Abstract
Changes occurring in freshwater ecosystems seem to be fundamental in the development of all microorganisms, including those pathogenic to fish. This has been especially evident in recent years during which dynamic variations in bacterial fish pathology have been observed. Gram-negative bacteria commonly known to be pathogenic to fish, like Aeromonas spp., Flavobacterium spp., Pseudomonas spp., and Shewanella putrefaciens are replaced by other species, which until now have not been known to be virulent or even conditionally pathogenic to fish. Nowadays, among these other species Acinetobacter spp., Plesiomonas shigelloides, Sphingomonas paucimobilis, and Stenotrophomonas maltophilia are the most frequently isolated from fish exhibiting clinical signs of disease. Two Gram-positive bacteria have become pathogens of particular importance in fish pathology in Poland: Lactococcus garviae and Streptococcus iniae. In addition, infections caused by the Gram-positive bacterium Kocuria rhizophila have appeared in recent years. This bacterium has not been known until now to be pathogenic to fish. Therefore, this infection could be called an emergent disease.
Keywords: freshwater fish, bacterial fish diseases, Poland
Introduction
Water is an environment in which other organisms live besides fish, including many species of saprophyte bacteria inhabiting sediments and plants, as well as phyto- and zooplankton. Some of them colonise the skin, gills, and digestive tract of fish, living there as commensals, supporting digestion and having a beneficial effect on the immune system of these animals. These microorganisms could also threaten fish health and therefore are referred to as conditionally pathogenic. Interactions between fish, bacteria, and diseases are the subject of many studies conducted around the world. The great interest aroused by these topics indicates their important role in fish pathology. However, the development of disease is a complicated process, dependent not only on bacteria being capable of causing health disorders, but also on the immune status of fish, environmental conditions, and virulence of the disease agent. Therefore, changes occurring in freshwater ecosystems seem to be fundamental in the development of any disease, including emerging ones (18).
The development of a particular fish disease depends largely on the climate conditions, prevailing in a given zone, region, or country. This means that dissimilar health problems occur in fish cultured in the Mediterranean Sea, in continental Europe and in Northern European countries. For example, in Scandinavian countries, one of the biggest health problems is posed by invasion of copepods and amoebic gill disease (AGD). Meanwhile, in Central Europe, infections caused by Aeromonas spp. are the most common among bacterial fish diseases, causing motile aeromonas septicaemia (MAS), motile aeromonas infection (MAI), and furunculosis. Infections caused by Flavobacterium spp. are also very often observed (30). In Poland, the epizootic situation regarding bacterial diseases in fish does not significantly differ from those presented by other continental European countries. Nevertheless, during recent years dynamic changes in the pathology of bacterial freshwater fish diseases have been observed. Gram-negative bacteria established in fact as pathogenic to fish, like Aeromonas spp., are displaced by infections caused by other species, hitherto not known to be pathogenic or even conditionally pathogenic to ichthyic species. Fish exhibiting clinical signs of the diseases often harbour the following microorganisms: Acinetobacter spp., Kocuria spp., Plesiomonas shigelloides, Sphingomonas paucimobilis, and Stenotrophomonas maltophilia.
The aim of this article is to present new potential bacteriological agents isolated from fish, which can cause diseases in freshwater fish cultures.
Commonly known bacterial infections occurring in freshwater fish
Aeromonas infections. Almost every year health disorders in freshwater fish are recorded on many farms in Poland. The causes of the diseases may be various factors; however, the most important among bacterial infections are those caused by motile Aeromonas: A. hydrophila, A. sobria, and A. caviae. Clinical symptoms observed during health disorders vary depending on the type of disease. In cases of MAI, skin ulceration and gill and fin lesions are most frequently noticed. The disease can also become a systemic infection, and then it is called MAS. The other psychrophilic Aeromonas, Aeromonas salmonicida subsp. salmonicida, causes furunculosis in salmonids, which is manifested in ulcers on the skin. During infection, high mortalities reaching about 80% of stock are also observed (3).
Aeromonas spp. are commonly found in various environments including water, and therefore fish are constantly exposed to bacteria. Interaction with bacteria is especially dangerous under conditions of stress, which include unfavourable environmental conditions as well as human intervention in catching, sorting, and transporting of the fish. Aeromonas species are also part of the physiological microflora of the fish intestine (3).
