Abstract
Background
The tropical climate of the Philippines and the high population of dogs, particularly in cities, favors the life-cycle of the brown dog tick, Rhipicephalus sanguineus (sensu lato), a vector of several canine tick-borne pathogens (TBPs) including zoonotic Rickettsia spp. Suspected cases of infections are commonly encountered in veterinary clinics, but the specific TBPs are rarely identified. Furthermore, infection with Rickettsia is not being clinically examined in dogs. In this study, the occurrence of TBPs in blood and ticks collected from household and impounded dogs in highly populated areas of the Philippines, Metro Manila, and the nearby province of Laguna, was examined.
Results
A total of 248 blood samples and 157 tick samples were subjected to PCR. First, samples were screened using primers for Anaplasma/Ehrlichia spp. and Babesia/Hepatozoon spp. Those that turned positive were further subjected to species-specific PCR. Rickettsia spp. were also detected through a nested PCR. Of the 248 blood samples, 56 (22.6%) were positive for Anaplasma/Ehrlichia spp., while 19 (7.6%) were positive for Babesia/Hepatozoon spp. Species-specific PCR revealed that 61 (23.4%) had a single TBP, with Ehrlichia canis being detected in 39 (15.7%) dogs, while 14 (5.6%) dogs were positive for different combinations of two to four TBPs. Rickettsia infection was detected in 6 (2.4%) dogs. In tick samples, 8 (3.2%) were positive for Ehrlichia/Anaplasma spp., while only 1 (0.63%) was positive for Babesia/Hepatozoon spp. As in the blood samples, E. canis was the most detected, being found in 5 (2%) samples. No tick samples tested positive for Rickettsia spp.
Conclusion
Ehrlichia canis is the most common TBP affecting dogs in the Philippines. Co-infection with TBPs is quite common, hence testing for multiple TBPs is necessary. Through nested PCR, Rickettsia infection was detected in dogs, and to the authors’ knowledge, this study provides the first molecular evidence of Rickettsia infection in dogs in the Philippines.
Keywords: Canine tick-borne pathogens, Rhipicephalus sanguineus (sensu lato), Philippines, Southeast Asia
Background
Ticks are known to be capable of transmitting more pathogens to humans and animals than any other arthropod [1]. Tick-borne pathogens (TBPs) cause critical infections that are potentially fatal. The incidence of tick-borne diseases (TBDs) has been reported to have increased worldwide in recent years, seriously threatening human and animal health. As with other vector-borne diseases, the complex epidemiology of TBDs makes control difficult [2]. Knowledge of the occurrence and distribution of TBPs in human and animal populations, as well as in the tick vector in various geographical areas, is critical for their control.
The brown dog tick Rhipicephalus sanguineus is the most widely distributed tick, prevalent throughout the year in tropical and subtropical areas [3]. It is known to be a vector of several canine pathogens including rickettsiae Ehrlichia canis and Anaplasma platys and protozoans Babesia vogeli and Hepatozoon canis, as well as zoonotic Rickettsia species [4]. Rhipicephalus sanguineus is a three-host tick, dropping from its host after each blood meal and molting in the environment to the next stage. Thus, this tick can utilize a different host for every blood meal, and therefore has a higher chance of spreading pathogens it might carry to other hosts. A single tick can be a vector for several pathogens and can transmit those pathogens simultaneously in a single blood meal [5]. Thus, concurrent infection with different TBPs can occur in dogs in endemic areas, especially in dogs heavily infested with ticks.
Among the common tick-borne bacterial diseases of dogs in Southeast Asia are canine monocytic ehrlichiosis (CME) caused by E. canis and canine infectious cyclic thrombocytopenia caused by A. platys. CME causes more severe clinical signs, but the common feature of these two diseases is thrombocytopenia [6]. Meanwhile, the tick-borne protozoan parasites of dogs are Babesia and Hepatozoon species, the latter being transmitted when the tick vector is ingested by the dog rather than through the tick’s blood meal as all the other TBPs. Anemia is a common finding in uncomplicated babesiosis and H. canis infection [7, 8]. Additionally, several Rickettsia species belonging to the spotted fever group can infect dogs. These include R. rickettsii (the cause of Rocky Mountain spotted fever), R. conorii (the cause of Mediterranean spotted fever), R. parkeri, and R. massiliae; all of these are known to be zoonotic [9]. Rickettsia rickettsii causes a potentially fatal disease in dogs, while the disease due to other species are either rarely reported or unknown [9].
