Abstract
Purpose
The purpose of this study was to investigate the effect of reducing diabetes-induced lysyl oxidase (LOX) overexpression on vascular cell apoptosis and blood–retinal barrier (BRB) characteristics in diabetic rats.
Methods
Nondiabetic rats, diabetic rats, and diabetic rats intravitreally (IV) injected with LOX siRNA or scrambled (scram) siRNA were used in the study. One month after the onset of diabetes, intravitreal injections were initiated at monthly intervals for up to three times. At the end of study, retinal capillary networks were isolated, stained with periodic acid-Schiff (PAS) and hematoxylin, and assessed for acellular capillaries (AC) and pericyte loss (PL). To assess vascular leakage, extravasation of FITC-dextran was evaluated in retinal capillaries after tail vein injection of FITC-dextran. Western blot analysis was performed to determine retinal LOX level and confirm LOX downregulation via LOX siRNA intravitreal injection.
Results
LOX expression was significantly upregulated in retinas of diabetic rats compared with that of nondiabetic rats. Diabetic rats injected with LOX siRNA showed a significant decrease in retinal LOX expression compared with those of diabetic rats or scram siRNA-injected rats. In diabetic retinas, AC and PL were significantly increased compared with those of nondiabetic retinas. Importantly, diabetic rats treated with LOX siRNA exhibited a significant decrease in AC and PL counts compared with those of untreated diabetic rats. Furthermore, diabetic rats treated with LOX siRNA showed significant decrease in retinal vascular permeability compared with that of untreated diabetic rats.
Conclusions
Findings suggest LOX siRNA intravitreal injection may be effective against diabetes-induced LOX overexpression in preventing apoptosis and vascular leakage associated with diabetic retinopathy.
Keywords: lysyl oxidase, apoptosis, hyperglycemia, vascular leakage
Diabetic retinopathy (DR) is the leading cause of blindness in the working age population.1,2 Currently, there is no cure for this ocular complication. Although anti-VEGF treatments are effective,3 they have a limited duration of efficacy, and pan-retinal photocoagulation has side effects that may not be effective for all DR patients.4 Therefore, the need for a robust, sustained approach to prevent vascular lesions would be extremely beneficial.
Retinal capillary basement membrane (BM) thickening had been observed long ago in diabetes5–7 and has been found to be associated with the pathogenesis of DR8–10; however, the consequences of this ultrastructural alteration in DR are only beginning to be understood. In particular, vascular BM thickening has been shown to contribute to the development of acellular capillaries (AC), pericyte loss (PL),11 and excess permeability12 during the development and progression of DR. Although it is paradoxical that thickened BMs are associated with increased retinal vascular leakage, current understanding suggests that altered cross-linking of BM components can affect the assembly and architecture of the thickened BM and thereby compromise BRB characteristics.13,14
Lysyl oxidase (LOX) is a cross-linking enzyme that plays a critical role in the development, maturation, and functionality of the vascular BM. LOX-mediated catalysis of oxidative deamination of lysyl residues from collagen and elastin to allysine is an essential step in this process.15 The aldehyde products then undergo spontaneous condensation reactions to form covalent cross-links in extracellular matrices (ECM).16 However, excess LOX-mediated cross-linking could lead to the formation of overly compact collagen fibril assemblages, leading to increased interfibrillar space and allowing excess permeability.17,18 Additionally, ECM overaccumulation and stiffening of BM could contribute to compromised BM ultrastructure and functionality.19,20
We previously demonstrated that a high glucose (HG) condition induces LOX overexpression in rat retinal endothelial cells (RRECs) and in retinas of diabetic rodents.13 Additionally, LOX activity is upregulated in RRECs grown in HG condition.13 Importantly, HG reduces AKT activation in RRECs, which could trigger apoptosis in these cells.21 Our recent observation indicated that, in diabetic mice, increased LOX levels were associated with decreased AKT activity and increased Bax and caspase-3 activity.21 Therefore, in this study, we investigated the effects of inhibiting diabetes-induced LOX overexpression in rats via intravitreal injections of LOX siRNA and determined whether this approach could prevent the development of AC, PL, and vascular permeability associated with DR.