According to the data collected during the last five years in Poland, health disorders caused by Aeromonas species were mostly observed in carp (Cyprinus carpio L.) and were usually manifested by skin lesions (MAI) in the form of ulceration (Fig. 1). Fish mortalities were also observed (26, data not published). Co-infections with other microorganisms like Pseudomonas spp., Shewanella putrefaciens, Acinetobacter spp. or Stenotrophomonas maltophilia were also found.
Fig. 1.

Skin infection with Aeromonas sobria in carp (Cyprinus carpio L.) (photo: A. Pękala-Safińska)
Pseudomonas infections. Pseudomonas spp. are widespread in the environment, forming a very large group of microorganisms. These psychrophilic bacteria develop well at low temperatures and are the dominant microflora. At higher temperatures (above 10ºC), they are quickly replaced by competing, mesophilic microorganisms, including bacteria of the genus Aeromonas.
P. fluorescens is the most important species in fish pathology and is very often associated with skin (Fig. 2) and fin disease. In some trout farms, throughout the year regardless of the water temperature, infections with P. fluorescens can cause sudden mortality, reaching even 100% of the rainbow trout population (data not published). Such a rapid disease course may be the effect of mutations, resulting in the appearance of a new property allowing the bacteria to adapt to new environmental conditions.
Fig. 2.
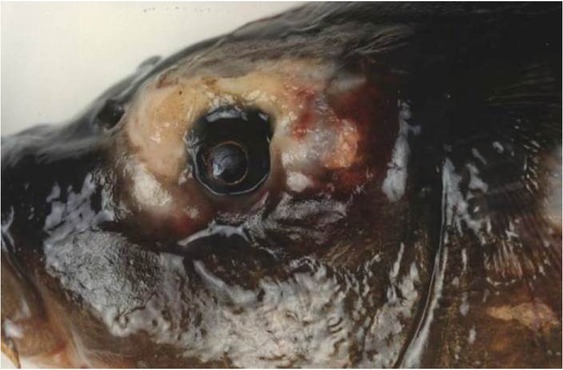
Skin infection with Pseudomonas fluorescens in carp (Cyprinus carpio L.) (photo: A. Kozińska)
Other Pseudomonas (P. putida or P. luteola) are often isolated from internal organs of fish; however, these species are mostly an accompanying microflora (23). Pseudomonas spp. can cause strawberry disease in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) (Fig. 3) and tench (Tinca tinca). Similar systemic infections with typical symptoms of septicaemia were also observed in crucian carp (Carassius carassius) and silver carp (Carassius gibelio) (1, 9, 23).
Fig. 3.
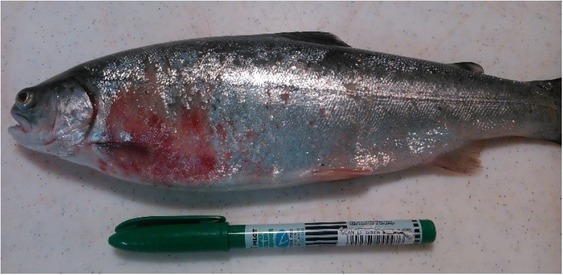
Strawberry disease/infection with Pseudomonas spp. in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) (photo: A. Kozińska, A. Pękala-Safińska)
Flavobacterium infections. Flavobacterium spp. naturally occur in the aquatic environment. These bacteria are also a part of the physiological gill microflora of healthy fish. However, the reservoirs of these bacteria as a source of potential disorders for fish have not been yet established (3). Flavobacteriosis is caused by three species of the genus Flavobacterium: F. columnare, which is the aetiological agent of columnaris disease, F. branchiophilum causing bacterial gill disease (BGD), and F. psychrophilum, which is associated with cold water disease (CWD) or rainbow trout fry syndrome (RTFS). Health disorders in cyprinids are caused by the first two species, while F. psychrophilum is mainly isolated from salmonids, although this species may also cause dangerous infections in cyprinids (28). Each of these diseases can be severe, and mortality can be 50% or even occasionally 80% of the fish population.
Relatively often during the last ten years, Flavobacterium spp. have been isolated from salmonids and cyprinids on Polish farms where clinical symptoms of the disease were observed in the fish (25) (Figs 4 and 5). The first documented outbreak of CWD in Poland was found in rainbow trout (4).
Fig. 4.