The tropical climate of Southeast Asian countries, which include the Philippines, the presence of stray or neglected companion animals, and the high popularity of dog ownership all contribute to favorable conditions for tick survival and reproduction, leading to enhanced transmission of TBPs [10]. The growing popularity of dog ownership in the Philippines resulted in an increased dog population particularly in big cities, including the country’s capital region, Metro Manila, and the nearby province of Laguna. In many communities, dogs are allowed to roam freely outdoors. Throughout the year, veterinarians in these areas encounter dogs showing clinical manifestations suggestive of TBDs, which include bleeding tendencies, anemia, and thrombocytopenia. CME or babesiosis is usually suspected in those cases; however, the specific etiologic agent is rarely identified due to limitations of the diagnostic tests performed. Moreover, the occurrence of Rickettsia infection in dogs and dog ticks is not examined. Thus, the Philippines still lacks a moderate amount of epidemiological data regarding the geographical occurrence of TBPs. There are several reports on detection of TBPs such as A. platys, E. canis, B. vogeli and H. canis using PCR in some parts of the country, such as Cebu [11–13], Nueva Ecija [14], and some parts of Metro Manila [15, 16], but these studies examined only a small number of pet dogs within small geographical locations. Here we investigated the occurrence of the commonly reported canine TBPs and Rickettsia spp. in household dogs presented to veterinary clinics and hospitals and in impounded dogs, as well as in R. sanguineus (sensu lato) ticks collected from those dogs, from 12 areas in Metro Manila and Laguna, Philippines.
Methods
Geographical area, study population and sample collection
This study included cities in the southern part of Metro Manila (Pasay, Taguig, Parañaque, Las Piñas and Muntinlupa) and cities or large municipalities in Laguna (San Pedro, Biñan, Santa Rosa, Calamba, Los Baños, San Pablo and Pagsanjan) located between 14°3'N and 14°32'N, and 121°0'E and 121°27'E (Fig. 1). Samples of blood and, if present, ticks were collected from pet dogs and impounded stray or abandoned dogs regardless of breed, age and sex. Samples from household dogs were obtained in cooperation with veterinary clinics and hospitals located in the selected cities and municipalities, while a samples from stray or abandoned dogs were obtained from a dog pound in Laguna. The selection criteria for the dogs were: (i) a recent history of or existing tick infestation; (ii) the presence of clinical signs suggestive of tick-borne infection; (iii) those previously suspected to have any tick-borne disease and currently undergoing treatment; and (iv) those that have apparently recovered from any tick-borne disease. A questionnaire was used to obtain pertinent information on the dogs, including the breed, sex, age, chief complaint upon clinical presentation, medical history, vitals and presenting clinical signs if any. The nature of confinement (i.e. indoor or outdoor), presence of other dogs in the household, and medications given prior to clinical presentation were also noted.
Fig. 1.
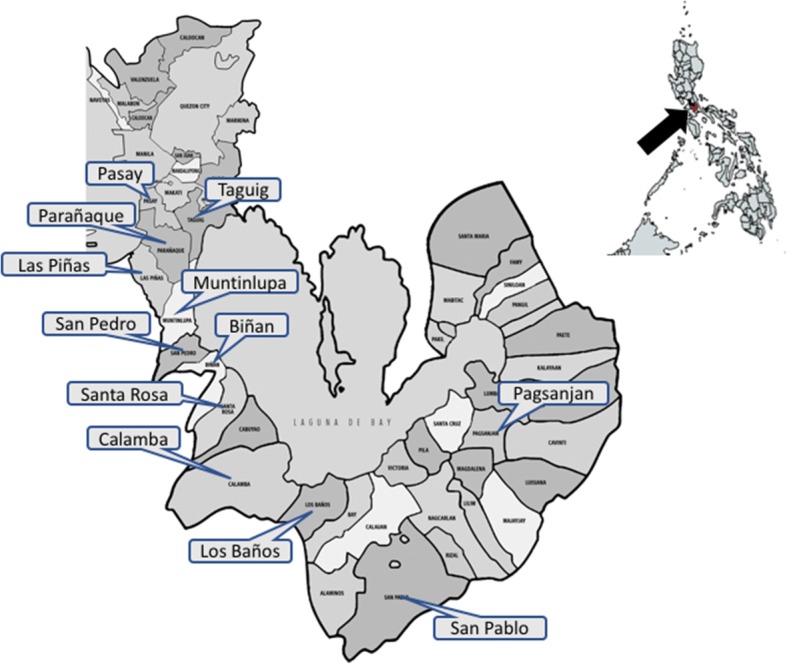
Map of the area of study in the Philippines. The arrow in the smaller map of the Philippines indicates the location of Metro Manila and Laguna. The enlarged map of Metro Manila and Laguna shows the 12 cities and municipalities (labelled) from which the samples were obtained
The veterinarians in participating veterinary clinics and hospitals agreed to collect the blood and tick samples, while veterinary students collected samples from dogs in the dog pound. At least 0.5 ml of blood was collected from each dog using a 3 ml syringe with a 23G needle and then placed in a 1.5 ml sterile EDTA coated tube. Each dog was also inspected for ticks and if present, 2–5 partially fed to fully engorged ticks were manually detached and placed in 1.5 ml tubes. All samples were kept in a freezer (-20 °C) in the clinics or hospitals until retrieval for processing in the laboratory.
Tick identification and pooling
Ticks were examined under a stereomicroscope and identified based on morphology [17, 18], then sorted according to developmental stage and sex. Pooling was done on nymphs or male ticks from the same dog while engorged female ticks were placed individually in 1.5 ml tubes. After the addition of 70% ethanol, tick samples were stored at -20 °C until DNA extraction.