Methods
Animals
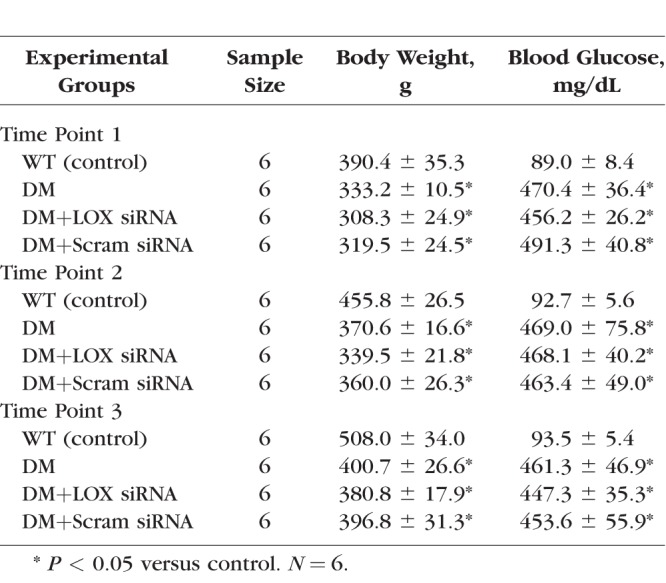
All animal studies were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. For each of the three time points, 24 Sprague-Dawley rats were assigned to four groups with six animals per group: nondiabetic (WT), diabetic (DM), diabetic intravitreally injected with LOX siRNA (DM+LOX siRNA), and diabetic intravitreally injected with scrambled siRNA (DM+Scram siRNA). Diabetes was induced by intraperitoneal injection of streptozotocin (55 mg/kg body weight), and blood glucose levels were checked 2 to 3 days after injection to confirm diabetic status of the rats (>250 mg/dL). Diabetic rats were administered neutral protamine hagedorn (NPH) insulin injections as needed to achieve slow weight gain without preventing hyperglycemia. Fasting blood glucose levels were measured two to three times weekly, and at the time of death, blood samples were collected from the animals to assess hemoglobin A1c (HbA1c; WT = 5.9 ± 0.5%, DM = 10.6 ± 1.8%, DM+LOX siRNA = 9.9 ± 0.3%, and DM+Scram siRNA = 11.1 ± 1.3%). At 1 month after the onset of diabetes, two groups of rats, DM+LOX siRNA and DM+Scram siRNA, received monthly intravitreal injections of 3 μM LOX siRNA or scram siRNA, respectively, for one, two, or three intravitreal injections. Animals that received intravitreal injections and were killed after 1 month represent time point 1. Second intravitreal injections were administered to the rest of the animals, and those killed after 1 month represent time point 2. The remaining animals received a third intravitreal injection and were killed after 1 month, and they represent time point 3. The Table shows the average body weights and blood glucose levels of all animals at the end of the study for all three time points. Intravitreal injections were performed in the pars plana region with a syringe attached to a 30-gauge needle; the volume of freshly prepared siRNA was ∼10 μL. Repeat intravitreal injections were well tolerated by the animals based on routine ocular examination. LOX siRNA used in this study specifically targeted two sequences of the mature rat LOX gene (5′-CUGAAUCAGACUACAGUA-3′ and 5′-ACAAGTACTCCGACGACAA-3′) that do not share homology with each other.
Table.
Average Measurements of Body Weight and Blood Glucose Levels in Nondiabetic Rats, Diabetic Rats, Diabetic Rats + LOX siRNA, and Diabetic Rats + Scrambled siRNA at Time of Euthanization in Time Point 1 (2 Months of Diabetes), Time Point 2 (3 Months of Diabetes), and Time Point 3 (4 Months of Diabetes)
Western Blot
To extract retinal total protein, rat retinas from all experimental groups were isolated from enucleated eyes and placed in an ice-cold buffer containing 25 mM Tris (pH 7.4), 1 mM EDTA, and 0.1% Triton X-100 (Sigma-Aldrich Corp., St. Louis, MO, USA). Retinal protein samples were then homogenized and centrifuged at 13,000g for 20 minutes at 4°C. Protein concentration for each sample was measured by bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Western blot analysis was performed with 20 μg protein per lane against protein molecular weight standards (Bio-Rad, Hercules, CA, USA) in a 10% SDS-polyacrylamide gel. After electrophoresis, the protein was transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) according to Towbin's procedure22 using a semidry apparatus. The membrane was blocked with 5% nonfat dry milk for 2 hours and then exposed to polyclonal LOX antibody (1:2000, catalog no. NB110-59729; Novus, Littleton, CO, USA) overnight at 4°C. The following day, the membrane was washed with Tris-buffered saline containing 0.1% Tween-20 (TTBS) and then incubated with AP-conjugated anti-rabbit IgG secondary antibody (1:3000, catalog no. 7054; Cell Signaling, Danvers, MA, USA) for 1 hour at room temperature. The membrane was washed again with TTBS and then exposed to Immun-star chemiluminescent substrate (Bio-Rad) to detect protein signals on X-ray film (CL-Xposure Film; Thermo Scientific, Waltham, MA, USA). The membrane was then stripped of LOX primary antibody (Restore Western Blot Stripping Buffer; Thermo Scientific) and reprobed with β-actin antibody (1:1000, catalog no. 4967; Cell Signaling) to confirm equal loading of protein in the gel lanes and to correct Western blot signals. ImageJ software (developed by W. Rasband; National Institutes of Health, Bethesda, MD ,USA) was used to analyze densitometric values of Western blot signals.
