s004030100224]. [PMID: 11480588]. [DOI] [PubMed] [Google Scholar]
- 21.Torcia M., Bracci-Laudiero L., Lucibello M., Nencioni L., Labardi D., Rubartelli A., Cozzolino F., Aloe L., Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85(3):345–356. doi: 10.1016/s0092-8674(00)81113-7. [http://dx.doi.org/10. 1016/S0092-8674(00)81113-7]. [PMID: 8616890]. [DOI] [PubMed] [Google Scholar]
- 22.Aloe L., Bracci-Laudiero L., Alleva E., Lambiase A., Micera A., Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc. Natl. Acad. Sci. USA. 1994;91(22):10440–10444. doi: 10.1073/pnas.91.22.10440. [http://dx.doi.org/10.1073/ pnas.91.22.10440]. [PMID: 7937971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger E.A., Shooter E.M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc. Natl. Acad. Sci. USA. 1977;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [http://dx.doi.org/ 10.1073/pnas.74.9.3647]. [PMID: 269420]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hempstead B.L. Deciphering proneurotrophin actions. Handb. Exp. Pharmacol. 2014;220:17–32. doi: 10.1007/978-3-642-45106-5_2. [http://dx.doi.org/10.1007/978-3-642-45106-5_2]. [PMID: 24668468]. [DOI] [PubMed] [Google Scholar]
- 25.Paoletti F., Covaceuszach S., Konarev P.V., Gonfloni S., Malerba F., Schwarz E., Svergun D.I., Cattaneo A., Lamba D. Intrinsic structural disorder of mouse proNGF. Proteins. 2009;75(4):990–1009. doi: 10.1002/prot.22311. [http://dx.doi.org/10.1002/prot.22311]. [PMID: 19089979]. [DOI] [PubMed] [Google Scholar]
- 26.Edwards R.H., Selby M.J., Rutter W.J. Differential RNA splicing predicts two distinct nerve growth factor precursors. Nature. 1986;319(6056):784–787. doi: 10.1038/319784a0. [http://dx.doi.org/10.1038/319784a0]. [PMID: 2419763]. [DOI] [PubMed] [Google Scholar]
- 27.Racke M.M., Mason P.J., Johnson M.P., Brankamp R.G., Linnik M.D. Demonstration of a second pharmacologically active promoter region in the NGF gene that induces transcription at exon 3. Brain Res. Mol. Brain Res. 1996;41(1-2):192–199. doi: 10.1016/0169-328x(96)00096-4. [http://dx. doi.org/10.1016/0169-328X(96)00096-4]. [PMID: 8883952]. [DOI] [PubMed] [Google Scholar]
- 28.De Nadai T., Marchetti L., Di Rienzo C., Calvello M., Signore G., Di Matteo P., Gobbo F., Turturro S., Meucci S., Viegi A., Beltram F., Luin S., Cattaneo A. Precursor and mature NGF live tracking: one versus many at a time in the axons. Sci. Rep. 2016;6:20272. doi: 10.1038/srep20272. [http://dx.doi.org/10.1038/srep20272]. [PMID: 26829890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masoudi R., Ioannou M.S., Coughlin M.D., Pagadala P., Neet K.E., Clewes O., Allen S.J., Dawbarn D., Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J. Biol. Chem. 2009;284(27):18424–18433. doi: 10.1074/jbc.M109.007104. [http://dx.doi.org/10.1074/jbc.M109.007104]. [PMID: 19389705]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nykjaer A., Lee R., Teng K.K., Jansen P., Madsen P., Nielsen M.S., Jacobsen C., Kliemannel M., Schwarz E., Willnow T.E., Hempstead B.L., Petersen C.M. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. doi: 10.1038/nature02319. [http://dx.doi.org/10.1038/nature02319]. [PMID: 14985763]. [DOI] [PubMed] [Google Scholar]
- 31.Abuaisha B.B., Costanzi J.B., Boulton A.J. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res. Clin. Pract. 1998;39(2):115–121. doi: 10.1016/s0168-8227(97)00123-x. [http://dx.doi.org/10.1016/S0168-8227(97)00123-X]. [PMID: 9597381]. [DOI] [PubMed] [Google Scholar]
- 32.Amendola T., Aloe L. Developmental expression of nerve growth factor in the eye of rats affected by inherited retinopathy: correlative aspects with retinal structural degeneration. Arch. Ital. Biol. 2002;140(2):81–90. [PMID: 12004645]. [PubMed] [Google Scholar]
- 33.Abelli L., Geppetti P., Maggi C.A. Relative contribution of sympathetic and sensory nerves to thermal nociception and tissue trophism in rats. Neuroscience. 1993;57(3):739–745. doi: 10.1016/0306-4522(93)90020-g. [http://dx.doi. org/10.1016/0306-4522(93)90020-G]. [PMID: 7508578]. [DOI] [PubMed] [Google Scholar]
- 34.Whittemore S.R., Seiger A. The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res. 1987;434(4):439–464. doi: 10.1016/0165-0173(87)90008-7. [http://dx. doi.org/10.1016/0165-0173(87)90008-7]. [PMID: 2825921]. [DOI] [PubMed] [Google Scholar]
- 35.Seiler M., Schwab M.E. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984;300(1):33–39. doi: 10.1016/0006-8993(84)91338-6. [http://dx.doi.org/10.1016/0006-8993(84)91338-6]. [PMID: 6203605]. [DOI] [PubMed] [Google Scholar]
