Abstract
Background:
To date, no drugs have been approved for gambling disorder. Numerous publications have described the value of opioid antagonists. Indeed, the mesocorticolimbic dopaminergic pathway has been suggested as the underlying cause of reward-seeking behaviour, and it is modulated by the opioid system.
Objective:
This study aims to evaluate the relevance of opioid antagonists for treating GD.
Method:
A systematic literature review was conducted. A search of the PubMed electronic database, PsycINFO and the Cochrane Systematic Review Database without any limits was performed.
Results:
There is little information concerning the effects of opioid antagonists on GD. The total search with “nalmefene and gambling” without any limits revealed only 11 articles. The search with “naltrexone and gambling” without any limits gener-ated 47 articles. Nevertheless, the best available data support the use of opioid antagonists, particularly in individuals with a history of alcohol use disorder or strong gambling urges.
Conclusion:
Future trials are still needed. Indeed, opioid antagonists effectiveness has been investigated in only a limited number of patients, clinical trials do not reflect the heterogeneity of GD and there is little knowledge of the predictive factors of response to treatments. Moreover, differential affinity to nalmefene for kappa receptors may be associated with a particular effect in a yet to be defined addiction phenotype. Head to head comparisons between naltrexone and nalmefene would be helpful in combining other medication or psychotherapy. The identification of subgroups of patients that are more likely to benefit from opioid antagonists should be a goal
Keywords: Nalmefene, naltrexone, gambling disorder, opioid antagonists, pathological gambling, addictive disorders
1. INTRODUCTION
1.1. Gambling Disorder, the First Described Behavioural Addiction, Shares Clinical and Neurobiological Patterns with SUD
Gambling disorder (GD), the new term for pathological gambling (PG), is now classified in the DSM-5 as a behavioural addiction. It is characterized by persistent and recurrent problematic gambling behaviour, leading to clinically significant impairment or distress [1]. Negative consequences include a high rate of suicide attempts, job loss, marital and family difficulties, legal problems and criminal behaviour.
The similarities between GD and substance use disorders (SUD) [2] led to a reclassification of GD together with SUD in the new section in the DSM-5 titled “substance-related and addictive disorders”. Some diagnostic criteria are common between SUD and GD, such as tolerance, repeated failures to stop the behaviour (or consumption), withdrawal and loss of control. Numerous recent studies have evaluated the underlying neurobiological basis of GD and highlighted its similarities with SUD [3, 4]. The potential for the mesocorticolimbic pathway to mediate reward, learning and salience has been reported across different addictions [5]. Indeed, this “reward system” refers to a group of structures that are activated by rewarding or reinforcing stimuli (e.g. gambling or using addictive drugs). Gambling (like using drugs) triggers the release of dopamine, which produces a feeling of well-being. Dopamine is purported to reinforce the rewarding nature of behaviours or drugs of abuse by signalling for the forthcoming reward [9]. Dopamine dysregulation has been implicated in addiction, as well as the provocative syndrome in Parkinson’s disease where medications acting on dopamine receptors lead to the emergence of impulse control disorders, including GD. Neuroimaging studies have shown reward-related deficits in both GD and SUD [6-8]. Similar clinical traits have also been highlighted, such as a heightened impulsivity. Impulsivity has been associated with opioid system disruptions [3].
Dopaminergic pathways are under the modulation of the opioid system. Opioid enhance the release of dopamine in the nucleus accumbens and the ventral pallidum through disinhibiting GABA input into the dopamine neurons of the ventral tegmental area. Dopamine function within these regions has been implicated in the subjective experience of pleasure and urges. Opioids are well known to play an essential role; the mu opioid receptors (MOR), the delta opioid receptors (DOR) and the kappa opioid receptors (KOR) are heterogeneously distributed into the brain. Opioid receptors, especially the MOR, are present in the majority of structures associated with the mesolimbic and mesocortical pathways (which together constitute the major pathway of the reward system) [9].
MOR are expressed in all areas of the brain associated with circuits of addiction [9]. In the ventral tegmental area, MOR are found on the GABA inhibitory neurons that inhibit the mesolimbic dopaminergic neurons that project in the nucleus accumbens. Ultimately, MOR activation disinhibits dopamine neurons through GABA neurons inhibition. DOR are less extensively located (compared with MOR and KOR) in the brain. Their role in the addiction process is more debate [9]. They are thought to be implicated in a degree of tolerance following opioid exposure. KOR seem to have an important role in addiction due to their high expression in mesolimbic and mesocortical regions; activation of the KOR has been implicated in the negative reinforcement aspects of numerous drug addiction [10, 11]. They are thought to act in an opposing manner to that of the MOR. Activation of KOR leads to negative emotional-like states: KOR agonists are dysphoric [12]. KOR antagonists produce antidepressant-like effects [12] and reverse anxiety-like behaviour associated with drug withdrawal in animals [12]. It had been proved that in certain addictions, like cocaine use disorder, there is a considerable increase in KOR in the nucleus accumbens [13].
Nutt highlighted the complexity between the opioid and dopamine systems and their relationship to impulsivity in alcohol use disorders [9]. The effects of opiate antagonist on impulsivity may be differentiated by dopamine levels, with MOR antagonist administration resulting in increased impulsivity in high dopamine individuals, and decreased impulsivity in low dopamine individuals. Moreover, it seems that activation of the MOR increase motor impulsivity and DOR decrease motor impulsivity. Antagonism of the opioid system may differentially impact dopamine levels based on the proportion of the opioid receptors. The effects of KOR antagonism vary as a function of dependence [10].
1.2. Numerous Drugs have been Tested for Treating Pathological Gambling (PG)
At present, no pharmacological treatment has been approved for GD treatment. However, the following medications have been tested:
Selective serotonin reuptake inhibitors (fluvoxamine, paroxetine, citalopram, and escitalopram) and clomipramine have been tested based on the hypothesis that serotonin dysfunction could be a potential mediator of gambling problems. Preclinical studies have demonstrated a correlation between a low level of serotonin in the central nervous system and the suppression of inhibitory responses. Clinical studies have suggested an association between impulsivity and serotoninergic dysregulation, and the serotoninergic system is thought to be involved in the aetiology of GD [14, 15].
Atypical antipsychotics have been tested (that act as dopamine antagonists), which is consistent with the implication of dopamine function in the subjective experience of pleasure and urges. They demonstrated similar reductions in gambling behaviour and gambling urges than placebo.
Mood stabilizers (lithium and carbamazepine) have been tested based on the observation that the clinical features of GD resemble those of mood disorders, particularly bipolar disorder.
Memantine, a non-competitive antagonist of NMDA glutamate receptors, is thought to reduce glutamate excitability. It is used in the treatment of Alzheimer’s disease and has demonstrated efficacy in treating alcoholism and therefore may have efficacy in treating GD as it shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling [16].
Opioid antagonists have been tested based on the hypothesis that the mesocorticolimbic dopaminergic reward system, which influences the rewarding and reinforcing behaviours involved in substance abuse, has also been implicated in GD. Opioid antagonists inhibit the release of dopamine in the nucleus accumbens and ventral pallidum through disinhibiting GABA input into the dopamine neurons of the ventral tegmental area. Opioid antagonists are thought to decrease dopamine neurotransmission in the nucleus accumbens and the motivational neurocircuitry, reducing gambling excitement and craving.
However, as described in the paragraph 1.1, a large body of evidence exists to support the complexity of the opioid receptors implication in addiction.
At this time, psychosocial approaches are the first choice of treatment for GD [17]. For pharmacological treatment options, we currently rely on off-label use of medications and have to evaluate the empirical basis for considering these medications as treatment. Opiate antagonists are currently considered as the most promising medication for treating GD, but there remains a lack of knowledge of which opiate antagonist to use, at which dose and for which patients.
1.3. Opiate Antagonists, the Most Studied Medications in GD
Evidence on the use of opioid antagonists in alcohol dependence has led to the hypothesis of their effectiveness for treating behavioural addictions [18-21]. The best available data support the use of both naltrexone and nalmefene.
Naltrexone is a mu delta kappa antagonist, and nalmefene is a mu delta antagonist and kappa partial agonist. The lack of potential hepatotoxicity for nalmefene may present a marked advantage over other opioid receptor antagonists. The effect of naltrexone (and probably nalmefene) is due to its ability to modulate the effects of the nucleus opioid neurons on the ventral tegmental area/mesolimbic dopamine circuitry, decreasing urges to engage in addictive behaviours and increasing periods of abstinence. Moreover, O’Brien et al. (2011) highlighted that long-term opioid blockade with naltrexone may affect the hedonic response, suggesting that naltrexone may lower the pleasure associated with alcohol use and gambling compared with a variety of other activities [22]. Despite the lack of data and study limitations, naltrexone and nalmefene are currently the only evidence-based pharmacological treatments for GD.
1.4. Evidence-based Efficacy, but what is the Relevance?
All articles exploring opioid antagonists in behavioural addiction, whether they are original studies or reviews, highlighted many weaknesses with regard to the evaluation or extrapolation of results, and there is a lack of studies with different designs. Indeed, psychopharmacology research is one of the most challenging areas in medicine because the degree of experimental variability is high.