Retinal Trypsin Digestion and Staining
To analyze the retinal vasculature for AC and PL, retinal trypsin digestion (RTD) was performed as described23 with slight modifications. Enucleated eyes were briefly fixed in 10% formalin, and intact retinas were then dissected out and subjected to 3% trypsin digestion at 37°C for 2 to 3 hours with gentle shaking. Under a dissecting microscope, the nonvascular retinal mass was removed from the vascular network and then mounted on a silane-coated slide. The slide was immersed in 0.5% periodic acid (Sigma-Aldrich) for 10 minutes, rinsed in dH2O, and exposed to Schiff's reagent for 45 minutes (Electron Microscopy Sciences, Hatfield, PA, USA). After another dH2O rinse, the slide was stained with hematoxylin for 20 minutes and rinsed again with dH2O. The slide was subjected to dehydration with an ethanol gradient, cleared with xylene, and mounted in mounting medium (Permount; Fisher Scientific, Pittsburgh, PA, USA). Ten representative images of the vascular network under the 20× objective were photographed using a digital camera (Nikon DS-FI1; Nikon Instruments, Melville, NY, USA), and the images were analyzed for AC and PL based on prominent histologic characteristics. ACs, by definition, are capillaries that have lost both endothelial cells and pericytes and represent a tubular structure constituted of basement membrane. Typically, ACs become obliterated, appearing thinner with diminished capillary caliber and ultimately reaching a “thread-like” structure before complete obliteration. Pericytes, on the other hand, leave behind an empty shell of their nuclear body, which used to contain DNA and other nuclear material when they were live. These empty shells represent a telltale signature of diabetic retinopathy known as pericyte ghosts. AC and PL counts are based on these histologic criteria.
In Vivo Permeability Assay
To assess blood–retinal barrier (BRB) permeability, the in vivo permeability (IVP) assay was performed following our procedure as described with minor modifications.12 Tail vein injections at 50 mg/kg body weight of FITC-dextran (FITC-Dex) were performed in all groups. After 20 minutes, the animals were euthanized in a chamber via CO2 inhalation. Afterward, eyes were enucleated and immediately placed in 10% formalin. One retina from each animal was analyzed for vascular leakage based on FITC-dextran extravasation under fluorescence microscopy. Ten representative images were digitally captured using a 20× objective, and areas of extravasation from retinal capillaries were analyzed using ImageJ (National Institutes of Health). At the time of death, plasma samples were taken from each animal, and the plasma fluorescence intensity was used to normalize for retinal fluorescence intensity representing extravasation. The average retinal fluorescence intensity was normalized to the plasma fluorescence intensity of each animal taken at the time of death. Data are shown as mean fluorescence intensity of retinal capillaries with extravasated areas divided by plasma fluorescence intensity.