- 36.Korsching S., Auburger G., Heumann R., Scott J., Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985;4(6):1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [http://dx.doi.org/10.1002/j.1460-2075. 1985.tb03791.x]. [PMID: 2411537]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gage F.H., Batchelor P., Chen K.S., Chin D., Higgins G.A., Koh S., Deputy S., Rosenberg M.B., Fischer W., Bjorklund A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989;2(2):1177–1184. doi: 10.1016/0896-6273(89)90184-0. [http://dx.doi.org/10.1016/0896-6273(89)90184-0]. [PMID: 2576209]. [DOI] [PubMed] [Google Scholar]
- 38.Martínez H.J., Dreyfus C.F., Jonakait G.M., Black I.B. Nerve growth factor promotes cholinergic development in brain striatal cultures. Proc. Natl. Acad. Sci. USA. 1985;82(22):7777–7781. doi: 10.1073/pnas.82.22.7777. [http://dx.doi.org/10.1073/pnas.82.22.7777]. [PMID: 3865196]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aloe L., Alleva E., De Simone R. Changes of NGF level in mouse hypothalamus following intermale aggressive behaviour: biological and immunohistochemical evidence. Behav. Brain Res. 1990;39(1):53–61. doi: 10.1016/0166-4328(90)90120-4. [http://dx.doi.org/10.1016/0166-4328(90) 90120-4]. [PMID: 2202329]. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs R.B., McCabe J.T., Buck C.R., Chao M.V., Pfaff D.W. Expression of NGF receptor in the rat forebrain detected with in situ hybridization and immunohistochemistry. Brain Res. Mol. Brain Res. 1989;6(4):275–287. doi: 10.1016/0169-328x(89)90073-9. [http://dx.doi.org/10.1016/0169-328X(89)90073-9]. [PMID: 2556618]. [DOI] [PubMed] [Google Scholar]
- 41.Cuello A.C., Bruno M.A., Allard S., Leon W., Iulita M.F. Cholinergic involvement in Alzheimer’s disease. A link with NGF maturation and degradation. J. Mol. Neurosci. 2010;40(1-2):230–235. doi: 10.1007/s12031-009-9238-z. [http://dx.doi.org/10.1007/s12031-009-9238-z]. [PMID: 19680822]. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer E.L., Gattaz W.F. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl.) 2008;198(1):1–27. doi: 10.1007/s00213-008-1092-0. [http://dx.doi.org/10.1007/s00213-008-1092-0]. [PMID: 18392810]. [DOI] [PubMed] [Google Scholar]
- 43.Olson L., Nordberg A., von Holst H., Bäckman L., Ebendal T., Alafuzoff I., Amberla K., Hartvig P., Herlitz A., Lilja A. Nerve growth factor affects 11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report). J. Neural Transm. Park. Dis. Dement. Sect. 1992;4(1):79–95. doi: 10.1007/BF02257624. [http://dx.doi.org/10.1007/BF02257624]. [PMID: 1540306]. [DOI] [PubMed] [Google Scholar]
- 44.Aloe L., Rocco M.L., Bianchi P., Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Transl. Med. 2012;10(1):239. doi: 10.1186/1479-5876-10-239. [http://dx.doi.org/10.1186/1479-5876-10-239]. [PMID: 23190582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson L., Backlund E.O., Ebendal T., Freedman R., Hamberger B., Hansson P., Hoffer B., Lindblom U., Meyerson B., Strömberg I. Intraputaminal infusion of nerve growth factor to support adrenal medullary autografts in Parkinson’s disease. One-year follow-up of first clinical trial. Arch. Neurol. 1991;48(4):373–381. doi: 10.1001/archneur.1991.00530160037011. [http://dx.doi.org/10.1001/archneur.1991.00530160037011]. [PMID: 2012510]. [DOI] [PubMed] [Google Scholar]
- 46.Eriksdotter Jönhagen M., Nordberg A., Amberla K., Bäckman L., Ebendal T., Meyerson B., Olson L. Seiger; Shigeta, M.; Theodorsson, E.; Viitanen, M.; Winblad, B.; Wahlund, L.O. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1998;9(5):246–257. doi: 10.1159/000017069. [http://dx.doi.org/10.1159/000017069]. [PMID: 9701676]. [DOI] [PubMed] [Google Scholar]
- 47.Apfel S.C. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int. Rev. Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [http://dx. doi.org/10.1016/S0074-7742(02)50083-0]. [PMID: 12198818]. [DOI] [PubMed] [Google Scholar]
- 48.Apfel S.C., Kessler J.A., Adornato B.T., Litchy W.J., Sanders C., Rask C.A. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology. 1998;51(3):695–702. doi: 10.1212/wnl.51.3.695. [http://dx.doi.org/10.1212/WNL.51.3.695]. [PMID: 9748012]. [DOI] [PubMed] [Google Scholar]
- 49.Apfel S.C., Schwartz S., Adornato B.T., Freeman R., Biton V., Rendell M., Vinik A., Giuliani M., Stevens J.C., Barbano R., Dyck P.J. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284(17):2215–2221. doi: 10.1001/jama.284.17.2215. [http://dx.doi.org/10.1001/jama.284.17.2215]. [PMID: 11056593]. [DOI] [PubMed] [Google Scholar]
- 50.Petty B.G., Cornblath D.R., Adornato B.T., Chaudhry V., Flexner C., Wachsman M., Sinicropi D., Burton L.E., Peroutka S.J. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann. Neurol. 1994;36(2):244–246. doi: 10.1002/ana.410360221. [http://dx.doi.org/10.1002/ana.410360221]. [PMID: 8053664]. [DOI] [PubMed] [Google Scholar]
- 51.Rogers B. C. 1996.