In 2016, the creation of an international research network was initiated by the Nantes University Hospital Addictology department with the participation of the Nantes University Hospital Pharmacology department. These two departments are specialized and renowned for their expertise in treating GD and in neuro-psycho-pharmacology, respectively. The IGNACE (International Gambling Network for Adapted Care Elaboration) network aims to conduct stratified-medicine research for gambling disorder under naturalistic conditions. A multimodal treatment programme will include psychotherapy as a common treatment line that is supplemented or not by other innovative treatment strategies, including opioid antagonists. In this context, a review of the relevance of these drugs in GD treatment is a prerequisite. Below, a review is provided of all studies / follow up / clinical practice / case reports / reviews / meta-analyses / opinion statements related to opioid antagonist used in the treatment of GD.
2. MATERIALS AND METHODS
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [23]. A search using MEDLINE, PubMed, PsycINFO, and the Cochrane Systematic Review
Database was performed without any limits. The search terms were a combination of medical subject heading (MeSH) terms and keywords, including “gambling and nalmefene” and “gambling and naltrexone”.
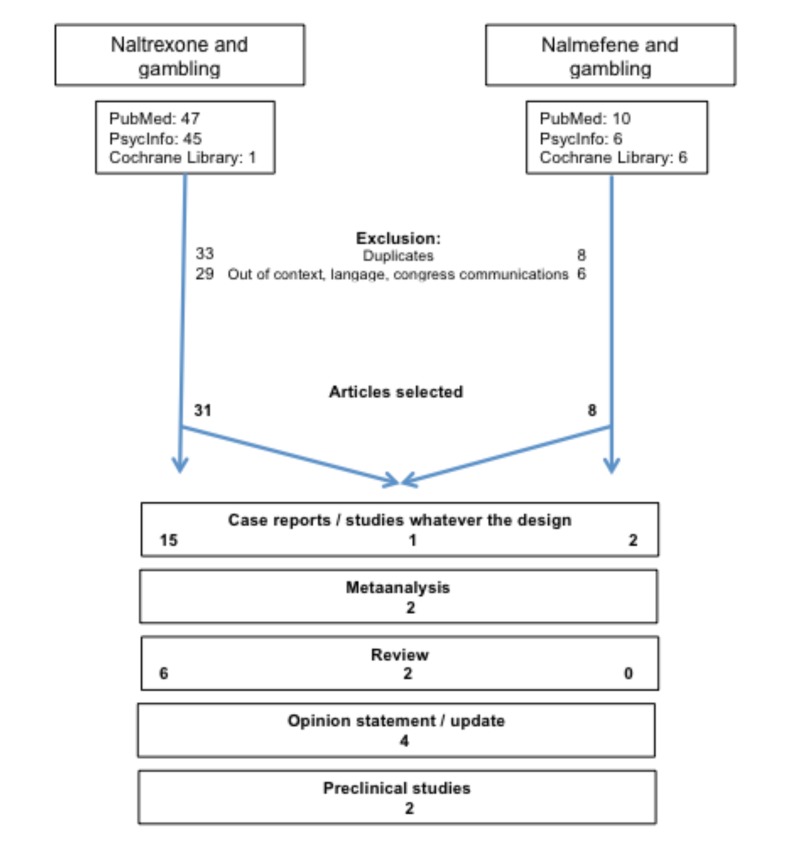
A manual search and screening of the bibliographies of selected articles was performed, in addition to the computerized search. The search strategy is summarized in Fig. (1). Extracted data included the following clinical and pharmacological considerations: the drug used, study sample size, dosage and duration, inclusion criteria, primary outcome and tolerance. The main results are presented in tables that summarize the effects of naltrexone and nalmefene on GD.
Fig. (1).
Flow chart of the search.
3. RESULTS
Thirty-four articles were considered for analysis.
3.1. Studies/Case Reports/Meta-analyses using Opiate Antagonists
The results are presented in Table 1 [20, 21, 24-40].
Table1.
GSAS (Gambling symptoms assessment scale); CGI (clinical global impression); SOGS (south Oaks Gambling Screen); PG-YBOCS (pathological gambling adaptation of Yale-Brown Obsessive compulsive scale); HDRS (Hamilton Depression Rating Scale), HARS (Hamiton Anxiety Scale); GAF (Global assessment of functioning); VAS (Visual analog scale).
| Author | Year | Drug | Type of Study | Number of Subjects |
Dosage
Length |
Primary Outcome Measure | Inclusion Criteria | Results | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crockford | 1998 | Naltrexone | Case report | Man 49 Alcohol dependence |
Naltrexone 50mg four weeks | By the end of 48h, cessation in his craving for alcohol and gambling | No relapse during 4 weeks on naltrexone | - | |||||||||||||||||||||
| Kim | 1998 | Naltrexone | Case reports | 15 patients Up to 9 months |
50mg was not effective; higher doses were required | improvement | Maintained abstinence during the nine months | Naltrexone appears to reduce urge-related symptoms | |||||||||||||||||||||
| Kim and Grant | 2001 | Naltrexone | Open label trial | 17 (7 men, 10 W) 14 completers |
Flexible doses, 6 weeks: 25mg two days, 50mg for the rest of the first week, titration 50mg/week until improvement or adverse effects (max 250mg) average dose 157mg/day |
CGI-PT; CGI-MD; G-SAS Therapeutic effect was noted at the fourth week |
>18 years old, Diagnosis of PG (DSM-IV), no other axis I diagnosis, psychotropic drugs free period of 4 weeks, SOGS>=5, HDRS<=16, HARS<16, normal complete blood count and liver function tests | Significant improvement on all the measures across all subjects (one to four week), included gambling urge strength, frequency and duration; gambling thought frequency and duration; subjective distress and amount of money lost; CGI scores diminished Adverse effects: nausea (47%) diarrhea (41%) drowsiness (38%) insomnia (38%) |
|||||||||||||||||||||
| Kim | 2001 | Naltrexone | Double blind, placebo controlled | 83 45 completers were analyzed, 20 naltrexone and 25 placebo (naltrexone 100mg/d for at least 2 weeks) |
Start 25mg/day, and titration until maximum symptom improvement or 250mg/day Mean dose 188mg/day 11 weeks |
PG-CGI-PT; PG-CGI-MD; G-SAS | >18 years old Diagnosis of PG (DSM-IV), no other axis I diagnosis and exclusion of severe personality disorders, psychotropic drugs free period of 2 weeks, SOGS>=5, HDRS<=16, HARS<16, normal complete blood count and liver function tests |
Naltrexone is significantly superior to placebo after 12 weeks according to all measures Based on GSAS: Very much improved: 55% versus 12 for placebo Much improved: 20% versus 12% Minimally improved: 10% versus 40% Nausea (45%), dry mouth (40%), vivid dreams (40%) Effectiveness was higher in subjects with more pronounced impulsivity at baseline (higher intensity of gambling urges) compared with low and moderate urges at baseline evaluation |
|||||||||||||||||||||
| Grant | 2002 | All pharmacological agents, psychotherapy possible | Real life clinical practice evaluation | 50 outpatients | Current treatments + treatment for gambling symptoms | Response to treatments | DSM-IV pathological gambling | 78% achieved response to medication treatment 90.9% for naloxone 45.5% for SSRI Patients with poorer social and occupational functioning due to urges and thoughts about gambling were less likely to respond to medication |
|||||||||||||||||||||
| Author | Year | Drug | Type of Study | Number of Subjects |
Dosage Length |
Primary Outcome Measure | Inclusion Criteria | Results | |||||||||||||||||||||
| Grant | 2004 | All pharmacological agents, psychotherapy possible | Real life clinical practice evaluation | 14 patients>60 years old | Current treatments | Much or very much improvement on the PG-CGI was defined as “responder” to pharmacotherapy | DSM-IV pathological gambling | 8/14 responders 2/4 with naltrexone 4/7 with antidepressants 2 responds with antidepressant with naloxone (among the 5 patients non responders in monotherapy) |
|||||||||||||||||||||
| Dannon | 2005 | Naltrexone Bupropion |
Open label randomized |
36 19,13c 17,12c |
25-150/day 150-450/day 12 weeks |
Full response: absence of gambling behavior for two weeks together with improvement on the clinical global impression improvement scale | SOGS at least 5, DSM IV criteria for PG and age between 18 and 65 Exclusion: comorbid diagnosis on Axes I and II, abnormal liver function tests, history of seizure disorder |
Full response: 10/13 for naltrexone and 9/12 bupropion No difference between arms No placebo controlled |
|||||||||||||||||||||
| Dannon | 2007 | Follow up after fluvoxamine, topiramate, bupropion or naltrexone | Naturalistic 12 months follow up study | 43 patients responders to one of 4 drug in previous studies | Patients maintained treatments of previous studies for a additional 3 months and then medication was discontinued | Abstinence: no gambling behavior during the month preceding the follow up visit | SOGS at least 5, DSM IV criteria for PG and age between 18 ans 65 Exclusion: comorbid diagnosis on Axes I and II, abnormal liver function tests, history of seizure disorder |
During the medication free 6 months period, relapse occurred: - 3/6 in fluvoxamine group - 3/9 in topiramate group - 7/18 in bupropion group - 4/10 in naltrexone group |
|||||||||||||||||||||
| Grant | 2008 | naltrexone | Randomized Double blind placebo controlled | 77 49 completers 36 (62.1%) in the NTX and 13 (68.4%) in the placebo group |
50-100-150mg/day 18 weeks |
PG-YBOCS, urge and behavior subscales of the PG-YBOCS, G-SAS, CGI-S | >18 years old Diagnosis of PG (DSM-IV),G-SAS>2 on item 1, SOGS>=5,gambling behavior within 2 weeks prior to enrollment Exclusion bipolar disorder I or II and substance use disorders, necessity of psychotropic medication, HAM-D or HAM-A>26 |
Naltrexone is significantly superior to placebo (greater reductions in PG-YBOCS total scores, gambling urges and gambling behaviors. Greater improvement in overall gambling severity CGI-S 23 subjects (39.7%) in naltrexone group stop gambling for one month, only 2 (10.5%) in the placebo group No difference between the various doses No differences between groups regarding adverse effects |
|||||||||||||||||||||
| Toneatto | 2009 | Naltrexone + cognitive behavioral therapy (seven sessions) | Double blind placebo controlled | 52 38 completers |
100+/-59 Mean dose 59mg/day 12 weeks |