Flavobacterium infection in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) (photo: A. Kozińska, A. Pękala-Safińska)
Fig. 5.

Rainbow trout fry infected with Flavobacterium psychrophilum (photo: A. Pękala-Safińska)
Acinetobacter infections. Bacterial fish diseases are generally caused by conditionally pathogenic microorganisms. One of them is Acinetobacter spp., which is widely dispersed in nature, including the aquatic environment. In recent years in Poland, these microorganisms have been isolated from trout and carp relatively often (Fig. 6). Detection was in fish in which disease symptoms were observed in different seasons, most frequently in May and September (27). Clinically, depigmentation of the skin, loss of scales, exophthalmia with congestion of the eye, and gill petechiae were observed in infected trout. Haemorrhages in the skin and gill congestion were noted in infected carp, and post-mortem examination showed intestinal inflammation in both fish species. Disease symptoms were accompanied by mortalities, ranging from 5% to 20% (27).
Fig. 6.

Infection with Acinetobacter spp. in carp (Cyprinus carpio L.) (photo: A. Pękala-Safińska)
Infection with Acinetobacter spp. in most cases is mixed with other bacterial infection, mainly from the genus Aeromonas or Chryseobacterium spp.; however, the dominant flora in bacteriological examinations belonged to the genus Acinetobacter. It is worth noting that Acinetobacter spp. are generally regarded as carriers of antimicrobial resistance genes. Therefore, they can be very important in the spread of drug resistance in the environment (29).
Shewanella putrefaciens infections. For over 10 years, shewanelloses have been serious diseases of freshwater cultured fish. The aetiological agent, Shewanella putrefaciens, is a halophilic bacterium and very well known as an important microorganism of the food spoilage process. It mainly putrefies fish stored at low temperatures, but also poultry meat and beef products (6, 15). This bacterium has also been isolated from marine and brackish waters, as well as from marine fish (2). The first bacterium isolations from diseased freshwater fish were described by Kozińska and Pękala (24). Infection rapidly spread and health disorders were noticed among different fish species, both cultured and ornamental: common carp (Cyprinus carpio L.), rainbow trout (Oncorhynchus mykiss, Walbaum, 1792), European eel (Anguilla anguilla), brown trout (Salmo trutta m. trutta), silver carp (Hypophthalmichthys molitrix), European whitefish (Coregonus lavaretus), sander (Sander lucioperca), ide (Leuciscus idus), common roach (Rutilus rutilus), zebrafish (Brachydanio rerio), slender krib (Pelvicachromis taeniatus), least killifish (Heterandria formosa), and koi carp (Cyprinus carpio L.) (36, 37).
Health disorders in cultured fish were noted mainly in spring, when water temperature rose to 7ºC–10ºC (34). Clinical signs, prevalently lethargy, darkening of the skin, skin lesions, and ulceration, have been observed in infected fish (Fig. 7). In post-mortem examination haemorrhage in the kidneys and spleen was noticed. The range of mortality varied from 40% to 85% in different fish species (34, 36).
Fig. 7.

Shewanella putrefaciens infection in carp (Cyprinus carpio L.) (photo: A. Kozińska, A. Pękala-Safińska)
Fish infection with Gram-positive bacteria. In recent years, the number of infections caused by Gram-positive bacteria, especially in rainbow trout, has increased significantly in Poland (26). Among many species of Gram-positive microorganisms, two of them are of particular importance in the pathology of bacterial fish diseases: Lactococcus garviae and Streptococcus iniae. Both bacterial species cause serious health disorders in different species of freshwater and marine fish: rainbow trout and other salmonids from the Oncorhynchus family, eels, and fish belonging to the Ictaluridae (catfish) and Cichlidae (tilapia) families (3).
The source of bacterial infection for fish can be both water and sediments (32). Although bacteria might be present in the environment and also in fish throughout the year, their related disorders occur during summer when the water temperature increases and reaches optimal values in the range of 18ºC–25ºC. Therefore, it is assumed that the temperature as well as the sanitary state of the aquatic environment are the most important factors conducive to the occurrence of disease symptoms (3).