- 52.Oderfeld-Nowak B., Bacia A. Expression of astroglial nerve growth factor in damaged brain. Acta Neurobiol. Exp. (Warsz.) 1994;54(2):73–80. [PMID: 8053415]. [PubMed] [Google Scholar]
- 53.Pan W., Banks W.A., Kastin A.J. Permeability of the blood-brain barrier to neurotrophins. Brain Res. 1998;788(1-2):87–94. doi: 10.1016/s0006-8993(97)01525-4. [http://dx.doi.org/10.1016/S0006-8993(97)01525-4]. [PMID: 9554964]. [DOI] [PubMed] [Google Scholar]
- 54.Olson L. NGF and the treatment of Alzheimer’s disease. Exp. Neurol. 1993;124(1):5–15. doi: 10.1006/exnr.1993.1167. [http://dx.doi.org/10.1006/exnr.1993. 1167]. [PMID: 8282080]. [DOI] [PubMed] [Google Scholar]
- 55.Malcangio M., Garrett N.E., Cruwys S., Tomlinson D.R. Expression of astroglial nerve growth factor in damaged brain. Acta Neurobiol. Exp. (Warsz.) 1994;54(2):73–80. [PubMed] [Google Scholar]
- 56.Lv Q., Lan W., Sun W., Ye R., Fan X., Ma M., Yin Q., Jiang Y., Xu G., Dai J., Guo R., Liu X. Intranasal nerve growth factor attenuates tau phosphorylation in brain after traumatic brain injury in rats. J. Neurol. Sci. 2014;345(1-2):48–55. doi: 10.1016/j.jns.2014.06.037. [http://dx.doi.org/10. 1016/j.jns.2014.06.037]. [PMID: 25128470]. [DOI] [PubMed] [Google Scholar]
- 57.Thorne R.G., Frey W.H., II Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin. Pharmacokinet. 2001;40(12):907–946. doi: 10.2165/00003088-200140120-00003. [http://dx.doi.org/10.2165/ 00003088-200140120-00003]. [PMID: 11735609]. [DOI] [PubMed] [Google Scholar]
- 58.Tian L., Guo R., Yue X., Lv Q., Ye X., Wang Z., Chen Z., Wu B., Xu G., Liu X. Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [http://dx.doi.org/10.1016/ j.brainres.2011.12.059]. [PMID: 22284619]. [DOI] [PubMed] [Google Scholar]
- 59.Zhu W., Cheng S., Xu G., Ma M., Zhou Z., Liu D., Liu X. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv. 2011;18(5):338–343. doi: 10.3109/10717544.2011.557785. [http://dx.doi.org/10.3109/10717544.2011.557785]. [PMID: 21348576]. [DOI] [PubMed] [Google Scholar]
- 60.Di Fausto V., Fiore M., Tirassa P., Lambiase A., Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur. J. Neurosci. 2007;26(9):2473–2480. doi: 10.1111/j.1460-9568.2007.05883.x. [http://dx.doi.org/10.1111/j.1460-9568.2007.05883.x]. [PMID: 17970722]. [DOI] [PubMed] [Google Scholar]
- 61.Lambiase A., Pagani L., Di Fausto V., Sposato V., Coassin M., Bonini S., Aloe L. Nerve growth factor eye drop administrated on the ocular surface of rodents affects the nucleus basalis and septum: biochemical and structural evidence. Brain Res. 2007;1127(1):45–51. doi: 10.1016/j.brainres.2006.09.102. [http://dx.doi.org/10.1016/j.brainres.2006.09.102]. [PMID: 17113055]. [DOI] [PubMed] [Google Scholar]
- 62.Holtzman D.M., Sheldon R.A., Jaffe W., Cheng Y., Ferriero D.M. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann. Neurol. 1996;39(1):114–122. doi: 10.1002/ana.410390117. [http://dx.doi.org/10.1002/ana.410390117]. [PMID: 8572656]. [DOI] [PubMed] [Google Scholar]
- 63.Chiaretti A., Piastra M., Polidori G., Di Rocco C., Caresta E., Antonelli A., Amendola T., Aloe L. Correlation between neurotrophic factor expression and outcome of children with severe traumatic brain injury. Intensive Care Med. 2003;29(8):1329–1338. doi: 10.1007/s00134-003-1852-6. [http://dx.doi.org/10.1007/s00134-003-1852-6]. [PMID: 12845427]. [DOI] [PubMed] [Google Scholar]
- 64.Chiaretti A., Genovese O., Riccardi R., Di Rocco C., Di Giuda D., Mariotti P., Pulitanò S., Piastra M., Polidori G., Colafati G.S., Aloe L. Intraventricular nerve growth factor infusion: a possible treatment for neurological deficits following hypoxic-ischemic brain injury in infants. Neurol. Res. 2005;27(7):741–746. doi: 10.1179/016164105X35611. [http://dx.doi.org/10.1179/016164105X35611]. [PMID: 16197811]. [DOI] [PubMed] [Google Scholar]
- 65.Chiaretti A., Antonelli A., Genovese O., Fernandez E., Giuda D., Mariotti P., Riccardi R. Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic-ischemic brain injury. Neurol. Res. 2008;30(3):223–228. doi: 10.1179/016164107X247948. [http://dx.doi.org/10.1179/ 016164107X247948]. [PMID: 18282376]. [DOI] [PubMed] [Google Scholar]