Frequency of gambling episodes and frequency and quantity of alcohol consumption | Diagnosis criteria for alcohol use disorder (abuse, dependence) and pathological gambling Exclusion of any other psychoactive substances |
Naltrexone is not significantly superior to placebo Naltrexone +CBT and placebo +CBT both improved Majority of the sample (80% of placebo and 63% of naltrexone) presented no adverse effects The most common: nausea (14.8% for naltrexone and 4% for placebo) |
|||||||||||||||||||||
| Lahti | 2010 | Naltrexone | Open label | 39 | 50mg as needed (when craving to gamble) 16 weeks |
PG-YBOCS | DSMIV criteria and SOGS | A significant decrease in reported obsessive compulsive gambling symptoms | |||||||||||||||||||||
| Author | Year | Drug | Type of Study | Number of Subjects |
Dosage Length |
Primary Outcome Measure | Inclusion Criteria | Results | |||||||||||||||||||||
| Rosenberg | 2013 | Four different drugs: naltrexone, topiramate, bupropion, escitalopram | Randomized | 78 34 dropped out during the first 2 years and one more during the 2 years after |
2 years with additional 2 years follow up with no medications | Hamilton depression rating scale, HARS, GAS, VAS to measure general well being | DSM IV criteria for PG | Significant improvement in all groups with a predominant effectiveness for patients with naltrexone Naltrexone treated group have a significant lower dropout rate, a lower HAMD in comparison to the bupropion group, a lower HARS/escitalopram and topiramate groups, and a higher VAS scores/bupropion and topiramate groups |
|||||||||||||||||||||
| Porchet | 2013 | Naltrexone Haloperidol or placebo |
Randomized double blind placebo controlled | 62 | 50mg/day 2mg/day |
Slot machine task with concurrent psychophysiological monitoring, dosage of prolactin (marker of dopamine tone) 2.5 hours after intake | 18_49 years Past year gambling involvement and at least 5 lifetime gambling experiences Exclusion substance abuse (heavy smoking too>10cig/day), mental health problems, probable pathological gambling |
Naltrexone is functionally more active on the modulation of gambling distortions compared to both haloperidol and placebo Prolactin increase in the naltrexone group compared with placebo. Haloperidol do not differ from placebo implying that naltrexone but not haloperidol may be active at this dose This support opioid modulation during gambling like tasks but did not support that naltrexone may act to ameliorate cognitive distortions |
|||||||||||||||||||||
| Yoon | 2013 | naltrexone | Case report | Man 58 | 200mg/day followed by monthly injection 380mg | Craving to gamble | Alcohol dependence, depression and GD following pramipexole treatment for restless leg syndrome | No compliance with oral daily treatment Improvement with monthly injection |
|||||||||||||||||||||
| Kovanen | 2016 | naltrexone | Randomized double blind placebo controlled | 101 69 completers |
50mg as needed (when planning to gamble or when experiencing a strong urge to gamble) 20 weeks |
PG-YBOCS, gambling related outcome measures included thoughts/urges and behavior subscales of PG-YBOCS, RAND-36 scale of emotional well-being and social functioning | SOGS revised score of 5 or more, Age>18, DSMIV criteria for GD, ability to speak Finnish Exclusion of severe depression, bipolar disorder, suicide risk, medical conditions, use of opioid agonists or antagonists |
No difference between naltrexone and placebo groups First study to evaluate whether a polymorphism of the opioid receptor mu 1 (OPRM1 A118G) gene was implicated in moderating treatment response in naltrexone therapy for PG The rate of response did not differ between groups, although emotional well-being increased in PG patients with a AA genotype of the OPRM1 A118G polymophism |
|||||||||||||||||||||
| Author | Year | Drug | Type of Study | Number of Subjects |
Dosage Length |
Primary Outcome Measure | Inclusion Criteria | Results | |||||||||||||||||||||
| Grant | 2006 | Nalmefene | Randomized Double blind placebo controlled | 207 73 completers |
25-50-100mg/day 16 weeks |
PG-YBOCS | DSMIV criteria for PG, age>18 years Minimum score of 5 on the south oaks gambling screen, at least moderate urges to gamble within the week before entry (score >=2) on the gambling symptom assessment scale, and gambling behavior within the two weeks before enrollment Exclusion current axis I disorder (SCID), bipolar disorder or any psychotic disorder (SCID) and substance use disorders, all concomitant use of psychotropic medication |
Nalmefene was superior to placebo in illness specific and global outcome measures. Nalmefene 25 and 50mg/day improved compared to placebo in primary measure (PG-YBOCS). Treatment response: CGI improvement score of 2 (much improved) or 1 (very much improved) were considered responders: 59.2% of the subjects receiving 25mg compared to 34% of those receiving placebo Discontinuation rates were higher among 50 and 100 daily than for 25mg Adverse effects like nausea were the most reason for drop out among 50 and 100mg compared to placebo |
|||||||||||||||||||||
| Grant | 2010 | Nalmefene | Randomized Double blind placebo controlled | 233 126 completers 46/74 placebo, 44/77 nalmefene 20mg/day; 36/82 nalmefene 40mg/day |
20-40mg/day | PG-YBOCS | Minimum score of >=21 on the PG-YBOCS, minimum score of >=5 on the SDS and gambling in the month prior Exclusion Axis I disorders and individuals seeking psychotherapy |
ITT nalmefene no different from placebo; post hoc analyses (patients who received a full titration of medication for at least one week; due to the fact that patients who discontinued the trial dropped out before the 20-40 regimen was reach): Nalmefene 40mg/day is superior to placebo on the main outcome measure, particularly on the urges to gamble. Response was defined as a decrease of>35% in the PG-YBOCS | |||||||||||||||||||||
| Grant | 2008 | Nalmefene or naltrexone |
Using randomized double blind placebo controlled studies to examine predictors of medication outcome | 284 treated in one of two study grant 2006 and grant 2008 | 50-100mg/day 16 weeks 100-150mg/day 18 weeks |
PG-YBOCS with positive response defined as >=35% reduction in the score for at least one month by study endpoint | Subjects treated in one of two trials (16 weeks nalmefene or 18 weeks naltrexone (Grant 2006 and Grant 2008) |
Family history of alcoholism predict response to opiate antagonists in PG Baseline urges to gamble did not predict treatment response for the entire sample, when analyzed by medication dose, baseline urges were associated, on a trend level, with response to higher doses of opiate antagonists Younger subjects were more likely to respond to placebo First study examining predictors of medication treatment outcome |
|||||||||||||||||||||
| Pallesen | 2007 | All pharmacological agents | Meta analysis | 16 studies involving 597 subjects | Means and SD for gambling related outcome measures were compiled at 2 points in time, baseline and posttreatment | Pharmacological interventions in PG with outcomes pertaining to gambling | Pharmacological interventions were more effective than no treatment or placebo. The magnitude of effects sizes at posttreatment was lower in studies using a placebo controlled condition compared with studies without any control conditions. No differences between the 3 main classes of pharmacological interventions (antidepressants, opiate antagonists and mood stabilizers) |
||||||||||||||||||||||
| Author | Year | Drug | Type of Study | Number of Subjects |
Dosage Length |
Primary Outcome Measure | Inclusion Criteria | Results | |||||||||||||||||||||
| Bartley | 2013 | All pharmacological agents | Meta analysis | 14 trials involving 1024 participants | Fixed-effects model used to calculate the standardized mean difference of the benefit of medication (stratified by class) compared to placebo | Randomized double blind placebo controlled trials examining pharmacological treatment for PG | Benefits of antidepressants, antipsychotics and anticonvulsants were not statistically greater than placebo. In contrast, opioid antagonists were associated with a small improvement in the severity of PG symptoms Warning: the lack of ITT reporting is considered as a limitation for the interpretation of the results. This is particularly problematic given the high short term placebo response seen in most of the treatments trials |
||||||||||||||||||||||
Among the selected articles on naltrexone, 3 were case reports, 3 open label trials, 2 real-life clinical practice evaluations (one of older pathological gamblers), 1 follow up, 1 comparison between different drugs and 5 double-blind, placebo controlled studies.