Statistical Analysis
All data are expressed as mean ± SD. Values from the wild-type groups were normalized to 100%, and values from all other groups are expressed as percentages of the wild type. Comparisons between groups were performed using 1-way ANOVA followed by Bonferroni's post hoc test. A level of P < 0.05 was considered statistically significant.
Results
Effects of LOX siRNA on Diabetes-induced LOX Protein Overexpression in Rat Retinas
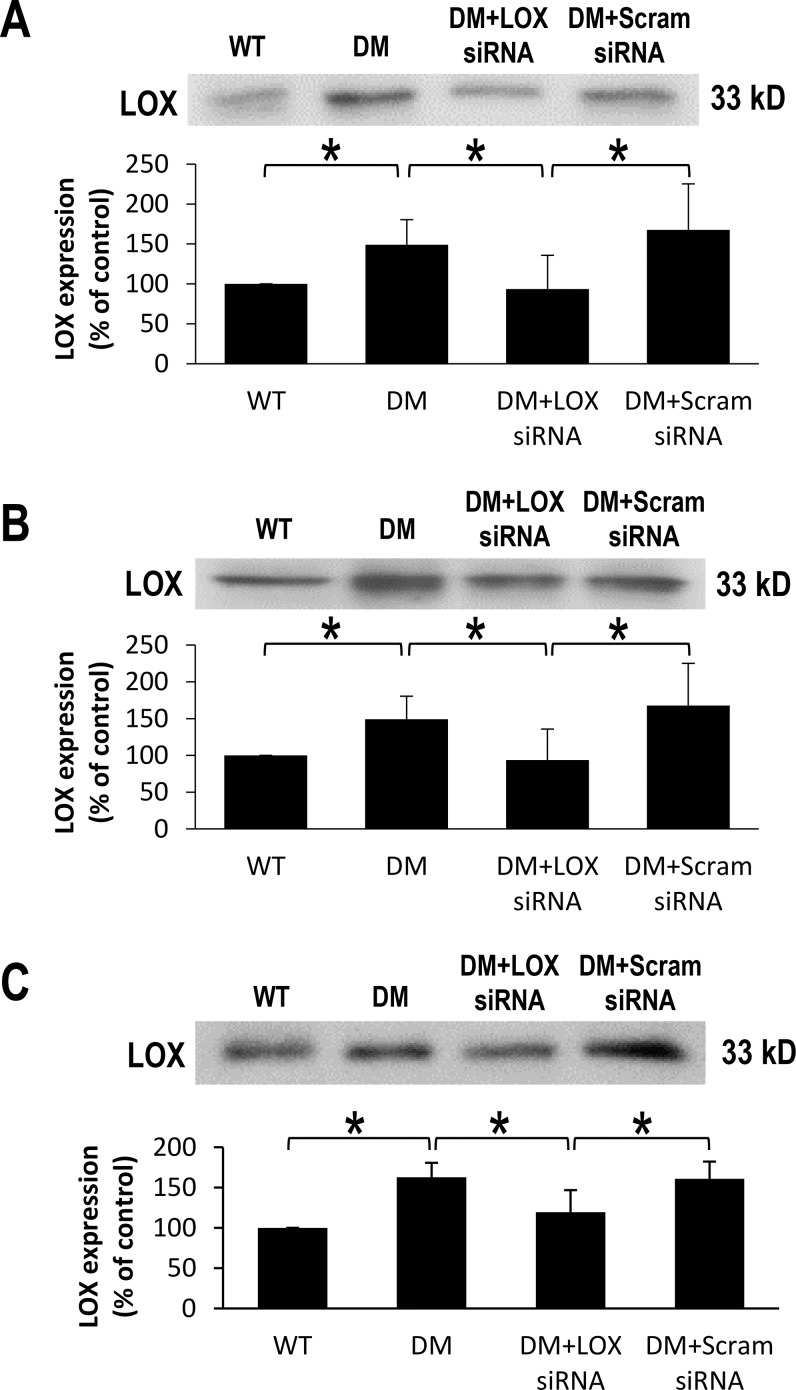
At 2, 3, and 4 months after the onset of diabetes, retinal LOX protein expression levels were significantly increased (151 ± 31% of WT, 159 ± 23% of WT, and 163 ± 18% of WT, respectively; P < 0.05; n = 6; Figs. 1A–1C) compared with those of nondiabetic rats. Importantly, LOX protein levels were significantly decreased in diabetic rat retinas that received LOX siRNA compared with those of diabetic rats across all three time points (94 ± 42% of WT, 124 ± 15% of WT, and 120 ± 27% of WT, respectively; P < 0.05; n = 6; Figs. 1A–1C). As expected, diabetic rat retinas that received scrambled siRNA did not demonstrate a significant change in LOX protein expression compared with those of untreated diabetic rats.
Figure 1.
Effects of diabetes and LOX siRNA on LOX expression in rat retinas after one, two, or three IV injections. Representative Western blot images show (A) 2, (B) 3, and (C) 4 months after the onset of diabetes significantly upregulates LOX expression. Graphical illustration of cumulative data shows that administration of LOX siRNA normalizes LOX expression in diabetic rat retinas. All data have been normalized using β-actin correction. Data are expressed as mean ± SD. *WT versus DM; DM versus DM+LOX siRNA; DM+LOX siRNA versus DM+Scram siRNA; P < 0.05; n = 6.
Effects of LOX siRNA on Acellular Capillaries and Pericyte Loss in Diabetic Rats
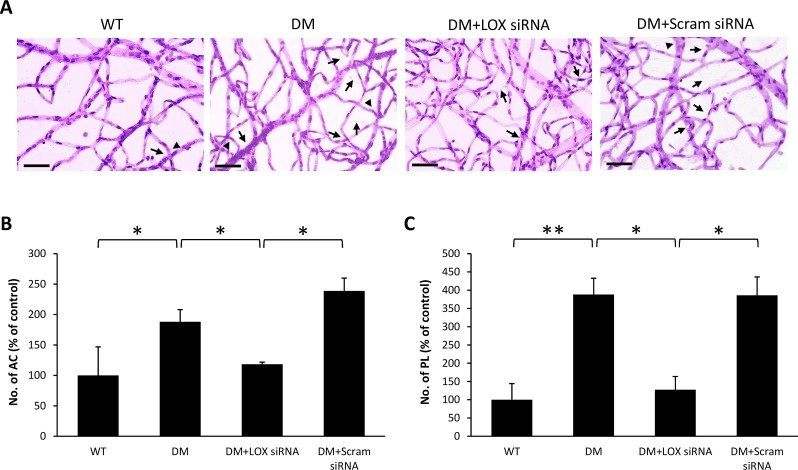
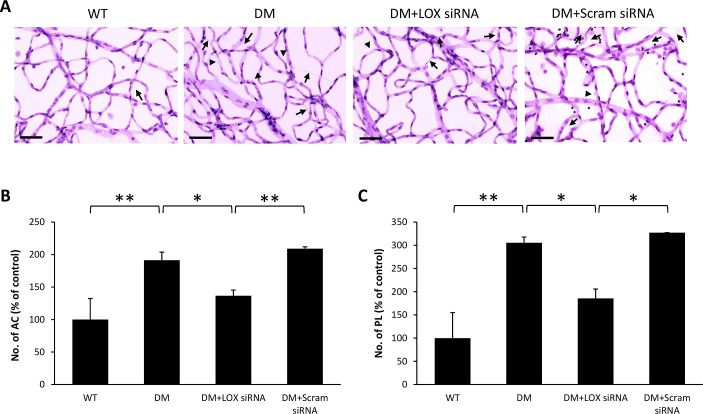
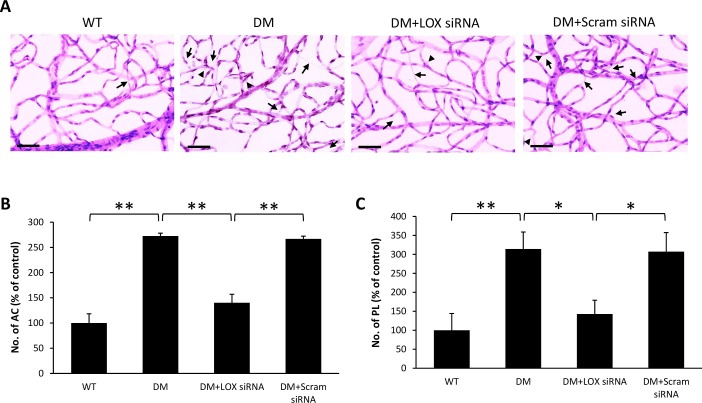