DNA extraction
The extraction of DNA from blood samples was performed using a commercial DNA extraction kit (innuPREP® DNA Mini Kit, Analytik Jena, Jena, Germany) following the manufacturer’s protocol with some modifications in the initial steps. The extraction of DNA from ticks was done using the alkali neutralization method described by Takano et al. [19] with some modifications. Briefly, after removal of ethanol and washing with phosphate-buffered saline, 200 μl or 500 μl of 25 mM NaOH was added directly to pooled nymphs and male ticks or to an engorged female tick, respectively. The ticks were crushed thoroughly using sterile tube pestles, and then the tubes were placed in a boiling water bath for 10 min. After cooling, 16 μl or 40 μl of 1M Tris-HCl was added to nymph and male tick samples or to engorged female tick samples, respectively. The supernatant was recovered after centrifugation at 20,000× g for 5 min. All DNA samples were stored at 4 °C until use.
PCR detection and sequence analysis
Prior to detection of pathogens in the samples, the control genes actin and mitochondrial 16S rRNA (mt-rrs) were first detected from blood and tick DNA samples, respectively, through end-point PCR. After positive confirmation of the control genes, nested PCR targeting the groEL of Ehrlichia/Anaplasma spp., and the 18S rRNA of Babesia/Hepatozoon spp., was performed. DNA samples that showed positive bands for Ehrlichia/Anaplasma spp. or Babesia/Hepatozoon spp. were further subjected to end-point PCR using species-specific primers, while nested PCR targeting the gltA gene was also done to detect Rickettsia. All of the primers used in this study and their respective annealing temperature and expected product size are listed in Table 1. PCR mixtures (Tks Gflex™, Takara, Shiga, Japan) were prepared following the manufacturer’s recommendation. PCR products were loaded into 2% agarose gel in TAE buffer and then visualized after staining with ethidium bromide. For sequence analysis, selected positive amplicons were purified after excision from the agarose gel using Nucleospin® gel and PCR clean-up kit (Macherey-Nagel, Leicestershire, England) following the manufacturer’s protocol. Additionally, sequencing of the mt-rrs from 10 engorged tick samples was performed to further confirm the identity of the ticks. After obtaining the sequence readings, sequences were compared to reported isolates using the Basic Local Alignment Search Tool (BLAST) of the U.S. National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1.
Primers used in the detection of various tick-borne pathogens and control genes
| Organism | Target gene | Primer name | Sequence (5'-3') | Annealing T (°C) | Product size (bp) | Reference |
|---|---|---|---|---|---|---|
| Anaplasma/Ehrlichia spp. | groEL | gro607F | GAAGATGCWGTWGGWTGTACKGC | 57 | 664 | [35] |
| gro1294R | AGMGCTTCWCCTTCWACRTCYTC | |||||
| gro677F | ATTACTCAGAGTGCTTCTCARTG | 57 | 315 | |||
| gro1121R | TGCATACCRTCAGTYTTTTCAAC | |||||
| Anaplasma platys | groESL | PLA-HS475F | AAGGCGAAAGAAGCAGTCTTA | 63 | 513 | [36] |
| PLAT-HS1198R | CATAGTCTGAAGTGGAGGAC | |||||
| Ehrlichia canis | gltA | EcanisFw | TTATCTGTTTGTGTTATATAAGC | 53 | 1372 | [37] |
| EcanisRev | CAGTACCTATGCATATCAATCC | |||||
| Rickettsia spp. | gltA | CS2d | ATGACCAATGAAAATAATAAT | 52 | 1250 | [38] |
| CSEndr | CTTATACTCTCTATGTACA | |||||
| RpCs877p | GGGGGCCTGCTCACGGCGG | 52 | 341 | |||
| RpCs1258n | ATTGCAAAAAGTACAGTGAAC | |||||
| Babesia/Hepatozoon | 18S rRNA | piro18S F1 | GGTGAAACTGCGAATGGCTC | 55 | 1500 | This study |
| piro18S R1 | AAGTGATAAGGTTCACAAAACTT | |||||
| piro18S F2 | TGGCTCATTACAACAGTTATA | 53 | ||||
| piro18S R2 | CGGTCCGAATAATTCACC | |||||
| Babesia canis | 18S rRNA | BcanisF | GTTTATTAGTTTGAAACCCGC | 59 | 456 | [39] |
| BcanisR | GAACTCGAAAAAGCCAAACGA | |||||
| Hepatozoon canis | 18S rRNA | HepF | ATACATGAGCAAAATCTCAAC | 50 | 666 | [23] |
| HepR | CTTATTATTCCATGCTGCAG | |||||
| Tick control | mt-rrs | mt-rrsF | CTGCTCAATGATTTTTTAAATTGCTGTGG | 56 | 460 | [40] |
| mt-rrsR | CCGGTCTGAACTCAGATCAAGTA | |||||
| Blood control | actin | Actin-F | CGCACCACCGGCATCGTGAT | 65 | 227 | [41] |
| Actin-R | TCCAGGGCCACGTAGCAGAG |
Abbreviation: T temperature
Data analysis
Based on the information obtained from the questionnaire, dogs were then classified according to age group (puppy, juvenile or adult) and sex. The presenting clinical signs were also noted. After PCR, the detection rate for each pathogen was calculated by dividing the number of positive samples by the total number of samples and then expressed as percentage. The occurrence of TBP infection with regard to dog origin, age and sex was also calculated, and then Chi-square analysis at a 95% confidence interval (α = 0.05) was performed using the online software WinEpi® to determine the presence of association. The detection of multiple pathogens (co-infection) in blood samples was also noted.