- 66.Chiaretti A., Conti G., Falsini B., Buonsenso D., Crasti M., Manni L., Soligo M., Fantacci C., Genovese O., Calcagni M.L., Di Giuda D., Mattoli M.V., Cocciolillo F., Ferrara P., Ruggiero A., Staccioli S., Colafati G.S., Riccardi R. Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: A case report. Brain Inj. 2017;31(11):1538–1547. doi: 10.1080/02699052.2017.1376760. [http://dx.doi.org/10.1080/02699052. 2017.1376760]. [PMID: 28972396]. [DOI] [PubMed] [Google Scholar]
- 67.Li A.K., Koroly M.J., Schattenkerk M.E., Malt R.A., Young M. Nerve growth factor: acceleration of the rate of wound healing in mice. Proc. Natl. Acad. Sci. USA. 1980;77(7):4379–4381. doi: 10.1073/pnas.77.7.4379. [http:// dx.doi.org/10.1073/pnas.77.7.4379]. [PMID: 6933491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Marco E., Marchisio P.C., Bondanza S., Franzi A.T., Cancedda R., De Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J. Biol. Chem. 1991;266(32):21718–21722. [PMID: 1718982]. [PubMed] [Google Scholar]
- 69.Matsuda H., Koyama H., Sato H., Sawada J., Itakura A., Tanaka A., Matsumoto M., Konno K., Ushio H., Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J. Exp. Med. 1998;187(3):297–306. doi: 10.1084/jem.187.3.297. [http://dx.doi.org/10.1084/jem.187.3.297]. [PMID: 9449710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J.C., Lin B.B., Hu H.W., Lin C., Jin W.Y., Zhang F.B., Zhu Y.A., Lu C.J., Wei X.J., Chen R.J. NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. BioMed Res. Int. 2014;2014:547187. doi: 10.1155/2014/547187. [PMID: 25006578]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernabei R., Landi F., Bonini S., Onder G., Lambiase A., Pola R., Aloe L. Effect of topical application of nerve-growth factor on pressure ulcers. Lancet. 1999;354(9175):307. doi: 10.1016/S0140-6736(99)02784-1. [http://dx.doi.org/ 10.1016/S0140-6736(99)02784-1]. [PMID: 10440316]. [DOI] [PubMed] [Google Scholar]
- 72.Landi F., Aloe L., Russo A., Cesari M., Onder G., Bonini S., Carbonin P.U., Bernabei R. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann. Intern. Med. 2003;139(8):635–641. doi: 10.7326/0003-4819-139-8-200310210-00006. [http://dx.doi.org/10.7326/0003-4819-139-8-200310210-00006]. [PMID: 14568851]. [DOI] [PubMed] [Google Scholar]
- 73.Tuveri M., Generini S., Matucci-Cerinic M., Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356(9243):1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [http://dx.doi. org/10.1016/S0140-6736(00)03212-8]. [PMID: 11095266]. [DOI] [PubMed] [Google Scholar]
- 74.Generini S., Tuveri M.A., Matucci Cerinic M., Mastinu F., Manni L., Aloe L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp. Clin. Endocrinol. Diabetes. 2004;112(9):542–544. doi: 10.1055/s-2004-821313. [http://dx.doi.org/ 10.1055/s-2004-821313]. [PMID: 15505764]. [DOI] [PubMed] [Google Scholar]
- 75.Rukwied R., Mayer A., Kluschina O., Obreja O., Schley M., Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148(3):407–413. doi: 10.1016/j.pain.2009.11.022. [http://dx.doi.org/10.1016/j.pain.2009.11. 022]. [PMID: 20022698]. [DOI] [PubMed] [Google Scholar]
- 76.McArthur J.C., Yiannoutsos C., Simpson D.M., Adornato B.T., Singer E.J., Hollander H., Marra C., Rubin M., Cohen B.A., Tucker T., Navia B.A., Schifitto G., Katzenstein D., Rask C., Zaborski L., Smith M.E., Shriver S., Millar L., Clifford D.B., Karalnik I.J. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54(5):1080–1088. doi: 10.1212/wnl.54.5.1080. [http://dx. doi.org/10.1212/WNL.54.5.1080]. [PMID: 10720278]. [DOI] [PubMed] [Google Scholar]
- 77.Peters E.M., Raap U., Welker P., Tanaka A., Matsuda H., Pavlovic-Masnicosa S., Hendrix S., Pincelli C. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm. Metab. Res. 2007;39(2):110–124. doi: 10.1055/s-2007-961812. [http://dx.doi.org/ 10.1055/s-2007-961812]. [PMID: 17326007]. [DOI] [PubMed] [Google Scholar]
- 78.Palazzo E., Marconi A., Truzzi F., Dallaglio K., Petrachi T., Humbert P., Schnebert S., Perrier E., Dumas M., Pincelli C. Role of neurotrophins on dermal fibroblast survival and differentiation. J. Cell. Physiol. 2012;227(3):1017–1025. doi: 10.1002/jcp.22811. [http://dx.doi.org/ 10.1002/jcp.22811]. [PMID: 21503896]. [DOI] [PubMed] [Google Scholar]