RTD analysis demonstrated a significant increase in the number of ACs (188 ± 20% of WT, 191 ± 12% of WT, and 272 ± 6% of WT, respectively; P < 0.05; n = 6; Figs. 2B, 3B, 4B) and PL (191 ± 12% of WT, 305 ± 12% of WT, and 314 ± 44% of WT, respectively; P < 0.05; n = 6; Figs. 2C, 3C, 4C) in diabetic rat retinas compared with those of nondiabetic rats across all three time points. Interestingly, retinas of diabetic rats that received LOX siRNA exhibited reduced number of ACs (118 ± 4% of WT, 136 ± 9% of WT, and 140 ± 17% of WT, respectively, P < 0.05; n = 6; Figs. 2B, 3B, 4B) and PL (187 ± 25% of WT, 185 ± 20% of WT, and 143 ± 36% of WT, respectively, P < 0.05; n = 6; Figs. 2C, 3C, 4C) compared with those of untreated diabetic rats across all three time points. Rats intravitreally injected with scrambled siRNA did not exhibit a significant difference in the number of ACs and PL compared with those of untreated diabetic rats.
Figure 2.
Effects of diabetes and LOX siRNA on AC and PL development after one IV injection. (A) Representative RTD images show the number of AC (long arrows) and PL (arrowheads) is increased in the retinas of rats with 2 months of diabetes compared with those of wild-type rats. Scale bar denotes 20 μm. Graphical illustration of cumulative data shows one IV injection of LOX siRNA significantly reduced the number of (B) AC and (C) PL. Data are expressed as mean ± SD. *P < 0.05, n = 6. **P < 0.01, n = 6.
Figure 3.
Effects of diabetes and LOX siRNA on AC and PL development after two IV injections. (A) Representative RTD images show the number of AC (long arrows) and PL (arrowheads) is increased in the retinas of rats with 3 months of diabetes compared with those of wild-type rats. Scale bar denotes 20 μm. Graphical illustration of cumulative data shows two IV injections of LOX siRNA significantly reduced the number of (B) AC and (C) PL. Data are expressed as mean ± SD. *P < 0.05, n = 6. **P < 0.01, n = 6.
Figure 4.
Effects of diabetes and LOX siRNA on AC and PL development after three IV injections. (A) Representative RTD images show the number of AC (long arrows) and PL (arrowheads) is increased in the retinas of rats with 4 months of diabetes compared with those of wild-type rats. Scale bar denotes 20 μm. Graphical illustration of cumulative data shows three IV injections of LOX siRNA significantly reduced the number of (B) AC and (C) PL. Data are expressed as mean ± SD. *P < 0.05, n = 6. **P < 0.01, n = 6.
Effects of LOX siRNA on Retinal Capillary Leakage in Diabetic Rats
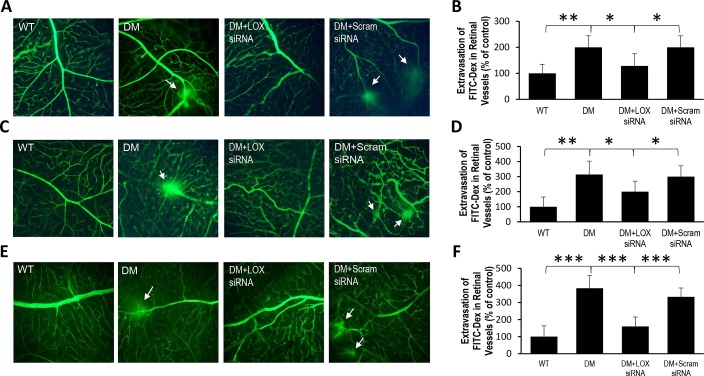
Retinal capillary networks of diabetic rats exhibited significantly increased vascular leakage compared with those of nondiabetic rats across all three time points (200 ± 44% of WT, 314 ± 89% of WT, and 383 ± 75% of WT, respectively; P < 0.01; n = 6; Figs. 5B, 5D, 5F). Importantly, vascular permeability was significantly reduced in rats intravitreally injected with LOX siRNA (128 ± 47% of WT, 200 ± 70% of WT, 166 ± 52% of WT, respectively; P < 0.05; n = 6, Figs. 5B, 5D, 5F), whereas scrambled siRNA injection yielded no significant effect.