Results
Data on sample collection
A total of 248 canine blood samples were obtained from 12 locations in southern Metro Manila and Laguna, 198 of which came from a total of 33 participating veterinary clinics and hospitals, while 50 came from a dog pound in Los Baños, Laguna. Although not all dogs had complete information in the provided questionnaires, the available history was still analyzed for grouping according to age and sex. The majority of the sampled dogs were adults (61.3%) and male (56.9%). Not all of the dogs were showing clinical signs at the time of sample collection, but the most common clinical signs indicated in the questionnaire were inappetence, lethargy, fever, pale mucus membranes, bloody diarrhea, epistaxis, hematuria, jaundice and vomiting. Only 90 dogs had ticks at the time of blood collection. A total of 157 tick samples were prepared for DNA extraction after identification and sorting. All ticks were morphologically identified to be R. sanguineus (sensu lato), 112 (71.3%) of which were females, 27 (17.2%) were males, and 18 (11.5%) were nymphs.
PCR results
The control genes actin and mt-rrs were successfully detected in all blood and tick DNA samples, respectively. Following nested PCR using screening primers, the DNA of bacterial pathogens Ehrlichia/Anaplasma spp. was detected in 56 (22.6%) blood samples, while DNA of protozoan pathogens Babesia/Hepatozoon spp. was detected in 19 (7.6%) blood samples. The result of species-specific PCR of blood samples that turned positive for screening primers is summarized in Table 2. Ehrlichia canis was the most commonly detected TBP (49 dogs, 22.6%), while Rickettsia and H. canis were the least detected (6 dogs, 2.4%). Analysis of TBP occurrence showed that 61 (24.6%) dogs were positive for a single pathogen, of which the majority were infected with E. canis (39 dogs, 15.7%), followed by B. vogeli (4%), A. platys (2.4%), Rickettsia spp. (1.6%) and Hepatozoon spp. (1.2%) (Table 3). Multiple pathogens were detected in 14 dogs, which indicate co-infection. Two TBPs of different combinations were detected in 10 dogs (4%), half of which were positive for both E. canis and A. platys. Interestingly, three dogs (1.2%) were positive for two different combinations of three TBPs, while one (0.4%) was positive for four TBPs.
Table 2.
Number of positive canine blood and tick samples for tick-borne pathogens (TBPs) that were detected using PCR
| TBPs | Blood samples (n = 248) (%)a | Tick samples (n = 157) (%)a |
|---|---|---|
| Ehrlichia canis | 49 (19.8) | 5 (3.2) |
| Anaplasma platys | 15 (6.0) | 1 (0.6) |
| Rickettsia spp. | 6 (2.4) | 0 |
| Babesia vogeli | 17 (6.8) | 1 (0.6) |
| Hepatozoon canis | 6 (2.4) | 1 (0.6) |
aPercentages were calculated based on the total number of tested blood and tick samples
Table 3.
Analysis of occurrence of tick-borne pathogens (TBPs) in dogs based on PCR results
| TBPs detected | No. of dogs (%)a |
|---|---|
| 1 TBP only | 61 (24.6) |
| Ehrlichia canis | 39 (15.7) |
| Anaplasma platys | 6 (2.4) |
| Rickettsia spp. | 4 (1.6) |
| Babesia vogeli | 10 (4.0) |
| Hepatozoon canis | 2 (1.2) |
| 2 TBPs | 10 (4.0) |
| E. canis and A. platys | 5 (2.0) |
| E. canis and Rickettsia sp. | 1 (0.4) |
| A. platys and Rickettsia sp. | 1 (0.4) |
| A. platys and B. vogeli | 1 (0.4) |
| B. vogeli and H. canis | 2 (0.8) |
| 3 TBPs | 3 (1.2) |
| E. canis, A. platys and B. vogeli | 2 (0.8) |
| E. canis, B. vogeli and H. canis | 1 (0.4) |
| 4 TBPs | 1 (0.4) |
| E. canis, A. platys, B. vogeli and H. canis | 1 (0.4) |
| Total number of dogs with TBPs | 75 (30.2) |
aPercentages were calculated based on the total number of tested dogs (n = 248)
With regard to the 157 tick samples, 8 (3.2%) and 1 (0.64%) were positive for Ehrlichia/Anaplasma spp. and Babesia/Hepatozoon spp., respectively (Table 2). Species-specific PCR showed that E. canis was the most detected (5 or 2%) in tick samples. One tick sample was positive for Ehrlichia, Babesia and Hepatozoon. All TBP-positive tick samples were engorged females. Rickettsia was not detected in any of the tick samples.