Table 2 summarizes the 5 double-blind, placebo controlled studies. Among them, one study was based on effects of naltrexone on gambling behaviour in a slot machine task. The others spanned 3 to 6 months. Pathological gamblers without any comorbidities were included, except in one study; Toneatto et al. (2009) included a population of pathological gamblers with comorbid alcohol use disorder. Two studies showed differences between naltrexone and placebo, but the rate of completion was low. Toneatto et al. (2009) failed to demonstrate the superiority of naltrexone compared with placebo and used the frequency of gambling episodes as a primary outcome measure. The adverse event profile of naltrexone was the same across studies and principally included nausea.
Table 2.
Summary of the double blind placebo controlled study for naltrexone in pathological gambling.
| Study | Diagnostic Inclusion |
Exclusion Criteria
Personality Disorders and Axis I Disorders, |
Length (Weeks) | Dosage | N/completers | Efficacy/assessment | Tolerance |
|---|---|---|---|---|---|---|---|
| Kim 2001 | DSM IV | Yes | 11 | Mean 188mg/day | 83/45 | Yes CGI patient and clinician rated, G-SAS | Nausea, dry mouth, vivid dream, elevated transaminases |
| Grant 2008 | DSM IV | Yes | 18 | 50 -100- 150mg/day | 77/49 | Yes CGI PG-YBOCS No difference between doses |
No difference |
| Toneatto 2009 | DSM IV for pathological gambling and alcohol use disorder | No | 12 | Mean 59mg/day + cognitive behavioral therapy |
52/38 | No on frequency of gambling episode | Nausea |
| Porchet 2013 | 5 lifetime gambling experience | Yes | Slot machine task | 50mg/day Other group 2mg/day haloperidol |
62 | Yes on modulation of gambling distortions | |
| Kovanen 2016 | DSM IV | Yes | 20 | 50mg as needed | 101/69 | No PG-YBOCS | No difference |
The results of the studies suggested a beneficial effect of naltrexone in different designs with the same primary outcome measure as in the clinical trials. Only two randomized, placebo controlled trials were performed to test the efficacy of nalmefene in pathological gamblers without any comorbidities (Table 3). One concluded nalmefene was superior to placebo. The other did not show any efficacy in intention to treat (ITT) analysis. Post hoc analysis in patients who received a full medication titration concluded the superiority of 40 mg/day nalmefene compared to placebo.
Table 3.
Summary of the double blind placebo controlled study for nalmefene in pathological gambling.
| Study | Diagnostic Inclusion | Exclusion Criteria Personality Disorders and Axis I Disorders | Length (Weeks) | Dosage | N/completers | Efficacy/assessment | Tolerance |
|---|---|---|---|---|---|---|---|
| Grant 2006 | DSM IV | Yes | 16 | 25 – 50 – 100mg/day | 207/73 | Yes PG-YBOCS, CGI | Nausea Discontinuation rates were higher among 50 and 100mg/day |
| Grant 2010 | Diagnosis of pathological gambling | Yes | 20 – 40mg/day | 233/126 | Only in post hoc analysis (patients who received a full titration of medication for at least one week) |
Finally, Grant et al. [34] were the first to explore predictors of therapeutic success using pathological gamblers enrolled in two randomized, double-blind, placebo controlled studies with nalmefene and naltrexone. A family history of alcoholism was a predictor of response. Baseline urges to gamble did not predict treatment response for the entire sample, but when analysed according to the medication dose, baseline urges tended to be associated with response to higher doses of opiate antagonists. Younger subjects were more likely to respond to placebo.
The meta-analysis of Pallesen et al. [38], which analysed all types of pharmacological interventions in GD with outcomes pertaining to gambling, concluded that these interventions were more effective than no treatment or placebo. The magnitude of the effect sizes at posttreatment was lower in studies using a placebo controlled condition compared to in studies without any control conditions. No difference was found between the 3 main classes of pharmacological interventions (antidepressants, opiate antagonists and mood stabilizers).
Finally, a meta-analysis [35] of all randomized, double-blind, placebo controlled trials examining pharmacological treatment for GD was conducted. The authors highlighted that the benefits of antidepressants, antipsychotics and anticonvulsants were not statistically higher than that of placebo. In contrast, opioid antagonists were associated with a small improvement in the severity of GD symptoms. Nevertheless, the lack of ITT reporting is considered a limitation on the interpretation of the results. This is particularly problematic given the high short-term placebo response observed in most of the treatments trials.
3.2. Reviews
Piquet-Pessoa et al. (2016) reviewed the use of opioid antagonists in a variety of addictive-like disorders, including GD, kleptomania, hypersexual disorder, compulsive buying, food addiction and body focused behaviours (skin picking and trichotillomania) [5]. Concerning GD, they concluded that despite limitations, opioid antagonists are the only pharmacological treatment that have proved efficacy, and they highlighted the need for further studies with different designs.
In a recent review chapter on the treatment of impulse control disorders, Grant et al. (2015) noted the importance of opioid therapy for GD, but they also emphasized the limitations of the studies, including the low number of randomized, placebo controlled studies, lack of association of other drugs and/or cognitive behavioural therapy (CBT), inappropriate outcome measures, variations in the dose, absence of long-term evaluation, and exclusion of co-occurring psychiatric conditions [41]. These limitations were previously mentioned [42, 43] and are particularly problematic given that an association with other treatments (medications or CBT) and co-occurring psychiatric disorders is very common in individuals with GD, restricting the scope of application in real-life clinical use. Aboujaoude et al. (2015) recently performed a review providing an assessment of the therapeutic role of naltrexone across the addictions spectrum. Among the 39 clinical trials they found, only 2 were on GD. Despite limitations, the data showed consistency in favour of the relative efficacy and safety of naltrexone use [3].
Dowling et al. (2016) performed a review identifying possible interventions for comorbid GD and psychiatric disorders. They concluded that despite understanding the heterogeneity in GD, there is very little evidence on which to base treatment recommendations for different subpopulations of gamblers given their psychiatric comorbidities [44].
Leung et al. (2009) performed a review on pharmacological and non-pharmacological treatment of GD. They also highlighted the low number of randomized controlled studies, and they underlined that CBT, which is currently considered as the gold standard for GD treatment, failed to produce superior outcomes compared with other less costly methods, such as gamblers anonymous and brief interventions [45].
According to the review by Hollander et al. (2016), the pharmacological treatments of PG have demonstrated short-term efficacy in sub-samples of adult treatment-seeking pathological gamblers. They insisted on the frequent comorbidities with bipolar spectrum disorders, SUD and attention deficit/hyperactivity disorder and on the influence of these comorbidities on treatment response [46].
3.3. Others
3.3.1. Opinion Statement/update
Taminga et al. (2006) explained the efficacy of opioid antagonist treatment in GD by focusing on the addiction instead of on the gambling activity [47]. Yip et al. (2014) assumed that opioidergic agents may be the most effective pharmacotherapy for GD, which may not be the case in all individuals [14]. They suggested that opioid antagonists may be most effective agents for treating GD in individuals with a co-occurring SUD or with a family history of alcoholism. In an update in 2013, Bullock and Potenza described published case studies and open-label studies, which can widen the scope compared to clinical trials [48]. Bosco et al. (2012) reported cases of three Parkinson’s disease patients who developed PG after the use of dopamine agonist drugs. They were not improved after the reduction or discontinuation of their medication and displayed a poor response to serotonin reuptake inhibitors, while treatment with naltrexone achieved GD remission [49].
3.3.2. Animal Studies [50, 51]
Currently no animal models exist of GD per se, only models of gambling proneness based on individual differences between choosing advantageous and disadvantageous options in gambling-like decision-making tasks described in specific literature. In these models, rodents are in general given the choice between options that produce an immediate large reward or small reward; the large reward option has more punishments (e.g. longer delays) than the small reward option, leading thus to fewer rewards per session. Accordingly, these tasks require that animals inhibit to choose for the “tempting” immediate high reward option, because the smaller reward option produces the highest number of rewards per session. Using one of these rodent Gambling Tasks (rGT), Di Ciano and Le Foll [50] observed that some rats made fewer advantageous options than others.
In humans participating in such gambling tasks, individuals with GD often choose the tempting option more often than matched controls [52]. Thus, the right may potentially provide a good model for assessing gambling proneness. It was found that naltrexone improved performance in the rGT in the subset of rats that more often chose the tempting disadvantageous choice at baseline. In mice, no effect of naltrexone was found, but the authors of this study did not differentiate their subjects according to baseline responding [51]. Although Di Ciano and Le Foll suggested that the effect of naltrexone was not due to effects on impulsivity, the mouse study showed effects of naltrexone on impulsivity. Whether these differences are due to species differences is open for further study. Overall these rodent data underline that opioid antagonists, such as naltrexone, may be of interest for treating GD.
4. DISCUSSION
4.1. Are Opiate Antagonists Efficient in GD Treatment?
There are very few studies with a high level of proof evaluating the use of opioid antagonists in GD treatment. In their recent naltrexone clinical review on all types of addiction, Aboujaoude et al. (2016) evaluated 39 placebo controlled randomized clinical trials, but only 2 were on GD [24, 32]. If we apply the very stringent and rigorous criteria of empirically validated treatments, treatments must be demonstrated as efficacious in randomized controlled clinical trials in a minimum of two studies conducted by two independent teams; if not, the treatment should be labelled as possibly efficacious [42].