Figure 5.
Effects of diabetes and LOX siRNA on vascular permeability after one, two, or three IV injections. (A) Representative IVP image shows vascular permeability significantly increases 2 months after the onset of diabetes. (B) Graphical illustration of cumulative data shows LOX siRNA significantly reduced vascular leakage after one IV injection. Data are expressed as mean ± SD. **WT versus DM; P < 0.01, n = 6. *DM versus DM+LOX siRNA; DM+LOX siRNA versus DM+Scram; P < 0.05, n = 6. (C) Representative IVP image shows vascular permeability significantly increases 3 months after the onset of diabetes. (D) Graphical illustration of cumulative data shows LOX siRNA significantly reduced vascular leakage after two IV injections. Data are expressed as mean ± SD. **WT versus DM; P < 0.01, n = 6. *DM versus DM+LOX siRNA; DM+LOX siRNA versus DM+Scram; P < 0.05, n = 6. (E) Representative IVP image shows vascular permeability significantly increases 4 months after the onset of diabetes. (F) Graphical illustration of cumulative data shows LOX siRNA significantly reduced vascular leakage after three IV injections. Data are expressed as mean ± SD. ***WT versus DM; DM versus DM+LOX siRNA; DM+LOX siRNA versus DM+Scram; P < 0.001, n = 6.
Discussion
The results from the present study indicate that diabetes-induced LOX overexpression is linked to the development of retinal vascular cell apoptosis and increased vascular permeability. To our knowledge, this is the first study showing downregulation of LOX level using LOX siRNA approach is sufficient to inhibit vascular cell loss and capillary leakage in the diabetic retinas. Additionally, we observed that the beneficial effect of siRNA treatment could be seen not only on a short-term basis but also with a regimen involving long-term treatment. Overall, the intravitreal LOX siRNA approach appears to be an effective strategy for modulating LOX gene overexpression and preventing vascular lesions in the diabetic rat retinas.
Although the exact mechanism underlying LOX overexpression-mediated vascular cell loss is not well understood, our previous observation suggests LOX overexpression decreases AKT phosphorylation, which can trigger apoptosis.21 In line with our observation that HG induces LOX overexpression and reduces cell proliferation,13,21 other studies have also indicated that LOX overexpression inhibits cell proliferation, promotes apoptosis,24 and reduces cell growth.25 These findings support our recent observation that decreased LOX level in the retinas of LOX+/− mice may have protective effects against diabetes-induced retinal vascular lesions.21 Taken together, these findings are only beginning to shed light into mechanistic insights involving abnormal LOX expression and its role in promoting vascular cell death in the diabetic retina.
Limited information is available regarding LOX expression in the retina in diabetic patients. Our recent work examining vitreous samples from diabetic patients with advanced diabetic retinopathy showed significant increase in LOX levels compared with those of nondiabetic individuals.26 Although it is currently unknown whether LOX expression is altered in diabetic conditions in retinal cell types other than the vascular cells, LOX was found to be abundantly expressed in the inner surface of the pigmented epithelia in the photoreceptor layer.27 A study reported that LOX was overexpressed at the mRNA level in RPECs grown under high glucose condition.28 Further studies are necessary to elucidate the effects of high glucose– or diabetes-induced LOX changes in other retinal cell types.
Previously we observed that retinal endothelial cells grown in high glucose condition overexpress LOX, which contributes, at least in part, to increased cell monolayer permeability.13 Here we show that by reducing diabetes-induced retinal LOX overexpression, excess vascular permeability can be ameliorated in the retinas of diabetic rats. When retinal vascular BM structure is compromised, it is known to contribute to vascular leakage in DR.13,20,29,30 By reducing LOX overexpression, it could improve BM structural integrity and thereby decrease vascular permeability. In line with our observation, advanced glycation endproducts, which are prominent inducers of DR pathogenesis,31–33 have been shown to upregulate LOX expression34 and induce excess cross-linking of collagen, compromise BM architecture,14 and promote increased vascular permeability.35
In addition to increasing vascular cell loss and vascular permeability, LOX overexpression has been observed to promote the synthesis of LOX pro-peptide, which in turn, influences activation of focal adhesion kinase (FAK)36 and RAS-mediated signaling pathways,37,38 leading to NF-κB inactivation to consequently promote apoptotic cell death.39,40 A recent study found that LOX overexpression induced BM stiffening, which contributed to endothelial cell activation and leukocyte accumulation in diabetic mouse retinas.19 Additionally, LOX inhibition has also been observed to prevent macrophage infiltration in inflammatory-stage lung tissue to prevent further inflammation.41
In the current study, we sought to deliver LOX siRNA through the intravitreal injection route because this is known to be the most efficient route in achieving the highest concentration and efficacy. However, siRNA delivered into the posterior chamber is known to deplete via diffusion through the BRB into the systemic circulation. Currently, this rate of depletion for siRNA is not known. Whereas intravitreally delivered LOX siRNA could ultimately find its way into the systemic circulation, the amount would likely be quite low given that only ∼400 ng is being delivered through intravitreal administration. In a clinical trial using intravitreally delivered siRNA, no systemic toxicity or inflammation was observed.42 VEGF-A siRNA delivered intravitreally localizes to the retina and distributes throughout the eye.43 Because all siRNAs are of similar sizes (21 to 23 nucleotides),44 the ocular distribution pattern after intravitreal injection is likely to be similar for LOX siRNA. Currently, there are no available data on the effects of LOX siRNA on other cell types in the eye and of systemic effects of LOX siRNA delivered via intravitreal injection.