Occurrence of TBPs with regards to some host attributes
The presence of TBPs in household and impounded dogs in different age groups (puppy, juvenile and adult) and of different sexes was compared (Table 4). A significantly higher number of household dogs presented to veterinary clinics were infected with at least one TBP (37.9%; χ2 = 12.163, df = 1, P < 0.001), whereas only 10% of dogs from the dog pound tested positive for any TBP. Chi-square analysis showed a positive association of TBP occurrence with age (χ2 = 11.082, df = 2, P = 0.004). The detection was highest in puppies, with 18 of 37 (48.6%) puppies testing positive for at least one TBP. Interestingly, five puppies had concurrent infection with two TBPs, and four were infected with Rickettsia sp. The next highest positive detection for at least one TBP was in juveniles (37.3%), and lowest was in adults (26.3%). With regard to sex, more females (37.5%) were found infected with at least one TBP than males (25%), also having a positive association according to Chi-square analysis (χ2 = 4.473, df = 1, P = 0.034).
Table 4.
Occurrence of TBPs with regard to host attributes. Chi-square analysis determined the presence of association, and P-values are shown
| Attribute | No. of dogs | No. (%) infected with at least one TBP | P-value |
|---|---|---|---|
| Origin | <0.001* | ||
| Dog pound | 50 | 5 (10.0) | |
| Household | 198 | 70 (28.2) | |
| Age | 0.004* | ||
| Puppy (< 1 year) | 37 | 18 (48.6) | |
| Juvenile (1–3 years) | 59 | 22 (37.3) | |
| Adult (> 3 years) | 152 | 35 (23.0) | |
| Sex | 0.034* | ||
| Male | 144 | 36 (25.0) | |
| Female | 104 | 39 (37.5) | |
*P < 0.05
Sequence analysis
BLAST analysis of mt-rrs from 10 engorged ticks showed that all share 97–100% identity with reported mt-rrs sequences of R. sanguineus (GenBank: MF351574.1, KC170744.1, AY883868.1). For each genus of TBP detected, at least three positive amplicons were subjected to sequencing and BLAST analysis. All obtained sequences for each pathogen were 100% homologous and were found to share 96–100% identity with reported E. canis (GenBank: CP025749.1, KU765198.1, AY647155.1), A. platys (GenBank: KY425417.1, AY077621.1, KU765205.1), B. vogeli (GenBank: LC331058.1, KU361222.1, KU361220.1) and H. canis (GenBank: KC138532.2, AF176835.1, KX818220.1) isolates. For Rickettsia, all positive amplicons share 99% sequence identity with the reported isolates of Rickettsia spp. such as R. japonica (GenBank: DQ909073.1) and R. raoultii (GenBank: KR265323.1). Representative sequences were deposited in GenBank under the accession numbers LC428206 (E. canis), LC428207 (A. platys), LC428209 (B. vogeli), LC428208 (H. canis) and LC428132 (Rickettsia sp.).
Discussion
The TBPs of dogs investigated in this study have recently become a major focus worldwide because of their significance in canine health and potential zoonotic transmission. Dogs have been in close contact with humans for a long time; thus, humans are at risk particularly for zoonotic TBPs. Furthermore, there is the threat of TBPs becoming established in new geographical areas due to the pet trade, which is made easier through the advent of social media and other online platforms, and increased international mobility [14]. TBPs can cause higher rates of morbidity and fatality in non-endemic areas. In this regard, it is the responsibility of veterinarians to monitor TBPs for effective disease surveillance and to execute strategies that will help eliminate or control the spread of these pathogens. In this study, PCR, which is proven to be highly sensitive, was used in detecting TBPs believed to be common not only in the Philippines but also in other Southeast Asian countries.
Only a few studies have been done on the occurrence of TBPs in dogs in the Philippines. Some of them utilized conventional microscopic examination of blood smears [16, 20]. While this technique is simple and inexpensive, it has been proven to have low sensitivity, especially in finding E. canis and A. platys [6]. Some serological studies on TBPs have been also conducted [21, 22], and serologically based commercial kits are widely used in veterinary clinics nowadays; however, among the limitations of detecting antibodies is the inability to differentiate past from on-going infection due to persistence of the antibodies.
In the last decade, studies on the molecular detection of TBPs in dogs through PCR have been increasing. However, this generated little epidemiological data in the Philippines because surveys were limited to small areas of the country. Furthermore, previous studies mostly included pet dogs that showed clinical signs and presented in a few veterinary clinics or hospitals. Our current study covered several big cities in Metro Manila, the capital region of the Philippines, as well as cities and municipalities in the nearby province of Laguna, and included both apparently healthy and sick dogs. Additionally, aside from pet dogs brought to veterinary clinics and hospitals, impounded stray or abandoned dogs were included in the study. Because stray dogs are neglected, they play a significant role in maintenance of different TBPs [10, 23–25].