A meta-analysis provided little data to suggest the efficacy of any pharmacological treatment in GD. Nevertheless, opiate antagonists provided a small but significant benefit compared to placebo [35]. Continued research is needed to understand the real benefit of opiate antagonists for GD treatment, but conducting these trials is challenging for various reasons. One is the inadequacy of the initial approach of applying a drug already used to treat addiction in the treatment of GD. GD is hypothesized to be a “natural addiction” that is characterized by compulsive consumption of a natural reward, i.e., free of the neurotoxic effects of psychoactive substance consumption. The expected impact of opiate antagonist use for GD is supposed to be focused on the underlying addictive vulnerability rather than on the observable gambling behaviour by reducing the dopamine neurotransmission in the reward circuitry. This hypothesis is supported by the observation that GD can be induced by dopaminergic therapies, especially in the framework of Parkinson’s disease treatment [53]. Therefore, dopamine is involved in the mechanism of GD and addiction in general [47]. Opioid antagonists could thus be helpful in GD treatment, but we must consider that this effect is modest and focus on the addictive vulnerability in general instead of specifically on GD; also, the efficacy might be restricted to a sub-group of patients.
4.2. Studies have many Methodological Limitations
The first major limitation of the studies on GD concerns the inclusion criteria, which are related to the definition of PG or GD. The diagnostic criteria for GD in the DSM-5 differ from those for PG in the DSM-IV [54]; the criterion “commission of gambling related illegal acts” has been removed, and the number of criteria needed for a diagnosis of GD has been decreased to four criteria (from five). While retrospective analysis suggests that this change will have relatively little impact on the prevalence of the disorder, one could ask if the populations studied will remain constant. Moreover, heterogeneous samples of gamblers could have been included in many studies, but the type of problem gamblers being treated was not described. Indeed, pathological gamblers are different in terms of their demographics, clinical features and type of gambling [55]. An important improvement in research in this field would be to compare the relative efficiency of opioid antagonists between distinct subtypes of individuals with GD.
A second major limitation is the exclusion of patients with psychiatric comorbidities. In all studies on this topic, patients with psychiatric comorbidities have been excluded (except for one study performed on patients with alcohol addiction). In the review identifying possible interventions for comorbid problematic gambling and psychiatric disorder, we found very few studies, and there is little evidence for treating subpopulations with comorbidities. Studies on patients with comorbid conditions are necessary because comorbid patients represent the majority of GD patients in “real life”, and there are relationships between GD and comorbid symptomatology. Moreover, there is no systematic contraindication between opiate antagonists and the pharmacological treatment of comorbidities. Nevertheless, Grant conducted a follow-up study of 14 older pathological gamblers under “real life” conditions, who were treated with pharmacotherapy for comorbid problems while they received treatment for PG. He found that the percentage of patients who responded to medication appeared to reflect the pharmacological response reported in studies in which the comorbidities were excluded. Given the small sample size and evaluated subgroup (older patients), the results should be interpreted with caution.
When comorbid conditions are not assessed, the results may not be generalizable to the larger population of pathological gamblers. Of note, this population has demonstrated higher rates (ranging from two to three times) of alcoholism and other substance abuse compared to the general population [37]. Moreover, the onset of SUD appeared to generally predate the onset of the gambling problem, and alcohol may trigger excessive gambling through disinhibition, poor judgement and susceptibility to social influences. However, most of studies have excluded patients with addictive comorbidities. Toneatto et al. reported a study using naltrexone together with cognitive behavioural therapy in comorbid patients with alcohol use disorder and PG. No group differences were observed. Nevertheless, this study used behavioural measures of the target behaviour (alcohol and gambling), which are less commonly employed to assess clinical efficacy, instead of measures on the symptomatology of addiction.
A recommendation for future research would be to include samples of “real-life” patients (i.e., patients who are representative of those in clinical settings).
Another limitation is the small number of subjects; the sample sizes are often too small to avoid type II errors. Even the inclusion of 30 participants per group only provides a 50:50 chance of discovering a medium sized effect [42]. Conducting randomized controlled clinical trials on larger samples would increase the proof level of studies assessing the efficacy of opioid antagonists.
The primary outcome measures are also questionable. For most studies, the Yale-Brown Obsessive-Compulsive Scale (PG-YBOCS) [46] and Gambling Symptom Assessment Scale (G-SAS) [56] were chosen as the primary outcome measures of improvement. These instruments are both reliable and valid in assessing changes in symptoms during a drug treatment study. The PG-YBOCS was developed to measure the severity and change in the severity of PG symptoms. It is a 10-item clinician-administered questionnaire assessing gambling thoughts and urges on the one hand and gambling-related behaviour on the other hand. The G-GAS is a 12-item self-rated scale designed to assess the gambling symptom severity and change during treatment. All items ask for an average symptom based on the past 7 days. The PG-YBOCS and the G-SAS are sensitive to short-term changes in PG severity, which is expected in clinical trials. However, PG is characterized by a long-term evolution. Therefore, treatments are expected to improve the symptoms in the short-term and to significantly and durably reduce the negative consequences of GD. Using the DSM-5 diagnostic criteria as the primary outcome measure could be a suitable option, which requires assessment of the patients over a longer period. It seems that primary outcome criteria influence the reported efficacy of naltrexone. In the study using the frequency of gambling as the primary outcome measure, no difference was observed between groups [37]. Therefore, pragmatic criteria are needed in the studies, preferably in long-term studies, while standardized and valid questionnaires seem to be more adapted to shorter studies and clinical trials.
Few studies assessed gambling behaviour in sufficient detail to allow for consideration of the full range of outcomes instead of restricted outcome categories based on instruments. These variables had to be evaluated at baseline. Short-term objectives of clinical trials could be based on behavioural control or withdrawal; however, when the objective is to assess the long-term efficacy, studies have to include evaluation of maintenance of the result for several months or years. The data regarding the pharmacotherapy of PG are supported by short- or intermediate-term studies. Given the limitations of short-term medication trials for PG, studies addressing the question of whether medication treatment can lead to long-term remission of diagnostic criteria are clearly needed.
Rosenberg et al. [30] reported the longest study, which was performed over two years, using naltrexone compared to topiramate, bupropion and escitalopram. Naltrexone seemed to provide the best results, but only 48 patients completed the study, and it is difficult to say whether the improvement resulted from the psychosocial effect of abstinence from gambling, the biological effect of the medications, or a placebo effect because there was no placebo group [30]. Dannon et al. [31] described a naturalistic drug-free follow up of 6 months of medication in 43 male pathological gamblers who had been full responders to one of the 4 drug treatments in a 6-month trial of topiramate, bupropion, naltrexone or fluvoxamine [27, 57]. In this study, relapse was observed in 33 to 50% of the patients, which depended on the drug group. No significant differences in the long-term outcome among the 4 medication groups were found in this study. Due to the low number of subjects and the study design, further studies are needed to confirm these findings [31]. For future research on the efficacy of opioid antagonists or other medications for treating GD, studies should include a long-term primary outcome measure or improvement in the addiction severity, as well as secondary outcomes based on the translation of effects in everyday life (quality of life, reduction of damage, etc.).
A final limitation concerned the double-blind, placebo controlled studies in this context, where the integrity of the double-blind may have been threatened by side effects (particularly nausea). The authors did not report any assessment of whether participants were able to determine the group to which they were assigned. It is possible that the beneficial effect of opiate antagonists was mediated by attrition bias based on the participants’ perceptions of receiving the active medication. To control for this limitation, future studies should include an assessment of the patient’s perception about the group to which he or she has been assigned.
4.3. Pharmacological Aspects
Some pharmacological parameters have to be discussed:
Dose: The study by Grant on nalmefene suggested that the medication dose may be an important consideration in achieving symptom control [25]. Indeed, only participants who achieved a full titration of medication for at least one week had greater reductions in the primary outcome measure. These results must be interpreted with caution because they correspond to a post hoc analysis and not an ITT analysis. The duration of drug intake is also very different between studies and should be assessed and standardized across studies.
Compliance: Yoon et al. highlighted the importance of patient compliance in the treatment success. Monthly injections could be an interesting approach for increasing treatment adherence [58].
Safety: The most common side effect of opioid antagonists was nausea, but they also may cause dizziness, insomnia and headaches. In addition, naltrexone, not nalmefene, has been associated with dose-dependent hepatotoxicity [41,49]. Although FDA labelling includes a boxed warning about hepatocellular injury, these effects seem to be associated with long-term use or higher doses than the FDA-approved dose. However, in studies on individuals with GD, the treatment seems to be well tolerated. Kim et al. (2006) concluded that the long-term use of high doses of naltrexone was safe (no significant increase in transaminases between pre-therapy/post-therapy) in patients with impulse control disorders (otherwise healthy) who restricted their intakes of acetaminophen, aspirin and non-steroidal anti-inflammatory drugs [59].
Drug interaction: Opioid antagonists have no abuse potential, which is a significant advantage for addiction treatment. Nevertheless, it could precipitate withdrawal in patients taking prescribed opiate medications or with illicit opioid use. Given that addictive comorbidities are frequent in GD individuals, a careful SUD history is imperative prior to treatment initiation. Indeed, it would not be efficient to give opioid treatment to a patient who is already receiving an opioid antagonist.