The clinical symptoms of L. garviae and Str. iniae infections are usually similar and vary only slightly, depending on the fish species. In both infections, a typical clinical sign is exophthalmia. Furthermore, darkening of the skin, anal oedema, petechiae in the eyes, on the gill covers, and at the base of the fins are observed. Anatomopathological examination showed haemorrhages in the swim bladder, liver, spleen, and kidney, as well as stomach inflammation. During the process of disease development, the initial phase of infection is very characteristic. Then, fish exhibit nervous whirling rapid movements from the bottom to the surface or in the opposite direction, resulting from meningitis or encephalitis (12, 13).
In Poland, streptococcosis was not diagnosed until 2010, when cases of salmonids infections were described for the first time by Grawiński (16). Since then, a few outbreaks of streptococcosis have been observed in salmonid farms each year, always during summer (26, data not published).
Emerging potential pathogens of freshwater fish
Serious new bacterial infections of freshwater fish have been observed for the last few years in Poland. These disorders can be described as emerging diseases. In order to define a disease as emerging, it must be possible to show that the disease or infection has appeared in a population for the first time, or may have existed previously, but is rapidly increasing in incidence or geographic range, or manifests itself in a new way (31). An example of such an emerging disease could be shewanellosis, a disease which appeared for the first time in 2004, causing serious health disorders in carp and rainbow trout (24). Then it spread rapidly to different fish species in different countries (34, 36, 37).
Considering these criteria, a few new infections or pathogens can be distinguished which potentially threaten fish health. Among Gram-negative bacteria isolated from both healthy and diseased fish, Plesiomonas shigelloides and Stenotrophomonas maltophilia have been diagnosed very often recently in our laboratory.
Plesiomonas shigelloides infections. Plesiomonas shigelloides, a microorganism belonging to the Enterobacteriaceae family, has been considered a potential pathogenic agent for humans and animals. So far, only a few cases of health disorders observed in fish have been described in connection with isolation of P. shigelloides. Some of them inflicted high mortalities in salmonids, reaching 40% of the stock (8, 41). Among the clinical symptoms, cachexia of fish and redness of the anus were noted. Post-mortem examinations showed the presence of punctate petechiae on the peritoneum and the presence of exudative fluid in the body cavity. Bacterial examination of samples collected from diseased fish showed growth of P. shigelloides in monoculture or co-infections with Flavobacterium sp. and Aeromonas hydrophila (8, 41).
P. shigelloides was also isolated from African catfish (Heterobranchus bidorsalis), eels (Anguilla anguilla), and sturgeon (Acipenser sturio) (22). Symptoms of infection were similar to those described in rainbow trout and included reddening of the anus, petechial haemorrhages in the peritoneum, and accumulation of ascetic fluid in the peritoneal cavity. However, it is worth noting that P. shigelloides is considered a part of the physiological microflora of fish intestines (40). These data have been confirmed by the authors of this article during their experimental studies on sturgeons (data not published). In addition, P. shigelloides is often isolated from fish showing nonspecific clinical symptoms related to fish mortality. Bacteriological examination showed diverse microflora isolated from collected samples.
Stenotrophomonas maltophilia infections. Stenotrophomonas maltophilia is another species of bacteria currently often isolated from freshwater fish. This microorganism was considered a ubiquitous environmental bacterium isolated from bottom sediments as well as freshwater and salt water (11, 19). In a terrestrial environment, this bacterium was isolated from industrial and agricultural soils (38) and plant tissues (39). S. maltophilia is also involved in the degradation of various xenobiotic compounds and therefore plays an important role in biological purification processes (10). Due to its ability to produce phytohormones, this bacterium is used as a plant growth promoter and a biological agent for combating plant pathogens (33). S. maltophilia is currently classified as a multidrug-resistant microorganism which has internal resistance to a wide range of antibacterial agents. It is mainly associated with respiratory diseases of humans (7).
Available literature indicates only a few publications regarding infections of fish caused by S. maltophilia. Among them, the most important are those describing outbreaks of the disease observed in African catfish (Heterobranchus bidorsalis) and channel catfish (Ictalurus punctatus) (14, 17). During clinical examination of the fish, in both cases lethargy, depigmentation of the skin, focal haemorrhages and petechiae, as well as oedema in the body cavity were observed. Post-mortem studies showed congestion of internal organs, petechiae on their surface, and intestines filled with gases. The mortality rate was 20% of the stock. These syndromes and health disorders were defined as infectious intussusception syndrome (IIS) (14).