- 79.Aich A., Afrin L.B., Gupta K. Mast cell-mediated mechanisms of nociception. Int. J. Mol. Sci. 2015;16(12):29069–29092. doi: 10.3390/ijms161226151. [http://dx.doi.org/10.3390/ijms161226151]. [PMID: 26690128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonini S., Aloe L., Bonini S., Rama P., Lamagna A., Lambiase A. Nerve growth factor (NGF): an important molecule for trophism and healing of the ocular surface . Adv Exp Med Biol. 2002;506 (Pt A ): 531 –7. doi: 10.1007/978-1-4615-0717-8_75. [DOI] [PubMed] [Google Scholar]
- 81.Lambiase A., Rama P., Bonini S., Caprioglio G., Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998;338(17):1174–1180. doi: 10.1056/NEJM199804233381702. [http://dx.doi. org/10.1056/NEJM199804233381702]. [PMID: 9554857]. [DOI] [PubMed] [Google Scholar]
- 82.Di Girolamo N., Sarris M., Chui J., Cheema H., Coroneo M.T., Wakefield D. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J. Cell. Mol. Med. 2008;12(6B):2799–2811. doi: 10.1111/j.1582-4934.2008.00290.x. [http://dx.doi.org/10.1111/j.1582-4934. 2008.00290.x]. [PMID: 19210757]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lambiase A., Bonini S., Manni L., Ghinelli E., Tirassa P., Rama P., Aloe L. Intraocular production and release of nerve growth factor after iridectomy. Invest. Ophthalmol. Vis. Sci. 2002;43(7):2334–2340. [PMID: 12091435]. [PubMed] [Google Scholar]
- 84.Lee H.K., Lee K.S., Kim H.C., Lee S.H., Kim E.K. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am. J. Ophthalmol. 2005;139(6):965–971. doi: 10.1016/j.ajo.2004.12.051. [http://dx.doi.org/10.1016/j.ajo.2004.12.051]. [PMID: 15953424]. [DOI] [PubMed] [Google Scholar]
- 85.Ghinelli E., Aloe L., Cortes M., Micera A., Lambiase A., Bonini S. Nerve growth factor (NGF) and lenses: effects of NGF in an in vitro rat model of cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241(10):845–851. doi: 10.1007/s00417-003-0733-6. [http://dx.doi.org/10.1007/s00417-003-0733-6]. [PMID: 13680251]. [DOI] [PubMed] [Google Scholar]
- 86.Muzi S., Colafrancesco V., Sornelli F., Mantelli F., Lambiase A., Aloe L. Nerve growth factor in the developing and adult lacrimal glands of rat with and without inherited retinitis pigmentosa. Cornea. 2010;29(10):1163–1168. doi: 10.1097/ICO.0b013e3181d3d3f9. [http://dx.doi.org/10.1097/ICO. 0b013e3181d3d3f9]. [PMID: 20595895]. [DOI] [PubMed] [Google Scholar]
- 87.Lambiase A., Mantelli F., Sacchetti M., Rossi S., Aloe L., Bonini S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011;149(2):283–292. doi: 10.4449/aib.v149i2.1363. [PMID: 21702001]. [DOI] [PubMed] [Google Scholar]
- 88.Lambiase A., Tirassa P., Micera A., Aloe L., Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest. Ophthalmol. Vis. Sci. 2005;46(10):3800–3806. doi: 10.1167/iovs.05-0301. [http://dx.doi.org/10.1167/iovs.05-0301]. [PMID: 16186366]. [DOI] [PubMed] [Google Scholar]
- 89.Lambiase A., Bonini S., Micera A., Rama P., Bonini S., Aloe L. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest. Ophthalmol. Vis. Sci. 1998;39(7):1272–1275. [PMID: 9620090]. [PubMed] [Google Scholar]
- 90.Amendola T., Fiore M., Aloe L. Postnatal changes in nerve growth factor and brain derived neurotrophic factor levels in the retina, visual cortex, and geniculate nucleus in rats with retinitis pigmentosa. Neurosci. Lett. 2003;345(1):37–40. doi: 10.1016/s0304-3940(03)00491-9. [http://dx. doi.org/10.1016/S0304-3940(03)00491-9]. [PMID: 12809983]. [DOI] [PubMed] [Google Scholar]
- 91.Pizzorusso T., Fagiolini M., Gianfranceschi L., Porciatti V., Maffei L. Role of neurotrophins in the development and plasticity of the visual system: experiments on dark rearing. Int. J. Psychophysiol. 2000;35(2-3):189–196. doi: 10.1016/s0167-8760(99)00053-7. [http://dx.doi.org/10.1016/S0167-8760(99)00053-7]. [PMID: 10677647]. [DOI] [PubMed] [Google Scholar]
- 92.Micera A., Lambiase A., Aloe L., Bonini S., Levi-Schaffer F., Bonini S. Nerve growth factor involvement in the visual system: implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev. 2004;15(6):411–417. doi: 10.1016/j.cytogfr.2004.09.003. [http://dx.doi.org/ 10.1016/j.cytogfr.2004.09.003]. [PMID: 15561599]. [DOI] [PubMed] [Google Scholar]