Our results showed that E. canis is the most detected pathogen in blood and tick samples. The detection rate of E. canis in blood samples obtained in this study is higher than that in previous reports in the Philippines that used PCR, which ranged between 2–10% [11, 14, 15], but is similar to that in the reported detection rate in other Southeast Asian countries [23, 26]. The difference between our results and those of previous studies in the Philippines could be attributed to the difference in the number of dogs and the selection criteria, the geographical area, and the targeted gene. A previous study that utilized a commercial antibody test kit reported 95% seropositivity for E. canis in blood of dogs from Metro Manila [21]. Meanwhile, the detection rates for A. platys, B. vogeli and H. canis are close to those in previous PCR-based studies in the country [11, 14, 15].
Another significant finding in this study is the detection of Rickettsia sp. in some dogs. Rickettsia sp. was detected singly in four dogs and in concurrent infection with species of Anaplasma or Ehrlichia in two other dogs, all of which are household pets presented to veterinary clinics/hospitals. Unfortunately, the clinical signs were not indicated in the information for those dogs. There are only a few reports on Rickettsia infection in dogs in Southeast Asia [26, 27], including the Philippines; hence, very little is known about the occurrence of this zoonotic TBP in the country. Seropositivity to R. prowazekii, R. rickettsii, and R. canadensis has been reported in dogs from Thailand, but none were found positive for any Rickettsia spp. using PCR [27]. Recently, R. felis was detected in 11 of 101 dogs from Cambodia using nested PCR [26]. There were only two published reports in the Philippines on the detection of antibodies against Rickettsia spp. in animals and humans [28, 29]. Camer et al. [28] reported detection of antibodies against R. japonica in dogs from Luzon Island of the Philippines. The sequence analysis of Rickettsia sp.-positive amplicons in this study suggests that dogs are infected with R. japonica. To our knowledge, this is the first molecular evidence for the presence of Rickettsia in the Philippines.
All Rickettsia species known to infect dogs are zoonotic [9]; hence our findings should raise an alarm because of the risk to humans. Infection with E. canis in humans has been also reported, in which E. canis DNA was detected through PCR from human patients who showed clinical manifestations of human monocytic ehrlichiosis [30]. Many pet owners in the Philippines keep their dogs indoors, and some are in close contact, even sleeping with their dogs. The risk to humans is augmented by the possibility that R. japonica infection in dogs may go unnoticed, as shown by a previous study wherein dogs experimentally infected with R. japonica showed non-specific clinical signs, such as fever, anorexia and depression, if immunosuppressed, but not if immunocompetent [31]. Further studies on Rickettsia infection in dogs, involving geographical distribution, clinical features, transmission and risk factors, should be performed to promote control of the potential threat to public health.
Concurrent infection with two or more TBPs was fairly common in tested dogs, observed in 22 (8.9%) individuals. The presence of co-infection may result in greater pathogenicity and more complications. Thus, the clinical signs exhibited by the affected dog may be variable [5]. Unfortunately, the incomplete questionnaire returned to us by the participating veterinary clinics/hospitals limited our analysis. Nevertheless, our results emphasize the importance of testing for more than one TBP, especially because the line of treatment for rickettsial and protozoan pathogens is different.
Analysis of some host attributes showed that household dogs, puppies and females have a higher tendency to become infected with TBPs. The majority of dogs included in this study were household pets with existing or a history of tick infestation, which may explain the higher detection rate as compared to dogs from the dog pound, among which only a few had tick infestation at the time of sample collection. Additionally, the brown dog tick R. sanguineus is nidicolous and endophilic, preferring to live indoors in close contact with its host [3]. Thus, a household dog staying in one place with a high population of ticks has a higher chance of getting infested repeatedly than does a stray dog that moves from place to place. Nevertheless, stray dogs can be vessels for ticks and spread them from one place to another, thereby playing a role in the spread of TBPs. The higher tendency of puppies to become infected with TBPs could be due to their greater susceptibility to tick infestation and heavier tick burden than older dogs [32]. Our results showed that five puppies were co-infected with two TBPs, which may produce more severe clinical manifestations [33]. The higher detection of TBPs in female dogs could be due to their sedentary habit [34]. Female dogs staying for a long time in areas with high tick population have higher chances of being infested repeatedly with ticks, hence also higher risk of getting infected with TBPs.
Conclusions
This study showed that E. canis is highly endemic in the Philippines and concurrent infection with other TBPs is quite common. To the authors’ knowledge, this study also provides the first molecular evidence of Rickettsia infection in dogs in the Philippines. Taken together, these results emphasize the need to test for multiple TBPs in dogs suspected of infection to facilitate appropriate treatment and to raise awareness of the threat to public health. Further investigation is needed to examine the epidemiology and clinical features of rickettsiosis in dogs in the Philippines.
Acknowledgments
The authors are grateful to the veterinarians and staff of participating veterinary clinics and hospital and to the volunteers of the Los Baños municipal dog pound. The authors also thank Fernando P. Micosa for preparing the map.
Funding
This study is supported by the University of the Philippines Balik PhD grant and the Japan Society for Promotion of Science (15H05264). The funding bodies have no role in the design and conduct of the study.
Availability of data and materials
The data supporting the conclusions in this study are included in the article.
Abbreviations
- BLAST
Basic Local Alignment Search Tool
- CME
Canine monocytic ehrlichiosis
- TBD
Tick-borne disease
- TBP
Tick-borne pathogen.