Presently in Poland, S. maltophilia is very often isolated from the internal organs and skin of fish exhibiting non-specific symptoms of the disease. The health disorders described above are sometimes accompanied by fish mortality (data not published). S. maltophilia is often accompanied by numerous and diverse microflora when isolated. Therefore, the interpretation of the results of bacteriological examinations, and consequently, the determination of the impact of S. maltophilia on fish health could be difficult, and further studies are needed.
Kocuria rhizophila infections. An excellent example of an emerging pathogen for fish is Kocuria rhizophila. This is a completely new bacterium in the context of the group with proven pathogenic potential to salmonids (35).
Kocuria spp. are acknowledged skin commensals in mammals, also isolated from various environments like marine sediment, chicken meat, fresh water, or food (5, 21). These bacteria were also isolated from the trout gut where they composed the physiological microflora (20). The presence of Kocuria spp. in the internal organs of clinically healthy Baltic cod (Ghadus morhua) was confirmed by the author's own research (data not published).
During infections with Kocuria rhizophila, abnormal mortality (approximately 50% of the stock) was accompanied by pathological changes in external tissues and internal organs. Clinically, exophthalmia, swollen abdomen, increased skin melanisation as well as skin petechiae and focal lesions were often seen in moribund fish (Fig. 8). Post-mortem examinations showed inflammation of the intestine, liver congestion, and haemorrhages in the tail muscles, mainly in the caudal part (35). On account of the presented data, as well as the lack of information in available literature regarding the health impact of Kocuria spp. on fish, it should be assumed that this microorganism is conditionally pathogenic to fish.
Fig. 8.

Exophthalmia observed in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) during infection with Kocuria rhizophila (photo: A. Pękala-Safińska)
The appearance of a new species of microorganism or differentiation of extant bacteria is a natural consequence of the variability in a given ecosystem, affected by many factors. However, the appearance of new microorganisms in the environment is a natural consequence of its variability. The question remains open how these microorganisms will affect animals’ and people’s health in the future and in which direction their further development will go.
Footnotes
Conflict of Interests Statement: The author declares that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This work was financed by Statutory acivity of the National Veterinary Research Institute in Pulawy.
Animal Rights Statement: None required.
References
- 1.Ahne W., Popp W., Hoffman R.. Pseudomonas fluorescens as a pathogen of tench Tinca tinca. Bull Eur Ass Fish Pathol. 1982;4:56–57. [Google Scholar]
- 2.Al-Harbi A.H., Naim Uddin M.. Bacterial diversity of tilapia Oreochromis niloticus cultured in brackish water in Saudi Arabia. Aquaculture. 2005;250:566–572. [Google Scholar]
- 3.Austin B., Austin D.A. Bacterial fish pathogens. Disease of farmed and wild fish, 6th ed. Springer International Publishing Switzerland; 2016. [Google Scholar]
- 4.Bernad A., Terech-Majewska E., Siwicki A.K. Siwicki A.K., Szweda W. Ochrona zdrowia ryb – aktualne problemy. IRS, Olsztyn; 2004. Choroby zimnej wody – przypadek w jednym z gospodarstw rybackich na terenie województwa warmińsko-mazurskiego; p. 168. Edited by. [Google Scholar]
- 5.Becker K., Rutsch F., Uekotter A., Kipp F., Konig J., Marquardt T., Peters G., von Eiff C.. Kocuria rhizophila adds to the emerging spectrum of micrococcal species involved in human infections. J Clin Microbiol. 2008;46:3537–3539. doi: 10.1128/JCM.00823-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borch E., Kant-Muermans M.L., Blixt Y.. Bacterial spoilage of meat and cured meat products. Int Food Microbiol. 1996;33:103–120. doi: 10.1016/0168-1605(96)01135-x. [DOI] [PubMed] [Google Scholar]