- 93.Carmignoto G., Maffei L., Candeo P., Canella R., Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J. Neurosci. 1989;9(4):1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [http://dx.doi.org/10.1523/JNEUROSCI.09-04-01263.1989]. [PMID: 2467970]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambiase A., Manni L., Bonini S., Rama P., Micera A., Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Vis. Sci. 2000;41(5):1063–1069. [PMID: 10752942]. [PubMed] [Google Scholar]
- 95.Bonini S., Lambiase A., Rama P., Caprioglio G., Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–1351. doi: 10.1016/s0161-6420(00)00163-9. [http://dx.doi.org/ 10.1016/S0161-6420(00)00163-9]. [PMID: 10889110]. [DOI] [PubMed] [Google Scholar]
- 96.Lambiase A., Manni L., Rama P., Bonini S. Clinical application of nerve growth factor on human corneal ulcer. Arch. Ital. Biol. 2003;141(2-3):141–148. [PMID: 12825325]. [PubMed] [Google Scholar]
- 97.Lawrenson J.G. Corneal sensitivity in health and disease. Ophthalmic Physiol. Opt. 1997;17(Suppl. 1):S17–S22. doi: 10.1016/s0275-5408(96)00078-6. [http://dx.doi. org/10.1016/S0275-5408(96)00078-6]. [PMID: 9219676]. [DOI] [PubMed] [Google Scholar]
- 98.Mensher J.H. Corneal nerves. Surv. Ophthalmol. 1974;19(1):1–18. [PMID: 4140578]. [PubMed] [Google Scholar]
- 99.Coassin M., Lambiase A., Costa N., De Gregorio A., Sgrulletta R., Sacchetti M., Aloe L., Bonini S. Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243(2):151–155. doi: 10.1007/s00417-004-0955-2. [http://dx.doi.org/ 10.1007/s00417-004-0955-2]. [PMID: 15650854]. [DOI] [PubMed] [Google Scholar]
- 100.Lambiase A., Aloe L., Centofanti M., Parisi V., Báo S.N., Mantelli F., Colafrancesco V., Manni G.L., Bucci M.G., Bonini S., Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA. 2009;106(32):13469–13474. doi: 10.1073/pnas.0906678106. [http://dx.doi.org/10.1073/pnas.0906678106]. [PMID: 19805021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambiase A., Coassin M., Sposato V., Micera A., Sacchetti M., Bonini S., Aloe L. NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies. Pharmacol. Res. 2007;56(1):65–69. doi: 10.1016/j.phrs.2007.03.007. [http://dx.doi.org/10.1016/j.phrs. 2007.03.007]. [PMID: 17512750]. [DOI] [PubMed] [Google Scholar]
- 102.Lambiase A., Mantelli F., Bonini S. Nerve growth factor eye drops to treat glaucoma. Drug News Perspect. 2010;23(6):361–367. doi: 10.1358/dnp.2010.23.6.1472299. [PMID: 20697603]. [DOI] [PubMed] [Google Scholar]
- 103.Colafrancesco V., Parisi V., Sposato V., Rossi S., Russo M.A., Coassin M., Lambiase A., Aloe L. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J. Glaucoma. 2011;20(2):100–108. doi: 10.1097/IJG.0b013e3181d787e5. [http:// dx.doi.org/10.1097/IJG.0b013e3181d787e5]. [PMID: 20436364]. [DOI] [PubMed] [Google Scholar]
- 104.Turner J.E., Delaney R.K. Retinal ganglion cell response to axotomy and nerve growth factor antiserum in the regenerating visual system of the newt (Notophthalmus viridescens): an ultrastructural morphometric analysis. Brain Res. 1979;177(1):35–47. doi: 10.1016/0006-8993(79)90916-8. [http://dx.doi.org/10.1016/0006-8993(79)90916-8]. [PMID: 497824]. [DOI] [PubMed] [Google Scholar]
- 105.Yip H.K., Johnson E.M., Jr Retrograde transport of nerve growth factor in lesioned goldfish retinal ganglion cells. J. Neurosci. 1983;3(11):2172–2182. doi: 10.1523/JNEUROSCI.03-11-02172.1983. [http://dx.doi.org/10.1523/JNEUROSCI.03-11-02172.1983]. [PMID: 6631475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lambiase A., Aloe L. Nerve growth factor delays retinal degeneration in C3H mice. Graefes Arch. Clin. Exp. Ophthalmol. 1996;234(Suppl. 1):S96–S100. doi: 10.1007/BF02343055. [http://dx.doi.org/10.1007/BF02343055]. [PMID: 8871157]. [DOI] [PubMed] [Google Scholar]
- 107.Lenzi L., Coassin M., Lambiase A., Bonini S., Amendola T., Aloe L. Effect of exogenous administration of nerve growth factor in the retina of rats with inherited retinitis pigmentosa. Vision Res. 2005;45(12):1491–1500. doi: 10.1016/j.visres.2004.12.020. [http://dx.doi.org/10.1016/j.visres.2004. 12.020]. [PMID: 15781068]. [DOI] [PubMed] [Google Scholar]
- 108.Lambiase A., Centofanti M., Micera A., Manni G.L., Mattei E., De Gregorio A., de Feo G., Bucci M.G., Aloe L. Nerve growth factor (NGF) reduces and NGF antibody exacerbates retinal damage induced in rabbit by experimental ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235(12):780–785. doi: 10.1007/BF02332863. [http://dx. doi.org/10.1007/BF02332863]. [PMID: 9439971]. [DOI] [PubMed] [Google Scholar]