Authors’ contributions
RLG, BPD, MA, TM and TT designed the study. RLG, AALM, SLDD and KBC coordinated sample collection. RLG, AALM, SLDD, IPMA, KACS and KBC performed experiments. RLG, BPD, MA, TM and TT analyzed the data. RLG wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Collection procedures in dogs were approved by the Institutional Animal Care and Use Committee of the College of Veterinary Medicine, University of the Philippines Los Baños. Consent of the dog owners was sought, with their signature in the informed consent form obtained prior to the collection of samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Remil L. Galay, Email: rlgalay@up.edu.ph
Anna Angelica L. Manalo, Email: annangelica.manalo2012@gmail.com
Sidney Lyndon D. Dolores, Email: sddolores@up.edu.ph
Irene Pearl M. Aguilar, Email: imaguilar@up.edu.ph
Kristina Andrea C. Sandalo, Email: kacsandalo@gmail.com
Kathlyn B. Cruz, Email: kbcruz1102@gmail.com
Billy P. Divina, Email: bpdivina@up.edu.ph
Masako Andoh, Email: masako@vet.kagoshima-u.ac.jp.
Tatsunori Masatani, Email: masatani@vet.kagoshima-u.ac.jp.
Tetsuya Tanaka, Email: k6199431@kadai.jp.
References
- 1.Baneth G. Tick-borne infections of animals and humans: a common ground. Int J Parasitol. 2014;44:591–596. doi: 10.1016/j.ijpara.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi: 10.1186/1756-3305-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, Solano-Gallego L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. 2015;8:75. doi: 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matijatko V, Torti M, Schetters TP. Canine babesiosis in Europe: how many diseases? Trends Parasitol. 2012;28:99–105. doi: 10.1016/j.pt.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Baneth G. Perspectives on canine and feline hepatozoonosis. Vet Parasitol. 2011;181:3–11. doi: 10.1016/j.vetpar.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Chomel B. Tick-borne infections in dogs-an emerging infectious threat. Vet Parasitol. 2011;179:294–301. doi: 10.1016/j.vetpar.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Irwin PJ, Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004;20:27–34. doi: 10.1016/j.pt.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Ybañez RHD, Ybañez AP, Arnado LLA, Belarmino LMP, Malingin KGF, Cabilete PBC, et al. Detection of Ehrlichia, Anaplasma, and Babesia spp. in dogs of Cebu, Philippines. Vet World. 2018;11:14–19. doi: 10.14202/vetworld.2018.14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ybañez AP, Ybañez RHD, Yokoyama N, Inokuma H. Multiple infections of Anaplasma platys variants in Philippine dogs. Vet World. 2016;9:1456–1460. doi: 10.14202/vetworld.2016.1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ybañez AP, Ybañez RHD, Talle MG, Liu M, Moumouni PFA, Xuan X. First report on Babesia vogeli infection in dogs in the Philippines. Parasitol Int. 2017;66:813–815. doi: 10.1016/j.parint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Corales J, Viloria V, Venturina V, Mingala C. The prevalence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs in Nueva Ecija, Philippines based on multiplex polymerase chain reaction (mPCR) assay. Ann Parasitol. 2014;60:267–272. [PubMed] [Google Scholar]
- 15.Adao D, Herrera C, Galarion L, Bolo N, Carlos RS, Carlos ET, et al. Detection and molecular characterization of Hepatozoon canis, Babesia vogeli, Ehrlichia canis, and Anaplasma platys in dogs from Metro Manila, Philippines. Korean J Vet Res. 2017;57:79–88. [Google Scholar]
- 16.Baticados AM, Baticados WN, Villarba LA, Carlos ET, Carlos S, Fajardo PV. PCR assay and microscopy for examination of mixed Ehrlichia canis and Babesia spp. infection in Bomb Sniffing dogs and other canines in the National Capital Region, Philippines. Eurasian J Vet Sci. 2011;27:111–6.