Placebo effect: High placebo response rates are observed, particularly in short-term trials. All studies with opiate antagonists used a one week placebo lead-in phase. Conducting trials with longer placebo lead-in periods would be helpful. A high placebo response may indicate that nonspecific factors have beneficial effects [60]. In particular, asking subjects to be more aware of their behaviours may act as cognitive behavioural therapy. Moreover, the strength of the therapeutic connection between the investigator and subject is a potentially important nonspecific factor. Pallesen et al. [38] concluded that pharmacological interventions were more effective than no treatment or placebo, and the magnitude of the effect sizes at posttreatment was lower in studies using a placebo controlled condition compared to studies without any control conditions [38]. This suggests the importance of an added placebo effect in PG treatment. Together with the conclusions of the Pallesen meta-analysis, this is in favour of the importance of CBT in treating PG irrespective of the added pharmacological approach.
4.4. Nalmefene or Naltrexone?
Nalmefene is an opioid antagonist that is not associated with liver toxicity. In a study by Grant and colleagues, it seems that dropout rates were related to the dose [33]. Although optimal dosing and titration of nalmefene cannot be determined from that study, it seems that low doses and a slow titration should be applied. Nalmefene was approved in Europe in 2013, and it has demonstrated benefits in a pharmacovigilance survey in France since September 2014. In the first pharmacovigilance assessment in December 2015, the French Health Product safety agency (ANSM) reported an adverse effect profile corresponding to expectation, except for suicidal thoughts and cutaneous effects, which need to be monitored.
Nalmefene and naltrexone are two different drugs: naltrexone is a mu delta kappa antagonist, and nalmefene is a mu delta antagonist and kappa partial agonist. We highlight the complexity of the interaction between dopaminergic and opioid pathways, and the importance of the KOR. Further clinical studies are needed to explore the reality of the difference in clinical response between these two drugs in GD treatment.
4.5. Future Directions
The use of animal models should be considered for assessing GD treatment. The observation that naltrexone improved choice behaviour in the subset of rats that made fewer advantageous choices at baseline [50] suggests that the rGT could be used as a pre-clinical method for screening potential drugs on gambling proneness. Medication development and approval are difficult in the framework of GD due to the lack of animal models for GD that would allow for preclinical screening of efficacy and tolerance. Such an animal model could thus offer interesting new directions for finding novel molecules to treat GD.
The simultaneous use of more than one active drug and/or non-pharmacological approaches should be explored. Current evidence implicates multiple neurotransmitter systems in the physiopathology of GD. This evidence may underlie the necessity of using a wide range of psychopharmacological agents to treat PG. Dopamine dysregulation has been especially implicated in Parkinson’s disease, and medications acting at the dopamine receptors are linked to the emergence of PG as a side effect. Even though in a study of recreational gamblers, haloperidol had few effects on the gambling tasks, but more studies are warranted [29].
The role of erroneous thoughts – e.g., important cognitive distortions – during gambling seems to be important and should be considered during treatment. The level of distorted thinking is elevated in people with PG, which is targeted by CBT. Nevertheless, the neurobiological mechanism underlying these distortions has been studied scarcely [29, 61] and could represent a future target for pharmacological treatments. Psychosocial treatments involve multiple different options, including brief intervention and individual or group CBT. All have demonstrated benefits in treating GD, and the most widely studied approach has been CBT [41]. Evaluation of the potential action of medications on cognitive distortions may be an interesting future direction for pharmacological studies on GD.
Control groups other than placebo would be useful. Head to head comparisons between naltrexone and nalmefene could also be useful because the differential affinity of nalmefene for kappa receptors and its resulting effect on the hypothalamic-pituitary-adrenal axis may be associated with a specific effect in a yet undefined addiction phenotype. Concerning primary outcome measures, it might help to develop precise outcome measures based on detailed information collected on the frequency and patterns of gambling behaviour before, during and after treatment.
A future essential direction would be to focus on subtypes of PG rather than considering problem gamblers to be a homogeneous population. This approach could increase the power and clinical applicability of the findings. Indeed, including different types of gamblers in a study introduces excessive variance and may bias the results. Until now, studies have been designed with inclusion of heterogeneous gamblers meeting DSM criteria who do not have psychiatric or addictive comorbidities. It could be helpful to select a more representative, homogeneous sample of gamblers without excluding comorbidities because the participants would better match real life conditions. Typologies of gamblers could be based on the type of gambling (online or offline; slot machine, lotteries, betting or poker), associated psychiatric comorbidities (mood disorders, addictive disorders, anxiety disorders, etc.), personality profile (impulsivity or self-esteem), level of gambling urges, etc. These different improvements in medication studies for GD could help identify subgroups of patients with greater responses to treatment. Previous studies have tried to characterize responses in specific populations, such as older pathological gamblers [39], but the factors mediating the response to various GD treatments, including medications, are still unknown.
Finally, genetic studies could offer important new findings in identifying responsive patients. Emerging data suggest that naltrexone’s pharmacological effect could be modified by the A118G polymorphism in the opioid receptor mu1 (OPRM1) gene. A meta-analysis suggested that the G allele lowers relapse rates of heavy drinking in response to naltrexone treatment. The role of the OPRM1 polymorphism has also been suggested in PG treatment [28]. Kovanen et al. [28] found no difference between naltrexone and placebo in a double-blind study on PG, but in an exploratory analysis within a subgroup of subjects with the AA genotype of OPRM1 polymorphism, naltrexone improved participants’ emotional well-being. In accordance with these results, the AA genotype has previously been linked to beneficial treatment response in alcoholism treatment. These results remain controversial, as they are inconclusive, and they should be interpreted with caution.
CONCLUSION
A priority objective for future studies on GD treatment should be to determine predictive factors for response to different treatment strategies or combinations of treatment strategies, following the precision medicine approach. Opiate antagonists could be one of these treatment options and larger clinical trials are needed in order to prove the promising effects of earlier studies. Opiate antagonists should be considered as a single pharmacological intervention alone or in combination with other pharmacological interventions. A combination with psychotherapeutic treatment is preferred, given the higher effect sizes for behavioural interventions. New directions should involve pragmatic studies, real life conditions and cluster-specific analytical methodologies to identify groups of responding patients and to characterize their profiles. It remains challenging to improve the management of pathological gamblers.
Consent for Publication
Not applicable.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kalapatapu R.K., Sullivan M.A. Prescription use disorders in older adults. Am. J. Addict. 2010;19(6):515–522. doi: 10.1111/j.1521-0391.2010.00080.x. [http://dx.doi. org/10.1111/j.1521-0391.2010.00080.x]. [PMID: 20958847]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leeman R.F., Potenza M.N. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl.) 2012;219(2):469–490. doi: 10.1007/s00213-011-2550-7. [http://dx.doi.org/10.1007/s00213-011-2550-7]. [PMID: 22057662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aboujaoude E., Salame W.O. Natrexone: a pan-addiction treatment? CNS Drugs. 2016;30(8):719–733. doi: 10.1007/s40263-016-0373-0. [http://dx.doi.org/10. 1007/s40263-016-0373-0]. [PMID: 27401883]. [DOI] [PubMed] [Google Scholar]