Clinical trials have been conducted examining the effects of siRNA as a treatment modality for ocular diseases42,45–48 such as diabetic macular edema42 and AMD.48 The results of these trials have shown promise, in particular, the combined approach using ranibizumab and PF-655 siRNA, a synthetic siRNA inhibiting the expression of RTP801, a stress-inducible adaptor protein that inactivates the cell regulatory mammalian target of Rapamycin (mTOR) pathway, was found to be more effective than ranibizumab alone against neovascular AMD.48 Therefore, the beneficial effects of LOX siRNA in reducing vascular lesions in the diabetic retina could also be applicable as a supplemental approach for treatment of DR. Although long-term siRNA treatment has not yet fully been explored, in line with previous studies,49,50 findings from the current study suggest sustained LOX siRNA administration as a gene modulatory approach in retinas of diabetic tissues could be possible. Taken together, the findings suggest abnormal LOX expression is a potential therapeutic target for preventing retinal vascular lesions in DR.
Acknowledgments
This study was presented in part at the 77th Scientific Sessions of the American Diabetes Association, San Diego, California, 2017.
Supported by National Eye Institute Grant EY025528 (to SR).
Disclosure: B. Song, None; D. Kim, None; N.-H. Nguyen, None; S. Roy, None
References
- 1.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 2.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57:347–370. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28:510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank RN. Retinal laser photocoagulation: benefits and risks. Vision Res. 1980;20:1073–1081. doi: 10.1016/0042-6989(80)90044-9. [DOI] [PubMed] [Google Scholar]
- 5.Kozak WM, Marker NA, Elmer KK. Effects of aldose reductase inhibition on the retina and health indices of streptozotocin-diabetic rats. Doc Ophthalmol. 1986;64:355–377. doi: 10.1007/BF00212059. [DOI] [PubMed] [Google Scholar]
- 6.Naccarato R, Maschio G, Sirigu F, et al. The muscle in diabetes mellitus. A histologic (light and electron microscope) and biochemical study by means of needle biopsy. Virchows Arch B Cell Pathol. 1970;4:283–293. [PubMed] [Google Scholar]
- 7.Ashton N. Vascular changes in diabetes with particular reference to the retinal vessels: preliminary report. Br J Ophthalmol. 1949;33:407–420. doi: 10.1136/bjo.33.7.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy S, Cagliero E, Lorenzi M. Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci. 1996;37:258–266. [PubMed] [Google Scholar]
- 9.Roy S, Maiello M, Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest. 1994;93:438–442. doi: 10.1172/JCI116979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth T, Podesta F, Stepp MA, Boeri D, Lorenzi M. Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci U S A. 1993;90:9640–9644. doi: 10.1073/pnas.90.20.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. 2003;52:1229–1234. doi: 10.2337/diabetes.52.5.1229. [DOI] [PubMed] [Google Scholar]
- 12.Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- 13.Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59:3159–3166. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defronzo R. Diabetic nephropathy. In: Porte D, Sherwin RS, editors. Diabetes Mellitus. Stamford, CT: Appleton & Lange; 1997. pp. 971–1008. [Google Scholar]
- 15.Siegel RC, Pinnell SR, Martin GR. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970;9:4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 16.Rucker RB, Kosonen T, Clegg MS, et al. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr. 1998;67:996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- 17.Ortolan EV, Spadella CT, Caramori C, Machado JL, Gregorio EA, Rabello K. Microscopic, morphometric and ultrastructural analysis of anastomotic healing in the intestine of normal and diabetic rats. Exp Clin Endocrinol Diabetes. 2008;116:198–202. doi: 10.1055/s-2007-993147. [DOI] [PubMed] [Google Scholar]
- 18.Grant WP, Sullivan R, Sonenshine DE, et al. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36:272–278. doi: 10.1016/s1067-2516(97)80072-5. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Scott HA, Monickaraj F, et al. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2016;30:601–611. doi: 10.1096/fj.15-277962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA, Roy S. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res. 2011;36:747–753. doi: 10.3109/02713683.2011.585735. [DOI] [PubMed] [Google Scholar]
- 21.Kim D, Mecham RP, Trackman PC, Roy S. Downregulation of lysyl oxidase protects retinal endothelial cells from high glucose-induced apoptosis. Invest Ophthalmol Vis Sci. 2017;58:2725–2731. doi: 10.1167/iovs.16-21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Wang B, Xu Y. Expression of lysyl oxidase in human osteosarcoma and its clinical significance: a tumor suppressive role of LOX in human osteosarcoma cells. Int J Oncol. 2013;43:1578–1586. doi: 10.3892/ijo.2013.2067. [DOI] [PubMed] [Google Scholar]
- 25.Sung FL, Cui Y, Hui EP, et al. Silencing of hypoxia-inducible tumor suppressor lysyl oxidase gene by promoter methylation activates carbonic anhydrase IX in nasopharyngeal carcinoma. Am J Cancer Res. 2014;4:789–800. [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian M, Stein T, Ness S, Siegel N, Roy S. Increased levels of lysyl oxidase in the vitreous humor of diabetic patients with advanced diabetic retinopathy. Biennial Meeting of the International Society for Eye Research September 9–13, 2018; Belfast, Northern Ireland, UK. Poster presented at.