- 7.Brooke J.S.. Stenotrophomonas maltophilia an emerging global opportunistic pathogen. Clin Microbiol. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz J.M., Saraiva A., Eiras J.C., Branco R., Sousa J.C.. An outbreak of Plesiomonas shigelloides in farmed rainbow trout, Salmo gairdneri Richardson, in Portugal. Bull Eur Ass Fish Pathol. 1986;6:20–22. [Google Scholar]
- 9.Csaba G.Y., Prigli M., Békési L., Kováacs-Gayer E., Bajmócy E., Fazekas B. Oláh J., Molnár K., Jeney S. Fish pathogens and environment in European polyculture. Fisheries Research Institute; Szarvas: 1981. Septicemia in silver carp Hypophthalmichthys molitrix Val.) and bighead Aristichthys nobilis Rich.) caused by Pseudomonas fluorescens; pp. 111–123. edited by. [Google Scholar]
- 10.Dubey K.K., Fulekar M.H.. Chlorpyrifos bioremediation in Pennisetum rhizosphere by a novel potential degrader Stenotrophomonas maltophilia. MHF ENV20. World J Microbiol Biotechnol. 2012;28:1715–1725. doi: 10.1007/s11274-011-0982-1. [DOI] [PubMed] [Google Scholar]
- 11.Dungan R.S., Yates S.R., Frankenberger Jr W.T.. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ Microbiol. 2003;5:287–95. doi: 10.1046/j.1462-2920.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 12.Eldar A., Bejerano Y., Bercovier H.. Streptococcus shiloi and Streptococcus difficile two new streptococcal species causing a meningoencephalitis in fish. Curr Microbiol. 1994;28:139–143. [Google Scholar]
- 13.Eldar A., Ghittino C.. Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss similar, but different diseases. Dis Aquat Organ. 1999;36:227–231. doi: 10.3354/dao036227. [DOI] [PubMed] [Google Scholar]
- 14.Geng Y., Wang K., Chen D., Huang X., He M., Yin Z.. Stenotrophomonas maltophila an emerging opportunist pathogen for cultured channel catfish, Ictalurus punctatus in China. Aquaculture. 2010;308:132–135. [Google Scholar]
- 15.Gennari M., Campanini R.. Isolation and characterization of Shewanella putrefaciens from fresh and spoiled fish, fresh and spoiled meat, dairy products, water and soil. Industrial Alimentaria. 1991;30:965–976. [Google Scholar]
- 16.Grawiński E.. Less notorious diseases of salmonids in Northern Poland territory. Życie Wet. 2010;85:522–528. [Google Scholar]
- 17.Jawahar A.T., Pradipta P., Adikesavalu H., Avijit P., Sayani B. Stenotrophomonas maltophila as an opportunistic pathogen in cultured African catfish Clarias gariepinus. Aquaculture 2016. Vol. 450. Burchell; 1822. pp. 168–172. [Google Scholar]
- 18.Johnson P.J., Paull S.H.. The ecology and emergence of diseases in fresh waters. Freshwater Biol. 2011;56:638–657. [Google Scholar]
- 19.Juhnke M.E., des Jardin E.. Selective medium for isolation of Xanthomonas maltophilia from soil and rhizosphere environments. Appl Environ Microbiol. 1989;55:747–750. doi: 10.1128/aem.55.3.747-750.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D.H., Brunt J., Austin B.. Microbial diversity of intestinal contents and mucus in rainbow trout Oncorhynchus mykiss. J Appl Microbiol. 2007;102:1654–1664. doi: 10.1111/j.1365-2672.2006.03185.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.B., Nedashkovskaya O.I., Mikhailov V.V., Han S.K., Kim K.O., Rhee M.S., Bae K.S.. Kocuria marina sp. nov., a novel actinobacterium isolated from marine sediment. Int J Syst Evol Micr. 2004;54:1617–1620. doi: 10.1099/ijs.0.02742-0. [DOI] [PubMed] [Google Scholar]
- 22.Klein B.U., Kleingeld D.W., Bohm K.H.. First isolation of Plesiomonas shigelloides from samples of cultured fish in Germany. Bull Eur Ass Fish Pathol. 1993;13:70–72. [Google Scholar]
- 23.Kozińska A.. Atypical cases of disorders in cyprinid wintering caused by Pseudomonas fluorescens infections. Bull Eur Ass Fish Pathol. 1999;19:216–220. [Google Scholar]