- 4.de Ruiter M.B., Oosterlaan J., Veltman D.J., van den Brink W., Goudriaan A.E. Similar hypo responsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend. 2012;121(1-2):81–89. doi: 10.1016/j.drugalcdep.2011.08.010. [http://dx.doi.org/10.1016/j.drugalcdep.2011.08.010]. [PMID: 21893386]. [DOI] [PubMed] [Google Scholar]
- 5.Piquet-Pessôa M., Fontenelle L.F. Opioid antagonists in broadly defined behavioral addictions: a narrative review. Expert Opin. Pharmacother. 2016;17(6):835–844. doi: 10.1517/14656566.2016.1145660. [http://dx.doi.org/10.1517/ 14656566.2016.1145660]. [PMID: 26798982]. [DOI] [PubMed] [Google Scholar]
- 6.Meng Y.J., Deng W., Wang H.Y., Guo W.J., Li T., Lam C., Lin X. Reward pathway dysfunction in gambling disorder: A meta-analysis of functional magnetic resonance imaging studies. Behav. Brain Res. 2014;275:243–251. doi: 10.1016/j.bbr.2014.08.057. [http://dx.doi.org/10.1016/ j.bbr.2014.08.057]. [PMID: 25205368]. [DOI] [PubMed] [Google Scholar]
- 7.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [http://dx.doi.org/ 10.1038/npp.2009.110]. [PMID: 19710631]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzkin J.C., Baskin-Sommers A., Newman J.P., Kiehl K.A., Koenigs M. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum. Brain Mapp. 2014;35(9):4282–4292. doi: 10.1002/hbm.22474. [http://dx.doi. org/10.1002/hbm.22474]. [PMID: 24510765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nutt D.J. The role of the opioid system in alcohol dependence. J. Psychopharmacol. (Oxford) 2014;28(1):8–22. doi: 10.1177/0269881113504017. [http://dx.doi.org/ 10.1177/0269881113504017]. [PMID: 24048097]. [DOI] [PubMed] [Google Scholar]
- 10.Henderson-Redmond A., Czachowski C. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and long evans rats. Psychopharmacology (Berl.) 2014;231(22):4309–4321. doi: 10.1007/s00213-014-3571-9. [http://dx.doi.org/10. 1007/s00213-014-3571-9]. [PMID: 24770627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Leri F. 2016. [Google Scholar]
- 12.Schlosburg J.E., Whitfield T.W., Jr, Park P.E., Crawford E.F., George O., Vendruscolo L.F., Koob G.F. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J. Neurosci. 2013;33(49):19384–19392. doi: 10.1523/JNEUROSCI.1979-13.2013. [http:// dx.doi.org/10.1523/JNEUROSCI.1979-13.2013]. [PMID: 24305833]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosshans M., Mutschler J., Kiefer F. Treatment of cocaine craving with as-needed nalmefene, a partial κ opioid receptor agonist: first clinical experience. Int. Clin. Psychopharmacol. 2015;30(4):237–238. doi: 10.1097/YIC.0000000000000069. [http://dx.doi.org/10.1097/YIC.0000000000000069]. [PMID: 25647453]. [DOI] [PubMed] [Google Scholar]
- 14.Yip S.W., Potenza M.N. Treatment of gambling disorders. Curr. Treat. Options Psychiatry. 2014;1(2):189–203. doi: 10.1007/s40501-014-0014-5. [http://dx.doi.org/ 10.1007/s40501-014-0014-5]. [PMID: 24904757]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollander E., Buchalter A.J., DeCaria C.M. Pathological gambling. Psychiatr. Clin. North Am. 2000;23(3):629–642. doi: 10.1016/s0193-953x(05)70185-4. [http:// dx.doi.org/10.1016/S0193-953X(05)70185-4]. [PMID: 10986732]. [DOI] [PubMed] [Google Scholar]
- 16.Grant J.E., Chamberlain S.R., Odlaug B.L., Potenza M.N., Kim S.W. Memantine shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling: a pilot study. Psychopharmacology (Berl.) 2010;212(4):603–612. doi: 10.1007/s00213-010-1994-5. [http://dx.doi. org/10.1007/s00213-010-1994-5]. [PMID: 20721537]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant J.E., Odlaug B.L., Schreiber L.R. Pharmacological treatments in pathological gambling. Br. J. Clin. Pharmacol. 2014;77(2):375–381. doi: 10.1111/j.1365-2125.2012.04457.x. [http://dx.doi.org/10.1111/j.1365-2125.2012. 04457.x]. [PMID: 22979951]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modesto-Lowe V., Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp. Clin. Psychopharmacol. 2002;10(3):213–227. doi: 10.1037//1064-1297.10.3.213. [http://dx.doi.org/10.1037/1064-1297.10.3.213]. [PMID: 12233982]. [DOI] [PubMed] [Google Scholar]
- 19.Kim S.W., Grant J.E., Grosz R.L. Pathological gambling. Current status and new treatments. Minn. Med. 2002;85(7):48–50, 52. [PMID: 12152529]. [PubMed] [Google Scholar]
- 20.Kim S.W. Opioid antagonists in the treatment of impulse-control disorders. J. Clin. Psychiatry. 1998;59(4):159–164. [http://dx.doi. org/10.4088/JCP.v59n0403]. [PMID: 9590665]. [PubMed] [Google Scholar]
- 21.Crockford D.N., el-Guebaly N. Naltrexone in the treatment of pathological gambling and alcohol dependence. Can. J. Psychiatry. 1998;43(1):86. [PMID: 9494755]. [PubMed] [Google Scholar]
- 22.O’Brien C.P., Gastfriend D.R., Forman R.F., Schweizer E., Pettinati H.M. Long-term opioid blockade and hedonic response: preliminary data from two open-label extension studies with extended-release naltrexone. Am. J. Addict. 2011;20(2):106–112. doi: 10.1111/j.1521-0391.2010.00107.x. [PMID: 21314752]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [http://dx. doi.org/10.1016/j.jclinepi.2009.06.005]. [PMID: 19631508]. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.W., Grant J.E., Adson D.E., Shin Y.C. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol. Psychiatry. 2001;49(11):914–921. doi: 10.1016/s0006-3223(01)01079-4. [http:// dx.doi.org/10.1016/S0006-3223(01)01079-4]. [PMID: 11377409]. [DOI] [PubMed] [Google Scholar]
- 25.Grant J.E., Odlaug B.L., Potenza M.N., Hollander E., Kim S.W. Nalmefene in the treatment of pathological gambling: multicentre, double-blind, placebo-controlled study. Br. J. Psychiatry. 2010;197(4):330–331. doi: 10.1192/bjp.bp.110.078105. [http://dx.doi.org/10.1192/bjp.bp.110.078105]. [PMID: 20884959]. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.W., Grant J.E. An open naltrexone treatment study in pathological gambling disorder. Int. Clin. Psychopharmacol. 2001;16(5):285–289. doi: 10.1097/00004850-200109000-00006. [http://dx.doi.org/10.1097/00004850-200109000-00006]. [PMID: 11552772]. [DOI] [PubMed] [Google Scholar]
- 27.Dannon P.N., Lowengrub K., Musin E., Gonopolski Y., Kotler M. Sustained-release bupropion versus naltrexone in the treatment of pathological gambling: a preliminary blind-rater study. J. Clin. Psychopharmacol. 2005;25(6):593–596. doi: 10.1097/01.jcp.0000186867.90289.ed. [http://dx.doi.org/10. 1097/01.jcp.0000186867.90289.ed]. [PMID: 16282845]. [DOI] [PubMed] [Google Scholar]
- 28.Kovanen L., Basnet S., Castrén S., Pankakoski M., Saarikoski S.T., Partonen T., Alho H., Lahti T. A randomised, double-blind, placebo-controlled trial of as-needed Naltrexone in the treatment of pathological gambling. Eur. Addict. Res. 2016;22(2):70–79. doi: 10.1159/000435876. [http:// dx.doi.org/10.1159/000435876]. [PMID: 26339899]. [DOI] [PubMed] [Google Scholar]
- 29.Porchet R.I., Boekhoudt L., Studer B., Gandamaneni P.K., Rani N., Binnamangala S., Müller U., Clark L. Opioidergic and dopaminergic manipulation of gambling tendencies: a preliminary study in male recreational gamblers. Front. Behav. Neurosci. 2013;7:138. doi: 10.3389/fnbeh.2013.00138. [http://dx.doi.org/10.3389/fnbeh.2013.00138]. [PMID: 24109443]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg O., Dinur L.K., Dannon P.N. Four-year follow-up study of pharmacological treatment in pathological gamblers. Clin. Neuropharmacol. 2013;36(2):42–45. doi: 10.1097/WNF.0b013e31828740ea. [http://dx.doi.org/10.1097/ WNF.0b013e31828740ea]. [PMID: 23503545]. [DOI] [PubMed] [Google Scholar]
- 31.Dannon P.N., Lowengrub K., Musin E., Gonopolsky Y., Kotler M. 12-month follow-up study of drug treatment in pathological gamblers: a primary outcome study. J. Clin. Psychopharmacol. 2007;27(6):620–624. doi: 10.1097/jcp.0b013e31815a4400. [http://dx.doi.org/10.1097/jcp.0b013e31815a 4400]. [PMID: 18004130]. [DOI] [PubMed] [Google Scholar]
- 32.Grant J.E., Kim S.W., Hartman B.K. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J. Clin. Psychiatry. 2008;69(5):783–789. doi: 10.4088/jcp.v69n0511. [http://dx.doi.org/10.4088/JCP.v69n0511]. [PMID: 18384246]. [DOI] [PubMed] [Google Scholar]
- 33.Grant J.E., Potenza M.N., Hollander E., Cunningham-Williams R., Nurminen T., Smits G., Kallio A. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am. J. Psychiatry. 2006;163(2):303–312. doi: 10.1176/appi.ajp.163.2.303. [http://dx.doi. org/10.1176/appi.ajp.163.2.303]. [PMID: 16449486]. [DOI] [PubMed] [Google Scholar]