- 17.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–85. [DOI] [PubMed]
- 18.Barker S, Walker A. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa. 2014;3816:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Takano A, Toyomane K, Konnai S, Ohashi K, Nakao M, Ito T, et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One. 2014;9:e104532. doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baticados A, Baticados W, Carlos E, Villarba L, Subiaga S, Magcalas J. Parasitological detection and molecular evidence of Hepatozoon canis from canines in Manila, Philippines. Vet Med Res Rep. 2011;1:7–10. doi: 10.2147/VMRR.S16529. [DOI] [Google Scholar]
- 21.Baticados A, Baticados W. Serological evidence for Ehrlichia canis exposure in military dogs and other canines in Metropolitan Manila, Philippines. Israel J Vet Med. 2011;66:151–156. [Google Scholar]
- 22.Ybañez AP, Ybañez RHD, Villavelez RR, Malingin HPF, Barrameda DNM, Naquila SV, Olimpos SMB. Retrospective analyses of dogs found serologically positive for Ehrlichia canis in Cebu, Philippines from 2003 to 2014. Vet World. 2016;9:43–47. doi: 10.14202/vetworld.2016.43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piratae S, Pimpjong K, Vaisusuk K, Chatan W. Molecular detection of Ehrlichia canis, Hepatozoon canis and Babesia canis vogeli in stray dogs in Mahasarakham province, Thailand. Ann Parasitol. 2015;61:183–187. doi: 10.17420/ap6103.05. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Ruttayaporn N, Saechan V, Jirapattharasate C, Vudriko P, Moumouni PFA, et al. Molecular survey of canine vector-borne diseases in stray dogs in Thailand. Parasitol Int. 2016;65:357–361. doi: 10.1016/j.parint.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Jittapalapong S, Rungphisutthipongse O, Maruyama S, Schaefer JJ, Stich RW. Detection of Hepatozoon canis in stray dogs and cats in Bangkok, Thailand. Ann N Y Acad Sci. 2006;1081:479–488. doi: 10.1196/annals.1373.071. [DOI] [PubMed] [Google Scholar]
- 26.Inpankaew T, Hii SF, Chimnoi W, Traub RJ. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasit Vectors. 2016;9:253. doi: 10.1186/s13071-016-1552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suksawat J, Xuejie Y, Hancock SI, Hegarty BC, Nilkumhang P, Breitschwerdt EB. Serologic and molecular evidence of coinfection with multiple vector-borne pathogens in dogs from Thailand. J Vet Intern Med. 2001;15:453–462. doi: 10.1111/j.1939-1676.2001.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 28.Camer GA, Masangkay J, Satoh H, Okabayashi T, Norizuki S, Motoi Y, et al. Prevalence of spotted fever rickettsial antibodies in dogs and rodents in the Philippines. Jpn J Infect Dis. 2000;4:162–163. [PubMed] [Google Scholar]
- 29.Camer GA, Alejandria M. Amor M, Satoh H, Muramatsu Y, Ueno H, Morita C. Detection of antibodies against spotted fever group Rickettsia (SFGR), typhus group Rickettsia (TGR), and Coxiella burnetii in human febrile patients in the Philippines. Jpn J Infect Dis. 2003;56:26–28. [PubMed] [Google Scholar]
- 30.Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 31.Inokuma H, Matsuda H, Sakamoto L, Tagawa M, Matsumoto K. Evaluation of Rickettsia japonica pathogenesis and reservoir potential in dogs by experimental inoculation and epidemiologic survey. Clin Vaccine Immunol. 2011;18:161–166. doi: 10.1128/CVI.00369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray J, Dantas-Torres F, Estrada-Peña A, Levin M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171–180. doi: 10.1016/j.ttbdis.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Gal A, Harrus S, Arcoh I, Lavy E, Aizenberg I, Mekuzas-Yisaschar Y, Baneth G. Coinfection with multiple tick-borne and intestinal parasites in a 6-week-old dog. Can Vet J. 2007;48:619–622. [PMC free article] [PubMed] [Google Scholar]
- 34.Konto M, Biu AA, Ahmed MI, Charles S. Prevalence and seasonal abundance of ticks on dogs and the role of Rhipicephalus sanguineus in transmitting Babesia species in Maidugiri, north-eastern Nigeria. Vet World. 2014;7:119–124. doi: 10.14202/vetworld.2014.119-124. [DOI] [Google Scholar]
- 35.Andoh M, Sakata A, Takano A, Kawabata H, Fujita H, Une Y, et al. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PLoS One. 2015;10:e0133700. doi: 10.1371/journal.pone.0133700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inokuma H, Fujii K, Okuda M, Onishi T, Beaufils JP, Raoult D, Brouqui P. Determination of the nucleotide sequences of heat shock operon groESL and the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys for phylogenetic and diagnostic studies. Clin Diagn Lab Immunol. 2002;9:1132–1136. doi: 10.1128/CDLI.9.5.1132-1136.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inokuma H, Brouqui P, Drancourt M, Raoult D. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J Clin Microbiol. 2001;39:3031–3039. doi: 10.1128/JCM.39.9.3031-3039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mediannikov OY, Sidelnikov Y, Ivanov L, Mokretsova E, Fournier P-E, Tarasevich I, Raoult D. Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerging Infect Dis. 2004;10:810–817. doi: 10.3201/eid1005.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inokuma H, Yoshizaki Y, Matsumoto K, Okuda M, Onishi T, Nakagome K, et al. Molecular survey of Babesia infection in dogs in Okinawa, Japan. Vet Parasitol. 2004;121:341–346. doi: 10.1016/j.vetpar.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Ushijima Y, Oliver JH, Keirans JE, Tsurumi M, Kawabata H, Watanabe H, Fukunaga M. Mitochondrial sequence variation in Carlos capensis (Neumann), a parasite of seabirds, collected on Torishima Island in Japan. J Parasitol. 2003;89:196–198. doi: 10.1645/0022-3395(2003)089[0196:MSVICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Belotindos LP, Lazaro JV, Villanueva MA, Mingala CN. Molecular detection and characterization of Theileria species in the Philippines. Acta Parasitol. 2014;59:448–453. doi: 10.2478/s11686-014-0256-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions in this study are included in the article.