- 24.Kozińska A., Pękala A.. First isolation of Shewanella putrefaciens from freshwater fish – a potential new pathogen of the fish. Bull Eur Ass Fish Pathol. 2004;24:199–203. [Google Scholar]
- 25.Kozińska A., Pękala A.. Various cases of flavobacteriosis in trout and carp cultured in Poland. Med Weter. 2007;63:858–863. [Google Scholar]
- 26.Kozińska A., Pękala A.. Występowanie infekcyjnych i inwazyjnych chorób ryb w Polsce w świetle najnowszych badań. Edited by Kozińska A., Pękala A., PIWet-PIB, Pulawy. 2013 [Google Scholar]
- 27.Kozińska A., Paździor E., Pękala A., Niemczuk W.. Acinetobacter johnsonii and Acinetobacter lwoffii – the emerging fish pathogens. Bull Vet Inst Pulawy. 2014;58:193–199. [Google Scholar]
- 28.Lehmann J., Mock D., Stürenberg F.J., Bernardet J.F.. First isolation of Cytophaga psychrophila from a systemic disease in ell and cyprinids. Dis Aquat Organ. 1991;10:217–220. [Google Scholar]
- 29.Manchanda V., Sanchaita S., Singh N.P.. Multidrug Resistant Acinetobacter. J Glob Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olesen N.J., Vendramin N. 20th Annual workshop of the National Reference Laboratories for fish diseases. National Veterinary Institute, Technical University of Denmark; Copenhagen, Denmark: 2016. Overview of the disease situation and surveillance in Europe in 2015; pp. 13–15. [Google Scholar]
- 31.Okamura B., Feist S.W.. Emerging diseases in freshwater systems. Freshwater Biol. 2011;56:627–637. [Google Scholar]
- 32.Park K.H., Kato H., Nakai T., Muroga K.. Phage typing of Lactococcus garvieae (formerly Enterococcus seriolicida a pathogen of cultured yellowtail. Fisheries Sci (Tokyo) 1998;64:62–64. [Google Scholar]
- 33.Peralta K.D., Araya T., Valenzuela S., Sossa K., Martínez M., Pena-Cortes H.. Production of phytohormones, siderophores and population fluctuation of two root-promoting rhizobacteria in Eucalyptus globulus cuttings. World J Microbiol Biotechnol. 2012;28:2003–2014. doi: 10.1007/s11274-012-1003-8. [DOI] [PubMed] [Google Scholar]
- 34.Pękala A., Kozińska A., Paździor E., Głowacka H.. Phenotypical and genotypical characterization of Shewanella putrefaciens strains isolated from diseased freshwater fish. J Fish Dis. 2015;38:283–293. doi: 10.1111/jfd.12231. [DOI] [PubMed] [Google Scholar]
- 35.Pękala A., Paździor E., Antychowicz J., Bernad A., Głowacka H., Więcek B., Niemczuk W.. Kocuria rhizophila and Micrococcus luteus as emerging opportunist pathogens in brown trout Salmo trutta Linnaeus, 1758 and rainbow trout Oncorhynchus mykiss Walbaum, 1792) Aquaculture 2018. 486:285–289. [Google Scholar]
- 36.Qin L., Zhang X., Bi K.. A new pathogen of gibel carp Carassius auratus gibelio-Shewanella putrefaciens. Wei Sheng Wu Xue Bao. 2012;52:558–565. [PubMed] [Google Scholar]
- 37.Rusev V., Rusenova N., Simeonov R., Stratev D.. Staphylococcus warneri and Shewanella putrefaciens co-infection in Siberian sturgeon Acipenser baerii and hybrid sturgeon Husohuso x Acipenser baerii. J Microbiol Exp. 2016;3:1–4. [Google Scholar]
- 38.Sturz A.V., Matheson B.G., Arsenault W., Kimpinski J., Christie B.R.. Weeds as a source of plant growth promoting rhizobacteria in agricultural soils. Can J Microbiol. 2001;47:1013–1024. doi: 10.1139/w01-110. [DOI] [PubMed] [Google Scholar]
- 39.Taghavi S., Garafola C., Monchy S., Newman L., Hoffman A., Weyens N.. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol. 2009;75:748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandepitte J., Van Damme L., Fofana Y., Desmyter J.. Edwardsiella tarda and Plesiomonas shigelloides. Their role as diarrhoeic agents and their epidemiology Edwardsiella tarda et Plesiomonas shigelloides Leur role comme agents de diarrhées et leur épidémiologie.) Bull Soc Pathol Exot. 1980;73:139–149. [PubMed] [Google Scholar]
- 41.Vladik P., Vitovec J.. Plesiomonas shigelloides in rainbow trout septicaemia. Vet Med (Praha) 1974;19:297–300. [PubMed] [Google Scholar]