- 27.Hayashi K, Fong KS, Mercier F, Boyd CD, Csiszar K, Hayashi M. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004;35:845–855. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 28.Coral K, Madhavan J, Pukhraj R, Angayarkanni N. High glucose induced differential expression of lysyl oxidase and its isoform in ARPE-19 cells. Curr Eye Res. 2013;38:194–203. doi: 10.3109/02713683.2012.720341. [DOI] [PubMed] [Google Scholar]
- 29.Roy S, Kim D, Hernandez C, Simo R, Roy S. Beneficial effects of fenofibric acid on overexpression of extracellular matrix components, COX-2, and impairment of endothelial permeability associated with diabetic retinopathy. Exp Eye Res. 2015;140:124–129. doi: 10.1016/j.exer.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trudeau K, Roy S, Guo W, et al. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52:6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammes HP, Alt A, Niwa T, et al. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia. 1999;42:728–736. doi: 10.1007/s001250051221. [DOI] [PubMed] [Google Scholar]
- 32.Katagiri M, Shoji J, Inada N, Kato S, Kitano S, Uchigata Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int Ophthalmol. 2018;38:607–615. doi: 10.1007/s10792-017-0499-1. [DOI] [PubMed] [Google Scholar]
- 33.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75:95–108. doi: 10.1016/s0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 34.Adamopoulos C, Piperi C, Gargalionis AN, et al. Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-kappaB and JNK-AP-1 signaling pathways. Cell Mol Life Sci. 2016;73:1685–1698. doi: 10.1007/s00018-015-2091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd-White J, Williams JC., Jr Effect of cross-linking on matrix permeability. A model for AGE-modified basement membranes. Diabetes. 1996;45:348–353. doi: 10.2337/diab.45.3.348. [DOI] [PubMed] [Google Scholar]
- 36.Nareshkumar RN, Sulochana KN, Coral K. Inhibition of angiogenesis in endothelial cells by Human Lysyl oxidase propeptide. Sci Rep. 2018;8:10426. doi: 10.1038/s41598-018-28745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S, Zhao Y, Imai M, et al. Inhibition of CIN85-mediated invasion by a novel SH3 domain binding motif in the lysyl oxidase propeptide. PLoS One. 2013;8:e77288. doi: 10.1371/journal.pone.0077288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Min C, Vora SR, Trackman PC, Sonenshein GE, Kirsch KH. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J Biol Chem. 2009;284:1385–1393. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeay S, Pianetti S, Kagan HM, Sonenshein GE. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Molec Cell Biol. 2003;23:2251–2263. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Gu X, Ma Y, et al. Nna1 mediates Purkinje cell dendritic development via lysyl oxidase propeptide and NF-kappaB signaling. Neuron. 2010;68:45–60. doi: 10.1016/j.neuron.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng T, Liu Q, Zhang R, et al. Lysyl oxidase promotes bleomycin-induced lung fibrosis through modulating inflammation. J Mol Cell Biol. 2014;6:506–515. doi: 10.1093/jmcb/mju039. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen QD, Schachar RA, Nduaka CI, et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest Ophthalmol Vis Sci. 2012;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 43.Garba AO, Mousa SA. Bevasiranib for the treatment of wet, age-related macular degeneration. Ophthalmol Eye Dis. 2010;2:75–83. doi: 10.4137/OED.S4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser PK, Symons RC, Shah SM, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150:33–39. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Montanes J, Sadaba B, Ruz V, et al. Phase I clinical trial of SYL040012, a small interfering RNA targeting beta-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther. 2014;22:226–232. doi: 10.1038/mt.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen QD, Schachar RA, Nduaka CI, et al. Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond) 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen QD, Schachar RA, Nduaka CI, et al. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study) Ophthalmology. 2012;119:1867–1873. doi: 10.1016/j.ophtha.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 49.Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther. 2012;23:356–366. doi: 10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy S, Nasser S, Yee M, Graves DT, Roy S. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis. 2011;17:3166–3174. [PMC free article] [PubMed] [Google Scholar]