- 34.Grant J.E., Kim S.W., Hollander E., Potenza M.N. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl.) 2008;200(4):521–527. doi: 10.1007/s00213-008-1235-3. [http://dx.doi.org/10.1007/s00213-008-1235-3]. [PMID: 18581096]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartley C.A., Bloch M.H. Meta-analysis: pharmacological treatment of pathological gambling. Expert Rev. Neurother. 2013;13(8):887–894. doi: 10.1586/14737175.2013.814938. [http://dx.doi.org/10.1586/14737175.2013.814938]. [PMID: 23952195]. [DOI] [PubMed] [Google Scholar]
- 36.Lahti T., Halme J.T., Pankakoski M., Sinclair D., Alho H. Treatment of pathological gambling with naltrexone pharmacotherapy and brief intervention: a pilot study. Psychopharmacol. Bull. 2010;43(3):35–44. [PMID: 21150845]. [PubMed] [Google Scholar]
- 37.Toneatto T., Brands B., Selby P. A randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of concurrent alcohol use disorder and pathological gambling. Am. J. Addict. 2009;18(3):219–225. doi: 10.1080/10550490902787007. [http://dx.doi.org/10.1080/10550490902787007]. [PMID: 19340640]. [DOI] [PubMed] [Google Scholar]
- 38.Pallesen S., Molde H., Arnestad H.M., Laberg J.C., Skutle A., Iversen E., Støylen I.J., Kvale G., Holsten F. Outcome of pharmacological treatments of pathological gambling: a review and meta-analysis. J. Clin. Psychopharmacol. 2007;27(4):357–364. doi: 10.1097/jcp.013e3180dcc304d. [http://dx.doi.org/10.1097/jcp.013e3180dcc304d]. [PMID: 17632219]. [DOI] [PubMed] [Google Scholar]
- 39.Grant J.E., Grosz R. Pharmacotherapy outcome in older pathological gamblers: a preliminary investigation. J. Geriatr. Psychiatry Neurol. 2004;17(1):9–12. doi: 10.1177/0891988703262000. [http://dx.doi.org/10.1177/ 0891988703262000]. [PMID: 15018691]. [DOI] [PubMed] [Google Scholar]
- 40.Grant J.E., Kim S.W. Effectiveness of pharmacotherapy for pathological gambling: a chart review. Ann. Clin. Psychiatry. 2002;14(3):155–161. doi: 10.1023/a:1021186519864. [http://dx.doi.org/10.3109/10401230209147452]. [PMID: 12585565]. [DOI] [PubMed] [Google Scholar]
- 41.Grant J.E., Odlaug B.L., Potenza M.N. 2015. Treatments for gambling disorder and impulse control disorders. [Google Scholar]
- 42.Toneatto T., Ladoceur R. Treatment of pathological gambling: a critical review of the literature. Psychol. Addict. Behav. 2003;17(4):284–292. doi: 10.1037/0893-164X.17.4.284. [http://dx.doi.org/10.1037/0893-164X.17.4.284]. [PMID: 14640824]. [DOI] [PubMed] [Google Scholar]
- 43.Petry N.M., Armentano C. Prevalence, assessment, and treatment of pathological gambling: a review. Psychiatr. Serv. 1999;50(8):1021–1027. doi: 10.1176/ps.50.8.1021. [http://dx.doi.org/10.1176/ps.50.8.1021]. [PMID: 10445649]. [DOI] [PubMed] [Google Scholar]
- 44.Dowling N.A., Merkouris S.S., Lorains F.K. Interventions for comorbid problem gambling and psychiatric disorders: Advancing a developing field of research. Addict. Behav. 2016;58:21–30. doi: 10.1016/j.addbeh.2016.02.012. [http://dx.doi.org/10.1016/j.addbeh.2016.02.012]. [PMID: 26900888]. [DOI] [PubMed] [Google Scholar]
- 45.Leung K.S., Cottler L.B. Treatment of pathological gambling. Curr. Opin. Psychiatry. 2009;22(1):69–74. doi: 10.1097/YCO.0b013e32831575d9. [http://dx.doi.org/10. 1097/YCO.0b013e32831575d9]. [PMID: 19122538]. [DOI] [PubMed] [Google Scholar]
- 46.Hollander E., Sood E., Pallanti S., Baldini-Rossi N., Baker B. Pharmacological treatments of pathological gambling. J. Gambl. Stud. 2005;21(1):99–110. doi: 10.1007/s10899-004-1932-8. [http://dx.doi.org/10.1007/s10899-004-1932-8]. [PMID: 15789195]. [DOI] [PubMed] [Google Scholar]
- 47.Tamminga C.A., Nestler E.J. Pathological gambling: focusing on the addiction, not the activity. Am. J. Psychiatry. 2006;163(2):180–181. doi: 10.1176/appi.ajp.163.2.180. [http://dx.doi.org/10.1176/appi.ajp.163.2.180]. [PMID: 16449466]. [DOI] [PubMed] [Google Scholar]
- 48.Bullock S.A., Potenza M.N. Update on the pharmacological treatment of pathological gambling. Curr. Psychopharmacol. 2013;2(3):204–211. doi: 10.2174/22115560113029990008. [http://dx.doi.org/10.2174/22115560113029990008]. [PMID: 25383315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosco D., Plastino M., Colica C., Bosco F., Arianna S., Vecchio A., Galati F., Cristiano D., Consoli A., Consoli D. Opioid antagonist naltrexone for the treatment of pathological gambling in Parkinson disease. Clin. Neuropharmacol. 2012;35(3):118–120. doi: 10.1097/WNF.0b013e31824d529b. [http://dx.doi.org/10.1097/WNF.0b013e31824d529b]. [PMID: 22426027]. [DOI] [PubMed] [Google Scholar]
- 50.Di Ciano P., Le Foll B. Evaluating the impact of naltrexone on the rat gambling Task to test its predictive validity for gambling disorder. PLoS One. 2016;11(5):e0155604. doi: 10.1371/journal.pone.0155604. [http://dx.doi.org/10.1371/ journal.pone.0155604]. [PMID: 27191857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez-Roige S., Ripley T.L., Stephens D.N. Alleviating waiting impulsivity and perseverative responding by μ-opioid receptor antagonism in two inbred mouse strains. Psychopharmacology (Berl.) 2015;232(8):1483–1492. doi: 10.1007/s00213-014-3786-9. [http://dx.doi.org/10.1007/s00213-014-3786-9]. [PMID: 25381183]. [DOI] [PubMed] [Google Scholar]
- 52.van den Bos R., Koot S., de Visser L. A rodent version of the Iowa Gambling Task: 7 years of progress. Front. Psychol. 2014;5:203. doi: 10.3389/fpsyg.2014.00203. [PMID: 24672498]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grall-Bronnec M., Sauvaget A., Perrouin F., Leboucher J., Etcheverrigaray F., Challet-Bouju G., Gaboriau L., Derkinderen P., Jolliet P., Victorri-Vigneau C. Pathological gambling associated with aripiprazole or dopamine replacement therapy: Do patients share the same features? A review. J. Clin. Psychopharmacol. 2016;36(1):63–70. doi: 10.1097/JCP.0000000000000444. [http://dx.doi.org/10.1097/JCP.0000000000000444]. [PMID: 26658263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petry N.M., Blanco C., Stinchfield R., Volberg R. An empirical evaluation of proposed changes for gambling diagnosis in the DSM-5. Addiction. 2013;108(3):575–581. doi: 10.1111/j.1360-0443.2012.04087.x. [http://dx.doi.org/10. 1111/j.1360-0443.2012.04087.x]. [PMID: 22994319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant J.E., Kim S.W. Demographic and clinical features of 131 adult pathological gamblers. J. Clin. Psychiatry. 2001;62(12):957–962. doi: 10.4088/jcp.v62n1207. [http://dx.doi.org/10.4088/JCP.v62n1207]. [PMID: 11780876]. [DOI] [PubMed] [Google Scholar]
- 56.Kim S.W., Grant J.E., Potenza M.N., Blanco C., Hollander E. The Gambling Symptom Assessment Scale (G-SAS): a reliability and validity study. Psychiatry Res. 2009;166(1):76–84. doi: 10.1016/j.psychres.2007.11.008. [http:// dx.doi.org/10.1016/j.psychres.2007.11.008]. [PMID: 19200607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dannon P.N., Lowengrub K., Gonopolski Y., Musin E., Kotler M. Topiramate versus fluvoxamine in the treatment of pathological gambling: a randomized, blind-rater comparison study. Clin. Neuropharmacol. 2005;28(1):6–10. doi: 10.1097/01.wnf.0000152623.46474.07. [http://dx.doi.org/10.1097/ 01.wnf.0000152623.46474.07]. [PMID: 15711432]. [DOI] [PubMed] [Google Scholar]
- 58.Yoon G., Kim S.W. Monthly injectable naltrexone for pathological gambling. Am. J. Psychiatry. 2013;170(6):682–683. doi: 10.1176/appi.ajp.2013.12111469. [http://dx. doi.org/10.1176/appi.ajp.2013.12111469]. [PMID: 23732971]. [DOI] [PubMed] [Google Scholar]
- 59.Kim S.W., Grant J.E., Yoon G., Williams K.A., Remmel R.P. Safety of high-dose naltrexone treatment: hepatic transaminase profiles among outpatients. Clin. Neuropharmacol. 2006;29(2):77–79. doi: 10.1097/00002826-200603000-00004. [http://dx.doi.org/10.1097/00002826-200603000-00004]. [PMID: 16614539]. [DOI] [PubMed] [Google Scholar]
- 60.Grant J.E., Kim S.W., Potenza M.N., Blanco C., Ibanez A., Stevens L., Hektner J.M., Zaninelli R. Paroxetine treatment of pathological gambling: a multi-centre randomized controlled trial. Int. Clin. Psychopharmacol. 2003;18(4):243–249. doi: 10.1097/00004850-200307000-00007. [http://dx.doi. org/10.1097/00004850-200307000-00007]. [PMID: 12817159]. [DOI] [PubMed] [Google Scholar]
- 61.Clark L., Studer B., Bruss J., Tranel D., Bechara A. Damage to insula abolishes cognitive distortions during simulated gambling. Proc. Natl. Acad. Sci. USA. 2014;111(16):6098–6103. doi: 10.1073/pnas.1322295111. [http://dx. doi.org/10.1073/pnas.1322295111]. [PMID: 24711387]. [DOI] [PMC free article] [PubMed] [Google Scholar]