- 109.Sposato V., Parisi V., Manni L., Antonucci M.T., Di Fausto V., Sornelli F., Aloe L. Glaucoma alters the expression of NGF and NGF receptors in visual cortex and geniculate nucleus of rats: effect of eye NGF application. Vision Res. 2009;49(1):54–63. doi: 10.1016/j.visres.2008.09.024. [http://dx.doi.org/10.1016/j.visres.2008.09.024]. [PMID: 18938194]. [DOI] [PubMed] [Google Scholar]
- 110.Faktorovich E.G., Steinberg R.H., Yasumura D., Matthes M.T., LaVail M.M. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347(6288):83–86. doi: 10.1038/347083a0. [http://dx.doi.org/10.1038/347083a0]. [PMID: 2168521]. [DOI] [PubMed] [Google Scholar]
- 111.D’Cruz P.M., Yasumura D., Weir J., Matthes M.T., Abderrahim H., LaVail M.M., Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [http://dx.doi.org/10.1093/hmg/9.4.645]. [PMID: 10699188]. [DOI] [PubMed] [Google Scholar]
- 112.Strauss O., Stumpff F., Mergler S., Wienrich M., Wiederholt M. The royal college of surgeons rat: an animal model for inherited retinal degeneration with a still unknown genetic defect. Acta Anat. (Basel) 1998;162(2-3):101–111. doi: 10.1159/000046474. [http://dx.doi.org/10.1159/ 000046474]. [PMID: 9831756]. [DOI] [PubMed] [Google Scholar]
- 113.Falsini B., Iarossi G., Chiaretti A., Ruggiero A., Manni L., Galli-Resta L., Corbo G., Abed E. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J. Transl. Med. 2016;14(1):8. doi: 10.1186/s12967-015-0750-3. [http://dx.doi.org/10.1186/s12967-015-0750-3]. [PMID: 26748988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberti G., Mantelli F., Macchi I., Massaro-Giordano M., Centofanti M. Nerve growth factor modulation of retinal ganglion cell physiology. J. Cell. Physiol. 2014;229(9):1130–1133. doi: 10.1002/jcp.24573. [http://dx. doi.org/10.1002/jcp.24573]. [PMID: 24501088]. [DOI] [PubMed] [Google Scholar]
- 115.Mehta S. Age-related macular degeneration. Prim. Care. 2015;42(3):377–391. doi: 10.1016/j.pop.2015.05.009. [http://dx.doi.org/10.1016/j.pop.2015.05.009]. [PMID: 26319344]. [DOI] [PubMed] [Google Scholar]
- 116.Lambiase A., Coassin M., Tirassa P., Mantelli F., Aloe L. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report. Ann. Ist. Super. Sanita. 2009;45(4):439–442. doi: 10.1590/s0021-25712009000400014. [PMID: 20061666]. [DOI] [PubMed] [Google Scholar]
- 117.Rasool N., Odel J.G., Kazim M. Optic pathway glioma of childhood. Curr. Opin. Ophthalmol. 2017;28(3):289–295. doi: 10.1097/ICU.0000000000000370. [http://dx. doi.org/10.1097/ICU.0000000000000370]. [PMID: 28257299]. [DOI] [PubMed] [Google Scholar]
- 118.Chiaretti A., Falsini B., Servidei S., Marangoni D., Pierri F., Riccardi R. Nerve growth factor eye drop administration improves visual function in a patient with optic glioma. Neurorehabil. Neural Repair. 2011;25(4):386–390. doi: 10.1177/1545968310395601. [http://dx.doi.org/10.1177/ 1545968310395601]. [PMID: 21343523]. [DOI] [PubMed] [Google Scholar]
- 119.Falsini B., Chiaretti A., Barone G., Piccardi M., Pierri F., Colosimo C., Lazzareschi I., Ruggiero A., Parisi V., Fadda A., Balestrazzi E., Riccardi R. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil. Neural Repair. 2011;25(6):512–520. doi: 10.1177/1545968310397201. [http://dx.doi.org/10.1177/1545968310397201]. [PMID: 21444653]. [DOI] [PubMed] [Google Scholar]
- 120.Falsini B., Chiaretti A., Rizzo D., Piccardi M., Ruggiero A., Manni L., Soligo M., Dickmann A., Federici M., Salerni A., Timelli L., Guglielmi G., Lazzareschi I., Caldarelli M., Galli-Resta L., Colosimo C., Riccardi R. Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain. 2016;139(Pt 2):404–414. doi: 10.1093/brain/awv366. [http://dx. doi.org/10.1093/brain/awv366]. [PMID: 26767384]. [DOI] [PubMed] [Google Scholar]
- 121.Neeper S.A., Gómez-Pinilla F., Choi J., Cotman C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1-2):49–56. [http://dx.doi.org/10.1016/0006-8993(96)00273-9]. [PMID: 8836544]. [PubMed] [Google Scholar]
- 122.Stener-Victorin E., Lundeberg T., Waldenström U., Manni L., Aloe L., Gunnarsson S., Janson P.O. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol. Reprod. 2000;63(5):1497–1503. doi: 10.1095/biolreprod63.5.1497. [http://dx.doi.org/10.1095/biolreprod63.5. 1497]. [PMID: 11058557]. [DOI] [PubMed] [Google Scholar]
- 123.Soligo M., Nori S.L., Protto V., Florenzano F., Manni L. Acupuncture and neurotrophin modulation. Int. Rev. Neurobiol. 2013;111:91–124. doi: 10.1016/B978-0-12-411545-3.00005-5. [http://dx.doi.org/10.1016/B978-0-12-411545-3.00005-5]. [PMID: 24215919]. [DOI] [PubMed] [Google Scholar]
- 124.Andersson S., Lundeberg T. Acupuncture--from empiricism to science: functional background to acupuncture effects in pain and disease. Med. Hypotheses. 1995;45(3):271–281. doi: 10.1016/0306-9877(95)90117-5. [http://dx.doi.org/ 10.1016/0306-9877(95)90117-5]. [PMID: 8569551]. [DOI] [PubMed] [Google Scholar]
- 125.Acupuncture: National institutes of health consensus development conference statement. Dermatol. Nurs. 2000;12(2):126–133. [PMID: 11271061]. [PubMed] [Google Scholar]
- 126.Manni L., Albanesi M., Guaragna M., Barbaro Paparo S., Aloe L. Neurotrophins and acupuncture. Auton. Neurosci. 2010;157(1-2):9–17. doi: 10.1016/j.autneu.2010.03.020. [http://dx.doi.org/10.1016/j.autneu.2010.03.020]. [PMID: 20451467]. [DOI] [PubMed] [Google Scholar]
- 127.Soligo M., Piccinin S., Protto V., Gelfo F., De Stefano M.E., Florenzano F., Berretta E., Petrosini L., Nisticò R., Manni L. Recovery of hippocampal functions and modulation of muscarinic response by electroacupuncture in young diabetic rats. Sci. Rep. 2017;7(1):9077. doi: 10.1038/s41598-017-08556-z. [http://dx.doi.org/10.1038/s41598-017-08556-z]. [PMID: 28831054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pagani L., Manni L., Aloe L. Effects of electroacupuncture on retinal nerve growth factor and brain-derived neurotrophic factor expression in a rat model of retinitis pigmentosa. Brain Res. 2006;1092(1):198–206. doi: 10.1016/j.brainres.2006.03.074. [http://dx.doi.org/10.1016/j.brainres.2006.03.074]. [PMID: 16696953]. [DOI] [PubMed] [Google Scholar]
- 129.Manni L., Florenzano F., Aloe L. Electroacupuncture counteracts the development of thermal hyperalgesia and the alteration of nerve growth factor and sensory neuromodulators induced by streptozotocin in adult rats. Diabetologia. 2011;54(7):1900–1908. doi: 10.1007/s00125-011-2117-5. [http://dx.doi.org/10.1007/s00125-011-2117-5]. [PMID: 21431457]. [DOI] [PubMed] [Google Scholar]
- 130.Manni L., Aloe L., Fiore M. Changes in cognition induced by social isolation in the mouse are restored by electro-acupuncture. Physiol. Behav. 2009;98(5):537–542. doi: 10.1016/j.physbeh.2009.08.011. [http://dx.doi.org/10.1016/ j.physbeh.2009.08.011]. [PMID: 19733189]. [DOI] [PubMed] [Google Scholar]
- 131.Soligo M., Protto V., Florenzano F., Bracci-Laudiero L., De Benedetti F., Chiaretti A., Manni L. The mature/pro nerve growth factor ratio is decreased in the brain of diabetic rats: Analysis by ELISA methods. Brain Res. 2015;1624:455–468. doi: 10.1016/j.brainres.2015.08.005. [http://dx.doi. org/10.1016/j.brainres.2015.08.005]. [PMID: 26282349]. [DOI] [PubMed] [Google Scholar]
- 132.Bruno M.A., Cuello A.C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA. 2006;103(17):6735–6740. doi: 10.1073/pnas.0510645103. [http://dx.doi.org/10.1073/pnas. 0510645103]. [PMID: 16618925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Epperly T., Dunay M.A., Boice J.L. Alzheimer disease: Pharmacologic and nonpharmacologic therapies for cognitive and functional symptoms. Am. Fam. Physician. 2017;95(12):771–778. [PMID: 28671413]. [PubMed] [Google Scholar]
- 134.Fisher A., Heldman E., Gurwitz D., Haring R., Karton Y., Meshulam H., Pittel Z., Marciano D., Brandeis R., Sadot E., Barg Y., Pinkas-Kramarski R., Vogel Z., Ginzburg I., Treves T.A., Verchovsky R., Klimowsky S., Korczyn A.D. M1 agonists for the treatment of Alzheimer’s disease. Novel properties and clinical update. Ann. N. Y. Acad. Sci. 1996;777:189–196. doi: 10.1111/j.1749-6632.1996.tb34418.x. [http:// dx.doi.org/10.1111/j.1749-6632.1996.tb34418.x]. [PMID: 8624083]. [DOI] [PubMed] [Google Scholar]
- 135.Bracci Laudiero M.L., Manni L. In: NGF and Immune Regulation.Handbook of Neurotoxicity; Kostrzewa, R.M. Verlag S., editor. 2013. [Google Scholar]